Abstract
Aims
Human laboratory studies have contributed extensively in the research and development of novel medications to treat alcohol use disorder (AUD). Alcohol tolerance may represent one additional variable that can be utilized to expand the understanding of the AUD wide phenotypic profile and provide support to the medication development process. Tolerance is characterized as an individual’s subjective response to alcohol and has been recognized as a predictor of AUD progression. Tolerance can be evaluated both by self-reported response (e.g. assessments) and objective measurements (e.g. motor impairment); as such, it represents an exploitable variable in the field of alcohol research.
Methods
This Narrative Review focuses on the use of alcohol tolerance, specifically within alcohol laboratory studies, for medication development. It seeks to identify a research gap and a research opportunity in clinical studies to evaluate biobehavioral responses captured in order to develop medications to treat AUD.
Results
Alcohol tolerance may provide additional information on the safety and tolerability of medications to treat AUD, in particular, when novel medications are co-administered with alcohol within the AUD population.
Conclusions
As such, alcohol tolerance represents an additional outcome that may be included in randomized clinical trial (RCT) protocols designed for developing AUD pharmacotherapies.
INTRODUCTION ON ALCOHOL TOLERACE AS A BIOBEHAVIORAL MEASURE IN HUMAN LABORATORY STUDIES
Human laboratory studies have provided an opportunity to utilize sophisticated paradigms from preclinical models that may elucidate not only neurobiological pathways in the development of alcohol use disorder (AUD), but may help to explain the mechanism of action of pharmacophores that can be utilized to treat AUD. Because subjective response to alcohol is a strong predictor of AUD, medication development has focused on drugs that can either mitigate the pleasant (e.g. naltrexone) (King et al., 1997), intensify the unpleasant (e.g. varenicline) (Childs et al., 2012) or amplify (e.g. baclofen) (Farokhnia et al., 2018) the effects of alcohol. One additional variable that can be utilized to expand the understanding of the diverse AUD phenotypic profile, modulate subjective response and contribute to the medication development process is alcohol tolerance.
Acute alcohol tolerance is measured as a decrease in self-response to alcohol, regardless of changes in blood alcohol concentration (Martin and Moss, 1993). As for many addictive substances (opioids, stimulants, etc.), alcohol tolerance develops when individuals require higher doses of alcohol to achieve its original and pleasurable effect. As such, tolerance is recognized as one of the most established theories of alcohol adaptation (Tabakoff and Hoffman, 1988).
In the field of alcohol administration research, tolerance is a key component of alcohol preference and subjective response (Waller et al., 1983; Gatto et al., 1987). Alcohol tolerance is measured using subjective intoxication ratings and motor coordination effects (Beirness and Vogel-Sprott, 1984; Fillmore et al., 2005), both critical pharmacodynamics parameters that can be evaluated within a laboratory setting. Interestingly, to ensure the safety and tolerability of medications used to treat AUD, biobehavioral responses should be evaluated when co-administered with alcohol. As recommended by the FDA (FDA, 2015), these outcomes need to be measured in randomized clinical trials (RCTs) and are critical for developing AUD pharmacotherapies.
ACUTE, RAPID, CHRONIC AND METABOLIC ALCOHOL TOLERANCE
Alcohol tolerance has been defined in several ways; for review, see (Kalant, 1998). Acute tolerance refers to tolerance developed during an alcohol administration protocol (either by drinking, vapor, or intravenous administration). There are several validated measures to assess acute tolerance in preclinical models including discriminative effects (Hiltunen and Jarbe, 1990), operant behavior (Hiltunen and Jarbe, 1992), motor impairment (Tullis et al., 1977) and hypnosis (Darbra et al., 2002). Human laboratory studies have also developed paradigms to assess acute alcohol tolerance by measuring behavioral control (Fillmore et al., 2005) and motor coordination in binge drinkers (Fillmore and Weafer, 2012).
Rapid tolerance is described as the process that occurs after a second alcohol exposure followed by complete clearance of the first dose (with the second dose occurring within 8–24 hours after the first) (Khanna et al., 1996). Rapid alcohol tolerance has been evaluated using fly (Geer et al., 1988) and rodent models which demonstrated similar mechanisms to chronic tolerance, the critical component for the development of AUD (Khanna et al., 1996); for review, see (Haass-Koffler et al., 2019).
Chronic tolerance is achieved by numerous and abundant alcohol exposures and leads to central nervous system (CNS) adaptations. Chronic tolerance however, in addition to CNS adaptation, produces metabolic adaptation, which is characterized by an increase in alcohol metabolism and rate of blood ethanol clearance (Cederbaum, 2012). As such, in addition to functional or pharmacodynamics tolerance, metabolic tolerance produces changes in alcohol pharmacokinetics due to changes in catabolism or drug distribution. Given the complexity of testing different forms of alcohol tolerance, interdisciplinary approaches with pharmacodynamics assessments and pharmacokinetic measures are critical to engendering paradigms for the development of medications to treat AUD (FDA, 2015).
CLINICAL PERSPECTIVE OF ALCOHOL TOLERANCE IN THE DSM-5 AND ICD-10 CLASSIFICATION
Tolerance, along with legal problems, was considered for removal within the latest version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (APA, 2013). Tolerance, however, represents a unique feature that does not overlap with other criteria in the DSM-5 (Hasin et al., 2013). As such, it was kept in order to retain diagnostic accuracy and expand medical treatment for ‘diagnostic orphans’ or patients that would not be included in the binary diagnosis (dependence and abuse) of the DSM-IV (Haass-Koffler and Kenna, 2013; Edwards et al., 2013).
While tolerance is not included in the diagnosis code system within the International Classification of Diseases (ICD), it is used to describe the features of alcoholism. According to the ICD-10 (Tenth Revision, Clinical Modification), as well as in the previous ICD versions, alcoholism is a disease characterized by four main features: craving, loss of control, physical dependence and tolerance (the need to drink greater amounts of alcohol in order to feel the same effect).
Tolerance is a well-recognized risk factor for developing AUD (APA, 2013). For example, the development of acute tolerance is affected by a family history of alcoholism (FHA) (Wetherill et al., 2012) and individual drinking history (King et al., 2014). Individuals whose family possesses a FHA reported significantly lower self-ratings of subjective alcohol intoxication than individuals without a FHA (Schuckit, 1984). Individuals with a FHA present an accentuated response during the rising blood alcohol curve (i.e. acute sensitization) and an attenuated response during the falling blood alcohol curve (i.e. acute tolerance) (Newlin and Thomson, 1991) (Fig. 1). Thus, individuals who have a FHA tend to be more sensitive and less tolerant to the pleasant and rewarding effects and less sensitive and more tolerant to the unpleasant effects of alcohol. Similar have also demonstrated that there is a marked acute tolerance towards the impairing effects of alcohol on motor coordination in binge drinkers (at-risk drinkers) compared to nonbinge drinkers (nonrisk drinkers) (Fillmore and Weafer, 2012).
Fig. 1.
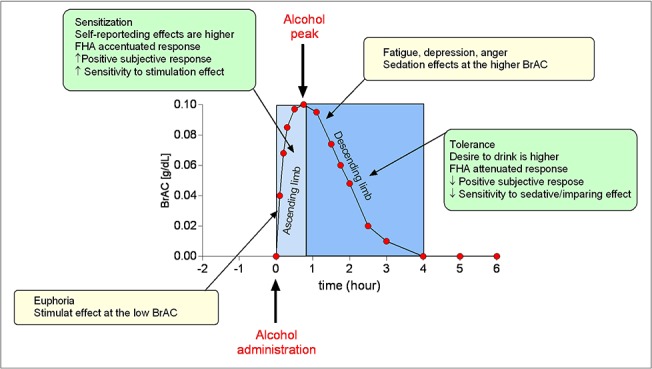
Alcohol-related biobehavioral and mood response within the biphasic effect of alcohol. Alcohol-related biobehavioral response (green boxes): ascending limb (greater positive subjective response and increased sensitivity to stimulating effects due to alcohol); descending limb (lower negative subjective response and decreased sensitivity to sedative or impairing effects due to alcohol). Mood-related response (yellow boxes): euphoria with stimulation at the low BAC level; fatigue, depression, anger with sedation at the higher BAC level. Hypothetical BAC curve after one standard drink designed to reach to 0.1 g/dl, with peak reached between 30 and 90 min after alcohol administration.
The proposed changes of the DSM-5 highlighted many problems and illustrated that further studies are warranted to address issues for which less data are available. Evaluating the process of the development of tolerance may not only represent an important tool to formulate the diagnosis of AUD, but may also be useful to develop and deliver individualized pharmacotherapies for specific subtypes of individuals with AUD.
MEASURES OF ALCOHOL TOLERANCE IN HUMAN LABORATORY STUDIES
The Biphasic Alcohol Effects Scale (BAES) was formulated in order to better assess the pharmacological and behavioral effects of alcohol (Martin et al., 1993b) in an acute alcohol administration paradigm. The BAES is a unipolar 14-item scale that rates alcohol’s stimulant and sedative effects (Martin et al., 1993b). The scale assesses subjective experiences of alcohol stimulation (e.g. elated, energized, excited, stimulated and vigorous) and sedation (e.g. difficulty concentrating, down, heavy head, sedated, slow thoughts and sluggish) and can be paired to more objective responses from alcohol-induced motor function (Brumback et al., 2007). The BAES was developed as a predictive tool to evaluate drinking behaviors in heavy and binge drinkers over time (Martin et al., 1993a; King et al., 2014). The reduced impairment at a given blood alcohol concentration on the ascending versus the descending limb of the curve suggests that the reduction might be due to some adaptive process occurring during physiological exposure to the drug over time (Fillmore et al., 2005).
The BAES is based on the Mellanby effect. This reaction refers to a behavioral impairment at a given blood alcohol concentration level in which the blood alcohol level is greater when rising than when it is falling (Mellanby, 1919). The Mellanby measure is the most widely used acute tolerance measure in human research (Holland and Ferner, 2017). This procedure in a human laboratory study allows researchers to control for breath alcohol concentration (BrAC) while assessing alcohol’s effects over time (Haass-Koffler et al., 2017). However, it is potentially confounded by differences in the change in direction of BrACs on the two limbs of the blood alcohol curve (Martin and Moss, 1993).
Several methods measuring acute tolerance that can be integrated with the BAES are available. In all cases, there is an attempt to control for changes in blood alcohol concentrations that occur physiologically in a time-dependent manner. The BAES is widely used to assess the pharmacodynamics effect of alcohol when it is co-administrated with a medication; for example, see: (King et al., 1997; Covault et al., 2014; Haass-Koffler et al., 2015, 2017).
Acute tolerance can also be measured through a steady-state blood alcohol concentration method. The clamp of intravenous alcohol infusion has been designed to avoid fluctuation of blood alcohol concentration, and to study acute tolerance compared to impaired behavior within a standardized function of time, (O'connor et al., 1998). Through this measure, subjects are kept at a constant blood alcohol concentration value and changes in the effect of alcohol are measured over time.
Other measurements used to assess changes in blood alcohol concentration throughout time include the Area Under the Curve (AUC) and the slope function. The AUC measure uses computed intoxication and blood alcohol concentrations as integrated measures over time, computed separately for each limb of the blood alcohol curve. These ratios describe the amount of intoxication that occurs per unit of alcohol concentration (Haass-Koffler et al., 2017). If acute tolerance occurs, this ratio should be greater on the ascending compared to the descending limb of the blood alcohol curve. This method offers a significant advantage from the Mellanby measure, which uses less data than the AUC measure and requires matched ascending/descending limb data points.
The slope function proposes that acute tolerance increases in a linear trend over time during exposure to alcohol and that this effect can be measured using an output function that relates blood alcohol concentrations and intoxication. Acute tolerance is captured using the rate of the increase of this function (Martin and Moss, 1993). The shape of the slope function measured during ascending blood alcohol concentrations may differ across subjects; however, this result is representative with regard to the shape of the slope function during the descending limb only (Martin and Moss, 1993). Values are lower when there is a greater alcohol effect relative to blood alcohol concentration and are higher when there is less alcohol effect relative to blood alcohol concentration. If acute tolerance occurs, values from the output function will increase over time (i.e. the slope will be positive). In addition, sharp slopes represent greater rates of acute tolerance. Each variable discussed above requires that blood alcohol concentrations and alcohol response measures are taken simultaneously at multiple time points across the blood alcohol curve.
Acute sensitization (increase in effect over time relative to blood alcohol concentrations) may occur during early ascending blood alcohol concentrations. The Mellanby method and AUC measure assess both acute sensitization and acute tolerance, rather than solely acute tolerance; a diagram of a hypothetical BrAC AUC is depicted in Fig. 1. Given the potential importance of both acute sensitization, tolerance and limb effects in determining drinking behaviors, as well as the lack of association of the slope function measure with the other measures, researchers should use both the AUC and the slope function measure when examining individual differences in acute tolerance (Martin and Moss, 1993), particularly when alcohol and a study medication are co-administered in a laboratory setting.
Additional measures that may lead to acute tolerance, such as subjective intoxication and motor performance, should be considered (Martin and Moss, 1993). For example, changes in the pleasurable and negative effects of drinking (measured by the BAES) can be paired to changes in cognitive performance (as measured by the digit symbol substitution test, DSST) (Wechsler, 2012) and in reaction time (as measured by a continuous performance test) (Conners et al., 2003). The DSST is impaired by low doses of alcohol. Within a human laboratory study evaluating an AUD medication, DSST can be used as a sensitive measure to determine whether or not a medication enhances alcohol impairment (Brumback et al., 2007). The CPT is a computer delivered neuropsychological test that measures sustained attention and vigilance, requiring speed and accuracy on a sustained reaction time task (Conners et al., 2003). Similar to outcomes of the DSST, the CPT is impaired by low doses of alcohol and can be used as a sensitive measure to estimate whether or not a medication enhances alcohol impairment (Swift et al., 1994). Additional useful assessments that can be integrated in the BAES scale include: the cued go/no-go reaction time (Mellanby, 1919; Holland and Ferner, 2017), subjective intoxication rate (Fillmore et al., 2005) and or session preference (i.e. session in which they felt less/more drunk and session in which they liked more) (Haass-Koffler et al., 2015).
INFLUENCING FACTORS FOR MEASURES OF ALCOHOL TOLERANCE IN HUMAN LABORATORY STUDIES
A correct interpretation of acute tolerance within the laboratory should take into account several influencing factors. Environmental stimuli could influence tolerance. Sex represents an additional factor which should not be ignored due to the fact that differences in the development of tolerance are observed between the sexes. Each factor contributes to the rewarding effects of alcohol in terms of individual subjective responses to alcohol behaviors (i.e. feeling more pleasant than unpleasant effects), which in turn can influence alcohol-related behaviors. Therefore, it is crucial that these factors are considered in the research and development of AUD medications.
Environment in the laboratory
The intensity of the impairing effects of alcohol on behavior has an environmental basis (Vogel-Sprott, 1992). Interestingly, environment markedly affects tolerance and represents an important factor for alcohol consumption in the laboratory. Learning among drug tolerance, originating from environmental cues, has been examined from the perspective of either operant or classical conditioning; furthermore, tolerance has been found to be influenced by both learning procedures (Beirness and Vogel-Sprott, 1984). Alcohol administration should occur within a laboratory setting that reflects the natural environment in which individuals predominantly consume alcohol, as tolerance is heavily influenced by environmental consequences of drug-compensatory performance (Vogel-Sprott and Sdao-Jarvie, 1989). To date, many RCTs that test medications for treating AUD have been performed in bar-laboratory settings among individuals with AUD who are currently seeking treatment (Kenna et al., 2016) and among those who are nontreatment seeking (Haass-Koffler et al., 2018).
Sex differences
In preclinical research, both male and female rats portrayed marked sensitivity to the anticonvulsant effects of alcohol administration. Female rats, however, exhibited tolerance to alcohol-induced motor impairment after one day of withdrawal, while male rats developed tolerance after 3 days (Koirala et al., 2008). Ovariectomized female rats exhibited the greatest anticonvulsant response to acute alcohol administration (Koirala et al., 2008). Such findings suggest that different responses to acute alcohol administration, regardless of hormonal status, are determined by multiple neuroadaptations.
Exploration of human studies suggests that most of the inconsistency in sensitivity and behavior when assessing acute tolerance is influenced more so by genetic variability (age, height, weight and drinking history) rather than sex (Wilson and Plomin, 1985). Furthermore, sex differences were not observed when assessing performance of the cued go/no-go task model (Fillmore et al., 2005).
The reported difference in alcohol-related response (decline of cognitive function, memory, attention and reaction time) can be simplified by examining pharmacokinetic profiles (such as bioavailability and rate of elimination). However, some conflicting results can arise when assessing the effect of the menstrual cycle on pharmacokinetics parameters. One study in particular reported that elevated estrogen levels appear to increase hepatic alcohol dehydrogenase activity, whereas lower estrogen levels tend to decrease hepatic alcohol dehydrogenase activity; for review, see: (Mumenthaler et al., 1999).
A significant interaction between the menstrual cycle phase and tolerance level was found; during the ovulation phase of the menstrual cycle, high tolerant women were significantly less accurate than low tolerant women in estimating alcohol’s effects (Hay et al., 1984). Also, a trend approaching significance suggested that with an increasing blood alcohol level, women in the oral contraceptive group were more accurate in self-reporting intoxication (Hay et al., 1984). Although more studies are needed to explore sex difference in alcohol tolerance, it appears that hormonal fluctuations and the role of sex hormones could influence alcohol metabolism in women and consequently, sensitivity and tolerance to alcohol.
Subjective intoxicating response
Subjective response to alcohol reflects individual differences in sensitivity to the pharmacological effects of alcohol. Prior to investigations on acute tolerance, studies on subjective response to alcohol have been conducted to detect predictors of AUD risk (Schuckit and Smith, 1996).
The low level response model (LLR) proposed that subjects who are less responsive to the sedative effect of alcohol present a greater risk of developing an AUD (Schuckit, 1994). The LLR model was recognized as a robust predictive factor to develop alcohol dependence beyond other well-known variables such as social environment, alcohol expectancies, age of drinking onset, typical alcohol use and body mass index (Trim et al., 2009). However, when examining tolerance as a predictor of excessive drinking, the LLR model does not explain the increase in an individual’s motivation to take higher doses of alcohol (Ray et al., 2016). Furthermore, studies on the LLR focused mostly on the effects on the descending limb of the blood alcohol concentration, accounting only for the sedative (i.e. negative) effects of alcohol (Schuckit et al., 2005).
The differentiator model was developed based upon evidence that the effects of alcohol are biphasic (Newlin and Thomson, 1990) (Fig. 1). Subjective response has been characterized by ‘factor-items’ as measured by self-report assessments, one factor includes stimulation and other pleasant effects; the second factor includes sedation and unpleasant effects; the third one consists of alleviation of tension and negative mood (Ray et al., 2016) in conjunction with craving as a fourth component (Bujarski et al., 2015). Craving is associated with subjective response and has the potential to make individuals less sensitive to the effects of tolerance than the magnitude of subjective responses themselves (Bujarski and Ray, 2014; Bujarski et al., 2015).
Self-reporting effects are perceived higher on the ascending limb, while desire to drink tends to take precedence on the descending limb, within consistent estimates of intoxication level. The differential effect that alcohol has on the ascending and descending limbs has also been observed using measures of mood. During the ascending limb, most subjects describe themselves as euphoric; conversely, they feel opposite effects during the descending limb (i.e. they feel tired, depressed or angry) (Ekman et al., 1964; Babor et al., 1983; Lukas et al., 1986). Generally, stimulant effects of alcohol occur at a relatively low blood alcohol concentration on the ascending limb (Tabakoff and Hoffman, 1988). Sedative effects usually occur at higher blood alcohol concentrations on the descending limb and result in negative correlations with drinking practices (Goldberg, 1943; O'Malley and Maisto, 1984). Tolerance to alcohol sedation is involved through mechanisms of heavy alcohol intake (Fig. 1) (Tabakoff and Hoffman, 1988).
Validated self-report scales have emerged as primary predictors of alcohol risk (Heath and Martin, 1991; Trim et al., 2009). The Subjective High Assessment Scale (SHAS), as a measure of subjective response (Judd et al., 1977), aims to identify subjective responses as risk factors for the development of AUD (Schuckit, 1994). Subjective response has been identified as the single strongest predictor of AUD (Schuckit et al., 1988). However, not all the items are clearly related to stimulation or sedation (Martin et al., 1993b). Nevertheless, the scale seems to be critically sensitive to the sedative/unpleasant effects of alcohol (Ray et al., 2009; Bujarski et al., 2015). Subjects who reported lower levels of subjective intoxication with less behavioral impairment were more likely to underestimate their blood alcohol concentrations than subjects who reported higher levels of subjective intoxication and greater behavioral impairment (Martin et al., 1991). In addition, accuracy in self-reporting was more precise on the ascending limb, compared to the peak blood alcohol concentration and the descending limb. It appears that cues depicting the effects of alcohol rapidly became unavailable on the descending limb, which may contribute to decisions concerning further alcohol consumption and driving after drinking (Martin et al., 1991).
FUTURE DIRECTIONS AND FINAL REMARKS
Subjective responses to alcohol have been identified as potential biobehavioral targets for the treatment of AUD; however, alcohol tolerance has not extensively evaluated within the context of a human laboratory for medication development. Several models exist that aim to accurately measure alcohol tolerance on a variety of levels (behavioral and environmental) and in varied forms (self-reporting and biobehavioral). However, it is crucial to use existing measures on alcohol tolerance and to develop new paradigms that are able to integrate multiple approaches to identify research gaps and research opportunities in order to continue developing medications to treat AUD.
One significant gap that was illustrated though this Narrative Review is the lack of research on sex differences among alcohol tolerance. While it appears common knowledge that women become more impaired than men after drinking similar quantities of alcohol, there is not a systematic research approach that can evaluate sex differences in alcohol tolerance neuroadaptation. Historically, this lack of knowledge was justified by the fact that only 2% of women are heavy drinkers, compared with 9% of men (Abuse and Administration, 1998); however, risks of developing AUD within females are on the rise (White et al., 2015; Slade et al., 2016).
Alcohol tolerance, when utilizing this measure as a subjective response to the effects of alcohol, does not account for discrepancies between cognitive or behavioral impairment and sedation. For example, in many individuals under stress or with an underlying anxiety disorder, these negative symptoms can be recognized as positive responses (e.g. relaxing and calming). This observation suggests that future research is needed to evaluate alcohol tolerance under stress conditions.
Medications approved by the FDA (naltrexone, acamprosate and disulfiram) and medications repurposed to treat AUD (e.g. topiramate, varenicline, baclofen, and gabapentin) have been inquired in relation to their actions towards targeting and modifying subjective response (Ray et al., 2010). Methods used to assess alcohol tolerance within the AUD population have the potential to measure the efficacy of these pharmacological treatments (Ray et al., 2016).
While more work must be done in order to systematically evaluate parameters of intoxication and impairment due to the amount of alcohol intake, acute alcohol tolerance represents an important variable in the development of novel pharmacotherapies for AUD, and should be included as an additional outcome in RCTs. As recommended by the FDA (FDA, 2015), and exemplified in previous research, biobehavioral responses need to be evaluated when medications are co-administered with alcohol in order to develop the most precise pharmacotherapies to target the diverse AUD spectrum.
Funding
Dr. Haass-Koffler was supported by the National Institute on Alcohol Abuse and Alcoholism (K01AA023867; R01AA027760) and in part by the National Institute of General Medical Sciences (NIGMS), Center of Biomedical Research Excellence (COBRE, P20 GM130414). Dr. Perciballi was supported by Sapienza, University of Rome, Italy. We thank Z. Brown for her contribution to the editing of the manuscript.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Substance Abuse and Mental Health Services Administration (1998) Preliminary Results from the 1997 National Household Survey on Drug Abuse. Rockville, MD: National Clearinghouse for Alcohol and Drug Information. [Google Scholar]
- APA (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Association, Washington, DC. [Google Scholar]
- Babor TF, Berglas S, Mendelson JH et al. (1983) Alcohol, affect, and the disinhibition of verbal behavior. Psychopharmacology (Berl) 80:53–60. [DOI] [PubMed] [Google Scholar]
- Beirness D, Vogel-Sprott M (1984) Alcohol tolerance in social drinkers: operant and classical conditioning effects. Psychopharmacology (Berl) 84:393–7. [DOI] [PubMed] [Google Scholar]
- Brumback T, Cao D, King A (2007) Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend 91:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Roche DJ et al. (2015) Factor structure of subjective responses to alcohol in light and heavy drinkers. Alcohol Clin Exp Res 39:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA (2014) Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob's allostatic model in humans. Drug Alcohol Depend 140:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI. (2012) Alcohol metabolism. Clin Liver Dis 16:667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC et al. (2012) Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res 36:906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, Angold A et al. (2003) Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol 31:555–62. [DOI] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R et al. (2014) Dutasteride reduces alcohol's sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl) 231:3609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbra S, Prat G, Pallares M et al. (2002) Tolerance and sensitization to the hypnotic effects of alcohol induced by chronic voluntary alcohol intake in rats. J Psychopharmacol 16:79–83. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Gillespie NA, Aggen SH et al. (2013) Assessment of a modified DSM-5 diagnosis of alcohol use disorder in a genetically informative population. Alcohol Clin Exp Res 37:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman G, Frankenhaeuser M, Goldberg L et al. (1964) Subjective and objective effects of alcohol as functions of dosage and time. Psychopharmacologia 6:399–409. [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Deschaine SL, Sadighi A et al. (2018) A deeper insight into how GABA-B receptor agonism via baclofen may affect alcohol seeking and consumption: lessons learned from a human laboratory investigation. Mol Psychiatry. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FADA (2015) Alcoholism: Developing Drugs for Treatment. Center for Drug Evaluation and Research (CDER). Rockville, MD. [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM (2005) Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol 66:663–72. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J (2012) Acute tolerance to alcohol in at-risk binge drinkers. Psychol Addict Behav 26:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Murphy J, Waller M et al. (1987) Chronic ethanol tolerance through free-choice drinking in the P line of alcohol-preferring rats. Pharmacol Biochem Behav. 28:111–5. [DOI] [PubMed] [Google Scholar]
- Geer BW, Mckechnie SW, Bentley MM et al. (1988) Induction of alcohol dehydrogenase by ethanol in Drosophila melanogaster. J Nutr 118:398–407. [DOI] [PubMed] [Google Scholar]
- Goldberg L. (1943) Quantitative Studies on Alcohol Tolerance in Man. The Influence of Ethyl Alcohol on Sensory, Motor and Psychological Functions Referred to Blood Alcohol in Normal and Habituated Individuals. Acta Physiologica Scandinavica, Vol. 5. [Google Scholar]
- Haass-Koffler CL, Akhlaghi F, Swift RM et al. (2017) Altering ethanol pharmacokinetics to treat alcohol use disorder: can you teach an old dog new tricks? J Psychopharmacol 31:812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Cannella N, Ciccocioppo R (2019) Translational dynamics of alcohol tolerance between preclinical models and human laboratory studies. Exp Clin Psychopharmacol , under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Goodyear K, Zywiak WH et al. (2018) Comparing and combining Topiramate and Aripiprazole on alcohol-related outcomes in a human laboratory study. Alcohol Alcohol 53:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Kenna GA (2013) Bacchus by Caravaggio as the visual diagnosis of alcohol use disorder from the fifth edition of the diagnostic and statistical manual of mental disorders (DSM-5). Front Psychiatry 4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Davidson D et al. (2015) Effects of idazoxan on alcohol pharmacokinetics and intoxication: a preliminary human laboratory study. Alcohol Clin Exp Res 39:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M et al. (2013) DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170:834–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay WM, Nathan PE, Heermans HW et al. (1984) Menstrual cycle, tolerance and blood alcohol level discrimination ability. Addict Behav 9:67–77. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG (1991) Intoxication after an acute dose of alcohol: an assessment of its association with alcohol consumption patterns by using twin data. Alcohol Clin Exp Res 15:122–8. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU (1990) Acute tolerance to ethanol using drug discrimination and open-field procedures in rats. Psychopharmacology (Berl) 102:207–12. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU (1992) Acute and chronic ethanol tolerance: operant behaviour in naive and ethanol tolerant rats. Psychopharmacology (Berl) 107:511–6. [DOI] [PubMed] [Google Scholar]
- Holland MG, Ferner RE (2017) A systematic review of the evidence for acute tolerance to alcohol—the Mellanby effect. Clin Toxicol (Phila) 55:545–56. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard RB, Huey LY et al. (1977) Lithium carbonate and ethanol induced "highs" in normal subjects. Arch Gen Psychiatry 34:463–7. [DOI] [PubMed] [Google Scholar]
- Kalant H. (1998) Research on tolerance: what can we learn from history? Alcohol Clin Exp Res 22:67–76. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH et al. (2016) Role of the alpha1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol 21:904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna JM, Chau A, Shah G (1996) Characterization of the phenomenon of rapid tolerance to ethanol. Alcohol 13:621–8. [DOI] [PubMed] [Google Scholar]
- King AC, Mcnamara PJ, Hasin DS et al. (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry 75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A et al. (1997) Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 129:15–22. [DOI] [PubMed] [Google Scholar]
- Koirala B, Alele PE, Devaud LL (2008) Influence of hormonal status on behavioral responses to an acute ethanol challenge during ethanol withdrawal in male and female rats. Pharmacol Biochem Behav 90:691–700. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Benedikt RA (1986) Instrumental analysis of ethanol-induced intoxication in human males. Psychopharmacology (Berl) 89:8–13. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE et al. (1993a) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–6. [DOI] [PubMed] [Google Scholar]
- Martin CS, Moss HB (1993) Measurement of acute tolerance to alcohol in human subjects. Alcohol Clin Exp Res 17:211–6. [DOI] [PubMed] [Google Scholar]
- Martin CS, Rose RJ, Obremski KM (1991) Estimation of blood alcohol concentrations in young male drinkers. Alcohol Clin Exp Res 15:494–9. [DOI] [PubMed] [Google Scholar]
- Mellanby E. (1919) Alcohol: Its Absorption into and Disappearance from the Blood Under Different Conditions. The University Press, Oxford, UK. [Google Scholar]
- Mumenthaler MS, Taylor JL, O'HARA R et al. (1999) Gender differences in moderate drinking effects. Alcohol Res Health 23:55–64. [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB (1990) Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108:383–402. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB (1991) Chronic tolerance and sensitization to alcohol in sons of alcoholics. Alcohol Clin Exp Res 15:399–405. [DOI] [PubMed] [Google Scholar]
- O'CONNOR S, Morzorati S, Christian J et al. (1998) Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. 22:202–10. [PubMed] [Google Scholar]
- O'Malley SS, Maisto SA (1984) Factors affecting the perception of intoxication: dose, tolerance, and setting. Addict Behav 9:111–20. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Roche DJ (2016) Subjective response to alcohol as a research domain criterion. Alcohol Clin Exp Res 40:6–17. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M (2010) Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des 16:2149–58. [DOI] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Leventhal A et al. (2009) Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res 33:2154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151:184–9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Risch SC, Gold EO (1988) Alcohol consumption, ACTH level, and family history of alcoholism. Am J Psychiatry 145:1391–5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL (1996) An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 53:202–10. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Beltran I et al. (2005) Performance of a self-report measure of the level of response to alcohol in 12- to 13-year-old adolescents. J Stud Alcohol 66:452–8. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. (1984) Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry, 41:879–84. [DOI] [PubMed] [Google Scholar]
- Slade T, Chapman C, Swift W et al. (2016) Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: Systematic review and metaregression. BMJ Open 6:e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O et al. (1994) Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry 151:1463–7. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL (1988) Tolerance and the etiology of alcoholism: hypothesis and mechanism. Alcohol Clin Exp Res 12:184–6. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL (2009) The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res 33:1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis KV, Sargent WQ, Simpson JR et al. (1977) An animal model for the measurement of acute tolerance to ethanol. Life Sci 20:875–82. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. (1992) Alcohol Tolerance and Social Drinking: Learning the Consequences. Guilford Press. [Google Scholar]
- Vogel-Sprott M, Sdao-Jarvie K (1989) Learning alcohol tolerance: the contribution of response expectancies. Psychopharmacology (Berl) 98:289–96. [DOI] [PubMed] [Google Scholar]
- Waller M, Mcbride W, Lumeng L et al. (1983) Initial sensitivity and acute tolerance to ethanol in the P and NP lines of rats. 19:683–6. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2012) Wechsler Preschool and Primary Scale of Intelligence, 4th edn. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wetherill L, Morzorati SL, Foroud T et al. (2012) Subjective perceptions associated with the ascending and descending slopes of breath alcohol exposure vary with recent drinking history. Alcohol Clin Exp Res 36:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Castle IJ, Chen CM et al. (2015) Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcohol Clin Exp Res 39:1712–26. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Plomin R (1985) Individual differences in sensitivity and tolerance to alcohol. Soc Biol 32:162–84.Wilson JR & Plomin R. 1985. Individual differences in sensitivity and tolerance to alcohol. Soc Biol, 32, 162–84. [DOI] [PubMed] [Google Scholar]


