Abstract
A 42-year-old Hispanic female underwent intravitreal autologous adipose-tissue derived stem cell injection to her left eye in the Dominican Republic for treatment of retinitis pigmentosa associated with Usher Syndrome. Prior to intravitreal injection, the patient's best-corrected-visual-acuity (BCVA) was 1/200. The patient experienced decreased vision gradually over a 3-month period. The patient presented with no light perception (NLP) vision with a total funnel retinal detachment, as well as hyphema, iris neovascularization, and nearly 360 posterior synechiae of the iris to the lens capsule. The patient suffered from ocular pain with an intraocular pressure (IOP) of 37 mm Hg. Transcleral cyclophotocoagulation was performed. The IOP was 6 mm Hg six weeks after treatment and the patient was pain free.
Keywords: Stem cells, Autologous stem cell transplantation, Retinitis pigmentosa, Neovascular glaucoma, US stem cell clinics, Intravitreal injection of stem cells, Usher syndrome
1. Introduction
The hype in stem cell treatment for numerous medical disorders has caused a surge in “stem cell” clinics in the United States and internationally. Those clinics offer unproven and unregulated “stem cell” therapies without the oversight of regulatory agencies, such as the U.S. Food and Drug Administration (FDA). Although there are ongoing FDA-regulated clinical trials studying the use of stem cell technology for ocular diseases, no FDA-approved stem cell treatments exist for any ocular conditions. Here, we report a case of neovascular glaucoma (NVG) following intravitreal injection of autologous adipose tissue-derived “stem cell” performed in the Dominican Republic by a U.S. licensed family medicine physician for the treatment of retinitis pigmentosa.
1.1. Case report
A 42-year-old Nicaraguan female was diagnosed at the Bascom Palmer Eye Institute (BPEI) with retinitis pigmentosa. Her best-corrected visual acuity was 20/70 OD and 1/200 OS. Five months later the patient elected to undergo autologous adipose tissue-derived stem cell injection (AASCI) in her left eye in the Dominican Republic by a family medicine doctor licensed in Florida. Five months after AASCI, she presented to the emergency service at BPEI for evaluation of eye pain and progressive vision loss over 3 months in the left eye. Per the patient, she received an intravitreal injection of stem cells in the left eye. Cells were prepared from adipose tissue aspirate (liposuction) obtained on the same day of injection. Details of preparation and quality assurance are proprietary and were not made available to the patient. The patient has a past medical history of Usher syndrome. The family history is remarkable for retinitis pigmentosa in a brother and glaucoma in a maternal aunt. Visual acuity was 20/200 PH 20/70 OD and NLP OS. Intraocular pressure (IOP) was 12 OD mm Hg and 31 OS mm Hg. Pupils were equally reactive with no afferent pupillary defect detected. Ophthalmic exam was significant for pallor of the optic nerve, attenuated retinal vessels, loss of foveal reflex, and peripheral pigmentary retinal degeneration in the right eye. In the left eye, the patient exhibited clear cornea, but with trace injection of the conjunctiva, 3+ inflammatory or red blood cells in the anterior chamber, approximately 360° posterior synechie sparing the 4 o'clock meridian, and 1+ vitreous cells. No view to the posterior pole was possible. B-scan revealed mild vitreous opacities and a funnel shaped, total retinal detachment (Fig. 1). Prednisolone acetate every 1 hour and dorzolamide-timolol and brimonidine twice per day was initiated in the left eye.
Fig. 1.
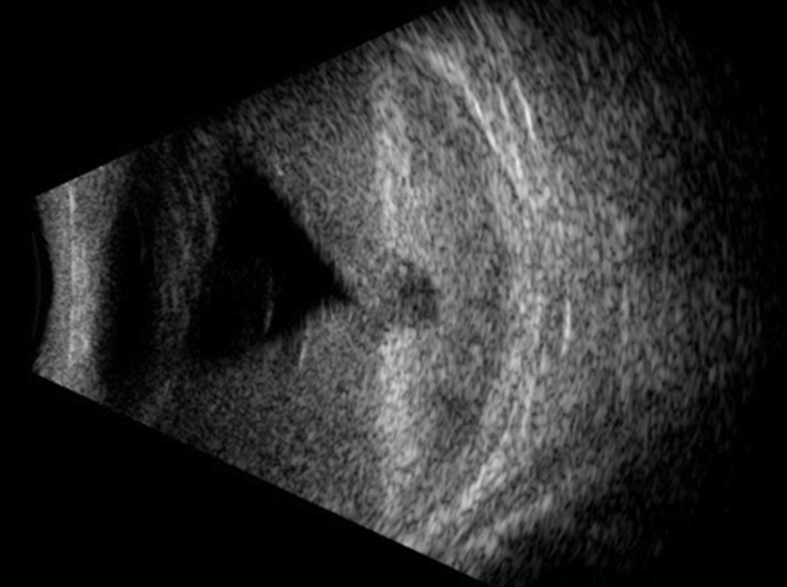
Vitreous opacities and funnel shaped, total retinal detachment of the left eye.
The patient returned to clinic the following day with similar vision. Her IOP OS had decreased to 15 mm Hg. Three weeks later, the patient had follow-up in retina clinic with an IOP of 37 mm Hg in the left eye. The patient has not been using her dorzolamide-timolol. However, the exam was now remarkable for red blood cells in the anterior chamber as well as 360-degree of iris neovascularization. Examination of the posterior pole disclosed vitreous hemorrhage. The patient was advised to continue aqueous suppressant glaucoma eyedrop medications. The patient returned to clinic 3 weeks subsequently with eye pain and an IOP of 34 mm Hg.
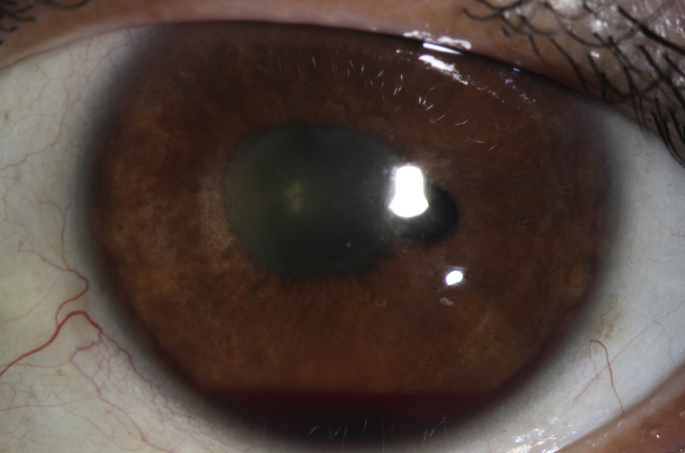
The patient was referred to glaucoma service for evaluation of neovascular glaucoma in the left eye. Glaucoma examination was remarkable for 1.5 mm layered hyphema in addition to the previous exam findings (Fig. 2). The patient continued to have pain on glaucoma medications. Gonioscopy was deferred secondary to hyphema and pain. Since the eye had NLP vision, the options of enucleation versus transcleral cyclophotocoaguation (TS-CPC) were discussed. The patient refused enucleation and elected to undergo CPC. The patient received intravitreal injection of bevacizumab (0.5 mL of 1.25mg dose) and started on atropine twice per day and prednisolone acetate 4 times per day in the left eye.
Fig. 2.
Left eye with hyphema, posterior synechiae sparing 4 o'clock.
The patient underwent TS-CPC in the left eye two weeks later. The slow burn CPC settings were used at 1250 mW, 4 seconds with 20 applications applied over 360°. On post-operative day one, the IOP was 6 mm Hg. Patient was asked to withhold IOP lowering medications and initiated on prednisolone acetate every 1 hour while awake and atropine twice per day. On post-op week 1, patient denied eye pain and was comfortable. Her IOP was 4 mm Hg. Six weeks after CPC, the patient continued to be pain free with an IOP of 6 mm Hg and a deep-formed anterior chamber. Iris neovascularization, and the hyphema had regressed. Gonioscopy revealed 360° of peripheral anterior synechiae. At 4-month post-operative follow-up, patient continued to have neovascularization of the iris but intraocular pressure was maintained at 2. Prednisolone acetate was tapered and atropine was stopped. Referral was made to low vision service to assist her activities of daily living.
2. Discussion
Autologous adipose tissue-derived stem cell injection has been increasing in frequency and is a growing trend in the United States due to a surge in the number of for-profit “stem cell” clinics.1 In a phase one/two clinic trial, stem cell transplantation of retinal pigment epithelial cells led to improvement of vision and vision-related quality of life measures for Stargardt's disease and age-related macular degeneration.2 However, no stem cell therapy has been approved by the FDA for any ocular conditions at this time. Despite FDA statements on the need for oversight and warnings to consumers, multiple “stem cell” clinics in the United States and around the world continue to offer unproven treatments.3
Retinal detachment following sub-retinal injection of autologous bone marrow-derived stem cells was first reported by Leung et al., in 2016 on a patient with Stargardt's macular dystrophy.4 Boudreault et al. reported a case of peribulbar stem cell injection in a patient with retinitis pigmentosa that resulted in central retinal artery occlusion and subsequently led to severe vision loss.5 In 2017, Kuriyan et al. reported a case series of six eyes in three patients who were treated at a “stem cell” clinic in Florida for macular degeneration. These patients developed profound vision loss ranging from 20/200 to no light perception after treatment. Five out of 6 injected eyes developed a combined tractional/rhegmatogenous retinal detachment with proliferative vitreoretinopathy in a delayed manner ranging from 3 days to 38 days. One eye resulted in retinal atrophy.6 Saraf et al. reported similar complications in a patient who underwent bilateral intravitreal injection of stem cell in Georgia for macular degeneration.7 Rong et al. reported similar complications in a patient who underwent intravitreal autologous adipose-tissue derived stem cell injection in his right eye for retinitis pigmentosa.8
To our knowledge this is the first reported case of a patient developing glaucoma after intravitreal injection of autologous adipose tissue-derived stem cell injection for Usher syndrome associated retinitis pigmentosa. Diagnosis of uveitis-glaucoma-hyphema (UGH) syndrome was also considered. At initial presentation it was not clear whether the patient had inflammatory or red blood cells in the anterior chamber. On subsequent examination, the acute pathology appears to have been hemorrhage from neovascularization in the setting of a chronic retina detachment. UGH syndrome arises from repetitive mechanical iris trauma by a malpositioned or subluxed intraocular lens (IOL).10 Patient was phakic and ultrasonagraphy did not suggest such a lens malposition.
Neovascular glaucoma is frequently observed in patients with ischemic diseases such as diabetic retinopathy, central retinal vein occlusion, central retinal artery, sickle-cell retinopathy, and retinopathy of prematurity. One can postulate in this case that the injection of “stem cells” may have caused a retinal artery or vein occlusion, which subsequently resulted in neovascularization secondary to ischemia. Cells and flare at initial presentation is likely a result of inflammatory response to the adipose stem cell injection. Chronic inflammation is also another stimulus for neovascularization.
Another postulated mechanism is that “stem cells” may settle on the retinal surface and differentiate improperly into myofibroblast-like cells which may increase oxygen demands, inducing ischemic microvascular changes which subsequently leads to neovascularization, in addition to tractional retinal detachment. As for the mechanism behind delayed tractional detachment, Kuriyan et al. proposed “stem cells” themselves form tractional scaffolds rather than the injection technique causing retinal detachment, especially since retinal detachment was often delayed and time is needed for cellular proliferation. “Stem cells” that may improperly differentiate into myofibroblast-like cells may produce contractile membranes similar to proliferative vitreoretinopathy.5
3. Conclusions
The prevalence of “stem cell” clinics is a rising trend in the United States and around the world.9 Although there have been advances in treatment of macular degeneration and Stargardt's macular dystrophy using human embryonic stem cell-derived retinal pigment epithelium, the poor outcome and the “stem cell” clinic direct-to-consumer advertisement for therapies without FDA outsight is concerning. Improved patient education on evidence-based treatments as well as the danger of undergoing unproven therapy such as “stem cell” injection is important to decrease the incidence of complications from stem cell transplantation.
Patient consent
The patient consent for research is included in the Bascom Palmer Eye Institute general consent as well as surgery consent.
Declaration of competing interest
The authors declared that they have no conflicts of interest.
Acknowledgments and disclosures
The Bascom Palmer Eye Institute is supported by NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant. R.K. Lee is supported by the Walter G. Ross Foundation. T.A. Albini is supported by the Klorfine Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2020.100647.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Turner L. US stem cell clinics, patient safety, and the FDA. Trends Mol Med. 2015;21(5):271–273. doi: 10.1016/j.molmed.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz S.D., Regillo C.D., Lam B.L. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase ½ studies. Lancet. 2015;385(9967):509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of health and human services food and Drug administration (FDA) 2017. https://www.fda.gov/forconsumers/consumerupdates/ucm286155.htm Nov [cited 2018, May 29]. Available from:
- 4.Leung E.H., Flynn H.W., Jr., Albini T.A. Retinal detachment after subretinal stem cell transplantation. Ophthalmic Surg Lasers Imag Retina. 2016;47(6):600–601. doi: 10.3928/23258160-20160601-16. [DOI] [PubMed] [Google Scholar]
- 5.Boudreault K., Justus S., Lee W., Mahajan V.B., Tsang S.H. JAMA Ophthalmol. 2016;134(6):711–712. doi: 10.1001/jamaophthalmol.2016.0803. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyan A.E., Albini T.A., Towsend J.H. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376(11):1047–1053. doi: 10.1056/NEJMoa1609583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraf S.S., Cunningham M.A., Kuriyan Bilateral retinal detahcments after intravitreal injection of adipose-derived ‘stem cells’ in a patient with exudative macular degeneration. Ophthalmic Surg Lasers Imag Retina. 2017;48(9):772–775. doi: 10.3928/23258160-20170829-16. [DOI] [PubMed] [Google Scholar]
- 8.Rong A.J., Lam B.L., Ansari Z.A. Vision loss secondary to autologous adipose stem cell injections: a rising problem. JAMA Ophthalmol. 2018;138(1):97–99. doi: 10.1001/jamaophthalmol.2017.5453. [DOI] [PubMed] [Google Scholar]
- 9.Berger I., Ahmed A., Bansal A. Global distribution of businesses marketing stem cell-based interventions. Cell Stem Cell. 2016;19(2):158–162. doi: 10.1016/j.stem.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Zemba M., Camburu G. Uveitis-glaucoma-hyphaema syndrome. General review. Rom J Ophthalmol. 2017;61(1):11–17. doi: 10.22336/rjo.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.