The authors regret that the original paper was published with an error in the supplementary data (Table 1). In order to identify tumor-secreted factors that contribute to cardiac atrophy under the condition of cancer cachexia, in the original study, we performed a differential secretome analysis comparing cell conditioned media from cachexia-inducing C26 colon carcinoma cells and non-cachexia-inducing MC38 colon carcinoma cells. Secreted proteins which were at least 2-fold more abundantly secreted from C26 cells were selected for further functional validation. Functional validation was performed by overexpressing candidate proteins in HEK293A cells and collecting the candidate-enriched cell conditioned media for assaying their atrophy-inducing potential on primary neonatal rat cardiomyocytes.
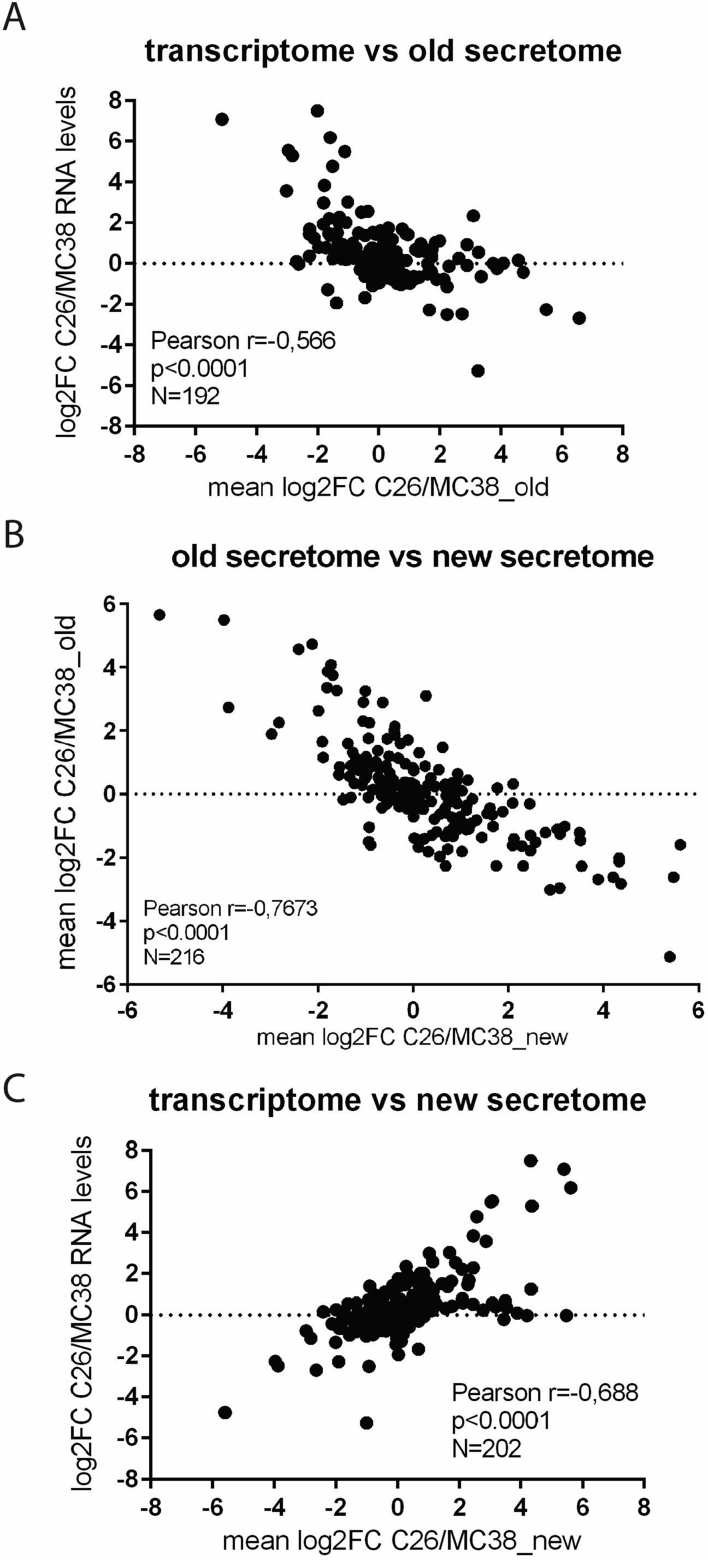
In the context of subsequent studies focusing on different aspects of cancer-induced cachexia, we performed a differential transcriptomics analysis comparing RNAseq data from C26 and MC38 cells. While differential protein secretion does not necessarily need to be fully reflected by corresponding differences at the level of gene expression, we were still surprised about the lack of congruency between the corresponding regulation of gene expression and protein secretion. Surprisingly, for the overlap of genes and proteins being differentially regulated between C26 and MC38 cells (regardless of the direction of change), we found a significant negative correlation between differential gene expression and differential protein secretion (Corrigendum Figure 1A). In order to elucidate the basis of this unexpected finding, we decided to repeat the differential secretome analysis in the same manner as it has been performed in the original study. Notably, the comparison between the secretome analyses (old vs new) revealed a remarkably strong negative correlation concerning the difference in protein secretion between C26 and MC38 cells (Corrigendum Figure 1B). Furthermore, when we then compared the new secretome analysis with the differences in the transcription between C26 and MC38 cells, there was a highly significant positive correlation (Corrigendum Figure 1C). The found consistency for the differences between the cell lines at distinct levels of regulation (transcription vs secretion) suggested that these datasets were correctly associated, in contrast to the previous comparison with the original differential secretome analysis. Taken together, these new analyses strongly indicate that in the original (old) secretome analysis, a swap in the sample allocation must have occurred, either during sample preparation, the subsequent proteomic or data analysis. Despite extensive evaluation of the respective experiment records, it was not possible to detect at which exact point in the course of the experimental work this mistake was made.
Corrigendum Figure 1.
Correlation analyses of the different datasets comparing C26 and MC38 colon carcinoma cells. Correlation between (A) differentially expressed genes determined by RNAseq and the original (old) differential secretome analysis, (B) the original differential secretome analysis (old) and the new differential secretome analysis (new), (C) differentially expressed genes and the original (old) differential secretome analysis. Data are depicted as log2-fold changes (log2FC). Statistical analysis was performed by correlation analysis using Graphpad prism. N numbers, Pearson correlation coefficients (r) and statistical significance levels (p) are depicted in the graphs.
Remarkably enough, the high-throughput functional validation of 109 candidates performed in the original study (original manuscript Figure 3C), using selected candidates now considered to be more abundantly secreted from non-cachexia-inducing MC38 instead of cachexia-inducing C26 cells, still revealed a set of candidates showing the expected atrophy effects upon treatment of primary cardiomyocytes with the respective candidate-enriched conditioned media. These effects were comparable to the effects of C26 conditioned medium on cardiomyocyte atrophy (original Figure 2A and B) and were therefore applied as primary selection criterion for putative cachexokines (mediators of cachexia). Additionally, further analysis selected a subset of 7 candidates which, similar to C26 conditioned medium, increased the fatty acid oxidation rate in neonatal rat cardiomyocytes treated with candidate-enriched medium (original Figure 4A). We speculate that the high-throughput functional analysis, albeit being based on an erroneous initial secretome analysis, contained a sufficient high number of protein candidates in order to contain protein factors which eventually turned out to be still relevant with respect to their capability to mediate a specific component of the cachexia phenotype (cardiomyocyte atrophy). This is exemplified by the main candidate Ataxin-10 (Atxn10), for which we could confirm elevated levels in different models of experimental cachexia, including C26 tumor-bearing mice but not in MC38 tumor-bearing mice (original Figure 5A–D). In a model pancreatic of cancer based on orthotopic cell implantation, circulating Atxn10 levels closely correlated with the degree of weight loss (Figure 5E and F). Finally, we found Atxn10 levels to be elevated in cancer patients with cachexia compared to weight-stable patients (Figure 5G). Therefore, we would like to emphasize that the majority of data provided in the publication is still correct. We apologize for any inconvenience that might have resulted from providing incorrect differential secretome data as supplemental material of the study and now provide the data of the new and correct differential secretome analysis (Corrigendum supplemental data).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.02.013.
Contributor Information
Johannes Backs, Email: johannes.backs@med.uni-heidelberg.de.
Stephan Herzig, Email: stephan.herzig@helmholtz-muenchen.de.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.