Abstract
Purpose
To describe the diagnosis and management of a patient with primary open angle glaucoma (POAG) who developed suprachoroidal hemorrhage (SCH) after micropulse cyclophotocoagulation (MPCPC) therapy.
Observations
A 77 year-old Caucasian man with end-stage POAG and multiple medical comorbidities including coronary artery disease on anticoagulation presented with 2 days of episodic, severe left eye pain 2 weeks after undergoing MPCPC diode in the left eye. His visual acuity was count fingers at 2 feet and his intraocular pressure (IOP) was 44. He had a shallow anterior chamber that was open to trabecular meshwork on gonioscopy. His dilated fundus exam showed large, dome-shaped hemorrhagic choroidals, which were confirmed on ultrasound. He was medically managed with analgesics, cycloplegics, topical steroids and IOP lowering medications. He was closely followed with serial b-scans and the SCH decreased in size without surgical intervention, however, his visual acuity did not improve from presentation.
Conclusions and importance
MPCPC diode has been increasingly used in refractory glaucoma and is considered to be a relatively safe procedure. Suprachoroidal hemorrhage has not yet been reported after MPCPC diode. This case demonstrates how devastating complications such as SCH can still occur with lower energy CPC therapy especially in the setting of post-procedural hypotony, and emphasizes the importance of prevention especially in high-risk patients.
Keywords: Glaucoma, Suprachoroidal hemorrhage, Micropulse cyclophotocoagulation diode
1. Introduction
Suprachoroidal hemorrhage is a rare but devastating complication of intraocular surgery that may occur intra-operatively1,2 or during the post-operative period.3,4 Delayed suprachoroidal hemorrhage (DSCH) has been reported to occur after various glaucoma filtering procedures, mainly trabeculectomy and tube shunt procedures, with an incidence rate of 0.7–6.0%.4, 5, 6, 7, 8 DSCH is extremely rare after transcleral cyclophotocoagulation (CPC), with only a few case reports in the literature.9,10
More recently, micropulse cyclophotocoagulation (MPCPC) diode has gained popularity for its efficacy and safety. Unlike transcleral CPC, which delivers energy continuously, MPCPC delivers shorter, repetitive pulses of energy with rest periods resulting in lower risk of post-operative complications such as hypotony, anterior chamber (AC) inflammation, scleral thinning, and phthisis bulbi.11 Suprachoroidal hemorrhage has not previously been reported after MPCPC diode. We describe the first reported case of DSCH after MPCPC diode therapy.
2. Case report
A 77-year-old Caucasian man with a history of end-stage primary open angle glaucoma (POAG) presented to the emergency department with 2 days of episodic severe left eye pain 2 weeks after undergoing MPCPC therapy in the left eye. In addition to POAG, he had cataract extraction with placement of anterior chamber intraocular lens (ACIOL) OD and posterior chamber intraocular lens (PCIOL) OS, and thyroid eye disease (TED) status post radioactive iodide ablation and orbital decompression and strabismus surgery in both eyes. His past medical history included type 2 diabetes mellitus, hypertension, atrial fibrillation controlled with a pacemaker and on ribaroxaban (xarelto), and coronary artery disease (CAD) status post multiple bypass graft surgeries and stent placements.
Our patient had a 15-year history of progressive POAG treated with topical and oral IOP lowering medications as well as selective trabeculoplasty (SLT) twice in right eye and once in left eye. After routine follow up, patient was found to have progression on visual fields in both eyes despite relatively low intraocular pressure (IOP) (12–15 OU) on maximum tolerated therapy of dorzolamide/timolol BID OU and latanoprost qHS OU. The patient had documented allergies to brimonidine and oral methazolamide. At this time, exam of the right eye was notable for IOP of 12–15 with VA of 20/30 and a cup to disc (CDR) of 0.90. His left eye exam was notable for IOP of 12–14 with VA of 20/50 and CDR of 0.90. The 10-2 Humphrey visual fields in both eyes showed dense superior and inferior arcuate defects with preservation of small central islands in both eyes, with MD of −21.22dB OD and −19.81dB OS. Due to the presence of significant lid retraction from TED, incisional glaucoma surgery was not advisable. (Patient was followed by an oculoplastic specialist for TED, which was inactive for years.) Thus MPCPC diode was recommended for both eyes.
The patient underwent MPCPC twice in the right eye using 810nm infrared diode laser at 2000mW with 31.3% duty cycle. This laser was applied for 90 sec inferiorly and 90 sec superiorly avoiding the 3 and 9 o'clock areas. The procedure was performed without any complications (post-first procedure OD IOP 12–15, VA 20/40; post-second procedure OD IOP 6–9, VA 20/70). Two months later, the patient underwent MPCPC in the left eye (with the same laser settings used in the right eye as described above). At his 1-week follow up appointment, his VA was counting fingers at 2 feet with pinhole to 20/200 and IOP of 6. His left eye exam at this time was notable for diffuse punctate epithelial keratopathy (PEK), deep and quiet AC, and optic nerve with CDR of 0.95.
At presentation to the emergency room 2 weeks after the procedure, he had a stable vision of CF at 2 feet but was found to have an IOP of 44 OS. Slit lamp exam of the left eye revealed lagophthalmos, lid retraction and scleral show, 1+ injection, diffuse PEK and PCIOL. His AC was shallow with 2+ flare and angles were open to trabecular meshwork on goniosocopy. On dilated fundus exam, he had large dome-shaped non-kissing hemorrhagic choroidals. Views of the macula and optic nerve were preserved and appeared similar to prior exams. B-scan ultrasonography showed highly reflective, large bullous choroidal detachments in all quadrants, with dense underlying opacities and pockets of low reflectivity consistent with suprachoroidal hemorrhage without evidence of retinal detachment (RD) (Fig. 1). His blood pressure (BP) was well controlled and measured 130/73 in the ED.
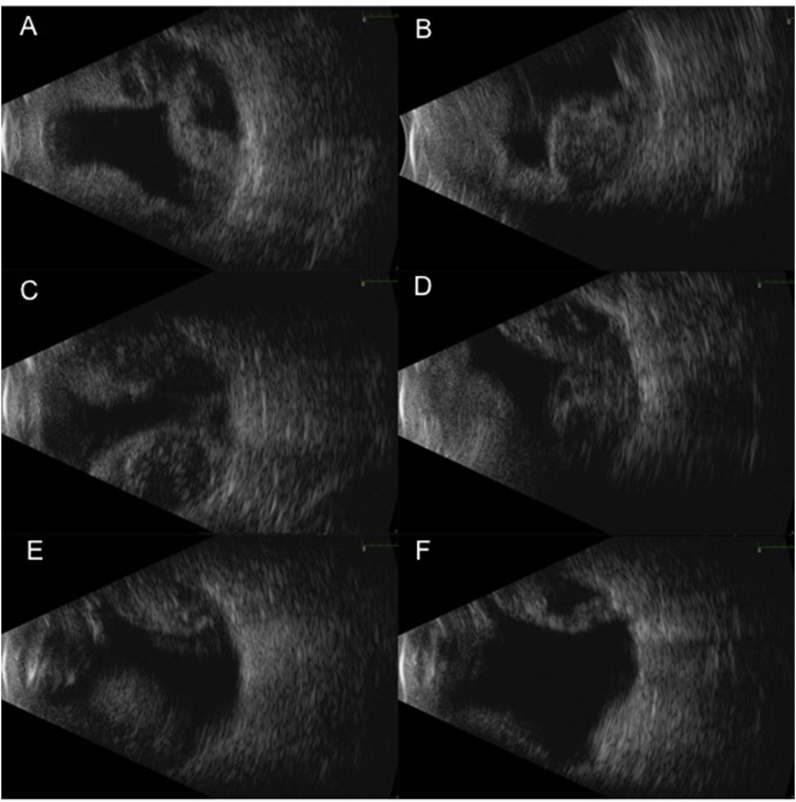
Fig. 1.
B-scan ultrasound of left eye at presentation shows highly reflective, large, bullous, appositional membranes in all quadrants (1A transverse 12 o'clock, 1B transverse 3 o'clock, 1C transverse 6 o'clock, 1D transverse 9 o'clock) with peripheral apposition, with dense underlying opacities with pockets of low reflectivity consistent with a suprachoroidal hemorrhage. There was no central apposition, and macula and optic nerve were visualized on exam (1E macula view, 1F optic nerve view). There was no evidence of retinal detachments seen.
He was started on analgesics and atropine for pain management, prednisolone acetate drops for intraocular inflammation, and oral acetazolamide and topical IOP lowering medications to lower pressure. Xarelto was continued due to risk of systemic thromboembolic events, and oral steroids were deferred due to his history of diabetes mellitus. Our patient's pain improved significantly after two days and IOP decreased to single digits. He was referred to the retina service and monitored with serial ultrasounds for possible drainage of choroidals (Fig. 2). During the first week of follow up, SCH was noted to increase in size and views of optic nerve and macula were obstructed (Fig. 3). This was thought to be due to repeat hemorrhage in the setting of low IOP (7 mmHg). Subsequently, acetazolamide as well as topical IOP lowering medications were stopped. At this time, there were areas of appositional choroidal hemorrhage noted on b-scan (Fig. 2B and C). Given patient's frail medical condition, the decision was made to observe for resorption of hemorrhage. He was also noted to have worsening epithelial defect and keratopathy, and was started on erythromycin ointment and patched.
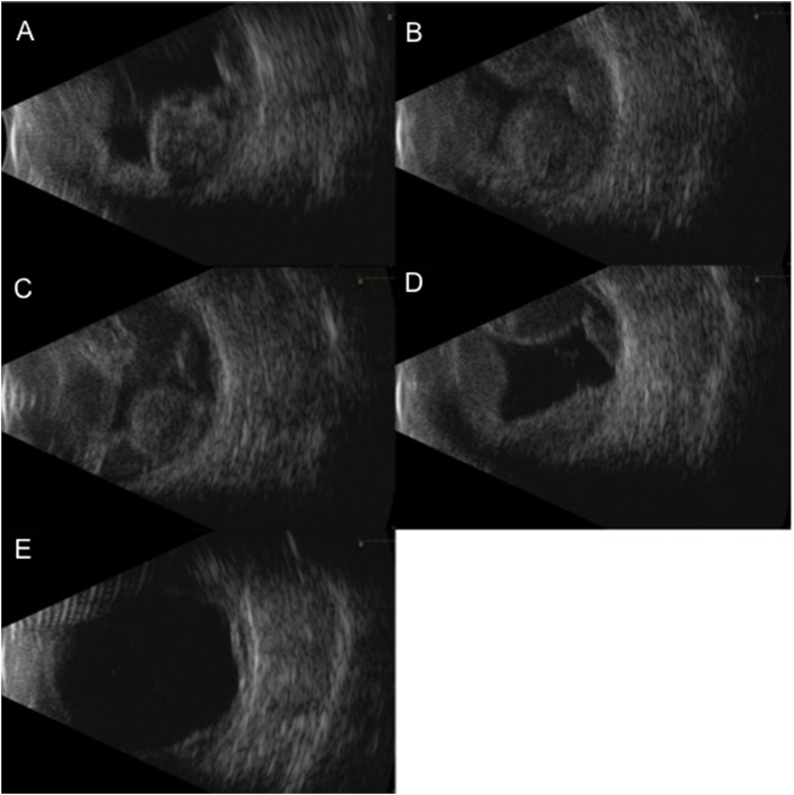
Fig. 2.
Serial B-scan ultrasounds of the inferotemporal quadrant show increase in size of hemorrhage between time of presentation (2A) and subsequent follow up 4 days (2B) and 1 week (2C) later. Patient was observed with serial b-scans, which showed decrease in size of hemorrhage at one month (2D) and two months (2E) after presentation.
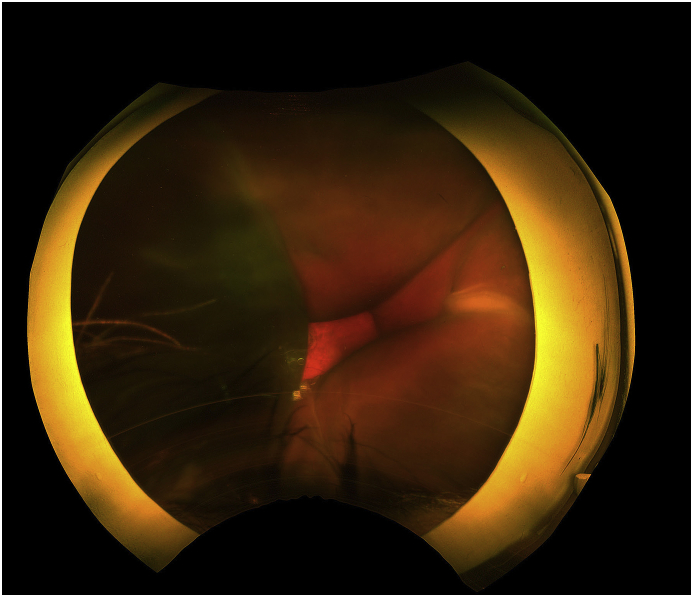
Fig. 3.
Fundus photo of the left eye taken four days after presentation shows multiple, dark choroidal elevations that were peripherally appositional and centrally non-appositional, consistent with hemorrhagic choroidals.
Approximately 1 month after presentation, his IOP increased to low teens and the size of the SCH decreased without surgical intervention (Fig. 2D). His visual acuity 1 month after presentation was hand-motions and his IOP was undetectable. At 2 months after presentation, his VA improved to count fingers at 2 feet but his IOP remained undetectable. At this time, he was found to have improvement in his keratopathy as well as improvement of choroidal hemorrhage allowing for visualization of the optic nerve and macula upon fundus examination. B-scan showed large but less thickened bullous membranes that were clearing and no longer appositional (Fig. 2E). His IOP remained low likely due to detachment of the ciliary body and poor aqueous production following the extensive choroidal detachments.
3. Discussion
Suprachoroidal hemorrhage is hypothesized to be caused by rupture of the short or long posterior ciliary arteries with resultant accumulation of blood in the suprachoroidal space.5 These arteries can rupture as a result of direct trauma or hypotony, which can stretch and shear vessels. Patients usually present with severe episodic pain, decreased vision, high IOP and a shallow AC. Dilated fundus exam can show smooth convex lobes of choroidal hemorrhage that appear darker in nature compared to choroidal serous detachments and are delineated by fixation of the choroid to vortex veins. B-scan ultrasonography can be helpful for diagnosis and for differentiation of SCH from choroidal effusions and RDs, as well as monitoring for progression or resolution.
Delayed suprachoroidal hemorrhage is relatively rare after glaucoma surgery; however, it has been associated with tube shunt and trabeculectomy procedures. One retrospective study found that the occurrence of DSCH to be highest for non-valved and valved glaucoma drainage device procedures (8.3% and 2.9% respectively), followed by trabeculectomy with and without antifibrotic agents (1.1% and 0.5% respectively), and lastly, the lowest for continuous CPC diode laser therapy (0.3%).9 The Tube versus Trabeculectomy (TVT) study reported rates of SCH to be 3% after trabeculectomy and 2% after Baerveldt implants.7 DSCH is extremely rare after continuous transscleral CPC therapy with only three cases reported in the literature.9,10 To the best of our knowledge, this is the first report of DSCH after MPCPC therapy. The indications for CPC include eyes in which trabeculectomy or drainage devices have a high risk of failure or have already failed, eyes with uncontrolled IOP and minimal useful vision, painful eyes with minimal or no useful vision, and patients who are poor surgical candidates due to other ocular or medical comorbidities.12 There are no specific guidelines about the therapeutic window or IOP range in which diode therapy should be utilized.
Settings for diode cyclophotocoagulation may differ between studies and providers. In general, MPCPC differs from continuous CPC therapy in that it delivers short bursts of energy (eg 0.5 ms) followed by rest periods that allow adjacent tissues to cool off. Unlike continue CPC diode, MPCPC is thought to result in minimal tissue disruption and delivers less energy per session. One randomized study by Aquino MC et al., compared the efficacy and safety of continuous vs. micropulse CPC diode in refractory glaucoma.11 In this study, the settings for continuous CPC with G probe was 1.5–2W, 2 seconds exposure time per burn, 20–28 burns per eye, delivering 60–112 J per treatment, while the settings for micropulse was 2W applied for 100s treatment time, consisting of on settings for 0.5 ms and off settings for 1.1 ms, delivering a total of 62.6 J.11 This study reported similar median IOP reduction between the two groups, with similar success rates at 18 months. However, the ocular complication rate was higher in the continuous group, with 7 cases of prolonged inflammation, 5 cases of hypotony, 1 case of phthisis bulbi compared to only 1 case of prolonged inflammation in the micropulse group.11 Again, no sight threatening complications were reported in the micropulse group. In general, the laser energy for MPCPC diode is set to 2000mW but there is a lack of consensus on the appropriate treatment duration or amount. Sanchez et al., reviewed the literature and found that ideal parameters of 112–150 total Joules would lead to 30% IOP reduction with few/no complications.13 Our patient received a total of 113 J in both eyes, which is similar to what other groups have reported but lower than the settings described by Aquino MC et al.13 14
A major hypothesis for the development of SCH is high pre-operative IOP followed by acute drop in IOP after the procedure.7 Interestingly, our patient did not have an acute drop in IOP since his initial IOP was low. According to a case-control study by Jeganathan VSE et al., there was a higher odds of SCH with post-op hypotony (OR 2.7).9 In our patient, post-op hypotony was likely a major contributing factor in the development of SCH. Other reported risk factors include aphakia (OR 3.6), prior intraocular surgery such as penetrating keratoplasty and pars plana vitrectomy (OR 4.4), systemic hypertension (>150 mmHg; OR 2.4), anticoagulation (OR 4.6), ischemic heart disease (OR 3.6) and respiratory disease (OR 3.2).9 While our patient's BP was controlled at the time of presentation to the ED, spontaneous SCH has been reported in patients with malignant hypertension.15 Furthermore, spontaneous SCH has been associated with anticoagulants,16,17 raising the possibility that Xarelto could have triggered the SCH in our patient. However, our patient was on chronic anticoagulation therapy and only developed SCH in the left eye in the setting of hypotony following the cyclodestructive procedure, but not the right eye. Given the multiple risk factors present in our patient, it is difficult to ascertain the cause and effect nature of the MPCPC diode and SCH. Regardless, this case demonstrates that non-incisional procedures such as MPCPC do not safeguard against SCH especially in settings of post-procedural hypotony and systemic risk factors.
Our case had several unique factors. First, MPCPC diode was performed in the right eye by the same surgeon and with the same settings only 2 months prior to the left eye. The right eye had a remote history of complicated cataract extraction requiring anterior vitrectomy and implantation of an ACIOL, whereas cataract extraction was uncomplicated in the left eye with implantation of PCIOL into the capsular bag. Thus it is not clear why our patient developed SCH in the left eye, when the right eye had similar pre-operative and post-operative IOPs with the added risk factors of the prior surgery and aphakia. One hypothesis is that our patient was coughing and/or straining around the time of the left eye procedure, which may have triggered the SCH. There are several reports of anticoagulation and valsalva maneuvers triggering spontaneous SCH.18, 19, 20 This is supported by the fact that the size of our patient's SCH increased before it decreased (as seen in Fig. 2) likely due to repeat hemorrhage from hypotony and unintentional valsalva. Second, he had a history of thyroid eye disease (TED). While it is possible that elevated episcleral pressure contributed to severity of glaucoma, it is unlikely that TED contributed to SCH as it was inactive at the time of the diode treatments. Lastly, our patient developed keratopathy possibly from a combination of severe dry eye, lagophthalmos from TED, and MPCPC-related keratopathy, which limited his visual potential. There have been recent reports of cases of neurotrophic keratopathy following MPCPC,14 which is likely due to its effect on corneal innervation.
Overall, the visual prognosis of SCH is poor, especially if hemorrhage involved all four quadrants or it was associated with RD.21,22 One study reported final visual acuity of 20/200 in 34% of eyes,22 while another study reported final visual acuity of no light perception in 23% of eyes.21 Because SCH is associated with poor outcomes, reducing the above mentioned risk factors and preventing of SCH is very important in preoperative glaucoma management. Pre-operative management includes lowering pre-operative IOP, optimizing medical health and stopping anticoagulation if risks outweighs its benefits.5 In general, there are no clear evidence about the benefits of holding anticoagulation with or without bridging therapy prior to glaucoma surgery, and it is generally recommended that decision to hold anticoagulation should be done in consultation with the patient's primary care physician (PCP) given the potential adverse consequence of stopping the medication.23 Because our patient's risk of systemic thromboembolic events associated with stopping anticoagulation outweighed the risk of the development of and worsening of suprachoroidal hemorrhage, we continued Xarelto as we had discussed with his PCP. Especially in patients on anti-coagulation, careful surgical technique should be used to minimize hypotony during the intraoperative period. Lastly, post-operative management involves avoidance of medications that can cause hypotony as well as counseling the patient on avoidance of valsalva maneuvers (including coughing, straining and sexual activity), which have been associated with SCH as well as pre-retinal, intraretinal and vitreous hemorrhage.5,19,24
The post-operative management of DSCH includes medical therapy, observation and surgical drainage. Pain can be controlled with analgesics and cycloplegics, and IOP is slowly lowered using topical and oral IOP lowering medications. Topical and systemic steroids can be used to decrease inflammation, although we deferred oral steroids due to medical concerns. Some patients may have spontaneous resolution of hemorrhage while others may need surgical drainage. The timing and indication for surgical drainage of SCH remains controversial, and it is not known whether early drainage or conservative management results in better visual outcomes.5,9 A few studies have reported improvement in visual outcomes after early surgical drainage,25,26 while others have reported good outcomes with delayed drainage.27 These differences may be due to patient-related variables such as size and complexity of the hemorrhage, as well as the surgeon's preferences for management.9 For those with persistent hemorrhage, surgery is usually delayed 1–3 weeks in order to allow for liquefaction of clots, which can facilitate drainage, however it is not known whether complete liquefaction is necessary for successful drainage.5,9,27,28 Reported indications for drainage include high IOP, and presence of appositional choroidals, vitreous hemorrhage, retinal detachment, and expulsion of intraocular content.29,30 Surgery typically involves posterior sclerotomies and drainage alone or in combination of pars plana vitrectomy.31
We monitored our patient with serial ultrasounds for clot lysis and spontaneous resolution. We were more conservative in our management because our patient was frail, had poor visual potential as a result of end-stage glaucoma, did not desire aggressive management, and was overall comfortable and without pain. Eventually, the hemorrhage decreased in size without surgical intervention (Fig. 3), however his IOP remained low and his visual acuity did not improve significantly.
4. Conclusion
Suprachoroidal hemorrhage is a rare complication after intraocular surgery that is associated with poor visual outcomes. MPCPC diode is considered to be a relatively safe and efficacious procedure that has not yet previously been associated with SCH. This case report demonstrates that SCH can occur after micropulse cyclophotocoagulation diode therapy especially in high-risk eyes. Furthermore, this case illustrates the importance of taking measures such as reducing laser diode energy in patients with lower pre-treatment IOP, stopping anticoagulation if medically able, optimizing systemic risk factors, and counseling patients on hypotony precautions to prevent this devastating complication.
Patient consent
Patient consent was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Declaration of competing interest
No funding or grant support. The authors have no financial disclosures or conflicts of interest related to this manuscript. All authors attest that they meet the current ICMJE criteria for authorship.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2020.100659.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ling R., Cole M., James C., Kamalarajah S., Foot B., Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88:478–480. doi: 10.1136/bjo.2003.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speaker M.G., Guerriero P.N., Met J.A., Coad C.T., Berger A., Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98:202–209. doi: 10.1016/s0161-6420(91)32316-9. ; discussion 210. [DOI] [PubMed] [Google Scholar]
- 3.Canning C.R., Lavin M., McCartney A.C., Hitchings R.A., Gregor Z.J. Delayed suprachoroidal haemorrhage after glaucoma operations. Eye. 1989;3(Pt 3):327–331. doi: 10.1038/eye.1989.47. [DOI] [PubMed] [Google Scholar]
- 4.Tuli S.S., WuDunn D., Ciulla T.A., Cantor L.B. Delayed suprachoroidal hemorrhage after glaucoma filtration procedures. Ophthalmology. 2001;108:1808–1811. doi: 10.1016/s0161-6420(01)00763-1. [DOI] [PubMed] [Google Scholar]
- 5.Schrieber C., Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol. 2015;26:134–142. doi: 10.1097/ICU.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri K., Schwartz S.G., Kishor K.S. Incidence of postoperative suprachoroidal hemorrhage after glaucoma filtration surgeries in the United States. Clin Ophthalmol. 2015;9:579–584. doi: 10.2147/OPTH.S78359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gedde S.J., Herndon L.W., Brandt J.D., Budenz D.L., Feuer W.J., Schiffman J.C. Postoperative complications in the Tube versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814 e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Givens K., Shields M.B. Suprachoroidal hemorrhage after glaucoma filtering surgery. Am J Ophthalmol. 1987;103:689–694. doi: 10.1016/s0002-9394(14)74331-4. [DOI] [PubMed] [Google Scholar]
- 9.Jeganathan V.S., Ghosh S., Ruddle J.B., Gupta V., Coote M.A., Crowston J.G. Risk factors for delayed suprachoroidal haemorrhage following glaucoma surgery. Br J Ophthalmol. 2008;92:1393–1396. doi: 10.1136/bjo.2008.141689. [DOI] [PubMed] [Google Scholar]
- 10.Tay E., Aung T., Murdoch I. Suprachoroidal haemorrhage: a rare complication of cyclodiode laser therapy. Eye. 2006;20:625–627. doi: 10.1038/sj.eye.6701948. [DOI] [PubMed] [Google Scholar]
- 11.Aquino M.C., Barton K., Tan A.M. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43:40–46. doi: 10.1111/ceo.12360. [DOI] [PubMed] [Google Scholar]
- 12.Pastor S.A., Singh K., Lee D.A. Cyclophotocoagulation: a report by the American academy of ophthalmology. Ophthalmology. 2001;108:2130–2138. doi: 10.1016/s0161-6420(01)00889-2. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez F.G., Peirano-Bonomi J.C., Grippo T.M. Micropulse transscleral cyclophotocoagulation: a hypothesis for the ideal parameters. Med Hypothesis, Discov Innovation Ophthalmol J. 2018;7:94–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Perez C.I., Han Y., Rose-Nussbaumer J., Ou Y., Hsia Y.C. Neurotrophic keratitis after micropulse transscleral diode laser cyclophotocoagulation. American Journal Of Ophthalmology Case Reports. 2019;15 doi: 10.1016/j.ajoc.2019.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung A.Y., David J.A., Ober M.D. Spontaneous bilateral hemorrhagic choroidal detachments associated with malignant hypertension. Retin Cases Brief Rep. 2017;11:175–179. doi: 10.1097/ICB.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 16.Chandra A., Barsam A., Hugkulstone C. A spontaneous suprachoroidal haemorrhage: a case report. Cases journal. 2009;2:185. doi: 10.1186/1757-1626-2-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knox F.A., Johnston P.B. Spontaneous suprachoroidal haemorrhage in a patient with age-related macular degeneration on excessive anticoagulation therapy. Eye. 2002;16:669–670. doi: 10.1038/sj.eye.6700109. [DOI] [PubMed] [Google Scholar]
- 18.Marous C.L., Sioufi K., Shields C.L., Mashayekhi A., Shields J.A. Coughing-induced suprachoroidal hemorrhage simulating melanoma in two cases. Retin Cases Brief Rep. 2018;12(4):336–341. doi: 10.1097/ICB.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao S.F., Shih M.H., Huang F.C. Spontaneous suprachoroidal hemorrhage: case report and review of the literature. Taiwan Journal of Ophthalmology. 2016;6:36–41. doi: 10.1016/j.tjo.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammam T., Madhavan C. Spontaneous suprachoroidal haemorrhage following a valsalva manoeuvre. Eye. 2003;17:261–262. doi: 10.1038/sj.eye.6700298. [DOI] [PubMed] [Google Scholar]
- 21.Wirostko W.J., Han D.P., Mieler W.F., Pulido J.S., Connor T.B., Jr., Kuhn E. Suprachoroidal hemorrhage: outcome of surgical management according to hemorrhage severity. Ophthalmology. 1998;105:2271–2275. doi: 10.1016/S0161-6420(98)91228-3. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds M.G., Haimovici R., Flynn H.W., Jr., DiBernardo C., Byrne S.F., Feuer W. Suprachoroidal hemorrhage. Clinical features and results of secondary surgical management. Ophthalmology. 1993;100:460–465. [PubMed] [Google Scholar]
- 23.Rahman S.I., Turalba A. Anticoagulation in glaucoma surgery. Semin Ophthalmol. 2018;33:108–111. doi: 10.1080/08820538.2017.1353828. [DOI] [PubMed] [Google Scholar]
- 24.Al Rubaie K., Arevalo J.F. Valsalva retinopathy associated with sexual activity. Case Reports In Medicine. 2014;2014 doi: 10.1155/2014/524286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrams G.W., Thomas M.A., Williams G.A., Burton T.C. Management of postoperative suprachoroidal hemorrhage with continuous-infusion air pump. Arch Ophthalmol. 1986;104:1455–1458. doi: 10.1001/archopht.1986.01050220049024. [DOI] [PubMed] [Google Scholar]
- 26.Frenkel R.E., Shin D.H. Prevention and management of delayed suprachoroidal hemorrhage after filtration surgery. Arch Ophthalmol. 1986;104:1459–1463. doi: 10.1001/archopht.1986.01050220053025. [DOI] [PubMed] [Google Scholar]
- 27.Chu T.G., Green R.L. Suprachoroidal hemorrhage. Surv Ophthalmol. 1999;43:471–486. doi: 10.1016/s0039-6257(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 28.Lakhanpal V., Schocket S.S., Elman M.J., Nirankari V.S. A new modified vitreoretinal surgical approach in the management of massive suprachoroidal hemorrhage. Ophthalmology. 1989;96:793–800. doi: 10.1016/s0161-6420(89)32819-3. [DOI] [PubMed] [Google Scholar]
- 29.Meier P., Wiedemann P. Massive suprachoroidal hemorrhage: secondary treatment and outcome. Graefe’s Arch Clin Exp Ophthalmol. 2000;238:28–32. doi: 10.1007/s004170050005. [DOI] [PubMed] [Google Scholar]
- 30.Learned D., Eliott D. Management of delayed suprachoroidal hemorrhage after glaucoma surgery. Semin Ophthalmol. 2018;33:59–63. doi: 10.1080/08820538.2017.1353814. [DOI] [PubMed] [Google Scholar]
- 31.Lavinsky F., Moisseiev J., Levkovitch-Verbin H. The surgical management of massive intraoperative and postoperative suprachoroidal hemorrhage: anatomic and functional outcomes. Arq Bras Oftalmol. 2013;76:212–214. doi: 10.1590/s0004-27492013000400003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.