Abstract
Purpose
The use of platelet-rich plasma (PRP) to treat canine osteoarthritis has gained support within the scientific community. PRP effects on pain control for degenerative joint disease induced by naturally occurring cranial cruciate ligament instability are limited, particularly in a cohort of dogs with chronic instability and osteoarthritis (>12 months), representing a commonly encountered clinical population that often defaults to medical management. The goal of this study was to assess the effects of a single intra-articular PRP injection into an effected stifle in this cohort, to assess response to treatment, quantitative kinetic data as it relates to percent body weight for peak vertical force (PVF) and vertical impulse (VI) were collected, and symmetry indices related to PVF were determined.
Methods
Twelve dogs with unilateral or bilateral osteoarthritis with ruptured, non-stabilized cranial cruciate ligaments over 12 months duration were identified. Unilateral injections of 2.5 mL of a PRP preparation into the most severely affected stifle based on kinetic analysis was performed. Repeat pressure-sensitive walkway analysis was conducted monthly for 3 months. Peak vertical force (PVF) and vertical impulse (VI) were normalized to body weight and identified in all four limbs. Previously published symmetry indices regarding PVF were calculated, comparing the treated limb with the contralateral limb, ipsilateral forelimb, and contralateral forelimb.
Results
After treatment, hind limb symmetry index (SI) regarding PVF showed improved symmetry, suggesting more weight placement at all-time points after injection of the most affected limb (p < 0.01). Further, PVF asymmetry indices assessing contralateral fore (CFL) and hind limb (CHL) as well as ipsilateral forelimb (IFL) revealing a significant decrease from baseline for CHL at week 4 (p = 0.02), but not weeks 8 and 12. The CFL showed decreased differences in symmetry from baseline at each time point (p = 0.03). There were no statistically significant changes in PVF or VI over time in treated dogs.
Conclusion
A single injection of PRP improved kinetics for minimally 4 weeks with some data suggesting an effect for up to 12 weeks. Therefore, PRP might be a viable therapeutic option for instability and inflammation associated with chronic osteoarthritis due to cranial cruciate ligament disease in the non-surgical patient.
Keywords: platelet, stifle, canine, osteoarthritis and cruciate
Introduction
Osteoarthritis (OA) is the most common form of arthritis and affects over 20% of the canine population at some point during a dog’s lifetime.1 Commonly, cranial cruciate ligament disease leads to osteoarthritis of the stifles accounting for billions of annual dollars in medical and surgical treatments.2,3 Nonsurgical treatment was common in 2004 and accounted for approximately 2.8 million dollars in costs with an individual mean of $240 per dog reported.3 Such costs and numbers for non-surgical management are likely under-reported and may reflect patients that are precluded from surgery due to access, finances, or comorbidities. Common non-surgical treatments include non-steroidal anti–inflammatory drugs (NSAIDs), weight management, joint supplements, and physiotherapy.4–6 Because NSAIDs may have potentially serious adverse effects, are contraindicated in many patients with a history of intolerance or certain comorbidities, and might not provide sufficient pain control, other analgesic options deserve exploration.7–9
Orthobiologic products (such as mesenchymal stem cells, bone marrow aspirate concentrate, and platelets rich products) are showing promise as safe and effective treatments for musculoskeletal diseases including osteoarthritis in people and animals.10–18 Platelets possess anabolic and anti–inflammatory properties attributed to the delivery of various growth factors and cell signaling molecules that can modulate inflammation and promote tissue healing.19,20 Because various methods exist to create different platelet-derived orthobiologics, we define platelet-rich plasma (PRP) as any product in which the concentration of platelets is increased relative to whole blood and partially or fully isolated from leukocytes and red cells within a plasma medium.15,20-22 More recently, the use of platelet-rich products has been shown to help alleviate pain and/or improve function including objective gait analysis in dogs with both naturally occurring and experimental models of OA.13–16,23
We hypothesized that a single intra-articular injection of pure PRP (no white or red cells within the product) into the more severely affected stifle would cause substantial positive changes in kinetic gait symmetry in dogs with unilateral or bilateral chronic osteoarthritis attributed to long-term (>12 months) naturally occurring cranial cruciate ligament disease. A validated pressure-sensitive walkway system was employed at baseline and every 4 weeks for 12 weeks post-treatment to determine peak vertical force (PF) and vertical impulse (VI) normalized to body weight in all limbs and calculate selected modified symmetry indices based on prior literature from PVF.24–27
Materials and Methods
The study population consisted of client-owned dogs presenting to Cornell University Hospital for Animals for evaluation and treatment of a lameness with a 12-month or longer history of cranial cruciate ligament disease in one or both stifles. All dogs were confirmed to have cranial cruciate rupture through sedated physical examination, revealing instability through positive tibial compression and cranial drawer tests. Additionally, radiographic changes consistent with long-term cruciate insufficiency were confirmed and included effusion, moderate to severe radiographic degenerative joint disease, and evidence of medial buttress. Orthopedic and neurologic exams helped exclude other contributing causes of lameness. Each dog had an initial complete blood count ([CBC] Bayer Advia 120, Siemens Corp., New York, NY, USA) and serum chemistry analysis (Hitachi 911, Roche Diagnostics, Indianapolis, IN, USA) performed to rule out any underlying metabolic or organ dysfunction that might preclude enrollment.
All clients consented to the study procedure prior to enrollment, which was approved by the Cornell University Animal Care and Use Committee (2016-0036), and before any procedures informed consent with signature was completed, with all procedures following best practice guidelines. During the trial, dogs were only allowed to receive NSAIDs, fish oil, and/or glucosamine/chondroitin sulfate without any change in these medications, and must have been on these medications for at least 6 weeks prior to the 12-week study period as standard of care for the disease. Dogs were excluded if they had evidence of kidney, uncontrolled endocrine, neurologic, or neoplastic disease; if they had a temperament not suited for gaiting on a lead; or were undergoing physical therapy. Every dog was fed its regular commercial dry and/or wet diet with no changes during the trial. All dogs had musculoskeletal examinations at each visit to determine if any changes in lameness of pain were evident that might preclude them from continuation in the study.
The preparation of PRP was accomplished using a modified protocol of a commercial kit previously described.22 Modification consisted of taking the 4 ml recovered from the kit and placing this leukocyte rich PRP into a 4 mL citrate vacutainer and centrifugation at 1250 g for 3 mins in the manufacturer’s centrifuge (Harvest, Terumo Inc., Lakewood CO, USA). This final spin caused complete sedimentation of the white and red blood cells allowing platelets to remain in the supernatant plasma, which was collected for injection. One-half of a milliliter of the pure PRP was saved for an automated complete blood count in conjunction with assessment of the whole blood to assess relative platelet enrichment. 2.5 mL of the PRP was then injected into the stifle of the hind limb exhibiting the lowest measured baseline peak vertical force (PVF) as a % body weight distribution during initial symmetry assessment.
Prior to sedation for PRP delivery, all dogs were trotted across a pressure-sensitive walkway (Tekscan Walkway 2.0, Boston, MA) at a natural velocity between 1.4 and 2.3 m/s for a total of 5 valid passes (as defined by Lascelles and colleagues 2006) assessing a minimum total of 15 footfalls per each hind limb. This was repeated 4, 8 and 12 weeks after PRP delivery. Passes on the walkway were considered valid if they were within 0.3 m/s of the mean speed of the baseline data collected and video imagery confirmed appropriate body posture and direction with foot strikes falling within the recording area of the walkway—similar to other recently published protocols collecting both peak vertical force (PVF) and vertical impulse (VI) data.24,25
A symmetry index for PVF was then derived from the hind limb data as described by Budsberg et al26. The symmetry index (SI) calculations were represented by the more affected hind limb PVF divided by the contralateral hind limb PVF providing an SI value less than one at the initial visit. For all follow-up visits, the numerator and denominator limbs remained the same allowing for symmetry values that could potentially be greater than one since many of our patients were bilaterally affected.
A second method of evaluation of symmetry was employed based on the prior work of Bockstahler et al27. This method examined the relative PVF forces of a single limb as it relates to the other three limbs and is defined as an Asymmetry Index (ASI). Therefore, overall peak vertical force differences were calculated for each respective limb in relationship to the treated limb to assess for decreases or increases in (ASI) for each limb with zero representing exact symmetry. This method separates it from the SI of Budsberg and colleagues as it accounts for load redistribution to other limbs besides the contralateral paired limb. It is important to note that paired forelimbs and paired hindlimbs should be relatively symmetrical and a value of near-zero in a normal dog whereas a forelimb paired with a hindlimb should provide a calculation that is not near zero (approximately 25–30% different) in a normal dog. Deviations from absolute symmetry are expressed as a percentage between paired limbs and might be tracked as change over time compared to baseline.27 Again, the limb variables remained in the same position in the equation, regardless of whether the most lame hindlimb changed during the course of the study. Because the forces from the more sound limb were subtracted from the forces of the lame limb for consistency with the original study, all derived values were multiplied by negative 1 so that the ASI of the baseline lame limb was represented by positive values for the sake of presentation.
Equations are as follows (LH represents the lamest hindlimb at baseline exam):
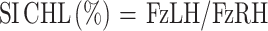 |
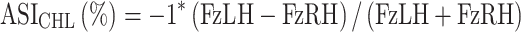 |
where ASI CHL is the ASI of the given parameter of the paired hindlimb with FzLH being the lame or treated hindlimb and the FzRH being the less affected hindlimb that was not treated.
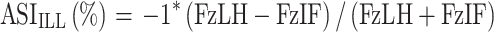 |
where ASI ILL is the ASI of the given parameter of the ipsilateral forelimb with FzLH being the lame or treated hindlimb and the FzIF being the ipsilateral forelimb,
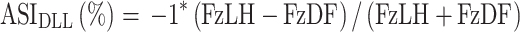 |
where ASI DLL is the ASI of the given parameter of the diagonal forelimb with FzLH being the lame or treated hindlimb and the FzDL being the diagonal forelimb.
Statistical Analysis
D’Agostino normality testing was used to assess normality for SI, ASI, PVF and VI data. For repeated measures data that were not normally distributed a non-parametric Friedman’s analysis was utilized (SI, ASI), while one-way analysis of variance with repeated measures (PVF, VI) was utilized for normally distributed data. Dunn’s post hoc testing was used to assess differences from baseline for all time points for both ASI and SI calculations when significances were noted. Wilcoxon-signed rank was used to assess platelet concentrating ability, and weights at the beginning and end of the trial. A p-value of less than 0.05 was deemed significant for all testing. All statistics and graphs were generated using Graphpad Prism (Graphpad Prism 6.0, LaJolla, CA, USA).
Results
Twelve dogs were enrolled in the study based on history, radiographic evidence of severe osteoarthritis in one or both stifle joints, cranial draw tests, development of medial buttress and pronounced periarticular fibrosis and osteophytosis were observed in all dogs. Radiographic evidence of moderate to severe osteoarthritis with tibia caudal osteophytosis was observed in all dogs. Nine of the 12 dogs had bilateral cranial cruciate ligament disease with subsequent degenerative joint disease of the stifle. At no time during the trial did an owner discuss any acute lameness, nor did evaluation reveal a meniscal “click” or severe pain found that might be attributed to developing meniscal pathology. Subsequent examinations during follow up revealed no new orthopedic or neurological findings compared to baseline. Dog mean weights prior to injection were 28.0 ± 7.0 kg, and the mean weight at week 12 was 28.3 ± 6.8 kg showing no significant difference (p = 0.44; Table 1). The average whole blood platelet concentration was 241 ± 31 thou/ul and the PRP concentrations were approximately sixfold higher with a mean of 1557 ± 492 (p < 0.01; Table 1) with complete removal of red and white blood cells.
Table 1.
Breed, Age, Gender, Weight (kg), Duration of Cruciate Deficit, Whole Blood and PRP Platelet Concentrations and Limb(s) Affected of the 12 Dogs Enrolled Dogs
| Breed | Age | Gender | Begin Weight | End Weight | WB Platelet | PRP Platelet | Disease Duration | Uni/Bilateral Affected |
|---|---|---|---|---|---|---|---|---|
| Foxhound | 9 | FS | 23.9 | 24.5 | 277 | 2359 | 16 mos | Bilateral |
| Foxhound | 11 | MC | 32.9 | 35.9 | 229 | 1348 | >24 mos | Bilateral |
| Pitbull | 5 | FS | 29.8 | 30.3 | 277 | 1910 | 14 mos | Bilateral |
| Mixed | 6 | MC | 22.0 | 23.3 | 254 | 1063 | >24 mos | Unilateral -R |
| Pitbull | 9 | FS | 34.5 | 34.3 | 275 | 1964 | >24 mos | Bilateral |
| Mixed | 6 | FS | 22.6 | 23.0 | 232 | 1339 | >24 mos | Bilateral |
| Mixed | 11 | FS | 36.6 | 35.7 | 210 | 1290 | >24 mos | Bilateral |
| Boxer | 4 | MC | 21.2 | 22.4 | 270 | 2146 | >24 mos | Unilateral -R |
| Doberman | 9 | FS | 35.5 | 33.7 | 250 | 2029 | >24 mos | Unilateral - L |
| Pitbull | 12 | FS | 34.9 | 34.3 | 228 | 1202 | >24 mos | Bilateral |
| Beagle | 8 | MC | 15.2 | 14.3 | 179 | 858 | >24 mos | Bilateral |
| GSP | 12 | FS | 27.2 | 28.1 | 213 | 1182 | >24 mos | Bilateral |
Abbreviations: GSP, German Shorthair Pointer; Pitbull, American Staffordshire Terrier; WB, whole blood; PRP, platelet-rich plasma.
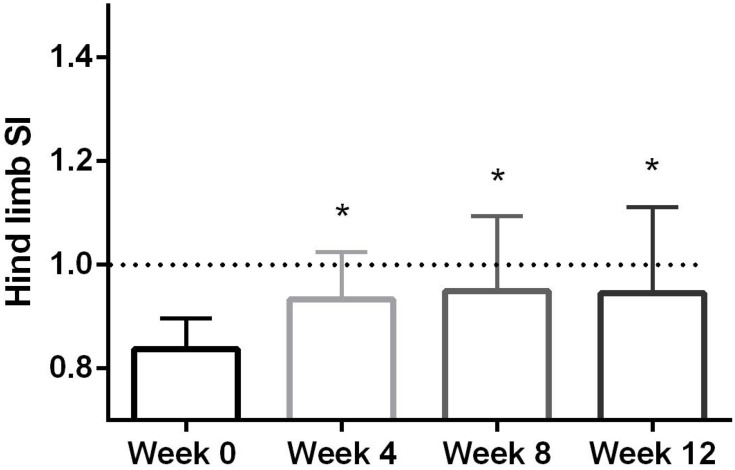
The hind limb SI evaluations had a median (range) pre-PRP injection was 0.84 (0.74–0.92), while on week 4 the median was significantly higher 0.94 (0.77–1.09) (p < 0.01; Figure 1). By week 8, the median SI was also significantly elevated at 0.96 (0.77–1.31) (p < 0.01), with the final 12-week evaluation being significantly higher than baseline at 0.91 (0.8–1.37) (p < 0.01; Figure 1).
Figure 1.
Column plot of hind limb mean symmetry index (SI) representing the mean and standard deviation. Dotted line at 1.0 represents perfect symmetry. *Indicates a significant difference (p < 0.01) between baseline symmetry index before PRP injection into worse affected stifle at each time point.
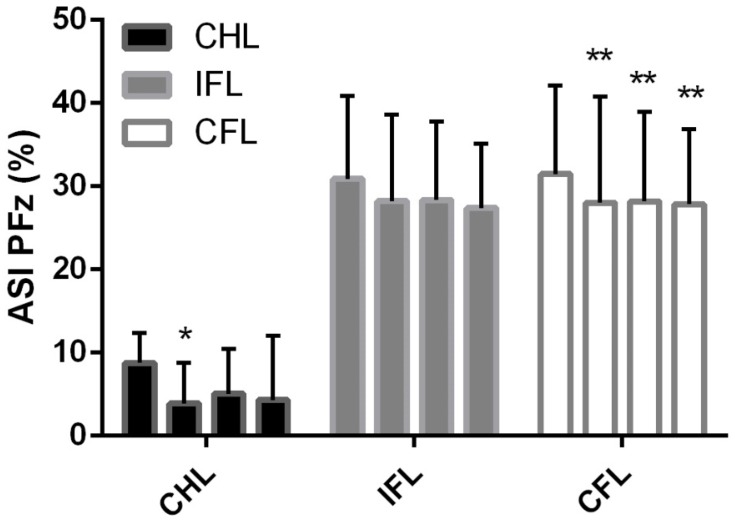
When assessing the ASI over time the hind limb asymmetry index decreased toward zero and was significant for week 4 only (Table 2 & Figure 2; p=0.02). The ipsilateral forelimb ASI showed no significant changes over time, while the contralateral forelimb ASI showed a significant change toward zero from baseline which persisted over all time points (Table 2 and Figure 2; p = 0.03).
Table 2.
Mean and Standard Deviation Asymmetry Index (ASI) Calculations for PVF Based on Treated Hind Limb for the Contralateral Hind Limb (CHL), Ipsilateral Fore Limb, and Contralateral Fore Limb at Weeks 0, 4, 8 and 12
| CHL | IFL | CFL | |
|---|---|---|---|
| Week 0 | 8.3 ± 3.6 | 30.8 ± 10.0 | 31.4 ± 10.6 |
| Week 4 | 3.8 ± 4.9 * | 28.2 ± 10.4 | 28.0 ± 12.7 * |
| Week 8 | 5.0 ± 5.4 | 28.3 ± 9.4 | 28.2 ± 10.8 * |
| Week 12 | 4.3 ± 7.7 | 27.3 ± 7.7 | 27.8 ± 9.0 * |
*Indicates a statistically significant difference from week 0 for the prospective limbs (p < 0.05).
Figure 2.
Bar graph plot with mean and standard deviation of contralateral hind limb (CHL), ipsilateral forelimb (IFL) and contralateral forelimb (CFL) intervals with zero representing absolute symmetry of all limbs at a trot. *Indicates a significant difference (p = 0.02) between baseline asymmetry index (ASI) before PRP injection into worse affected stifle at week 4 for CHL, **Indicates a significant difference (p < 0.03) between baseline ASI before PRP injection into worse affected stifle at week 4, 8 and 12 for CFL.
PVF and VI changed on the treated hind limb, showing an increase in weight-bearing forces at all-time points after treatment; however, these differences were not significant (Tables 3 and 4, respectively). Conversely, the contralateral hind limb showed a decrease in PVF and VI from week 0 to week 12, yet this was not deemed significant either. Similarly, both the IFL and CFL showed decreases over time which was not statistically significant (Tables 3 and 4, respectively).
Table 3.
Mean and Standard Deviation Peak Vertical Force Calculations as a Percentage of Body Weight Based on Treated Hind Limb for the Treated Affected Hind Limb (AHL), Contralateral Hind Limb (CHL), Ipsilateral Fore Limb (IFL), and Contralateral Fore Limb (CFL) at Weeks 0, 4, 8 and 12
| AHL | CHL | IFL | CFL | |
|---|---|---|---|---|
| Week 0 | 44.3 ± 8.3 | 52.8 ± 7.7 | 85.4 ± 20.6 | 86.7 ± 17.6 |
| Week 4 | 45.9 ± 10.9 | 49.4 ± 8.5 | 83.1 ± 22.6 | 83.6 ± 19.4 |
| Week 8 | 46.1 ± 8.0 | 49.5 ± 10.3 | 83.1 ± 19.4 | 84.7 ± 18.9 |
| Week 12 | 45.3 ± 9.1 | 49.5 ± 10.9 | 81.7 ± 21.1 | 80.9 ± 21.9 |
Table 4.
Mean and Standard Deviation of Vertical Impulse Calculations as a Percentage of Body Weight per Second Based on Treated Affected Hind Limb (AHL), Contralateral Hind Limb (CHL), Ipsilateral Fore Limb (IFL), and Contralateral Fore Limb (CFL) at Weeks 0, 4, 8 and 12
| AFL | CHL | IFL | CFL | |
|---|---|---|---|---|
| Week 0 | 5.0 ± 0.9 | 6.4 ± 1.0 | 11.3 ± 2.8 | 11.5 ± 2.5 |
| Week 4 | 5.5 ± 1.2 | 6.1 ± 1.5 | 11.3 ± 2.4 | 11.4 ± 2.5 |
| Week 8 | 5.4 ± 0.8 | 5.8 ± 1.4 | 11.1 ± 2.3 | 11.0 ± 2.3 |
| Week 12 | 5.3 ± 0.9 | 6.0 ± 1.0 | 10.9 ± 2.0 | 10.9 ± 1.8 |
Discussion
Using a single intra-articular pure PRP product, we demonstrated improvements in symmetry as derived from measured PVFs in dogs over a 3-month period. Such changes are reflective of an improvement in weight-bearing forces of the treated limb over time. Intra-articular injection of PRP is associated with molecular and clinical benefits attributed to the release of anabolic growth factors and other mediators that might reduce local inflammation and improve clinical signs.12,18,19 Consensus is lacking regarding the optimal cellular compositions of PRP (including platelet recovery); however, it is generally theorized that reducing inflammatory leukocytes, especially granulocytes, might be of more benefit when targeting osteoarthritis.28–30 Other canine OA studies have demonstrated a positive clinical response of about 3 months from single intra-articular PRP administration.13,16,23 The type of PRP collection, preparation, method of delivery, species, frequency of delivery, duration of the study, methods of assessment, target joint, underlying pathophysiology, severity and chronicity of disease represent the numerous variables confounding comparisons amongst or within studies. Regardless of composition and concentrations of platelets, our data support other previous findings regarding the use of platelet-rich plasma in both naturally occurring OA due to chronic instability of the stifle.
The Pond-Nuki dog research model is well established in dogs and requires a traumatic severing of the cranial cruciate ligament to induce mechanical stifle instability and subsequent OA. Although the model may not accurately portray the degenerative pathophysiology of naturally occurring canine cranial cruciate disease, it is reflective of generation of OA in a mechanically unstable stifle, similar to our clinical participants, and has been used to study PRP. In a Pond-Nuki model, a series of five intra-articular injections of PRP were compared to control injections of saline and showed improvements in comfort and decreases in inflammation lasting approximately 6 months.15,31 Repeat delivery of the PRP and its relatively earlier PRP intervention over the course of the disease process further distinguishes this study from ours. Yun et al31 similarly demonstrated the benefit of multiple PRP injections in a Pond Nuki OA model regarding subjective lameness scoring, histopathology, inflammation, and mechanical cartilage testing.
The use of PRP in naturally occurring canine cranial cruciate ligament disease with osteoarthritis has also been explored. A randomized controlled trial examined a PRP product delivered by intra-articular administration at the time of cranial cruciate ligament stabilization surgery and then twice following surgery suggested improved recovery. In this study, an improvement in the ground reaction force of the treated limb was noted 90 days after surgery when compared to the control group.16 This study was similar to ours in that the disease was naturally occurring; however, the product; stifle stability; and timing and frequency of administration differed. Lastly, a non-randomized trial investigating the use of PRP on medically managed cranial cruciate ligament disease in Bulldogs showed ground reaction forces positively responded in the treated limbs over 180 days.14 Unlike our study, this trial did not account for rescue analgesic use, or changes in orthopedic or neurological status during repeat kinetic data collections. Additionally, this study utilized only Bulldogs, and PRP was delivered once weekly for 4 weeks. Weekly injections may be impractical for the typical dog owner and had minimal (although significant) positive changes on PVF at day 180 compared to days 60 and 90 where improvement was more dramatic. The use of Bulldog’s in this study lacks the heterogeneity observed across breeds in load distribution due to the unique conformation that likely leads to greater forelimbs loading.32,33
Similar to these latter publications, we chose to analyze the symmetries of ground reaction forces over time as our outcome measurement. The primary kinetic variable of peak vertical force as a percentage of body weight (PVF) and its subsequently used to calculated symmetry indices which has been shown to have the least trial variability and better accuracy in diagnosing lameness in dogs.26,33-35 This consensus was reflected in our data. Although not significant, when examining the PVF and VI data (Tables 3 and 4, respectively) it is evident that there were improvements in the affected limbs as they uniformly remained higher than baseline forces and impulse generated over the 12-week period, while the peak force and impulse on the contralateral and diagonal contralateral limbs decreased uniformly. We, therefore, incorporated two different, but validated methods of calculating asymmetry in our study group from PVFs: one equation through Budsberg (SI) and another through Bockstahler (ASI).26,27
Both SIs and ASIs between paired hindlimbs showed a significant change toward being more symmetrical by week 4 after treatment (Figure 1, Table 2). The SIs between hind limbs maintained this significance for up to 12 weeks after treatment with PRP. Similarly, the ASIs comparing the treated hindlimb to the contralateral forelimb also showed significant change toward symmetry at week 4 and maintained this difference over 12 weeks. Bockstahler et al have shown redistribution of weight from a lame forelimb to the contralateral hindlimb during a symmetric gait.27 A similar, but opposite, partial redistribution of weight from a hindlimb lameness to the forelimbs has been previously confirmed.36,37 Since 9/12 dogs in this study suffered from bilateral hindlimb lameness, the typical redistribution of the majority of weight from the lamer limb to the contralateral hindlimb appears altered or different. When examining the ASI, redistribution to the contralateral forelimb appears to be more sensitive to change; and remains significant over the 12-week trial, while the ASI weight redistribution between hindlimbs is also present for 12 weeks, but is not significant for the full 12-week period. This suggests that dogs with bilateral hindlimb lameness may prefer to redistribute a greater percentage of weight to the forelimbs compared to the contralateral hindlimb when compared to dogs with a single hindlimb lameness. Adding further support to this theory, our baseline ASI between the ipsilateral forelimbs and treated hindlimb was greater (32%) than the healthy dog population (22–27%) that Bockstahler et al previously described.27 These findings highlight the importance of assessing symmetry in multiple limb pairs, especially as a multi-limbed lameness may confound the expected compensatory force redistributions of single-limb lameness and employing multiple methods to calculate symmetry may improve sensitivity to detecting changes.
The use of objective kinetic analysis, particularly in multi-limb lameness patients, has its limitations. For example, SI calculations assume that only one limb is affected by lameness and that resolution of this lameness would result in normal force distributions of all limbs for a given healthy dog. Because 9/12 dogs in our study had bilaterally stifle disease, a dramatic improvement in the treated limb could result in a shift of the SI so that the contralateral hindlimb becomes identified as the lamer limb. In this situation, the SI would reflect values greater than 1 and the ASI would have an inverse (negative) sign. Furthermore, if disease worsened on the contralateral limb, load redistribution to the treated limb may occur, making the treatment falsely appear efficacious and/or making the contralateral limb lamer than the treated limb. Four dogs experienced this shift; however, the injected limb was always clinically better than the contralateral limb at each time point. A common example of a disease acutely worsening within the contralateral stifle is meniscal injury; however, given the chronicity of cruciate insufficiency in our population and the lack of supportive findings on clinical examination, it was deemed unlikely, but surely a limitation to this study. Furthermore, all dogs with unilateral lameness (3) showed significant improvement in their SI and ASI values across all time points.
Although it might be considered another potential limitation of our study, we chose not to include client-specific outcome measurements (CSOMs) because we lacked a placebo control group to weigh outcomes against. Subjective scoring has been associated with a high positive placebo effect in veterinary studies of osteoarthritis, rendering outcome interpretation challenging.38 CSOMs are likely further confounded when animals are experiencing multi-site lameness and pain while treatment is isolated to a single site, such as seen in 75% of our study population. Regardless, client perception of improvement needs further investigation with a greater population of unilateral cruciate disease.
The duration of positive effects when using PRP treatment remains ambiguous, but appear to diminish over time in people.12,19 We observed variation in the duration of response to treatment within our study population. Two dogs exhibited clinical improvement for only 4 weeks, while the other 10 dogs maintained a better SI compared to baseline throughout the 12-week follow up period. We can speculate that other factors not examined might be associated with a better clinical response in some individuals than others. For example, we did not examine tissue samples from joints or grade the degree of osteoarthritis or cruciate disease. Therefore, it is entirely possible that joint health status as defined by structural, mechanical, molecular and cytokine properties could influence response.19,20
Despite some limitations, our study further supports the use of PRP in dogs with chronic cranial cruciate ligament disease and OA. Our study is unique in that we explored the effects of a single PRP injection in the stifles of a mixed-dog population with long-term, naturally occurring, medically managed cranial cruciate ligament disease. Furthermore, our pure PRP product differentiates it from those used in prior studies as we achieved an approximately sixfold increase in platelets without any leukocyte or red cell component. In conclusion, these findings are promising for the treatment of osteoarthritis and/or medically managed cranial cruciate ligament disease in patients that are not surgical candidates for stabilization of their stifle, NSAID use is contra-indicated, or traditional management is insufficient. Regardless, more randomized controlled trials are warranted with larger patient populations and better methods of objectively assessing multi-limb lameness.
Acknowledgments
The authors would like to thank Erin Berthelsen for technical support during collection of the data and Dr. Katherine Walden for data collection and record-keeping for patients during this trial.
Funding Statement
This work was performed using an internal grant from Cornell University Department of Clinical Sciences and donated Kits from Terumo Corporation (Harvest 30 PRP kits).
Abbreviations
LH, lame hind limb identified at baseline and treated; RH, contralateral hind limb to treated limb; IF, ipsilateral Forelimb to treated limb; CF, contralateral forelimb to treated limb; Fz, peak vertical force normalized to % body weight; SI, symmetry index for PVF based on Budsberg et al;26 ASI, asymmetry index for PVF based on Backstahler et al27.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Marshall W, Bockstahler B, Hulse D, Carmichael S. A review of osteoarthritis and obesity: current understanding of the relationship and benefit of obesity treatment and prevention in the dog. Vet Comp Ortho Traum. 2009;22:339–345. doi: 10.3415/VCOT-08-08-0069 [DOI] [PubMed] [Google Scholar]
- 2.Hayashi K, Manley PA, Muir P. Cranial cruciate ligament pathophysiology in dogs with cruciate disease: a review. J Amer Anim Hosp Assoc. 2004;40(5):385–390. doi: 10.5326/0400385 [DOI] [PubMed] [Google Scholar]
- 3.Wilke VL, Robinson DA, Evans RB, Rothschild MF, Conzemius MG. Estimate of the annual economic impact of treatment of cranial cruciate ligament injury in dogs in the United States. J Amer Vet Med Assoc. 2005;227:1604–1607. doi: 10.2460/javma.2005.227.1604 [DOI] [PubMed] [Google Scholar]
- 4.Frye CW, Shmalberg JW, Wakshlag JJ. Obesity, exercise and orthopedic disease. Vet Clinics NA. 2016;46(5):831–841. [DOI] [PubMed] [Google Scholar]
- 5.Duerr FM, Martin KW, Rishniw M, Palmer RH, Selmic LE. Treatment of canine cranial cruciate ligament disease. Vet Comp Ortho Traum. 2014;27(6):478–483. [DOI] [PubMed] [Google Scholar]
- 6.Vasseur PB. Clinical results following non-operative management for rupture of the cranial cruciate ligament in dogs. Vet Surg. 1984;13(4):243–246. [Google Scholar]
- 7.Hunt JR, Dean RS, Davis GN, Murrell JC. An analysis of the relative frequencies of reported adverse events associated with NSAID administration in dogs and cats in the United Kingdom. Vet J. 2015;206(2):183–190. [DOI] [PubMed] [Google Scholar]
- 8.Innes JF, Clayton J, Lascelles BD. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec. 2010;166(8):226–230. [DOI] [PubMed] [Google Scholar]
- 9.Lascelles BD, McFarland JM, Swann H. Guidelines for safe and effective use of NSAIDs in dogs. Vet Therap. 2005;6(3):237–251. [PubMed] [Google Scholar]
- 10.Whitney KE, Liebowitz A, Bolia IK, et al. Current perspectives on biological approaches for osteoarthritis. Ann N Y Acad Sci. 2017;1410:26–43. doi: 10.1111/nyas.2017.1410.issue-1 [DOI] [PubMed] [Google Scholar]
- 11.Block TJ, Garza JR. Regenerative cells for the management of osteoarthritis and joint disorders: a concise literature review. Aesthet Surg J. 2017;37:S9–S15. doi: 10.1093/asj/sjx015 [DOI] [PubMed] [Google Scholar]
- 12.Laver L, Marom N, Dnyanesh L, Mei-dan O, Espregueira-mendes J, Gobbi A. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage. 2017;8:341–364. doi: 10.1177/1947603516670709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahie MA, Ortolano GA, Guercio V, et al. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J Amer Vet Med Assoc. 2013;243:1291–1297. doi: 10.2460/javma.243.9.1291 [DOI] [PubMed] [Google Scholar]
- 14.Vilar JM, Manera ME, Santana A, et al. Effect of leukocyte-reduced platelet-rich plasma on osteoarthritis caused by cranial cruciate ligament rupture: a canine gait analysis model. PLoS One. 2018;12:e0194752, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook JL, Smith PA, Bozynski CC, et al. Multiple injections of leuko reduced platelet rich plasma reduce pain and functional impairment in a canine model of ACL and meniscal deficiency. J Ortho Res. 2016;34:607–615. doi: 10.1002/jor.23054 [DOI] [PubMed] [Google Scholar]
- 16.Silva RF, Camona JU, Rexende CMF. Intra-articular injections of autologous platelet concentrates in dogs with surgical reparation of cranial cruciate ligament rupture. Vet Comp Ortho Traum. 2013;26:285–290. doi: 10.3415/VCOT-12-06-0075 [DOI] [PubMed] [Google Scholar]
- 17.Koch TG, Berg LC, Betts DH. Current and future regenerative medicine—principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Canad Vet J. 2009;50(2):155–165. [PMC free article] [PubMed] [Google Scholar]
- 18.Dhillon MS, Behera P, Patel S, Shetty V. Orthobiologics and platelet rich plasma. Ind J Orthopaed. 2014;48(1):1–8. doi: 10.4103/0019-5413.125477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarrel TM, Mall NA, Lee AS, Cole BJ, Butty DC, Fortier LA. Considerations for the use of platelet-rich plasma in orthopedics. Sports Med. 2014;44:1025–1036. doi: 10.1007/s40279-014-0195-5 [DOI] [PubMed] [Google Scholar]
- 20.Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339–346. doi: 10.1177/0363546516665809 [DOI] [PubMed] [Google Scholar]
- 21.Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11:583–592. doi: 10.1007/s12178-018-9524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye CW, Enders A, Brooks MJ, Struble AM, Wakshlag JJ. Assessment of canine autologous platelet-rich plasma produced with a commercial centrifugation and platelet recovery kit. Vet Comp Ortho Traum. 2016;29:14–19. doi: 10.3415/VCOT-15-03-0046 [DOI] [PubMed] [Google Scholar]
- 23.Franklin SP, Cook JL. Prospective trial of autologous conditioned plasma versus hyaluronan plus corticosteroid for elbow osteoarthritis in dogs. Canad Vet J. 2013;54(9):881. [PMC free article] [PubMed] [Google Scholar]
- 24.Lascelles BD, Roe SC, Smith E, et al. Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. Am J Vet Res. 2006;67:277–282. doi: 10.2460/ajvr.67.2.277 [DOI] [PubMed] [Google Scholar]
- 25.Baltzer WI, Smith-ostrin S, Warnock JJ, Ruaux CG. Evaluation of the clinical effects of diet and physical rehabilitation in dogs following tibial plateau leveling osteotomy. J Am Vet Med Assoc. 2018;252:686–700. doi: 10.2460/javma.252.6.686 [DOI] [PubMed] [Google Scholar]
- 26.Budsberg SC, Jevens DJ, Brown J, Foutz TL, Decamp CE, Reece L. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am J Vet Res. 1993;54:1569–1574. [PubMed] [Google Scholar]
- 27.Bockstahler BA, Vorbornik A, Muller M, Peham C. Compensatory load redistribution in naturally occurring osteoarthritis of the elbow joint and induced weight-bearing lameness of the forelimbs compared to the clinically sound dog. Vet J. 2009;180:202–212. doi: 10.1016/j.tvjl.2007.12.025 [DOI] [PubMed] [Google Scholar]
- 28.Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Amer J Sports Med. 2014;42(5):1204–1210. doi: 10.1177/0363546514525593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarrel T, Fortier L. Temporal growth factor release from platelet‐rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthopaed Res. 2009;27(8):1033–1042. doi: 10.1002/jor.v27:8 [DOI] [PubMed] [Google Scholar]
- 30.Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800. doi: 10.1177/0363546515580787 [DOI] [PubMed] [Google Scholar]
- 31.Yun S, Ku SK, Kwon YS. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthopaed Sur Res. 2016;11(1):9. doi: 10.1186/s13018-016-0342-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voss K, Wiestner T, Geleandro L, Hassig M, Montavon PM. Effect of dog breed and body conformation on vertical ground reaction forces, impulses and stance times. Vet Comp Ortho Traum. 2011;24:106–112. doi: 10.3415/VCOT-10-06-0098 [DOI] [PubMed] [Google Scholar]
- 33.Krotscheck U, Todhunter RJ, Nelson SA, Sutter NB, Mohammed HO. Precision and accuracy of ground reaction force normalization in a heterogeneous population of dogs. Vet Surg. 2014;43(4):437–445. doi: 10.1111/j.1532-950X.2014.12176.x [DOI] [PubMed] [Google Scholar]
- 34.Fanchon L, Grandjean D. Accuracy of asymmetry indices of ground reaction forces for diagnosis of hind limb lameness in dogs. Am J Vet Res. 2007;68(10):1089–1094. doi: 10.2460/ajvr.68.10.1089 [DOI] [PubMed] [Google Scholar]
- 35.Oosterlinck M, Bosmans T, Gasthuys F, et al. Accuracy of pressure plate kinetic asymmetry indices and their correlation with visual gait assessment scores in lame and nonlame dogs. Am J Vet Res. 2011;72(6):820–825. doi: 10.2460/ajvr.72.6.820 [DOI] [PubMed] [Google Scholar]
- 36.Katic N, Bockstahler BA, Mueller M, Peham C. Fourier analysis of vertical ground reaction forces in dogs with unilateral hind limb lameness caused by degenerative disease of the hip joint and in dogs without lameness. Am J Vet Res. 2009;70(1):118–126. doi: 10.2460/ajvr.70.1.118 [DOI] [PubMed] [Google Scholar]
- 37.Fischer S, Anders A, Nolte I, Schilling N. Compensatory load redistribution in walking and trotting dogs with hind limb lameness. Vet J. 2013;197(3):746–752. [DOI] [PubMed] [Google Scholar]
- 38.Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc. 2012;241:1314–1319. [DOI] [PubMed] [Google Scholar]