Abstract
Purpose
To describe a patient with an amelanotic choroidal melanoma, originally misdiagnosed as a choroidal granuloma, following his systemic diagnosis of tattoo-associated sarcoidosis.
Observations
The amelanotic choroidal tumor, suspected to be a granuloma, failed initial steroid treatment. Full-thickness chorioretinal biopsy demonstrated histologic presence of uveal melanoma and tumor genetics via GEP analysis demonstrated a PRAME negative, Class 1A lesion. The amelanotic choroidal melanoma was treated successfully with I-125 plaque brachytherapy.
Conclusion and importance
Despite a systemic diagnosis which predisposes a patient to uveal granuloma, amelanotic choroidal melanomas can still occur and should be considered. The association of uveal melanoma and sarcoidosis remains rare and of unclear significance.
Keywords: Uveal melanoma, Eye, Tumor, Sarcoidosis
1. Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy; however, it is rare with an estimated incidence of 5–6 cases per million population per year.1,2 UM has a higher prevalence in older, non-Hispanic, Caucasian men,3 but can present in younger individuals nonetheless. Metastasis is highly associated with tumor size, location, and genetic characteristics.4
The differential diagnosis for an amelanotic choroidal tumor is broad including both malignant and benign diagnoses: amelanotic melanoma, nevus, metastasis, hemangioma, peripheral exudative hemorrhagic chorioretinopathy (PEHCR), sclerochoroidal (SC) calcification, osteoma, granuloma, lymphoma, solitary idiopathic choroiditis (SIC). Patient characteristics such as sex, age, laterality, and base size can help narrow the differential, with melanoma occurring typically in older individuals and metastasis occurring primarily in patients with a known history of systemic cancer. Amelanotic choroidal melanomas are more likely to be unilateral with a larger basal diameter, subretinal fluid, and a hollow appearance on ultrasound. Despite the predilection for older individuals, young males with an amelanotic choroidal tumors with a base ≥8 mm most likely will present with melanoma (80%), hemangioma (8%), or metastasis (5%).5 However, preexisting systemic diagnoses can bias the differential such as sarcoidosis with an associated choroidal granuloma.
Tattoo-associated sarcoidosis has a male predominance, asymptomatic and nonspecific presentation, with dermatopathologic diagnosis based on the relative abundance of epithelioid histiocytes, giant cell infiltration, and the number of foreign bodies.6 Several lines of evidence support the hypothesis that tattoo ingredients or metabolites may be immunogenic agents that promote autoimmunity in those with genetic predisposition to developing sarcoidosis. Tattoo ingredients are poorly regulated with variable pharmacokinetic properties dependent on tattoo color. This may explain the predominance of sarcoidosis in red and black tattoos.6 For example, sarcoidosis may account for roughly 30% of papulo-nodular tattoo reactions in black tattoos, and newer tattoos may induce widespread reactions in other black tattoos of the same individual.7 The chemical composition of tattoo dyes includes agents that are potent immunotoxins, and may be chemically unstable when exposed to UV radiation.8 Proteins such as metallothionein, which can bind to metal elements in tattoo dyes, may play a role in tattoo granuloma formation.9 Furthermore, though intended to stay in the skin, tattoo ingredients may be carried by blood or lymphatics to other organs such as the liver.10 In fact, half a century ago, tattoo sarcoidosis and uveitis were hypothesized to be caused by allergens absorbed from tattoos which accumulate in the uvea,11 though this has not been corroborated. Interestingly, emerging cases with granulomatous tattoo reactions and associated uveitis, without evidence of systemic sarcoidosis, suggest distinct categories of “Tattoo sarcoidosis and uveitis” and “Tattoo Granulomas with Uveitis or TAGU”.12
The presence of tattoo-associated sarcoidosis complicates the interpretation and management of concomitant ocular pathology. Since choroidal granuloma can be the initial presentation of systemic sarcoidosis13 a trial of systemic corticosteroids is a less-invasive and practical approach to differentiating choroidal sarcoid granuloma from malignancy.14 Ocular manifestations occur in 25–80% of patients with sarcoidosis, and of those with ocular involvement 30% involve in the posterior segment.15 Early estimates suggest only 5% of patients with ocular sarcoid have posterior segment findings in the absence of anterior chamber disease,16 however, this finding may be limited to anterior uveitis in association with chorioretinitis, periphlebitis, or chorioretinal nodules in ocular sarcoid. More recent work suggests patients with acute choroidal granulomas from sarcoidosis are in fact more likely to present without associated anterior uveitis.17 The authors were surprised by the biopsy results of melanoma, because of the high pretest probability for choroidal granuloma in this patient with an amelanotic choroidal lesion and a pre-existing diagnosis of tattoo-associated sarcoidosis.
2. Case report
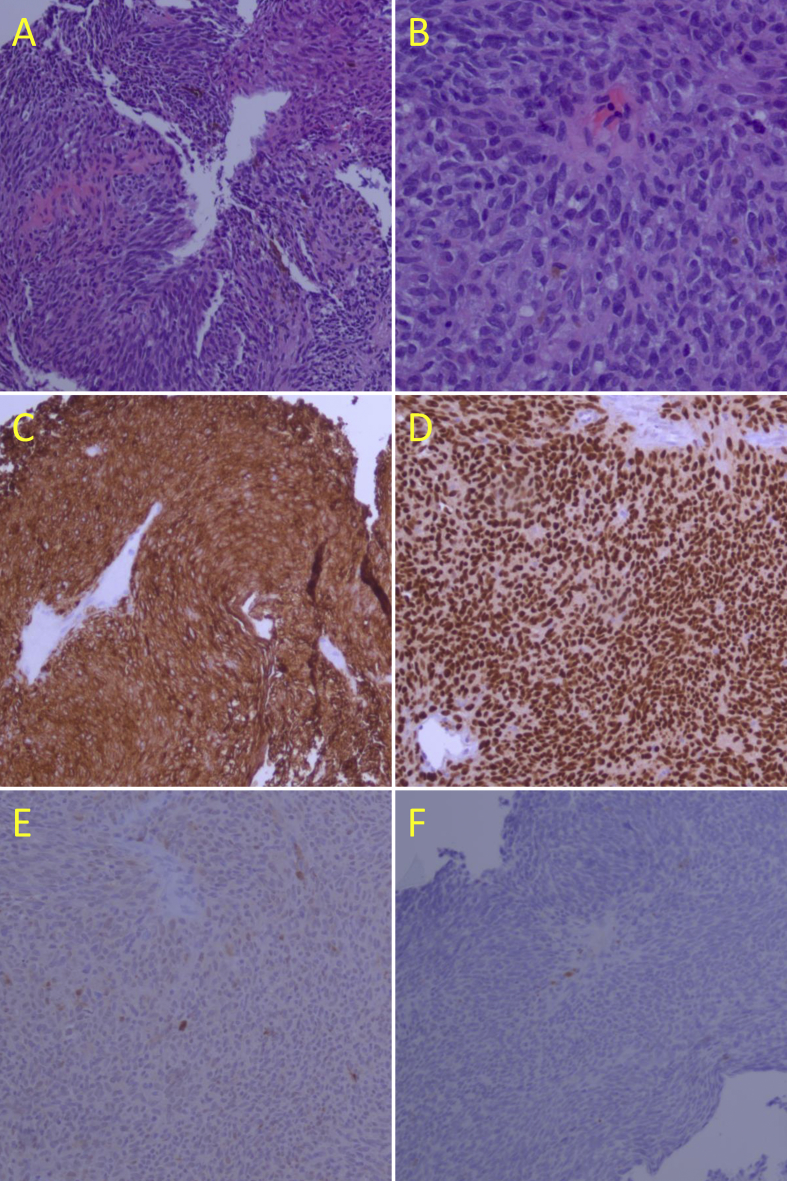
A thirty-three-year-old Caucasian male presented with an amelanotic choroidal mass in his amblyopic right eye with an associated asymptomatic exudative retinal detachment, with concern for ocular sarcoidosis in the right eye. Three months prior, he had noticed a bump in his right arm within the area of a large tattoo. This was later diagnosed as dermatologic sarcoid following a full thickness skin biopsy. The patient had concomitant lung disease and was placed on oral corticosteroid therapy. A routine asymptomatic complete eye examination demonstrated a choroidal mass for which he was referred for ocular oncologic opinion. Intraocular pressure was 11 mmHg in the right eye and 13 mmHg in the left eye. Best-corrected visual acuity was 20/70+ with no improvement on pinhole in the right eye, and 20/40 in left eye. The anterior segment was without abnormalities bilaterally. Examination of the posterior segment in the left eye was unremarkable. The posterior segment in the right eye had no vitritis. There was a subretinal mass 1 mm from the foveola with overlying fluid seen in fundus photography in (Fig. 1). There was an inferotemporal associated serous retinal detachment affecting the fovea. OCT of the macula of the right eye showed retinal elevation consistent with subretinal fluid over the lesion with a foveal thickness of 234 μm. Autofluorescence from orange pigment was increased peripheral to the lesion in the right eye, with fluorescein angiography showing consistent early leakage from the mass. Posterior segment ultrasound demonstrated an acoustically hollow choroidal tumor with a base of 9.1 mm × 8.2 mm and a thickness of 2.6mm. There was an associated retinal detachment observed on ultrasound. Based on the prior history of sarcoidosis, the patient was given 80mg prednisone daily to treat a presumptive choroidal granuloma. Over the next month, visual acuity and posterior fundus examination were largely unchanged, with worsening ocular discomfort and increased subretinal fluid. Due to the lack of resolution of the choroidal granuloma with steroid treatment, the patient was offered chorioretinal biopsy with partial resection of the tumor down to bare sclera. Cytopathology revealed atypical spindle cells consistent with melanoma. Immunohistochemical staining showed tumor cells positive for SOX-10, Melan-A, patchy HMB45, weak and patchy S100 staining, with negative AE1/AE3, SMA, p63, consistent with malignant melanoma (Fig. 2). The specimen was hypocellular; however, flow cytometry was not concerning for lymphoproliferative etiology (small population of B cells with no evidence of monoclonality, normal kappa:lambda ratio, no CD5 or CD 10 co-expression; few T cells with normal expression of pan T-cell antigens and normal CD4:CD8 ratio). Genetic analysis was performed at the time of biopsy on the unlikely chance that melanoma was diagnosed pathologically. The tumor had a molecular signature consistent with Class 1A melanoma (DecisionDx-UM, Castle Biosciences, Phoenix AZ). Treatment options including enucleation, episcleral plaque therapy, and proton beam therapy were discussed, and the patient elected for brachytherapy. An I-125 plaque designed to deliver 85 Gy to the tumor apex was placed with the help of Plaque Simulator software. The plaque was removed after 72 hours and proliferative vitreoretinopathy was managed with repeat vitrectomy, inferior retinectomy, endolaser and silicone oil infusion. Several days after plaque removal, visual acuity in right eye was counting fingers, with normal intraocular pressure and no residual pain. No metastases secondary to the uveal melanoma have been identified one year following radiation, and the patient's systemic corticosteroid therapy has been tapered.
Fig. 1.
Compilation of clinical images. A. Wide-angle Zeiss Clarus photograph demonstrating an inferotemporal subretinal amelanotic or partially pigmented choroidal lesion and associated exudative detachment. B. Wide-angle late phase Optos fluorescein angiograph demonstrating intense intrinsic hyperfluorescence of the lesion consistent with leakage. C. Late phase Heidelberg indocyanine green angiography demonstrates low intrinsic ICG fluorescence compared to background choroidal vasculature. D. Wide-field montage Zeiss Clarus autofluorescence demonstrates increased autofluorescence at the nasal and inferior edges of the lesion consistent with orange pigment. E. Posterior segment ultrasound demonstrates a dome-shaped choroidal lesion with low to medium echogenicity and an inferior exudative detachment. F. Superior-inferior oriented optical coherence tomography demonstrates a choroidal lesion with an overlying exudative detachment.
Fig. 2.
A. Low magnification H and E stain demonstrating palisading spindle and epithelioid cells with nests of brown pigment. B. 20X magnification H and E image demonstrates mostly spindle cells with rare inflammatory cells and rare mitotic figures with scattered pigment. C. Low magnification Melan-A immunostain shows intense uptake. D. Low magnification Sox-10 immunostain shows intense uptake. E. Low magnification S-100 immunostain demonstrates minimal uptake. F. Low magnification P63 immunostain demonstrates minimal uptake.
3. Discussion
Uveal melanoma has been associated with myriad epidemiological factors18 including familial cancer susceptibility,19,20 nevi,21 and xeroderma pigmentosum.22 Despite these dermatological associations, the links between choroidal melanoma and systemic conditions such as sarcoidosis, are as yet incompletely defined. To our knowledge, this is the first case of amelanotic melanoma occurring in a patient with known tattoo-associated sarcoidosis. Amelanotic choroidal tumors have a broad differential which complicates diagnosis in management. In this patient, prior history of sarcoidosis misdirected the initial diagnosis towards ocular granuloma. Diagnostic uncertainty in these cases is further complicated by documented associations between sarcoidosis and various hematologic and solid tumor malignancies including skin melanoma and nonmelanoma skin cancers.23 Moreover, patients with malignancy can have “sarcoid reactions” in which non-caseating epithelioid-cell granulomas are found in lymph nodes draining malignant tumors.24 There are reports of systemic malignancies predating ocular sarcoid-like reactions, systemic malignancy found after diagnosis of ocular sarcoid-like reactions, and ocular malignancy predating systemic sarcoid-like reaction.25 These findings suggest some malignancies may lead to paraneoplastic syndromes which promote formation of noncaseating granulomas. Equally likely is a shared genetic predisposition to developing both sarcoidosis and malignancy. For example, a gene from the PRAME family, implicated as an independent prognostic in uveal melanoma26 was identified via whole-exome sequencing as a commonly linked gene in familial sarcoidosis.27 In a similar vein, vitreous fluid of eyes with uveal melanoma may have upregulated cytokines and chemokines28,29 which could prime the complex immune cell dysregulation behind sarcoidosis. The presence of toxic compounds and potential carcinogens in tattoo ingredients8 may have promoted both autoimmune and carcinogenic changes in this patient. Ultimately, the association of uveal melanoma and sarcoidosis remains rare and of unclear significance, and the associated tattoo reaction here further muddies the water.
4. Conclusions
The association between sarcoidosis and systemic malignancy was speculated over one century ago.30,31 Despite this early hypothesis, the association remains unclear. In the current case, the short three-month window between the diagnosis of sarcoid and the discovery of a choroidal melanoma obscured the conclusion that sarcoidosis directly preceded the development of the neoplasm. Nevertheless, this case highlights the importance of a broad differential ophthalmic diagnosis in patients with amelanotic choroidal tumors despite a pre-existing systemic condition such as sarcoidosis.
Patient consent
The patient consented to publication of the case in writing. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: STB, ALB, DAR.
Acknowledgements
None.
References
- 1.Egan K.M., Seddon J.M., Glynn R.J., Gragoudas E.S., Albert D.M. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- 2.Singh A.D., Turell M.E., Topham A.K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmol. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Nichols E.E., Richmond A., Daniels A.B. Disparities in uveal melanoma: patient characteristics. Semin Ophthalmol. 2016;31:296–303. doi: 10.3109/08820538.2016.1154176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields C.L., Furuta M., Thangappan A. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 5.Welch R.J., Newman J.H., Honig S.E. Choroidal amelanotic tumours: clinical differentiation of benign from malignant lesions in 5586 cases. Br J Ophthalmol. 2020;104(2):194–201. doi: 10.1136/bjophthalmol-2018-313680. [DOI] [PubMed] [Google Scholar]
- 6.Kluger N. Sarcoidosis on tattoos: a review of the literature from 1939 to 2011. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:86–102. [PubMed] [Google Scholar]
- 7.Sepehri M., Hutton Carlsen K., Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679–686. doi: 10.1159/000453315. [DOI] [PubMed] [Google Scholar]
- 8.Bäumler W. Absorption, distribution, metabolism and excretion of tattoo colorants and ingredients in mouse and man: the known and the unknown. Curr Probl Dermatol. 2015;48:176–184. doi: 10.1159/000369222. [DOI] [PubMed] [Google Scholar]
- 9.Hanada K., Hashimoto I. Metallothionein expression in tattooed skin. Br J Dermatol. 1998;138:359–360. doi: 10.1046/j.1365-2133.1998.02094.x. [DOI] [PubMed] [Google Scholar]
- 10.Sepehri M., Sejersen T., Qvortrup K., Lerche C.M., Serup J. Tattoo pigments are observed in the kupffer cells of the liver indicating blood-borne distribution of tattoo ink. Dermatology. 2017;233:86–93. doi: 10.1159/000468149. [DOI] [PubMed] [Google Scholar]
- 11.Rorsman H., Brehmer-Andersson E., Dahlquist I. Tattoo granuloma and uveitis. Lancet. 1969;2:27–28. doi: 10.1016/s0140-6736(69)92600-2. [DOI] [PubMed] [Google Scholar]
- 12.Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852–1861. doi: 10.1111/jdv.15070. [DOI] [PubMed] [Google Scholar]
- 13.Verma A., Biswas J. Choroidal granuloma as an initial manifestation of systemic sarcoidosis. Int Ophthalmol. 2010;30:603–606. doi: 10.1007/s10792-009-9328-5. [DOI] [PubMed] [Google Scholar]
- 14.Turkoglu E.B., Lally S.E., Shields C.L. Choroidal sarcoid granuloma simulating prostate carcinoma metastasis. Retin Cases Brief Rep. 2017;11(Suppl 1):S226–S228. doi: 10.1097/ICB.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 15.Bonfioli A.A., Orefice F. Sarcoidosis. Semin Ophthalmol. 2005;20:177–182. doi: 10.1080/08820530500231938. [DOI] [PubMed] [Google Scholar]
- 16.Obenauf C.D., Shaw H.E., Sydnor C.F., Klintworth G.K. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–655. doi: 10.1016/0002-9394(78)90184-8. [DOI] [PubMed] [Google Scholar]
- 17.Desai U.R., Tawansy K.A., Joondeph B.C., Schiffman R.M. Choroidal granulomas in systemic sarcoidosis. Retina. 2001;21:40–47. doi: 10.1097/00006982-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Nichols E.E., Richmond A., Daniels A.B. Tumor characteristics, genetics, management, and the risk of metastasis in uveal melanoma. Semin Ophthalmol. 2016;31:304–309. doi: 10.3109/08820538.2016.1154175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A.D., Shields C.L., De Potter P. Familial uveal melanoma. Clinical observations on 56 patients. Arch Ophthalmol. 1996;114:392–399. doi: 10.1001/archopht.1996.01100130388005. [DOI] [PubMed] [Google Scholar]
- 20.van Hees C.L., Jager M.J., Bleeker J.C., Kemme H., Bergman W. Occurrence of cutaneous and uveal melanoma in patients with uveal melanoma and their first degree relatives. Melanoma Res. 1998;8:175–180. doi: 10.1097/00008390-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Weis E., Shah C.P., Lajous M., Shields J.A., Shields C.L. The association of cutaneous and iris nevi with uveal melanoma: a meta-analysis. Ophthalmol. 2009;116:536–543. doi: 10.1016/j.ophtha.2008.10.008. .e2. [DOI] [PubMed] [Google Scholar]
- 22.Johnson M.W., Skuta G.L., Kincaid M.C., Nelson C.C., Wolter J.R. Malignant melanoma of the iris in xeroderma pigmentosum. Arch Ophthalmol. 1989;107:402–407. doi: 10.1001/archopht.1989.01070010412036. [DOI] [PubMed] [Google Scholar]
- 23.Cohen P.R., Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326–333. doi: 10.1016/j.clindermatol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Brincker H. Sarcoid reactions in malignant tumours. Canc Treat Rev. 1986;13:147–156. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 25.Balasubramaniam S.C., Salomão D.R., Davies J.B. Paraneoplastic sarcoid-like reactions and the eye. Retina. 2015;35:789–797. doi: 10.1097/IAE.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 26.Field M.G., Decatur C.L., Kurtenbach S. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Canc Res. 2016;22:1234–1242. doi: 10.1158/1078-0432.CCR-15-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishore A., Petersen B.S., Nutsua M. Whole-exome sequencing identifies rare genetic variations in German families with pulmonary sarcoidosis. Hum Genet. 2018;137:705–716. doi: 10.1007/s00439-018-1915-y. [DOI] [PubMed] [Google Scholar]
- 28.Nagarkatti-Gude N., Bronkhorst I.H., van Duinen S.G., Luyten G.P., Jager M.J. Cytokines and chemokines in the vitreous fluid of eyes with uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53:6748–6755. doi: 10.1167/iovs.12-10123. [DOI] [PubMed] [Google Scholar]
- 29.Dunavoelgyi R., Funk M., Sacu S. Intraocular activation of angiogenic and inflammatory pathways in uveal melanoma. Retina. 2012;32:1373–1384. doi: 10.1097/IAE.0b013e318239e299. [DOI] [PubMed] [Google Scholar]
- 30.Herkheimer G. Uber Karzinom und Tuberkulose. Z Tuberk. 1917;27:251–258. [Google Scholar]
- 31.Pautrier L. Cas extraordinaire de sarcoides dermiques noueuses disseminées. Bull Soc Franc Derm Syph. 1934;41:1233–1252. [Google Scholar]