Abstract
A relationship has been reported between myelodysplastic syndrome (MDS) and autoimmune disease. Behçet's disease is a multisystem inflammatory disorder with mucocutaneous, articular, gastrointestinal, neurological, and vascular manifestations. The co-occurrence of MDS with trisomy 8 and Behçet's-like disease was recently demonstrated. We herein describe a case that shows the relationship between the acquisition of trisomy 8 and occurrence of Behçet's-like disease. Immune dysregulation and altered T-cell hemostasis play an important role in the pathogenesis of Behçet's-like disease and MDS with trisomy 8.
Keywords: Behçet's disease, Myelodysplastic syndrome (MDS), Trisomy 8
Myelodysplastic syndrome (MDS) is a clonal hematopoietic stem cell disorder that presents with ineffective hematopoiesis, increased bone marrow cellularity, myeloid lineage dysplasia, and peripheral cytopenia with an increased risk of acute myeloid leukemia. MDS can be associated with autoimmune and inflammatory manifestations which can be various and challenge the patient therapy [1].
Behçet's disease is an inflammatory disorder of unknown etiology that is characterized by recurrent oral aphthous and genital ulcers, uveitis, and skin lesions. Abnormal neutrophil functions, the overproduction of inflammatory cytokines, and immunological abnormalities have been implicated in the etiology of Behçet's disease.
A number of case reports have shown a relationship between Behçet's-like disease and MDS with trisomy 8 [2,3]; however, an etiological link has not yet been demonstrated. Trisomy 8 is associated with significant CD8+ T-cell expansion, which preferentially suppresses hematopoietic cell growth [4]. Trisomy 8 is also associated with the overexpression of WT1, and T-cell immune responses to this antigen suggest a role for WT1 in triggering T-cell-mediated immune suppression in MDS [5]. A recent study reported higher serum levels of soluble interleukin 2 receptor (sIL2R), interferon (IFN)-γ, interleukin (IL)-1β, IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor in a Behçet's-like disease associated MDS patient with trisomy 8 during the active phase [6]. The production of pro-inflammatory cytokines and reactive oxygen species may be contributing factors to the symptoms of BD. We herein describe the occurrence of Behçet's-like disease in a MDS patient that was associated with the acquisition of trisomy 8.
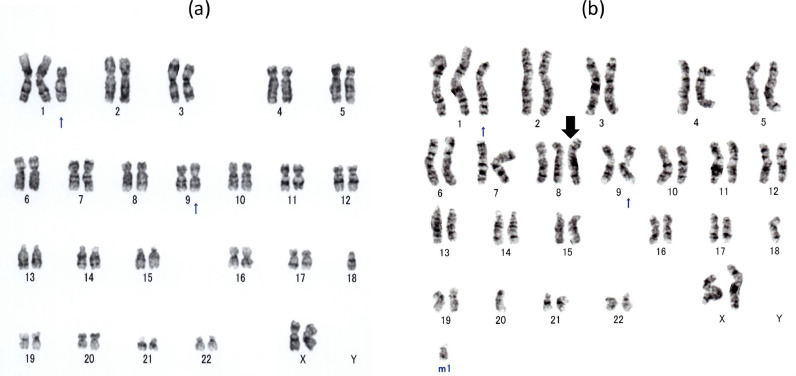
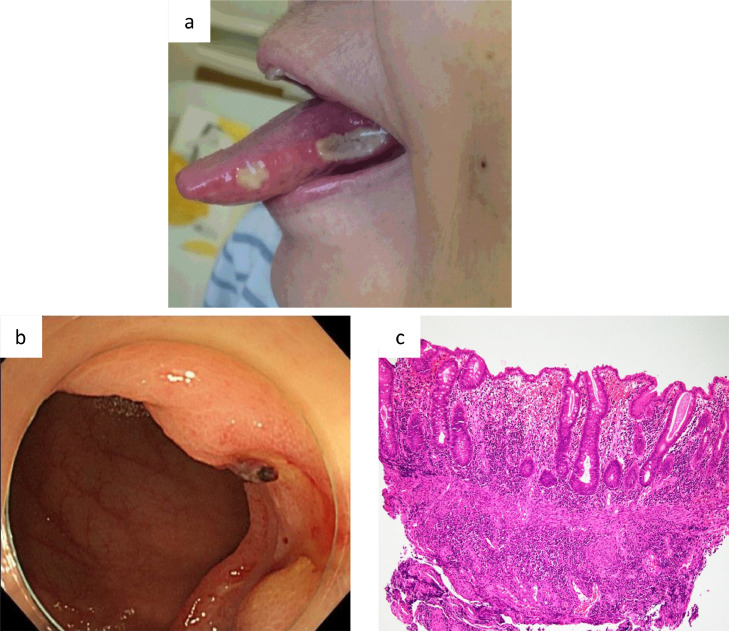
A 73-year-old Japanese woman was referred to our hospital with bicytopenia (white blood cell (WBC) count, 2.3 × 109/L; neutrophil cell count, 1.6 × 109/L; red blood cell (RBC) count, 446 × 1010/L; hemoglobin (Hb) concentration, 13.5 g/dL; and platelet count, 69 × 109/L). The patient was negative for serum rheumatoid factor and antibodies including antinuclear, anti-SS-A, anti-SS-B, anti-DNA, anti-Sm, and anti-RNP antibodies. Her serum Wilm's tumor (WT1) mRNA level was 2 × 102 copy/μg (normal range; <5 × 101 copy/μg). A bone marrow examination showed normal cellularity with dysplasia in both the myeloid and megakaryocytic series, but no increase in blasts. The immunophenotyping of bone marrow lymphocytes showed a normal CD4:CD8 ratio (0.8 (36.9:47.9) (normal range; 0.4–2.3)). Neither nuclear p53 nor CD34 immunostaining was detected in hematopoietic cells. A karyotic analysis showed 47, XX, der (1:18)(q10;q10), inv (9) (p12q13) (14/20 cells), 46, XX, inv (9) (p12q13) (6/20 cells) (Fig. 1a). The patient was diagnosed with refractory cytopenia with multi-lineage dysplasia (RCMD) (International Prognostic Scoring System: intermediate-1). She had not been receiving any medication because she declined treatment. When she was 77 years old, she was admitted to our hospital for high grade fever, lower abdominal pain, and melena. Laboratory tests revealed the following: WBC count, 1.8 × 109/L; neutrophil cell count, 1.4 × 109/L; RBC count, 216 × 1010/L; Hb concentration, 5.7 g/dL; and platelet count, 53 × 109/L. Her C-reactive protein (CRP) level was 2.49 mg/dL (normal range: lower than 0.30 mg/dL), sIL2R level was 2152 U/ml (normal range: 145–519 U/ml), and serum WT1 mRNA level was 2.4 × 103 copy/μg. An endoscopic examination of her gastrointestinal tract revealed multiple ulcers in the ileocecal region. She was negative for HLA-B51. A repeat marrow examination showed normal cellular marrow with erythroid and megakaryocytic dysplasia, but no increase in blasts. The immunophenotyping of bone marrow MNCs showed a reversed CD4:CD8 ratio (0.3 (21.9:69.6)). Immunostaining revealed some p53-positive cells, but no proliferation of CD34-positive cells. A karyotic analysis showed 47, XX, +1, der (1:18)(q10;q10), +8, inv (9)(p12q13), −20 (12/20 cells), 46, XX, inv (9) (p12q13) (8/ 20 cells) (Fig. 1b). The patient was diagnosed Behçet's-like disease associated with MDS (RCMD) because of the presence of at least two international Behçet's criteria, and a combination of gastrointestinal involvement in the absence of other autoimmune/inflammatory disorders and granuloma from digestive biopsies. Prednisolone, mesalazine and colchicine were administered; however, melena did not improve and she was dependent on red blood cell transfusion. Thus, infliximab therapy was initiated. Although the attenuation of intestinal Behçet's-like symptoms was temporarily achieved, she was admitted to our hospital for high grade fever, lower abdominal pain, and melena after 4 cycles of infliximab. On admission, her temperature was 39.4 °C and oral ulcers were observed (Fig. 2a). Laboratory tests revealed the following: WBC count, 1.5 × 109/L; neutrophil cell count, 1.3 × 109/L; RBC count, 307 × 1010/L; Hb concentration, 8.7 g/dL; and platelet count, 49 × 109/L. Her CRP level was 10.5 mg/dL. Her sIL2R level was 8840 U/ml and serum WT1 mRNA level was 4.6 × 103 copy/μg. An endoscopic examination of her gastrointestinal tract revealed multiple ulcers in the ileocecal region (Fig. 2b). Intestinal ulcer biopsy showed non-specific inflammation (Fig. 2c). A repeat marrow examination showed normal cellular marrow with trilineage dysplasia, but no increase in blasts or other abnormal cells. Strong nuclear p53 and CD34 immunostaining was detected in 5 and 0% of hematopoietic cells, respectively. The administration of azacitidine (75 mg/m2 on days 1–7 every 28 days, subcutaneously) was initiated. Her oral ulcers gradually improved and melena disappeared, and her CRP and sIL2R levels decreased (0.5 mg/dL and 1250 U/ml, respectively). However, after 2 cycles of azacitidine, she died of sepsis.
Fig. 1.
(a) A karyotic analysis at the diagnosis of MDS showed 47, XX, der (1:18)(q10;q10), inv (9) (p12q13). (b) A karyotic analysis at the occurrence of Behçet's disease in this patient showed acquired +8.
Fig. 2.
(a) Oral ulcers developed. (b) An endoscopic examination of the gastrointestinal tract revealed multiple ulcers in the ileocecal region. (c) Intestinal ulcer biopsy showed non-specific inflammation (hematoxylin and eosin staining, × 100).
Various autoimmune diseases have been reported to be associated with MDS and the excessive production of inflammatory cytokines and abnormal neutrophil functions have been reported. Accumulating evidence has shown that marrow failure in some MDS is associated with autoimmunity, T-cell-mediated myelosuppression, and cytokine-induced cytopenias. Abnormal neutrophil functions, the overproduction of inflammatory cytokines, and other immunological abnormalities are also observed in BD. The majority of Behçet's-like disease associated with MDS cases have been classified as intestinal symptom, particularly ileocecal involvement, and trisomy 8 is a very common chromosomal abnormality. Chen et al. analyzed gene expression patterns in purified CD34-positive hematopoietic progenitor cells obtained from MDS patients with trisomy 8 or monosomy 7 [7]. In cases of trisomy 8, up-regulated genes included those involved primarily in immune and inflammatory responses, whereas genes up-regulated in cells with monosomy 7 did not, but did include those involved in leukemia transformation and apoptosis.
Chen et al. analyzed various gene expression patterns with MDS and trisomy 8 using a microarray analysis, and observed the higher gene expression of several cytokines, including transforming growth factor (TGF)-β, IFN-β2, IL-6, and IL-7R, which are all involved in immune activity and inflammation [7]. Behçet's disease is a chronic inflammatory disease with abnormalities in inflammatory cytokines, including IL-1β, IL-6, IL-8, IL-17, IL-18, tumor necrosis factor (TNF)-α, and IFN-γ. Hasegawa et al. reported higher serum levels of sIL-2R, IFN-γ, IL-1β, IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor in a Behçet's-like disease associated MDS patient with trisomy 8 during the active phase [6].
Trisomy 8 is associated with significant CD8 T-cell expansion [4]. The trisomy 8 marker allows T-cell interactions with normal and MDS cells to be separately identified and their specificity defined in vitro. Sloand et al. showed that cytotoxic T cells specifically targeted trisomy 8-bearing cells [4]. Since WT1 is overexpressed on CD34 cells in many patients with MDS, it is an obvious candidate antigen for an MDS-specific T-cell response and MDS patients with trisomy 8 overexpress WT1. The relationship between T-cell interactions and trisomy 8 may be involved in the pathogenesis of Behçet's-like disease associated with MDS in patients with trisomy 8. The present case showed a relationship between the occurrence of Behçet's-like disease and acquisition of trisomy 8. The number of CD8 cells in BM and the level of sIL2R increased when the patient showed Behçet's-like symptoms.
Recently Wesner et al. analyzed 11 cases of trisomy 8-MDS associated with Behçet's-like disease. In their report, overall survival and progression to acute myeloid leukemia remained similar with and without inflammatory disease and only the response to hematological therapy conferred an improvement survival [8]. A retrospective study described on the efficacy of azacitidine in the treatment of autoimmune diseases associated with MDS and showed that the use of azacitidine was efficacious for controlling autoimmune disease symptoms and reducing steroid doses [9]. Sanchez-Abarca et al. showed that azacitidine inhibited the proliferation and activation of T cells and decreased the production of proinflammatory cytokines, and this effect was not attributable to the proapoptotic effects of the drug, but to the down-regulation of genes that induce cell growth arrest [10]. In our case, oral ulcers and melena were ameliorated after azacitidine therapy. Azacitidine may suppress the up-regulated genes involved in immune and inflammatory responses, thereby controlling Behçet's-like disease.
To the best of our knowledge, this is the first case report of the acquisition of trisomy 8 associated with Behçet's-like disease in MDS. This case showed that T-cell interactions and trisomy 8 may be related to the pathogenesis of Behçet's-like disease and pancytopenia. Further studies are warranted to clarify the relationship between Behçet's-like disease and MDS with trisomy 8.
References
- 1.Mekinian A., Grignano E., Braun T. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology. 2016;55(2):291–300. doi: 10.1093/rheumatology/kev294. [DOI] [PubMed] [Google Scholar]
- 2.Tomonari A., Tojo A., Takahashi T. Resolution of Behçet’s disease after HLA-mismatched unrelated cord blood transplantation for myelodysplastic syndrome. Ann. Hematol. 2004;83(7):464–466. doi: 10.1007/s00277-003-0819-6. [DOI] [PubMed] [Google Scholar]
- 3.Nonami A., Takenaka K., Sumida C. Successful treatment of myelodysplastic syndrome (MDS)-related intestinal Behçet’s disease by up-front cord blood transplantation. Intern. Med. 2007;46(20):1753–1756. doi: 10.2169/internalmedicine.46.0291. [DOI] [PubMed] [Google Scholar]
- 4.Sloand E.M., Mainwaring L., Fuhrer M. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106(3):841–851. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloand E.M., Melenhorst J.J., Tucker Z.C. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011;117(9):2691–2699. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa H., Iwamasa K., Hatta N. Behçet’s disease associated with myelodysplastic syndrome with elevated levels of inflammatory cytokines. Mod. Rheumatol. 2003;13(4):350–355. doi: 10.3109/s10165-003-0245-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen G., Zeng W., Miyazato A. Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood. 2004;104(13):4210–4218. doi: 10.1182/blood-2004-01-0103. [DOI] [PubMed] [Google Scholar]
- 8.Wesner N., Drevon L., Guedon A. Gastrointestinal Behcet's-like disease with myelodysplastic neoplasms with trisomy 8: a French case series and literature review. Leuk Lymphoma. 2019;60(7):1782–1788. doi: 10.1080/10428194.2018.1542152. [DOI] [PubMed] [Google Scholar]
- 9.Fraison J.B., Mekinian A., Grignano E. Efficacy of Azacitidine in autoimmune and inflammatory disorders associated with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk. Res. 2016;43:13–17. doi: 10.1016/j.leukres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Abarca L.I., Gutierrez-Cosio S., Santamaría C. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115(1):107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]