Summary
During neurodevelopment, the growth cone deciphers directional information from extracellular guidance cues presented as shallow concentration gradients via signal amplification. However, it remains unclear how the growth cone controls this amplification process during its navigation through an environment in which basal cue concentrations vary widely. Here, we identified inositol 1,4,5-trisphosphate (IP3) receptor type 3 as a regulator of axonal sensitivity to guidance cues in vitro and in vivo. Growth cones lacking the type 3 subunit are hypersensitive to nerve growth factor (NGF), an IP3-dependent attractive cue, and incapable of turning toward normal concentration ranges of NGF to which wild-type growth cones respond. This is due to globally, but not asymmetrically, activated Ca2+ signaling in the hypersensitive growth cones. Remarkably, lower NGF concentrations can polarize growth cones for turning if IP3 receptor type 3 is deficient. These data suggest a subtype-specific IP3 receptor function in sensitivity adjustment during axon navigation.
Subject Areas: Biological Sciences, Neuroscience, Molecular Neuroscience, Cellular Neuroscience
Graphical Abstract
Highlights
-
•
IP3 receptor type 3 (IP3R3) controls axonal sensitivity to IP3-based guidance cues
-
•
IP3R3−/− growth cones are not attracted to NGF due to global Ca2+ responses
-
•
Lower NGF concentrations can polarize IP3R3−/− growth cones for attractive turning
-
•
NGF knockdown in vivo can revert abnormal trajectory of IP3R3−/− axons
Biological Sciences; Neuroscience; Molecular Neuroscience; Cellular Neuroscience
Introduction
During neurodevelopment, an axon in search of its appropriate target relies on the navigational activity of its distal end called the growth cone, which senses molecular guidance cues in the immediate environment and makes path-finding decisions at choice points. These guidance cues can be presented as a shallow gradient among a noisy background, with spatial and temporal variations in cue concentrations. Consequently, the growth cone needs to not only amplify guidance signals but also adjust its sensitivity as it migrates through different segments along the chemical gradient. One important challenge for understanding axon guidance is to decipher whether and how intracellular second messengers, critical components mediating signal amplification and growth cone polarization for turning, can also control its sensitivity to guidance signals.
Both attractive and repulsive cues instruct growth cone turning through, in most cases, asymmetric Ca2+ elevations with higher Ca2+ concentrations on the side of the growth cone facing the source of the cues (reviewed in Gomez and Zheng, 2006). Whether the growth cone turns toward the higher Ca2+ side (attraction) or the lower Ca2+ side (repulsion) depends, in principle, on the source of Ca2+ signals: Ca2+ release from the ER triggers attraction, whereas Ca2+ influx through plasma membrane channels induces repulsion (reviewed in Tojima et al., 2011). Inositol 1,4,5-trisphosphate (IP3) is one important second messenger produced from membrane phospholipids and, upon binding to tetrameric IP3 receptors (IP3Rs) on the ER, can elicit Ca2+ release from the ER into the cytosol (reviewed in Mikoshiba, 2007). This process is commonly termed IP3-induced Ca2+ release (IICR).
In the skin, nerve growth factor (NGF) produced by epidermal keratinocytes (Botchkarev et al., 2006) participates in target field innervation by sensory neurons (Albers et al., 1994, Patel et al., 2000). Also, in vitro studies demonstrated that NGF acts as an attractive axon guidance cue through binding to tropomyosin receptor kinase A (TrkA) receptor and activating phospholipase C, an enzyme that catalyzes the hydrolytic conversion of membrane phospholipids into diacylglycerol and IP3 (Gallo et al., 1997, Ming et al., 1999). Our subsequent work showed that, in a growth cone migrating in an extracellular NGF gradient, phospholipase-C-dependent production of IP3 and ensuing IICR occurs on the side of the growth cone facing higher NGF concentrations (Akiyama et al., 2009) and that this asymmetric IICR causes polarized membrane dynamics leading to attractive axon turning toward NGF (Akiyama and Kamiguchi, 2010). In this way, IP3-induced asymmetric Ca2+ signals across the growth cone mediate attractive guidance responses to physiological cues such as NGF.
Although a growth cone can adjust its sensitivity to a wide range of guidance cue concentrations via multiple mechanisms such as receptor internalization and turnover (reviewed in Gallo and Letourneau, 2002, Ming et al., 2002, Piper et al., 2005), a potential role for second messenger signaling in this adjustment process remains to be determined. In the current study, we investigate NGF-dependent axon guidance using mice lacking each of the three IP3R subunits identified in mammals—type 1 (Furuichi et al., 1989), type 2 (Südhof et al., 1991), and type 3 (Blondel et al., 1993), abbreviated respectively as IP3R1, IP3R2, and IP3R3. These subunits can form a homo- or heterotetrameric IP3R that acts as a functional Ca2+ channel upon IP3 binding to each subunit (Maes et al., 2001, Monkawa et al., 1995, Nucifora et al., 1996, Wojcikiewicz and He, 1995). Navigational behavior of dorsal root ganglion (DRG) neurons derived from IP3R1 knockout (R1KO) and IP3R2 knockout (R2KO) mice are indistinguishable from that of wild-type (WT) neurons. However, DRG neuronal growth cones of IP3R3 knockout (R3KO) mice cannot respond properly to normal concentration ranges of NGF in vitro and in the skin in vivo, most likely because they are hypersensitive to IP3 and therefore exhibit global Ca2+ elevations in response to NGF gradients. These data raise the possibility that growth cones could adjust their sensitivity at the IP3R level such that they continue to produce polarized signals even in the presence of local variations in guidance cue concentrations.
Results
Subtype-Specific IP3R Involvement in Growth Cone Turning Responses
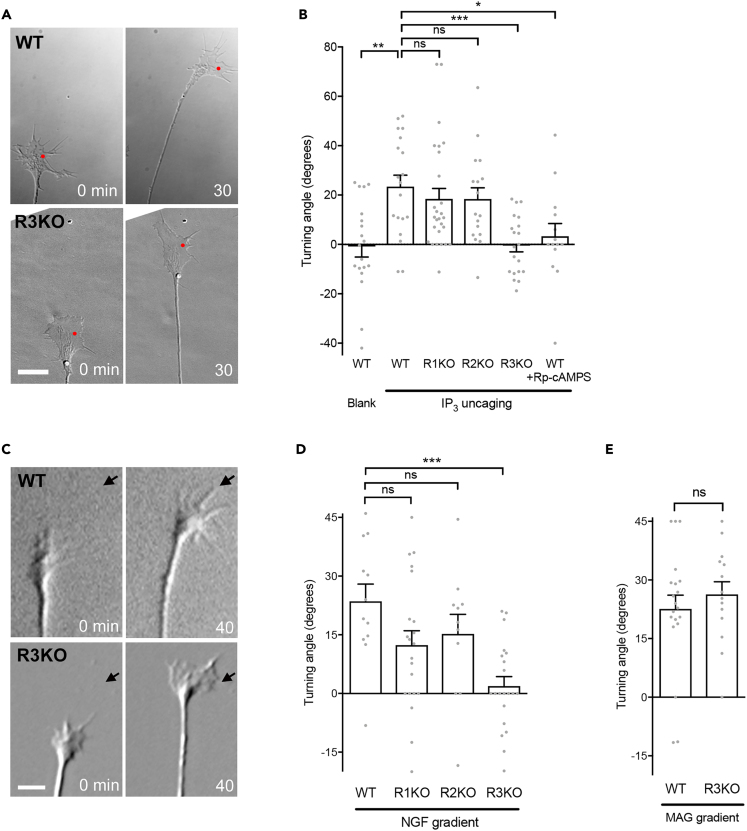
Although it is known that asymmetric IICR across the growth cone plays a crucial role in axon guidance mediated by extracellular cues such as NGF, which one(s) of the three mammalian IP3R subtypes participates in this process remains unclear. We employed focal laser-induced photolysis (FLIP) of caged IP3 to generate spatially localized IP3 signals in growth cones derived from IP3R-subtype-specific knockout mice. For loading, DRG neurons were incubated with 0.5 μM solution of caged IP3. In our previous study using chicken neurons (Akiyama et al., 2009), FLIP of caged IP3 resulted in a sustained increase in IP3 and its consequent Ca2+ elevation on the side of the growth cone receiving laser irradiation (“near side”). These asymmetric signals caused growth cone attractive turning toward higher IP3 and Ca2+.
In the current experiments, DRG growth cones from WT mice also turned attractively toward higher IP3, whereas control growth cones that had not been loaded with caged IP3 showed no detectable turning after laser irradiation (Figures 1A and 1B). As the occurrence of IICR depends on cytosolic levels of cyclic adenosine monophosphate (cAMP), chicken neurons cultured on a laminin substrate in our previous study, which caused a reduction in cAMP levels in growth cones, failed to respond to IP3 signals (Akiyama et al., 2009). Consistently, IP3-induced turning of mouse neuronal growth cones was also dependent on cAMP because the cAMP antagonist Rp-cAMPS (20 μM) blocked the effect of IP3 uncaging (Figure 1B). Analyses of neurons derived from IP3R-subtype-specific knockout mice showed that R1KO or R2KO growth cones turned toward higher IP3 (Figure 1B). By contrast, R3KO growth cones showed no significant turning (Figures 1A and 1B), indicating that IP3R3 is necessary for turning responses to IP3 under this experimental condition.
Figure 1.
Subtype-Specific IP3R Involvement in Growth Cone Turning Responses
(A) Caged IP3 was photolyzed at the red spots to generate spatially localized IP3 elevations. Shown are representative growth cones of WT (upper panels) and R3KO (lower panels) neurons at the beginning (0 min) and end (30 min) of repetitive FLIP. Scale bar, 10 μm.
(B) Mean turning angles of WT and IP3R subtype-specific knockout growth cones 30 min after the onset of repetitive FLIP, with or without (“Blank”) preloading of caged IP3. The rightmost bar indicates data from WT growth cones in the presence of bath-applied Rp-cAMPS (20 μM). Positive angles represent attractive turning toward the side with laser irradiation. Bars represent mean ± SEM, with each gray dot indicating turning angle of individual growth cones in this experiment. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, not significant; Dunnett's test.
(C) WT and R3KO growth cones immediately before and 40 min after the onset of repetitive NGF ejection from the direction indicated by the arrows. Scale bar, 10 μm.
(D and E) Average turning angles of WT and IP3R subtype-specific knockout growth cones 40 min after the start of repetitive ejection of NGF (D) or MAG (E). Bars represent mean ± SEM, with each gray dot indicating turning angle of individual growth cones in this experiment. ∗∗∗p < 0.001; ns, not significant; Dunnett's test (D) or Student's t test (E).
See also Figure S1.
We next tested whether an extracellular NGF gradient was attractive to DRG growth cones from WT and each of the three knockout mice. In these assays, NGF concentrations near the growth cones were approximately 0.1% of in-pipette concentration of 20 μg/mL (Lohof et al., 1992). The NGF gradient attracted WT, R1KO and R2KO growth cones, but not R3KO growth cones (Figures 1C and 1D). The migration speed of growth cones in NGF gradients was not affected by the loss of each IP3R subtype (Figure S1). Because attractive turning responses of WT and R1KO growth cones may potentially be different quantitatively, we calculated Cohen's d between WT and R1KO to be 0.23 for IP3 uncaging (Figure 1B) and 0.69 for NGF gradients (Figure 1D), suggesting that the effect of R1KO is small to moderate. Nonetheless, we concluded that, at least qualitatively, R1KO growth cones are indistinguishable from WT growth cones in their responses to IP3 and NGF signals. As a control to show that R3KO growth cones were still able to turn, we examined the effect of myelin-associated glycoprotein (MAG) known to attract nascent axons via Ca2+ release from the ER through another class of Ca2+ channels, ryanodine receptors (Henley et al., 2004, Tojima et al., 2014). Consistent with such differences in the requirement of ion channels mediating Ca2+ release, MAG caused attractive turning of R3KO growth cones (Figure 1E). Therefore, the loss of IP3R3 rendered growth cones unresponsive to an IP3R-based guidance cue such as NGF.
Although IP3R1 and IP3R3 are expressed by neurons, IP3R2 is detectable only in glial cells in the nervous system (Sharp et al., 1999, Taylor et al., 1999). Consistent with this glial-specific expression pattern of IP3R2, our results showed that IP3R2 was dispensable for IP3-dependent growth cone turning. Therefore, we decided to leave out R2KO mice in subsequent experiments.
Growth Cone Turning Responses Correlate with Ca2+ Signal Asymmetry
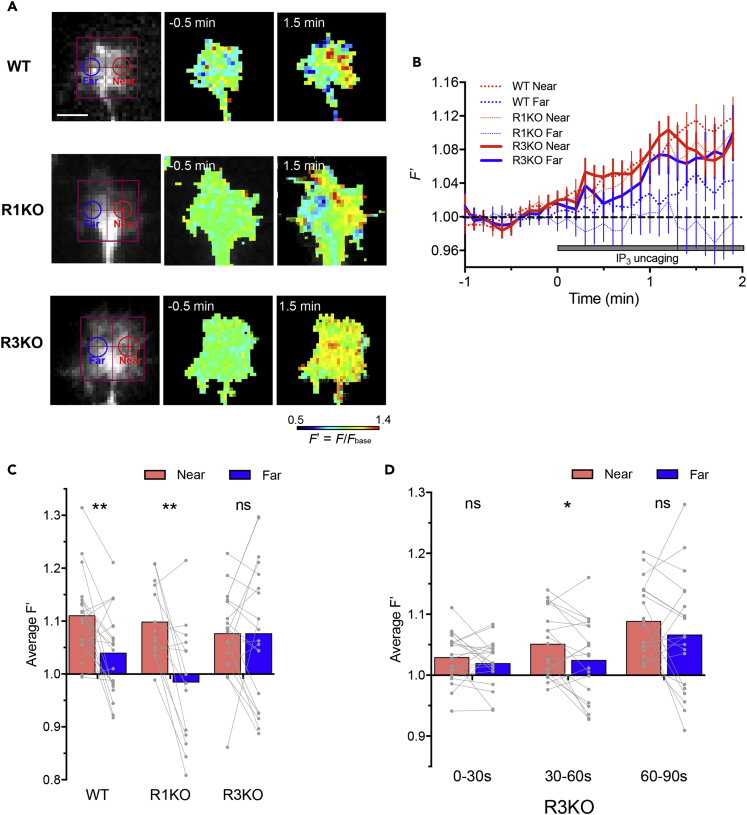
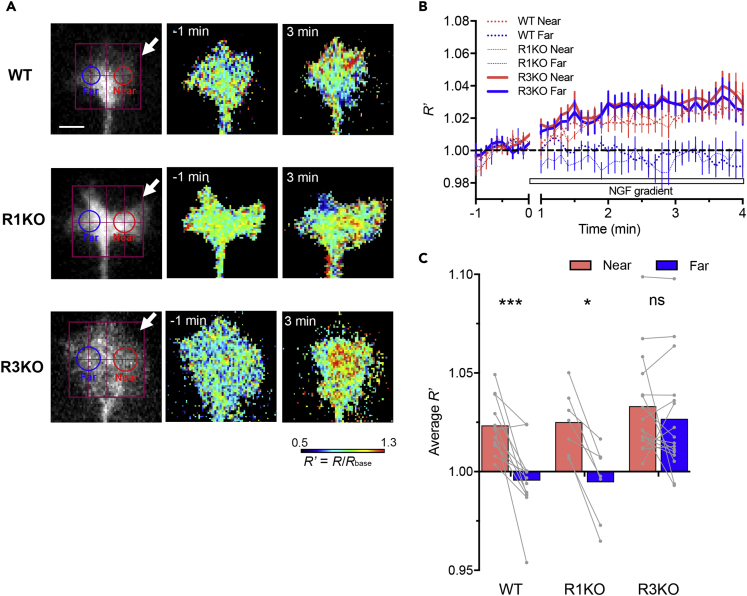
To investigate a correlation between growth cone turning and intracellular signaling asymmetry, we monitored spatiotemporal dynamics of Ca2+ in response to localized IP3 signals. In WT and R1KO growth cones, IP3 uncaging resulted in Ca2+ elevations on the near side but no detectable Ca2+ increases on the opposite “far” side (Figures 2A–2C). Such asymmetric Ca2+ signals are consistent with the ability of these growth cones to turn toward the side with IP3 production. By contrast, R3KO growth cones showed widespread increases in Ca2+ spanning both the near and far sides after localized IP3 uncaging on the near side (Figures 2A–2C). To capture a potential timepoint when Ca2+ gradients could be formed in R3KO growth cones, we compared Ca2+ levels between the near and far sides during earlier periods after IP3uncaging. As shown in Figure 2D, localized IP3 production caused a transient asymmetry in Ca2+ concentrations across the R3KO growth cone at 30–60 s but could not sustain the Ca2+ gradient after 1 min of the onset of IP3 uncaging (Figures 2C and 2D), suggesting that R3KO growth cones have symmetric IICR on the timescale for axon turning processes. We also tested whether extracellular NGF gradients cause similar spatiotemporal dynamics of Ca2+ in growth cones, using ratiometric Oregon Green 488 BAPTA-1 (OGB-1)–Fura-red (FR) imaging as a measure of Ca2+ levels. NGF gradients induced Ca2+ elevations only on the near side of WT and R1KO growth cones but caused widespread Ca2+ increases on both sides of R3KO growth cones (Figure 3). These results suggest that the failure of R3KO growth cones to turn toward NGF-induced IP3 signals may be due to symmetric Ca2+ elevations, a distribution pattern that is unlikely to act as a polarizing signal.
Figure 2.
Spatiotemporal Dynamics of Ca2+ in Growth Cones Responding to Localized IP3 Signals
(A) Pseudo-color Ca2+ images in WT, R1KO, and R3KO growth cones 0.5 min before and 1.5 min after the onset of repetitive FLIP of caged IP3. The red crosshairs represent the sites of laser irradiation. Relative changes in Fluo-8H fluorescence (F/Fbase, defined as F′) were used as a measure of cytosolic Ca2+ levels. The near side ROI (red) was defined as a circular region whose center corresponded to the site of laser irradiation and whose diameter equaled to one-third of the width of each growth cone. The far side ROI (blue) was defined as a circular region of the same diameter that was placed on the center of the far-side half of each growth cone. Scale bar, 5 μm.
(B) Time course changes in F′ in the near (red line) and far (blue line) ROIs positioned on WT (dotted line), R1KO (finely dotted line), and R3KO (solid line) growth cones. Data are represented as mean ± SEM.
(C and D) The mean amplitude of F′ during 90–120 s (C) and earlier periods (D) after the onset of repetitive FLIP shown in (B). Data on WT, R1KO, and R3KO neurons were included in (C), and data on only R3KO neurons in (D). Each gray line connecting two dots represents data from the near and far ROIs of a single growth cone, and each colored bar represents the mean. ∗p < 0.05; ∗∗p < 0.01; ns, not significant; paired t test.
See also Figure S2.
Figure 3.
Spatiotemporal Dynamics of Ca2+ in Growth Cones Responding to NGF Gradients
(A) Pseudo-color Ca2+ images in WT, R1KO, and R3KO growth cones 1 min before and 3.5 min after the onset of repetitive NGF ejection from the direction indicated by the white arrows. The growth cones were preloaded with a ratiometric pair of calcium indicators, OGB-1 and FR, and the OGB-1/FR emission ratio (FOGB-1/FFR, defined as R) was determined. Shown are relative changes in R (R/Rbase, defined as R′) used as a measure of cytosolic Ca2+ levels. The near (red) and far (blue) ROIs were defined as described in Figure 2. Scale bar, 5 μm.
(B) Time course changes in R′ in the near (red line) and far (blue line) ROIs positioned on WT (dotted line), R1KO (finely dotted line), and R3KO (solid line) growth cones. Data from the first minute after the start of NGF ejection were excluded because of a lack of stable NGF gradients. Data are represented as mean ± SEM.
(C) The mean amplitude of R′ over the last 1 min of repetitive NGF ejection shown in (B). Each gray line connecting two dots represents data from the near and far ROIs of a single growth cone, and each colored bar represents the mean. ∗p < 0.05; ∗∗∗p < 0.001; ns, not significant; Wilcoxon matched pairs signed rank test.
See also Figure S2.
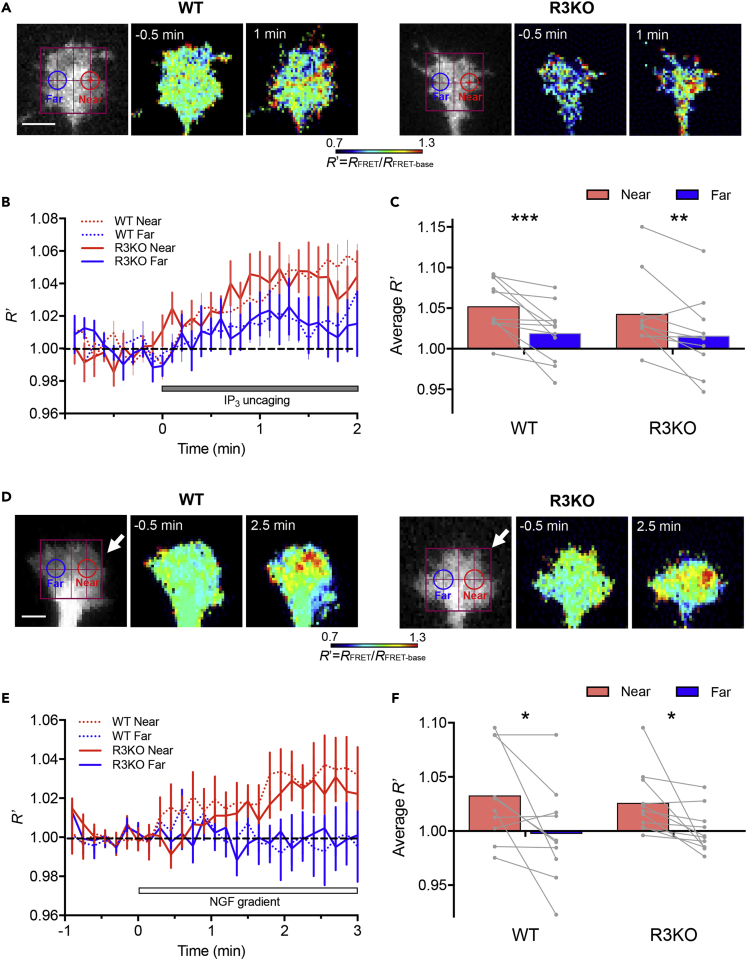
One possible explanation for the lack of Ca2+ signal asymmetry in R3KO growth cones is widespread distribution of IP3 due to faster diffusion or slower degradation of IP3. To test for this possibility, we monitored spatiotemporal dynamics of IP3 that had been photo-released from caged IP3, using the IP3 sensor IRIS-2.3 (Matsu-ura et al., 2019). This sensor contains enhanced green fluorescent protein (EGFP) and HaloTag- tetramethylrhodamine (TMR), and the efficiency of fluorescence resonance energy transfer (FRET) from EGFP to TMR decreases upon IP3 binding to IRIS-2.3. Therefore, we calculated the inverse FRET ratio in IRIS-2.3, i.e., the ratio of EGFP emission compared with TMR emission (FEGFP/FTMR) as a measure of IP3 levels. In both WT and R3KO neurons, IP3uncaging on one side of the growth cone resulted in asymmetric increases in IP3, with IP3 levels on the near side being significantly higher than those on the far side (Figures 4A–4C). Similarly, NGF gradients induced IP3 elevations only on the near sides of both WT and R3KO growth cones (Figures 4D–4F). These data indicate that spatiotemporal dynamics of IP3 is indistinguishable between WT and R3KO growth cones and that the lack of Ca2+ signal asymmetry in R3KO growth cones is not attributable to altered IP3 dynamics.
Figure 4.
Spatiotemporal Dynamics of IP3 in Growth Cones Responding to Localized IP3 Signals or NGF Gradients
(A) Pseudo-color IP3 images in WT and R3KO growth cones 0.5 min before and 1 min after the onset of repetitive FLIP of caged IP3. Each growth cone was transfected with the FRET-based IP3 sensor IRIS-2.3 containing EGFP and HaloTag-TMR, and the ratio of EGFP emission compared with TMR emission (FEGFP/FTMR, defined as RFRET) was determined. Shown are relative changes in RFRET (RFRET/RFRET-base, defined as R′) used as a measure of IP3 levels. The red crosshair indicates the site of laser irradiation. The near (red) and far (blue) ROIs were defined as described in Figure 2. Scale bar, 5 μm.
(B) Time course changes in R′ in the near (red line) and far (blue line) ROIs positioned on WT (dotted line) and R3KO (solid line) growth cones.
(C) The mean amplitude of R′ over the last 30 s of repetitive FLIP shown in (B). ∗∗p < 0.01; ∗∗∗p < 0.001, Wilcoxon matched pairs signed rank test.
(D) Pseudo-color IP3 images in WT and R3KO growth cones 0.5 min before and 2.5 min after the onset of repetitive NGF ejection from the direction indicated by the white arrows. Scale bar, 5 μm.
(E) Time course changes in R′ in the near (red line) and far (blue line) ROIs positioned on WT (dotted line) and R3KO (solid line) growth cones.
(F) The mean amplitude of R′ over the last 1 min of repetitive NGF ejection. ∗p < 0.05; paired t test.
In (B and E), data are represented as mean ± SEM. In (C and F), each gray line connecting two dots represents data from the near and far ROIs of a single growth cone, and each colored bar represents the mean.
Another possible explanation for the lack of Ca2+ signal asymmetry in R3KO growth cones is increased expression of NGF downstream signaling components such as TrkA and IP3R1, in which the far side of R3KO growth cones could respond to lower concentrations of NGF. However, these possibilities are unlikely because we could not detect any substantial increase in the amount of TrkA and IP3R1 expressed in DRGs of R3KO mice (Figure S2).
R3KO Growth Cones Are Hypersensitive to IP3-Based Guidance Signals
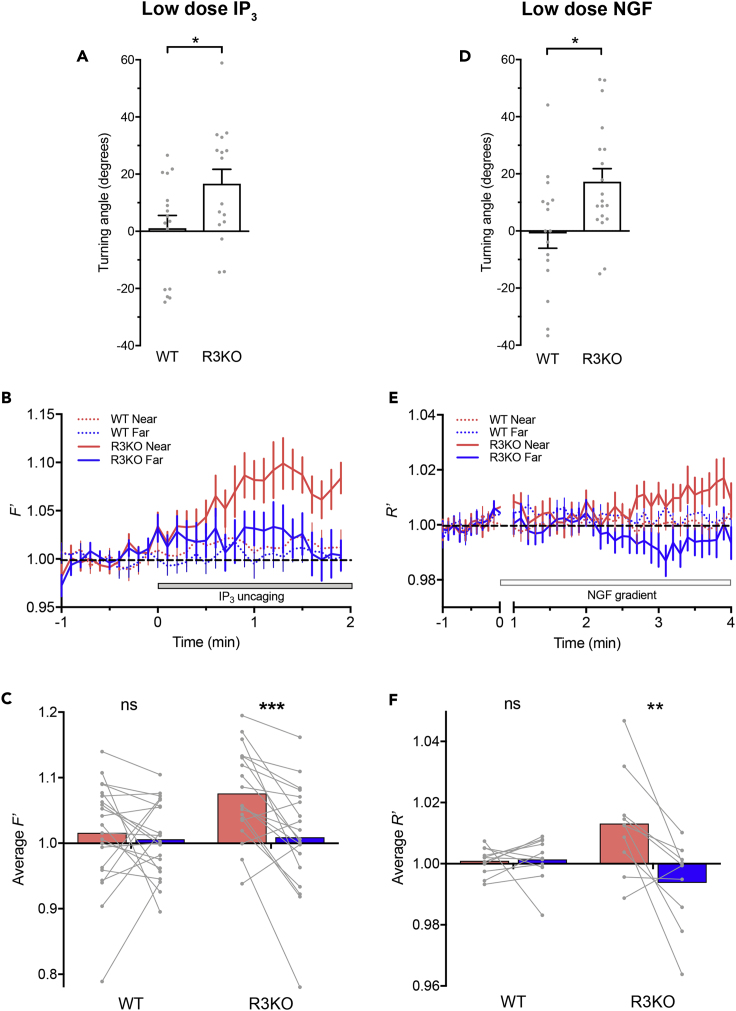
In R3KO growth cones, symmetric Ca2+ elevations in spite of asymmetric IP3 signals suggest the presence of hypersensitive IP3Rs that can be maximally activated by physiological IP3 signals, such as those in the growth cone near side, and also generate substantial Ca2+ release in response to low levels of IP3, such as those in the growth cone far side. To test for this hypothesis, we monitored Ca2+ responses to low-amplitude IP3 signals generated by FLIP of a smaller amount of caged IP3, i.e., 0.1 μM in contrast to 0.5 μM in previous experiments (Figures 1A, 1B, and 2). These low-amplitude IP3 signals attracted R3KO growth cones but had no detectable effect on directional preference of WT growth cones (Figure 5A). Consistent with these turning responses, R3KO growth cones exhibited asymmetric Ca2+ elevations with higher Ca2+ on the side with IP3 uncaging, whereas WT growth cones showed no detectable Ca2+ increases presumably because the amplitude of IP3 signals were below the threshold for IICR (Figures 5B and 5C).
Figure 5.
R3KO Growth Cones Are Hypersensitive to IP3-Based Guidance Signals
Shown are growth cone responses to lower doses of IP3 (A–C) and NGF (D–F).
(A) Average turning angles of WT and R3KO growth cones 30 min after the onset of repetitive FLIP of a smaller amount of caged IP3 (0.1 μM in contrast to 0.5 μM in Figure 1B). ∗p < 0.05; student's t test.
(B) Time course changes in F′, calculated as F/Fbase of Fluo-8H fluorescence, in the near (red line) and far (blue line) ROIs positioned on WT (dotted line) and R3KO (solid line) growth cones in response to repetitive FLIP of a smaller amount of caged IP3 (0.1 μM in contrast to 0.5 μM in Figure 2B).
(C) The mean amplitude of F′ over the last 30 s of repetitive FLIP shown in (B). ∗∗∗p < 0.001; ns, not significant; paired t test.
(D) Average turning angles of WT and R3KO growth cones 40 min after the start of repetitive ejection of NGF. In-pipette concentration of NGF was 0.4 μg/mL in contrast to 20 μg/mL in Figure 1D. ∗p < 0.05; student's t test.
(E) Time course changes in R′, calculated as R/Rbase of a ratiometric pair of OGB-1 and FR, in the near (red line) and far (blue line) ROIs positioned on WT (dotted line) and R3KO (solid line) growth cones in response to NGF gradients. In-pipette concentration of NGF was 0.4 μg/mL NGF in contrast to 20 μg/mL in Figure 3B.
(F) The mean amplitude of R′ over the last 1 min of repetitive NGF ejection shown in (E). ∗∗p < 0.01; ns, not significant; Wilcoxon matched pairs signed rank test. In (A and D), bars represent mean ± SEM, with each gray dot indicating turning angle of individual growth cones in this experiment.
In (B and E), data are represented as mean ± SEM. In (C and F), each gray line connecting two dots represents data from the near and far ROIs of a single growth cone, and each colored bar represents the mean.
We also examined the effect of lower-concentration NGF gradients, i.e., 0.4 μg/mL NGF in pipette in contrast to 20 μg/mL in previous experiments (Figures 1C, 1D, and 3). These NGF gradients induced neither Ca2+ elevations nor turning responses in WT growth cones but caused asymmetric Ca2+ signals and attractive turning responses in R3KO growth cones (Figures 5D–5F). These data support our notion that R3KO growth cones are hypersensitive to IP3-based guidance cues such as NGF.
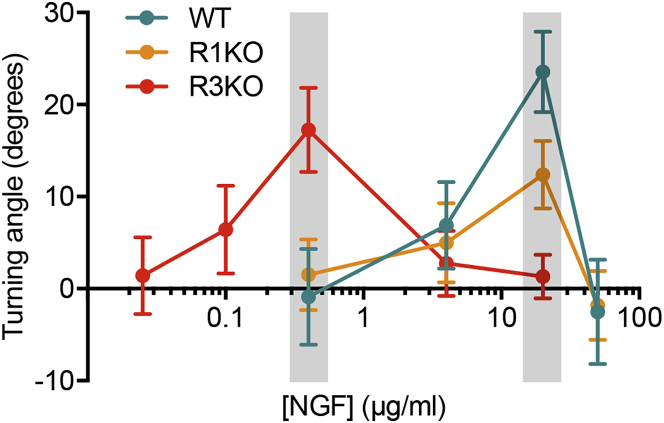
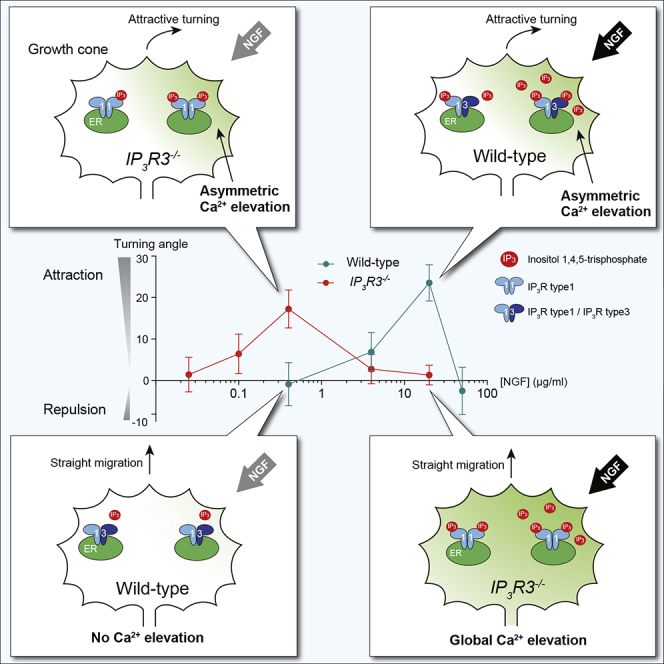
To determine growth cone sensitivity more comprehensively, we analyzed the effect of other NGF concentrations on axon turning responses using WT- and IP3R-subtype-specific knockout neurons. Dose-response curves were generated by plotting axon turning angles against log-scale NGF concentrations in micropipette (Figure 6). WT and R1KO growth cones were responsive to similar concentration ranges of NGF, but the curve for R3KO growth cones shifted to lower concentration ranges of NGF. Collectively, our results suggest that growth cones can respond to different concentration ranges of IP3-based guidance cues depending on whether IP3R3 participates in the generation of IICR.
Figure 6.
Optimal Concentration Ranges of NGF for Growth Cone Attractive Responses
Turning angles of WT, R1KO, and R3KO growth cones were determined 40 min after the start of repetitive ejection of various concentrations of NGF. Dose-response curves were generated by plotting the average angles against the log-scale of NGF concentration in the pipette. Data points in gray boxes are the same as those presented in Figures 1D and 5D. The number of growth cones assayed for each concentration was 11–22. Data are represented as mean ± SEM.
If R3KO axons are hypersensitive, partial inhibition of a remaining IP3R subtype in these axons, IP3R1, should restore their sensitivity to NGF into normal ranges. It is known that cAMP-dependent phosphorylation of IP3R1 is necessary for IICR (Nakade et al., 1994) and that the cAMP antagonist Rp-cAMPS blocks axonal responses to IP3-mediated guidance signals such as NGF (Akiyama et al., 2009). Therefore, instead of a commonly used Rp-cAMPS concentration of 20 μM, we treated R3KO neurons with 0.4 μM Rp-cAMPS that corresponded to 5% of a reported Ki of 8 μM (Van Haastert et al., 1984). Such mild treatment caused R3KO axons to recover attractive turning responses to a normal concentration range of NGF (20 μg/mL in pipette): turning angle (mean ± SEM) = 1.7° ± 3.0° (19 untreated axons) versus 10.5° ± 2.7° (19 Rp-cAMPS-treated axons), p < 0.05; student's t test. These data further support our notion that IP3R sensitivity, which can be regulated by cAMP-dependent phosphorylation, is an important determinant of optimal concentration ranges of NGF for axon guidance.
NGF Downregulation Restores R3KO Axon Trajectory in the Skin
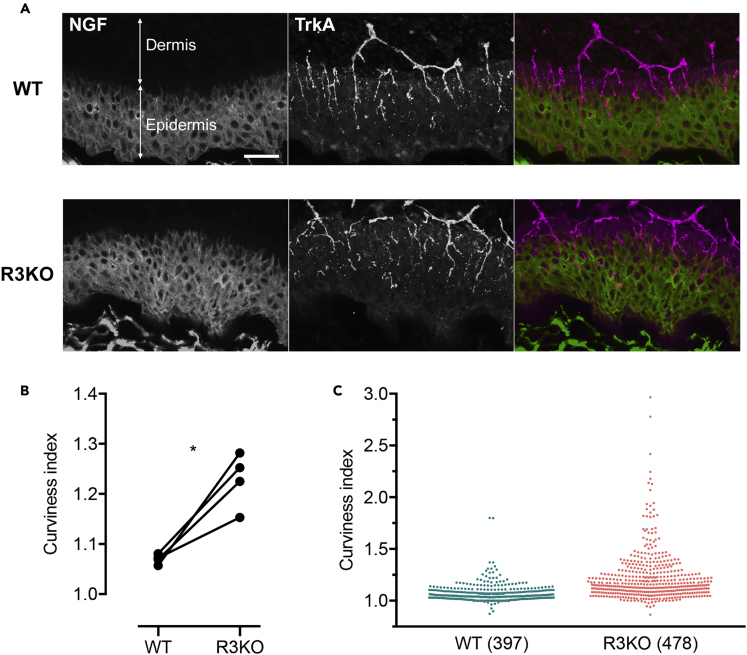
To validate in vivo physiological significance of our findings, we compared projection patterns of NGF-responsive TrkA-expressing sensory axons in the hindpaw skin of WT versus R3KO mice. Sensory axons in the hindpaw skin is one of the most well-studied models, and axon projection patterns are relatively easy to quantify (Patel et al., 2000, Wang et al., 2013). We analyzed four pairs of WT/R3KO littermates from IP3R3 heterozygous-heterozygous mating. The border between epidermis and dermis was evident because the epidermis except for its basal layer, but not the dermis, is immunopositive for NGF (Botchkarev et al., 2006). We observed that TrkA-positive axons in the epidermis of WT and R3KO mice had different morphologies: WT axons were straighter and well organized (relatively evenly spaced), whereas R3KO axons were undulating and appeared to follow meandering paths (Figure 7A). Such abnormal trajectories of R3KO axons in the skin were also observed in cleared whole-mount tissues where TrkA-positive axons were three-dimensionally reconstructed (Videos S1 and S2). To quantify axon morphological characteristics on skin sections, we employed a “curviness index” of each TrkA-positive axon in the NGF-positive epidermis (see Transparent Methods). The curviness index of 1 indicates the completely straight axon trajectory, whereas larger values represent more curly axons. As shown in Figures 7B and 7C, R3KO axons had significantly larger curviness index than WT axons, confirming our observation that R3KO axons have abnormal trajectories in the skin.
Figure 7.
Trajectory of TrkA-Positive Axons in the Skin Epidermis
(A) Sagittal tissue sections of the hindpaw plantar surface from WT and R3KO animals. The sections were immunostained for NGF and TrkA. The superimposed images show NGF in green and TrkA in magenta. Scale bar, 50 μm.
(B) Trajectory of TrkA-positive axons was represented as “curviness index”—the length of each entire axon segment within the NGF-positive area divided by the straight-line distance between the two ends of that segment. Four pairs of WT and R3KO littermates were analyzed. The indexes of TrkA axons were averaged in each animal and compared between R3KO and its WT littermate. ∗p < 0.05; paired t test.
(C) The curviness indexes of all axons from the four pairs of animals analyzed in (B) were pooled and presented as scatterplots. Each dot corresponds to a single axon segment (n = 397 for WT, n = 478 for R3KO).
See also Figures S3 and S4.
Three-dimensional animation of reconstructed trajectories of WT axons in the skin. The arrowheads indicate representative TrkA-labeled axons extending into the epidermis, i.e., in the direction of smaller scale values on the z-axis.
Three-dimensional animation of reconstructed trajectories of R3KO axons in the skin. The arrowheads indicate representative TrkA-labeled axons extending into the epidermis, i.e., in the direction of smaller scale values on the z-axis.
To test whether the observed abnormality of R3KO axons in the skin is due to their intrinsic characteristics, we cultured dissociated WT and R3KO DRG neurons in vitro and compared their axon morphologies. We confirmed that the lack of IP3R3 did not cause any innate growth defects or curliness in DRG axons (Figure S3), excluding the possibility that the undulated morphology of R3KO axons in vivo is due to any intrinsic defects in directional growth. Furthermore, because skin keratinocytes from WT and R3KO mice release comparable amounts of NGF (Figure S4A), it is unlikely that a drastic alteration in the level of epidermal NGF in R3KO mice causes TrkA axons to meander in the skin.
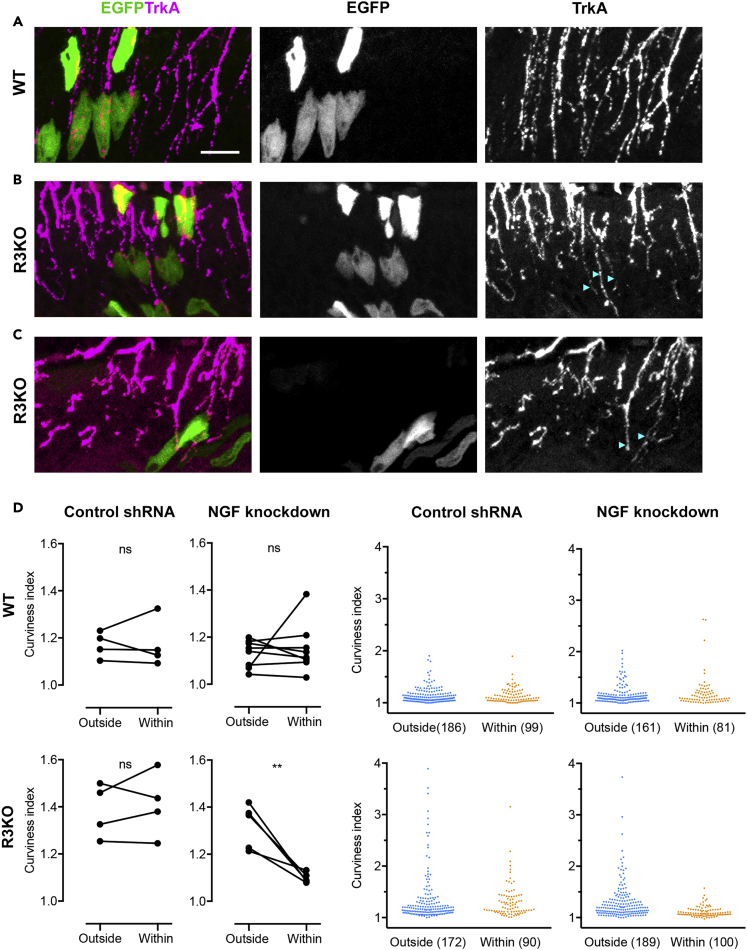
If the curly phenotype of R3KO axons is due to their hypersensitivity that renders growth cones irresponsive to epidermal NGF, then this phenotype should be reverted back to normal trajectory when epidermal NGF levels are lowered. To control NGF expression in the epidermis, we obtained short hairpin RNA (shRNA) against NGF and confirmed its knocking down effect using cultured primary keratinocytes (Figure S4B). We then analyzed the morphology of TrkA axons in the epidermal area transfected with either NGF shRNA or control shRNA plasmid (Figures 8A–8C). The curviness index of R3KO axons was shifted toward 1 in the epidermal area transfected with NGF shRNA, but not with control shRNA (Figure 8D), indicating that NGF downregulation can antagonize the effect of IP3R3 deficiency on the trajectory of TrkA axons. By contrast, NGF downregulation did not significantly affect the trajectory of TrkA axons in WT mice (Figure 8D). These findings are consistent with our model that the lack of IP3R3 subunit causes DRG sensory axons to be hypersensitive to NGF and therefore unable to extend properly into the epidermis.
Figure 8.
NGF Downregulation Restores R3KO Axon Trajectory in the Skin
(A) Example of Wthindpaw epidermis transfected with control shRNA plasmid. (Left) Superimposition of EGFP (green) and TrkA (magenta). (Middle) EGFP-positive areas representing successful transfection and long-term integration of the shRNA sequence into the genome of skin epidermal cells (mostly keratinocytes). (Right) Immunofluorescence of TrkA axons in the epidermis. Scale bar, 20 μm.
(B and C) Two examples of R3KO hindpaw epidermis (taken from the same animal at different locations) with EGFP fluorescence representing areas of shRNA-mediated NGF downregulation. TrkA axons contacting EGFP-positive areas are straighter (arrowheads) compared with axons in remote areas. Color images show EGFP (green) and TrkA (magenta).
(D) Curviness indexes of WT (top) and R3KO (bottom) axons within (“within EGFP area”) or outside (“outside EGFP area”) EGFP-positive areas. EGFP delineates areas transfected with either control or NGF shRNA. Left four panels: each line representing a difference in the average curviness index between the two areas of one animal. ∗∗p < 0.01; ns, not significant; paired t test. Right four panels: scatterplots in which each dot represents the curviness index of a single axon segment. The numbers in parentheses indicate the numbers of axon segments analyzed.
See also Figure S4.
Discussion
We identified the IP3R3 subunit as a regulator of growth cone sensitivity to guidance signals in vitro and in vivo and demonstrated the correlation between IICR asymmetry and turning competency when encountering various concentration ranges of the IP3-based guidance cue NGF. Compared with WT growth cones that respond to “normal” concentrations of NGF, IP3R3 deficiency renders growth cones hypersensitive, in which the growth cones are either able to detect lower than “normal” concentrations of NGF or unable to respond to “normal” concentrations of NGF because intracellular second messenger signaling is saturated and symmetric across the growth cone axis. Our FLIP experiments using different concentrations of caged IP3 showed that IP3R3-dependent adjustment of growth cone sensitivity occurs at the level of IP3 detection, suggesting that functional tetrameric IP3Rs composed without the type 3 subunit are hypersensitive and can mediate IICR in response to low levels of IP3.
One prominent difference among the three IP3R subtypes is their affinities for IP3. IP3R3 is known to have lower affinity compared with IP3R1 and IP3R2 (Iwai et al., 2005, Newton et al., 1994, Wojcikiewicz and Luo, 1998). Because IP3 affinity of each IP3R subtype is tightly correlated with its IP3 sensitivity (Wojcikiewicz and Luo, 1998), the three subtypes can be activated to different extent within certain ranges of IP3 concentrations in the cytosol. In other words, IP3R3 should be least sensitive to increases in cytosolic IP3 concentrations and requires relatively high levels of IP3 for its activation. WT neurons express IP3R1 and IP3R3 but not IP3R2, and it has been shown that IP3R1 and IP3R3 can form a heterotetrameric IP3R (Miyakawa et al., 1999). Based on our data and previous findings, we propose a model of how IP3R3 controls growth cone sensitivity to guidance signals (Figure S5). In WT growth cones, a homo- or heterotetrameric IP3R comprising IP3R3 may be activated only where there is a substantial amount of IP3 such as the growth cone near side. By contrast, in R3KO growth cones that also lack the expression of glial IP3R2, a homotetrameric IP3R composed solely of the high-affinity IP3R1 can mediate substantial IICR even where there is a small amount of IP3 such as the growth cone far side (Figure S5).
Other mechanisms have been suggested as to how growth cones can adjust their sensitivities to guidance cues, e.g., endocytosis-dependent desensitization and protein-synthesis-dependent resensitization (Ming et al., 2002, Piper et al., 2005). These mechanisms are broadly referred to as growth cone adaptation. One group of researchers, however, has proposed an alternative but not exclusive mechanism that relies on the growth cone's preexisting architecture: through spatiotemporal averaging of guidance cue-receptor binding along the growth cone circumference (Rosoff et al., 2004, Xu et al., 2005). This group used both in vitro NGF-elicited DRG axon turning and computer modeling to show that adaptation was not necessary to explain the long-term growth cone responses to gradients of guidance cues. Due to the stochastic nature of ligand-receptor binding, when NGF-receptor binding signals were pooled spatially over the growth cone circumference, and also pooled temporally, growth cones were in theory able to sense the difference in NGF concentration even in shallow gradients and respond correctly by turning.
Although we demonstrated that growth cone sensitivity can be controlled by intracellular signaling at the IP3R level, it remains unclear how this regulation occurs under physiological conditions. One possible mechanism is phosphorylation of IP3R subunits (reviewed in Mikoshiba, 2007, Shah et al., 2015, Vanderheyden et al., 2009). For example, cAMP-dependent protein kinase A (PKA) phosphorylates IP3R3 at three known sites: serines 916, 934, and 1832, in contrast to only one for each of IP3R1 or IP3R2. Interestingly, PKA phosphorylation of IP3R3 can result in enhanced (Chaloux et al., 2007, Dyer et al., 2003, Wojcikiewicz and Luo, 1998) or decreased (Giovannucci et al., 2000, Straub et al., 2002) sensitivity to IP3, depending on cell type. In DT40 cells, PKA activation results in decreased IICR, but this regulation is not dependent on the three PKA phosphorylation sites on IP3R3 (Soulsby and Wojcikiewicz, 2007). These findings are consistent with the hypothesis that intracellular signaling components such as PKA regulate IP3R3 activity via multiple mechanisms and that these mechanisms may control the overall activity of tetrameric IP3Rs given the particular importance of IP3R3 for sensitivity regulation.
In the skin, NGF is expressed and secreted by epidermal keratinocytes (Botchkarev et al., 2006). Although not as clear as in vitro, NGF may serve axon guidance functions in vivo. Rather than acting as a long-range guidance cue, NGF is mainly responsible for the final stages of axon path-finding and target innervation because cutaneous innervation in the hindlimb is lost in the absence of NGF-TrkA signaling (Patel et al., 2000). Moreover, NGF can direct axon growth in transplants after injury to central and peripheral nervous systems (Hu et al., 2010, Ziemba et al., 2008). In ex utero slice cultures of mouse embryos, ectopic NGF is able to attract spinal nerve growth to abnormal regions (Tucker et al., 2001). These findings suggest that growing axons in vivo can use NGF as a positional cue to reach specific regions or cells, such as TrkA axons extending into the epidermis where keratinocytes secrete NGF.
Our in vivo data indicated that the presence of specific IP3R subtype is important for the formation of normal axon trajectory during skin development. It is well documented that defective guidance during development, e.g., via eliminating the expression of particular guidance cues, causes the axons to display wavy, meandering, and disorderly paths of growth compared with the highly organized and well-defined paths of normal growth (Bentley and Toroian-Raymond, 1986, Enriquez-Barreto et al., 2012, Honig et al., 2002). In our data, R3KO mice had curly sensory axons in the skin epidermis compared with the straighter axons in WT mice. We interpreted this phenotype as an axon guidance defect and proposed that the abnormality is due to the axons' inability to respond properly to the level of NGF in the epidermis. Further experiments showed that downregulating NGF levels can ameliorate the defect in R3KO mice. This is most likely because lower NGF levels match the sensitivity range of hypersensitive IP3Rs in R3KO growth cones such that R3KO axons are guided correctly to their targets. These findings strongly support our model that, depending on the involvement of IP3R3, growth cones respond to different concentration ranges of IP3-based guidance signals.
In conclusion, we identified IP3R3 as an important subunit in keeping the sensitivity of functional IP3R within a range that matches the availability of IP3-based guidance cues in the environment. This IP3R3-based sensitivity regulation is necessary for the production of asymmetric Ca2+ signals in DRG growth cones exposed to different concentration ranges of NGF. Other types of axons should also navigate long distances during development through different segments along the chemical gradient of guidance cues and therefore be equipped with similar machineries for sensitivity adjustment. Future studies should address the broader verification of IP3R subtype-dependent regulation of growth cone sensitivity in developing and regenerating axons.
Limitations of the Study
Although this paper showed that IP3R3 regulates growth cone sensitivity to IP3-based guidance signals such as NGF, it remains unclear how this IP3R3-dependent adjustment can be tuned during axonal navigation in vivo. Because each WT axon follows a well-organized and almost straight trajectory toward the epidermis in spite of varying expression levels of NGF (Figures 7 and 8), this system may be suitable for investigating mechanisms underlying IP3R3-dependent tuning of axon guidance in vivo. Further studies are needed to monitor NGF downstream signaling in individual axons such as TrkA phosphorylation and to investigate its causative relationship with regulated expression and/or activity of IP3R3.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research B 19H03332 and AMED-CREST JP18gm0910006 to H.K. and Japan Society for the Promotion of Science International Fellowship to C.C. We are grateful to Y. Takahashi for the Tol2 vectors. We thank our laboratory members, T. Hida and H. Ito, for their assistance with this research, A.T. Guy for critical reading of this manuscript, and the RIKEN Center for Brain Science's Research Resources Division for experimental instruments.
Author Contributions
C.C. performed the experiments in Figures 1, 2, 3, 4, 5, 6, 7, 8, and S1–S3 and wrote the manuscript. N.O. performed the experiments in Figures 4, 8, and S4. H.A. contributed to Figures 1, 2, 3, and 4. T.F. provided axon growth data in the manuscript. M.I. performed the experiments in Videos S1 and S2. T.M. and K.M. designed and generated IRIS2.3. T.S. provided guidance for Figure 8. H.K. designed the research project, directed the experiments, and wrote the manuscript with C.C.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100963.
Supplemental Information
References
- Akiyama H., Kamiguchi H. Phosphatidylinositol 3-kinase facilitates microtubule-dependent membrane transport for neuronal growth cone guidance. J. Biol. Chem. 2010;285:41740–41748. doi: 10.1074/jbc.M110.156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Matsu-ura T., Mikoshiba K., Kamiguchi H. Control of neuronal growth cone navigation by asymmetric inositol 1,4,5-trisphosphate signals. Sci. Signal. 2009;2:ra34. doi: 10.1126/scisignal.2000196. [DOI] [PubMed] [Google Scholar]
- Albers K.M., Wright D.E., Davis B.M. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J. Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D., Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G.I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 1993;268:11356–11363. [PubMed] [Google Scholar]
- Botchkarev V.A., Yaar M., Peters E.M., Raychaudhuri S.P., Botchkareva N.V., Marconi A., Raychaudhuri S.K., Paus R., Pincelli C. Neurotrophins in skin biology and pathology. J. Invest. Dermatol. 2006;126:1719–1727. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- Chaloux B., Caron A.Z., Guillemette G. Protein kinase A increases the binding affinity and the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor type 3 in RINm5F cells. Biol. Cell. 2007;99:379–388. doi: 10.1042/BC20060121. [DOI] [PubMed] [Google Scholar]
- Dyer J.L., Mobasheri H., Lea E.J.A., Dawson A.P., Michelangeli F. Differential effect of PKA on the Ca2+ release kinetics of the type I and III InsP3 receptors. Biochem. Biophys. Res. Commun. 2003;302:121–126. doi: 10.1016/s0006-291x(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Enriquez-Barreto L., Palazzetti C., Brennaman L.H., Maness P.F., Fairen A. Neural cell adhesion molecule, NCAM, regulates thalamocortical axon pathfinding and the organization of the cortical somatosensory representation in mouse. Front. Mol. Neurosci. 2012;5:76. doi: 10.3389/fnmol.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Gallo G., Lefcort F.B., Letourneau P.C. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J. Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G., Letourneau P. Axon guidance: proteins turnover in turning growth cones. Curr. Biol. 2002;12:R560–R562. doi: 10.1016/s0960-9822(02)01054-0. [DOI] [PubMed] [Google Scholar]
- Giovannucci D.R., Groblewski G.E., Sneyd J., Yule D.I. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J. Biol. Chem. 2000;275:33704–33711. doi: 10.1074/jbc.M004278200. [DOI] [PubMed] [Google Scholar]
- Gomez T.M., Zheng J.Q. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Henley J.R., Huang K.H., Wang D., Poo M.M. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig M.G., Camilli S.J., Xue Q.S. Effects of L1 blockade on sensory axon outgrowth and pathfinding in the chick hindlimb. Dev. Biol. 2002;243:137–154. doi: 10.1006/dbio.2001.0556. [DOI] [PubMed] [Google Scholar]
- Hu X., Cai J., Yang J., Smith G.M. Sensory axon targeting is increased by NGF gene therapy within the lesioned adult femoral nerve. Exp. Neurol. 2010;223:153–165. doi: 10.1016/j.expneurol.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J. Biol. Chem. 2005;280:10305–10317. doi: 10.1074/jbc.M413824200. [DOI] [PubMed] [Google Scholar]
- Lohof A.M., Quillan M., Dan Y., Poo M.M. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes K., Missiaen L., Parys J.B., De Smet P., Sienaert I., Waelkens E., Callewaert G., De Smedt H. Mapping of the ATP-binding sites on inositol 1,4,5-trisphosphate receptor type 1 and type 3 homotetramers by controlled proteolysis and photoaffinity labeling. J. Biol. Chem. 2001;276:3492–3497. doi: 10.1074/jbc.M006082200. [DOI] [PubMed] [Google Scholar]
- Matsu-ura T., Shirakawa H., Suzuki K.G.N., Miyamoto A., Sugiura K., Michikawa T., Kusumi A., Mikoshiba K. Dual-FRET imaging of IP3 and Ca2+ revealed Ca2+-induced IP3 production maintains long lasting Ca2+ oscillations in fertilized mouse eggs. Sci. Rep. 2019;9:4829. doi: 10.1038/s41598-019-40931-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J. Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- Ming G., Song H., Berninger B., Inagaki N., Tessier-Lavigne M., Poo M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Ming G.L., Wong S.T., Henley J., Yuan X.B., Song H.J., Spitzer N.C., Poo M.M. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosaki T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkawa T., Miyawaki A., Sugiyama T., Yoneshima H., Yamamoto-Hino M., Furuichi T., Saruta T., Hasegawa M., Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J. Biol. Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- Nakade S., Rhee S.K., Hamanaka H., Mikoshiba K. Cyclic AMP-dependent phosphorylation of an immunoaffinity-purified homotetrameric inositol 1,4,5-trisphosphate receptor (type I) increases Ca2+ flux in reconstituted lipid vesicles. J. Biol. Chem. 1994;269:6735–6742. [PubMed] [Google Scholar]
- Newton C.L., Mignery G.A., Südhof T.C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J. Biol. Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- Nucifora F.C., Jr., Sharp A.H., Milgram S.L., Ross C.A. Inositol 1,4,5-trisphosphate receptors in endocrine cells: localization and association in hetero- and homotetramers. Mol. Biol. Cell. 1996;7:949–960. doi: 10.1091/mbc.7.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T.D., Jackman A., Rice F.L., Kucera J., Snider W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Piper M., Salih S., Weinl C., Holt C.E., Harris W.A. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat. Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff W.J., Urbach J.S., Esrick M.A., McAllister R.G., Richards L.J., Goodhill G.J. A new chemotaxis assay shows the extreme sensitivity of axons to molecular gradients. Nat. Neurosci. 2004;7:678–682. doi: 10.1038/nn1259. [DOI] [PubMed] [Google Scholar]
- Shah S.Z.A., Zhao D., Khan S.H., Yang L. Regulatory mechanisms of endoplasmic reticulum resident IP3 receptors. J. Mol. Neurosci. 2015;56:938–948. doi: 10.1007/s12031-015-0551-4. [DOI] [PubMed] [Google Scholar]
- Sharp A.H., Nucifora F.C., Jr., Blondel O., Sheppard C.A., Zhang C., Snyder S.H., Russell J.T., Ryugo D.K., Ross C.A. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J. Comp. Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- Soulsby M.D., Wojcikiewicz R.J. Calcium mobilization via type III inositol 1,4,5-trisphosphate receptors is not altered by PKA-mediated phosphorylation of serines 916, 934, and 1832. Cell Calcium. 2007;42:261–270. doi: 10.1016/j.ceca.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub S.V., Giovannucci D.R., Bruce J.I., Yule D.I. A role for phosphorylation of inositol 1,4,5-trisphosphate receptors in defining calcium signals induced by Peptide agonists in pancreatic acinar cells. J. Biol. Chem. 2002;277:31949–31956. doi: 10.1074/jbc.M204318200. [DOI] [PubMed] [Google Scholar]
- Südhof T.C., Newton C.L., Archer B.T., 3rd, Ushkaryov Y.A., Mignery G.A. Structure of a novel InsP3 receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Genazzani A.A., Morris S.A. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- Tojima T., Hines J.H., Henley J.R., Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T., Itofusa R., Kamiguchi H. Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. J. Neurosci. 2014;34:7165–7178. doi: 10.1523/JNEUROSCI.5261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K.L., Meyer M., Barde Y.A. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Van Haastert P.J., Van Driel R., Jastorff B., Baraniak J., Stec W.J., De Wit R.J. Competitive cAMP antagonists for cAMP-receptor proteins. J. Biol. Chem. 1984;259:10020–10024. [PubMed] [Google Scholar]
- Vanderheyden V., Devogelaere B., Missiaen L., De Smedt H., Bultynck G., Parys J.B. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Jing X., DeBerry J.J., Schwartz E.S., Molliver D.C., Albers K.M., Davis B.M. Neurturin overexpression in skin enhances expression of TRPM8 in cutaneous sensory neurons and leads to behavioral sensitivity to cool and menthol. J. Neurosci. 2013;33:2060–2070. doi: 10.1523/JNEUROSCI.4012-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz R.J., He Y. Type I, II and III inositol 1,4,5-trisphosphate receptor co-immunoprecipitation as evidence for the existence of heterotetrameric receptor complexes. Biochem. Biophys. Res.Commun. 1995;213:334–341. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J., Luo S.G. Differences among type I, II, and III inositol-1,4,5-trisphosphate receptors in ligand-binding affinity influence the sensitivity of calcium stores to inositol-1,4,5-trisphosphate. Mol. Pharmacol. 1998;53:656–662. doi: 10.1124/mol.53.4.656. [DOI] [PubMed] [Google Scholar]
- Xu J., Rosoff W.J., Urbach J.S., Goodhill G.J. Adaptation is not required to explain the long-term response of axons to molecular gradients. Development. 2005;132:4545–4552. doi: 10.1242/dev.02029. [DOI] [PubMed] [Google Scholar]
- Ziemba K.S., Chaudhry N., Rabchevsky A.G., Jin Y., Smith G.M. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J. Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional animation of reconstructed trajectories of WT axons in the skin. The arrowheads indicate representative TrkA-labeled axons extending into the epidermis, i.e., in the direction of smaller scale values on the z-axis.
Three-dimensional animation of reconstructed trajectories of R3KO axons in the skin. The arrowheads indicate representative TrkA-labeled axons extending into the epidermis, i.e., in the direction of smaller scale values on the z-axis.