Summary
Regulation of cell division orientation controls the spatial distribution of cells during development and is essential for one-directional tissue transformation, such as elongation. However, little is known about whether it plays a role in other types of tissue morphogenesis. Using an ascidian Halocynthia roretzi, we found that differently oriented cell divisions in the epidermis of the future trunk (anterior) and tail (posterior) regions create an hourglass-like epithelial bending between the two regions to shape the tailbud embryo. Our results show that posterior epidermal cells are polarized with dynein protein anteriorly localized, undergo dynein-dependent spindle rotation, and divide along the anteroposterior axis. This cell division facilitates constriction around the embryo's circumference only in the posterior region and epithelial bending formation. Our findings, therefore, provide an important insight into the role of oriented cell division in tissue morphogenesis.
Subject Areas: Organizational Aspects of Cell Biology, Developmental Biology, Embryology
Graphical Abstract

Highlights
-
•
Differently oriented cell division creates epithelial bending to shape the tail bud
-
•
Epidermal cells divide in different orientations in two different adjacent regions
-
•
Posterior cells undergo dynein-dependent spindle rotation and divide along A-P axis
-
•
Dynein is enriched in the anterior surface of the posterior cells
Organizational Aspects of Cell Biology; Developmental Biology; Embryology
Introduction
During animal development, tissues, organs, and organisms are shaped into specific structures. Elucidating the mechanisms of these dynamic processes at multiple levels—molecules, cells, tissues, and organisms—is an important and fundamental issue in developmental biology. Although the structures of animals show considerable variation in shape, recent research has demonstrated the involvement of some fundamental mechanisms in the process of creating tissues, organs, and organisms across animal species. An example of this is epithelial bending or invagination, which is found in several cell morphogenetic events including gastrulation in Drosophila, Xenopus, and sea urchin (Sawyer et al., 2010, Davidson, 2012), and formation of the tracheal system, dorsal transverse fold, and leg epithelial fold in Drosophila embryos (Kondo and Hayashi, 2013, Wang et al., 2012, Ambrosini et al., 2019). However, interestingly, studies have shown that epithelial bending is driven by different cellular mechanisms, including apical constriction (Sawyer et al., 2010, Martin and Goldstein, 2014), mitotic cell rounding (Kondo and Hayashi, 2013), differential positioning of adherence junctions (Wang et al., 2012), and an actomyosin structure connecting the apical surface to the nucleus, which is anchored to the basal side in apoptotic cells (Ambrosini et al., 2019).
Regulation of cell division orientation, a well-known mechanism for proper distribution of determinants during asymmetric cell divisions (Bergstralh et al., 2017, di Pietro et al., 2016), is also essential for tissue morphogenesis such as tissue elongation and thickening (Panousopoulou and Green, 2014, da Silva and Vincent, 2007, Lechler and Fuchs, 2005). This is achieved via controlling the organized spatial distribution of cells following the oriented cell divisions. Three main rules apply to regulation of cell division orientation. The first is the Hertwig's rule, wherein cells divide along the longest axis of the cell; this is frequently observed in epithelial cell divisions (Hertwig, 1884, Brun-Usan et al., 2017, Minc et al., 2011, Minc and Piel, 2012). The second is the Sachs' rule (Saches, 1878), typically found in early embryonic cell divisions during cleavage stages (Guerrier, 1970, Meshcheryakov and Beloussov, 1975), wherein cells divide in a direction perpendicular to the orientation of the previous division. The third is the cell polarization rule (Brun-Usan et al., 2017, Morin and Bellaïche, 2011), wherein cells are polarized either by external cues or localized intrinsic factors and divide according to the polarity. One of the molecular bases of such cell polarization is the core cortical machinery that orients mitotic spindles and is known to function in asymmetric cell divisions, including those of Drosophila neuroblasts (di Pietro et al., 2016) and the Caenorhabditis elegans P1 cell of the two-cell embryo (Grill and Hyman, 2005, Rose and Gönczy, 2014). The core machinery comprises Mud, Pins, and Gαi in Drosophila (Bowman et al., 2006, Schaefer et al., 2000); LIN-5, GPR1/2, and GOA-1/GPA-16 in C. elegans (Srinivasan et al., 2003, Colombo et al., 2003); and NuMA, LGN, and Gαi in vertebrates (Du and Macara, 2004, Peyre et al., 2011). These proteins form a complex with the dynein motor at the cell cortex, thereby exerting a pulling force on the astral microtubules by moving along them until the spindle is properly aligned (di Pietro et al., 2016, Bergstralh et al., 2017).
Ascidians are marine invertebrates that belong to the phylum Chordata. They are useful model systems for studying cellular behavior during morphogenesis in embryonic stages because of their invariant cell lineage and small number of cells constituting the embryo (Conklin, 1905, Nishida, 1986, Kumano and Nishida, 2007, Ogura and Sasakura, 2013). One remarkable morphogenetic process during ascidian embryogenesis, in addition to neural tube closure (Hashimoto et al., 2015) and endoderm invagination during gastrulation (Sherrard et al., 2010), is tail formation. In transitioning from the neurula to tailbud stages, a boundary between the future trunk and tail regions appears morphologically as an hourglass-like epithelial bending at the middle level of the embryo along the anteroposterior (A–P) axis (Hotta et al., 2007, Figure 1A). Following this, the tail region undergoes extensive elongation along the A–P axis (Hotta et al., 2007, Figure 1A). This derives mainly from cell rearrangements (Munro and Odell, 2002a, Munro and Odell, 2002b, Passamaneck et al., 2007), rather than cell proliferation, and the tail region ends up being several times longer than is the trunk region. This is in contrast with other animals including some annelid and vertebrate species, in which tail formation depends on proliferation of a small group of cells known as posterior growth zones or tailbuds, which are located at the posterior end of the embryo (Williams and Nagy, 2017, McGrew et al., 2008).
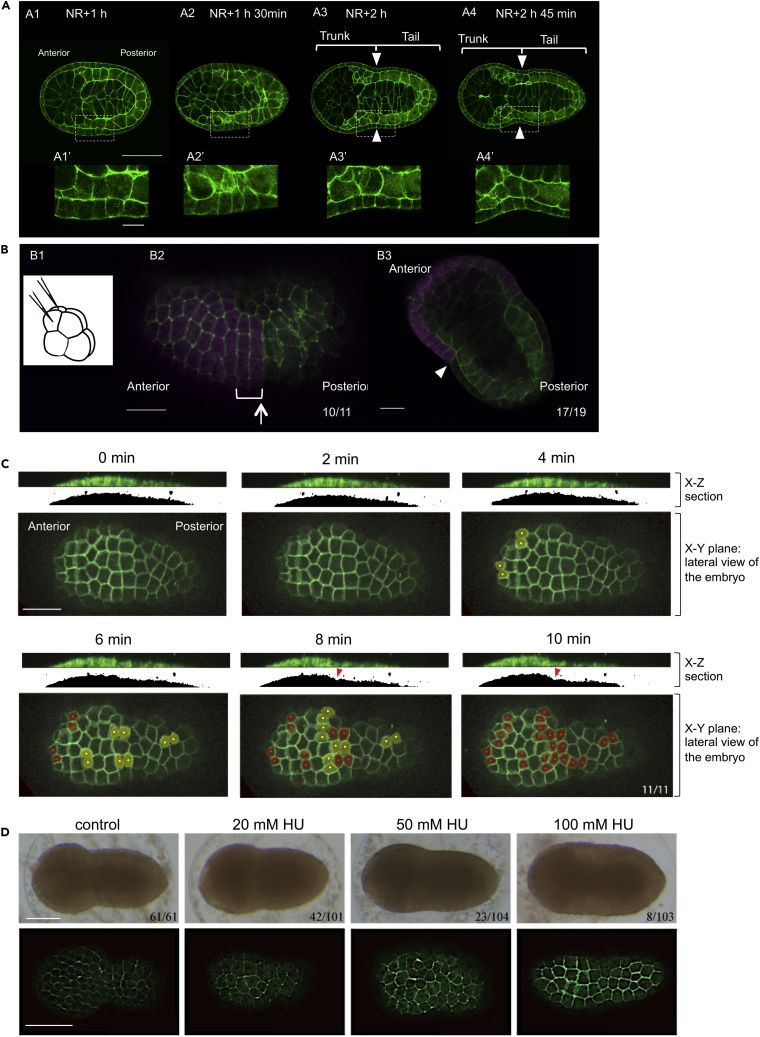
Figure 1.
Epithelial Bending Formation and Tailbud Shaping
(A) Phallacidin-stained embryos at the neurula through early tailbud stages. The image of each stage is a single focal plane, where the area of the embryo is at maximum. A1–A4 are viewed with anterior to the left. Close-up views of the dot-lined square in A1–A4 are shown at the bottom (A1′–A4′). (A1) At 1 h after the neurula rotation (NR+1 h) (Nishide et al., 2012), approximately 30 min before the start of the 11th epidermal cell divisions. (A2) At 1 h and 30 min after the neurula rotation (NR+1 h 30 min). (A3) 2 h after the neurula rotation (NR+2 h). (A4) 2 h and 45 min after the neurula rotation (NR+2 h 45 min). Arrowheads in A3 and A4 indicate the boundary between the trunk and tail regions. Scale bars, 100 μm (A1–A4), 20 μm (A1′–A4′).
(B1) Schematic diagram of eight-cell embryo injected for lineage trace. Lateral view, with anterior to the left. Fluorescent dye was microinjected into anterior ectodermal cells, named a4.2 cells, on both sides of the embryo. (B2) The descendants of a4.2 cells (magenta). Cell shape is visualized with Phallacidin (green). The boundary of anterior and posterior ectodermal lineages is indicated by an arrow. Rows of rectangular-shaped cells are indicated with white brackets. The digits in the bottom right-hand corner indicate the proportion of the embryo in which rows of rectangular-shaped cells were derived from a4.2 cells. Scale bar, 50 μm. (B3) Frontal section of an early tailbud embryo. The descendants of a4.2 cells (magenta), and cell shape is visualized with Phallacidin (green). The deepest position of the epithelial bending is indicated by an arrowhead. The digits in the bottom right-hand corner indicate the proportion of the embryo in which the lineage boundary corresponded to the deepest position of the epithelial bending. Scale bar, 50 μm.
(C) Snap shot images obtained from live confocal imaging showing the epidermal cell division. The cell membrane was labeled with PH-YFP. Anterior is to the left. In each time point, a section along the anterior–posterior axis (top, X–Z section) and lateral view of the embryo (bottom, X–Y section) are shown. Shadow images of the X–Z section are shown for clarification of the formation of epithelial bending (red arrowheads). The dividing and divided cells in the X–Y plane are indicated with yellow and red dots, respectively. The digits in the bottom right-hand corner at the 10 min image indicate the proportion of the embryo in which epidermal cell division accompanied formation of epithelial bending. Scale bar, 100 μm.
(D) Hydroxyurea (HU) treatment. The effect of HU on epidermal cell division (bottom) and formation of epithelial bending (top). The treated embryos were fixed and stained with Phallacidin. The number of embryos that form epithelial bending is shown in the bottom right. Scale bars, 100 μm.
In this study, we analyzed the molecular and cellular mechanisms that shape the tailbud embryo of the ascidian Halocynthia roretzi. We propose a model for epithelial bending and overall body shape mechanisms that require cell divisions oriented perpendicularly to each other in two different adjacent areas of the epithelial layer. We determined that epidermal cells in the anterior and future trunk region divided around the circumference of the embryo, whereas those in the posterior and future tail region divided along the A–P axis. We also found that the difference in division orientation between the anterior and posterior regions was established by dynein-mediated spindle rotation just before the cell divisions in the posterior cells, which resulted in its orientation along the A–P axis regardless of cell shape. Accordingly, this cell division results in constriction around the circumference of the embryo only in the posterior region and creates epithelial bending at the boundary of the anterior and posterior regions to shape the tailbud.
Results
Cell Morphology around the Future Epithelial Bending Site
To understand the cellular mechanisms by which the hourglass-like epithelial bending to shape the H. roretzi tailbud embryo is generated on the lateral side of the neurula embryo (Figure 1A), our first aim was to characterize the morphology of epidermal cells in the outermost and epithelial layer of the embryo during bending formation. Phallacidin staining of fixed neurula embryos revealed that epidermal cells around the future bending region exhibit rectangular shape, frequently arranged in two rows, with their long axes aligned around the circumference of the embryo (bracket in Figure 1B2). Cell lineage analyses, in which descendants of the anterior ectodermal cells named a4.2 were traced following injection of pairs of a4.2 cells with a fluorescent dye, Texas Red dextran, at the eight-cell stage (Figure 1B1), revealed that the ectodermal A–P lineage boundary, which is defined by the second cleavage after fertilization, coincides both with the posterior end of the rows of rectangular-shaped cells (90.9% [n = 11], arrow in Figure 1B2) and with the deepest point of the epithelial bending (89.5% [n = 19], arrowhead in Figure 1B3) following its formation.
Epidermal Cell Divisions during Epithelial Bending and Tailbud Shape Formation
We next carried out live-imaging analysis using PH-YFP encoding a fusion YFP fluorescent protein to a pleckstrin homology (PH) domain to visualize cell membranes (Tall et al., 2000, Passamaneck et al., 2007) of epidermal cells on the lateral side of the embryo. We found that the timing of the formation of epithelial bending (X-Z sections in Figure 1C) coincides with the timing of epidermal cell divisions (X-Y planes in Figure 1C). These cell divisions were the 11th to occur after fertilization (Figure S1). To examine whether the epidermal cell divisions are related causally to the formation of epithelial bending, we cultured embryos in the presence of Hydroxyurea (HU), a cell division inhibitor, beginning at approximately 1 h and 30 min prior to the cell divisions. We found that HU treatment inhibited both cell division (bottom in Figure 1D) and formation of epithelial bending/tailbud shaping (top in Figure 1D) in a concentration-dependent manner. However, we cannot rule out the possibility that the resultant larger cell size itself might have blocked the morphogenesis. We also cannot eliminate the possibility that this treatment affects other cells, especially those located inside the embryo; however, previous studies suggest that at least notochord and muscle cells, which constitute a large portion of the tail region, have already ceased their embryonic cell divisions before the late neurula stage (Nishida, 1987, Kuwajima et al., 2014).
Differently Oriented Cell Divisions between Anterior and Posterior Regions
We then tried to clarify the potential contribution of those epidermal cell divisions to the formation of epithelial bending and the tailbud shaping. Via live-imaging analysis with PH-YFP, we found that epidermal cells undergo differently oriented cell divisions in anterior and posterior regions on the lateral side of the embryo. Cells in the anterior region divide around the circumference of the embryo (red lines in Figure 2A), whereas cells in the posterior region divide along the A–P axis (white lines in Figure 2A), forming a clear boundary of division orientation around the bending forming region (Figure 2A, Video S1). To ascertain the precise location of the division orientation boundary, we took advantage of asymmetric inheritance of the injected mRNA and identified the ectodermal A–P lineage boundary by means of different cell membrane fluorescence intensity. We ascertained that the division orientation boundary is located one cell row anterior to the A–P lineage boundary and that the one cell row difference was invariant in nearly all the embryos observed (Figures 2B and 2C).
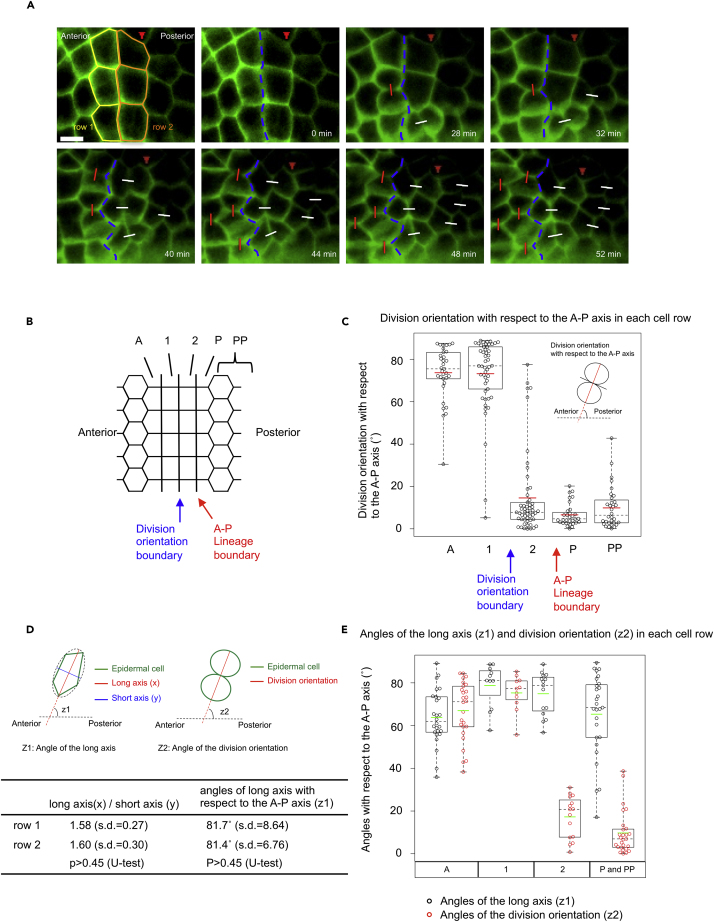
Figure 2.
Differently Oriented Cell Division with Clear Boundary
(A) Snap shot images from Video S1 showing the epidermal cells. Lateral side view. In the top left image, cell rows 1 and 2 (see below for the definition) are indicated by yellow and orange lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead. Division orientation boundary is indicated by blue broken lines. Division angles with respect to the A–P axis are indicated by white line (between 0° and 30°) and red line (between 61° and 90°). Sister cells are connected with white line and red line according to the division angles. Scale bar, 20 μm.
(B) Schematic diagram of epidermal cells on the lateral side of the embryo. The cell row located anterior to the division orientation boundary is indicated as row 1, that posterior to row 1 is indicated as row 2, that anterior to row 1 is indicated as row A, that posterior to row 2 is indicated as row P, and that posterior to row P is indicated as row PP.
(C) Division orientation with respect to the A–P axis. The inset in the graph demonstrates how to measure the angle of division orientation with respect to the A–P axis. Each circle represents a measurement of a single cell. The values were extracted from 41 cells/8 embryos for row A, 58 cells/9 embryos for row 1, 62 cells/9 embryos for row 2, 41 cells/6 embryos for row P, and 23 cells/3 embryos for row PP. Data are represented as individual values and boxplots. Means are indicated as red horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values.
(D) Schematic diagrams showing how the angles of the cell long axis (z1) and the division orientation axis (z2) are defined with respect to the A–P axis. At the bottom is a table describing the ratio of the long to the short axes (x/y) and the angle of the long axis of epidermal cells in rows 1 and 2 with respect to the A–P axis to evaluate cell geometry.
(E) The values of z1 (black circle) and z2 (red circle) were extracted from 26 cells/5 embryos for row A, 11 cells/6 embryos for row 1, 15 cells/6 embryos for row 2, and 27 cells/3 embryos for rows P and PP. Data are represented as individual values and boxplots. Means are indicated as green horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 61°–90°) are indicated by white and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Comparison of division orientations between different rows of the rectangular-shaped cells (rows 1 and 2 in Figures 2A and 2B) is especially interesting as they divided in directions nearly perpendicular to each other (red circles in the middle two columns of Figure 2E), even though their cell geometries are almost identical in terms of cell shape and long axis orientation (Figure 2D, black circles in the middle two columns of Figure 2E). The cells in row 1 divided along their long axes, whereas those in row 2 did not (Figure 2E). Our analyses of other cells also revealed that epidermal cells in a row anterior to row 1 (row A in Figure 2B) divide along their long axes (left-most column of Figure 2E), whereas epidermal cells in rows posterior to row 2 (rows P and PP in Figure 2B) do not (right-most column of Figure 2E). These findings suggest that cells in rows 2, P, and PP undergo cell divisions against the Hertwig's rule (Hertwig, 1884) and that a mechanism actively directs their division orientation so that they divide along the A–P axis.
Mitotic Spindle Rotation in Posterior Epidermal Cells
We next investigated how epidermal cells in the posterior region come to divide along the A–P axis. We conducted live-imaging analysis using MAP7-GFP, which is known to localize to the microtubules and spindle poles during mitosis (McDougall et al., 2015), together with PH-YFP, to visualize mitotic spindle orientation in epidermal cells. We found that the epidermal cells in rows 2, P, and PP undergo mitotic spindle rotation such that the spindles are oriented along the A–P axis before cell divisions (Figures 3A and 3B; Videos S2 and S3). In contrast, the spindles of the cells in row 1 remained oriented in the same direction and did not rotate (Figure 3A; Video S2). Notably, the mitotic spindles were aligned along the long axes of the cells prior to rotation (Figures 3A–3C). The spindles then rotated in either a clockwise or counterclockwise direction, with most of them achieving the reorientation via the shorter of the two rotation paths (76.3% [n = 38], Figure 3D).
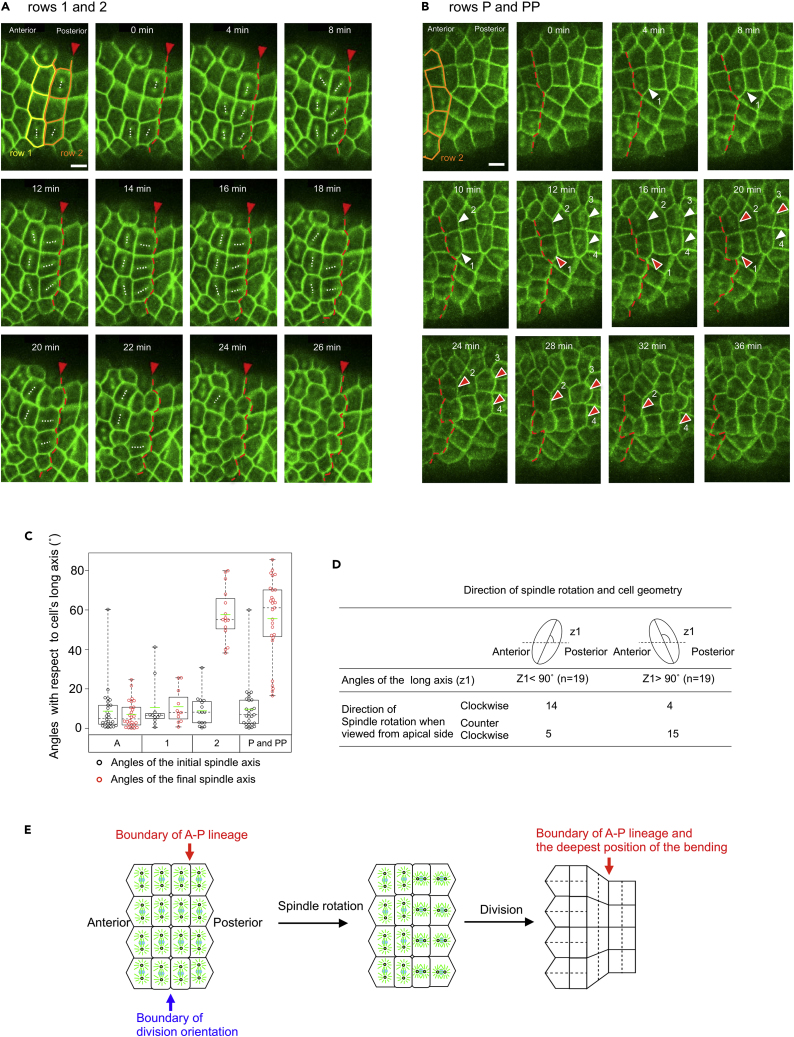
Figure 3.
Spindle Rotation before the Epidermal Cell Division
(A) Snap shot images from Video S2 showing the spindle orientation in rows 1 and 2. In the top left image, cell rows 1 and 2 are indicated by yellow and orange lines, respectively. Anterior is to the left, and the lineage boundary is indicated by red arrowhead and red broken lines. Orientation of the spindle is indicated with dotted white lines. 0 min indicates the start of the observation.
(B) Snap shot images from Video S3 showing the spindle rotation in rows P and PP. In the top left image, cell row 2 is indicated by orange line. Anterior is to the left, and the lineage boundary is indicated by red broken lines. Note that the lineage boundary becomes zigzag due to different cell division timing during the cell division period. 0 min indicates the start of the observation. White arrowheads indicate that the spindle is aligned along the circumference of the embryo, whereas red arrowheads indicate that the spindle is either rotating or aligned along the A–P axis. Scale bar, 20 μm (A and B).
(C) The angle of the spindle axis with respect to cell's long axis. Black circles and red circles indicate the initial and final angles, respectively. The values were extracted from 26 cells/5 embryos for row A, 11 cells/6 embryos for row 1, 15 cells/6 embryos for row 2, and 27 cells/3 embryos for rows P and PP. Data are represented as individual values and boxplots. Means are indicated as green horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values.
(D) Direction of spindle rotation in two cases: one is when the angle of the long axis was less than 90° (left); the other is when the angle was more than 90° (right).
(E) A model for the formation of epithelial bending during the 11th epidermal cell division. Mitotic spindle rotations in rows 2 and P create perpendicularly oriented cell division between the anterior and posterior regions and contribute to the formation of epithelial bending (see text for detail).
Anterior is to the left. In the first frame, cell rows 1 and 2 are indicated by yellow and orange lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. In the first frame, cell rows 2 is indicated by orange lines. White arrowheads indicate that the spindle is aligned along the circumference of the embryo, whereas red arrowheads indicate that the spindle is either rotating or aligned along the A–P axis.
Based on the above findings, we propose a model for the hourglass-like epithelial bending formation to shape the tailbud embryo of the ascidian H. roretzi (Figure 3E). Epidermal cells on the lateral side of the neurula embryo divide in a different orientation perpendicular to each other in the anterior and posterior regions after the collective and regional rotation of mitotic spindles in the posterior cells. This cell division with different division orientation facilitates constriction around the embryo's circumference only in the posterior region, with a lesser number of daughter cells distributed around the circumference than in the anterior region, which leads to the generation of a bending at the boundary between the anterior and posterior regions.
Dynein-Mediated Mitotic Spindle Rotation and Tailbud Shape Formation
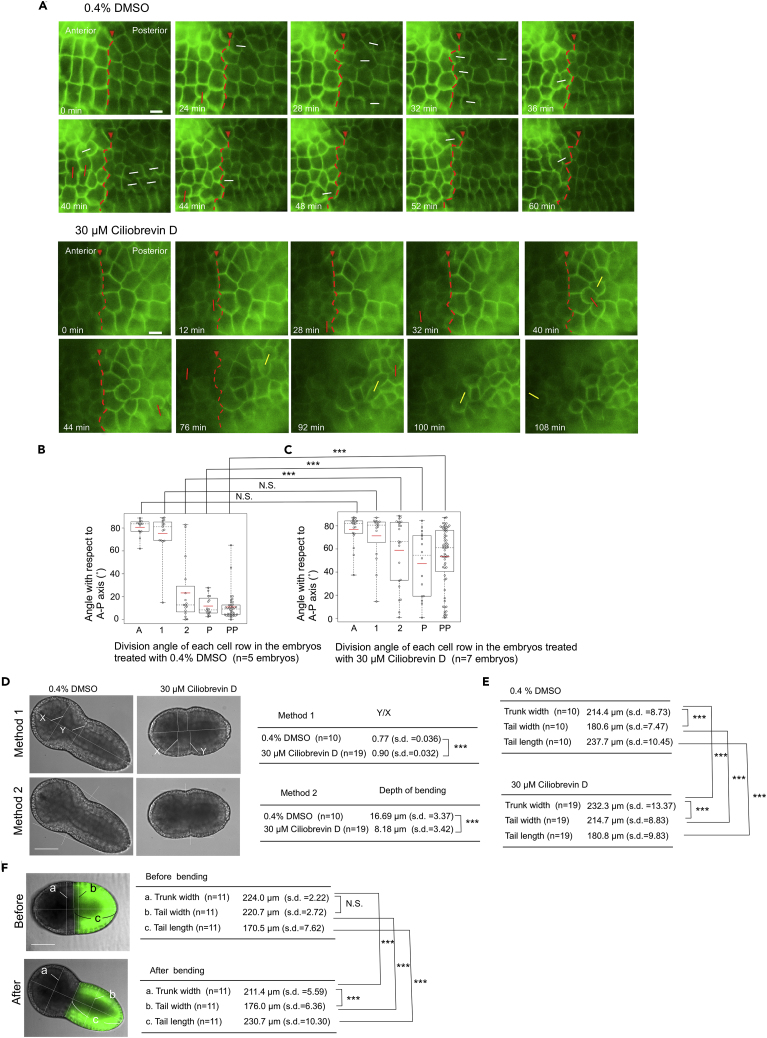
To evaluate our model, we tried to disrupt the mitotic spindle rotation pharmacologically and ascertain its effect on the formation of a thinner and longer posterior body part and epithelial bending for the tail bud shaping. Research has shown that a motor protein dynein localizes at a cell cortex and drives mitotic spindle rotation for determination of cell division orientation (Lu and Johnston, 2013, di Pietro et al., 2016). Therefore, we treated embryos with 30 μM of a dynein inhibitor, Ciliobrevin D (Firestone et al., 2012, Lecland and Lüders, 2014), for 1 h before the 11th ectodermal cell divisions. This abrogated the division orientation of the epidermal cells in rows 2, P, and PP and directed it in a more circumferential direction, increasing the angles with respect to the A–P axis when compared with control cells treated with DMSO (Figures 4A–4C; Videos S4 and S5). The division angles of the cells treated with Ciliobrevin D, especially those in rows P and PP, were distributed relatively evenly in a wide range between 0° and 90° (Figure 4C); this is likely because the angles of the cells' long axes in rows P and PP were distributed in a similar way (Figure 2E), along which the mitotic spindles were aligned originally (Figure 3C), and they divided according to Hertwig's rule. Intriguingly, the inhibitor-treated embryos did not have the normal shape of tailbud embryos and instead displayed a significantly reduced extent of epithelial bending, as evaluated using two different calculation methods (Figure 4D). In addition, these embryos had wider and shorter tails than did normal embryos (Figure 4E) (214.7 μm for the Ciliobrevin D-treated tail width versus 180.6 μm for control, p < 0.001, t test; 180.8 μm for the Ciliobrevin D-treated tail length versus 237.7 μm for control, p < 0.001, t test). In the normal epithelial bending formation, the tail becomes relatively thinner when compared with the trunk and longer (Figure 4F). We also noticed that, in the Ciliobrevin D-treated embryos, cell divisions were delayed (Figure S2). However, this delay is not likely a cause of the phenotype described above, because treatment with the inhibitor under different conditions (at 10 μM for 2 h) further delayed cell division but did not affect the shaping of the tailbud embryo (Figure S2).
Figure 4.
Disruption of the Orientation of Epidermal Cell Division and Epithelial Bending by Treatment with Dynein Inhibitor
(A) Division pattern of epidermal cells in the control embryo and embryo treated with Ciliobrevin D. Lineage boundary is indicated by red arrowhead and red broken lines. Snap shot images from Videos S4 and S5 showing the epidermal cell divisions. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown by white, yellow, and red lines, respectively. 0 min indicates the start of the observation. Scale bars, 20 μm.
(B and C) Angle of division orientation with respect to the A–P axis in DMSO-treated controls (B) and Ciliobrevin D-treated embryos (C). In the DMSO-treated controls (n = 5 embryos), the values were extracted from 14 cells for row A, 15 cells for row 1, 16 cells for row 2, 16 cells for row P, and 40 cells for row PP. Data are represented as individual values and boxplots. Means are indicated as red horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values. In the Ciliobrevin D-treated embryos (n = 7 embryos), the values were extracted from 20 cells for row A, 16 cells for row 1, 23 cells for row 2, 17 cells for row P, and 65 cells for row PP. Data are represented as individual values and boxplots. Means are indicated as red horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values. There is no statistically significant difference in the cell rows A and 1 between the DMSO-treated control and Ciliobrevin D-treated embryos; however, the angles of the cell rows 2, P, and PP of the Ciliobrevin D-treated embryos were significantly higher than those of the control embryos.
(D) Phenotype of Ciliobrevin D-treated embryos and quantitative evaluation of the epithelial bending. See Transparent Methods for the calculation methods. Table shows the results of calculations. N.S. and ∗∗∗ indicate p > 0.05 and p < 0.001, respectively (t test). Scale bar, 100 μm.
(E) The width of the trunk and tail regions and the tail length in the DMSO-treated control and Ciliobrevin D-treated embryos. See Transparent Methods for the calculation methods. N.S. and ∗∗∗ indicate p > 0.05 and p < 0.001, respectively (t test). Note that the trunk in the Ciliobrevin D-treated embryos is significantly wider than that in control embryos for unknown reason.
(F) Characterization of the morphogenetic change during the formation of epithelial bending. The width of the trunk and tail regions (ventral view) and the tail length before and after the epithelial bending formation. See Transparent Methods for the calculation methods. Table shows the results of calculations. N.S. and ∗∗∗ indicate p > 0.05 and p < 0.001, respectively (t test). Scale bar, 100 μm.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Along with Ciliobrevin D, we used another dynein inhibitor EHNA and observed its effect on the epithelial bending and tailbud shape formation. EHNA is reported to be less specific (Firestone et al., 2012), but it has been shown to disrupt mitotic spindle orientation in another ascidian species, Ciona intestinalis (Negishi and Yasuo, 2015). The EHNA treatment abrogated cell division orientation (Figures S3A and S3B; Video S6) in a manner similar to that with Ciliobrevin D treatment, with significantly less bending (Figure S3C) and wider and shorter tail (Figure S3D) when compared with control embryos; however, the morphologies of the resultant tailbud embryo seemed to differ between the two inhibitor treatments (Figures 4D and S3C). In fact, the EHNA-treated tail was significantly thinner and longer than the Ciliobrevin D-treated one (191.3 μm for the EHNA-treated tail width [Figure S3D] versus 214.7 μm for the Ciliobrevin D-treated tail width [Figure 4E], p < 0.001, t test; 215.0 μm for the EHNA-treated tail length [Figure S3D] versus 180.8 μm for the Ciliobrevin D-treated tail length [Figure 4E], p < 0.001, t test), possibly due to less inhibitory effect of EHNA on cell division orientation (a division angle of 42.2°on average with respect to the A–P axis for EHNA-treated cells of the row PP [Figure S3B] versus 53.3°for Ciliobrevin D-treated PP cells [Figure 4C] and/or to the non-specific effect of EHNA).
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
These results suggest that the mitotic spindle rotation is dynein dependent and required for the tailbud shaping.
Anterior Enrichment of Dynein Protein in Posterior Epidermal Cells
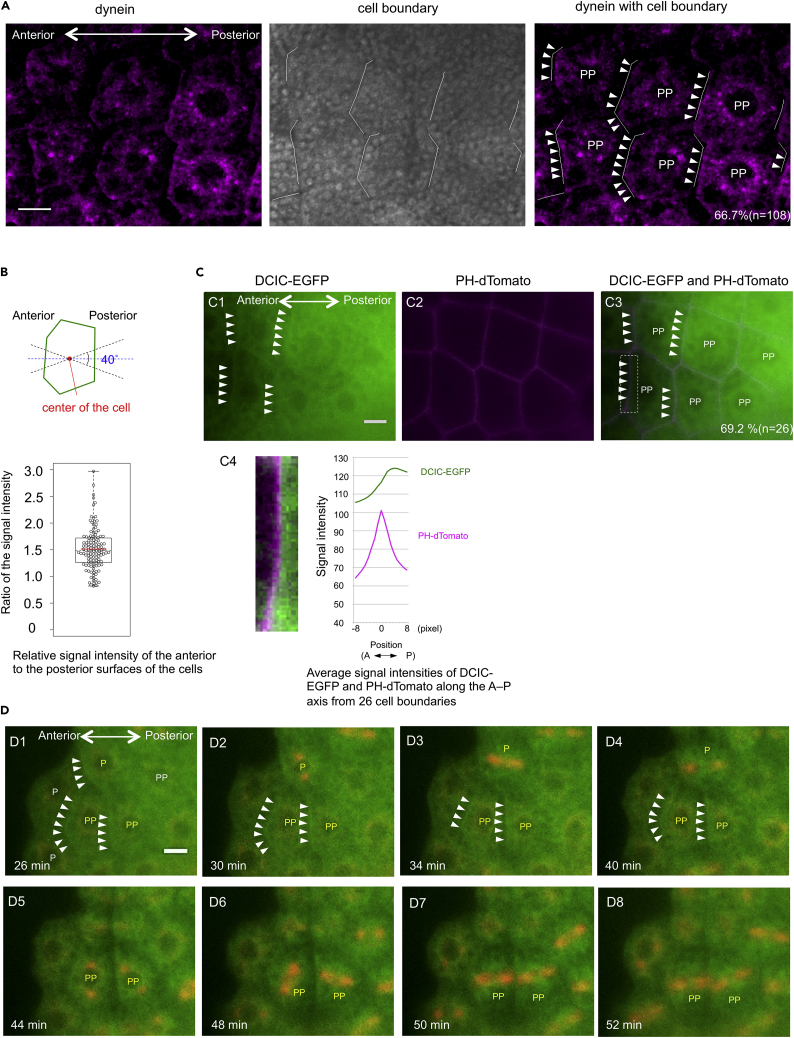
We then investigated whether dynein protein is enriched in a localized cell cortical domain of posterior epidermal cells, as is shown in several asymmetrically dividing cells undergoing mitotic spindle rotations (Lu and Johnston, 2013, di Pietro et al., 2016). To observe sub-cellular localization of dynein protein, we first performed antibody staining using anti-dynein heavy chain antibody, which has been used successfully to locate dynein protein in early embryonic cells of another ascidian species, C. intestinalis (Negishi and Yasuo, 2015). The antibody staining suggested that dynein protein is present at the cell cortex and the peri-nuclear region of posterior epidermal cells (Figure 5A). Significantly, signals for dynein at the cell cortex were more concentrated to the anterior edge of cells (Figure 5A). The average ratio of the anterior to the posterior dynein signal intensities was 1.51 (Figure 5B, n = 108, S.D. = 0.39). The anterior localization was also confirmed by fluorescent observation using a fusion protein of dynein cytoplasmic intermediate chain (DCIC) to EGFP and PH-dTomato (Figure 5C). Further confirmation of these findings was obtained by live-imaging analysis, wherein mRNAs encoding DCIC-EGFP and MAP7-mCherry were injected. EGFP signals accumulated at the cell cortex, presumably at the anterior cortex of the cell, given the earlier results, and in the peri-nuclear region (Figure 5D; Video S7). Notably, the EGFP signals at the cell cortex were evident (cells P and PP with arrowheads in Figure 5D; Video S7) before the mitotic spindle rotation occurs (cells P and PP with arrowheads in Figure 5D; Video S7). These results suggest that posterior epidermal cells are polarized along the A–P axis with dynein protein accumulating at the cells' anterior edges and that the protein drives mitotic spindle rotation prior to cell division. We detected fluorescent signals suggesting anteriorly concentrated dynein protein accumulation in cells in row PP from both the antibody staining (60.0% [n = 30], Figure S4) and the live-imaging analysis (59.1% [n = 22], Figure 5D and Video S7); however, the percentages were small for cells in rows 2 (18.2% [n = 11]) and P (7.7% [n = 13]) from the antibody staining. In most of the cells in rows 2 and P, the protein was present at the cell cortex and showed no obvious bias in its localization along the A–P axis (Figure S4).
Figure 5.
Enrichment of Dynein in the Anterior Surface of the Cells before the Spindle Rotation
(A) Images of anti-dynein antibody staining of the cells in the row PP are shown. Anterior is to the left. (Left) Dynein signal is observed at the cellular surface of the cells and around the nucleus. (Middle) Cell boundary (white line) is identified by separating cell-cell contact after squishing the specimen. (Right) A merged image of the left and middle images indicating the enriched cell cortex signal is from the anterior of the cells (arrowheads). Scale bar, 10 μm.
(B) Quantification of the dynein protein enrichment. (Top) Schematic diagram showing how to measure the signal intensity. (Bottom) Relative signal intensity of the anterior to the posterior surfaces of the cells. All the cells with cell boundaries identified by squishing were measured. See Transparent Methods for the calculation methods. Data are represented as individual values and boxplot. The mean is indicated as a red horizontal bar. The median is indicated with dashed line. Box indicates the second and third quartiles, and whiskers indicate the maximum and minimum values.
(C) Images of DCIC-EGFP and PH-dTomato fluorescent signals in the row PP cells. (C1) Dynein signal is observed in the surface of PP cells (arrowheads). Scale bar, 10 μm. (C2) PH-dTomato signal (magenta) is detected at the cell membrane of PP cells. (C3) A merged image of C1 and C2. (C4, left) A close-up view of the adjacent cell surfaces between PP cells (a dot-lined square in C3). Brightness and contrast were enhanced for visualization. (C4, right) Average signal intensities of DCIC-EGFP and PH-dTomato from 26 different cell surfaces. See Transparent Methods for the calculation method. Note that DCIC-EGFP signal was peaked posterior to the PH-dTomato peak, indicating that DCIC-EGFP signal is enriched in the anterior surface of the cell.
(D) Selected snapshot images from Video S7 showing the expression of DCIC-EGFP (for dynein, green) and MAP7-mCherry (for spindle orientation, red) in posterior epidermal cells. Anterior is to the left. P and PP represent cells in the rows P and PP, respectively. Cell boundaries between different rows are not straight perpendicular to the A–P axis due to different timing of cell divisions (also see Figure 3B). Enrichment of the dynein is observed in the anterior surface of the cells (arrowheads). Time after the start of observation is shown in the bottom left of each image. The yellow P and PP indicate the cells in which dynein is observed in the anterior surface before spindle rotation. Scale bar, 10 μm.
Anterior is to the left. The expression of DCIC-EGFP (for dynein, green) and MAP7-mCherry (for spindle orientation, red) are shown. Enrichment of the dynein is observed in the anterior surface of the cells (arrowheads in cells P and PP) before the spindle rotation.
Autonomous Cell Division Reorientation to the Epithelial Sheet
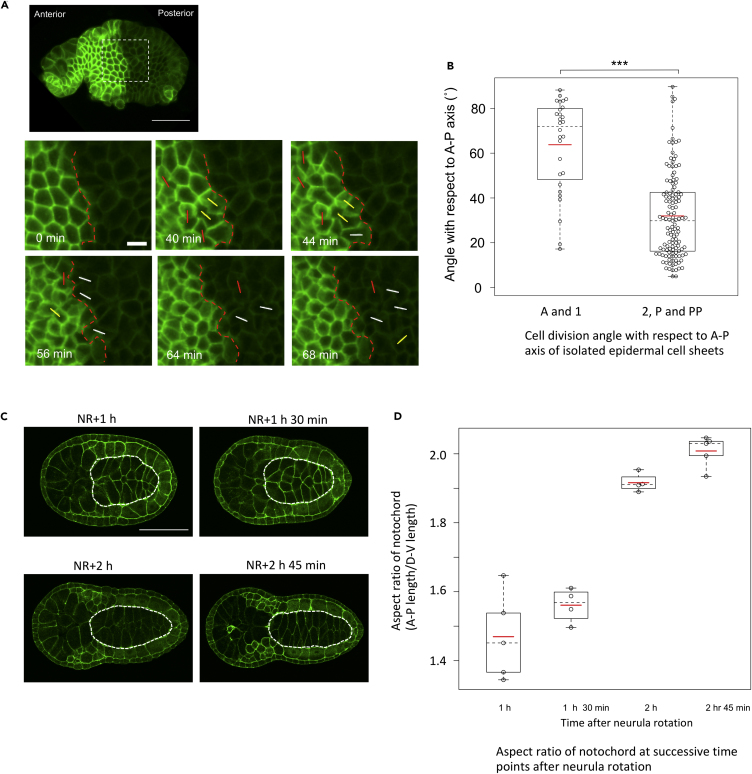
To obtain an insight into the mechanisms of cell division reorientation, we examined possible involvement of the underlying tissues such as muscle and notochord. We manually isolated the epidermal layer of the lateral side of the embryo from the underlying tissues approximately 40 min after neurula rotation (Nishide et al., 2012) (i.e., approximately 45 min before the cell divisions) and observed the patterns of division of epidermal cells in the isolated layer. We found that majority of the epidermal cells in rows 2, P, and PP in the isolated layer divided along the A–P axis, whereas the cells in rows A and 1 divided more in a circumferential direction (Figures 6A and 6B; Video S8), as observed in the intact embryos (Figure 2). Therefore, underlying tissues do not appear to affect the division reorientation of epidermal cells in the posterior region, at least after the 40-min mark after neurula rotation (45 min prior to the cell divisions). Notably, an initial step of convergent extension movement of the notochord occurred between 1 h 30 min and 2 h after neurula rotation (Figures 6C and 6D), eliminating the possibility that cell division orientation in the overlying epidermal cells is affected by physical force generated by the notochord extension along the A–P axis.
Figure 6.
Cell Division Pattern in Isolated Epidermal Cell Sheets
(A and B) Division pattern of the isolated epidermal cells. (A) Snap shots images from Video S8 showing epidermal cell division in isolated epidermal cell sheet. Anterior is to the left. The lineage boundary is indicated in red broken lines. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. Time after the start of observation is shown in the left bottom of each image. Scale bar, 20 μm. (B) Division angle with respect to the A–P axis in the cell rows A, 1 and 2, P and PP. Each dot represents a single observed cell. In the cell rows A and 1 (n = 5 embryos), the values were extracted from 28 cells. In the cell rows 2, P and PP (n = 5 embryos), the values were extracted from 79 cells. Data are represented as individual values and boxplots. Means are indicated as red horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values. The difference of the division angle between these two groups was statistically significant. ∗∗∗ indicates p < 0.001 (t test).
(C and D) Characterization of notochord convergent extension. (C) Images of Phallacidin-stained embryos at different stages from the neurula to the early tailbud stages (NR+1 h, NR+1 h 30 min, NR+2 h, NR+2 h 45 min). At each stage, the frontal section is shown with the notochord outlined by white broken lines. Anterior is to the left. (D) Aspect ratio of the notochord at each stage. Each circle represents one embryo. Data are represented as individual values and boxplots. Means are indicated as red horizontal bars. Medians are indicated with dashed lines. Boxes indicate second and third quartiles, and whiskers indicate the maximum and minimum values. Aspect ratio is increased highly from the NR+1 h 30 min to the NR+2 h.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively.
Discussion
In this study, we found a mechanism by which epithelial bending and overall tailbud body shape is generated during animal development. The mechanism for shaping an ascidian tailbud embryo centers on cell divisions, with division orientations between two adjacent regions of the epithelial layer directed perpendicular to each other. The differently oriented cell divisions in the adjacent regions produce a relative narrowing in one region and the layer ends up with a bending at their boundary.
Possible Other Mechanisms for the Epithelial Bending Formation
Although our present findings suggest that an ascidian tailbud embryo is shaped by the two-directional epithelial layer transformation by different cell division orientations, we cannot rule out the involvement of other mechanisms.
Initially, we suspected that an anisotropic apical constriction of epidermal cells at the future bending region may have a role in the formation of epithelial bending. The rectangular shape of the cells in that region (Figure 1B2) was reminiscent of the mesoderm precursor cells undergoing anisotropic apical constriction in the ventral midline of gastrulating Drosophila embryos (Dawes-Hoang et al., 2005, Fox and Peifer, 2007, Sawyer et al., 2010). However, for the following reasons, we concluded that this is not the case. First, in the frontal section of the embryos, the epidermal cells at the bending region did not display a wedge-like shape along the apico-basal axis (Figures 1A3 and 1A4). Second, the deepest point of the epithelial bending did not coincide with the center point of the rectangular-shaped cell rows (in this case, the interface of rows 1 and 2) (Figures 1B2 and 1B3). Coincidence with the center point would have been observed if bending is driven by anisotropic apical constriction.
Another mechanism that may contribute to the formation of epithelial bending is a shortening of the lateral sides of the epidermal cells along the apico-basal axis. A shortening of cells drives invagination of endoderm cells during gastrulation in another ascidian species (Sherrard et al., 2010). We observed that epidermal cells around the future bending site were extended along the apico-basal axis longer than were other epidermal cells located more anteriorly and posteriorly prior to the formation of bending (Figure 1A2) but their length was reduced to that of the other cells upon formation of the epithelial bending (Figures 1A3 and 1A4). This could explain why there was still a small degree of bending in embryos treated with Ciliobrevin D (Figure 4D). Moreover, EHNA treatment reduced epithelial bending by a significantly greater extent than did Ciliobrevin D according to one of our calculation methods (8.18 μm in Figure 4D [Ciliobrevin D] versus 6.34 μm in Figure S3C [EHNA] calculated by Method 2, p < 0.001 [t test]) despite the fact that it still generated longer and thinner tails than did Ciliobrevin D (Figures 4 and S3). EHNA has been reported to be less specific to dynein inhibition and to interfere with actin-dependent cellular processes (Schliwa et al., 1984). Consequently, the EHNA treatment may have affected the shortening of the epidermal cells through inhibiting actin function and reducing the extent of the epithelial bending.
It may also be possible that tissues underlying the epidermal layer, such as muscle and notochord, are involved in the formation of epithelial bending and the tailbud shaping. Although our isolation experiment of the epidermal layer suggests that epidermal cells undergo division reorientation without the underlying tissue (Figure 6A), it is still possible that underlying tissues are involved in ways other than regulation of cell division orientation. Suggestively, the boundary between underlying tissues, namely, mesenchyme and muscle, appears to coincide with the epithelial bending position.
Environmental Factors Required for the Tailbud Shaping
Our model for shaping the ascidian tailbud embryo (Figure 3E) would be valid if the field around the embryo's circumference is resistant to a tension generated in the posterior region by the differently oriented cell divisions in the two adjacent regions (Figure S5A, top). Accordingly, the constriction in the posterior region would properly take place as a result of the cell divisions. We speculate that the neural tube closure (Hashimoto et al., 2015, Ogura and Sasakura, 2016) could provide such a field, as we had found that the neural tube was nearly closed (two-thirds to three-quarters from the posterior end) when the epidermal cells divided (100% [n = 46]). If the neural tube is not closed, it is likely that the tension generated by the differently oriented cell division would not be propagated normally around the circumference of the embryo and posterior region would not be constricted (Figure S5A, bottom). Although our current approaches (see the Limitations of the Study section) are inconclusive, it is tempting to propose the importance of “a chain of morphogenetic events” for the correct shaping of individual tissues in the scale of the whole organism, in which one event (in this case, neural tube closure) influences another event (tailbud shaping) occurring at a different place and time. Recently, it has been reported that neural tube closure is necessary for correct orientation of cell division and epidermal stratification in mouse embryos (Box et al., 2019). Consequently, future studies will be required to examine whether the interactive regulation of spatially and temporally different morphogenetic events is a common characteristic in animal morphogenesis.
Determination of the Position of the Division Orientation Boundary
Our present findings suggest that determination of the position of the epithelial bending along the A–P axis in the neurula embryo relies on the position of the cell division orientation boundary. We speculate that the position of the division orientation boundary along the A–P axis is determined by the cell lineage, rather than by extracellular signals occurring at a later stage—such as antagonistic signals for patterning the neurula embryo along the A–P axis (Pasini et al., 2012)—because the division orientation boundary fell consistently at the same position with respect to the A–P lineage boundary in the ectoderm (i.e., one cell row away anteriorly) in different individuals (Figures 2B and 2C). As early as the second cell cleavage after fertilization that separates the A–P lineage, a difference could be made between the two lineages and memorized until it comes into effect at the late neurula stage. One candidate for the identity of the difference could be a member(s) of Postplasmic/Pem RNAs, a group of maternal mRNAs that are localized to the future posterior region of the egg and are inherited by posterior cells during cleavage stages (Makabe and Nishida, 2012, Sardet et al., 2005, Prodon et al., 2007). Although previous studies on Postplasmic/Pem RNAs have identified their function in determination of developmental fate (Nishida and Sawada, 2001, Nakamura et al., 2006) and regulation of cell division patterning (Negishi et al., 2007, Prodon et al., 2010) in the posterior-vegetal cells (mesoderm and endoderm), one member named Pem has been shown to repress anterior ectodermal genes, such as FoxA and SoxB1, in posterior-animal cells (ectoderm) at the eight-cell stage (Kumano et al., 2011).
Currently, it is also unclear as to how the division orientation boundary is placed one cell row anterior to the A–P lineage boundary. We determined that posterior epidermal cells are polarized along the A–P axis, and, therefore, planar cell polarity (PCP) pathway may contribute to establishing cell polarization. PCP is known to be propagated in the Drosophila wing disc, from the prospective proximal region to the prospective distal region, to pattern the orientation of wing hair (Ambegaonkar et al., 2012, Devenport, 2014). In the ascidian Ciona savignyi, a polarizing signal has been shown to be propagated from a notochord cell to establish PCP in an adjacent notochord cell located anteriorly (Kourakis et al., 2014). Based on these findings, we speculate that, in the H. roretzi neurula embryo, the epidermal cells in row 2, the only cells that undergo spindle rotations derived from the anterior ectoderm lineage, may receive a polarizing signal from the cells derived from the posterior ectoderm lineage. However, we observed that the majority of the epidermal cells in row PP are polarized in terms of localization of dynein protein (Figures 5 and S4; Video S7), whereas those in rows 2 and P are not (Figure S4). Therefore, the cells that share the boundary of the A–P lineages, both those in row 2 that share it posteriorly and those in row P that share it anteriorly, may behave differently owing to their contact with the different lineage cells across the boundary. However, they still undergo rotation of the mitotic spindle in a dynein-dependent manner (Figures 4A, 4B, S3A, and S3B; Videos S5 and S6).
Shaping of the Tailbud Embryo in Another Ascidian Species
The shape of the tailbud embryo is conserved in different ascidian species, with epithelial bending formed in the middle of the embryo along the A–P axis and the tail region becoming thinner and longer (Hotta et al., 2007). However, study has shown that most of the epidermal cells in both the anterior and posterior regions of the neurula embryo in the ascidian C. intestinalis type A (Currently C. robusta) divide along the A–P axis at their 11th divisions (Ogura et al., 2011, Negishi et al., 2016). In addition, live-imaging observation indicated that the timing of an epithelial bending to shape the tailbud embryo of Ciona seemed not to coincide with the 11th cell divisions (Negishi et al., 2016; Negishi, personal communication). Therefore, H. roretzi and C. intestinalis type A (Currently C. robusta) may use different mechanisms for shaping the tailbud embryo and may exemplify a phenomenon known as developmental system drift, whereby divergence of underlying molecular mechanisms occurs for conserved tissue development across species (Lemaire and Piette, 2015, True and Haag, 2001).
Limitations of the Study
As discussed above, the relationship between neural tube closure and tailbud shaping is still speculative. At present we have tried to address this issue by the following approaches.
We prevented the neural tube from closing by removing the chorion, as has been reported (Nishida and Satoh, 1985), at the late gastrula stage, and determined, via the four calculation methods described earlier, that such embryos failed to shape the normal tailbud embryos with a reduced extent of epithelial bending and wider and shorter tail (Figures S5B and S5C). However, these embryos exhibited the normal pattern of cell division—around the circumference of the embryo in the anterior versus along the A–P axis in the posterior regions (Figures S5D and S5E; Video S9)—suggesting that the failure is not due to an abrogated pattern of cell division.
Anterior is to the right. Division angles with respect to the A–P axis (0°–30°, 61°–90°) are indicated by white and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
We also tried to make the field around the embryo's circumference less resistant to a tension generated by cell division by culturing embryos in Ca2+-free sea water, thereby weakening cell-cell adhesion. From right after the neural tube had been closed to two-thirds but before cell division, embryos were cultured in the absence of Ca2+. These embryos exhibited a reduced extent of epithelial bending and wider and shorter tail (Figures S6A and S6B) but normal cell division orientations (Figures S6C and S6D).
These results support our speculation that a resistant field to the tension around the circumference of the embryo created by the neural tube closure could be necessary for shaping the epithelial bending and the tailbud embryo. However, non-specific effects cannot be ruled out in these experiments.
In another ascidian C. intestinalis type A (currently C. robusta), neural tube closure was successfully blocked by targeted overexpression of a dominant-negative form of RhoA (Hashimoto et al., 2015) and Cadherin 2 (Hashimoto and Munro, 2019) to dorsal midline cells including neural and epidermal cells flanking the boundary of the both cell types. Therefore, for the future prospective, it would be worthwhile to evaluate the epithelial bending and tailbud shape formation in H. roretzi embryos upon such more specific treatments to disrupt neural tube closure. These studies would shed light on an interactive regulation of spatially and temporally different morphogenetic events and its importance for tissue morphogenesis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Drs. Shigeo Hayashi and Kagayaki Kato for their technical advices for live imaging; Dr. Anna Di Gregorio for the Tbx6b>PH-YFP construct; Dr. Alex McDougall for the MAP7-GFP and PH-dTomato constructs; Dr. Takefumi Negishi for H2B-GFP construct and helpful discussion. This study was supported by The Sumitomo Foundation (G.K.), Yamada Science Foundation (G.K.), and Grant-in-Aid for Scientific Research (KAKENHI) on Innovative Areas (23111515 to G.K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. Also, this study was supported by Narishige Zoological Science Award (A.N.), and Grant-in-Aid for Scientific Research (KAKENHI) (19K06735 to A.N.), Japan Society for the Promotion of Science.
Author Contributions
G.K. provided supervision and conceptualization. A.N. and G.K. designed experiments. A.N. conducted most of the experiments and analyzed the results. A.N. wrote the manuscript, and A.N. and G. K. revised it. A.N. and G.K. secured the funding.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100964.
Supplemental Information
References
- Ambegaonkar A.A., Pan G., Mani M., Feng Y., Irvine K.D. Propagation of Dachsous-Fat planar cell polarity. Curr. Biol. 2012;22:1302–1308. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini A., Rayer M., Monier B., Suzanne M. Mechanical function of the nucleus in force generation during epithelial morphogenesis. Dev. Cell. 2019;50:1–15. doi: 10.1016/j.devcel.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstralh D.T., Dawney N.S., St Johnston D. Spindle orientation: a question of complex positioning. Development. 2017;144:1137–1145. doi: 10.1242/dev.140764. [DOI] [PubMed] [Google Scholar]
- Bowman S.K., Neumüller R.A., Novatchkova M., Du Q., Knoblich J.A. The Drosophila NuMA Homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell. 2006;10:731–742. doi: 10.1016/j.devcel.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Box K., Joyce B.W., Devenport D. Epithelial geometry regulates spindle orientation and progenitor fate during formation of the mammalian epidermis. Elife. 2019;8 doi: 10.7554/eLife.47102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun-Usan M., Marín-Riera M., Grande C., Truchado-Garcia M., Salazar-Ciudad I. A set of simple cell processes is sufficient to model spiral cleavage. Development. 2017;144:54–62. doi: 10.1242/dev.140285. [DOI] [PubMed] [Google Scholar]
- Colombo K., Grill S.W., Kimple R.J., Willard F.S., Siderovski D.P., Gönczy P. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science. 2003;300:1957–1961. doi: 10.1126/science.1084146. [DOI] [PubMed] [Google Scholar]
- Conklin E.G. The organization and cell lineage of the ascidian egg. J. Acad. Nat. Sci. (Phila.) 1905;13:1–119. [Google Scholar]
- da Silva S.M., Vincent J.P. Oriented cell divisions in the extending germband of Drosophila. Development. 2007;134:3049–3054. doi: 10.1242/dev.004911. [DOI] [PubMed] [Google Scholar]
- Davidson L.A. Epithelial machines that shape the embryo. Trends Cell Biol. 2012;22:82–87. doi: 10.1016/j.tcb.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes-Hoang R.E., Parmar K.M., Christiansen A.E., Phelps C.B., Brand A.H., Wieschaus E.F. Folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Devenport D. The cell biology of planar cell polarity. J. Cell Biol. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pietro F., Echard A., Morin X. Regulation of mitotic spindle orientation: an integrated view. EMBO Rep. 2016;17:1106–1130. doi: 10.15252/embr.201642292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q., Macara I.G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Firestone A.J., Weinger J.S., Maldonado M., Barlan K., Langston L.D., O’Donnell M., Gelfand V.I., Kapoor T.M., Chen J.K. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D.T., Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Grill S.W., Hyman A.A. Spindle positioning by cortical pulling forces. Dev. Cell. 2005;8:461–465. doi: 10.1016/j.devcel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Guerrier P. Les caracteres de la segmentation et la determination de la polarite dorsoventrale dans le developpement de quelques Spiralia I. Les formes a premier clivage egal. J. Embryol. Exp. Morphol. 1970;23:611–637. [PubMed] [Google Scholar]
- Hashimoto H., Robin F.B., Sherrard K.M., Munro E.M. Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev. Cell. 2015;32:241–255. doi: 10.1016/j.devcel.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Munro E. Differential expression of a classic Cadherin directs tissue-level contractile asymmetry during neural tube closure. Dev. Cell. 2019;51:158–172. doi: 10.1016/j.devcel.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig O. Das Problem der Befruchtung und der Isotropie des Eies. Eine Theorie der Vererbung. Jen. Z. Med. Naturwiss. 1884;18:276–318. [Google Scholar]
- Hotta K., Mitsuhara K., Takahashi H., Inaba K., Oka K., Gojobori T., Ikeo K. A web-based interactive developmental table for the ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Dev. Dyn. 2007;236:1790–1805. doi: 10.1002/dvdy.21188. [DOI] [PubMed] [Google Scholar]
- Kondo T., Hayashi S. Mitotic cell rounding accelerates epithelial invagination. Nature. 2013;494:125–129. doi: 10.1038/nature11792. [DOI] [PubMed] [Google Scholar]
- Kourakis M.J., Reeves W., Newman-Smith E., Maury B., Abdul-Wajid S., Smith W.C. A one-dimensional model of PCP signaling: polarized cell behavior in the notochord of the ascidian Ciona. Dev. Biol. 2014;395:120–130. doi: 10.1016/j.ydbio.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano G., Nishida H. Ascidian embryonic development: an emerging model system for the study of cell fate specification in chordates. Dev. Dyn. 2007;236:1732–1747. doi: 10.1002/dvdy.21108. [DOI] [PubMed] [Google Scholar]
- Kumano G., Takatori N., Negishi T., Takada T., Nishida H. A maternal factor unique to ascidians silences the germline via binding to P-TEFb and RNAP II regulation. Curr. Biol. 2011;21:1308–1313. doi: 10.1016/j.cub.2011.06.050. [DOI] [PubMed] [Google Scholar]
- Kuwajima M., Kumano G., Nishida H. Regulation of the number of cell division rounds by tissue-specific transcription factors and Cdk inhibitor during ascidian embryogenesis. PLoS One. 2014;9:e90188. doi: 10.1371/journal.pone.0090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T., Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecland N., Lüders J. The dynamics of microtubule minus ends in the human mitotic spindle. Nat. Cell Biol. 2014;16:770–778. doi: 10.1038/ncb2996. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Piette J. Tunicates: exploring the sea shores and roaming the open ocean. A tribute to Thomas Huxley. Open Biol. 2015;5:150053. doi: 10.1098/rsob.150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.S., Johnston C.A. Molecular pathways regulating mitotic spindle orientation in animal cells. Development. 2013;140:1843–1856. doi: 10.1242/dev.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabe K.W., Nishida H. Cytoplasmic localization and reorganization in ascidian eggs: role of postplasmic/PEMRNAs in axis formation and fate determination. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:501–518. doi: 10.1002/wdev.54. [DOI] [PubMed] [Google Scholar]
- Martin A.C., Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall A., Chenevert J., Prulière G., Costache V., Hébras C., Salez G., Dumollard R. Centrosomes and spindles in ascidian embryos and eggs. Methods Cell Biol. 2015;129:317–339. doi: 10.1016/bs.mcb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- McGrew M.J., Sherman A., Lillico S.G., Ellard F.M., Radcliffe P.A., Gilhooley H.J., Mitrophanous K.A., Cambray N., Wilson V., Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- Meshcheryakov V.N., Beloussov L.V. Asymmetrical rotations of blastomeres in early cleavage of gastropoda. Wilhelm Roux Arch. Dev. Biol. 1975;177:193–203. doi: 10.1007/BF00848080. [DOI] [PubMed] [Google Scholar]
- Minc N., Burgess D., Chang F. Influence of cell geometry on division-plane positioning. Cell. 2011;144:414–426. doi: 10.1016/j.cell.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc N., Piel M. Predicting division plane position and orientation. Trends Cell Biol. 2012;22:193–200. doi: 10.1016/j.tcb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Morin X., Bellaïche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Munro E.M., Odell G. Morphogenetic pattern formation during ascidian notochord formation is regulative and highly robust. Development. 2002;129:1–12. doi: 10.1242/dev.129.1.1. [DOI] [PubMed] [Google Scholar]
- Munro E.M., Odell G.M. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Makabe K.W., Nishida H. The functional analysis of Type I postplasmic/PEM mRNAs in embryos of the ascidian Halocynthia roretzi. Dev. Genes Evol. 2006;216:69–80. doi: 10.1007/s00427-005-0035-6. [DOI] [PubMed] [Google Scholar]
- Negishi T., Yasuo H. Distinct modes of mitotic spindle orientation align cells in the dorsal midline of ascidian embryos. Dev. Biol. 2015;408:66–78. doi: 10.1016/j.ydbio.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Negishi T., Takada T., Kawai N., Nishida H. Localized PEM mRNA and protein are involved in cleavage-plane orientation and unequal cell divisions in ascidians. Curr. Biol. 2007;17:1014–1025. doi: 10.1016/j.cub.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Negishi T., Miyazaki N., Murata K., Yasuo H., Ueno N. Physical association between a novel plasma-membrane structure and centrosome orients cell division. Elife. 2016;5 doi: 10.7554/eLife.16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H., Satoh N. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. II. The 16- and 32-cell stages. Dev. Biol. 1985;110:440–454. doi: 10.1016/0012-1606(85)90102-2. [DOI] [PubMed] [Google Scholar]
- Nishida H., Sawada K. macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature. 2001;409:724–729. doi: 10.1038/35055568. [DOI] [PubMed] [Google Scholar]
- Nishida H. Cell division pattern during gastrulation of the ascidian, Halocynthia roretzi. Dev. Growth Differ. 1986;28:191–201. doi: 10.1111/j.1440-169X.1986.00191.x. [DOI] [PubMed] [Google Scholar]
- Nishida H. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue restricted stage. Dev. Biol. 1987;121:526–541. doi: 10.1016/0012-1606(87)90188-6. [DOI] [PubMed] [Google Scholar]
- Nishide K., Mugitani M., Kumano G., Nishida H. Neurula rotation determines left-right asymmetry in ascidian tadpole larvae. Development. 2012;139:1467–1475. doi: 10.1242/dev.076083. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Sasakura Y. Ascidians as excellent models for studying cellular events in the chordate body plan. Biol. Bull. 2013;224:227–236. doi: 10.1086/BBLv224n3p227. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Sasakura Y. Developmental control of cell-cycle compensation provides a switch for patterned mitosis at the onset of chordate neurulation. Dev. Cell. 2016;37:148–161. doi: 10.1016/j.devcel.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Sakaue-Sawano A., Nakagawa M., Satoh N., Miyawaki A., Sasakura Y. Coordination of mitosis and morphogenesis: role of a prolonged G2 phase during chordate neurulation. Development. 2011;138:577–587. doi: 10.1242/dev.053132. [DOI] [PubMed] [Google Scholar]
- Panousopoulou E., Green J.B.A. Spindle orientation processes in epithelial growth and organisation. Semin. Cell Dev. Biol. 2014;34:124–132. doi: 10.1016/j.semcdb.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Pasini A., Manenti R., Rothbächer U., Lemaire P. Antagonizing retinoic acid and FGF/MAPK pathways control posterior body patterning in the invertebrate chordate Ciona intestinalis. PLoS One. 2012;7:e46193. doi: 10.1371/journal.pone.0046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamaneck Y.J., Hadjantonakis A.-K., Di Gregorio A. Dynamic and polarized muscle cell behaviors accompany tail morphogenesis in the ascidian Ciona intestinalis. PLoS One. 2007;2:e714. doi: 10.1371/journal.pone.0000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre E., Jaouen F., Saadaoui M., Haren L., Merdes A., Durbec P., Morin X. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J. Cell Biol. 2011;193:141–154. doi: 10.1083/jcb.201101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodon F., Yamada L., Shirae-Kurabayashi M., Nakamura Y., Sasakura Y. Postplasmic/PEM RNAs: a class of localized maternal mRNAs with multiple roles in cell polarity and development in ascidian embryos. Dev. Dyn. 2007;236:1698–1715. doi: 10.1002/dvdy.21109. [DOI] [PubMed] [Google Scholar]
- Prodon F., Chenevert J., Hébras C., Dumollard R., Faure E., Gonzalez-Garcia J., Nishida H., Sardet C., McDougall A. Dual mechanism controls asymmetric spindle position in ascidian germ cell precursors. Development. 2010;137:2011–2021. doi: 10.1242/dev.047845. [DOI] [PubMed] [Google Scholar]
- Rose L., Gönczy P. Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook. 2014:1–43. doi: 10.1895/wormbook.1.30.2. [DOI] [PubMed] [Google Scholar]
- Saches J. Vol. 2. Arbeiten des Botanisches Institut; 1878. pp. 46–104. (Uber die Anordnung der Zellen in jungsten Pflanzentheilen). [Google Scholar]
- Sardet C., Dru P., Prodon F. Maternal determinants and mRNAs in the cortex of ascidian oocytes, zygotes and embryos. Biol. Cell. 2005;97:35–49. doi: 10.1042/BC20040126. [DOI] [PubMed] [Google Scholar]
- Sawyer J.M., Harrell J.R., Shemer G., Sullivan-Brown J., Roh-Johnson M., Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Shevchenko A., Knoblich J.A. A protein complex containing Inscuteable and the Galpha-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr. Biol. 2000;10:353–362. doi: 10.1016/s0960-9822(00)00401-2. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Ezzell R.M., Euteneuer U. erythro-9-[3-(2-Hydroxynonyl)]adenine is an effective inhibitor of cell motility and actin assembly. Proc. Natl. Acad. Sci. U S A. 1984;81:6044–6048. doi: 10.1073/pnas.81.19.6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard K., Robin F., Lemaire P., Munro E. Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr. Biol. 2010;20:1499–1510. doi: 10.1016/j.cub.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan D.G., Fisk R.M., Xu H., van den Heuvel S. A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C. elegans. Genes Dev. 2003;17:1225–1239. doi: 10.1101/gad.1081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall E.G., Spector I., Pentyala S.N., Bitter I., Rebecchi M.J. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol. 2000;10:743–746. doi: 10.1016/s0960-9822(00)00541-8. [DOI] [PubMed] [Google Scholar]
- True J.R., Haag E.S. Developmental system drift and flexibility in evolutionary trajectories. Evol. Dev. 2001;3:109–119. doi: 10.1046/j.1525-142x.2001.003002109.x. [DOI] [PubMed] [Google Scholar]
- Wang Y.-C., Khan Z., Kaschube M., Wieschaus E.F. Differential positioning of adherens junctions is associated with initiation of epithelial folding. Nature. 2012;484:390–395. doi: 10.1038/nature10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.A., Nagy L.M. Linking gene regulation to cell behaviors in the posterior growth zone of sequentially segmenting arthropods. Arthropod Struct. Dev. 2017;46:380–394. doi: 10.1016/j.asd.2016.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 61°–90°) are indicated by white and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. In the first frame, cell rows 1 and 2 are indicated by yellow and orange lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. In the first frame, cell rows 2 is indicated by orange lines. White arrowheads indicate that the spindle is aligned along the circumference of the embryo, whereas red arrowheads indicate that the spindle is either rotating or aligned along the A–P axis.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.
Anterior is to the left. The expression of DCIC-EGFP (for dynein, green) and MAP7-mCherry (for spindle orientation, red) are shown. Enrichment of the dynein is observed in the anterior surface of the cells (arrowheads in cells P and PP) before the spindle rotation.
Anterior is to the left. Division angles with respect to the A–P axis (0°–30°, 31°–60°, 61°–90°) are shown with white, yellow, and red lines, respectively.
Anterior is to the right. Division angles with respect to the A–P axis (0°–30°, 61°–90°) are indicated by white and red lines, respectively. The boundary of the anterior and posterior ectodermal lineages is indicated by red arrowhead.