This study investigated the effect of Akkermansia muciniphila on fatty liver disease. Although some research about the effects of A. muciniphila on host health has been published, study of the relationship between A. muciniphila administration and fatty liver, as well as changes in the gut microbiota, has not been conducted. In this study, we demonstrated that A. muciniphila prevented fatty liver disease by regulation of the expression of genes that regulate fat synthesis and inflammation in the liver.
KEYWORDS: gut microbiota, fatty liver disease, Akkermansia muciniphila, obesity
ABSTRACT
The objective of this study was to elucidate the effect of intestinal Akkermansia muciniphila bacteria on fatty liver disease. Five-week-old C57BL/6N mice were administered either phosphate-buffered saline (PBS; control) or A. muciniphila at 108 to 109 CFU/ml, and were fed either a 45% fat diet (high-fat diet [HFD]) or a 10% fat diet (normal diet [ND]) for 10 weeks. After 10 weeks, the mice were euthanized, and blood and tissue samples, including adipose tissue, cecum, liver, and brain, were immediately collected. Biochemical and histological analyses were conducted, and the expression levels of related factors were compared to determine the antiobesity effects of Akkermansia muciniphila. The gut microbiome was analyzed in fecal samples. Oral administration of A. muciniphila significantly (P < 0.05) lowered serum triglyceride (TG) and alanine aminotransferase (ALT) levels in obese mice. Compared to the non-A. muciniphila-treated group, the expression of SREBP (regulator of TG synthesis in liver tissue) was decreased in the A. muciniphila-treated group. The expression of IL-6 in the liver of obese mice was decreased following the administration of A. muciniphila. Furthermore, alterations in the ratio of Firmicutes to Bacteroidetes and the decrease in bacterial diversity caused by the HFD were restored upon the administration of A. muciniphila. These results indicate that A. muciniphila prevents fatty liver disease in obese mice by regulating TG synthesis in the liver and maintaining gut homeostasis.
IMPORTANCE This study investigated the effect of Akkermansia muciniphila on fatty liver disease. Although some research about the effects of A. muciniphila on host health has been published, study of the relationship between A. muciniphila administration and fatty liver, as well as changes in the gut microbiota, has not been conducted. In this study, we demonstrated that A. muciniphila prevented fatty liver disease by regulation of the expression of genes that regulate fat synthesis and inflammation in the liver.
INTRODUCTION
The human body contains several types of microorganisms that comprise the microbiota (1). These microorganisms colonize every surface of the human body, including the skin, oral cavity, respiratory tract, urogenital tract, and gastrointestinal (GI) tract.
In the gut environment, the bacterial community affects metabolic processes such as digestion, fermentation, and the synthesis of vitamins (2, 3). Due to the physiological roles of the gut microbiota, changes in the composition of the gut microbiota are related to diseases, including obesity and diabetes. Many studies have been conducted in mice and humans to investigate this relationship (4, 5). The relationship between the bacterial species present in the gut microbiota and disease has been studied. Specifically, the protective effects exhibited by Bifidobacterium longum and Bifidobacterium thetaiotaomicron have been investigated previously (6).
Akkermansia muciniphila is one of the strains comprising the gut microbiota that colonizes the mucus layer and colon by degrading and utilizing mucin as an energy source (7). After birth, the number of A. muciniphila cells increases as the human grows, eventually consisting of 1 to 4% of the gut microbiota, and the bacterium produces short-chain fatty acids (SCFAs) by mucin degradation (7, 8). Production of these SCFAs, which include acetate, propionate, and butyrate, is known to have an important role in human health (9–11). In facts, some studies (12, 13) have suggested that A. muciniphila may be involved in obesity and intestinal immunity. Therefore, there is a growing interest in the effect exerted by A. muciniphila on host health (14, 15).
Fatty liver disease is the main cause of hepatitis, which is a health problem worldwide. Hepatic damage is caused by various factors, such as viral infections, alcohol consumption, and a high-fat diet (16). Liver disease is closely associated with changes in the composition of the gut microbiota. The maintenance of gut homeostasis is important for the host’s immunological status. It prevents leakage of bacterial products that may damage liver tissues (17).
Therefore, the objective of this study was to determine the correlations between gut microbiota at the bacterial species level and whether the consumption of a high-fat diet could induce liver damage. In addition, the role of A. muciniphila in liver damage was investigated.
RESULTS AND DISCUSSION
Obesity was induced in mice with a high-fat diet (HFD). A. muciniphila was then administered to the mice for 10 weeks. Weight gain (%) was higher (P < 0.05) in the HFD group than that in the normal diet (ND) group. However, no difference in weight gain was observed between the A. muciniphila-treated group and the non-A. muciniphila-treated group (Table 1). This result indicates that obesity—known to induce fatty liver disease—was successfully induced by an HFD and that A. muciniphila was not able to prevent weight gain.
TABLE 1.
Weight gain and food efficiency of the treated micea
| Treatment | % wt gain (mean ± SD) | % food efficiency (mean ± SD) |
|---|---|---|
| ND + PBS | 33.3 ± 5.4 B | 3.9 ± 0.6 B |
| ND + A. muciniphila | 36.0 ± 4.4 B | 4.2 ± 0.7 B |
| HFD + PBS | 55.3 ± 6.3 A | 7.2 ± 0.7 A |
| HFD + A. muciniphila | 50.0 ± 6.8 A | 6.3 ± 0.9 A |
Means with different letters in the same columns are significantly different (P < 0.05, n = 5).
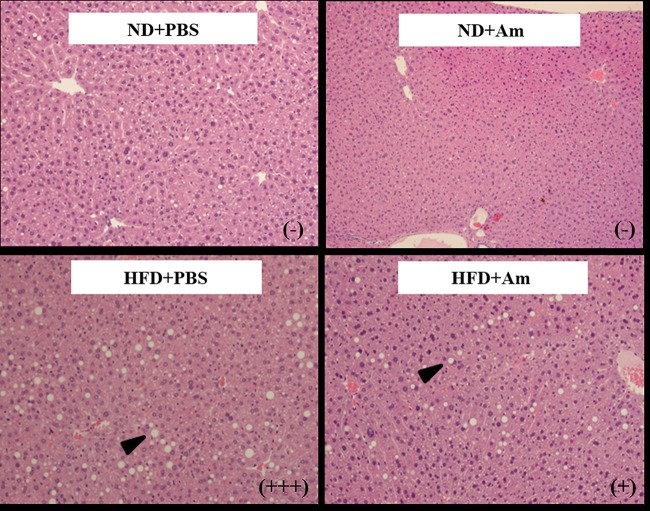
In the biochemical analysis of the serum samples, total cholesterol (T-chol) and triglyceride (TG) levels were higher (P < 0.05) in the HFD groups than those in the ND groups, as expected (Table 2). In the ND groups, serum TG levels were not significantly different between the phosphate-buffered saline (PBS)- and A. muciniphila-treated groups, although the serum TG levels were lower (P < 0.05) in the HFD + A. muciniphila group than in the HFD + PBS group (Table 2). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, which are indicators of liver damage, were higher in the HFD group than those in the ND group, and the values were lower (P < 0.05) in the HFD + A. muciniphila group (ALT, 41.8 U/liter; AST, 89.0 U/liter) than those in the HFD + PBS group (ALT, 53.1 U/liter; AST, 91.8 U/liter) (Table 2). These results indicate that A. muciniphila lowered serum TG and ALT levels in the HFD groups. Histological analysis of the liver tissue showed that the level of fatty changes in the liver were unremarkable or mild in the HFD + A. muciniphila group compared to that in the HFD + PBS group, which showed a mild or marked level of fatty changes (Fig. 1). The results of histological analysis and the low level of serum ALT in the HFD + A. muciniphila group suggest that A. muciniphila prevented liver damage from being caused by the HFD.
TABLE 2.
Levels of biochemical parameters in serum
| Treatment | Level (mean ± SD) ofa: |
||||
|---|---|---|---|---|---|
| T-chol (mg/dl) | TG (mg/dl) | ALT (U/liter) | AST (U/liter) | Glucose (mg/dl) | |
| ND + PBS | 145 ± 17 A | 96 ± 12 A | 31.5 ± 4.5 A | 78.5 ± 9.3 A | 211.2 ± 32.3 A |
| ND + A. muciniphila | 146 ± 14 A | 109 ± 16 A | 28.5 ± 2.2 A | 80.3 ± 4.2 A | 216.4 ± 33.3 A |
| HFD + PBS | 158 ± 13 C | 101 ± 13 C | 53.1 ± 12.0 C | 91.8 ± 20.9 C | 192.9 ± 28.6 C |
| HFD + A. muciniphila | 156 ± 10 C | 77 ± 12 D | 41.8 ± 4.5 D | 89.0 ± 6.9 C | 166.0 ± 32.4 C |
Means with different letters in the same column are significantly different (P < 0.05, n = 5). T-chol, total cholesterol; TG, triglyceride; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
FIG 1.
Histological analysis of liver tissues stained with hematoxylin and eosin (×100). ND+PBS, 10% fat diet with phosphate-buffered saline (PBS); ND+Am, 10% fat diet with Akkermansia muciniphila; HFD+PBS, 45% fat diet with PBS; and HFD+Am, 45% fat diet with A. muciniphila. Images are representative of two independent experiments. Levels of liver injury were determined based on the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND). Grades for fatty change are showed as symbols in parentheses, as follows: −, unremarkable; +, mild; +++, marked.
The gene expression levels of related factors were examined to investigate the mechanism of prevention of liver injury by A. muciniphila. The genes chREBP and SREBP are involved in triglyceride synthesis in the liver. Their expression levels are influenced by glucose homeostasis and insulin resistance. The liver also secretes inflammatory cytokines such as IL-6 in response to high-fat-diet-induced inflammation (18). Thus, the expression levels of chREBP and SREBP in liver tissues were examined. Expression levels of chREBP in the ND groups were lower (P < 0.05) than those in the HFD groups, although they were not different between the HFD + PBS and HFD + A. muciniphila groups (Fig. 2A). Expression levels of SREBP in the HFD groups were higher (P < 0.05) than those in the ND groups. They were lower (P < 0.05) in the HFD + A. muciniphila group than in the HFD + PBS group (Fig. 2B). Expression levels of IL-6 were lower (P < 0.05) in the HFD + A. muciniphila group than in the HFD + PBS group (Fig. 2C). These results indicate that HFD-induced liver damage is prevented by A. muciniphila administration via suppressing SREBP and IL-6 expression.
FIG 2.
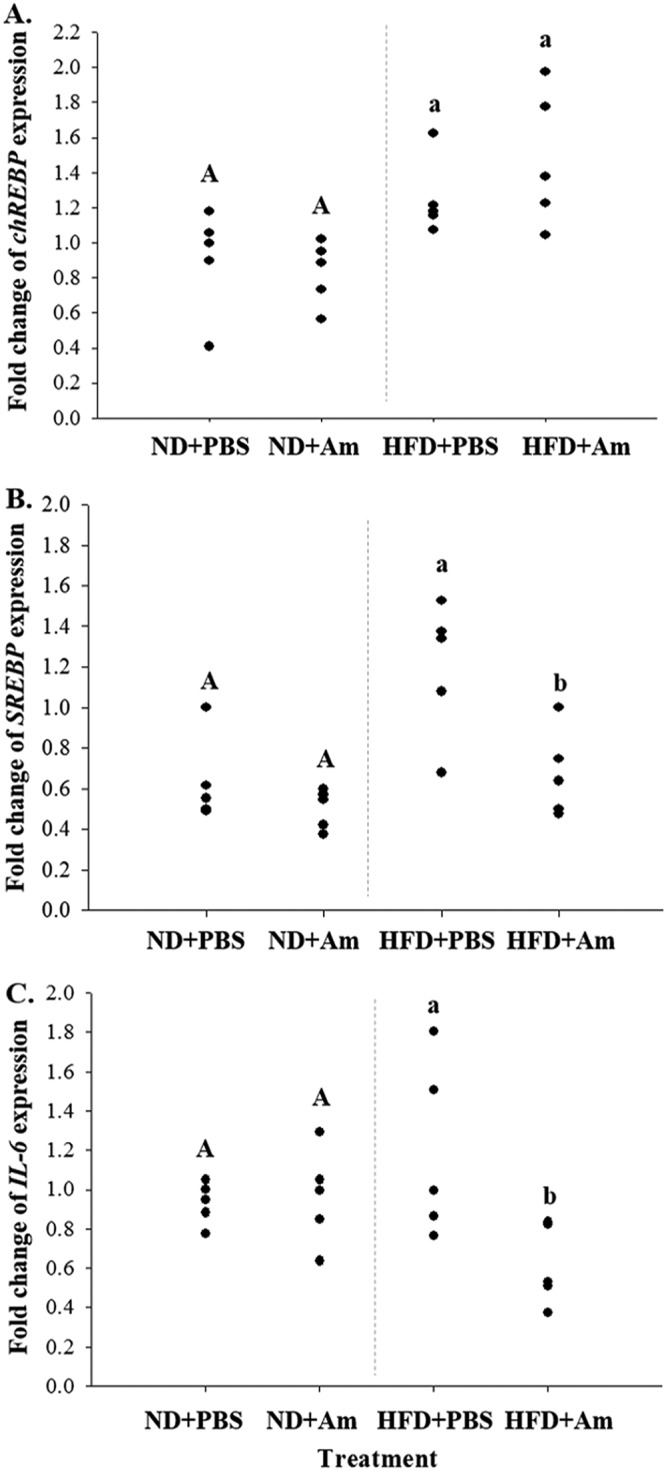
Relative expression levels of fat synthesis-related genes chREBP (A) and SREBP (B) and inflammatory gene IL-6 (C) in liver tissues. ND+PBS, 10% fat diet with phosphate-buffered saline (PBS); ND+Am, 10% fat diet with Akkermansia muciniphila; HFD+PBS, 45% fat diet with PBS; and HFD+Am, 45% fat diet with Akkermansia muciniphila. Means with different letters (A, a, and b) are significantly different from each other (P < 0.05; n = 5).
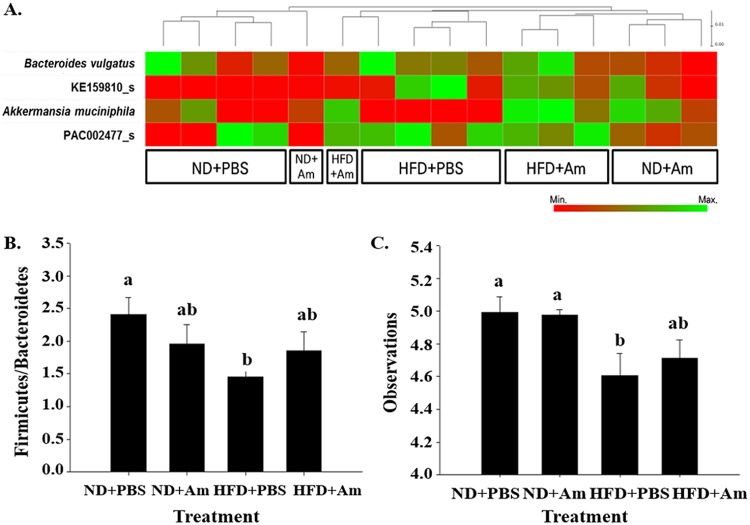
Dietary factors can significantly affect the composition of the gut microbiome. Changes in the microbial community are known to play an important role in disease development by altering the metabolic processes and/or the immune responses of the host (19). Continuous consumption of an HFD can induce changes in the gut microbiota. Impaired microbiota can lead to a microbial imbalance or dysbiosis (also called dysbacteriosis). Dysbiosis is known to increase gut permeability (17), which results in the leakage of bacterial products from the intestine to the liver, causing liver inflammation and damage. Therefore, the present study investigated the gut microbiota in A. muciniphila-treated mice. After quality trimming and removal of chimeric sequences, an average yield of 75,187 reads (ranging from 64,296 to 82,457) per barcoded sample was retained for downstream analysis (Fig. S1A). The rarefaction curves approached asymptotes for most samples, indicating that a reasonable number of tags were analyzed (Fig. S1B). In UniFrac analysis, the distance of the samples in the same treatment group was close, but not that in different treatment groups. This result shows that compositions of gut microbiota were differentiated by the treatments (Fig. S2A). Firmicutes and Bacteroidetes were the major phyla in the gut microbiota. The percentages of Bacteroidetes in the HFD + PBS and HFD + A. muciniphila groups were 32.2 to 44.6% and 24.0 to 38.9%, respectively, and those of Firmicutes were 49.2 to 57.4% and 50.0 to 63.7%, respectively (Fig. S2B). At the genus level, Pseudoflavonifractor, PAC000664_g, and Oscillibacter were prevalent in the gut microbiota. The proportion of Pseudoflavonifractor decreased in high-fat-diet-fed mice in a previous study (20), and the same result was observed in this study (Fig. S2C). The proportion of this bacterium was lower in the HFD + PBS group (10.6%) and in the HFD + A. muciniphila group (15.3%) compared to that in the ND + PBS group (18.0%). The composition of the gut microbiota at the species level was classified according to the treatments (Fig. 3A). Among the frequent species, the proportions of A. muciniphila were higher in the ND + PBS (1.9%), ND + A. muciniphila (4.0%), and HFD + A. muciniphila (6.3%) groups than in the HFD + PBS (0.0%) group. This result shows that the proportion of A. muciniphila was decreased by the high-fat diet, but it was recovered by A. muciniphila treatment.
FIG 3.
Analysis of the gut microbiome. Gut microbiome composition at the species level (A), ratio of Firmicutes to Bacteroidetes (B), and diversity of the gut microbiome (C). ND+PBS, 10% fat diet with phosphate-buffered saline (PBS); ND+Am, 10% fat diet with Akkermansia muciniphila; HFD+PBS, 45% fat diet with PBS; and HFD+Am, 45% fat diet with Akkermansia muciniphila. Means with different letters (a and b) are significantly different from each other (P < 0.05; n = 4).
A previous study reported that obese and lean mice have different Firmicutes to Bacteroidetes ratios (21). Changes in the ratio of these two phyla may alter the energy harvest from foods (4). Thus, the ratio of Firmicutes to Bacteroidetes was investigated in this study. The results showed that the ratio of Firmicutes to Bacteroidetes was lower (P < 0.05) in the HFD + PBS group than that in the ND group. The Firmicutes to Bacteroidetes ratio in the HFD + A. muciniphila group was higher than that in the HFD + PBS group but was similar to that in the ND group (Fig. 3B). Furthermore, the diversity of gut microbiota in the HFD + A. muciniphila group was higher than that in the HFD + PBS group (P < 0.05) (Fig. 3C). Rarefaction curve analysis also showed that the species richness of the non-A. muciniphila-treated group was lower than that of the A. muciniphila-treated group (Fig. S1B). This indicates that A. muciniphila may play a role in maintaining gut homeostasis by decreasing the ratio of Firmicutes to Bacteroidetes and restoring the diversity of the gut microbiota. This might be due to SCFAs such as propionic acid and acetic acid that are produced by A. muciniphila from degrading mucin. Hence, A. muciniphila treatment may increase the diversity of the gut microbiota. Lukovac et al. (22) and Derrien et al. (23) also suggested that this bacterium might affect the compositions of the gut microbiota.
The dysbiosis affects gut barrier functions such as the integrity of the gut barrier and gut permeability. The reduced integrity of the gut barrier triggered leakage of bacteria and lipopolysaccharide (LPS) from the gut to other organs. This translocation stimulated lipogenesis and inflammatory responses in the liver, inducing nonalcoholic fatty liver disease (24, 25). Therefore, increase in the diversity of the gut microbiota and gut homeostasis by A. muciniphila treatment may reduce the transfer of bacterial substances and may prevent development of fatty liver.
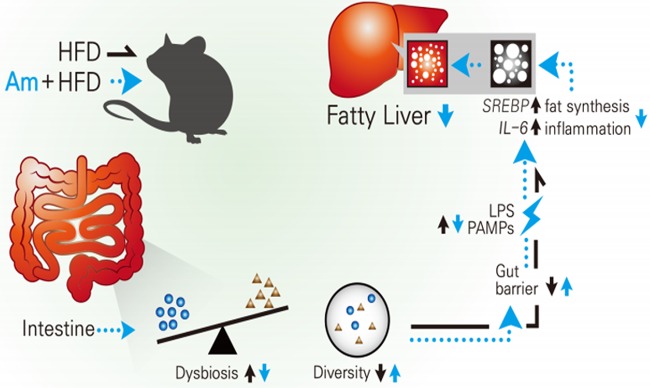
In conclusion, as described in Fig. 4, A. muciniphila treatment maintained gut homeostasis (maintaining balance and diversity), which improved the integrity of the gut barrier. Thus, the translocation of pathogen-associated molecular patterns (PAMPs) decreased, and it downregulated expression of genes SREBP and IL-6, which resulted in decreased lipid synthesis and inflammation in the liver, respectively. Therefore, A. muciniphila treatment prevented fatty liver disease in obese mice.
FIG 4.
Effect of Akkermansia muciniphila (Am) administration on the development of fatty liver. A high-fat diet (HFD; black line) causes dysbiosis (imbalance and decrease of diversity of the gut microbiota). It increases intestinal permeability and allows pathogen-associated molecular patterns (PAMPs; e.g., lipopolysaccharide [LPS] and lipid A) translocation. The materials from the intestine move to the liver and induce fat synthesis and inflammation, causing fatty liver. Akkermansia muciniphila administration with HFD (blue line) increased diversity and mitigated dysbiosis. These might induce maintenance of gut health and prevent the translocation of pathogenic materials from the intestine. In the liver, fat synthesis and inflammation were inhibited by decreases of SREBP and IL-6 expression, respectively. Akkermansia muciniphila administration eventually inhibited development of fatty liver, which is induced by an HFD.
MATERIALS AND METHODS
Preparation of bacterial strain.
A. muciniphila BAA-835 was grown in Difco brain heart infusion (BHI) broth (BD, Sparks, MD). After incubating at 37°C in an anaerobic chamber (5% CO2, 5% H2, and 90% N2; Coy Laboratory Products, Grass Lake, MI) for 48 h, A. muciniphila cells were harvested by centrifugation at 18,000 × g for 5 min at 4°C and resuspended in sterile phosphate-buffered saline (pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4·7H2O, 8.0 g of NaCl, and 0.2 g of KCl in 1 liter of distilled water). The harvested cells were mixed with glycerol (60%) at a ratio of 1:2 and stored at −70°C until further use. For further experiments, the glycerol stock of A. muciniphila was cultured in BHI broth containing 0.5% mucin from porcine stomach (Sigma-Aldrich, St. Louis, MO) and 0.05% cysteine in an anaerobic chamber at 37°C for 48 h. After incubation, the A. muciniphila cells were harvested by centrifugation at 13,000 rpm for 3 min at 4°C and resuspended in PBS to a concentration of 108 CFU/ml.
Animal treatment.
Five-week-old male C57BL/6N mice were purchased from OrientBio (Seongnam, South Korea) and housed in a controlled environment (26). After 1 week of adaptation, 20 mice were randomly divided into four groups as follows: ND + PBS (10% fat diet + PBS), ND + A. muciniphila (10% fat diet with A. muciniphila), HFD + PBS (45% fat diet + PBS), and HFD + A. muciniphila (45% fat diet with A. muciniphila). Five mice were assigned to each group. A. muciniphila cells (108 to 109 CFU/ml) suspended in PBS were administered to the mice. Mice in the ND + PBS and HFD + PBS groups were fed with the same volume of PBS by oral gavage. The treatment was continued for 10 weeks. The local Institutional Animal Care and Use Committee approved all experimental procedures (approval number SMWU-IACUC-1801-038). The mice were fasted for 18 h before sacrifice. Spleen, liver, white adipose tissue (WAT) from epididymal fat, brown adipose tissue (BAT), intestine, cecum, and colon were rapidly excised from each mouse and stored at −70°C for further experiments. Weights of the spleen, liver, and WAT were measured. Tissues were kept in RNAlater RNA stabilization reagent (Qiagen) for RNA extraction. For histological analysis, the tissues were fixed in 10% formalin solution. To compare the size of the WAT, tissues were photographed using a digital camera (NX-300M; Samsung, South Korea).
Weight gain and food intake efficiency.
During the treatment of the mice, food intake (in g) and weight (wt; in g) were measured. Weight gain (%) was calculated using the following equation: weight gain = [(wt10W − wt0w)/wt0w] × 100, where wt10W was the weight (g) at the 10th week and wt0w was the weight (g) before treatment. Food intake efficiency (%) was calculated using the following equation: food intake efficiency = [weight gain/food intake]) × 100.
Serum biochemical analysis.
The blood samples in serum-separating tubes (Microtainer; BD) were centrifuged at 5,000 rpm for 10 min at 4°C. The supernatants (sera) were transferred to sterile microcentrifuge tubes, immediately frozen using dry ice, and stored at −70°C until further experiments. The AST and ALT activities and TG and total cholesterol levels in the serum samples were measured using a BS-200E chemistry analyzer (Mindray, Shenzhen, China).
Histological analysis.
The liver tissue was fixed in paraffin and cut into 3-μm thick sections (27). The sections were stained with hematoxylin and eosin (H&E), and the stained sections were observed under a microscope. Lesions of the liver tissue were diagnosed based on the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND). The degree of fatty changes was determined by evaluating the numbers and sizes of lipid droplets in the hepatic cells. The grade for fatty change was shown as follows: −, unremarkable; +, mild; and +++, marked. Two samples from each group were used for this histological analysis, and representative images are presented.
Gut microbiota analysis.
DNA was extracted from the cecal samples using the QIAamp DNA stool minikit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Briefly, 200-mg portions of the cecal samples in 2-ml microcentrifuge tubes were homogenized in ASL buffer using a TissueLyser LT bead mill (Qiagen), followed by vortexing and heating at 70°C. After centrifugation, an InhibitEx tablet was added to the supernatant to remove inhibitors that can degrade DNA and inhibit enzymatic reactions. After treatment with proteinase K and AL buffer (Qiagen), the mixture was heated to 70°C. The lysates were mixed with ethanol, and the mixtures were loaded onto QIAamp spin columns, followed by centrifugation so that the DNA could bind to the membrane in the column. After washing, the DNA was finally eluted with 200 μl of AE buffer (Qiagen). The DNA samples were meta-analyzed using an Illumina MiSeq sequencing system (Illumina, San Diego, CA) according to the manufacturer’s instructions. The quality of raw reads was checked, and low-quality reads (Q < 25) were filtered using Trimmomatic 0.32 (Usadel Lab, Aachen, Germany) (28). Primers were trimmed at a similarity cutoff of 0.8. Nonspecific amplicons were detected by the HMMER hmmsearch program (EMBL-EBI, Hinxton, Cambridge, UK) with 16Ss rRNA profiles (29). The EzBioCloud database was used for taxonomic assignment using USEARCH (8.1.1861_i86linux32) (30). High-quality sequences were then clustered into operational taxonomic units (OTUs) using CD-HIT and UCLUST at a 97% similarity level. Composition of the gut microbiota was analyzed at the phylum, genus, and species levels (>1.0%). Alpha diversity indexes were compared, and beta diversity was determined by UniFrac distance.
Gene expression.
mRNA was extracted from WAT using the RNeasy lipid tissue minikit (Qiagen, Hilden, German), according to the manufacturer’s instructions. Briefly, 100 mg of tissue was obtained from the WAT samples and stored in RNAlater, and 30 mg portions were obtained from the liver, intestine, and colon samples and stored in RNAlater. The tissues were then transferred to 2-ml microcentrifuge tubes kept on ice and containing 5-mm-diameter stainless steel beads (Qiagen). QIAzol lysis reagent was added to the WAT samples, while RLT buffer was added to the other samples. The samples were lysed and homogenized with a TissueLyser LT bead mill (Qiagen) at 50 Hz for 5 min. The lysates were transferred to new microcentrifuge tubes and incubated at 25 ± 2°C for 5 min. Aliquots (200 μl) of chloroform were added to each tube, followed by thorough mixing. After incubating at room temperature for 2 to 3 min, the samples were centrifuged at 16,000 × g for 15 min at 4°C to obtain phase separation. The upper aqueous phase was transferred to new tubes, and equal volumes of 70% ethanol was added, followed by thorough mixing. The mixtures were immediately transferred to RNeasy minispin columns placed in 2-ml collection tubes. After centrifugation, the column was repeatedly washed with RW1 buffer and RPE buffer, followed by centrifugation. The flowthroughs were discarded. Finally, RNase-free water (50 μl) was directly added to each RNeasy spin column placed in a new 1.5-ml collection tube. The RNA elution step was accomplished by centrifuging the tube containing the column at 16,000 × g for 1 min. The extracted RNA was quantified using a Take3 microvolume plate and an Epoch microplate spectrophotometer (BioTek, Winooski, VT). The purity of each RNA sample was determined based on the A260/A280 ratio (1.8 to 2.1). The RNA samples were stored at −20°C until further use.
cDNAs were synthesized from the mRNAs using the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer’s instructions. Contaminating genomic DNA (gDNA) was removed from the mRNA (1 μg) samples by adding 2-μl aliquots of gDNA Wipeout buffer, followed by incubation at 42°C for 2 min. Aliquots (8 μl) of the mixture were added to the cDNA synthesis reactions, which were prepared as follows: 1 μl of Quantiscript reverse transcriptase (RT), 4 μl of 5× Quantiscript RT buffer, and 1 μl of RT primer mix. The mixtures were incubated at 42°C for 15 min, followed by incubation at 95°C for 3 min to inactivate Quantiscript reverse transcriptase.
Quantitative reverse transcriptase PCR (qPCR) mixtures were prepared by mixing 12.5 μl of 2× Rotor-Gene SYBR green PCR mastermix (Qiagen), 2.5 μl of forward primer (10 μM), 2.5 μl of reverse primer (10 μM), and 6.5 μl of RNase-free water in a qPCR tube (0.1-ml strip tubes; Qiagen). After 1 μl of template cDNA was added, the mixture was amplified using a Rotor-Gene Q instrument (Qiagen) with the following cycling conditions: 95°C for 5 min, followed by 35 cycles of 95°C for 5 s and 60°C for 10 s. The primer sequences used in this study are listed in Table 3. The relative gene expression levels were calculated using the threshold cycle (2−ΔΔCT) method (31).
TABLE 3.
Primer sequences used for qRT-PCR
| Gene | Reference | Primer type | Primer sequence (5′→3′) |
|---|---|---|---|
| chREBP | 32 | Forward | AGA TGG AGA ACC GAC GTA TCA |
| Reverse | ACT GAG CGT GCT GAC AAG TC | ||
| SREBP-1 | 33 | Forward | AAG CAA ATC ACT GAA GGA CCT GG |
| Reverse | AAA GAC AAG GGG CTA CTC TGG GAG | ||
| IL-6 | 34 | Forward | CCG CTA TGA AGT TCC TCT CTG C |
| Reverse | ATC CTC TGT GAA GTC TCC TCT CC | ||
| GAPDH | 35 | Forward | TCC TGC ACC AAC TGC TTA G |
| Reverse | TGC TTC ACC TTC TTG ATG TC |
Statistical analyses.
To analyze the results, SAS version 9.2 (SAS Institute, Cary, NC) was used. Means were compared using a pairwise t test at an α value of 0.05. Results of the biochemical parameters in serum and mRNA were analyzed separately for the ND and HFD groups.
Data availability.
The 16S rRNA sequence data sets generated and analyzed in this study were deposited in the NCBI database under BioProject accession number PRJNA593343.
Supplementary Material
ACKNOWLEDGMENT
We have no competing financial interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Park HE, Kim YJ, Do KH, Kim JK, Ham JS, Lee WK. 2018. Effects of queso blanco cheese containing Bifidobacterium longum KACC91563 on the intestinal microbiota and short chain fatty acid in healthy companion dogs. Korean J Food Sci Anim Resour 38:1261–1272. doi: 10.5851/kosfa.2018.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. 2011. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarner F, Malagelada JR. 2003. Gut flora in health and disease. Lancet 361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. 2014. Adiposity, gut microbiota and fecal short chain fatty acids are linked in adult humans. Nutr Diabetes 4:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Jia W, Li H, Zhao L, Nicholson JK. 2008. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov 7:123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 7.Derrien M, Vaughan EE, Plugge CM, de Vos WM. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 8.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. 2007. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol 73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, Oravecz-Wilson K, Wu SR, Sun Y, Rossi C, Fujiwara H, Byun J, Shono Y, Lindemans C, Calafiore M, Schmidt TM, Honda K, Young VB, Pennathur S, van den Brink M, Reddy P. 2016. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 17:505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. 2014. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 11.van Passel MWJ, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, Chain PS, Woyke T, Palva A, de Vos WM, Smidt H. 2011. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS One 6:e16876. doi: 10.1371/journal.pone.0016876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. 2014. Microbiota and diabetes: an evolving relationship. Gut 63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson CL, Onnerfalt J, Xu J, Molin G, Ahrne S, Thorngren-Jerneck K. 2012. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 20:2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 15.Rajilic-Stojanovic M, Shanahan F, Guarner F, de Vos WM. 2013. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis 19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. 2017. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol 8:1804. doi: 10.3389/fmicb.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. 2016. Non-alcoholic fatty liver and the gut microbiota. Mol Metab 5:782–794. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asaoka Y, Terai S, Sakaida I, Nishina H. 2013. The expanding role of fish models in understanding non-alcoholic fatty liver disease. Dis Model Mech 6:905–914. doi: 10.1242/dmm.011981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vajro P, Paolella G, Fasano A. 2013. Microbiota and gut-liver axis: a mini review on their influences on obesity and obesity related liver disease. J Pediatr Gastroenterol Nutr 56:461–468. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, Kim SE, Kim AR, Kang S, Park MY, Sung MK. 2019. Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice. BMC Microbiol 19:193. doi: 10.1186/s12866-019-1557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O'Toole PW, Cotter PD. 2012. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes 3:186–202. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. 2014. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 5:e01438-14. doi: 10.1128/mBio.01438-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, de Vos WM. 2011. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front Microbiol 2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai X, Wang B. 2015. Role of gut barrier function in the pathogenesis of nonalcoholic fatty liver disease. Gastroenterol Res Pract 2015:287348. doi: 10.1155/2015/287348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. 2013. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect 19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Huang E, Park S, Holzapfel W, Lim SD. 2018. Physiological characteristics and anti-obesity effect of Lactobacillus plantarum K10. Korean J Food Sci Anim Resour 38:554–569. doi: 10.5851/kosfa.2018.38.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Z, Mao C, Fu X, Ma M. 2019. High-density lipoprotein from egg yolk (EYHDL) improves dyslipidemia by mediating fatty acids metabolism in high fat diet-induced obese mice. Food Sci Anim Resour 39:179–196. doi: 10.5851/kosfa.2018.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Gao M, Bu L, Ma Y, Liu D. 2013. Concurrent activation of liver X receptor and peroxisome proliferator-activated receptor alpha exacerbates hepatic steatosis in high fat diet-induced obese mice. PLoS One 8:e65641. doi: 10.1371/journal.pone.0065641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schupp M, Chen F, Briggs ER, Rao S, Pelzmann HJ, Pessentheiner AR, Bogner-Strauss JG, Lazar MA, Baldwin D, Prokesch A. 2013. Metabolite and transcriptome analysis during fasting suggest a role for the p53-Ddit4 axis in major metabolic tissues. BMC Genomics 14:758. doi: 10.1186/1471-2164-14-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. 2012. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu SH, Wang B, Kutay H, Bid H, Shreve J, Zhang X, Costinean S, Bratasz A, Houghton P, Ghoshal K. 2013. Hepatic loss of miR-122 predisposes mice to hepatobiliary cyst and hepatocellular carcinoma upon diethylnitrosamine exposure. Am J Pathol 183:1719–1730. doi: 10.1016/j.ajpath.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA sequence data sets generated and analyzed in this study were deposited in the NCBI database under BioProject accession number PRJNA593343.