Abstract
Pre-term birth is a major health concern that occurs in approximately 10% of births worldwide. Despite high incidence rate, long-term consequences of pre-term birth remain unclear. Recent evidence suggests that elevated oxidative stress observed in pre-term born infants could persist into adulthood. Given that oxidative stress is known to play an important role in response to physical activity and hypoxia, we investigated whether oxidative stress responses to acute exercise in normoxia and hypoxia may be differently modulated in pre-term vs. full-term born adults. Twenty-two pre-term born and fifteen age-matched full-term born controls performed maximal incremental cycling tests in both normoxia (FiO2: 0.21) and normobaric hypoxia (FiO2: 0.13; simulated altitude of 3800 m) in blinded and randomized manner. Plasma levels of oxidative stress (advanced oxidation protein products [AOPP] and malondialdehyde), antioxidant (ferric reducing antioxidant power, glutathione peroxidase, catalase [CAT] and superoxide dismutase [SOD]) and nitrosative stress markers (nitrotyrosine, nitrite and total nitrite and nitrate [NOx]) were measured before and immediately after each test. AOPP (+24%, P<0.001), CAT (+38%, P<0.001) and SOD (+12%, P=0.018) and NOx (+17%, P=0.024) significantly increased in response to exercise independently of condition and birth status. No difference in response to acute exercise in normoxia was noted between pre-term and full-term born adults in any of measured markers. Hypoxic exposure during exercise resulted in significant increase in AOPP (+45%, P=0.008), CAT (+55%, P=0.019) and a trend for an increase in nitrite/nitrate content (+35%, P=0.107) only in full-term and not pre-term born individuals. These results suggest that prematurely born adult individuals exhibit higher resistance to oxidative stress response to exercise in hypoxia.
Keywords: Altitude, Exercise, Nitrosative stress, Normobaric hypoxia, Oxidative stress, Prematurity
Graphical abstract
Highlights
-
•
Oxidative stress and antioxidant activity are increased in full-term and pre-term adults following normoxic exercise.
-
•
Plasma AOPP, catalase and NOx levels are not increased in pre-term adults following an acute exercise in hypoxia.
-
•
Oxidative stress is differently regulated in pre-term adults during acute physical exercise under hypoxic condition.
-
•
Pre-term birth may have long term consequences concerning oxidative stress regulation especially during these conditions.
Abbreviations list
- AMS
acute mountain sickness
- AOPP
advanced oxidation protein products
- CAT
catalase
- DAN
2,3-diaminonaphtalene
- dSpO2
arterial blood desaturation
- dVE
ventilation variation
- FRAP
ferric reducing antioxidant power
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- HVR
hypoxic ventilatory response
- H2O2
hydrogen peroxide
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate hydrogen
- NOx
total nitrite and nitrate
- NTB
nitrobluetretrazolium
- PTB
pre-term birth
- O2•
superoxide anion
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SpO2
capillary oxygen saturation
- TBA
2-thiobarbituric
- VE
ventilation
- VO2
oxygen uptake
1. Introduction
Pre-term birth (PTB), defined as birth occurring prior to the 37th week of gestation, represents approximately 10% of the births worldwide [1] and is among the leading causes of death for the under-5 year olds [2]. However, continuous progress in neonatology during the last 50 years resulted in significantly greater survival rates [3], consequently leading to increased pre-term born population. A growing body of literature describes the long-term consequences of PTBs [4,5]. In particular, many of the physiological mechanisms linked to the detrimental consequences of PTB could be associated with elevated oxidative stress levels [6]. However, surprisingly only one study to date investigated the oxidative stress levels in adolescents (13–15 years) born very pre-term [7] and no data exists on the oxidative stress modulation in adults pre-term born [8].
Oxidative stress is defined as an imbalance between the generation of oxidant species, also termed reactive oxygen species (ROS), and antioxidants in favor of the former [9]. It is well established that many external factors can modulate oxidative stress level including physical exercise [10,11] and hypoxia [11,12]. In addition, the combination of both hypoxia and acute exercise is also known to have cumulative augmentative effect on oxidative stress [11,[13], [14], [15], [16]]. However, the deleterious outcomes related to hypoxia-induced increased oxidative stress remain controversial and might be limited to extreme altitude [16] or to pathological conditions [17]. Nevertheless, the oxidative stress responses to both, exercise [10] and hypoxia [18] seem to importantly modulate the physiological adaptation of humans to these two stimuli.
Several studies reported reduced physical capacity in normoxia in adults born pre-term compared to those born full-term [[19], [20], [21], [22], [23]]. Additionally, it has been shown that at rest, hypoxic ventilatory response (HVR), defined as the ability to change ventilation in function of blood oxygen saturation, was lower in adults born pre-term than in adults born full-term [24]. However, to our knowledge, only one study investigated physical performance under hypoxia in pre-term and reported no difference with adults born full-term [23]. Moreover, because of the previously reported higher oxidative stress levels in adults born pre-term [7] and the above mentioned importance of oxidative stress adaptation to chronic exercise and hypoxia, the interaction between these two factors warrants scrutiny.
Accordingly, we aimed to investigate the oxidative stress responses to acute exercise in normoxia and normobaric hypoxia in individuals born pre-term as compared to age and aerobic capacity matched controls born at full-term.
2. Material and methods
2.1. Participants
Collectively, thirty-seven healthy men volunteered and gave written informed consent to participate in this study. All participants were free of cardiorespiratory and haematological diseases and were not exposed to altitudes above 1500 m during the one-month period prior to the study. The baseline characteristics of the participants are outlined in Table 1. Twenty-two participants were born pre-term and 15 were born full-term. The experimental protocol was approved by the National Medical Ethics Committee of Slovenia and performed in accordance with the principles of the Declaration of Helsinki. The study was also pre-registered at ClinicalTrials.gov (NCT02780908) (https://clinicaltrials.gov/ct2/show/NCT02780908?term=NCT02780908&rank=1).
Table 1.
Baseline characteristics of the participants.
| Variable | Full-term (n = 15) |
Pre-term (n = 22) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 22 | 2 | 21 | 2 |
| Body mass (kg) | 73 | 6 | 69 | 7 |
| Height (cm) | 180 | 5 | 175*** | 7 |
| BMI (kg.m-2) | 23 | 2 | 23 | 3 |
| Gestational age (weeks) | 39 | 2 | 29*** | 3 |
| O2max (mL.kg-1.min-1) | 52 | 5 | 48 | 6 |
SD, standard deviation; BMI, body mass index; O2max, maximal oxygen consumption. ***P<0.0001 vs term.
2.2. Incremental exercise tests
The participants performed two graded exercise test on an electromagnetically braked cycle-ergometer (Ergo Bike Premium, Daum electronics, Fürth, Germany) under normoxic (FiO2=0.21; PiO2=147mmHg) and normobaric hypoxic (FiO2=0.13; PiO2=91mmHg) conditions in a randomized manner. They were blinded in regards to FiO2 of the gas mixture they were inspiring on both occasions. Both tests were performed at the same time of the day for each individual. The test protocol started at 60W with an increase of 40W every 2 min until volitional exhaustion. The normoxic and hypoxic tests were separated by exactly 7 days.
Throughout the tests the participants breathed through a facemask (Vmask, 7500 series, Hans Rudolph Inc., Shawnee, USA) and oxygen uptake (VO2) and ventilation (VE) were measured using a metabolic cart (Quark CPET, Cosmed, Rome, Italy). Capillary oxygen saturation (SpO2) was measured using a transcutaneous finger pulse oximetry device (Nellcor, BCI 3301, Boulder, USA).
2.3. Physiological response measurement
The methodological details of the cardiorespiratory measurements during both, normoxic and hypoxic incremental exercise tests are detailed elsewhere [25]. Arterial blood desaturation (dSpO2) was calculated as the difference between SpO2 measured at volitional exhaustion during the incremental test and the SpO2 at rest before beginning the test. The ventilation variation (dVE) was defined as the difference between the VE measured at volitional exhaustion during the incremental test and the VE at rest before beginning the test. HVR was computed as where represents the difference between ventilation at exhaustion in hypoxia and ventilation at rest in normoxia and represents the difference between SpO2 at exhaustion in hypoxia and SpO2 at rest in normoxia [26,27].
2.4. Blood sampling
Six mL of venous blood were obtained from the antecubital vein of the seated participants before and within 1 min following the incremental exercise. Blood samples were drawn into ethylenediaminetetraacetic acid blood collection tubes, centrifuged (10min at 3500rpm, 4 °C) and the plasma was aliquoted into three 1.5 mL cryotubes, which were immediately frozen to -20 °C and to -80 °C.
2.5. Biochemical analysis
All spectrophotometry and fluorometry measurements were performed with TECAN Infinite 2000 plate reader (Männedorf, Switzerland) as previously reported [28].
2.6. Oxidative stress markers
Advanced oxidation protein products (AOPP) levels were measured via spectrophotometry by reading at 340 nm 40 μL of plasma diluted in 200 μL of PBS 1X and with 20 μL of acetic acid (99–100%) in 96 well microtest plates. AOPP level was computed using chloramine-T standard solution, which absorb at 340 nm in presence of potassium iodide.
Malondialdehyde (MDA) level was determined by adding NaOH, 2-thiobarbituric acid (TBA) and HCl solutions to 50 μL of plasma. After 1 h at 100 °C, MDA form a complex with TBA that can be measured by spectrophotometry in 96 well microtest plates at 532 nm. MDA level was computed using 1,1,3,3-tetraethoxypropane as standard.
2.7. Antioxidant enzymes
Catalase activity was determined by measuring the kinetics of formaldehyde apparition formed by the reaction between methanol and hydrogen peroxide (H2O2), which is catalyzed by catalase. 30 μL of methanol (100%) and 20 μL of H2O2 solution (0.14%) were added to 20 μL of plasma diluted in 100 μL of PBS 1X in 96 well microtest plates. Twenty minutes later the reaction was stopped by adding 30 μL of potassium hydroxide solution (10.69mol·L-1). Formaldehyde was revealed by adding 30 μL of purpald solution (0.20mol·L-1) and its concentration was measured 5 min later by spectrophotometry at 540 nm and computed using formaldehyde standards.
Glutathione peroxidase (GPx) activity was assessed by measuring nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) consumption, which is proportional to GPx activity to reduce H2O2 in presence of glutathione reductase and reduced glutathione. Glutathione reductase, NADPH (10 mmol·L-1) and reduced glutathione solutions were added to 20 μL of plasma diluted in 200 μL of PBS 1X in 96 well microtest plates. 30 μL of H2O2 solution was then added and NADPH oxidation into NAD+ was measured during 5 min by spectrophotometry at 340 nm.
Superoxide dismutase (SOD) activity measurement was based on the higher affinity of SOD to react with superoxide anion (O2•-) than nitrobluetretrazolium (NTB), which produce detectable blue formazan. 250 μL of a solution containing NTB, trizmahydrochloride, diethylenetriaminepentaacetic acid and hypoxanthine is added to 20 μL of plasma in 96 well microtest plates. Then, 20 μL of xanthine oxidase (1.02U·mL-1) were added and react with hypoxantine to produce O2•-. Appearance of blue formazan is measured by spectrophotometry at 560 nm during 5 min. SOD activity is computed by subtracting the rate of blue formazan appearance with deproteinized plasma (blank) to those with plasma sample.
2.8. Ferric reducing antioxidant power
Ferric reducing antioxidant power (FRAP) was determined by measuring the ability of the plasma to reduce ferric into ferrous iron. 17 μL of ferric chloride solution (20 mmol·L-1), 17 μL of tripyridyltriazine solution (8 mmol·L-1) and 167 μL of acetate solution (300 mmol·L-1) were added to 20 μL of plasma diluted in 40 μL of H2O in 96 well microtest plates. Ferrous iron forms a complex with tripyridyltriazine at low pH that can be measured by spectrophotometry at 593 nm and computed with ferrous iron solution (FeSO4-7H2O) standards.
2.9. Metabolites of NO
Nitrite levels were detected by using 2,3-diaminonaphtalene (DAN) that fixes nitrite and emits at 450 nm after an excitation at 365nm. 18 μL of DAN solution containing DAN and HCl was added to 10 μL of plasma diluted in 90 μL of H2O in 96 well microtest plates. 10 min later, the reaction was stopped with 18 μL oh NaOH solution. Nitrite level was measured by fluorometry (excitation at 365 nm and emission at450 nm) and computed with NO2 standards.
To measure total nitrite and nitrate (NOx) level, nitrate was reduced into nitrite and nitrite was then measured as described above. 40 μL of nitrate reductase solution was added to 10 μL of plasma in 96 well microplates and 15 min later 50 μL of H2O was added. Then nitrite level is then determined as described above.
2.10. Statistics
R (version 3.3.2) with nlme [29] and multcomp [30] packages was used to perform linear mixed effects analyses with condition (normoxia vs. hypoxia), time (pre vs. post exercise) and birth (full-term vs. pre-term) and their interactions as fixed effects and a random effect for participants after checking for normality and homoscedasticity. Significance level was set a priori at P<0.05 and a tendency was set at P<0.1.
3. Results
3.1. Exercise cardio-respiratory responses
The general cardio-respiratory responses to normoxic and hypoxic exercise are detailed elsewhere [25] but are summarized in Table 2 for the convenience of the reader. Expectedly, dSpO2 was significantly higher during acute exercise in hypoxia than in normoxia independently of birth (3.5% during normoxic exercise vs. 21.6% during hypoxic exercise; pooled data; P<0.0001) but dSpO2 was lower in participants born pre-term than full-term (-22%, P<0.01) independently of the condition. Furthermore, dVE during an acute exercise was greater in hypoxia than in normoxia independently of birth (+11%, P<0.05) and tended to be lower in participants born pre-term than full-term in normoxia and hypoxia (P=0.09). However, there was no difference in exercise HVR between the full-term and pre-term born participants.
Table 2.
Physiological response to graded exercise in normoxia and hypoxia in adults born full-term or pre-term.
| Birth |
Full-term (n = 15) |
Pre-term (n = 22) |
P values |
||||
|---|---|---|---|---|---|---|---|
| Condition | Normoxia | Hypoxia | Normoxia | Hypoxia | Hypoxia | Birth | Birthhypoxia |
| O2peak (mL.kg-1.min-1) | 52 (5) | 45 (4) | 48 (6) | 42 (7) | <0.0001 | 0.08 | NS |
| PPO (W) | 322 (33) | 273 (28) | 272 (39) | 235 (36) | <0.0001 | 0.008 | 0.01 |
| dSpO2 (%) | 5.64 (4.14) | 23.71 (4.75) | 2.29 (2.69) | 21 (5.18) | <0.0001 | 0.006 | NS |
| dVE (L.min-1) | 110.1 (25.1) | 124.9 (26.2) | 99.5 (28.4) | 107.3 (31.2) | 0.049 | 0.09 | NS |
| HVR (L.min-1.%-1) | 5.44 (1.80) | 5.55 (1.98) | NS | ||||
Values are presented as means (SD). Abbreviations: O2peak, peak oxygen consumption; PPO, peak power output dSpO2, arterial blood oxygen desaturation; dVE, ventilation variation; HVR, hypoxic ventilatory response; NS, not significant. These results are detailed elsewhere [25].
3.2. Oxidative stress responses to exercise
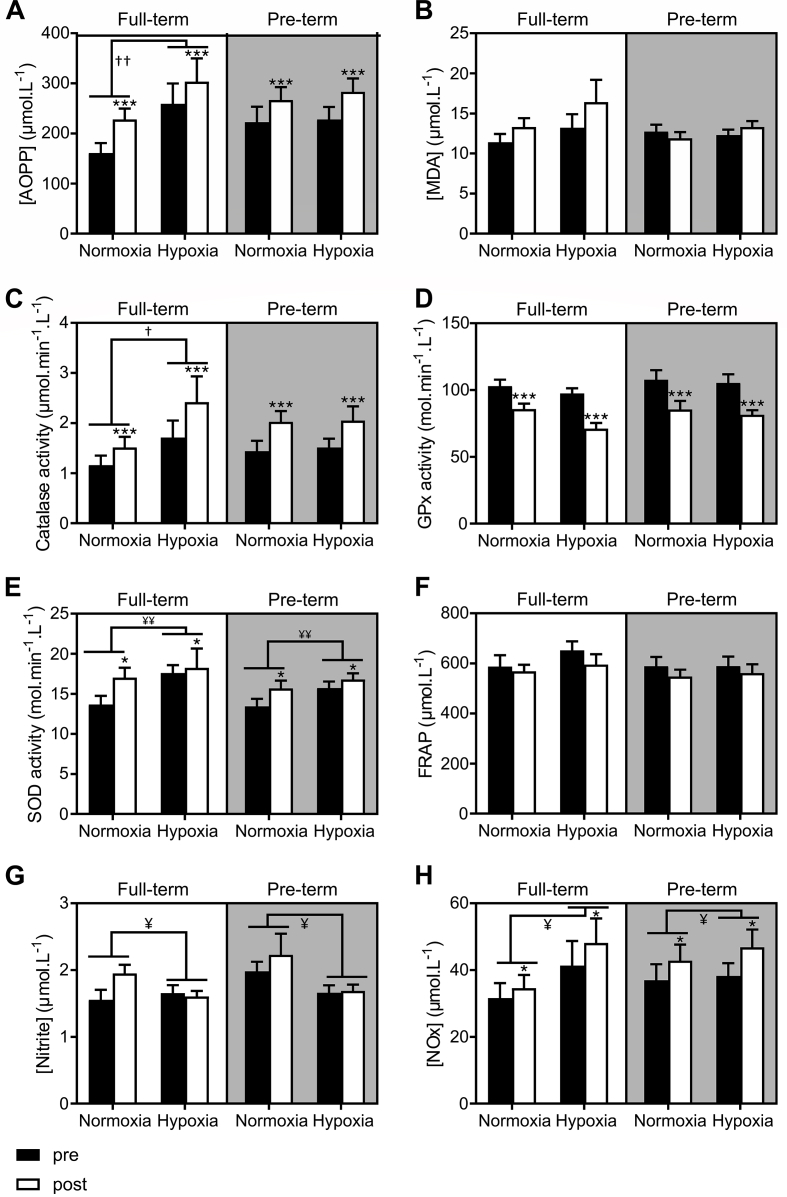
The responses of the measured oxidative stress and antioxidant markers to exercise are detailed in Table 3 and Fig. 1. While plasma AOPP significantly increased in response to exercise independently of the condition and/or birth status (+24%, P<0.001), the levels of MDA remained constant (P=0.182). Similarly, the catalase and SOD activity significantly increased in response to exercise regardless of birth and condition (+38%, P<0.001 and + 12%, P=0.018 respectively for catalase and SOD) while GPx activity significantly decreased following exercise (-22%, P<0.001). FRAP tended to decrease following exercise independently of the condition and birth status (P=0.087). Acute exercise also induced significantly increased NOx levels in both groups (+17%, P=0.024) but did not change the levels of nitrite.
Table 3.
P values for the main effects of condition (normoxia or hypoxia), birth (full-term or pre-term) and exercise (pre or post), and their interactions for oxidative or nitrosative stress plasma level.
| Effect | Oxidative stress markers | Antioxidant enzymes | NO metabolism markers | |||||
| AOPP | MDA | CAT | GPx | SOD | FRAP | Nitrite | NOx | |
| Condition | 0.003a | 0.115 | 0.024a | 0.091 | 0.007a | 0.278 | 0.015a | 0.023a |
| Birth | 0.735 | 0.319 | 0.854 | 0.278 | 0.237 | 0.449 | 0.119 | 0.687 |
| Exercise | <0.001a | 0.182 | <0.001a | <0.001a | 0.018a | 0.087 | 0.221 | 0.024a |
| Conditionbirth | 0.008a | 0.234 | 0.019a | 0.346 | 0.564 | 0.367 | 0.218 | 0.107 |
| Conditionexercise | 0.919 | 0.318 | 0.679 | 0.519 | 0.230 | 0.861 | 0.208 | 0.556 |
| Birthexercise | 0.832 | 0.140 | 0.912 | 0.858 | 0.823 | 0.937 | 0.898 | 0.663 |
| Conditionbirthexercise | 0.551 | 0.872 | 0.483 | 0.590 | 0.602 | 0.551 | 0.653 | 0.937 |
AOPP, advanced oxidation protein products; CAT, catalase activity; FRAP, ferric reducing antioxidant power; GPx, glutathione peroxidase activity; NOx, total nitrite and nitrate; SOD, superoxide dismutase activity.
Significant effects or interactions.
Fig. 1.
AOPP (A), MDA (B), catalase activity (C), GPx (D), SOD activity (E), FRAP (F), nitrite (G) and NOx (H) levels before (black columns) and immediately following exercise (white columns) under normoxic and hypoxic conditions in the full-term (n = 15) and pre-term born adults (n = 22). Data are expressed as means ± SEM. AOPP, advanced oxidation protein products; FRAP, ferric reducing antioxidant power; GPx, glutathione peroxidase; NOx, total nitrite and nitrate; pre, pre-exercise; post, post-exercise; SOD, superoxide dismutase.. Differences pre vs. post exercise independently of birth and hypoxic/normoxic conditions: ***P<0.001, **P<0.01, *P<0.05. Differences hypoxia vs. normoxia independently of exercise (pre/post): ††P<0.01, †P<0.05. Differences hypoxia vs. normoxia independently of exercise (pre/post) and birth: ¥ P<0.05, ¥¥ P<0.01.
3.3. Oxidative stress responses to hypoxia
The responses of antioxidant and oxidative stress markers to hypoxic exercise are detailed in Table 3 and Fig. 1. Plasma AOPP levels increased significantly in response to hypoxia only in the full-term born individuals (+45% in full-term vs +5% in pre-term, P=0.008) independently to exercise condition (i.e. exercise or rest) (Fig. 1A). However, no significant differences between MDA and FRAP responses to normoxic and hypoxic exercise were noted between full-term and pre-term born individuals. While the activity of SOD increased significantly in hypoxia compared to normoxia independently of birth and exercise condition (+14%, P=0.007), the catalase activity was only increased in hypoxia in the full-term born participants (+55% in full-term vs +3% in pre-term, P=0.019) independently to exercise condition (i.e. exercise or rest) (Fig. 1C). The GPx levels tended to decrease in response to hypoxia in both groups (P=0.091). Plasma nitrite levels were significantly lower in hypoxia than in normoxia (-16%, P=0.015) independently of birth and exercise condition (i.e. exercise or rest) whereas hypoxia-induced increase in NOx levels trend to be only noted in the full-term born individuals (+35% in full-term vs +5% in pre-term, P=0.107) (Fig. 1H).
4. Discussion
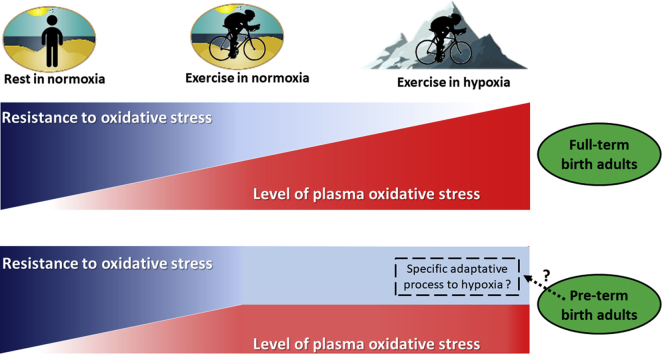
The present study aimed to investigate the potential differential oxidative and nitrosative stress responses to acute exercise in normoxia and hypoxia in individuals born pre-term as compared to matched controls born at full-term. Our data show a similar exercise-induced increase in oxidative stress and antioxidant activity in pre-term and full-term. In addition, and in contrast to full-term born adults, the plasma AOPP, catalase and NOx responses to hypoxia does not increase compared to normoxic conditions in adults born prematurely and suggest higher resistance to oxidative stress in this population.
4.1. Oxidative stress responses to exercise
As already demonstrated in adults born full-term [10,11], acute exercise resulted in increased oxidative stress markers and antioxidant enzymes activity in the pre-term born individuals. This is most likely due to exercise-induced ROS overproduction [10]. Independently of birth, we found that acute exercise augmented the plasma levels of AOPP, while no significant change was noted in MDA. The changes in MDA levels in response to acute graded exercise remains controversial [31]. Indeed, some studies reported increased level of plasma MDA in similar condition [13,[32], [33], [34], [35]] while others did not find any significant changes [14,[36], [37], [38]]. The observed non-significant increase in MDA (+9%, P=0.182) could be explained by the reported moderate sensibility and specificity of the employed MDA assay [39]. The catalase and SOD activity increased in response to exercise independently of birth, confirming the previously documented exercise-related upregulation of antioxidant enzymes activity [40]. However, in contrast to some studies [40,41], we observed a decreased GPx activity in response to exercise regardless of the birth status. Nevertheless, this could be explained by the fact that reduced glutathione (GSH) is a limiting substrate for GPx activity and that lower GSH content in erythrocytes in response to acute exercise has been shown [42]. Since no effect of prematurity has been observed in response to acute exercise regarding oxidative stress markers, further investigations are clearly warranted to determine whether adults born pre-term may also have the same oxidative stress responses than adults born full-term after prolonged, chronic or intermittent exercise training.
4.2. Oxidative stress responses to hypoxia
The additive effects of hypoxia during exercise are clearly reflected in the increased AOPP levels observed in the full-term born individuals. These findings also support a growing body of literature indicating that acute hypoxic exposure augments oxidative stress [13,14,28]. Interestingly, we failed to observe a concomitant significant increase in MDA (+10%, P=0.115) although differences between lipid and protein oxidation in response to hypoxia has been previously observed [14]. The observed higher SOD, catalase activities and NOx levels in hypoxia also lend further support to the hypothesis that both ROS production and antioxidant activity are increased under hypoxic conditions [11].
However, in contrast to full-term born individuals, the pre-term born group did not display the above noted AOPP increase (see Fig. 1). This suggests a beneficial adaptation to hypoxia at least on the redox balance level. This is further confirmed by the lack of catalase activity increase in response to hypoxia in adults born pre-term, even though an increase was noted in the full-term born group. In contrast to SOD and catalase, GPx activity was decreased regardless of the birth status. The response of GPx to hypoxic exercise could potentially be explained by a lower GSH content also reported in erythrocytes in response to altitude exposure [42]. Interestingly, we found that hypoxia-related NOx levels increase trend to occur only in full-term born individuals (+35% vs. +7% in participants born pre-term). This observation suggests that, like oxidative stress, the NO metabolism response to hypoxia may also be blunted in the pre-term born adult individuals.
The observed blunted oxidative stress and NO responses to hypoxia in the pre-term born individuals are quite surprising. Indeed, it has been shown that, the resting levels of 8-isoprostane are higher in exhaled breath condensate of adolescents (13–15 years) born very pre-term compared to those born at full-term [7] suggesting that a higher oxidative stress level could persist at adulthood in people born pre-term [8]. However, no significant differences were observed between the two groups in baseline oxidative stress markers. Nevertheless, and although we hypothesized that an exacerbated oxidative stress in response to both, hypoxia and graded exercise would be noted in the pre-term born individuals, it seems that prematurely born adults developed adaptive mechanisms to face up to high oxygen variations early in life, known to generate high amount of ROS, that may limit oxidative stress and NO metabolism to hypoxia. Whether this greater resistance to oxidative stress response to hypoxia could be beneficial or detrimental adaptive mechanism in the pre-term born adults needs to be determined.
4.3. Exercise cardio-respiratory responses
While Bates et al. observed a reduced HVR at rest in adults born pre-term [24], we confirmed their findings at rest [25] but not during exercise. This could mean that the stimulus of an incremental exercise on ventilation can outmatch/override the potential effect of hypoxia in the participants born pre-term. In any case, the blunted HVR at rest in participants born pre-term could be linked with the lower oxidative stress in response to hypoxia in participants born pre-term since a positive correlation between that changes in oxidative stress and HVR has previously been reported [43,44]. However, ventilatory response to hypoxia has also been related to the risk of altitude illness but with very limited sensitivity and specificity [27]. Interestingly, there seems to be no epidemiologic data on the incidence of acute mountain sickness (AMS) in the population born pre-term. Given the established influence and contribution of oxidative stress to AMS [45], our data provide strong impetus for future investigations on the AMS modulation in adults born pre-term since lower hypoxia-induced oxidative stress response was noted in this population. We should however be cautious regarding the direct translation of the present results to real high-altitude scenarios (hypobaric hypoxia) since we previously demonstrated that normobaric hypoxic exposure (i.e. simulated altitude) results in a lower oxidative stress increase than exposure to hypobaric hypoxia [28,46]. Nevertheless, since both groups were exposed to the same, normobaric hypoxic condition the observed difference between the two groups in oxidative stress responses provide important new insight into the consequence of prematurity.
5. Conclusions
In conclusion, similarly to the full-term born adults, the pre-term individuals displayed an increased oxidative stress, antioxidant activity and NO metabolism in response to acute exercise. However, and in contrast to the full-term born individuals, we report for the first time that hypoxia did not increase plasma AOPP, catalase and NOx levels in the pre-term born cohort suggesting that they developed adaptive processes that lead to greater resistance to oxidative stress in response to acute hypoxic exposure. Although we did not find significant difference in the exercise HRV in adults born pre-term, mechanisms regarding ventilatory and oxidative stress modulation in the pre-term born individuals warrants further scrutiny to expand our understanding.
Funding information
This study was funded by Slovene Research Agency (Grant No. J3-7536) and Ljubljana University Medical Centre (Grant No-TP20140088) grants.
Author contribution statement
GM, DO, TD and VP participated in design the study; AM, MM, TD and VN performed the experiments; MM, DO and TD included the patients; AM, GM, CF, TD and VP analyzed and interpreted the results; AM, TD and VP wrote the manuscript; AM, EG, TD and VP revised the manuscript; AM, EG, GM, DO, MM, CF, TD and VP reviewed manuscript drafts and edited the manuscript.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We would like to thank Quentin Barge, Hugo Catois and Elise Madeuf for their help with the assays. We would also like to acknowledge Mr. Miro Vrhovec for his indispensable technical assistance throughout the study. This work was funded by Slovene Research Agency (Grant No. J3-7536) and Ljubljana University Medical Centre (Grant Nr-TP20140088).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101497.
Contributor Information
Agnès Martin, Email: agnes.martin@ens-lyon.org.
Grégoire Millet, Email: gregoire.millet@unil.ch.
Damjan Osredkar, Email: damjan.osredkar@kclj.si.
Minca Mramor, Email: minca.mramor@ukclj.si.
Camille Faes, Email: camille.faes@univ-lyon1.fr.
Etienne Gouraud, Email: etienne.gouraud@univ-lyon1.fr.
Tadej Debevec, Email: tadej.debevec@fsp.uni-lj.si.
Vincent Pialoux, Email: vincent.pialoux@univ-lyon1.fr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.-B., Narwal R., Adler A., Vera Garcia C., Rohde S., Say L., Lawn J.E. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 3.Manley B.J., Doyle L.W., Davies M.W., Davis P.G. Fifty years in neonatology. J. Paediatr. Child Health. 2015;51:118–121. doi: 10.1111/jpc.12798. [DOI] [PubMed] [Google Scholar]
- 4.Raju T.N.K., Pemberton V.L., Saigal S., Blaisdell C.J., Moxey-Mims M., Buist S. Adults born preterm conference speakers and discussants, long-term healthcare outcomes of preterm birth: an executive summary of a conference sponsored by the national institutes of health. J. Pediatr. 2017;181:309–318.e1. doi: 10.1016/j.jpeds.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Raju T.N.K., Buist A.S., Blaisdell C.J., Moxey-Mims M., Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017;106:1409–1437. doi: 10.1111/apa.13880. [DOI] [PubMed] [Google Scholar]
- 6.Luo Z.C., Fraser W.D., Julien P., Deal C.L., Audibert F., Smith G.N., Xiong X., Walker M. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med. Hypotheses. 2006;66:38–44. doi: 10.1016/j.mehy.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Filippone M., Bonetto G., Corradi M., Frigo A.C., Baraldi E. Evidence of unexpected oxidative stress in airways of adolescents born very pre-term. Eur. Respir. J. 2012;40:1253–1259. doi: 10.1183/09031936.00185511. [DOI] [PubMed] [Google Scholar]
- 8.Martin A., Faes C., Debevec T., Rytz C., Millet G., Pialoux V. Preterm birth and oxidative stress: effects of acute physical exercise and hypoxia physiological responses. Redox Biology. 2018;17:315–322. doi: 10.1016/j.redox.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Powers S.K., Nelson W.B., Hudson M.B. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic. Biol. Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Debevec T., Millet G.P., Pialoux V. Hypoxia-induced oxidative stress modulation with physical activity. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magalhães J., Ascensão A., Viscor G., Soares J., Oliveira J., Marques F., Duarte J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat Space Environ. Med. 2004;75:16–22. [PubMed] [Google Scholar]
- 13.Bailey D.M., Davies B., Young I.S. Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin. Sci. 2001;101:465–475. [PubMed] [Google Scholar]
- 14.Pialoux V., Mounier R., Ponsot E., Rock E., Mazur A., Dufour S., Richard R., Richalet J.-P., Coudert J., Fellmann N. Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur. J. Clin. Nutr. 2006;60:1345–1354. doi: 10.1038/sj.ejcn.1602462. [DOI] [PubMed] [Google Scholar]
- 15.Woodside J.D.S., Gutowski M., Fall L., James P.E., McEneny J., Young I.S., Ogoh S., Bailey D.M. Systemic oxidative–nitrosative–inflammatory stress during acute exercise in hypoxia; implications for microvascular oxygenation and aerobic capacity. Exp. Physiol. 2014;99:1648–1662. doi: 10.1113/expphysiol.2014.081265. [DOI] [PubMed] [Google Scholar]
- 16.Quindry J., Dumke C., Slivka D., Ruby B. Impact of extreme exercise at high altitude on oxidative stress in humans. J. Physiol. 2016;594:5093–5104. doi: 10.1113/JP270651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey D.M., Rimoldi S.F., Rexhaj E., Pratali L., Salinas Salmòn C., Villena M., McEneny J., Young I.S., Nicod P., Allemann Y., Scherrer U., Sartori C. Oxidative-nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest. 2013;143:444–451. doi: 10.1378/chest.12-0728. [DOI] [PubMed] [Google Scholar]
- 18.Pialoux V., Mounier R., Brown A.D., Steinback C.D., Rawling J.M., Poulin M.J. Relationship between oxidative stress and HIF-1α mRNA during sustained hypoxia in humans. Free Radic. Biol. Med. 2009;46:321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Vrijlandt E.J.L.E., Gerritsen J., Boezen H.M., Grevink R.G., Duiverman E.J. Lung function and exercise capacity in young adults born prematurely. Am. J. Respir. Crit. Care Med. 2006;173:890–896. doi: 10.1164/rccm.200507-1140OC. [DOI] [PubMed] [Google Scholar]
- 20.Svedenkrans J., Henckel E., Kowalski J., Norman M., Bohlin K. Long-term impact of preterm birth on exercise capacity in healthy young men: a national population-based cohort study. PloS One. 2013;8 doi: 10.1371/journal.pone.0080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovering A.T., Laurie S.S., Elliott J.E., Beasley K.M., Yang X., Gust C.E., Mangum T.S., Goodman R.D., Hawn J.A., Gladstone I.M. Normal pulmonary gas exchange efficiency and absence of exercise-induced arterial hypoxemia in adults with bronchopulmonary dysplasia. J. Appl. Physiol. 2013;115:1050–1056. doi: 10.1152/japplphysiol.00592.2013. [DOI] [PubMed] [Google Scholar]
- 22.Clemm H.H., Vollsæter M., Røksund O.D., Eide G.E., Markestad T., Halvorsen T. Exercise capacity after extremely preterm birth. Development from adolescence to adulthood. Annals ATS. 2014;11:537–545. doi: 10.1513/AnnalsATS.201309-311OC. [DOI] [PubMed] [Google Scholar]
- 23.Farrell E.T., Bates M.L., Pegelow D.F., Palta M., Eickhoff J.C., O'Brien M.J., Eldridge M.W. Pulmonary gas exchange and exercise capacity in adults born preterm. Ann Am Thorac Soc. 2015;12:1130–1137. doi: 10.1513/AnnalsATS.201410-470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates M.L., Farrell E.T., Eldridge M.W. Abnormal ventilatory responses in adults born prematurely. N. Engl. J. Med. 2014;370:584–585. doi: 10.1056/NEJMc1311092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debevec T., Pialoux V., Millet G.P., Martin A., Mramor M., Osredkar D. Exercise overrides blunted hypoxic ventilatory response in prematurely born men. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathat C., Richalet J.-P., Herry J.-P., Larmignat P. Detection of high-risk subjects for high altitude diseases. Int. J. Sports Med. 1992;13:S76–S78. doi: 10.1055/s-2007-1024602. [DOI] [PubMed] [Google Scholar]
- 27.Bärtsch P., Swenson E.R. Acute high-altitude illnesses. N. Engl. J. Med. 2013;368:2294–2302. doi: 10.1056/NEJMcp1214870. [DOI] [PubMed] [Google Scholar]
- 28.Faiss R., Pialoux V., Sartori C., Faes C., DéRiaz O., Millet G.P. Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia. Med. Sci. Sports Exerc. 2013;45:253–260. doi: 10.1249/MSS.0b013e31826d5aa2. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro J., Bates D., DebRoy S., Sarkar D. R Package Version; 2009. Linear and Nonlinear Mixed Effects Models. 3–1. [Google Scholar]
- 30.Hothorn T., Bretz F., Westfall P., Heiberger R.M., Schuetzenmeister A., Scheibe S., Hothorn M.T. Project for Statistical Computing; Vienna, Austria: 2016. Package ‘multcomp,’ Simultaneous Inference in General Parametric Models.http://ftp5.gwdg.de/pub/misc/cran/web/packages/multcomp/multcomp.pdf [Google Scholar]
- 31.Clarkson P.M., Thompson H.S. Antioxidants: what role do they play in physical activity and health? Am. J. Clin. Nutr. 2000;72:637s–646s. doi: 10.1093/ajcn/72.2.637S. [DOI] [PubMed] [Google Scholar]
- 32.Lovlin R., Cottle W., Pyke I., Kavanagh M., Belcastro A.N. Are indices of free radical damage related to exercise intensity. Eur. J. Appl. Physiol. Occup. Physiol. 1987;56:313–316. doi: 10.1007/BF00690898. [DOI] [PubMed] [Google Scholar]
- 33.Sumida S., Tanaka K., Kitao H., Nakadomo F. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int. J. Biochem. 1989;21:835–838. doi: 10.1016/0020-711x(89)90280-2. [DOI] [PubMed] [Google Scholar]
- 34.Ashton T., Rowlands C.C., Jones E., Young I.S., Jackson S.K., Davies B., Peters J.R. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]
- 35.Ashton T., Young I.S., Peters J.R., Jones E., Jackson S.K., Davies B., Rowlands C.C. Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J. Appl. Physiol. 1999;87:2032–2036. doi: 10.1152/jappl.1999.87.6.2032. [DOI] [PubMed] [Google Scholar]
- 36.Viinikka L., Vuori J., Ylikorkala O. Lipid peroxides, prostacyclin, and thromboxane A2 in runners during acute exercise. Med. Sci. Sports Exerc. 1984;16:275–277. [PubMed] [Google Scholar]
- 37.Leaf D.A., Kleinman M.T., Hamilton M., Barstow T.J. The effect of exercise intensity on lipid peroxidation. Med. Sci. Sports Exerc. 1997;29:1036–1039. doi: 10.1097/00005768-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Quindry J.C., Stone W.L., King J., Broeder C.E. The effects of acute exercise on neutrophils and plasma oxidative stress. Med. Sci. Sports Exerc. 2003;35:1139–1145. doi: 10.1249/01.MSS.0000074568.82597.0B. [DOI] [PubMed] [Google Scholar]
- 39.Lefèvre G., Beljean-Leymarie M., Beyerle F., Bonnefont-Rousselot D., Cristol J.P., Thérond P., Torreilles J. [Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances] Ann. Biol. Clin. (Paris). 1998;56:305–319. [PubMed] [Google Scholar]
- 40.Berzosa C., Cebrián I., Fuentes-Broto L., Gómez-Trullén E., Piedrafita E., Martínez-Ballarín E., López-Pingarrón L., Reiter R.J., García J.J. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. J. Biomed. Biotechnol. 2011:1–7. doi: 10.1155/2011/540458. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elosua R., Molina L., Fito M., Arquer A., Sanchez-Quesada J.L., Covas M.I., Ordoñez-Llanos J., Marrugat J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–334. doi: 10.1016/s0021-9150(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 42.Joanny P., Steinberg J., Robach P., Richalet J.P., Gortan C., Gardette B., Jammes Y. Operation Everest III (Comex’97): the effect of simulated severe hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation. 2001;49:307–314. doi: 10.1016/s0300-9572(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 43.Pialoux V., Hanly P.J., Foster G.E., Brugniaux J.V., Beaudin A.E., Hartmann S.E., Pun M., Duggan C.T., Poulin M.J. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am. J. Respir. Crit. Care Med. 2009;180:1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 44.Mounier R., Amonchot A., Caillot N., Gladine C., Citron B., Bedu M., Chirico E., Coudert J., Pialoux V. Pulmonary arterial systolic pressure and susceptibility to high altitude pulmonary edema. Respir. Physiol. Neurobiol. 2011;179:294–299. doi: 10.1016/j.resp.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Bailey D.M., Davies B., Young I.S., Hullin D.A., Seddon P.S. A potential role for free radical-mediated skeletal muscle soreness in the pathophysiology of acute mountain sickness. Aviat Space Environ. Med. 2001;72:513–521. [PubMed] [Google Scholar]
- 46.Ribon A., Pialoux V., Saugy J.J., Rupp T., Faiss R., Debevec T., Millet G.P. Exposure to hypobaric hypoxia results in higher oxidative stress compared to normobaric hypoxia. Respir. Physiol. Neurobiol. 2016;223:23–27. doi: 10.1016/j.resp.2015.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.