Abstract
Background
Intra-articular injections of hyaluronic acid—also called viscosupplementation (VS)—is frequently used for the symptomatic treatment of knee osteoarthritis, a painful and debilitating long-term disease, affecting an important fraction of elderly populations. Severity of knee osteoarthritis is generally described by Kellgren-Lawrence (KL) radiological classification. VS has been widely studied in many clinical trials; however, the results are rarely analyzed in detail according to KL grade.
Method
A large, clinical, open-label study was performed in 2004–2007 on 1177 patients with knee osteoarthritis, each treated with VS consisting of 3 injections of Arthrum H 2% (LCA Pharmaceutical, Chartres, France). The characteristics of the patients at baseline included demographic profile, body mass index, KL grade, and clinical scores for pain and function using the Western Ontario and McMaster Universities index. Follow-up visits were at 3, 6, and 9 months after VS procedure. This large database was entirely reprocessed in 2019 to provide a separate analysis per KL grade, complemented by the assessment of the Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International rates (%) of responders to the treatment. The analysis was carried out for both intention-to-treat and per-protocol completer populations.
Results
A primary outcome in the intention-to-treat analysis, variations of the Western Ontario and McMaster Universities index pain subscore from inclusion to the end of the study were 19.8, 19.8, 17.8, and 14.2 for KL grade I to KL grade IV patients, respectively, on a 0 to 100 scale. In the per-protocol analysis, under the same conditions, the variations were 20.6, 19.9, 17.1, and 11.7. All results were significant (P < 0.001) and clinically relevant for each KL grade. Significant improvements were also observed for the Western Ontario and McMaster Universities index function subscore and for the other secondary outcomes. The Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International responders rate reached 72% to 82% for KL grade I through III patients at Month 6 and Month 9. For KL grade IV patients, the maximum rate reached was 47.7% at Month 6. There was evidence that KL grade is a critical parameter, particularly if KL grade IV is present. Other parameters such as gender, body mass index, and age were not identified as prognostic factors of response to VS based on χ2 and odds ratio (95% CI) testing.
Conclusions
Detailed analysis by KL grade supports that VS treatment with Arthrum H 2% applies to a large variety of patients with knee osteoarthritis.
Keywords: Knee osteoarthritis, hyaluronic acid, intra articular, viscosupplementation, Kellgren-Lawrence
Introduction
Osteoarthritis (OA) is a frequently painful disease affecting a large part of elderly populations. Based on data from the Institut National de la Statistique et des études économiques describing the French population aged 15 years or older on January 1, 2011, patients with symptomatic OA is estimated to be a large group of 7.2 million. Among them, 23.2% of people aged 65 years or older stated that they have knee OA, which represents about 2.6 million people in early 2019, for a total population close to 67 million.1
Treatments of knee OA are mostly symptomatic and, among them, intra-articular (IA) injections of hyaluronic acid (HA) or viscosupplementation (VS) have been widely used over the past decades. Numerous studies evaluated the effects of VS using different forms and regimens of HA.2,3 Among commercially available HAs, VS with Arthrum H 2% (LCA Pharmaceutical, Chartres, France) administered in 3 weekly injections demonstrated its effectiveness in improving pain and function of knee OA patients in several studies.4, 5, 6, 7 In addition, a large multicenter open study was conducted during 2004–2007 in 1177 treated patients assessed using the Western Ontario and MacMaster Universities, OA pain subscore (WOMAC A) and function subscore (WOMAC C) with a 0 to 4 Likert scale.8 When this study was initiated, its main objective was to consolidate the clinical results of this product in real-world conditions. It included a follow-up period extended up to 9 months, which is beyond the 3 to 6 months of follow-up of previous studies.
Because there is still a need to better identify the patients who will benefit the most from IA HA, we re-explored the data of this unpublished study, in a retrospective manner taking advantage of the large size of the population included. The objective was to assess whether 3 critical parameters in the progression of OA—Kellgren-Lawrence (KL) radiological grade for OA severity,9 age, and body mass index (BMI)—could also be identified as prognostic factors of therapeutic response rate. In addition, we calculated the rate of Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) responders among this large population managed in daily practice.10
Methods
Initial study design
The initial 2004–2007 study was designed as an observational multicenter cohort study with no comparator involved, meaning no randomization or concealment, and therefore allowing an open-label design. The investigators were 83 rheumatologists, 6 orthopedic surgeons, and 6 doctors of physical and rehabilitation medicine, all prescribing the product Arthrum H 2% in their current practice, in France. The patients were individually invited to participate in the study only after VS with this product was prescribed and their verbal consent was received. Patients could withdraw from the study at any time for any reason. In all cases, the French Social Security system covered costs related to OA diagnosis, HA product, and IA injections. During participation in the study, follow-up visits—additional to routine practice—were part of investigator fees provided by the sponsor of the study, and therefore free of charge for the patients. Patients received no fee or other financial advantage. Remuneration of the investigators was related to the time spent for data collection and no additional incentive or advantage was given.
The study design was in accordance with the Declaration of Helsinki (52nd revision, October 2000), which did not recommend the use of placebo control when enough efficacy evidence was available. Arthrum H 2% was indeed European community approval marked and has been available on the French market since the end of 1998. By 2004, nearly 3500 patients had already participated in previous clinical studies evaluating the effects of this specific product. In addition, there were several other published studies comparing competing IA HA products versus placebo.
Because the study aimed to evaluate the product in real-world conditions, investigators had no restrictions and were responsible for the indications of Arthrum H 2%, regardless of the severity of knee OA. Inclusion and exclusion criteria were based on the instructions for use of Arthrum H 2% and the French Good Clinical Practice, as detailed in Table 1. Specifically, no limit was fixed for age, KL grade, anteriority, concomitant or pre-existing treatment, or general health of the patient. The use of the product was performed in accordance with the information sheet. Case report forms were provided to the investigators. Patient ability to fill questionnaires was estimated by the investigator and the patient was asked to attend at least 2 of the 3 follow-up visits planned at Month 3, Month 6, and Month 9. No registration was requested by the French health authorities for this type of noninterventional cohort study. The data analyses, statistics and final 2007 clinical report were done by an independent clinical research organization.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Primary and secondary outcomes
The primary outcome was the WOMAC A with the objective of demonstrating a clinically relevant and significant improvement from baseline (Month 0) to the last visit (Month 9 or Month 6).
The secondary outcomes included the evolution at any time of all the indexes: WOMAC A, WOMAC C, influence on daily life (Agence nationale d'accréditation et d’évaluation en santé [ANAES] questions [replaced by the Haute autorité de santé in 2005]) and handicap assessment. The influence on concomitant treatments (corticosteroids and/or nonsteroidal anti-inflammatory drugs) was assessed through 1 final question.
Materials
The results obtained in 2007 have neither been presented to any scientific congress nor published in any medical journal. The analytical report was given to the health authority and this study is mentioned only in the Commission Nationale d'Evaluation des Dispositifs Médicaux et des Technologies de Santé advice about Arthrum, dated July 7, 2009.
During 2019, this large database (1177 patients) was first considered of interest because of the size of the population and duration of follow-up. Therefore, a new analysis was conducted, detailed per KL grade—as known at inclusion—and completed with the assessment of the OMERACT-OARSI responders,10 after recollecting each patient's results from the case report form. The pain and function assessments were available from the WOMAC A and C subscores. Each patient's global assessment was determined from the 3 ANAES questions related to quality of daily life: influence of OA on walking, on working, and on sleep. Each question was assessed on a 0 to 10 scale, at inclusion (ie, baseline) and each follow-up visit. To be suitable with OMERACT-OARSI responders, the WOMAC A and C scores and the combined ANAES scores were each converted to a 0 to 100 scale.
New analysis
The new analysis, including graphs, was performed using Excel (Microsoft, Redmond, Washington). MIX 2.0 software (BiostatXL, Mountain View, California) was used to compute the heterogeneity indicators and forest plots. Synthesis was done under fixed effects and inverse variance weighting.
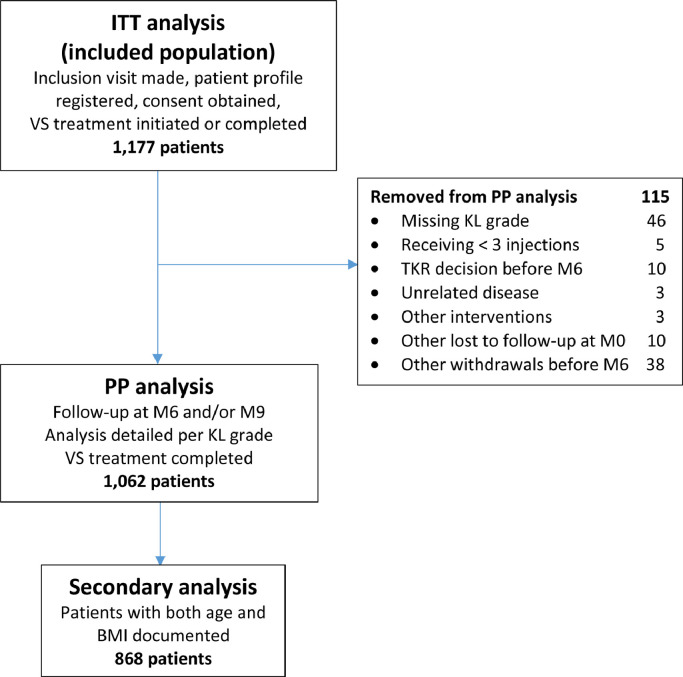
Our selection method is described in Figure 1, starting from a first analysis made for all included patients identified as the intent-to-treat (ITT) population because they received at least 1 injection. After removing patients with missing KL grade data; those who were lost to follow-up; or those who withdrew after Month 0 or Month 3 for any reason, including surgery procedures, a second analysis was performed on the per-protocol (PP) completer population.
Figure 1.
2019 reanalysis flow chart. KL = Kellgren-Lawrence radiologic osteoarthritis scale (grade I–IV); M = month. TKR = total knee replacement; VS = viscosupplementation; BMI = body mass index
The baseline KL grades were recorded for each patient to allow the detailed analysis for all criteria, from WOMAC results to OMERACT-OARSI responders. To assess precisely the primary and secondary outcomes in the ITT analysis, the final population at Month 9 was compared with baseline. In the PP analysis, to keep the same completed PP population from baseline to end of the study, results at Month 6 were accepted for patients not seen at Month 9 because it was defined in the protocol with 1 no-show visit accepted among the 3 time-point visits.
Finally, it appeared important to compare, within each KL subgroup, the OMERACT-OARSI responders and non responders to study potential relations with patient age and BMI. This was done in the secondary analysis after removal of patients with missing age or BMI. Several approaches were considered, first using scatter plots for each KL, and then making unilateral comparisons with χ2 test and odds ratio (OR).
Because different methods were used to collect and interpret the data, the populations may not be strictly identical to the 2007 analysis, where KL grade was only grouped as grade I + II and grade III + IV (unpublished results).
Potential bias
Comparing detailed KL subgroups results across the same observation time allowed detection of potential selection bias. Otherwise, heterogeneity indicators were used for the primary outcome (WOMAC A) and the secondary outcome (WOMAC C) in the score difference assessment from baseline (Month 0) to the last visit (Month 9 or Month 6). A comparison with the 2007 results allowed for control of this risk of bias. The influence of excluding patients from the PP analysis was also discussed, particularly with patients undergoing total knee replacement (TKR), during the 9-month observation period.
Potential role of age or BMI
To assess the potential role of age or BMI in relation to KL grade, a secondary analysis was performed. The fraction of the PP population with both age and BMI recorded was considered. The responders (%) correspond to the last visit at Month 9 or Month 6. Considering that age and BMI are continuous variables, we first used scatter plot graphs, 1 per KL grade, to represent each patient as a point by function of age (x-axis) and BMI (y-axis). Different coding distinguished the responders from the non responders. Correlation between age and BMI was assessed with R2 for each subgroup. Univariate analyses were intended for each potential factor based on the OR. The first analysis was performed for all KL grades together and the second analysis was detailed for each KL grade.
Results
Data collection
The flow chart (Figure 1) describes each of the populations analyzed. Among the ITT population of 1177 patients, 1062 completer patients were selected for the PP analysis, all seen at Month 6 or Month 9. The following patients were excluded from this analysis: 5 patients who received fewer than 3 injections of Arthrum, 46 patients without documented KL grade, 10 patients with TKR surgery decision before Month 6 and no further visits, 3 patients with diseases unrelated to OA (including 1 cancer and 1 death), 3 patients with intervention procedures (hip replacement, arthroscopy, and IA corticosteroid), 10 patients lost to follow-up at Month 0, and 38 other withdrawals before Month 6 (most on patient's decision). Altogether, the total of patients excluded for fewer than 3 injections and for medical or surgical reasons (related or unrelated to knee OA) was 21, representing 1.8% of the ITT population.
Patient profile
Patient profile is described in Table 2, for both the ITT population (1177 patients) and the PP population (1062 patients). Globally, in terms of age, gender, and BMI, there was no significant difference between these 2 populations. Also from this Table 2, there was no strong evidence of relation between BMI and KL grade. A wide age range was observed for each KL, with a large 53 to 62 years difference between the youngest and the oldest patient, making interpretation difficult. However, there could be a potential correlation between age and KL, as KL grade IV population was more than 10 years older than the KL grade I population. Obviously, this could just be an expression of the progressive character of OA disease. These points are analyzed further in the Results section.
Table 2.
Patient profile.*
| Patient | Total | KL radiologic osteoarthritis scale grade |
|||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| ITT population | 1177 | ||||
| KL distribution | 1131† | 146 (13) | 416 (37) | 422 (37) | 147 (13) |
| Women (%) | 754 (64) | 94 (64) | 260 (63) | 283 (67) | 88 (60) |
| Men (%) | 419 (36) | 52 (36) | 154 (37) | 138 (33) | 59 (40) |
| Age, y (SD) | 68.4 (10.6) | 61.9 (10.2) | 67.3 (10.4) | 70.2 (9.9) | 73.0 (9.7) |
| Minimum | 27 | 27 | 33 | 29 | 44 |
| Maximum | 97 | 83 | 95 | 91 | 97 |
| Body mass index (SD) | 27.5 (4.5) | 26.7 (3.7) | 27.0 (4.4) | 28.0 (4.7) | 28.3 (4.3) |
| Minimum | 16.2 | 19.3 | 16.6 | 16.6 | 16.2 |
| Maximum | 60.0 | 37.1 | 47.8 | 46.4 | 40.1 |
| PP population | |||||
| KL distribution | 1062 | 141 (13) | 396 (37) | 395 (37) | 130 (12) |
| Women (%) | 685 (65) | 91 (65) | 247(63) | 271 (69) | 76 (58) |
| Men (%) | 374 (35) | 50 (35) | 147 (37) | 123 (31) | 54 (42) |
| Age, y (SD) | 68.4 (10.5) | 62.3 (9.8) | 67.2 (10.4) | 70.2 (9.8) | 73.1 (10.0) |
| Minimum | 29 | 29 | 33 | 29 | 44 |
| Maximum | 97 | 83 | 95 | 91 | 97 |
| Body mass index (SD) | 27.5 (4.4) | 26.7 (3.7) | 26.9 (4.3) | 28.0 (4.7) | 28.1 (4.1) |
| Minimum | 16.2 | 19.3 | 16.6 | 16.6 | 16.2 |
| Maximum | 47.8 | 37.1 | 47.8 | 46.4 | 40.1 |
ITT = intention to treat; KL = Kellgren-Lawrence; PP = per protocol.
Values for KL and sex distributions are presented as n (%). Values for age and body mass index are presented as mean (SD).
After removal of 46 patients with unknown Kellgren-Lawrence radiologic osteoarthritis scale grade.
Primary outcome
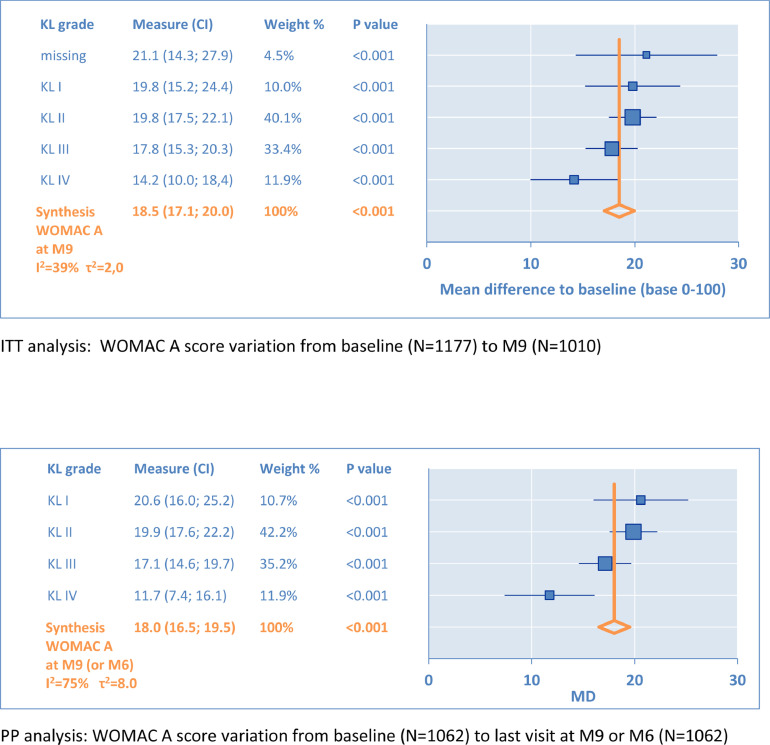
The variations of the WOMAC A subscore from baseline (Month 0) to the last visit are illustrated in Figure 2. The first forest plot gives results for ITT population and the second for the defined PP population. Each KL grade is detailed with 95% CI represented by horizontal bars. The y-axis (X = 0) corresponds to the baseline, and any positive point (X > 0) describes an improvement from baseline.
Figure 2.
Primary outcome: Western Ontario & McMaster Universities (WOMAC) A (pain subscale). KL = Kellgren-Lawrence radiologic osteoarthritis scale (grade I–IV); M = month; MD = mean difference.
From KL grade I to KL grade IV, respectively, the mean difference (MD) varied from 19.8 to 14.2 in ITT and from 20.6 to 11.7 in PP. The synthesis gave equivalent results for MD 18.5 (95% CI, 17.1–20.0) in ITT versus MD 18.0 (95% CI, 16.5–19.5) in PP. No significant difference was observed between the results of the 2 analyses.
All these WOMAC A changes to baseline were significant (P < 0.001), above the minimal clinically important difference for improvement, and above the smallest detectable difference, respectively, 7.5 and 8.1, defined by Angst et al.11 Also, these MDs were above the minimal perceptible clinical improvement of 9.7, as defined by Ehrich et al.12 In all cases, this indicated that the results for the primary outcome were clinically relevant and in favor of IA HA. In the ITT population, the effect size (ES) reached 1.07 (95% CI, 0.98–1.16), which is close to the standard mean difference found by Miller and Block13 for pain 1.14 (95% CI, 0.89–1.39) in a direct comparison to baseline, at 14 to 26 weeks.
Secondary outcomes
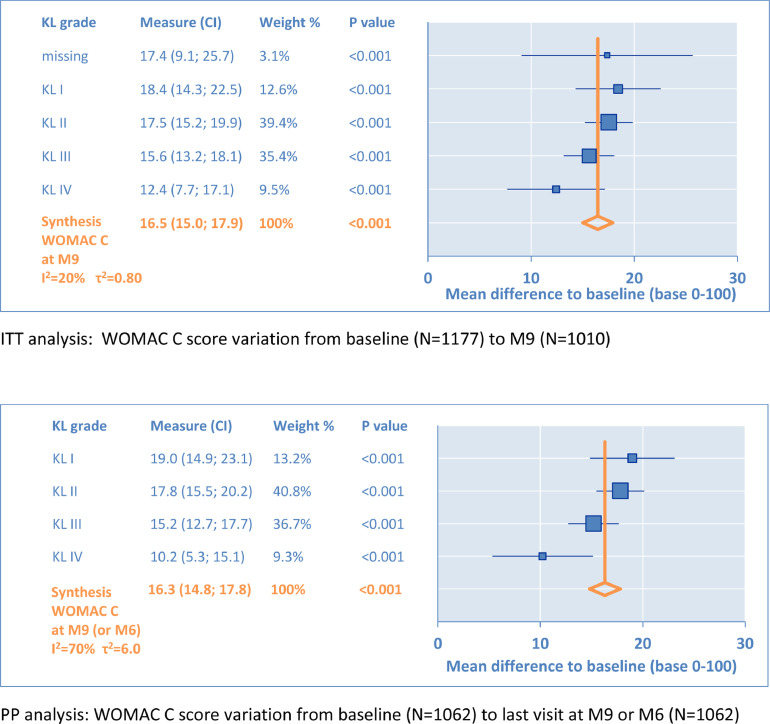
Under the same conditions, the variations of the WOMAC C subscore from baseline are illustrated in Figure 3. From KL grade I to KL grade IV, MD varied, respectively, from 18.4 to 12.4 in ITT and from 19.0 to 10.2 in PP. The synthesis was nearly identical with MD = 16.5 (95% CI, 15.0–17.9) in ITT versus MD = 16.3 (95% CI, 14.8–17.8) in PP. These changes from baseline were significant (P < 0.001) and all above the minimal clinically important difference, the smallest detectable difference and the minimal perceptible clinical improvement, respectively, defined at 6.7, 7.8, and 9.3 for the WOMAC C. Also, these differences were above the minimal clinically important improvement defined by Tubach et al14 in absolute change (9.1; 95% CI, 7.5–10.5) for the WOMAC C. As with the WOMAC A, the results for the WOMAC C were clinically relevant and in favor of IA HA. Similarly, ES reached 0.94 (95% CI, 0.85–1.03) in ITT, compared with ES = 1.07 (95% CI, 0.84–1.30) from Miller and Block13 for function in baseline comparison.
Figure 3.
Secondary outcome: Western Ontario & McMaster Universities (WOMAC) C (function subscale). KL = Kellgren-Lawrence radiologic osteoarthritis scale (grade I–IV); M = month.
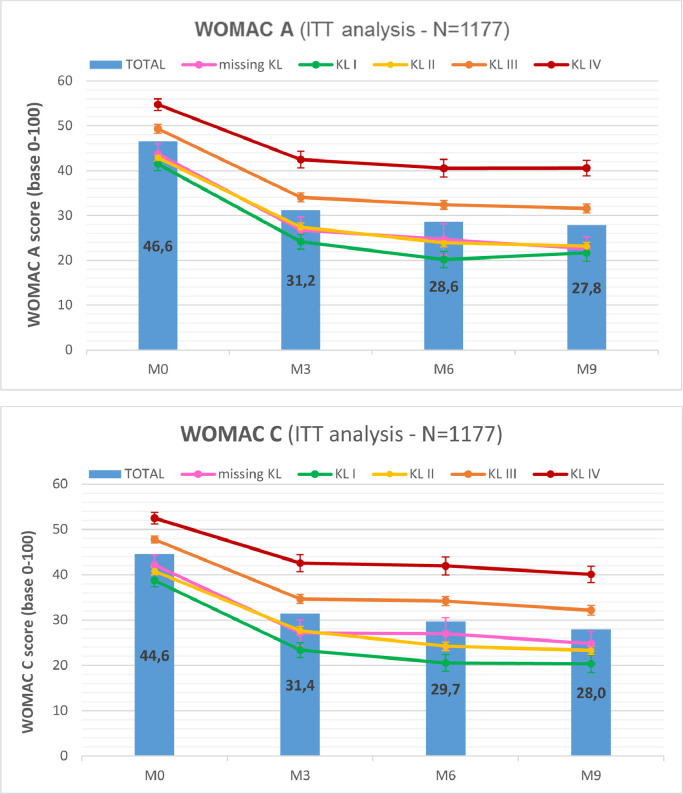
The WOMAC A and WOMAC C subscale scores and other quantitative results (ANAES questions) are all given on a 0 to 100 scale, and are presented in Table 3 for the ITT analysis. They were detailed by observation time (ie, Month 0, Month 3, Month 6, and Month 9) and the KL grade. At Month 6 or Month 9 for the WOMAC A in ITT, the improvement from baseline was important, reaching 21.3 (–51%) for KL grade I, 19.7 (–46%) for KL grade II, 17.7 (–36%) for KL grade III, and 14.2 (–26%) for KL grade IV. Under the same parameters, the improvement of the WOMAC C in ITT was 18.5 (–48%), 17.4 (–43%), 15.6 (–33%), and 12.4 (–24%), respectively. The evolutions of the WOMAC A and C scores from baseline (Month 0) were also represented in Figure 4, for the ITT population. Globally, all results were stable or very slightly improved from Month 6 to Month 9 for each KL grade.
Table 3.
Intention-to-treat analysis applying Western Ontario & McMaster Universities index for osteoarthritis symptoms assessment (WOMAC) subscales A (pain) and C (function) and Agence nationale d'accréditation et d’évaluation en santé (ANAES) scores.*
| KL grade | Score† | Time point (mo) |
|||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | ||
| Missing | n | 46 | 37 | 28 | 37 |
| WOMAC A | 43.7 (15.4) | 26.8 (17.7) | 24.6 (18.5) | 22.6 (16.1) | |
| WOMAC C | 42.2 (18.9) | 27.1 (18.6) | 27.0 (19.4) | 24.8 (19.5) | |
| ANAES 3 questions | 49.5 (14.7) | 34.5 (16.4) | 31.4 (16.9) | 30.3 (15.9) | |
| I | n | 146 | 127 | 99 | 131 |
| WOMAC A | 41.5 (17.3) | 24.1 (18.7) | 20.2 (18.4) | 21.7 (21.5) | |
| WOMAC C | 38.8 (17.4) | 23.4 (16.7) | 20.5 (17.8) | 20.3 (17.6) | |
| ANAES 3 questions | 43.9 (18.5) | 26.4 (17.8) | 23.9 (17.4) | 23.5 (17.8) | |
| II | n | 416 | 340 | 336 | 376 |
| WOMAC A | 42.9 (15.7) | 27.4 (16.8) | 23.9 (16.9) | 23.1 (17.1) | |
| WOMAC C | 40.8 (16.0) | 27.7 (17.3) | 24.2 (16.2) | 23.3 (17.3) | |
| ANAES 3 questions | 45.4 (16.4) | 28.8 (16.1) | 25.4 (15.2) | 24.4 (15.0) | |
| III | N | 422 | 345 | 334 | 351 |
| WOMAC A | 49.3 (15.4) | 34.0 (18.4) | 32.4 (18.5) | 31.6 (20.1) | |
| WOMAC C | 47.8 (15.6) | 34.7 (18.1) | 34.2 (18.5) | 32.2 (19.2) | |
| ANAES 3 questions | 51.2 (15.5) | 34.5 (17.4) | 32.7 (16.4) | 32.8 (17.6) | |
| IV | N | 147 | 121 | 107 | 115 |
| WOMAC A | 54.7 (15.6) | 42.4 (20.8) | 40.5 (20.4) | 40.6 (19.0) | |
| WOMAC C | 52.5 (18.5) | 42.6 (21.7) | 41.9 (21.9) | 40.1 (20.4) | |
| ANAES 3 questions | 52.5 (16.0) | 42.0 (19.2) | 40.9 (20.6) | 39.9 (19.2) | |
| All patients | n | 1177 | 970 | 904 | 1010 |
| WOMAC A | 46.6 (16.4) | 31.2 (19.0) | 28.6 (19.2) | 27.8 (19.9) | |
| WOMAC C | 44.6 (17.1) | 31.4 (19.0) | 29.7 (19.3) | 28.0 (19.5) | |
| ANAES 3 questions | 48.3 (16.5) | 32.4 (17.8) | 29.9 (17.5) | 29.2 (17.7) | |
ANAES = Agence nationale d‧accréditation et d'évaluation en santé; KL = Kellgren-Lawrence radiologic osteoarthritis scale grade (I–IV).
Replaced by Haute autorité de santé [French Health Authority] in 2005.
WOMAC and ANAES scale scores were 0 to 100. Values are presented as mean (SD).
Figure 4.
Intention-to-treat (ITT) analysis: Western Ontario & McMaster Universities (WOMAC) and outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) results. The first graph represents the WOMAC A (pain subscale) mean score evolution, with a continuous curve for each Kellgren-Lawrence (KL) radiologic osteoarthritis scale grade (I–IV), and vertical bars for the whole population. Error bars represent the standard error. Similarly, the second graph represents the WOMAC C (function subscale) score evolution. M = month.
Among 1037 ITT patients taking concomitant corticosteroids or nonsteroidal anti-inflammatory drug treatments, 63% were satisfied with medication intake reduction, whereas 21% remained totally unsatisfied.
Heterogeneity assessment
In the ITT analysis at end of study, the index I2 (ratio of heterogeneity across KL grade results) was moderate, 40% or 55% for the WOMAC A subscale, and 20% or 54% for the WOMAC C subscale, depending on assessment made from MD or ES, respectively. In identical conditions, the variance of the true effect index τ2 remained very low, at 0.015 for the WOMAC A and 0.013 for the WOMAC C, in dimensionless units used for ES assessment. So, a low variance (τ2 < 0.04) was observed between each KL grade study.
OMERACT-OARSI responders
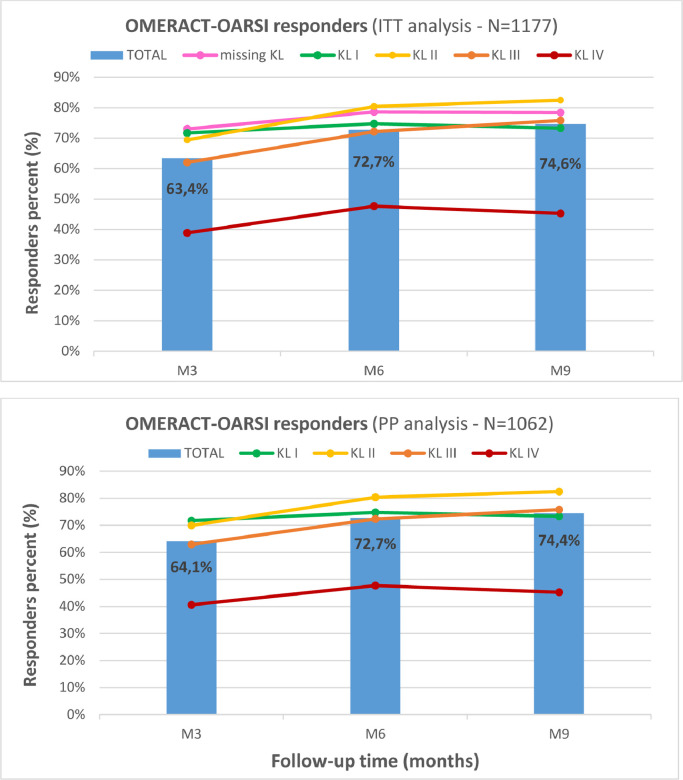
The percentage of patients who were OMERACT-OARSI responders are given for three populations in Table 4, and illustrated in Figure 5 for the ITT and PP populations. For combined KL grades and for each population subgroup, there was a significant increase in the responders (%) from Month 3 to Month 6, followed by a small increase from Month 6 to Month 9. There were significant differences in responders (%) between each KL grade subgroup. The highest rates above 80% were observed for KL II at Month 6 and Month 9. Conversely, the best rate with KL grade IV was 47.7% at Month 6. No selection bias was introduced by potential difference between the profiles of the studied populations because they were very close. This confirms KL grade to be the most determinant factor for the rate of responders.
Table 4.
Comparison to other Arthrum studies with various Kellgren-Lawrence radiologic osteoarthritis scale (KL) grade I through IV.*
| Study | n | Age, y | KL profile (%) |
Variation from baseline |
OMERACT-OARSI responders (%) | |||
|---|---|---|---|---|---|---|---|---|
| I & II | III | IV | WOMAC A | WOMAC C | ||||
| Arramon et al4 | 271 | 67.2 | 39 | 45 | 16 | 17.9 | 15.8 | NA |
| Germonville et al5 | 126 | 66.9 | 54 | 38 | 8 | 25.6 | 25.9 | 85.0 |
| Thomas et al6 | 202 | 65.6 | 54 | 46 | 0 | 22.3 | 18.8 | NA |
| Hilliquin et al7 | 182 | 45.0 | 62 | 34 | 4 | 20.1 | 17.0 | NA |
| Baron et al15 | 214 | 62.9 | 54 | 46 | 0 | 33.6 | 27.6 | 90.0 |
| Present study | 1177 | 68.4 | 50 | 37 | 13 | 18.0 | 14.9 | 72.7 |
NA = not available; OARSI = Osteoarthritis Research Society International; OMERACT = Outcome Measures in Rheumatoid Arthritis Clinical Trials; WOMAC = Western Ontario & McMaster Universities index for osteoarthritis symptoms assessment.
All results are related to knee osteoarthritis treatment with Arthrum (LCA Pharmaceutical, Chartres, France) products at 6-month follow-up. There were clear differences for the patients KL profiles between these trials. It was observed that the highest scores were obtained from studies with 0% to 8% patients at KL grade IV. None of the trials containing ≥12% KL grade IV had variations from baseline >20 for WOMAC A (pain subscore), >16 for WOMAC C (function subscore) (on a scale of 0–100), or a percentage of OMERACT-OARSI responders >75%. In the present study the percentage of responders increases to 76.0% at Month 6 and 78.3% at Month 9 after removal of KL grade IV and unspecified KL grade patients from the intention to treat population. The percentage of KL grade IV patients admitted may be critical to interpret clinical results.
Figure 5.
Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) responders. The graphs represent the evolution of the OMERACT-OARSI percentage of responders, for the intention-to-treat (ITT) and the per-protocol (PP) populations. Continuous curves represent each Kellgren-Lawrence (KL) radiologic osteoarthritis scale grade (I–IV), and vertical bars represent the whole population. The missing KL subgroup scores were close to KL grade II; their removal had minimal influence on the total result. M = month.
Role of KL grade, age, BMI, and other factors
The secondary analysis identified 862 patients with known KL grade, gender, age, and BMI (Figure 1).
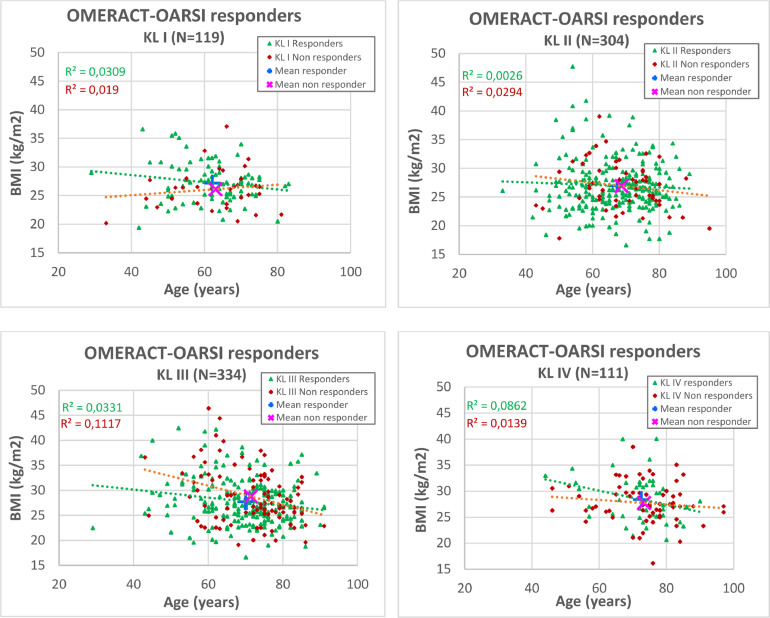
The scatter plots are given for each KL grade (Figure 6), describing age and BMI as independent parameters. No correlation was identified between BMI and age for OMERACT-OARSI responders or non responders subgroups (R2 ≤ 0.12). However, age and KL grade cannot be considered as totally independent variables because OA evolution was slow, as evidenced by the age mean difference of more than 10 years between KL grade I and KL grade IV (Table 2).
Figure 6.
Secondary analysis: Scatter plots. On each graph dedicated to a specific Kellgren-Lawrence (KL) radiologic osteoarthritis scale grade (I–IV), each patient was represented by 1 mark, as a function of age (x-axis) and body mass index (BMI) (y-axis), with a color differentiation between responders and nonresponders. The center of gravity of each subpopulation, was represented by + for the responders and by × for the non responders. The distance between these 2 symbols was always very small, illustrating that both age and BMI had no significant influence on the response. The linear curves and the associated R2 supported the absence of correlation between BMI and age. OMERACT-OARSI = Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International.
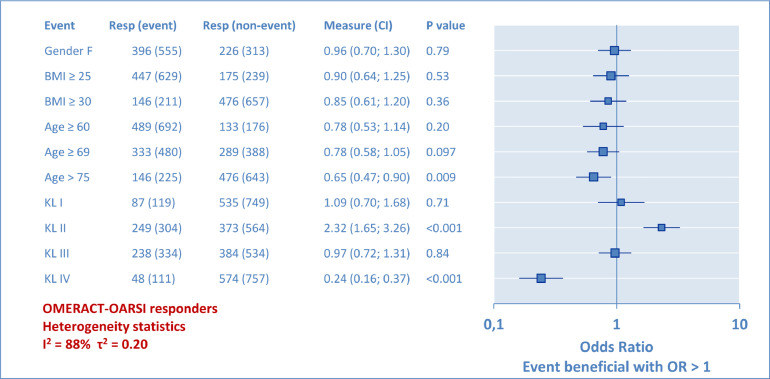
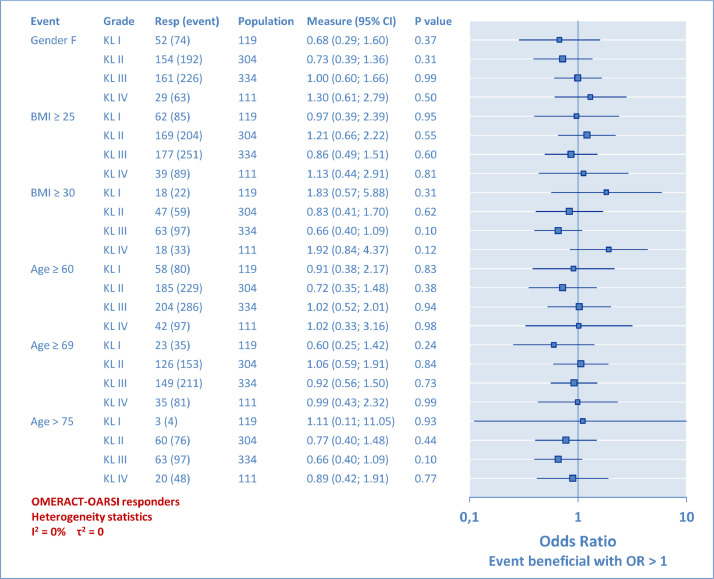
Univariate analyses with OR results are given in the form of a forest plot (Figure 7) for the whole population and for each KL grade (Figure 8). The attached tabulated data provides the OR scores (95% CI) and the P values. BMI was assessed to designate overweight (BMI ≥ 25) and obesity (BMI ≥ 30). Age incidence was assessed, first from below the first quartile (≥60 years), then over the mean (≥69 years), and finally over the third quartile (>75 years) of the studied population. From the global analysis in Figure 7, it was observed that KL grade plays the largest role, demonstrating the relative higher chance of success for the KL grade II, and the lowest for the KL grade IV. However, this method has limits because KL grade II, for instance, was compared with a group combining all other KL grades, which is not necessarily relevant. Therefore, the second analysis was performed per KL grade (Figure 8) to complete the information provided to the practitioner and to indicate if a second factor associated with a known KL grade can be determinant or not. The χ2 test results are not detailed here because they led to identical results for the P values. To summarize, at no moment were gender, overweight, obesity, or patient's age perceived to have any significant influence on the rate of OMERACT-OARSI responders in relation to the KL grade. For heterogeneity, a huge difference was observed between the 2 analyses, with I2 and τ2 varying from 88% and 0.20, respectively, in the whole population analysis, to 0 in the analysis detailed per KL grade. This suggests that pooling different KL results induced a lot of heterogeneity in VS trials.
Figure 7.
Secondary analysis: Odds ratio (OR). Univariate analysis of potential prognostic factors. OR compared the proportion of Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) responders between 1 part of the population (event) and the rest of the population (nonevent). In this forest plot, each OR result was represented as a point with the 95% CI interval as a horizontal bar. At OR = 1 there was no probability for the event to be beneficial or unfavorable (P = 1). Each event (left column) was individually analyzed. The Kellgren-Lawrence (KL) radiologic osteoarthritis scale grade (I–IV) had the greatest influence, with KL grade II (relatively) favorable and KL grade IV (relatively) unfavorable.
Figure 8.
Secondary analysis per Kellgren-Lawrence (KL) radiologic osteoarthritis scale grade (I–IV): Odds ratio (OR). Prognostic factors: gender, body mass index, and age. The large population (862 patients) allowed detailed analysis by KL subgroups. On the forest plot graph, each 95% CI included OR = 1, meaning no evidence of a relation for each test, confirmed with P > 0.05. The minimal statistical size was reached for each event except 1, because there were only 4 KL grade I patients older than age 75 years. The heterogeneity indicators I2 and τ2 have dropped to 0.
Incidence of patients with surgery procedure and other exclusions from PP analysis
There were a total of 18 patients with a decision for TKR surgery, taken between Month 0 and Month 9, all present in the ITT analysis. In the PP analysis, 7 patients were still present because their study was completed at Month 6, before the TKR decision. Other patients with interventions were removed from the PP analysis (Figure 1): 1 TKR patient who received only 2 injections, 10 others with TKR (3 lost after Month 0 and 7 seen at Month 3), 1 patient with arthroscopy (lost after Month 0), and 1 patient with osteotomy (seen at Month 6, but unknown KL grade). All patients with surgery seen at Month 3 were non responders.
The 46 patients with unknown KL grade performed similarly to a KL grade II as shown in Figures 4 and 5, and they have been removed from this rationale because 45 of them completed the study at Month 6 or Month 9.
The 21 patients excluded for medical or surgical reasons, as described in the Data Collection section, had a profile at inclusion (age = 73.0 years, BMI = 27.4, WOMAC A = 46.9, and WOMAC C = 45.8) that differs for age and KL grade IV (40%), but not for the WOMAC scores, with reference to Tables 2 and 3 (ITT population profile).
There were 38 patient withdrawals after Month 3. Among them, 7 were satisfied and 10 were unsatisfied with the VS treatment, whereas the remaining 21 withdrawals (patient's decision) included 13 responders and 8 non responders. Therefore, several withdrawals were clearly identified from patients improved with VS treatment, who just canceled the last visits for personal convenience. The remaining 10 lost-to-follow-up patients after Month 0 could not be analyzed, except from their profile at inclusion.
In summary, the 69 patients excluded from PP population were not specifically non responders, and their average profile at inclusion (age = 67.4 years, BMI = 28.5, WOMAC A = 45.8, and WOMAC C = 40.8) did not contribute to worse profile of the ITT population.
Discussion
Incidence of KL grade IV: Comparison with other trials results and interpretation
From our findings, the presence or absence of KL grade IV patients, has a clear influence on the clinical results. Taking the KL profile into account, this suggests comparisons should be made with other Arthrum H 2% trials4, 5, 6, 7 and with Arthrum Visc 75 (LCA Pharmaceutical),15 an alternative version of Arthrum that is designed for single injection. As described in Table 4, the Arthrum groups containing 12% KL grade IV or more, clearly offer less—but still relevant—improvements for the WOMAC subscores (pain and function) and for the percentage of OMERACT-OARSI responders, at Month 6.
Another important aspect of the KL grade IV knee OA is the level of response that can be expected to the VS treatment in comparison to the IA placebo. In the literature, IA placebo results are very scarce in KL grade IV knee OA. We only found 1 study by Blanco et al,16 a specific randomized controlled trial for VS in knee OA patients with 100% KL grade IV. At Month 6, the WOMAC subscores variations were 21.7 (–35%) for pain, and 24.7 (–39%) for function with IA HA, versus 11.2 (–17%) and 4.4 (–6%), respectively, for the IA placebo. These results support a large difference in favor of the IA HA. From there, one can estimate the average patient should be an OMERACT-OARSI responder if IA HA treated, and nonresponder if IA placebo treated. In other words, the IA placebo effect appears smaller in the presence of a KL grade IV, than with lower KL grades. This supports our opinion that in knee OA, the level of response given by an IA placebo should be identified as KL-dependent.17
About the prognostic factors of response to VS
From the OR assessment made in Figure 7, the most favorable patient profile clearly appears to be at KL grade II, preferably younger than age 60 years, which aligns with the findings of Maheu et al.18 However, our further analysis, detailed per KL grade and performed with a double approach—scatter plots then OR—did not reveal any significant relation between the gender, BMI, or age of the patient, and the response to the VS treatment. So, the VS treatment of older patients with higher KL grades should not be discouraged.
One previous study of the prognostic factors for the response to VS in knee OA,19 based on results obtained with Arthrum H 2%,5 did not reveal any relation with age or BMI. This is confirmed by the results of the present study in a much larger population, which does not support obesity as a risk factor, but only the KL grade IV in a relative proportion.
To summarize, the above results demonstrate that Arthrum H 2% (3 injection) offers a good prognosis of success of VS in a large diversity of OA profiles, from KL grade I through III, regardless of age or BMI. For the KL grade IV OA patients, there is still a chance of success, limited to a moderate improvement by the patient, and possibly a lower duration of treatment efficacy. It is not our purpose to discuss here the difficulties with the KL grade IV patients or the lack of alternative treatments, particularly for noncandidates for TKR surgery.
Limitations
There are certainly limitations with this post hoc retrospective analysis. However, our data are in the same magnitude of previously published data of controlled studies and the effects of this common bias are limited if anything. The second usual flaw of such a real-life study is the lack of an IA comparator and the open-label design; again, quantitative results on pain and function compared versus baseline were consistent with those reported by Miller et al13 in their large systematic review and meta-analysis of randomized, controlled trials supporting the reliability of our reported data. Also, the study was conducted on a large cohort population of more than 1000 patients, under real-world conditions, with a long follow-up extension of 9 months. Moreover, a unique analysis per KL grade was proposed, allowed by the large population size.
The clinical benefit of IA saline was assessed by Altman et al,20 who found standard mean difference = 0.61 (95% CI, 0.45–0.76) for pain improvement at long term (6–12 months). This result is clearly smaller than our primary outcome (ES = 1.07; 95% CI, 0.98–1.16) and this allows an estimate of ES = 0.46 versus IA placebo for Arthrum H 2% at the long term, which is relevant for the whole population. This result—ES = 0.46—appears to be coherent and slightly above the average found in several meta-analyses described by Maheu et al.18 That said, the lack of comparator results accurately described per KL grade for an IA placebo was a serious handicap to the interpretation of our results.
Attrition of the ITT population may be a source of bias because it was largely dependent on the patient's decision. This influence was moderate and ITT and PP analyses provided very close data, even when including 11 more patients with TKR in the ITT analysis. Therefore, the risk of selection bias seems very limited. Other aspects, such as tolerance, have not been considered in this new analysis.
Conclusions
Due to its large cohort size (more than 1000 patients), this long-term study allowed a detailed analysis of all KL grades versus baseline. As key results obtained here with the product Arthrum H 2% (3 injections regimen), the variation from baseline was clinically relevant at Month 6 and Month 9 for the WOMAC A (pain subscore) and for the WOMAC C (function subscore) at any KL grade. The rate of OMERACT-OARSI responders at Month 6 and Month 9 was high—from 72% to 82% for each KL grade from I to III. Gender, overweight, obesity, and age were not identified as prognostic factors of response to VS. Only KL grade was a sensitive factor, particularly with KL grade IV, which was relatively unfavorable. Globally, this study suggests that VS with Arthrum H 2% applies to a large diversity of patients with knee OA.
Acknowledgments
Acknowledgments
P. Vincent initiated the secondary analysis and participated actively at each step of the redaction of the article. T. Lucas de Couville participated to the secondary analysis, and to the redaction of the article. T. Thomas participated to the interpretation of the data, and to the conception and redaction of the article. The authors thank Jo-Ann Elicia West, MSc, an independent contractor in Cartigny L'Epinay, France, for providing editorial support, which was funded by LCA Pharmaceutical, Chartres, France, in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Conflicts of Interest
The original study and this new analysis of the study database were entirely sponsored by LCA Pharmaceutical, Chartres, France. P. Vincent and T. Lucas de Couville are employees of LCA Pharmaceutical. P. Vincent is also shareholder of LCA Pharmaceutical. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2020.100575.
Appendix. Supplementary materials
References
- 1.Institut de Recherche et de Documentation en Economie de Santé (IRDES): Premiers résultats de l'Enquête Santé Protection sociale2006 – http://www.irdes.fr/presse/communiques/DossierPresseESPS2006.pdf.
- 2.Richette P. Viscosupplémentation au genou – hyaluronan for knee osteoarthritis. Revue du rhumatisme monographies. 2016;83:158–161. [Google Scholar]
- 3.Maheu E., Rannou F., Reginster J.Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis : Evidence from real-life setting trials and surveys. Seminars in Arthritis and Rheumatism. 2016;45:528–533. doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Arramon J.Y., Bergamasco P., Blasco G., Vincent P. Intra-articular sodium hyaluronate (ARTHRUM H 2.0%) in the treatment of patients with knee osteoarthritis: a multi-centric, randomized, comparative trial. Minerva Ortopedica e Traumatologica. 2008;59(2):69–79. [Google Scholar]
- 5.Germonville T., Prudat M., Vincent P. Pain Care in Knee Osteoarthritis by Intra-Articular injections of Hyaluronic Acid (ARTHRUMⓇ H 2.0 %). A Randomized double-blind Controlled Trial versus another Hyaluronic Acid (HYALGANⓇ) Minerva Ortopedica e Traumatologica. 2015;66(6):235–253. [Google Scholar]
- 6.Thomas T., Amouroux F., Vincent P. Intra articular hyaluronic acid in the management of knee osteoarthritis: Pharmaco-economic study from the perspective of the national health insurance system. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0173683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilliquin P. Mesure de l'efficacité en vie réelle de trois injections intra-articulaires du produit de santé ARTHRUM H 2% chez les sujets de moins de 60 ans atteints de gonarthrose. Réflexions Rhumatologiques. 2017;195(21):30–32. [Google Scholar]
- 8.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with of the hip or knee. J Rheumatol. 1988;15(12):1833–1844. [PubMed] [Google Scholar]
- 9.Kellgren J.H., Lawrence J.S. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham T., Van der Heidje D., Altman R.D., Anderson J.J., Bellamy N., Hochberg M. OMERACT-OARSI Initiative: Osteoarthritis Research Society International set of responder criteria for clinical trials revisited. Osteoarthritis and Cartilage. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Angst F., Aeschlimann A., Stucki G. Smallest Detectable and Minimal Clinically Important Differences for rehabilitation intervention with their implication for required sample sizes unsing WOMAC and SF-36 Quality of Life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Care & Research. 2001;45:384–391. doi: 10.1002/1529-0131(200108)45:4%3C384::AID-ART352%3E3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Ehrich E.W., Davies G.M., Watson D.J., Bolognese J.A., Seidenberg B.C., Bellamy N. Minimal Perceptible Clinical Improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessment in patients with osteoarthritis. J Rheumatol. 2000 Nov;27(11):2635–2641. [PubMed] [Google Scholar]
- 13.Miller L.E., Block J.E. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthitis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tubach F., Ravaud P., Baron G., Falissard B., Logeart I., Bellamy N., Bombardier C., Felson D., Hochberg M., Van der Heije D., Dougados M. Evaluation of clinically relevant changes in patients reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron D., Flin C., Porterie J., Despaux J., Vincent P. Hyaluronic acid single injection in knee osteoarthritis : a multi-center open prospective study (ART-ONE 75) with placebo post-hoc comparison. Current Therapeutic Research. 2018;88C:35–46. doi: 10.1016/j.curtheres.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco F.J., Fernandez-Sueiro J.L., Pinto-Tasente J.A., Fernandez-Lopez J.C., Ramallal M., Freire A., Galdo F. Intra-articular hyaluronan treatment of patients with knee osteoarthritis waiting for replacement surgery. The Open Arthritis Journal. 2008;1:1–7. (Bentham Open) [Google Scholar]
- 17.Vincent P. Intra-articular (IA) hyaluronic acid (HA) in the symptomatic treatment of knee osteoarthritis (OA): a meta-analysis of single injection products (IA HA mono-injections) Current Therapeutic Research. March 2019 doi: 10.1016/j.curtheres.2019.02.003. available online 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitively include hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review – Seminars in Arthritis and Rheumatism, 48(4):563–572. doi: 10.1016/j.semarthrit.2018.06.002. [DOI] [PubMed]
- 19.Hilliquin P. Facteurs prédictifs de succès ou d’échec de la viscosupplémentation. Réflexions Rhumatologiques. 2017;188(21):43–45. [Google Scholar]
- 20.Altman R.D., Devji T., Bhandari M., Fierlinger A., Niazi F., Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Seminars in Arthritis and Rheumatism. 2016;46:151–159. doi: 10.1016/j.semarthrit.2016.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.