Abstract
OBJECTIVE
Nonalcoholic fatty liver disease (i.e., increased intrahepatic triglyceride [IHTG] content), predisposes to type 2 diabetes and cardiovascular disease. Adipose tissue lipolysis and hepatic de novo lipogenesis (DNL) are the main pathways contributing to IHTG. We hypothesized that dietary macronutrient composition influences the pathways, mediators, and magnitude of weight gain-induced changes in IHTG.
RESEARCH DESIGN AND METHODS
We overfed 38 overweight subjects (age 48 ± 2 years, BMI 31 ± 1 kg/m2, liver fat 4.7 ± 0.9%) 1,000 extra kcal/day of saturated (SAT) or unsaturated (UNSAT) fat or simple sugars (CARB) for 3 weeks. We measured IHTG (1H-MRS), pathways contributing to IHTG (lipolysis ([2H5]glycerol) and DNL (2H2O) basally and during euglycemic hyperinsulinemia), insulin resistance, endotoxemia, plasma ceramides, and adipose tissue gene expression at 0 and 3 weeks.
RESULTS
Overfeeding SAT increased IHTG more (+55%) than UNSAT (+15%, P < 0.05). CARB increased IHTG (+33%) by stimulating DNL (+98%). SAT significantly increased while UNSAT decreased lipolysis. SAT induced insulin resistance and endotoxemia and significantly increased multiple plasma ceramides. The diets had distinct effects on adipose tissue gene expression.
CONCLUSIONS
Macronutrient composition of excess energy influences pathways of IHTG: CARB increases DNL, while SAT increases and UNSAT decreases lipolysis. SAT induced the greatest increase in IHTG, insulin resistance, and harmful ceramides. Decreased intakes of SAT could be beneficial in reducing IHTG and the associated risk of diabetes.
Introduction
The rapid increase in the prevalence of obesity has led to a coepidemic of nonalcoholic fatty liver disease (NAFLD) (1). NAFLD is strongly associated with insulin resistance (IR) and predicts the development of type 2 diabetes and cardiovascular disease (1). Although obesity is its primary acquired cause, some subjects who gain weight do not develop NAFLD (1,2). Whether composition of the diet contributes to susceptibility of NAFLD is unclear. Saturated but not polyunsaturated fat has been reported to increase intrahepatic triglycerides (IHTGs) in young nonobese adults, despite similar weight gain (2). The epidemic of obesity has been attributed to an increased intake of simple sugars (3). However, no studies have compared the effects on IHTGs of overfeeding diets enriched in simple sugars or saturated or unsaturated fat.
Fatty acids (FAs) in IHTGs can originate from adipose tissue lipolysis, hepatic de novo lipogenesis (DNL), and dietary fat (4). Lipolysis provides most of the FAs used for synthesis of IHTGs (4). DNL produces exclusively saturated FAs (SFAs) from substrates such as simple sugars (4,5). Excess sugar intakes increase DNL and IHTG content in humans (5). In mice, a high-fat diet increases adipose tissue lipolysis (6). The pathway by which IHTGs are synthesized in response to overconsuming fat has not been studied in humans.
Although IHTGs are commonly associated with IR, triglycerides (TGs) themselves are inert and do not confer IR (7). We have previously shown in humans that IR cosegregates with hepatic ceramides, independent of IHTGs and obesity (7). Ceramides are synthesized de novo from SFAs, such as palmitate, and interfere with glucose metabolism by inhibiting insulin signaling (8) and by stimulating hepatic gluconeogenesis (9). Inflammatory mediators, such as endotoxins derived from gut bacteria, may induce IR by upregulating ceramide synthesis (10). In mice, saturated but not unsaturated fat feeding increases the proportion of endotoxin-containing bacteria in the gut, increases plasma concentrations of endotoxin, and induces IR (11,12). To our knowledge, there are no human data comparing the effects of hypercaloric diets enriched with saturated or unsaturated fat or simple sugars on IR, plasma ceramides, endotoxemia, and gut microbiota.
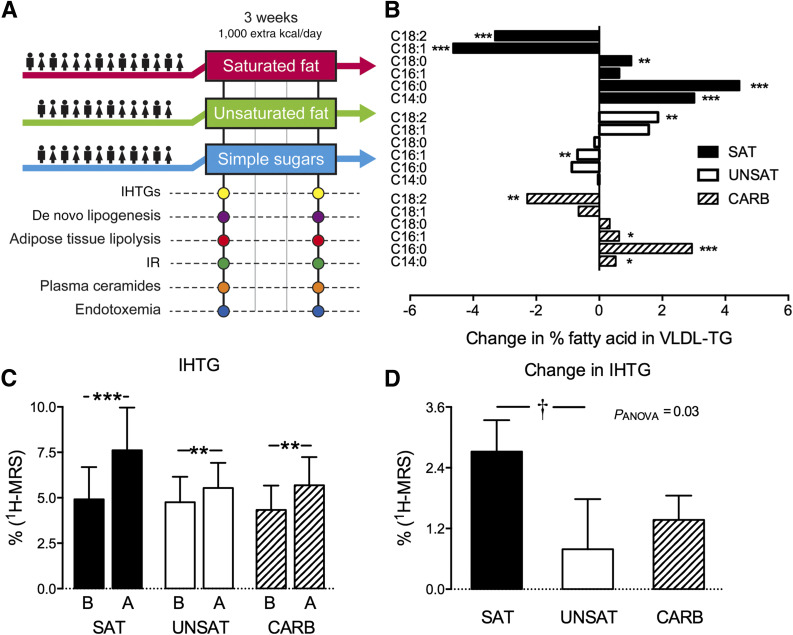
In the current study, we hypothesized that the metabolic effects of a hypercaloric diet depend on the macronutrient composition. Specifically, we hypothesized that 1) overconsumption of simple sugars stimulates DNL whereas saturated fat increases lipolysis, 2) overconsumption of simple sugars and saturated fat increases availability of SFAs and thereby ceramide synthesis, and 3) saturated but not unsaturated fat or simple sugar diets induce endotoxemia, which may further increase ceramide synthesis. Accordingly, we compared the effects of three hypercaloric (1,000 extra kcal/day for 3 weeks) diets on 1) IHTG content, 2) rates of DNL, 3) rates of lipolysis, 4) adipose tissue transcriptome, 5) plasma concentrations of ceramides, and 6) gut microbiota and a circulating marker of endotoxemia (Fig. 1A).
Figure 1.
Design of the study (A), overfeeding-induced changes in FA composition of VLDL-TG in the groups (B), IHTG before (B) and after (A) overfeeding (C), and changes in IHTG between the groups (D). The subjects were randomized into overfeeding saturated fat (SAT), unsaturated fat (UNSAT), or simple sugars (CARB) groups. All subjects underwent metabolic studies at baseline and after 3 weeks of overfeeding. At these visits, IHTG content was determined by 1H-MRS, hepatic DNL from 2H2O enrichment in VLDL-TG palmitate, adipose tissue lipolysis by [2H5]glycerol in the basal state and during euglycemic hyperinsulinemia, plasma ceramides by ultra high-performance liquid chromatography–mass spectrometry, and endotoxemia by fecal 16S rRNA and serum LBP-to-sCD14 ratio. In addition, adipose tissue transcriptome was determined by microarray. B: The x-axis shows the change in percentage FA in VLDL-TG after vs. before overfeeding, and the y-axis shows the specific FAs in VLDL-TG. Data are shown as mean ± SEM. *P < 0.05,**P < 0.01, and ***P < 0.001 within groups; †P < 0.05 between groups.
Research Design and Methods
Subjects
Subjects in this study (clinicaltrials.gov, NCT02133144) were recruited by newspaper advertisements or by contacting subjects who previously had participated in metabolic studies. Exclusion criteria included 1) type 1 or 2 diabetes, 2) preexisting autoimmune, viral, or drug-induced liver disease, 3) excessive use of alcohol (>20 g/day for women and >30 g/day for men), 4) evidence of any other acute or chronic disease, 5) extreme obesity (BMI ≥40 kg/m2), 6) use of drugs influencing glucose or lipid metabolism, and 7) pregnancy or lactation. All subjects suitable for the study based on a telephone interview were invited for a screening visit. The flowchart of study subjects is shown in Supplementary Fig. 1. The nature and potential risks of the study were explained to volunteers before obtaining written informed consent. The Helsinki University Hospital Ethics Committee approved the study protocol.
Study Design
The day before the metabolic study, a blood sample was taken for measurement of background enrichment of 2H in plasma water and VLDL-TG palmitate for measurement of DNL (Fig. 1A). Subjects then underwent measurement of IHTGs by proton MRS (1H-MRS). The following morning, subjects arrived at the clinical research unit after an overnight fast and after consuming deuterated water (2H2O) (3 g/kg body water) the evening before the study day to achieve a plasma water enrichment of 0.3% for the measurement of DNL. Fecal samples were self-collected and stored immediately at −20°C and within 24 h at −80°C until analysis. Body composition (InBody 720; BioSpace, Seoul, Korea), weight, and height were measured, and blood samples were taken for measurement of DNL and for liver function tests, fasting glucose, free FAs (FFAs), insulin, TGs, and total, HDL, and LDL cholesterol. Thereafter, a euglycemic-hyperinsulinemic clamp combined with infusion of [2H5]glycerol for measurement of lipolysis was performed.
After the baseline visit, the subjects were randomized to one of three groups to consume a hypercaloric (1,000 excess kcal/day) diet for 3 weeks, with excess energy originating predominantly from saturated fat (SAT, 76% from SFAs, 21% from monounsaturated FAs [MUFAs], and 3% from polyunsaturated FAs [PUFAs]), unsaturated fat (UNSAT, 57% from MUFAs, 22% from PUFAs, 21% from SFAs), or simple sugars (CARB, 100% simple sugars). The overfeeding diets were provided to participants and consisted in the SAT group of 30 g coconut oil, 40 g butter, and 100 g 40% fat containing blue cheese as extra energy per day; in the UNSAT group of 36 g olive oil, 26 g pesto, 54 g pecan nuts, and 20 g butter; and in the CARB group of 2.8 dL orange juice, 4.3 dL sugar-sweetened beverage, and 200 g candy. Of the overfeeding energy, 2%, 91%, and 7% came from carbohydrate, fat, and protein in the UNSAT group and 1%, 86%, and 13% from these sources in the SAT group, respectively, and 100% from simple sugars in the CARB group. After 3 weeks of consuming the hypercaloric diets, baseline measurements were repeated.
Adherence to the diets was reinforced by weekly contacts with the study dietitian and verified by 3-day dietary records, which were performed before and after 3 weeks on the diet, and by measuring the FA composition of fasting VLDL-TG as an objective biomarker of recent dietary FA intake (13). The food records were analyzed using the AivoDiet software (version 2.0.2.3; Aivo Finland, Turku, Finland).
Methods
The FA composition of VLDL-TG was assessed as described in the Supplementary Data. Respiratory gas exchange and rates of resting energy expenditure and substrate oxidation were recorded by indirect calorimetry as previously described (14). IHTG content was determined by 1H-MRS and visceral and subcutaneous fat by MRI using 1.5-T Siemens Avantofit as described (5). Fasting DNL was assessed based on the incorporation of deuterium from 2H2O in plasma water (Finnigan GasBench-II; Thermo Fisher Scientific, Loughborough, U.K.) into VLDL-TG palmitate using gas chromatography–mass spectrometry. Further details of DNL methodology are given in the Supplementary Data. Insulin action on serum FFAs and glycerol Ra were determined using the euglycemic-hyperinsulinemic clamp technique as previously described (5). The insulin infusion lasted 120 min (120–240), and the rate of the continuous insulin infusion was 0.4 mU/kg · min. Adipose tissue transcriptome and gut microbiota were analyzed as detailed in the Supplementary Data. Plasma ceramide analyses were performed as previously described (15). Serum lipopolysaccharide-binding protein (LBP) and soluble cluster of differentiation 14 (sCD14) were measured by quantitative ELISA using human LBP DuoSet and human CD14 DuoSet kits (R&D Systems, Minneapolis, MN). Plasma adiponectin (7) and other analytical procedures were assessed as previously described (15).
Power Calculation
IHTG was the primary outcome. We calculated from a previous study (2) that 12 subjects per group were needed to detect a 1.4% difference in change in liver fat between the three groups with an α of 0.05, β of 0.2, and SD of 1.0.
Statistics
Continuous variables were tested for normality using the Kolmogorov-Smirnov method. Changes between groups were compared using one-way ANOVA, followed by a Fisher least significant differences test to analyze the differences between groups. Nonparametric variables were log-transformed for analysis and back-transformed for presentation or were analyzed nonparametrically with the Kruskal-Wallis test. The paired Student t test was used to explore within-group effects of overfeeding. Categorical variables were analyzed with the Fisher exact test. Data are presented as the means with SDs for normally distributed variables and as medians (quartiles 1–3) for nonnormally distributed variables, unless otherwise specified. P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
Baseline studies were performed in 39 subjects, of which 38 completed the study. The SAT, UNSAT, and CARB groups were comparable with respect to age, sex, BMI, IHTG, body composition, and biochemical characteristics such as glucose, insulin, lipids, and liver enzymes (Table 1). Baseline composition of the diet (Supplementary Table 1) and the FA composition of VLDL-TG (Supplementary Table 2) were also comparable between the groups.
Table 1.
Baseline clinical characteristics of the study subjects according to diet group
| SAT | UNSAT | CARB | |
|---|---|---|---|
| Group size | 14 | 12 | 12 |
| Age (years) | 48 ± 8 | 52 ± 10 | 45 ± 10 |
| Sex | |||
| Women | 8 | 7 | 6 |
| Men | 6 | 5 | 6 |
| BMI (kg/m2) | 30 ± 6 | 31 ± 6 | 33 ± 6 |
| Fat free mass (kg) | 58.6 ± 9.7 | 60.2 ± 13.3 | 61.9 ± 12.3 |
| Liver fat by 1H-MRS (%) | 4.9 ± 6.6 | 4.8 ± 4.9 | 4.3 ± 4.7 |
| Adipose tissue by MRI (cm3) | |||
| Visceral | 1,940 ± 1,605 | 2,019 ± 1,328 | 2,014 ± 1,217 |
| Subcutaneous | 4,770 ± 2,152 | 4,732 ± 2,350 | 5,133 ± 2,154 |
| Waist circumference (cm) | 97 ± 17 | 98 ± 13 | 102 ± 12 |
| Waist-to-hip ratio | 0.90 ± 0.10 | 0.90 ± 0.07 | 0.92 ± 0.06 |
| LBP-to-sCD14 ratio | 4.3 ± 1.0 | 4.6 ± 0.9 | 4.9 ± 1.6 |
| fP-Glucose (mmol/L) | 5.6 ± 0.6 | 5.7 ± 0.6 | 5.9 ± 0.7 |
| Impaired fasting glucose (>5.6 mmol/L) | 8 | 6 | 8 |
| fS-Insulin (mU/L) | 8.1 (5.8–11.9) | 9.1 (6.5–14.6) | 10.3 (6.2–19.2) |
| HOMA-IR | 1.9 (1.3–3.2) | 2.3 (1.6–4.0) | 2.8 (1.7–5.0) |
| Blood pressure (mmHg) | |||
| Systolic | 133 ± 15 | 134 ± 17 | 139 ± 20 |
| Diastolic | 80 ± 11 | 83 ± 7 | 85 ± 13 |
| fP-TGs (mmol/L) | 1.1 ± 1.0 | 1.1 ± 0.4 | 1.4 ± 0.6 |
| fP-HDL cholesterol (mmol/L) | 1.62 ± 0.39 | 1.61 ± 0.47 | 1.53 ± 0.37 |
| fP-LDL cholesterol (mmol/L) | 3.2 ± 1.0 | 3.4 ± 0.8 | 3.5 ± 0.8 |
| fS-FFA (μmol/L) | 556 ± 217 | 610 ± 181 | 639 ± 225 |
| fP-Adiponectin (μg/mL) | 11.0 ± 4.3 | 10.5 ± 5.7 | 10.3 ± 5.8 |
| fP-Alanine aminotransferase (IU/L) | 28 ± 15 | 26 ± 9 | 24 ± 11 |
| fP-Aspartate aminotransferase (IU/L) | 26 ± 5 | 27 ± 7 | 26 ± 6 |
| PNPLA3 genotype at rs738409 | |||
| CC | 9 | 7 | 5 |
| CG | 5 | 4 | 4 |
| GG | 0 | 1 | 2 |
Data are n, mean ± SD, or median (25th–75th percentile), as appropriate. There were no significant differences in any variable between the groups using ANOVA, Kruskal-Wallis, and Fisher exact test, as appropriate. fP, fasting plasma; fS, fasting serum.
Compliance
Macronutrient Composition
At the end of the overfeeding period, fat comprised 60% (54–64) and 59% (53–61) of the total energy intake in the SAT and UNSAT groups, respectively. These percentages were twofold higher than in the CARB group (24% [20–26]) (Supplementary Table 1). Saturated fat intake was twofold higher in the SAT group (33% [28–36]) than in the UNSAT group (14% [14–18], P < 0.001). Monounsaturated (28% [23–30] UNSAT vs. 13% [12–15] SAT, P < 0.001) and polyunsaturated (11% [10–14] UNSAT vs. 5% [4–5] SAT, P < 0.001) fat intakes were twofold higher in the UNSAT than in the SAT group. The percentage of total energy intake from carbohydrate was 2.8-fold higher in the CARB group (64% [58–68]) than in the UNSAT (23% [19–29], P < 0.001) or the SAT (26% [23–32], P < 0.001) groups.
FA Composition of VLDL-TG
The FA composition of fasting plasma VLDL-TG was used to monitor compliance (Fig. 1B). During the SAT diet, the abundance of SFA in VLDL-TG increased significantly: 16:0 by 17% (26.2 ± 3.6 vs. 30.7 ± 3.6 mol% [P < 0.001], 18:0 (3.1 ± 1.2 vs. 4.1 ± 1.2 mol% [P < 0.01]), and 14:0 (1.8 ± 0.7 vs. 4.8 ± 1.7 mol% [P < 0.001]). The abundance of 18:2 decreased significantly during the SAT diet. During the UNSAT diet, 18:2 increased significantly (P < 0.01). During the CARB diet, the abundance of 16:0, 14:0, and 16:1 increased and 18:2 decreased significantly (P < 0.05) (Fig. 1B).
Body Weight and Composition
Body weight in all subjects increased by 1.4 ± 1.5% from 92.1 ± 17.0 to 93.3 ± 17.2 kg (P < 0.001). Changes in body weight averaged 1.4 ± 1.2 kg in the SAT, 0.9 ± 1.1 kg in the UNSAT, and 1.4 ± 1.6 kg in the CARB groups (P = NS). Visceral (1,940 ± 1,605 vs. 2,072 ± 1,674 cm3; 2,019 ± 1,328 vs. 2,080 ± 1,361 cm3; 2,014 ± 1,217 vs. 2,115 ± 1,351 cm3, before vs. after, respectively, in the SAT, UNSAT, and CARB groups) and subcutaneous (4,770 ± 2,152 vs. 4,906 ± 2,090 cm3; 4,732 ± 2,350 vs. 4,805 ± 2,473 cm3; 5,133 ± 2,154 vs. 5,204 ± 2,157 cm3, respectively) fat volumes tended to increase but did not change significantly during overfeeding.
Energy Expenditure and Substrate Oxidation Rates
As expected, overfeeding increased resting energy expenditure significantly from 7.49 ± 1.36 to 7.61 ± 1.48 MJ/day (P < 0.05) in all subjects. Rates of energy expenditure and substrate oxidation rates expressed per kilogram body weight or fat-free mass remained unchanged in all subjects, within and between groups (data not shown). Nonprotein respiratory quotient did not change with overfeeding (data not shown).
IHTGs
IHTGs increased by 55% (4.9 ± 6.6 vs. 7.6 ± 8.8%, P < 0.001) in the SAT group, by 15% (4.8 ± 4.9 vs. 5.5 ± 4.8%, P < 0.02) in the UNSAT group, and by 33% (4.3 ± 4.7 vs. 5.7 ± 5.4%, P < 0.02) in the CARB group (Fig. 1C). The increase was significantly greater in the SAT than the UNSAT group (P < 0.01) (Fig. 1D). This difference was independent of changes in body weight as determined by the significantly different intercepts of the linear regression lines between change in IHTGs and change in body weight in the SAT versus the UNSAT group (P < 0.05) (Supplementary Fig. 2).
Hepatic DNL
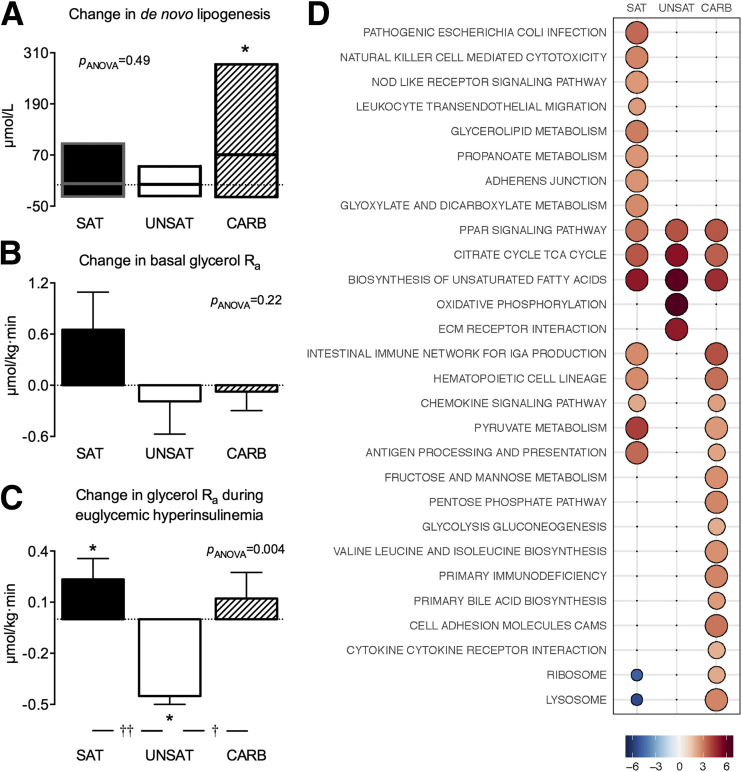
Hepatic DNL, as determined by the amount of newly synthesized palmitate in VLDL-TG, increased significantly during the CARB diet (96 [47–116] vs. 190 [61–303] µmol/L, P < 0.05) but not during the other diets (Fig. 2A).
Figure 2.
Overfeeding-induced changes in hepatic de novo lipogenesis (A), basal glycerol Ra (B), and glycerol Ra (C) during euglycemic hyperinsulinemia. The y-axes indicate the change of mean values after vs. before overfeeding within groups. Data are shown as medians (interquartile ranges) in panel A and as mean ± SEM in B and C. *P < 0.05 within group; †P < 0.05 and ††P < 0.01 between groups. D: Adipose tissue transcriptome. The bubble grid shows the reporter test statistics (proportional to size and color intensity) of relative gene expression after compared with before overfeeding. Only pathways significant in at least one diet are shown (<5% false discovery rate).
Lipolysis
Basal State
Fasting serum insulin increased significantly with overfeeding in the SAT group (8.1 [5.8–11.9] vs. 9.5 [5.9–11.9] mU/L, P < 0.05) but remained unchanged in the UNSAT (9.1 [6.5–14.6] vs. 9.5 [7.8–11.1] mU/L, P = NS) and CARB (10.3 [6.2–19.2] vs. 11.1 [6.9–23.8] mU/L, P = NS) groups. Basal whole-body glycerol Ra remained unchanged in all groups (Fig. 2B).
Euglycemic Hyperinsulinemia
During hyperinsulinemia, increases in serum insulin concentrations were similar before and after the diets (22 ± 7 vs. 22 ± 6 mU/L, P = NS), with no differences between the SAT, UNSAT, and CARB groups (data not shown). Whole-body glycerol Ra during euglycemic hyperinsulinemia compared with baseline increased in the SAT (2.08 ± 0.46 vs. 2.31 ± 0.59 μmol/kg · min, P < 0.05), decreased in the UNSAT (2.59 ± 0.87 vs. 2.14 ± 0.77 μmol/kg · min, P < 0.05), and remained unchanged in the CARB (2.15 ± 0.74 vs. 2.27 ± 0.64 μmol/kg · min, P = NS) group after overfeeding (Fig. 2C). Whole-body glycerol Ra during euglycemic hyperinsulinemia increased significantly more in the SAT compared with the UNSAT (P < 0.001) and in the CARB compared with the UNSAT (P < 0.01) group (Fig. 2C).
Serum FFAs
Serum free fatty acid concentrations, which reflect the net effects of lipolysis and lipogenesis, remained unchanged in the SAT group but decreased significantly during hyperinsulinemia by overfeeding in the UNSAT and CARB groups (Supplementary Fig. 3).
Adipose Tissue Transcriptome
Gene set analysis identified 28 reporter pathways of 134 curated Kyoto Encyclopedia of Genes and Genomes pathways at a 5% false discovery rate. The SAT and CARB diets changed 18 pathways, and the UNSAT diet changed 5 pathways, which were highly distinctive between the diets. Only three pathways overlapped between all diets (Fig. 2D). The pathways upregulated by the SAT diet included those related to inflammation, such as genes related to Escherichia coli infection, natural killer cell–mediated cytotoxicity, nucleotide-binding oligomerization domain-like receptor signaling, and leukocyte transendothelial migration, and to glycerolipid metabolism. The SAT and CARB diets shared some pathways related to inflammation, such as genes related to antigen processing, chemokine signaling, and hematopoietic cell lineage. In addition, the CARB diet induced pathways related to carbohydrate metabolism, such as fructose and mannose metabolism, pentose phosphate pathway, and glycolysis/gluconeogenesis. The UNSAT diet upregulated pathways related to oxidative phosphorylation and extracellular matrix.
IR and Plasma Ceramides
In the SAT group, HOMA-IR increased significantly by 23% (1.9 [1.3–3.2] vs. 2.2 [1.4–3.3], P < 0.05). Total plasma ceramide concentration also increased significantly by 49% (P < 0.001) in the SAT group. In contrast, there were no changes in plasma ceramides in the UNSAT or CARB groups. The increase in total plasma ceramides was significantly higher in the SAT compared with the UNSAT (P < 0.05) and the CARB (P < 0.001) groups. This difference was independent of changes in body weight as determined by the significantly different intercepts of the linear regression lines between change in total plasma ceramides and change in body weight in the SAT versus the UNSAT (P < 0.05) and in the SAT versus the CARB (P < 0.001) groups.
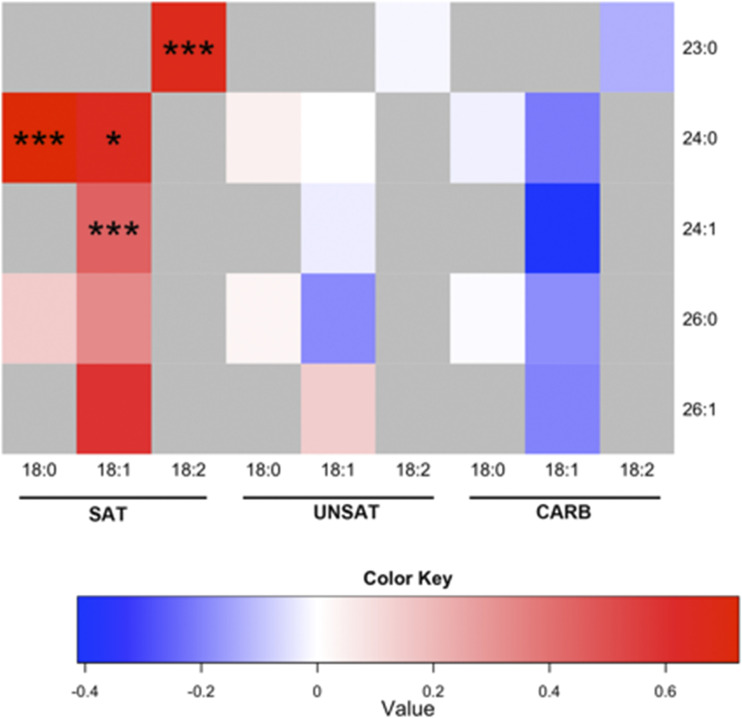
The increase in total plasma ceramide concentration in the SAT group was due to increases in several long-chain ceramides (Fig. 3 and Supplementary Table 3). Plasma concentrations of dihydroceramides (i.e., the precursors of ceramides in the de novo ceramide synthetic pathway, the species with 18:0 sphingoid base) were also increased in the SAT but not in the other groups (Fig. 3 and Supplementary Table 3).
Figure 3.
Overfeeding-induced changes in individual plasma ceramides in the groups. Each square in the heat map indicates the log2 of the ratio between mean concentrations after vs. before for an individual ceramide. The color key denotes the relationship between the color of the heat map and log2 of the ratio between the means, with 0 indicating no change. The y-axis denotes the fatty acyl chain structure (number of carbon atoms:number of double bonds), and the x-axis indicates the sphingoid base species. *P < 0.05; ***P < 0.001.
Gut Microbiota and LBP-to-sCD14 Ratio
Serum LBP-to-sCD14 ratio, a marker of endotoxemia (16), increased significantly (4.3 ± 1.0 vs. 4.7 ± 1.1 [P < 0.01]) in the SAT group but not in the other groups. The increase in LBP-to-sCD14 ratio was significantly higher in the SAT compared with UNSAT (P < 0.05) and CARB (P < 0.001) groups. We found 79% of the between-sample gut microbiota variation was explained by the subject (P < 0.001) (Supplementary Fig. 4), highlighting overall resilience of the individual-specific microbiota during overfeeding. We next analyzed individual taxa that were affected by overfeeding (Supplementary Fig. 5). The abundance of gram-negative Proteobacteria increased 3.6-fold during the SAT (P = 0.038) but not the other diets (Supplementary Fig. 6). Other bacterial families remained unchanged.
Plasma Lipids, Adiponectin, and Liver Enzymes
Baseline concentrations are reported in Table 1. Plasma HDL cholesterol increased significantly by 17% in the SAT (+0.3 ± 0.3 mmol/L, P < 0.01 for after vs. before) but not in the UNSAT (+0.1 ± 0.3 mmol/L) or CARB (−0.1 ± 0.2 mmol/L) groups. The increase in HDL cholesterol was significantly greater in the SAT than in the CARB group (P < 0.001). Plasma LDL cholesterol increased by 10% in the SAT group (+0.3 ± 0.4 mmol/L, P < 0.01) but remained unchanged in the UNSAT and CARB groups. No significant changes occurred in plasma TGs or adiponectin in the groups. Plasma alanine aminotransferase increased significantly in the SAT (28 ± 15 vs. 35 ± 18 IU/L, P < 0.05) but not in the UNSAT (26 ± 9 vs. 27 ± 5 IU/L) or CARB (24 ± 11 vs. 28 ± 18 IU/L) groups. Plasma aspartate aminotransferase increased in the SAT (26 ± 5 vs. 29 ± 6 IU/L, P < 0.05) but remained unchanged in the UNSAT and CARB groups.
Conclusions
NAFLD has been shown to predict type 2 diabetes and cardiovascular disease in multiple studies, even independent of obesity (1), and also to increase the risk of progressive liver disease (17). It is therefore interesting to compare effects of different diets on liver fat content and understand the underlying mechanisms. We examined whether provision of excess calories as saturated (SAT) or unsaturated (UNSAT) fats or simple sugars (CARB) influences the metabolic response to overfeeding in overweight subjects. All overfeeding diets increased IHTGs. The SAT diet induced a greater increase in IHTGs than the UNSAT diet. The composition of the diet altered sources of excess IHTGs. The SAT diet increased lipolysis, whereas the CARB diet stimulated DNL. The SAT but not the other diets increased multiple plasma ceramides, which increase the risk of cardiovascular disease independent of LDL cholesterol (18).
Compliance
The FA composition of liver TG is similar to that in VLDL (4). We monitored compliance by analyzing changes in FA composition of VLDL-TG. The SAT diet increased SFA, and the UNSAT diet increased PUFA (Fig. 1B). The CARB diet also increased SFAs, which are exclusive products of DNL (5). These data demonstrate that subjects were compliant and are novel in showing that different diets have distinct effects on hepatic FA composition as determined from that in VLDL-TG. Weight gain averaged 1.2 kg in all subjects overeating 1,000 kcal for 3 weeks. This weight gain is consistent with the gain of 1.6 kg observed by Risérus and colleagues (2) in healthy subjects overeating 600 kcal/day for 7 weeks but less than the gain of 2.3 kg observed by Harris et al. (19) during the first 3 weeks of overfeeding 1,000 kcal/day. As in the current study, interindividual variation in the latter study was large and ranged from 1 to 7 kg after 8 weeks (19). We also observed slightly but not significantly less weight gain in subjects fed the UNSAT diet compared with the other diets. However, the important key findings (i.e., the greater increase in liver fat, lipolysis, and ceramides by the SAT compared with the UNSAT diet) were independent of changes in body weight.
IHTG
Overfeeding increases IHTG (2,5,20). The SAT diet increased IHTG significantly more than the UNSAT diet in the face of similar energy excess and independent of changes in body weight. This is consistent with a previous study showing a greater increase in IHTG after overfeeding saturated than polyunsaturated fat (2). Similarly, an isocaloric saturated fat-enriched diet increases IHTG compared with polyunsaturated fat (21). High saturated fat intakes characterize subjects with NAFLD (22,23). Thus, diet composition influences IHTG, and saturated fat induces a greater accumulation of IHTG than unsaturated fat.
Pathways of IHTG
Regarding the mechanisms underlying increased IHTG during overfeeding, direct quantification of sources of FAs in IHTG using stable isotopes and liver biopsy specimens in subjects with elevated IHTG has shown that most IHTGs are derived from adipose tissue lipolysis (59%) and DNL (26%) (4). We found SAT increased and UNSAT decreased adipose tissue lipolysis. Lipolysis was traced using deuterated glycerol, which is not reesterified in adipose tissue due to a lack of glycerol kinase. This contrasts with serum FFA, the concentration of which reflects net effects of lipolysis and lipogenesis. Overfeeding decreased serum FFA, as reported previously (20). No studies have measured overfeeding effects on lipolysis in humans. High saturated fat–feeding in mice stimulates lipolysis via inflammatory mediators (6). In keeping with such data, we found upregulation of multiple inflammation-related pathways in the adipose tissue transcriptome. PUFAs can inhibit lipolysis by activation of G-protein–coupled receptor 120, which mediates anti-inflammatory and insulin-sensitizing effects (24,25). The opposite effects of the SAT and UNSAT diets on lipolysis, the major contributor to IHTG, could explain why the SAT diet increased IHTG to a greater extent than the UNSAT diet.
Overfeeding simple sugars stimulates DNL, which produces exclusively SFA in humans and occurs mainly in the liver (5). Consistent with these data, the CARB diet increased hepatic DNL and SFAs in VLDL-TG (Figs. 1B and 2A). The CARB diet also induced multiple pathways related to carbohydrate metabolism in the adipose tissue transcriptome (Fig. 2D).
IR and Its Mediators
The SAT diet induced IR. This is consistent with several studies showing that isocaloric substitution of saturated fat with monounsaturated (26,27) or polyunsaturated fat (28) or carbohydrates (27) ameliorates IR. A large prospective study recently reported intake of foods rich in SFA, such as butter and cheese, increased the risk of type 2 diabetes (29).
SAT but not the other diets increased multiple plasma ceramides. TGs themselves are inert and do not confer IR (7). Ceramides are key mediators of saturated fat-induced IR (8–10,30–33) in mice and are the most upregulated lipid species in inflamed adipose tissue in human NAFLD (34). SFAs and ceramides originating from de novo ceramide synthesis cosegregate with IR in human NAFLD (7). Saturated palmitoyl-CoA is an obligate precursor for the de novo pathway (8,30). The SAT diet thus may have induced IR via stimulating the de novo ceramide synthetic pathway in the liver. The SAT but not the other diets also increased markers of endotoxemia and upregulated genes related to gram-negative bacterial infection in adipose tissue. These changes could have contributed to SAT-induced lipolysis and de novo ceramide synthesis, because in mice, endotoxin induces both adipose tissue inflammation (11) and ceramide-dependent IR (10).
Limitations of our study include small sample size although the largest of its kind (35). Another limitation is that we did not assess physical activity objectively during the study. Including a control group being overfed their habitual diet might also have been helpful.
In conclusion, overfeeding saturated or unsaturated fat or simple sugars for 3 weeks increases IHTG, but its magnitude and the associated metabolic changes depend on the diet. Saturated fat induced the highest increase in IHTG by stimulating adipose tissue lipolysis, the major pathway of IHTG. Moreover, saturated fat induced IR and increased circulating concentrations of ceramides. In contrast to saturated fat, overfeeding of unsaturated fat led to a smaller increase in IHTG, decreased lipolysis, and no change in ceramides. Simple sugars increased IHTG by stimulating DNL. Consistent with current dietary recommendations (36–38), the current study shows that saturated fat is the most harmful dietary constituent regarding IHTG accumulation. Because NAFLD increases the risk of type 2 diabetes, avoidance of foods rich in saturated fats might also help in prevention of diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors gratefully acknowledge Anne Salo, Aila Karioja-Kallio, Päivi Ihamuotila, Pentti Pölönen, Leena Kaipiainen, and Raisa Harjula (Helsinki University Hospital) as well as Catriona Charlton (University of Oxford) for skillful technical assistance, A. Margot Umpleby (University of Surrey) for advice on stable isotope methodology, and Siiri Luukkonen (University of Oulu) for graphical assistance.
Funding. This study was supported by grants from Suomen Lääketieteen Säätiö (P.K.L.), Yrjö Jahnssonin Säätiö (P.K.L.), Emil Aaltosen Säätiö (P.K.L.), the Diabetes Research Foundation (P.K.L.), Helsingin Yliopisto (P.K.L. and A.S.), the Elucidating Pathways of Steatohepatitis consortium funded by the Horizon 2020 Framework Program of the European Union under grant Agreement EPoS 634413 (B.K., T.H., M.O., A.G., K.C., and H.Y.-J.), Ministero dell'Istruzione, dell'Università e della Ricerca and Consiglio Nazionale delle Ricerche (A.G.), PIA-F-Crin Force program (V.P. and K.C.), the British Heart Foundation Intermediate Fellowship in Basic Science (FS/11/18/28633) (L.H.), Suomen Akatemia (H.Y.-J.), the Sigrid Juselius Foundation (H.Y.-J.,), Evo (H.Y.-J.), the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF 115372, H.Y.-J.), and Novo Nordisk Foundation (H.Y.-J.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.K.L. contributed to conducting the clinical research; acquisition, analysis, and interpretation of data; and drafting and critical revision of the manuscript. S.S. contributed to study diet design, conducting the clinical research, and critical revision of the manuscript. Y.Z. and B.K. contributed to analysis and interpretation of the data and critical revision of the manuscript. A.A., L.A., S.L., V.P., M.G., C.J., A.H., N.L., H.G., M.O., T.H., M.O.-M., A.G., and K.C. contributed to acquisition of data and critical revision of the manuscript. A.S., A.R., and L.H. contributed to acquisition, analysis, and interpretation of data and to critical revision of the manuscript. H.Y.-J. contributed to study concept and design, analysis and interpretation of the data, drafting and critical revision of the manuscript, and study supervision. H.Y.-J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 52nd Annual Meeting of the European Association for the Study of Diabetes, Munich, Germany, 12–16 September 2016, and at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
Clinical trial reg. no. NCT02133144, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0071/-/DC1.
References
- 1.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014;2:901–910 [DOI] [PubMed] [Google Scholar]
- 2.Rosqvist F, Iggman D, Kullberg J, et al. . Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014;63:2356–2368 [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevastianova K, Santos A, Kotronen A, et al. . Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012;96:727–734 [DOI] [PubMed] [Google Scholar]
- 6.Wueest S, Item F, Lucchini FC, et al. . Mesenteric fat lipolysis mediates obesity-associated hepatic steatosis and insulin resistance. Diabetes 2016;65:140–148 [DOI] [PubMed] [Google Scholar]
- 7.Luukkonen PK, Zhou Y, Sädevirta S, et al. . Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 2016;64:1167–1175 [DOI] [PubMed] [Google Scholar]
- 8.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–594 [DOI] [PubMed] [Google Scholar]
- 9.Xie C, Jiang C, Shi J, et al. . An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 2017;66:613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland WL, Bikman BT, Wang LP, et al. . Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 2011;121:1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani PD, Amar J, Iglesias MA, et al. . Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 12.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 2015;22:658–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–380 [DOI] [PubMed] [Google Scholar]
- 14.Yki-Järvinen H, Puhakainen I, Saloranta C, Groop L, Taskinen MR. Demonstration of a novel feedback mechanism between FFA oxidation from intracellular and intravascular sources. Am J Physiol 1991;260:E680–E689 [DOI] [PubMed] [Google Scholar]
- 15.Luukkonen PK, Zhou Y, Nidhina Haridas PA, et al. . Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J Hepatol 2017;67:128–136 [DOI] [PubMed] [Google Scholar]
- 16.Laugerette F, Alligier M, Bastard JP, et al. . Overfeeding increases postprandial endotoxemia in men: inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol Nutr Food Res 2014;58:1513–1518 [DOI] [PubMed] [Google Scholar]
- 17.Dongiovanni P, Stender S, Pietrelli A, et al. . Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med 2018;283:356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers SA. Could ceramides become the new cholesterol? Cell Metab 2018;27:276–280 [DOI] [PubMed] [Google Scholar]
- 19.Harris AM, Jensen MD, Levine JA. Weekly changes in basal metabolic rate with eight weeks of overfeeding. Obesity (Silver Spring) 2006;14:690–695 [DOI] [PubMed] [Google Scholar]
- 20.Sobrecases H, Lê KA, Bortolotti M, et al. . Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 2010;36:244–246 [DOI] [PubMed] [Google Scholar]
- 21.Bjermo H, Iggman D, Kullberg J, et al. . Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012;95:1003–1012 [DOI] [PubMed] [Google Scholar]
- 22.Tiikkainen M, Bergholm R, Vehkavaara S, et al. . Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes 2003;52:701–707 [DOI] [PubMed] [Google Scholar]
- 23.Musso G, Gambino R, De Michieli F, et al. . Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003;37:909–916 [DOI] [PubMed] [Google Scholar]
- 24.Oh DY, Talukdar S, Bae EJ, et al. . GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Zhang X, Ma LJ, et al. . Omega-3 polyunsaturated fatty acids ameliorate ethanol-induced adipose hyperlipolysis: a mechanism for hepatoprotective effect against alcoholic liver disease. Biochim Biophys Acta 2017;1863:3190–3201 [DOI] [PubMed] [Google Scholar]
- 26.Vessby B, Uusitupa M, Hermansen K, et al.; KANWU Study . Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 2001;44:312–319 [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Jiménez F, López-Miranda J, Pinillos MD, et al. . A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetologia 2001;44:2038–2043 [DOI] [PubMed] [Google Scholar]
- 28.Summers LK, Fielding BA, Bradshaw HA, et al. . Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002;45:369–377 [DOI] [PubMed] [Google Scholar]
- 29.Guasch-Ferré M, Becerra-Tomás N, Ruiz-Canela M, et al. . Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr 2017;105:723–735 [DOI] [PubMed] [Google Scholar]
- 30.Holland WL, Brozinick JT, Wang LP, et al. . Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 31.Raichur S, Wang ST, Chan PW, et al. . CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 2014;20:687–695 [DOI] [PubMed] [Google Scholar]
- 32.Turpin SM, Nicholls HT, Willmes DM, et al. . Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 2014;20:678–686 [DOI] [PubMed] [Google Scholar]
- 33.Xia JY, Holland WL, Kusminski CM, et al. . Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab 2015;22:266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolak M, Westerbacka J, Velagapudi VR, et al. . Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 2007;56:1960–1968 [DOI] [PubMed] [Google Scholar]
- 35.Yki-Järvinen H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients 2015;7:9127–9138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015-2020 Dietary Guidelines for Americans, 8th ed. [Internet], 2015. Washington, DC, U.S. Department of Health and Human Services. Available from http://www.health.gov/DietaryGuidelines. Accessed 11 August 2017
- 37.American Diabetes Association Lifestyle management. Sec 4. In Standards of Medical Care in Diabetes–2018. Diabetes Care 2018;41(Suppl. 1):S38–S50 [DOI] [PubMed] [Google Scholar]
- 38.Sacks FM, Lichtenstein AH, Wu JHY, et al.; American Heart Association . Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 2017;136:e1–e23 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.