Abstract
A rapid electrochemical immunoassay method was developed to detect and measure stress biomarkers (cortisol and cortisone) in two biological samples (Zebrafish whole-body and artificial saliva). This methodology utilizes an immunoassay approach taking advantage of the lock and key mechanism that is related to the antibody-antigen interaction depending on the reliable immobilization of the antibody labelled with ferrocene tags (Ab-Fc) on a modified tin-doped indium oxide (ITO) electrode using electrochemical instrumentation to build a POC platform. The limit of detection (LOD) obtained for this biosensor was 1.03 pg ml−1 for cortisol and 0.68 pg ml−1 for cortisone, respectively. The correlation coefficient was 0.9852 and 0.9841 for cortisol and cortisone, respectively with a linear concentration from (0-50 ng ml−1) which covers the standard levels of stress hormones in both selected biological samples. The incubation time was investigated and 30 min was found to be the optimum incubation time. This time would be acceptable for the POC system as total process time can be determined within 35 min.
Keywords: Biomedical engineering, Electrochemistry, Biosensors, Immunoassay, Stress biomarkers, Point of care
Biomedical engineering; Electrochemistry; Biosensors; Immunoassay; Stress biomarkers; Point of care.
1. Introduction
With recent medical requirements and scientific developments, there has been a fast increase in the demand for healthcare and environmental monitoring systems to monitor specific biomarkers [1]. As a result, there is a need for the construction of a miniaturized, portable, automated and disposable to easily monitor a range of biochemical markers, such as point -of -care (POC) system, which can deliver a valuable health and environment informatics [2, 3]. In this work, a protocol was developed using lock and key mechanism that is related to antibody and antigen interaction due to the high sensitivity, selectivity and reproducibility provided from these immunosensors using electrochemical as the detection signal [4, 5]. In general, electrochemical method capital cost of batch fabrication is low, and owns the ability for miniaturization without loss of performance has elevated this method up to speed with diverse immunosensing to construct biosensors [6], consisting of a bioreceptor such as antibodies that employs the specific recognition to an antigen to measure the concentration or the presence of an analyte [7, 8] and a measurement transducers (i.e. electrochemical techniques) [9].
The focal point of this research focuses on the detection and measurement of Glucocorticoids (GCs) including cortisol and cortisone as an indication of stress using electrochemical immunoassay. This could be attributed to the fact that cortisol and cortisone hormones are considered as the most potent biomarker for stress in humans and receive the most attention in clinical and scientific research [10, 11].
Stress is defined as homeostasis state alteration due to extrinsic and intrinsic events, whether actual or perceived. This threatens is then counteracted by a cascade of physiologic responses to re-establish and maintain homeostasis in an automatic process known as the “fight-or-flight” reaction or the "stress response” [12, 13]. Chronic stress can be fatal to the body by causing stomach ulcers, fertility problems, cardiovascular disease, hypertension and chronic inflammation [14, 15, 16]. Extensive academic proof-of-concept studies have demonstrated the use of electrochemical detection in cortisol and cortisone immunoassay [17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. However, inherited limitation of sensitivity, selectivity figures, and strong acid or perchlorate environment used to enhanced electron transfer [31] incorporated with electrochemical detection has slightly restricted their use [32].
Within this project, the above method was adapted to immobilize cortisol and cortisone antibodies on the ITO electrode by carrying out an electrochemical immunoassay to detect and monitor the analyte in two biological samples (Zebrafish whole-body and artificial saliva). This was achieved by measuring the change in current using cyclic voltammetry and the more sensitive square wave techniques.
2. Experimental
2.1. Materials and instrumentation
Cortisone, Phosphate buffer saline tablet (PBS), Ferrocenecarboxylaldehyde (FcCHO), Potassium carbonate (K2CO3), Sodium borohydride (NaBH4), Hydrochloric acid (HCl), Potassium chloride (KCl), 4-Nitrobenzene diazonium tetra-fluoroborate, N-Hydroxysulfosuccinimide sodium salt (Sulfo-NHS), Tween 20, Bovine serum albumin (BSA), N, N-dimethylformamide (DMF), Ammonium nitrate, Potassium phosphate, Potassium citrate, Uric acid sodium salt, Urea, Lactic acid sodium salt, Bovine submaxillary gland mucin Type I–S, tricaine methanesulfonate (500 mg ml−1 MS-222) were purchased from Sigma-Aldrich, UK. Acetonitrile (ACN), Ethanol (EtOH), Sodium hydroxide (NaOH), Tertrabutylammonium perchlorate (TBAP) were obtained from Fisher Scientific, UK. N-(3-Dimethylaminopropyl)- N′-ethyl carbodiimide hydrochloride (EDC) was sourced from Fluka, UK. Anti-cortisol antibody, anti-cortisone antibody, cortisol (hydrocortisone) were obtained from Abcam biochemicals, UK.
Electrochemical measurements conducted with a standard three-electrode system using a Palm-sens Potentiostat (Palm Instruments, Netherlands) connected to the PSTrace 4.3 software. The electrochemical cell consists of a tin-doped indium oxide (ITO) coated glass electrode (Delta Technologies Limited, USA) which was the working electrode, a nickel wire as the counter electrode, as this enables more versatility with respect to the experimental setup and silver/silver chloride (Ag/AgCl) (3M NaCl) (ALVATEK) as the reference electrode (BSA, UK). All potentials were reported versus the Ag/AgCl reference electrode at room temperature.
3. Experimental procedures
3.1. Biosensor platform surface fabrication
The construction of a biosensor platform was done following Jwan et al group work [33]. The modification of an ITO electrode surface was done in two steps; deposition of nitrobenzene group to yield nitrobenzene-modified ITO electrode and nitrobenzene reduction to aniline. Subsequently, cortisol and cortisone antibodies were chemically labelled with a redox tag (ferrocene). A purification procedure was carried out to remove any ferrocene excess. The final solution was stored in the fridge (4 °C) until use. To enable antibodies electrochemical detection, the ferrocene-tagged antibody had to be immobilized on the ITO electrode surface using an activation buffer consisting of 2mM (N-(3-dimethyl aminopropyl)-N′-ethyl carbodiimide hydrochloride) (EDC) and 5 mM (N-hydroxysulfosuccinimide sodium salt) (sulfo-NHS) to activate the –COOH on the antibody facilitating the bonding to the electrode via an amide linkage. The resulted modified ITO electrode was used for subsequent experiments.
3.2. Optimization of the antigen incubation time
Cortisol (hydrocortisone) standard solution (50 ng ml−1) was selected to determine the optimum time for the antigen to be incubated with an immobilized antibody with a range of incubation times (10, 15, 20, 30, 45 and 60 min), and cyclic voltammetry was carried out.
3.3. Electrochemical immunoassay calibration graph
To build the calibration graph for the electrochemical immunoassay procedure, a stock solution of cortisol was prepared by dissolving 5 mg of cortisol (hydrocortisone) in ethanol and making the volume up to 50 ml in PBS (10 mM) to give a concentration of 100 ppm. Then increasing volumes of stock solutions were transferred into a series of 5 ml volumetric flask and made up the mark with PBS (10 mM) to prepare a set of working standards from (0.001–50 ng ml−1).
The modified electrodes were washed with PBS (10 mM) and the calibration standards (antigen) were added to each electrode individually. After an incubation period of 30 min, the measurement was made using cyclic voltammetry in PBS (10 mM) over 3 scans followed by square wave voltammetry also in PBS (10 mM) where the frequency was 25 Hz with an amplitude of 1 mV, the scan was between 0 V and +0.5 V. This procedure was repeated for cortisone.
3.4. Measurements of stress biomarkers in biological samples
Two biological samples were analyzed for stress biomarkers; Zebrafish whole-body: All experiments were ethically permitted and followed the HO schedule 1 method prepared by HO license holders. To avoid the fluctuation in cortisol occurs due to the natural circadian rhythms, experiments were done at approximately the same time of the day. Zebrafish were captured and euthanized with tricaine methanesulfonate (500 mg ml−1 MS-222). Whole-body cortisol extraction was performed according to Canavello et al [34] method. The yellowish lipid extract obtained was reconstituted with 1 ml of cortisol standard dissolved in phosphate buffer saline (PBS) and stored at 4 °C for 24 h. This procedure was compared with an alternative method that omits the use of the extraction procedure.
3.4.1. Artificial saliva
A recipe outlined by West et al. [35] was used to prepare an artificial human saliva sample which include sodium chloride (1.954 g L−1), ammonium nitrate (0.328 g L−1), potassium phosphate (0.639 g L−1), potassium chloride (0.202 g L−1), potassium citrate (0.308 g L−1), uric acid sodium salt (0.021 g L−1), urea (0.198 g L−1), lactic acid sodium salt (0.146 g L−1) and bovine submaxillary gland mucin Type I–S (30 g L−1), the solution was completed with deionized water. For all standard, there was a slight difference where they were made up in artificial saliva instead of PBS (10 mM). The electrochemical immunoassay was repeated using the above biological samples. All the above procedures were repeated for cortisone.
4. Results and discussion
4.1. Tin-doped indium oxide (ITO) electrode modification
In an effort to develop a reproducible methodology for cortisol and cortisone stress biomarkers measurements in biological samples, an electrochemical immunoassay protocol was introduced to immobilize cortisol and cortisone antibodies on a modified ITO electrode. The procedure is based on the immobilization of the ferrocene tagged antibody on the ITO electrode. To achieve this; the electrode was modified by electrochemical deposition of 4-nitrobenzene diazonium tetrafluoroborate on the conductive surface of an ITO electrode and sequentially an electrochemical reduction of the nitrobenzene group to an amine group was done. The immobilization protocol was elucidated and characterized using cyclic voltammetry as can be seen in Figures 1 and 2.
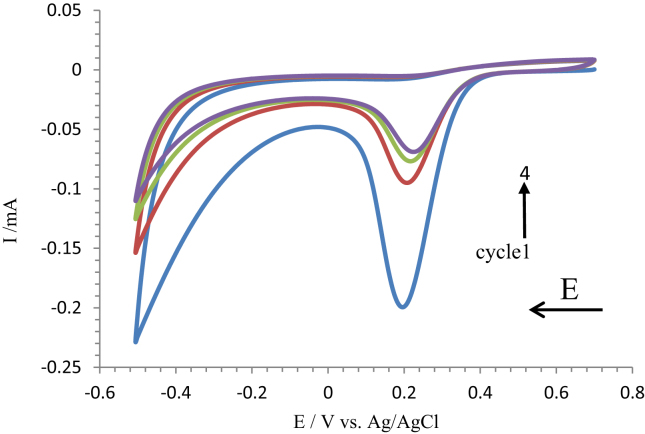
Figure 1.
Cyclic voltammograms of the deposition of nitrobenzene onto the ITO electrode using a deposition solution. The cyclic voltammetry run is performed over four scans with a scan rate of 0.1 V s−1 at room temperature.
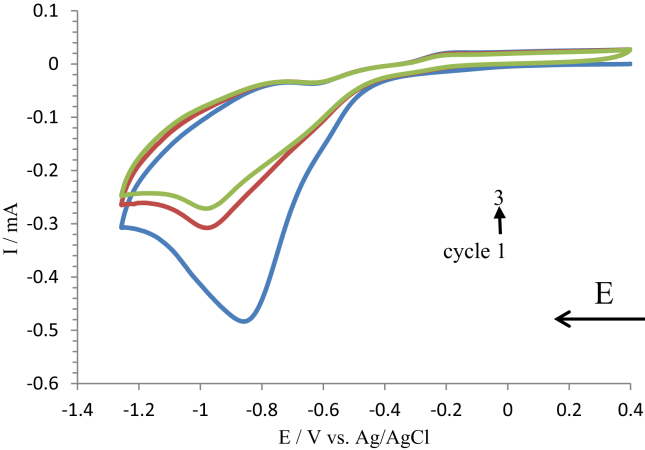
Figure 2.
Voltammograms of nitrobenzene reduction to aniline. The cyclic voltammetry was performed over three oxidation-reduction voltammetry cycle scans with a scan rate of 0.1 V s−1. All scans were in 90:10 0.1 M KCl: ethanol solution at room temperature.
Figure 1 illustrates the first cycle where there is a peak relating to an irreversible reduction at + 0.19 V. This can be attributed to the nitrobenzene deposition onto the electrode. It is assumed that after the first scan a monolayer is formed. The reduction of peaks after scans 2, 3 and 4 is due to the formation of a multilayer through the addition of nitrobenzene to the ortho position of the pre-deposited layer which blocks the electron transfer from the ITO electrode into the solution [36, 37].
The voltammograms in Figure 2 shows that for the first scan an irreversible reduction peak is obtained at - 0.87 V, which is attributed to the conversion of nitrobenzene to aniline after immersing the electrode into a solution of 0.1 M KCl: ethanol. Towards the completion of scan 1, there was an oxidation peak at - 0.3 V which can be attributed to the reverse step through a two electrons oxidation/reduction mechanism. For subsequent (2, 3, and 4) voltammograms there was a reduction due to self-inhibition through blockage of the electrode surface [38].
4.1.1. Ferrocene tagged antibody immobilization on modified ITO electrode surface
Bare antibodies give a negligible measurable electrochemical response over the addition of an antigen and therefore, the antibodies were chemically labelled with a redox tag. Ferrocenecarboxaldehyde (Fc-CHO) was utilized in a two-stage process; synthesis of Ab-Fc. This was achieved by the reaction between the Fc-CHO and the antibody free amino group to form an unstable Schiff base compound. This compound was further reduced by the addition of sodium borohydride to form a Schiff base [39]. This was followed by the immobilization of Fc-Cho onto the modified electrode. This was done by the addition of an activation solution consisting of EDC and Sulfo-NHS prepared in PBS, which was used as a linker to bind the antibody to the ITO electrode surface via an amide linkage. The EDC compound activates the acid group by forming o-acylisourea, then by using the NHS-Sulfo, a fast transformation of the NHS-ester occurs, which interacts with the amino group on the electrode to form the amide linkage [40]. This enables the acid groups residing at the base of the antibody heavy chain to bind to the electrode end up, i.e. in the desired position to interact with the antigen.
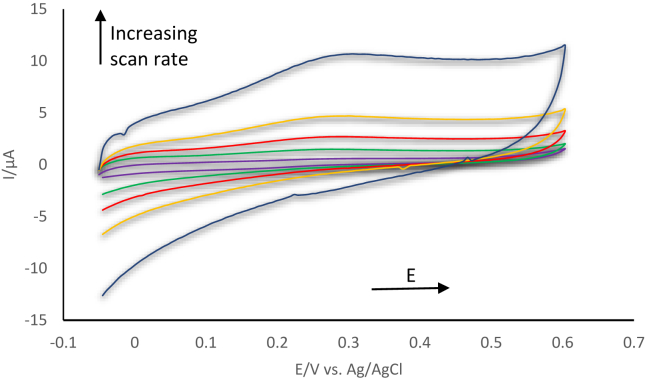
As can be seen from Figure 3; an increase in the scan rate leads to an increase in current and an obvious oxidation peak at + 0.25 V confirm the ferrocenium cation oxidation from ferrocene on the ITO electrode with a reduction peak at + 0.15 V that is not clearly visible compared to the oxidation peak. Slower scan rate causes slower analysis time and small signals while faster scan rate effects the electrode kinetic where ITO electrode resistance will contribute to the signal, therefore 100 mV s−1 was selected for all electrochemical immunoassay experiments.
Figure 3.
Cyclic voltammogram of ferrocene labelled anti-cortisol on a modified ITO electrode surface with various scan rates ranging from 10 mV s−1 to 1000 mV s−1.
4.2. Optimization of incubation time
To optimize the immunoassay protocol, the incubation time for the antigen needed to be studied (10, 15, 20, 30, 45 and 60 min) was chosen to keep a realistic time for point of care (POC) measurements.
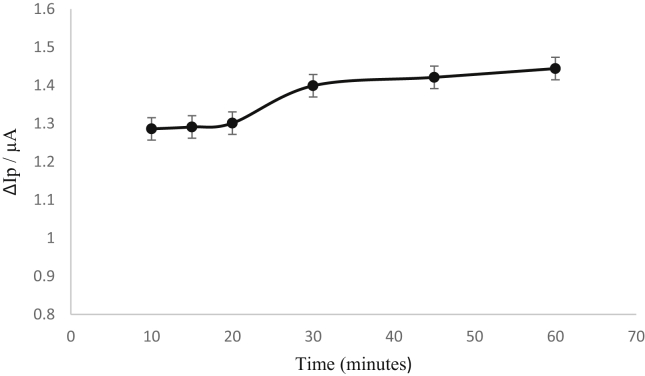
Figure 4 gives the effect of incubation time on the current signal, it can be concluded that there was an increase in the current signal until 30 min after which the current signal began to plateau. Therefore 30 min was selected as the optimum incubation time to carry out the electrochemical immunoassay.
Figure 4.
The graph indicates the change in current for 10, 15, 20, 30, 45 and 60 min incubation time for cortisol of 50 ng ml−1 respectively from cyclic voltammetry measurements. Scan rate of 100 mV s−1.
4.3. Construction of electrochemical calibration graphs for cortisol and cortisone
Calibrations graphs were constructed by the addition of different concentrations of the antigens using both CV and SWV techniques.
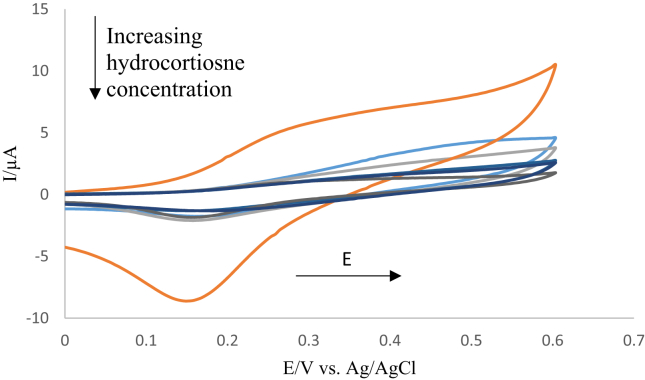
Figure 5 represents the change in current for cyclic voltammetry which corresponds to the various addition of cortisol solutions to the ferrocene tagged anti-cortisol antibody immobilized on the modified ITO electrode. This figure shows the orange line representing the blank (without the addition of standard cortisol), which appears clearly to have an oxidation and reduction peaks at + 0.25 V and +0.15 V respectively. A significant decrease in oxidation and reduction peaks were observed after the addition of cortisol and this continues as the cortisol concentrations increase. This is because antibody para top region binds to the antigen epitope region with the association of primarily electrostatic and hydrophobic interaction. In addition, van der Waals interaction and hydrogen bonding are involved in the antigen-antibody bound system. This leads to blocking out ferrocene moieties attached to the paratope where this causes an alteration in the ferrocene environment i.e. the ferrocene cannot further participate in potential voltammetry [40].
Figure 5.
Cyclic voltammograms of the cortisol standard solutions ranging from (0.001–50 ng ml−1) on the ferrocene tagged anti-cortisol antibody modified ITO electrode for an incubation time of 30 min, with the orange line corresponds to the blank. Scan rate 100 mV s−1 and the measurements were conducted in 10 mM PBS.
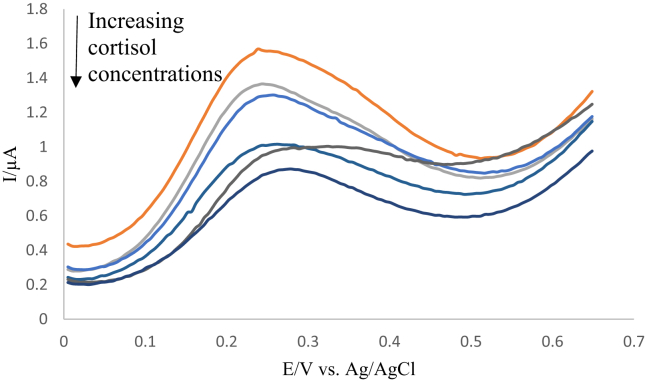
Figure 6 shows the change in current for the standards using square wave voltammetry. It is noticeable that there is a clear peak at + 0.22 V with a drop in the current signal as the cortisol concentrations increase. The signal change was more noticeable with square wave voltammetry compared to cyclic voltammetry, and although the CV technique offers full information about the system while behaviour the SWV technique has the advantage of high sensitivity [41]. Therefore the SWV technique was chosen for the construction of the calibration curves.
Figure 6.
Square wave voltammograms presenting the effect of cortisol standard solutions with concentrations ranging from (0.001–50 ng ml−1) on the current signal after the addition to the ferrocene tagged anti-cortisol antibody attached to the modified ITO electrode. These waves represent the current signal before subtracting from the blank. Measurements were carried out in 10 mM PBS. The orange line represents the blank prior to the addition of cortisol, frequency 25 Hz, amplitude 1 mV.
In order to determine the concentration of cortisol and cortisone in biological samples (Zebrafish whole-body and artificial saliva samples), calibrations graphs were built using the following concentrations (0.001, 0.005, 0.01, 0.1, 1, 10, 25, and 50 ng ml−1).
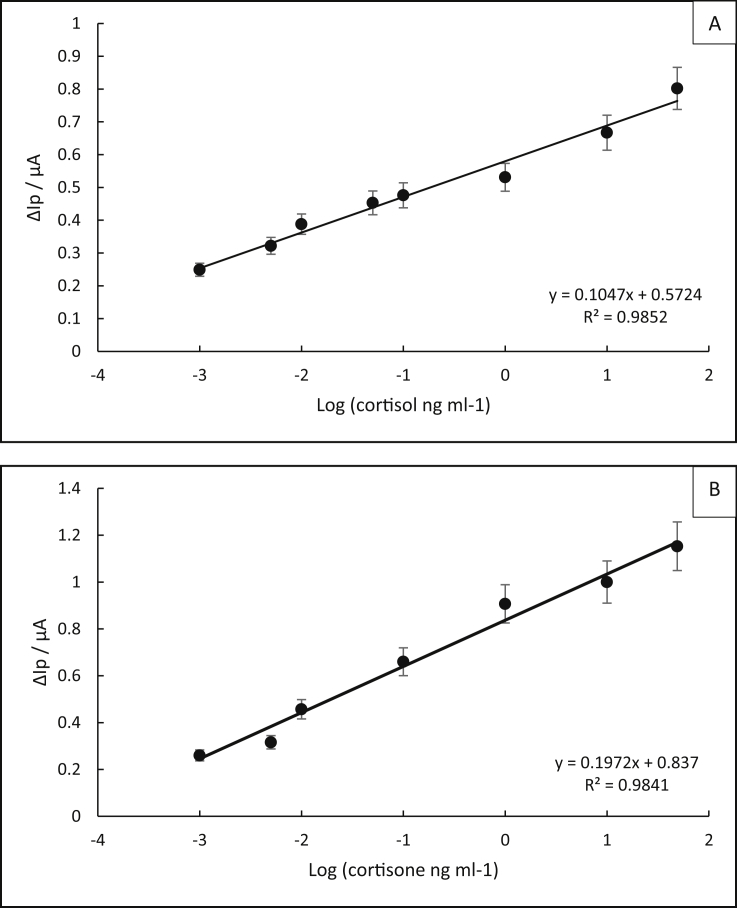
Figure 7 (A and B) shows the standard cortisol and cortisone calibration graphs using square wave voltammetry, where Δ peak current (ΔIp) is plotted against log of cortisol and cortisone concentrations. The plot shows a linear correlation trend between (0.001–50 ng ml−1) which is within the standard range for cortisol and cortisone in both Zebrafish whole-body sample and artificial saliva sample with a correlation coefficient 0.9852 for cortisol and slightly lower at 0.9841 for cortisone. The limit of detection (LOD) was calculated and found to be 1.03 pg ml−1 and 0.68 pg ml−1 for cortisol and cortisone, respectively.
Figure 7.
Calibration graph of (A) cortisol and (B) cortisone standard solutions, respectively with ΔIp current peak plotted against their concentrations. The error bar indicates the standard deviation (n = 3). The measurements were done using a square wave technique. The frequency of 25 Hz and an amplitude of 1 mV.
4.4. Determination of biological samples using electrochemical immunoassay
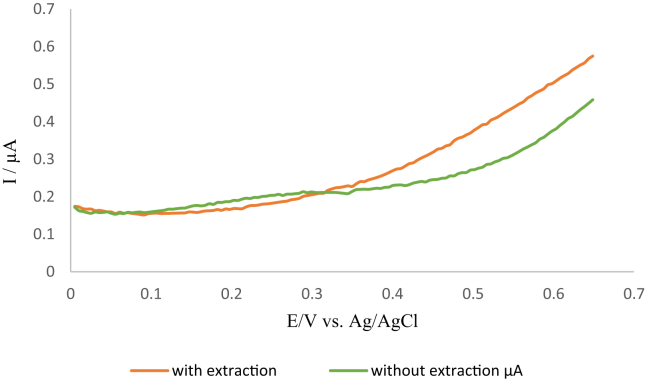
Having established the system applicability to carry out an electrochemical immunoassay, cortisol and cortisone levels were measured using the new platform in the Zebrafish whole-body sample. A comparison was made between measurements with and without the extraction procedure. The results obtained are shown in Figure 8.
Figure 8.
Square wave voltammograms showing the difference in current between the Zebrafish whole-body sample before and after extraction with diethyl ether.
As can be seen from the above figure the two square wave voltammograms differ from each other, however, at the peak potential +0.26 V there is a negligible difference for both square wave voltammograms which shows the sensitivity and selectivity of the used procedure and allows the uses of the sample without the extraction. Also, there was a shift in the peak current to +0.26 V from the observed from the buffer voltammogram at + 0.22 V, this can be attributed to the matrix used but the same trend is observed by decreasing the signal. Therefore and to minimize the number of steps which is one of the targets' aims of this study, the following experiments were done without the extraction.
Recovery and RSD values were calculated and summarized in Table 1 for both cortisol and cortisone.
Table 1.
The Recovery and RSD values of cortisol and cortisone standard solution spiked in the Zebrafish whole-body sample (n = 3).
| Analyte | Concentration added (ng ml−1) | Recovery % | RSD % |
|---|---|---|---|
| Cortisol | 50 ng ml−1 | 87.7 % | 1.12 % |
| Cortisone | 10 ng ml−1 | 82.6 % | 2.22 % |
From Table 1 cortisol recovery was 87.7 % and cortisone was 82.6 %. Also, the values of the RSD was 1.12 % for cortisol and 2.22 % for cortisone, respectively. These results show the applicability of the immunoassay protocol followed by square wave detection for the determination of stress hormones in the Zebrafish whole-body sample.
The other biological sample used to determine stress hormones were artificial saliva. The artificial saliva recipe was prepared and spiked with cortisol (10 ng ml−1) and cortisone (25 ng ml−1) and incubated for 30 min. These concentrations were chosen as they represent the maximum clinical concentration in artificial saliva to analyze the resulting data clearly.
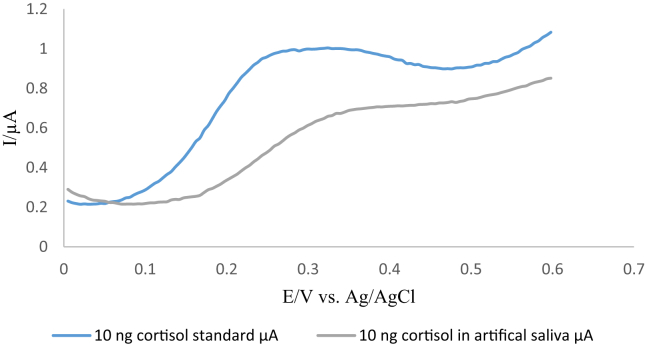
A comparison was carried out between the standard cortisol solution10 ng ml−1 and artificial saliva spiked with 10 ng ml−1 standard cortisol solution. Figure 9 shows that there was a shift in the peak from +0.22 V to +0.33 V resulted from the matrix effect.
Figure 9.
shows square wave voltammograms for the artificial saliva spiked with 10 ng ml−1 cortisol standard solution and compared it with 10 ng ml−1 cortisol standard prepared in PBS. The comparison shows the shift in the peak from +0.22 V to +0.33 V.
The possibility of stress hormones detection in a real sample using electrochemical immunoassay was achieved with a decrease in the current signal compared with the current related to the standard solution. A shift in the current voltammograms was observed which may attribute to the matrix effect. Table 2 summarizes the data obtained from cortisol and cortisone.
Table 2.
The recovery and RSD values of cortisol and cortisone standard solution spiked in the artificial saliva sample for three different electrodes.
| Analyte | Concentration added (ng ml−1) | Recovery % | RSD % |
|---|---|---|---|
| Cortisol | 10 ng ml−1 | 89.00 % | 1.25 % |
| Cortisone | 25 ng ml−1 | 88.49 % | 1.70 % |
The calculation gave a recovery of 89.00 % and 88.49 % for cortisol and cortisone, respectively. For the RSD % values, cortisol gave 1.25 % and more value for cortisone of 1.70 %. Interferences studies were carried out using a range of similar structures for both stress hormones by Jwan et al group showing a minor interference-effect. Also, A comparison study with standard Enzyme-Linked Immunosorbent Assay (ELISA) was done by Jwan et al group giving a good agreement with the new immobilization method [33].
5. Conclusions
A selective and specific method for the quantitative detection of cortisol and cortisone as a stress biomarkers using an immunoassay strategy with square wave voltammetry was developed. Two applications were investigated, firstly the quantitative determination of cortisol and cortisone in Zebrafish for low cost, rapid, environmental analysis. Secondly the detection of cortisol and cortisone in artificial saliva as an example of a point of care detection method. The limit of detection (LOD) obtained for this procedure was 1.03 pg ml−1 for cortisol and 0.68 pg ml−1 for cortisone, respectively. The correlation coefficient was 0.9852 and 0.9841 for cortisol and cortisone, respectively with a linear concentration from (0-50 ng ml−1). Owing to its merits, the new platform can employ for biomarkers monitoring and detection competing conventional technologies and achieving the same accuracy and sensitivity.
Compliance and ethical standards
This study was approved by the University of Hull ethics committee, and all the experiments were done in compliance with the HO schedule 1 methods prepared by HO licence holders.
Declarations
Author contribution statement
Jwan O. Abdulsattar, Gillian M. Greenway, Jay D. Wadhawan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Ministry of Higher Education and Scientific Research in Iraq.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Mustansiriyah University, Baghdad-Iraq for its support in the present work. We are grateful to all academics and staff in the School of Mathematics and Physical Sciences at the University of Hull, UK for their help.
References
- 1.Zarei M. Portable biosensing devices for point-of-care diagnostics: recent developments and applications. Trac. Trends Anal. Chem. 2017;91:26–41. 2017/06/01/ [Google Scholar]
- 2.Xu D., Huang X., Guo J., Ma X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018;110:78–88. doi: 10.1016/j.bios.2018.03.018. 2018/07/01/ [DOI] [PubMed] [Google Scholar]
- 3.Zarei M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017;98:494–506. doi: 10.1016/j.bios.2017.07.024. 2017/12/15/ [DOI] [PubMed] [Google Scholar]
- 4.Lin C.-C., Wang J.-H., Wu H.-W., Lee G.-B. Microfluidic immunoassays. JALA: J. Assoc. Lab. Autom. 2010;15(3):253–274. [Google Scholar]
- 5.Ahmed S.A., Laa M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: a review. RSC Adv. 2016;6:24995–25014. [Google Scholar]
- 6.Hervás M., López M.A., Escarpa A. Electrochemical immunosensing on board microfluidic chip platforms. Trac. Trends Anal. Chem. 2012;31:109–128. [Google Scholar]
- 7.Darwish I.A. Immunoassay methods and their applications in pharmaceutical analysis: basic methodology and recent advances. Int. J. Biomed. Sci. 2006;2(3):217–235. [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci F., Adornetto G., Palleschi G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta. 2012;84:74–83. 2012/12/01/ [Google Scholar]
- 9.Koivusalo AKaSG Mirkka. Sensors, transducers, and effectors that regulate cell size and shape. J. Biol. Chem. 2009;284:6595–6599. doi: 10.1074/jbc.R800049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad C.D. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19(6):395–412. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W., Kirschbaum C., Grass J., Stalder T. LC–MS based analysis of endogenous steroid hormones in human hair. J. Steroid Biochem. Mol. Biol. 2016;162:92–99. doi: 10.1016/j.jsbmb.2015.12.022. 2016/09/01/ [DOI] [PubMed] [Google Scholar]
- 12.Parks C.G., Miller D.B., McCanlies E.C., Cawthon R.M., Andrew M.E., DeRoo L.A. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiology Biomarkers. Prevention. 2009;18(2):551. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anne E., Kalomiris R.A.P., Kiel E.J. The relation between specific parenting behaviors and toddlers’ early anxious behaviors is moderated by toddler cortisol reactivity. J. Abnorm. Child Psychol. 2019;47(8):1367–1377. doi: 10.1007/s10802-019-00522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilliams T.G., Edwards L. Chronic stress and the HPA axis. Standard. 2010;(2):1–12. [Google Scholar]
- 15.Juster R.-P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. 2010/09/01/ [DOI] [PubMed] [Google Scholar]
- 16.Kajantie E., Räikkönen K. Early life predictors of the physiological stress response later in life. Neurosci. Biobehav. Rev. 2010;35(1):23–32. doi: 10.1016/j.neubiorev.2009.11.013. 2010/09/01/ [DOI] [PubMed] [Google Scholar]
- 17.Duan H., Wang L., Zhang L., Liu J., Zhang K., Wu J. The relationship between cortisol activity during cognitive task and posttraumatic stress symptom clusters. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0144315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno-Guzman M., Agüí L., Gonzalez-Cortes A., Yanez-Sedeno P., Pingarron J. Gold nanoparticles/carbon nanotubes/ionic liquid microsized paste electrode for the determination of cortisol and androsterone hormones. J. Solid State Electrochem. 2013;17(6):1591–1599. [Google Scholar]
- 19.Moreno-Guzmán M., González-Cortés A., Yáñez-Sedeño P., Pingarrón J.M. Multiplexed ultrasensitive determination of adrenocorticotropin and cortisol hormones at a dual electrochemical immunosensor. Electroanalysis. 2012;24(5):1100–1108. [Google Scholar]
- 20.Liu X., Zhao R., Mao W., Feng H., Liu X., Wong D.K. Detection of cortisol at a gold nanoparticle| Protein G–DTBP-scaffold modified electrochemical immunosensor. Analyst. 2011;136(24):5204–5210. doi: 10.1039/c1an15411g. [DOI] [PubMed] [Google Scholar]
- 21.Arya S.K., Chornokur G., Venugopal M., Bhansali S. Antibody functionalized interdigitated μ-electrode (IDμE) based impedimetric cortisol biosensor. Analyst. 2010;135(8):1941–1946. doi: 10.1039/c0an00242a. [DOI] [PubMed] [Google Scholar]
- 22.Hauer D., Weis F., Krauseneck T., Vogeser M., Schelling G., Roozendaal B. Traumatic memories, post-traumatic stress disorder and serum cortisol levels in long-term survivors of the acute respiratory distress syndrome. Brain Res. 2009;1293:114–120. doi: 10.1016/j.brainres.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Smajdor J., Piech R., Rumin M., Bator B.P. New high sensitive hydrocortisone determination by means of adsorptive stripping voltammetry on renewable mercury film silver based electrode. Electrochim. Acta. 2015;182:67–72. [Google Scholar]
- 24.Kim K.S., Lim S.R., Kim S.-E., Lee J.Y., Chung C.-H., Choe W.-S. Highly sensitive and selective electrochemical cortisol sensor using bifunctional protein interlayer-modified graphene electrodes. Sensor. Actuator. B Chem. 2016 [Google Scholar]
- 25.Vabbina P.K., Kaushik A., Pokhrel N., Bhansali S., Pala N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015;63:124–130. doi: 10.1016/j.bios.2014.07.026. 2015/01/15/ [DOI] [PubMed] [Google Scholar]
- 26.Vabbina P.K., Kaushik A., Tracy K., Bhansali S., Pala N. SPIE; 2014. Zinc Oxide Nanostructures for Electrochemical Cortisol Biosensing. 8. [Google Scholar]
- 27.Ueno Y., Furukawa K., Hayashi K., Takamura M., Hibino H., Tamechika E. Graphene-modified interdigitated array electrode: fabrication, characterization, and electrochemical immunoassay application. Anal. Sci. 2013;29(1):55–60. doi: 10.2116/analsci.29.55. [DOI] [PubMed] [Google Scholar]
- 28.Kämäräinen S., Mäki M., Tolonen T., Palleschi G., Virtanen V., Micheli L. Disposable Electrochemical Immunosensor for Cortisol Determination in Human Saliva. Talanta. 2018;188:50–57. doi: 10.1016/j.talanta.2018.05.039. 2018/10/01/ [DOI] [PubMed] [Google Scholar]
- 29.Venugopal M., Arya S.K., Chornokur G., Bhansali S. A realtime and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sensor Actuator Phys. 2011;172(1):154–160. doi: 10.1016/j.sna.2011.04.028. 2011/12/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aparajita SinghAjeet Kaushik RKaNB Electrochemical sensing of cortisol: a recent update. Appl. Biochem. Biotechnol. 2014;174(3):1115–1126. doi: 10.1007/s12010-014-0894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhulkar S., Alwarappan S., Liu G., Li C.-Z. Amperometric micro-immunosensor for the detection of tumor biomarker. Biosens. Bioelectron. 2009;24(12):3524–3530. doi: 10.1016/j.bios.2009.05.002. 2009/08/15/ [DOI] [PubMed] [Google Scholar]
- 32.Lazcka O., Del Campo F.J., Munoz F.X. Pathogen detection: a perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007;22(7):1205–1217. doi: 10.1016/j.bios.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Abdulsattar J.O., Greenway G.M. A sensitive chemiluminescence based immunoassay for the detection of cortisol and cortisone as stress biomarkers. J. Anal. Sci. Technol. 2019;10(1):34. 2019/12/06. [Google Scholar]
- 34.Canavello P.R., Cachat J.M., Beeson E.C., Laffoon A.L., Grimes C., Haymore W.A. Measuring endocrine (cortisol) responses of zebrafish to stress. Zebrafish neurobehavioral protocols. 2011:135–142. [Google Scholar]
- 35.West C.E., Hardcastle J.L., Compton R.G. Sono-electroanalytical determination of lead in saliva. Electroanalysis. 2002;14(21):1470–1478. [Google Scholar]
- 36.Wright K.J. Hull; 2015. Development towards a point-of-care system. To Monitor Pregnancy and Fertility Biomarkers. [Google Scholar]
- 37.Laforgue A., Addou T., Bélanger D. Characterization of the deposition of organic molecules at the surface of gold by the electrochemical reduction of aryldiazonium cations. Langmuir. 2005;21(15):6855–6865. doi: 10.1021/la047369c. 2005/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Gui A.L., Liu G., Chockalingam M., Le Saux G., Luais E., Harper J.B. A comparative study of electrochemical reduction of 4-nitrophenyl covalently grafted on gold and carbon. Electroanalysis. 2010;22(16):1824–1830. [Google Scholar]
- 39.Okochi M., Ohta H., Tanaka T., Matsunaga T. Electrochemical probe for on-chip type flow immunoassay: immunoglobulin G labeled with ferrocenecarboaldehyde. Biotechnol. Bioeng. 2005;90(1):14–19. doi: 10.1002/bit.20313. [DOI] [PubMed] [Google Scholar]
- 40.Dou Y.H., Haswell S.J., Greenman J., Wadhawan J. Voltammetric immunoassay for the detection of protein biomarkers. Electroanalysis. 2012;24(2):264–272. [Google Scholar]
- 41.Hoyos-Arbeláez J., Vázquez M., Contreras-Calderón J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: a review. Food Chem. 2017;221:1371–1381. doi: 10.1016/j.foodchem.2016.11.017. 2017/04/15/ [DOI] [PubMed] [Google Scholar]