Abstract
Objective
To investigate, in detail, the effects of rituximab (RTX), an off-label drug for treating multiple sclerosis (MS) disease on preventing and/or ameliorating experimental autoimmune encephalomyelitis (EAE).
Methods
Using bioinformatics analysis of publicly available transcriptomics data, we determined the accumulation of B cells, plasma cells and T cells in different compartments of multiple sclerosis patients (MS) and healthy individual brains. Based on these observations and on the literature search, we dosed RTX in EAE mice either orally, or injected intraperitoneally (IP). The latter route was used either prophylactically (asymptomatic stage; upon the induction of the disease), or therapeutically (acute stage; upon the appearance of the first sign of the disease). Further, we used RTX as a preventive drug either as a single agent or in combination with other routes of administration.
Results
Because no complete recovery was observed when RTX was used prophylactically or therapeutically, we devised another protocol of injecting this drug before the onset of the disease and designated this regiment as prevention. We demonstrated that the 20 μg/mouse prevention completely reduced the EAE clinical score, impaired infiltration of T and B cells into the perivascular space of mice brains, along with inhibiting the inflammation and demyelination. However, the 5 and 10 μg/mouse doses although reduced all aspects of inflammation in these mice, their effects were not as potent as the 20 μg/mouse RTX dose. Finally, we combined the 5 μg/mouse prevention treatment with either the prophylactic or therapeutic regimen and observed a robust effect.
Conclusion
We observed that combinatorial regimens resulted in further reduction of inflammation, T and B cell extravasation into the brains of EAE mice and improved the re-myelination.
Keywords: rituximab, inflammation, prevention, prophylactic, therapeutic, T cells, B cells, in silico, bioinformatics, immunohistochemistry
Introduction
Multiple Sclerosis (MS) is a devastating disease-causing inflammatory demyelination of the central nervous system (CNS) characterized by formation of isolated areas of inflammation called MS lesions.1 There are four key pathological characteristics of MS: (a) inflammation, that is typically believed to be the primary cause of the main events leading to CNS tissue damage, (b) demyelination, the hallmark of MS, in which the myelin sheath or the oligodendrocyte cell body destroyed via the inflammatory process; (c) axonal loss or damage; and (d) gliosis.
The most common form of MS is relapsing-remitting MS (RRMS), with relapses of disease separated by periods without clinical progression, which accounts for approximately 80% to 85% of initial diagnoses of MS.2 This is characterized by periods of active inflammation within the CNS, throughout that symptoms worsen, alternating with periods where symptoms are less acute. The time when symptoms worsen is called relapses or exacerbations. As a relapse ends, the severity of symptoms diminishes. The quiet periods between relapses are referred to as remissions, which could last for months or years before a relapse happens. On the other hand, primary progressive MS (PPMS), affecting approximately 15% of people with MS, where neurological deterioration is present from the onset of the disease.3
Infiltrating CD4+ and CD8+ T cells, B cells and macrophages are thought to be critical for disease development and progression. It is believed that T cells are activated in the periphery, and then enter the CNS through at least two distinct sites including the subarachnoid space via the choroid plexus, or through the perivascular space at the blood–brain barrier.4,5 On the other hand, B cells play an essential role in MS, both in humans and mouse models,6 and are considered very important therapeutic targets for this disease. Cells of the B lymphocyte lineage are found to persist in the inflamed MS central nervous system and occupy multiple sub-compartments. These include the CSF and parenchymal white matter lesions.6,7 Studies in animal models demonstrated a complicated role for B cells.8
Rituximab (Rituxan, RTX), a monoclonal antibody against CD20 expressed on naive B cells, works as a B cell depleting agent. According to research, long-term depletion of peripheral B cells via RTX is safe. The drug has been approved by the FDA for treating diseases such as non-Hodgkin’s lymphoma and rheumatoid arthritis,9 but sometimes it is prescribed as an “off-label” drug for MS patients. The first study of RTX, conducted in 5 people with PPMS, showed that most peripheral blood B cells were depleted up to 14 months post-treatment.10 A Phase I open study, designed to assess the drug safety and tolerability in RRMS did not reveal serious adverse effects.11 Clinical trials that followed showed that B cell depletion effectively suppressed MS disease activity. Rituximab also showed efficacy in ameliorating demyelinating disorders of the CNS.12 Intrathecal administration of RTX in progressive MS was well tolerated,13 demonstrating good tolerability and efficacy in cases of both relapsing and progressive forms of MS.14 The risk of John Cunningham (JC) virus and progressive multifocal leukoencephalopathy (PML) in MS patients was lowest in RTX-treated MS patients as compared to natalizumab, fingolimod or other disease-modifying therapy (DMT).15,16
In this work, we systematically investigated the effects of RTX on ameliorating EAE disorder. We utilized several treatment regimens, including prophylactic treatment using the drug orally or intraperitoneally, as well as using it as a therapeutic agent. Further, we devised previously unexplored mode of treatment, designated as “prevention”. Here, we used the drug long before inducing the diseases. Also, we utilized this regimen as combinatorial treatment with prophylactic or therapeutic regimen. In addition to the EAE clinical scores and mice body weight, we investigated the inflammation taking place in the brains of these mice. Based on our findings, we determine that prevention treatment is the best mode of therapy for these mice. Hence, these results are novel demonstrating that extended treatment with this drug might be safe for MS patients.
Materials and Methods
Using Publicly Available Data to Predict Immune Cells Infiltration in Brain of MS Patients
In order to understand the molecular basis of MS in human brains, we extracted publicly available RNAseq data (GSE123496) using geo omnibus platform (https://www.ncbi.nlm.nih.gov/geo/). The samples analyzed are fresh-frozen autopsy brain tissues from five MS female patients and five age-matched healthy females. Five different regions per sample were investigated including corpus callosum (CC), internal capsule (IC), hippocampus (HY), frontal cortex (FC), and parietal cortex (PC). The analysis was performed using AltAnalyze v.3.1.2 tool,17 to identify differentially expressed genes and pathways enrichment between the two groups.
In silico Prediction of the Percentage and Status of Immune Cells in Brain of MS Patients
As the top upregulated genes in MS patients compared to healthy individuals were related to immune cells, we used the transcriptomic data to predict the percentage and status of immune cells in the brains of MS patients compared to the healthy samples using CIBERSORT, a computational method for quantifying cell fractions from bulk tissue gene expression profiles.18
Induction of EAE in SJL Mice
All animal studies and procedures were approved by the University of Sharjah Animal care and use committee (ACUC) and following the UoS research policy and procedures for animal care. Mice were kept under pathogen-free conditions at the University of Sharjah. Female SJL/J mice (H-2ˢ, obtained from Charles River laboratories, Fairfield, USA), ages 4–6 weeks old were immunized subcutaneously (SC) with 200 μg of PLP139–151 peptide purchased from ABBIOTEC (San Diego, CA, USA) emulsified in complete Freund’s adjuvant containing 1 mg Mycobacterium tuberculosis (Sigma-Aldrich, Sharjah branch, UAE), at four sites in the right and left flanks. Following each injection, 200 ng of Bordetella pertussis toxin (EMD chemicals, Darmstadt, Germany) was injected intraperitoneal (IP) 0 and 48 hrs after immunization with the peptide. The animals were independently observed and monitored daily, and the EAE clinical score was measured according to the following scoring scheme: 0 = no clinical disease, 1 = tail flaccidity, 2 = hind limb weakness, 3 = hind limb paralysis, 4 = forelimb paralysis, and 5 = moribund or death, as described.19
Treatment of EAE Mice
Rituximab was obtained from Hoffmann-La Roche Ltd. (Basil, Switzerland). SJL/J mice were divided into several groups and each group consisted of 5 mice each. The first group where EAE was induced was left without treatment, and the other groups were treated as such:
Oral prophylactic: Mice were fed orally with 5 μg/mouse every 3 days since the initiation of the disease and until termination (Supplementary Figure 1A).
IP prophylactic: Mice were injected IP with 5 μg/mouse mice every 3 days since the initiation of the disease and until termination (Supplementary Figure 1B).
Therapeutic: Mice were injected IP with 5 μg/mouse mice every 3 days at the appearance of symptoms; usually 10–12 days after initiation of the disease and until termination (Supplementary Figure 1C).
Prevention: The mice were injected IP with 5, 10 or 20 μg/mouse of RTX at days −15, −10, −5, and 0. EAE was induced at day 0 (Supplementary Figure 1D).
Prevention plus prophylactic: The mice were injected IP with 5 μg/mouse RTX. On day 0, EAE was induced along with injection of 5 μg/mouse RTX at the onset of disease initiation and continued until termination with intervals of 3 days (Supplementary Figure 1E).
Prevention plus therapeutic: The mice were injected IP 5 μg/mouse RTX. On day 0, EAE was induced, and after the symptoms developed at day 10–12, mice were injected with 5 μg/mouse RTX every three days and continued until termination with intervals of 3 days (Supplementary Figure 1F).
For controls, EAE mice were injected with 1 μg IgG (R&D Systems, UK) IP every 3 days, or were orally gavaged every day with 1 mg monomethyl fumarate (MMF, Sigma-Aldrich) for the duration of the experiments, as previously described.19
Tissue Processing
Brains were collected from all mice and fixed in 10% Neutral buffered formalin (Thermo Scientific, Waltham, MA USA). Approximately after 2 days of fixation, tissues were processed with Excelsior AS Tissue Processor (Thermo Scientific) using routine overnight protocol. The tissues were embedded into paraffin-embedded blocks by TEC 2900 tissue embedding centre (Histo-Line laboratories, Pantigliate MI, Italy). FFPE tissues are then sectioned at 5 µM with HM 355S Automatic Microtome (Thermo Scientific) and collected on plain slides (Medix, Newbury Park, CA) for regular staining and charged slides (Thermo Scientific) for immunohistochemistry (IHC) staining.
Hematoxylin and Eosin Staining
The slides were incubated 2 times in xylene (Eurolab, Brussels, Belgium) for 10 mins each. They were then transferred to a series of ethanol (Honeywell, Charlotte, North Carolina, USA) for rehydration in the order of 100% ethanol 2 times, 90% ethanol, 70% ethanol of 5 min each. The slides were incubated in distilled water for 5 min and then incubated in Shandon Harris Hematoxylin (Thermo Scientific) for 3–7 min to stain the nuclei. The slides were then transferred to a staining jar with running tap water until the slides were clear. They were incubated in Shandon Eosin (Thermo Scientific) for 2–4 min and transferred to a series of alcohol for dehydration in the order of 70% ethanol, 90% ethanol and 100% ethanol 2 times of 2 min each. Finally, the slides were incubated for 5 min in xylene and tissues were mounted with xylene-based synthetic mount (Thermo Scientific) with cover slides. Hematoxylin and Eosin (H & E) stained images were captured with Olympus DP74 microscope digital camera attached to Olympus BX43 microscope.
Luxol Fast Blue Staining of Brain Sections
The Luxol fast blue Staining was performed according to manufacture protocol (Eurolab) to stain myelin sheath. Briefly, the slides were deparaffinized through 2 times incubation in xylene for 10 min each. They were transferred to a series of alcohol for rehydration in the order of 100% ethanol 2 times, 90% ethanol, 70% ethanol of 5 min each. The slides were hydrated by incubation in distilled water for 5 min and then incubated in Luxol fast blue solution overnight at 37°C to stain the myelin sheath. The stain was differentiated by dipping in lithium carbonate solution, followed by dipping in 70% ethanol and finally rinsed in distilled water. They were incubated in Cresyl Echt Violet for 2–5 min and then rinsed in distilled water. The slides were then transferred to a series of alcohol for dehydration in the order of 70% ethanol, 90% ethanol and 100% ethanol 2 times for 2 min each. Finally, the slides were incubated for 5 min in xylene and tissues were mounted with xylene-based synthetic mount (Thermo Scientific) with cover slides. Luxol Fast Blue stained images were captured with Olympus DP74 microscope digital camera attached to Olympus BX43 microscope.
Immunohistochemistry (IHC) Staining
The slides were incubated 2 times in xylene of 10 min each, and then transferred to a series of alcohol for rehydration in the order of 100% ethanol 2 times, 90% ethanol, 70% ethanol of 5 min each. They were incubated in distilled water for 5 min and then retrieved by microwave oven with Tris-EDTA buffer (pH 9). Once the slides were cooled down, peroxide blocking was performed by incubating the slides for 20 min at 0.3% hydrogen peroxide solution (Honeywell). The slides were washed in stormed tap water for 5 min and with distilled water for 1 min, then with PBS incubation for 5 min. They were incubated overnight at 4°C with anti-CD3 antibody at a dilution of 1:150 (Thermo Scientific), anti-CD19 antibody (Novus Biologicals, Centennial, CO, USA) at dilution of 1:800 in 1% BSA, or anti-CD20 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:100 in 1% BSA. After washing, they were incubated with secondary antibody goat anti-rabbit IgG HRP (Abcam, Cambridge, United Kingdom) at a dilution 1:20,000 in 1% BSA for CD3 staining, secondary antibody goat anti-rabbit IgG HRP (Abcam) at a dilution 1:1500 in 1% BSA solution for CD19 staining, or secondary antibody mouse-IgGκ- BP-HRP (Santa Cruz Biotechnology) at a dilution 1:50 in 1% BSA solution for anti-CD20 staining. DAB solution (Abcam) was added to the slides, incubated for 5–10 min for the color development, and the reaction was stopped by adding PBS. The slides were then washed with tap water and then distilled water for 5 min each and then counterstained with Hematoxylin. The slides were then transferred to series of alcohol for dehydration in the order of 70% ethanol, 90% ethanol and 100% ethanol 2 times for 2 min each. Finally, the slides were incubated for 5 min in xylene and tissues were mounted with xylene-based synthetic mount (Thermo Scientific) with cover slides. The images were captured with Olympus DP74 microscope digital camera attached to Olympus BX43 microscope.
Statistical Analysis
Significant values (P<0.05) were calculated using the Student’s t-test, or one-way ANOVA followed by Sidak’s test analysis calculated by Graphpad Prism 6 program (San Diego, CA, USA). Area under curve analysis was performed using the Graphpad Prism 6 program.
Results
Differential Accumulation of Immune Cells in the Brains of MS Patients
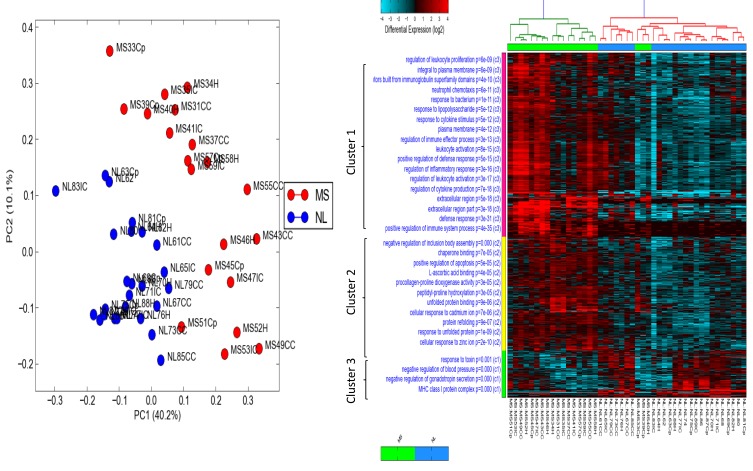
Principle component (PCA) and differential analysis heat map were performed on publicly available RNAseq data (GSE123496) to identify differentially expressed genes in MS brains compared to healthy individuals. PCA showed that MS samples were clustered away from healthy samples no matter what the anatomical region examined indicating a generalized change in the brain transcriptomes of MS patients (Figure 1A). In order to identify genes clusters that differentiate MS from healthy brains, heat map analysis of the top differentially expressed genes was generated as seen in Figure 1B. Three clusters were shown to be significantly different between the two groups, where most of immune cells and regulators of immune responses are found in cluster 1. This confirms the role of dysregulated immune response in the pathophysiology of MS disease regarding altered genes related to local immune response or those specific to immune cells infiltrating the diseased brains.
Figure 1.
Principle component analysis (PCA) and heat map for differentially expressed genes in brains of multiple sclerosis (MS) patients compared to healthy brains obtained from publicly available RNAseq data (GSE123496). (A) Differential principle component log fold analysis of gene samples clustered in MS patients in different brain regions (red dots) versus those expressed in normal healthy individuals brains (NL) represented with blue dots. (B) Heat map performed on publicly available RNAseq data (GSE123496). Differential analysis showed clearly that top genes that are upregulated in MS compared to healthy individuals were related to immune cells. Three gene clusters were observed; immune cells and their regulators are accumulated in cluster 1.
Abbreviations: PC 1,2, principle component 1,2; MS, multiple sclerosis; NL, normal individuals.
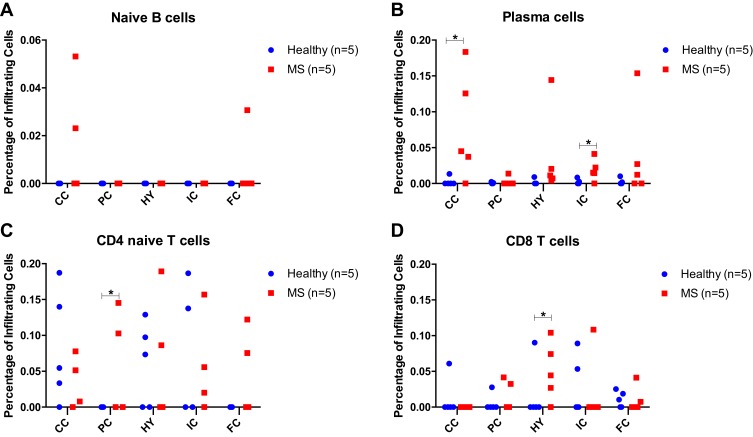
In silico estimation of immune cells and their activation status was performed using CIBERSORT tool and different regions of the brains were compared between healthy and MS patients. Plasma cells were significantly higher in corpus callosum (CC; p<0.05), and internal capsule (p<0.05) of MS patients when compared to healthy controls. Other notable differences were the percentage of CD4+ and CD8+ T cells, where CD4+ naïve T cells were higher in parietal cortex (PC) of MS patients compared to healthy (p<0.05), while CD8+ T cells were significantly higher in hippocampus (HY) of MS patients as compared to healthy individuals (p<0.05, Figure 2).
Figure 2.
Immune cells accumulation in the brains of multiple sclerosis (MS) patients vs healthy individuals using CIBERSORT tool. Red squares indicate MS patients, whereas blue circles indicate healthy brains. The percentage of (A) naïve B cells, (B) plasma cells, (C) naïve CD4+ or (D) naïve CD8+ cells infiltrating different regions in the brains of MS and healthy individual brains, are shown. *p<0.05 comparing cells infiltrating certain areas of the MS brains versus healthy controls.
Abbreviations: FC, frontal cortex; CC, corpus callosum; PC, parietal cortex; HY, hippocampus; IC, internal capsule.
Oral Prophylactic, IP Prophylactic or IP Therapeutic Regiment Treatments of EAE Mice with RTX
Due to the importance of B and T cells in exacerbating MS disease and based on the in silico analysis, we used RTX to treat mice with EAE. Because it has been previously reported that prophylactic (asymptomatic stage) oral administration of RTX reduced the clinical score in C57Bl/6J mice using MOG peptide,20 we sought to determine whether similar administration might ameliorate the clinical score in SJL/J mice injected with PLP. The EAE clinical score among untreated mice (gray line) and mice treated with 5 μg oral prophylactic RTX (red line) started at day 0 and continued every three days until termination. Data were collected from 5 mice in each group at any time point. The p values were provided from the results of the unpaired sample t-test. There was no statistically significant difference in EAE clinical score among EAE untreated group and oral prophylactic RTX-treated group (Supplementary Figure 2A). In fact, mice treated via this route lost weight as compared to untreated mice (Supplementary Figure 2B).
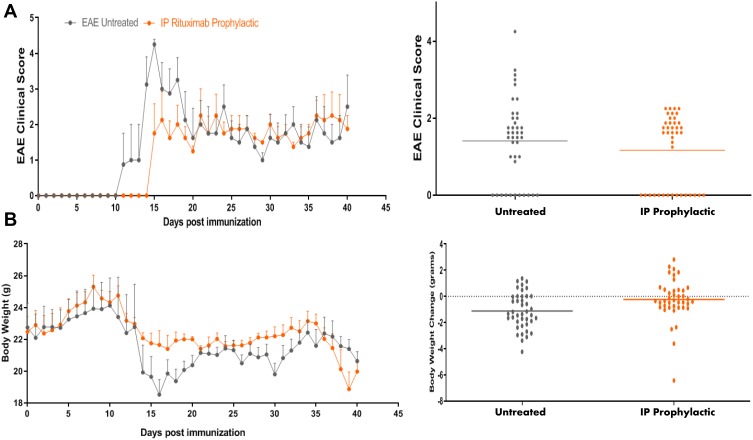
Next, we examined whether mice treated prophylactically via intraperitoneal (IP) administration of RTX might show better response than those treated orally. In this case, mice were injected IP with RTX at day 0 and continued every 3 days until termination. Although there was a trend in reducing the clinical score, this did not reach statistical significance when compared to untreated group (P = 0.0540, Figure 3A). However, bodyweight of IP prophylactic RTX-treated group was significantly increased compared to EAE untreated group (P <0.002, Figure 3B). As a control, IgG and monomethyl fumarate (MMF) were used as negative and positive control, respectively. As expected, MMF significantly reduced the EAE clinical score when compared to untreated mice or mice dosed with IgG antibody (Supplementary Figure 3). The effect of MMF supports our earlier findings showing that this molecule robustly ameliorated EAE clinical score in mice.19 In contrast, IgG dosing did not significantly affect the disease.
Figure 3.
Intraperitoneal (IP) prophylactic treatment with RTX. (A) SJL/J mice were induced to develop EAE (grey line). Mice also received 5 μg/mouse RTX IP at the start of disease induction (day 0) and continued every three days until the termination of the experiment (orange line). (B) Bodyweight of animals measured every day since the induction of the disease.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; RTX, rituximab.
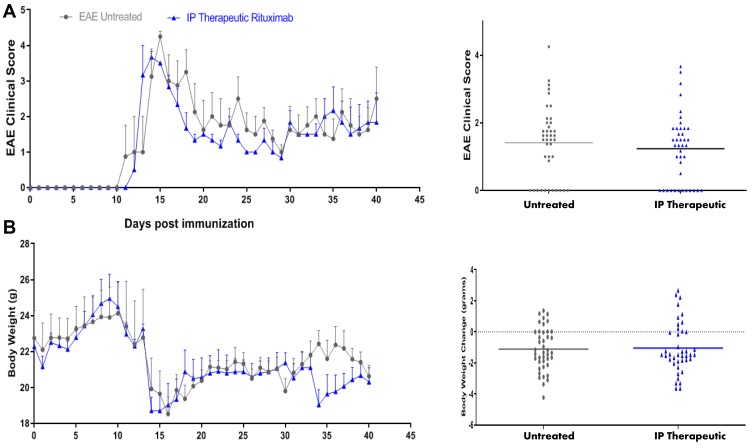
To investigate whether other modes of treatment with RTX might improve the clinical score, we treated the mice with RTX therapeutically (first sign of disease appearance; acute stage). Mice received 5 μg RTX at the first sign of symptoms, i.e. clinical score of 0.5–1, which always occurred between 10 and 12 days post injection of the peptide. These mice continued receiving 5 μg IP of RTX every three days until the end of the experiments. Comparison of the EAE clinical score among untreated mice (gray line) and mice treated with 5 μg RTX IP therapeutically (blue line) showed a trend of ameliorating the clinical score but there was no statistically significant difference in EAE clinical score among untreated group and IP therapeutic RTX-treated group (Figure 4A). Neither, we observed a significant improvement in body weight of these mice after IP therapeutic administration of RTX when compared to untreated mice (Figure 4B).
Figure 4.
Intraperitoneal (IP) therapeutic treatment with RTX. (A) SJL/J mice were induced to develop EAE (grey line). Mice also received 5 μg/mouse RTX IP upon first sign of disease appears usually on day 10 or 12 after disease induction, and continued every three days until the termination of the experiment (blue line). (B) Bodyweight of animals measured every day since the induction of the disease.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; RTX, rituximab.
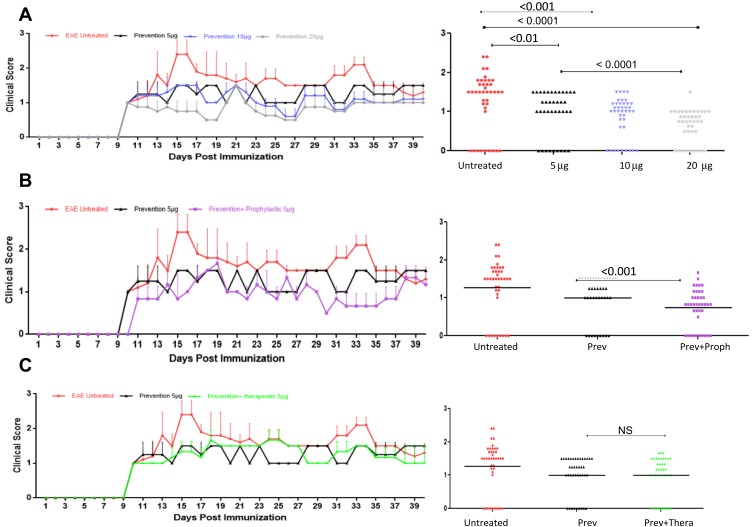
Because all classical treatments with RTX, i.e. prophylactic or therapeutic did not significantly reduce the clinical score of EAE mice, we performed a new therapeutic strategy for RTX. In these experiments, the mice were injected IP with RTX 5 times with different doses of the drug, i.e. 5, 10 or 20 μg IP at 3 days apart before inducing the diseases. We designated this treatment as prevention. Results in Figure 5A show comparison of the EAE clinical score among untreated mice and mice treated with 5 μg, 10 μg and 20 μg IP RTX for 15 days prior to inducing EAE. Data were collected from 5 mice in each group at any time point. The Figure also provides the area under curve for 45 days post immunization along with p values. These p values were provided from the results of one way-ANOVA. There was a statistically significant difference in EAE clinical score among EAE untreated group and IP prevention RTX-treated groups injected with 5, 10 or 20 μg (P ˂ 0.01, <0.001 and <0.0001, respectively, Figure 5A). When p values were compared among EAE clinical scores of mice receiving different doses, we observed that 20 μg/mice was the best dose that ameliorates the diseases, followed by 10 μg and then 5 μg/mouse, as shown in Figure 5A. In fact, prevention with 20 μg RTX was far superior to injecting 5 μg per mouse and showed statistically significant differences (P<0.0001, Figure 5A).
Figure 5.
RTX significantly ameliorates EAE clinical score when used preventively. (A) SJL/J female mice were either left untreated (red line), injected IP with 5 μg/mouse (black lines), 10 μg/mouse (blue lines) or 20 μg/mouse (grey line) on days −15, −10, −5 and 0. At this time, EAE was induced in these mice and clinical scores were measured every day until termination. Significant values shown in the right panels compared the EAE clinical scores among the various groups, using Sidak’s multiple comparison test. (B) In these experiments, the mice were treated preventively with 5 μg/mouse RTX on days −15,-10, −5 and 0. EAE was induced at day 0; this group is called the prevention group (black line). The second group received RTX as described in (A) but also received 5 μg/muse RTX, 3 days after induction and for the duration of the experiments with 3 days interval between injections; this group is called prevention plus prophylactic (purple line). Significant values shown in the right panels compared the EAE clinical scores among the various groups, using Sidak’s multiple comparison test. (C) In these experiments, the mice were treated preventively with 5 μg/mouse RTX on days −15, −10, −5 and 0. EAE was induced at day 0; this group is called the prevention group (black line). The second group received RTX as described in (A), but also received 5 μg/mouse RTX after the first sign of disease appearance, and for the duration of the experiments with 3 days interval between injections; this group is called prevention plus therapeutic (green line). Red lines in A and B indicate untreated mice. Significant values shown in the right panels compared the EAE clinical scores among the various groups, using Sidak’s multiple comparison test.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; RTX, rituximab; Prev, prevention; Proph, prophylactic; NS, not significant; IP, intraperitoneal.
Due to the nature of MS disease (relapsing/remitting), we hypothesized that a combination of prevention with either prophylactic or therapeutic treatment might highly improve the EAE disease. To perform these experiments, we used 5 μg RTX as a preventive agent; this dose was chosen because it did not give maximum reduction of EAE clinical score, when compared to 20 μg dose treatment. First, a combination of preventive RTX with IP prophylactic RTX was performed. Results show the comparison of the EAE clinical score among untreated mice, mice treated with 5 μg IP prevention RTX alone and mice treated prevention plus prophylactic for 40 days. A significant reduction in EAE clinical score was observed among mice treated preventively plus prophylactically, when compared to mice treated preventively alone (P<0.001, Figure 5B). Next, we combined prevention treatment with IP therapeutic treatment. There was no significant improvement when therapeutic regiment was combined with preventive regiment, when compared to 5 μg RTX/mouse used alone as a preventive drug (Figure 5C).
Histopathological Evaluation of the CNS Inflammation in EAE Mice and Comparison with Treated Mice
To correlate the clinical data with histological evidence, we examined these aspects of EAE inflammation in all types of treatments used in this study. These parameters include 1) H & E staining of the perivascular space (PVS) to demonstrate the inflammation; 2) Staining with anti-CD3 to determine the infiltration of T cells into the brain parenchyma; 3) Staining with anti-CD19 (or anti-CD20) to demonstrate the presence of B cells; and 4) Luxol staining to demonstrate the demyelination/remyelination.
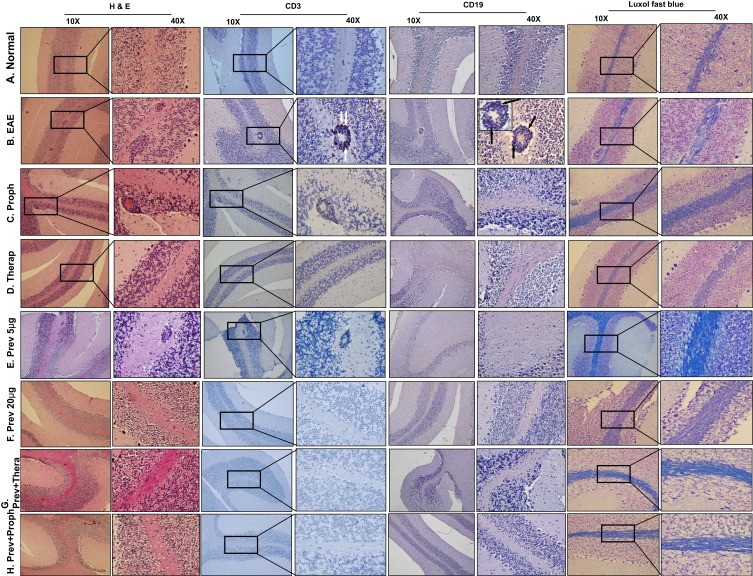
We focused on the perivascular space (PVS) of the brain because this is the vascularized area where autoreactive cells extravasate into the brain parenchyma.4,5 Normal mice treated with vehicle showed normal PVS with no T cell infiltration or demyelination as determined by H & E staining, shown in Figure 6A. In contrast, EAE mice showed significant demyelinating areas presented as less myelin density, in addition to areas of loss of myelination in the white matter, as determined by Luxol fast staining (Figure 6). This was accompanied by extensive infiltration of CD3+ T lymphocytes in the PVS (white arrows, CD3 40X, Figure 6B). B cells were also detected in the same areas as T cells, as determined by anti-CD19 or anti-CD20 staining (black arrows, CD19, Figure 6B). In addition, reactive gliosis was also observed in the EAE model as compared to normal mice. These features were more detectable in the cerebellum of the affected mice with high clinical scores, supporting the in silico analysis. In contrast, both 5 μg/mouse prophylactic (Figure 6C), and therapeutic (Figure 6D), RTX protocols differentially reduced CD3+ T lymphocytes infiltration, B cell accumulation, and repaired part of the demyelination in the EAE mice model. However, the level of CD3+ T lymphocyte positive cells reduction was more pronounced in the therapeutic group, particularly in terms of the presence of CD3+ T cells in the PVS (Figure 6D), as compared to prophylactic group (Figure 6C). Moreover, both groups still showed evidence of astrogliosis.
Figure 6.
Immunohistochemical examination of the brains of mice. (A) Brains of normal mice showing no sign of inflammation in the PVS (H & E staining), no CD3+ or CD19+ infiltration and no demyelination, as detected by Luxol fast staining. (B) Brains of EAE mice showing high inflammation with infiltration of CD3+ cells (white arrows), CD19+ (CD20+ staining is showing in the inset; black arrows), and high demyelination. (C) Prophylactic treatment of mice showing some inflammation in the PVS, T cell infiltration and some B cell infiltration, associated with much lower demyelination in the brains when compared to untreated EAE mice. (D) Brains of EAE mice treated therapeutically with RTX showing decreased inflammation in the PVS, few, if any, CD3+ or CD19+ infiltration, and improved demyelination. (E) The 5 μg/mouse RTX when used preventively, reduced inflammation in the PVS (H & E staining), but the PVS still showing infiltration of CD3+ cells and scattered B cells, improved but not reversal of demyelination (Luxol fast staining). (F) The 20 μg/mouse RTX used preventively completely resolved the inflammation, impaired T and B cells infiltration, and reversed the demyelination to almost normal level. (G) RTX used preventively at 5 μg/mouse plus prophylactically also at 5 μg/mouse significantly improved most signs of inflammation in the PVS and in the cortex of the brains. (H) RTX was used preventively and therapeutically at 5 μg/mouse each showing a complete reversal of PVS inflammation and brain demyelination. All sections were examined at 10X and highlighted at 40X magnification. Rectangles indicate PVS at 10X magnification.
Abbreviations: H&E, hematoxylin and eosin; EAE, experimental autoimmune encephalomyelitis; Proph, prophylactic; Prev, prevention; Therap, therapeutic; PVS, perivascular space; RTX, rituximab.
Preventive RTX Reduces Inflammation, T and B cell Infiltration, as Well as Inducing the Re-Myelination in EAE Mice in a Dose-Dependent Manner
Mice treated with RTX as a preventive drug were also evaluated for the extent of inflammation in the CNS. Using RTX at a low dose of 5 µg/mouse was sufficient to cause certain improvement in the PVS inflammation as determined by H & E stain and in the re-myelination, determined by Luxol fast staining (Figure 6E). However, CD3+ T cells were still apparent in the PVS of these mice (Figure 6E). Intriguingly, when using a high concentration of RTX, i.e. 20 µg, we observed a complete reduction of inflammation in the PVS. This was evident by the absence of CD3+ T lymphocytes or CD19+ B lymphocytes to levels similar to that observed in control mice, as well as the complete absence of demyelination areas (Figure 6F). This clearly demonstrates that administration of high concentration of RTX as a preventive drug can affectively protect mice from the development of the disease.
Further, we evaluated the inflammation in the brain parenchyma and the infiltration of T and B cells in mice receiving the low concentration of RTX, i.e. 5 μg along with prophylactic or therapeutic regimen. The combinatorial protocol of preventive RTX at low concentration with therapeutic regimen showed improved in many aspects of inflammation (Figure 6G). However, we observed that in certain areas of the brains, some signs of inflammation are still apparent (not shown). Intriguingly, a combination of prevention with prophylactic treatment caused complete abolishment of the inflammatory histopathological features of EAE. This was evident from H & E staining, CD3+ and CD19+ cell infiltration, as well as the extent of remyelination (Figure 6H).
Quantitation of T cells infiltrating the brains of mice showed a significant reduction of the number of T cells in the PVS of mice treated preventively with 20 μg/mouse RTX alone. Although the administration of 5 μg/mouse also significantly reduced the numbers of T cells, there was a robust and complete ablation of T cells upon combinatorial treatments (Supplementary Figure 4).
Discussion
There is presently no cure for multiple sclerosis, and current treatments can only help speed up recovery from attacks, modify the course of the disease and manage symptoms. The available disease-modifying therapies which work by modifying the activity of the immune system to slow the frequency and severity of attacks have been used to treat MS including RRMS, as well as progressive MS in those individuals who continue to experience relapses. The first line of treatment was represented by type 1 interferons (IFN) and glatiramer acetate (GA). Type I IFNs are natural antiviral molecules produced with immunoregulatory properties. GA, which was discovered due to studies in EAE,21 is a copolymer of four amino acids present in myelin basic protein, namely glutamic acid, lysine, alanine and tyrosine.
MS affects the brains from the onset of the disease, and it is imperative that therapy should start as soon as possible before damages occur in the whole brain. The FDA approved rituximab for lymphoma, leukemia, rheumatoid arthritis, but its use in MS is only off-label and depends on the agreement between the neurologists and their patients. Because RTX is used to deplete human B cells, the question arises of its suitability for utilization in mice. Indeed, there is 75% homology among mice and human CD20.22 Further, there is evidence in the literature providing strong support that RTX can be used to treat EAE mice. The work of Monson et al clearly showed that RTX ameliorates EAE in hCD20Tg mice.23 Intriguingly, James et al observed that radiolabeled RTX6 binds to B cells in the brain and spinal cord of huCD20tg EAE mice.7 To this end, we observed robust effects of RTX on T cells infiltration and consequent reduced inflammation, suggesting that RTX may target T cells in SJL/EAE model.
A study found that the drug performs better than other commonly used DMTs in patients with newly diagnosed RRMS.24 Other studies provided additional evidence that RTX is effective and relatively safe in this highly disabling condition.25,26 Switching to RTX from using other drugs also showed improvements of disease progression.27 However, no study to our knowledge described the effect of RTX as a preventive agent. Prevention is highly important, and it may save the patients lots of aggravated pain before the disease progresses. Also, the society will benefit from preventive procedures far more than using drugs for therapy. Prevention can be recommended to those patients who are susceptible to disease development based on genetic analysis and family history, or to those with cognitive decline which appears long before walking disability starts to be pronounced.28 Evidence indicate that patients who received interferon-beta had improvement in mortality if they start receiving the drug early.29 Consequently, it is highly important to understand how DMTs work and when is the best time of using them in order to efficiently interfere with disease progression. The argument then arises how would treatment with RTX which depletes B cells might result in better outcome after long-term treatment? In this regards, reports showed that RTX treatment is associated with immune reconstitution and B-cells recovery, which usually starts 6 months after therapy with complete recovery to normal levels in 9–12 months.30 Moreover, it was also demonstrated that patients who receive extended RTX treatment showed no evidence of increasing clinically relevant infection.30 Our study adds support to the concept of using this drug for extended time. This approach might help in the adoption of novel management strategies with the aim of sufficiently administrating RTX far enough in advances to prevent disease progression or relapse.
Recent advances in the field of bioinformatics provide strong support exploring certain mechanisms and activities that were difficult to understand using classical methods of investigation. We utilized in silico analysis to delineate the accumulation of immune cells, and observed that B cells, plasma cells, CD4+ and CD8+ T cells clustered in different areas of the brains. The in silico results demonstrate that these cells have different distributions in the brains of MS patients vs those of healthy individuals. For example, plasma cells differentially accumulate in the internal capsule, whereas CD4+ T cells are found in internal capsule or parietal cortex, and CD8+ cells in the hippocampus of MS patient brains, when compared to healthy rains. This method aids us prior to the in vivo experiments, in predicting the location of B and T cells in the brains of EAE mice before and after RTX treatment. The robust in silico method has been recently used to explore the role of various molecules in multiple sclerosis disease. For example, Fagone et al utilized in silico approach to delineate the role of macrophage migration inhibitory factor in MS etiopathogenesis.31 Similar method was used to investigate the role of IL-37 in MS patients, and how this cytokine might influence the disease before or after therapy.32 Also, the role of Tetraspanin-32 in MS was explored utilizing ex vivo and in silico analysis.33
Based on in silico analysis we performed immunohistochemical studies to corroborate the effects of RTX in ameliorating the EAE clinical scores with histological examination of brain tissues. We were intrigued by the effect of high dose, i.e. 20 μg/mouse of RTX in ablating inflammatory histological features examined in this study which include perivascular inflammation, infiltration of CD3+ and CD19+ cells and reversal of the de-myelination. This is corroborated with the ability of this dose of RTX to ameliorate the disease and to significantly reduce the clinical score in mice treated preventively. Because 20 μg/mouse might be a large dose and if given as a bolus injection or continuous infusion in humans might result in certain complications (although this has not been examined in our study), we hypothesized that using the low dose of RTX as a preventive agent which partially reduced the clinical score, in combination with either prophylactic or therapeutic regimen, might result in better amelioration than using each regimen alone. Intriguingly, such a strategy resulted in interesting and important findings. We observed that combining low dose of RTX as a preventive agent with low dose of the drug as prophylactic agent highly ameliorated the disease, corroborated with reduced inflammation in the PVS inflammation, CD3+ and CD19+ reduced infiltration as well as reversal of demyelination. These results mimic those observed when RTX was used at a high concentration as a preventive drug. Inflammation was also reduced when low dose preventive RTX was combined with low dose therapeutic RTX. Surprisingly, however, the latter did not translate into better clinical score when combined with RTX as a preventive agent. Although it has been previously reported that oral ingestion of RTX in EAE mice ameliorated the EAE clinical score along with reducing the levels of inflammatory cytokines such as IFN-γ, IL-12, IL-17 and TNF-α,20 we could not observe any effect when RTX was dosed orally. There are several reasons for this discrepancy, including the use of peptide and the strain of mice in the two studies. Alternatively, because RTX is an antibody and it might be difficult to reach the circulation or localize inside the brain.
Although there was a trend towards reducing the clinical score when RTX was used prophylactically or therapeutically, this did not reach statistical significance. It has been reported that different treatment regimens might have differential outcomes on disease progression. For example, prophylactic treatment of EAE mice with fingolimod resulted in better disease reduction than therapeutic regimen.34 Consequently, better interference strategies must be established in order to reach a successful outcome. Although RTX is an antibody against B cells, its effect on T cells is intriguing. The reduction in T cell infiltration into the PVS after RTX treatment could be due to the lack of B cells, which could act as antigen-presenting cells for T cells, or perhaps this drug may affect T cells recruitment and activation through paracrine fashion. It has been previously shown that T cells in the peripheral blood of healthy donors or rheumatoid arthritis patients express CD20 which may be depleted upon RTX therapy.35 The presence of CD20+ T cells was also observed in MS patients.36 Therefore, it was suggested that the effect of RTX may be due to depleting T cells.37 The fact that in our system, RTX reduced T cell infiltration and consequent inflammation suggests that this antibody might deplete T cells in SJL mice affected with EAE. Indeed, a very recent study showed that RTX depletes activated CD8+ T cells in MS patients.38 The nature of T cell targets for RTX in EAE mice is currently under investigation.
Conclusions
In summary and based on these findings, we recommend that RTX should be used preventively when early sign of disease development such as the appearance of cognitive disability in MS patients, occurred. Finally, the choice of using high concentration of this drug or low concentration combining with infusing low concentration at the onset of disease development will depend on the neurologist choice and the preference of MS patients. The combinatorial regimens can be utilized during unfortunate situations where the disease might develop even when the drug is used preventively. Our findings suggest that prevention plus prophylactic treatment may provide the best outcome.
Funding Statement
This work is supported by a grant from the University of Sharjah grant numbers 1701090222-P and 1701090223-P, and by Terry-Fox Foundation.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hestvik ALK. The double-edged sword of autoimmunity: lessons from multiple sclerosis. Toxins. 2010;2(4):856–877. doi: 10.3390/toxins2040856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hachim MY, Elemam NM, Maghazachi AA. The beneficial and debilitating effects of environmental and microbial toxins, drugs, organic solvents and heavy metals on the onset and progression of multiple sclerosis. Toxins. 2019;11(3):147–163. doi: 10.3390/toxins11030147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/WNL.46.4.907 [DOI] [PubMed] [Google Scholar]
- 4.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maghazachi AA. On the role of natural killer cells in neurodegenerative diseases. Toxins. 2013;5(2):363–375. doi: 10.3390/toxins5020363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michel L, Touil H, Pikor NB, Gommerman JL. B cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS-compartmentalized inflammation. Front Immunol. 2015;6:636. doi: 10.3389/fimmu.2015.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James ML, Hoehne A, Mayer AT, et al. Imaging B cells in a mouse model of multiple sclerosis using 64 Cu-rituximab PET. J Nucl Med. 2017;58(11):1845–1851. doi: 10.2967/jnumed.117.189597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez MI, Catalan-dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74(2):318–326. doi: 10.1016/j.cyto.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aloisi F, Pujol-borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786 [DOI] [PubMed] [Google Scholar]
- 10.Monson NL, Cravens PD, Frohman EM, Hawker K, Racke MK. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol. 2005;62:258–264. doi: 10.1001/archneur.62.2.258 [DOI] [PubMed] [Google Scholar]
- 11.Alcalá C, Gascón F, Pérez-miralles F, et al. Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol. 2018;265(7):1690–1697. doi: 10.1007/s00415-018-8899-3 [DOI] [PubMed] [Google Scholar]
- 12.Ineichen BV, Moridi T, Granberg T, Piehl F. Rituximab treatment for multiple sclerosis. Mult Scler. 2019;25:1352458519858604. [DOI] [PubMed] [Google Scholar]
- 13.Bergman J, Burman J, Gilthorpe JD, et al. Intrathecal treatment trial of rituximab in progressive MS: an open-label phase 1b study. Neurology. 2018;91(20):e1893–e1901. doi: 10.1212/WNL.0000000000006500 [DOI] [PubMed] [Google Scholar]
- 14.Alldredge B, Jordan A, Imitola J, Racke MK. Safety and efficacy of rituximab: experience of a single multiple sclerosis center. Clin Neuropharmacol. 2018;41(2):56–59. doi: 10.1097/WNF.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 15.Baber U, Bouley A, Egnor E, Sloane JA. Anti-JC virus antibody index changes in rituximab-treated multiple sclerosis patients. J Neurol. 2018;265(10):2342–2345. doi: 10.1007/s00415-018-8996-3 [DOI] [PubMed] [Google Scholar]
- 16.Oshima Y, Tanimoto T, Yuji K, Tojo A. Drug-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler. 2019;25(8):1141–1149. doi: 10.1177/1352458518786075 [DOI] [PubMed] [Google Scholar]
- 17.Emig D, Salomonis N, Baumbach J, Lengauer T, Conklin BR, Albrecht M. AltAnalyze and DomainGraph: analyzing and visualizing exon expression data. Nucleic Acids Res. 2010;38(Web Server issue):W755–W762. doi: 10.1093/nar/gkq405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Jaderi Z, Maghazachi AA. Vitamin D3 and monomethyl fumarate enhance natural killer cell lysis of dendritic cells and ameliorate the clinical score in mice suffering from experimental autoimmune encephalomyelitis. Toxins. 2015;7(11):4730–4744. doi: 10.3390/toxins7114730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brod SA. Ingested (oral) rituximab inhibits EAE. Cytokine. 2016;85:177–183. doi: 10.1016/j.cyto.2016.06.026 [DOI] [PubMed] [Google Scholar]
- 21.Lalive PH, Neuhaus O, Benkhoucha M, et al. Glatiramer acetate in the treatment of multiple sclerosis emerging concepts regarding its mechanism of action. J CNS Drugs. 2011;25(5):401–414. doi: 10.2165/11588120-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz K, Geisslinger G, Tegeder I. Monoclonal antibodies in preclinical EAE models of multiple sclerosis: a systematic review. Int J Mol Sci. 2017;18(9):E1992. doi: 10.3390/ijms18091992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monson NL, Cravens P, Hussain R, et al. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS One. 2011;6(2):e17103. doi: 10.1371/journal.pone.0017103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320–327. doi: 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’amico E, Zanghì A, Chisari CG, et al. Effectiveness and safety of Rituximab in demyelinating diseases spectrum: an Italian experience. Mult Scler Relat Disord. 2019;27:324–326. doi: 10.1016/j.msard.2018.09.041 [DOI] [PubMed] [Google Scholar]
- 26.Scotti B, Disanto G, Sacco R, Guigli M, Zecca C, Gobbi C. Effectiveness and safety of rituximab in multiple sclerosis: an observational study from southern Switzerland. PLoS One. 2018;13(5):e0197415. doi: 10.1371/journal.pone.0197415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Flon P, Söderström L, Laurell K, et al. Immunological profile in cerebrospinal fluid of patients with multiple sclerosis after treatment switch to rituximab and compared with healthy controls. PLoS One. 2018;13(2):e0192516. doi: 10.1371/journal.pone.0192516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2018;89(8):844–850. doi: 10.1136/jnnp-2017-317509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78(17):1315–1322. doi: 10.1212/WNL.0b013e3182535cf6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worch J, Makarova O, Burkhardt B. Immunreconstitution and infectious complications after rituximab treatment in children and adolescents: what do we know and what can we learn from adults? Cancers. 2015;7(1):305–328. doi: 10.3390/cancers7010305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagone P, Mazzon E, Cavalli E, et al. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: in silico and in vivo evidences. J Neuroimmunol. 2018;322:46–56. doi: 10.1016/j.jneuroim.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 32.Cavalli E, Mazzon E, Basile MS, et al. In Silico and in vivo analysis of IL37 in multiple sclerosis reveals its probable homeostatic role on the clinical activity, disability, and treatment with Fingolimod. Molecules. 2019;25(1):E20. doi: 10.3390/molecules25010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basile MS, Mazzon E, Mangano K, et al. Impaired expression of Tetraspanin 32 (TSPAN32) in memory T cells of patients with multiple sclerosis. Brain Sci. 2020;10(1):E52. doi: 10.3390/brainsci10010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonfiglio T, Olivero G, Merega E, et al. Prophylactic versus therapeutic fingolimod: restoration of presynaptic defects in mice suffering from experimental autoimmune encephalomyelitis. PLoS One. 2017;12(1):e0170825. doi: 10.1371/journal.pone.0170825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilk E, Witte T, Marquardt N, et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum. 2009;60:3563–3571. doi: 10.1002/art.24998 [DOI] [PubMed] [Google Scholar]
- 36.Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol. 2014;193:580–586. doi: 10.4049/jimmunol.1400118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agahozo MC, Peferoen L, Baker D, Amor S. CD20 therapies in multiple sclerosis and experimental autoimmune encephalomyelitis - targeting T or B cells? Mult Scler Relat Disord. 2016;9:110–117. doi: 10.1016/j.msard.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 38.Sabatino JJ, Wilson MR, Calabresi PA, Hauser SL, Schneck JP, Zamvil SS. Anti-CD20 therapy depletes activated myelin-specific CD8+ T cells in multiple sclerosis. Proc Natl Acad Sci USA. 2019;116:25800–25807. doi: 10.1073/pnas.1915309116 [DOI] [PMC free article] [PubMed] [Google Scholar]