Abstract
OBJECTIVE
Trimethylamine N-oxide (TMAO) is suggested as an independent gut microbiota–derived risk factor for cardiovascular and renal disease. We investigated associations between plasma TMAO concentrations and cardio-renal outcomes in a prospective study of individuals with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Plasma TMAO was measured at baseline in 1,159 individuals with type 1 diabetes (58% male, mean ± SD age 46 ± 13 years). End points were all-cause and cardiovascular mortality, cardiovascular disease (CVD), and renal events tracked from national registries. Associations between TMAO and end points were tested using Cox regression models.
RESULTS
After 15.0 (6.7–19.3) (median [interquartile range]) years of follow-up, we recorded all-cause and cardiovascular mortality (n = 363 and 120, respectively), combined CVD (n = 406), coronary outcome (myocardial infarction and coronary intervention) (n = 163), stroke (n = 115), hospitalization for heart failure (n = 81), and end-stage renal disease (n = 144). In univariate analyses, higher TMAO concentrations were associated with all end points (P ≤ 0.005). Except for stroke and heart failure, all end points remained significantly associated with higher TMAO concentrations after adjustment for conventional cardiovascular risk factors (P ≤ 0.003). After further adjustment for baseline estimated glomerular filtration rate (eGFR), results became insignificant for all end points. TMAO was inversely associated with baseline eGFR (R2 = 0.29; P < 0.001).
CONCLUSIONS
In individuals with type 1 diabetes, higher concentrations of plasma TMAO were associated with mortality, CVD events, and poor renal outcome, independent of conventional risk factors. However, the association became insignificant after further adjustment for baseline eGFR. This could reflect TMAO as a renal function marker or a risk factor for micro- and macrovascular complications mediated through impaired renal function.
Introduction
Individuals with type 1 diabetes are at increased risk of developing micro- and/or macrovascular complications, including heightened mortality (1). Although prevention and treatment of diabetic complications have improved, cardiovascular disease (CVD) and renal disease remain prevalent and often lethal (2). Conventional CVD risk factors and kidney function measures are important for risk stratification to guide treatment, and the pursuit for new risk prediction markers is important to enable earlier treatment for prevention of type 1 diabetes–related complications (1). Recently, the gut microbiota and its metabolites have gained interest as potential markers and mediators of disease, such as development of atherosclerosis and chronic kidney disease (CKD) (3,4).
Trimethylamine N-oxide (TMAO) is a metabolite (amine oxide) derived from a gut microbiota–dependent pathway using nutrients abundant in a Western diet, including phosphatidylcholine, choline, and carnitine (5). For example, dietary and biliary phosphatidylcholine, the major sources of choline in the gut, are metabolized by the gut microbiota to produce trimethylamine (TMA) as a waste product (5). TMA is highly permeable and readily enters into the portal circulation (6), whereupon it is converted in the liver to TMAO by enzymes of the flavin monooxygenase family (7). TMAO is mainly eliminated from the circulation through the kidneys.
TMAO has been proposed as a marker of heightened CVD risks in humans, also facilitating enhanced atherosclerosis in animal models (5,8,9). Moreover, studies suggest that TMAO is mechanistically linked to the pathogenesis of CVD, with alterations in tissue sterol metabolism and vascular cell activation of proinflammatory pathways (8,10). Circulating concentrations of plasma TMAO are higher in individuals with type 2 diabetes, and increased TMAO concentrations are independently associated with risk of CVD and its adverse events, independent of glycemic control (11).
To our knowledge, the relationship between plasma TMAO concentrations and the risks of adverse cardiovascular and renal events in individuals with type 1 diabetes has not yet been reported. Therefore, we investigated the additive prognostic value of the gut microbiota–dependent metabolite TMAO and incident CVD and renal complications, as well as mortality, in a well-characterized Danish cohort of individuals with type 1 diabetes (n = 1,159) with a long-term follow-up.
Research Design and Methods
Study Population
A total of 1,159 subjects with type 1 diabetes were recruited from the outpatient clinic at Steno Diabetes Center Copenhagen in Denmark. Baseline examination was performed between 1993 and 2001 and 2009 and 2011. The cohort was originally recruited with the purpose of studying development of diabetic late complications. In the first part (1993–2001), the original purpose was to study genetic risk factors related to late complications, and in the second part (2009–2011), the focus was to study associations between blood pressure and arterial stiffness for development of diabetic late complications. Details of the cohort and the original purposes have been published previously (12,13). The study conformed with the Declaration of Helsinki and was approved by the ethics committees in Denmark (journal number H-16027336). Written informed consent was obtained from each participant at study recruitment.
Measurement of Plasma TMAO Concentration
Plasma samples obtained at the cohort baseline evaluation were used for measuring circulating TMAO concentrations. Samples were collected in EDTA tubes, centrifuged, and stored immediately after collection in freezers at −80°C until analysis of all samples at the same time point. Plasma concentrations of TMAO were measured using stable isotope dilution liquid chromatography tandem mass spectrometry technique by the Lerner Research Institute and Heart and Vascular Institute, Cleveland Clinic, with lower and upper limits of quantification of 0.05 and >200 μmol/L, respectively. Quality control samples were run with each batch, and interbatch variation (expressed as coefficient of variation) was <10%.
Other Baseline and Laboratory Measurements
Medical history, venous blood samples, urine analyses, and vital sign variables were obtained for all subjects at baseline. The medical history, including information on history of CVD, was obtained through medical records and interview by the physician using the Rose angina questionnaire. Office blood pressure was calculated as the average of two measurements taken after 10-min rest using a validated device and an appropriate cuff size. Smoking status was obtained through a standardized questionnaire. Current smoking was defined as one or more cigarettes, cigars, or pipes per day. Cholesterol concentrations were determined in serum by standard methods. HbA1c was measured by high-performance liquid chromatography. Level of serum creatinine was determined by a modified Jaffe method until 1 September 2004 and, thereafter, by an enzymatic reaction (isotope dilution mass spectrometry). Therefore, the serum creatinine measurements before 1 September 2004 were transformed from Jaffe to isotope dilution mass spectrometry (14). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (15). A subset of 378 subjects with macroalbuminuria had 51chromium-EDTA (51Cr-EDTA) clearance glomerular filtration rate (GFR) measured at baseline and annually, with a median (interquartile range [IQR]) follow-up of 6.8 (3–12.9) years (16). Linear regression analysis of the GFR determinations in each individual were used to estimate the rate of renal function decline. The urinary albumin excretion rate (UAER) was measured in 24-h urine collections by an enzyme immunoassay. Subjects were categorized as 1) normoalbuminuric if UAER was <30 mg/day, 2) microalbuminuric if UAER was or previously had been recorded between 30 and 299 mg/day, and 3) macroalbuminuric if UAER was or previously had been recorded at ≥300 mg/day in two of three consecutive measurements. All subjects classified as normoalbuminuric did not have any history of micro- or macroalbuminuria before enrollment in the study.
Follow-up Data
We traced all individuals through the Danish National Health Registry and the Danish National Death Registry. Reporting of data to these registries is mandatory and has nationwide coverage and high validity (17,18). Data on morbidity were obtained from the Danish National Health Registry, where diagnoses from all hospital admissions and outpatient visits are reported according to the ICD-10 classification (www.who.int/classifications/icd/en) and procedural codes according to the Nordic Classification of Surgical Procedures (www.sst.dk). ICD-10 diagnoses were available from January 1994 because of changes in the diagnosis classification from ICD-8 to ICD-10. All data from the Danish National Health Registry were available until 31 December 2016. For deceased subjects, the date and cause of death were obtained from the Danish National Death Registry. Information on cause of death was available until 31 December 2015. All deaths were classified as CVD related unless an unequivocal noncardiovascular cause was reported, a previously recognized approach (19). Cause of death was unknown in 20 subjects (5.5% of all deaths). All subjects were followed until emigration, death, or 31 December 2016, whichever came first.
CVD end points were defined as 1) combined CVD comprising CVD-related death, ischemic heart disease (including nonfatal myocardial infarction [ICD-10 codes I20–I25]), nonfatal stroke, coronary interventions, or peripheral arterial interventions (including amputations); 2) coronary event (nonfatal and fatal myocardial infarction [ICD-10 codes I21–I24]) or coronary intervention (percutaneous arterial intervention or coronary bypass grafting [procedural codes KFNA–KFNG]); 3) nonfatal or fatal stroke (ICD-10 codes I61–I66); and 4) hospitalization as a result of heart failure (ICD-10 code I50) (see Supplementary Table 1 for procedural codes).
Development of end-stage renal disease (ESRD) was defined as CKD stage 5 (ICD-10 code N18.5), initiation of permanent dialysis, renal transplantation (see Supplementary Table 1 for procedural codes), eGFR <15 mL/min/1.73 m2, or death as a result of chronic renal failure. Information regarding cause of ESRD was not available. Measurement of plasma creatinine during follow-up was obtained from the local electronic laboratory system for calculation of eGFR with the Chronic Kidney Disease Epidemiology Collaboration equation. Subjects who experienced multiple events were followed in the analyses until occurrence of the first event within each end point.
Statistical Methods
All normally distributed variables are presented as mean ± SD and nonnormally distributed variables as median (IQR). Categorical variables are presented as total numbers with corresponding percentages. TMAO and UAER were not normally distributed and are summarized as medians with IQR and log2 transformed in all analyses. Baseline characteristics in quartiles of plasma concentrations of TMAO and in groups divided according to albuminuria level were compared using ANOVA for continuous variables and χ2 test for categorical variables.
Cox proportional hazards analyses were applied to compute hazard ratios (HRs) with 95% CIs per doubling of TMAO for all specified end points. First, we applied unadjusted models (model 1) to determine whether any association existed between TMAO and the predefined end points. The subsequent adjustment included age, sex, diabetes duration, HbA1c, systolic blood pressure, total cholesterol, smoking status, and UAER (model 2). To assess whether plasma concentration of TMAO was a predictor independent of renal function, adjustment for baseline eGFR was included in the final model (model 3). These analyses were applied in the total population and stratified in subjects with normo- and macroalbuminuria. Cox model assumption for proportional hazards was tested using Schoenfeld residuals plots and was fulfilled for all end points. Moreover, we tested the assumption of linearity of the logarithm of TMAO for the outcomes by plotting parameter estimates of quintiles versus means of each quintile and by demonstrating that parameter estimates of quintiles did not differ significantly from 0 in a model containing the continuous variable. For all end points, the assumption was fulfilled.
For consideration of power, a post hoc simulation–based power calculation was performed. The empirical distribution of the baseline dates was taken into account. For all-cause mortality, the test of no linear effect has a power of at least 80% for values of the true hazard rate outside the interval (0.86–1.15), and for combined CVD, the test of no linear effect has a power of at least 80% for values of the true hazard rate outside the interval (0.85–1.14).
To examine the potential added predictive ability of TMAO over traditional risk factors, we calculated the relative integrated discrimination index (rIDI). This measure is suggested as a powerful method to demonstrate improved diagnostic performance of a biomarker (20).
Moreover, we applied Kaplan-Meier survival function estimates and the log-rank test to compare the incident rates in quartiles of plasma concentrations of TMAO. Yearly change in eGFR (slopes) was calculated among 1,013 subjects on the basis of all the available measurements from outpatient visits during follow-up, with at least two measurements and a minimum follow-up duration of 3 years.
For database management and statistical analysis, we used SAS 9.4 software (SAS Institute, Cary, NC), and for estimating eGFR slopes, we used general linear modeling in R (www.r-project.org). Statistical significance was set at a two-sided α-level of 0.05.
Results
Baseline Characteristics
The population included 1,159 individuals (58% men) with mean ± SD age of 46 ± 13 years. Diabetes duration was 27 ± 12 years at baseline, and 128 (11.3%) had a history of CVD. The eGFR averaged 88.8 ± 26.1 mL/min/1.73 m2, and median (IQR) UAER was 23 (8–360) mg/day. The median plasma TMAO concentration was 5.7 (3.8–9.9) μmol/L. A total of 511 subjects (44%) were categorized as normoalbuminuric, 164 (14%) as microalbuminuric, and 484 (42%) as macroalbuminuric.
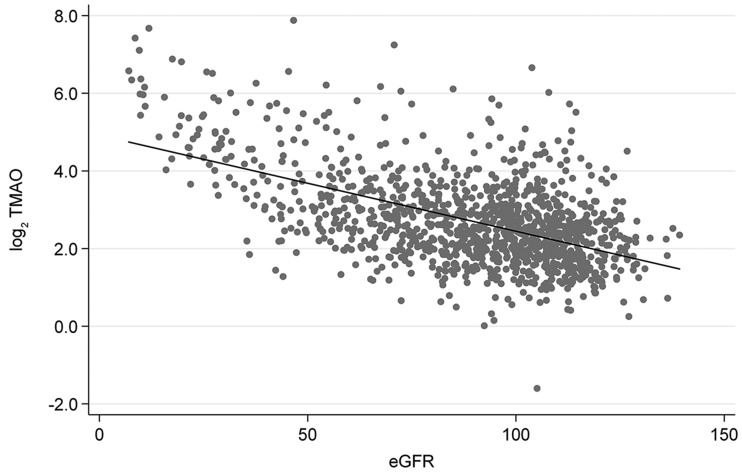
Table 1 shows the baseline characteristics by quartiles of TMAO. Across the quartiles, all characteristics were significantly different (P ≤ 0.036 for trend), except for the frequency of smokers (P = 0.27). Subjects with higher concentrations of plasma TMAO were older and had a lower eGFR and higher UAER than subjects with lower TMAO (Table 1). As shown in Supplementary Table 2, the baseline characteristics also differed significantly (P ≤ 0.005 for trend) among groups of albuminuria (normo-, micro-, and macroalbuminuria), except for age and BMI (P ≥ 0.11). Figure 1 illustrates the negative correlation between TMAO and eGFR (R2 = 0.29; P < 0.0001).
Table 1.
Clinical characteristics in the 1,159 individuals with type 1 diabetes overall and divided according to quartiles of plasma TMAO concentrations
| TMAO concentration |
||||||
|---|---|---|---|---|---|---|
| Overall | Quartile 1 (≤3.81 μmol/L) | Quartile 2 (>3.81 to ≤5.72 μmol/L) | Quartile 3 (>5.72 to ≤9.88 μmol/L) | Quartile 4 (>9.88 μmol/L) | P value | |
| Subjects, n | 1,159 | 291 | 289 | 290 | 289 | |
| Female | 491 (42.4) | 143 (49.1) | 123 (42.6) | 109 (37.6) | 116 (40.6) | 0.036 |
| Age (years) | 46.4 ± 13.0 | 42.2 ± 12.1 | 46.7 ± 13.1 | 47.8 ± 13.4 | 49.1 ± 12.3 | <0.001 |
| History of CVD | 128 (11.3) | 24 (8.3) | 27 (9.5) | 29 (10.4) | 48 (17.1) | 0.004 |
| Diabetes duration (years) | 27.2 ± 12.3 | 25.0 ± 11.6 | 28.1 ± 13.1 | 27.5 ± 12.1 | 28.4 ± 12.2 | 0.003 |
| eGFR (mL/min/1.73 m2) | 89 ± 26.1 | 102 ±17.0 | 95 ±19.6 | 89 ± 21.5 | 69 ± 31.3 | <0.001 |
| UAER (mg/day) | 23 (8–360) | 13 (7–70) | 20 (8–316) | 25 (6–285) | 133 (11–761) | <0.001 |
| Systolic blood pressure (mmHg) | 137 ± 20 | 131 ± 18 | 135 ± 19 | 138 ± 19 | 143 ± 23 | <0.001 |
| Diastolic blood pressure (mmHg) | 78 ± 11 | 76 ± 10 | 77 ± 10 | 78 ± 11 | 81 ± 12 | <0.001 |
| HbA1c (mmol/mol) | 71 ± 15.1 | 70 ± 13.6 | 70 ± 14.6 | 72 ± 14.7 | 74 ± 16.9 | <0.001 |
| HbA1c (%) | 8.7 ± 1.4 | 8.5 ± 1.2 | 8.6 ± 1.3 | 8.7 ± 1.3 | 8.9 ± 1.5 | <0.001 |
| Total cholesterol (mmol/L) | 5.1 ± 1.10 | 4.9 ± 0.99 | 5.1 ± 0.93 | 5.1 ± 1.22 | 5.3 ± 1.18 | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.5 | 24.2 ± 3.2 | 24.7 ± 3.4 | 24.7 ± 3.3 | 24.8 ± 3.9 | 0.036 |
| Smokers | 418 (37.2) | 111 (38.8) | 112 (39.6) | 106 (37.9) | 89 (32.3) | 0.27 |
Data are n (%), mean ± SD, or median (IQR) unless otherwise indicated. P values are for trend across quartiles.
Figure 1.
Linear regression of log2-transformed plasma TMAO concentration versus eGFR (mL/min/1.73 m2). TMAO was negatively associated with eGFR (R2 = 0.29; P < 0.001). Dots, individual measurements; line, regression line.
Incidence of Events
In the overall study population, the median (IQR) follow-up was 15.0 (6.7–19.3) years for mortality and 8.7 (5.1–16.3) years for the combined CVD end point. No subjects were lost to follow-up. A total of 363 subjects died (23.5 per 1,000 person-years), and 406 experienced a fatal or nonfatal CVD event (32.5 per 1,000 person-years). Mortality included 120 CVD deaths. Considering cause-specific first CVD events and the incidence of fatal and nonfatal coronary events in 163 subjects (11.7 per 1,000 person-years), stroke occurred in 115 (8.1 per 1,000 person-years) and heart failure in 81 (5.6 per 1,000 person-years) (Table 2). The median (IQR) follow-up for ESRD was 12.7 (5.6–17.3) years, and 144 subjects (10.7 per 1,000 person-years) reached ESRD (Table 2).
Table 2.
HRs relating plasma TMAO concentrations to mortality, cardiovascular end points, and ESRD
| All-cause mortality |
Cardiovascular mortality |
Combined CVD |
Coronary |
Stroke |
Heart failure |
ESRD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Total population (n = 1,159) | ||||||||||||||
| Events, n (%) | 363 (31.3) | 120 (10.4) | 406 (35.0) | 163 (14.1) | 115 (9.9) | 81 (7.0) | 144 (12.4) | |||||||
| Unadjusted | 1.36 (1.27–1.47) | <0.001 | 1.40 (1.23–1.58) | <0.001 | 1.31 (1.21–1.41) | <0.001 | 1.30 (1.17–1.46) | <0.001 | 1.22 (1.06–1.40) | 0.005 | 1.30 (1.12–1.53) | 0.001 | 1.86 (1.68–2.06) | <0.001 |
| Adjusted | 1.19 (1.09–1.29) | <0.001 | 1.21 (1.05–1.39) | 0.008 | 1.17 (1.07–1.27) | <0.001 | 1.21 (1.07–1.37) | 0.003 | 1.08 (0.93–1.27) | 0.33 | 1.12 (0.93–1.35) | 0.23 | 1.67 (1.47–1.96) | <0.001 |
| Adjusted + eGFR | 1.00 (0.90–1.12) | 0.98 | 1.00 (0.83–1.20) | 0.97 | 1.06 (0.95–1.18) | 0.31 | 1.03 (0.87–1.21) | 0.77 | 1.02 (0.84–1.24) | 0.84 | 1.00 (0.78–1.28) | 0.99 | 1.41 (0.97–1.35) | 0.12 |
| Macroalbuminuria (n = 484) | ||||||||||||||
| Events, n (%) | 246 (53.7) | 84 (17.4) | 251 (51.9) | 96 (19.8) | 75 (15.5) | 55 (11.4) | 141 (29.1) | |||||||
| Unadjusted | 1.28 (1.18–1.39) | <0.001 | 1.29 (1.13–1.49) | <0.001 | 1.25 (1.15–1.37) | <0.001 | 1.21 (1.05–1.38) | 0.007 | 1.16 (0.99–1.36) | 0.07 | 1.21 (1.01–1.45) | 0.04 | 1.59 (1.44–1.76) | <0.001 |
| Adjusted | 1.23 (1.13–1.35) | <0.001 | 1.22 (1.05–1.43) | 0.011 | 1.23 (1.12–1.35) | <0.001 | 1.21 (1.04–1.40) | 0.013 | 1.13 (0.96–1.34) | 0.15 | 1.12 (0.92–1.37) | 0.27 | 1.63 (1.43–1.85) | <0.001 |
| Adjusted + eGFR | 1.06 (0.94–1.21) | 0.36 | 0.99 (0.90–1.23) | 0.95 | 1.11 (0.98–1.26) | 0.093 | 0.99 (0.81–1.21) | 0.91 | 1.04 (0.84–1.30) | 0.71 | 1.08 (0.83–1.40) | 0.58 | 1.13 (0.96–1.34) | 0.14 |
| Normoalbuminuria (n = 511) | ||||||||||||||
| Events, n (%) | 97 (18.9) | 30 (5.9) | 109 (21.3) | 46 (9.0) | 26 (5.1) | 19 (3.7) | 1 | |||||||
| Unadjusted | 1.06 (0.86–1.30) | 0.60 | 1.16 (0.81–1.66) | 0.42 | 1.11 (0.92–1.34) | 0.30 | 1.32 (1.00–1.73) | 0.049 | 1.10 (0.74–1.62) | 0.64 | 1.09 (0.69–1.73) | 0.72 | — | — |
| Adjusted | 0.93 (0.73–1.18) | 0.54 | 1.11 (0.73–1.69) | 0.62 | 0.95 (0.77–1.17) | 0.61 | 1.20 (0.88–1.63) | 0.24 | 1.00 (0.61–1.50) | 0.86 | 0.93 (0.56–1.57) | 0.80 | — | — |
| Adjusted + eGFR | 0.89 (0.70–1.14) | 0.36 | 1.12 (0.74–1.50) | 0.59 | 0.91 (0.73–1.14) | 0.91 | 1.16 (0.83–1.63) | 0.38 | 0.98 (0.62–1.54) | 0.94 | 0.87 (0.48–1.60) | 0.87 | — | — |
Data express the risk per doubling in TMAO. Boldface indicates significance at P < 0.05. Combined CVD was cardiovascular death, ischemic heart disease (including myocardial infarction), stroke, coronary interventions, or peripheral arterial interventions (including amputations). Coronary outcome was myocardial infarction and coronary intervention. Adjustment included age, sex, diabetes duration, HbA1c, systolic blood pressure, total cholesterol, smoking status, and UAER.
TMAO and Associated Risk
The stepwise adjusted HRs with 95% CIs associated with a doubling of plasma TMAO concentration for all-cause mortality, cardiovascular mortality, fatal combined with nonfatal CVD events, and ESRD are shown in Table 2.
Mortality
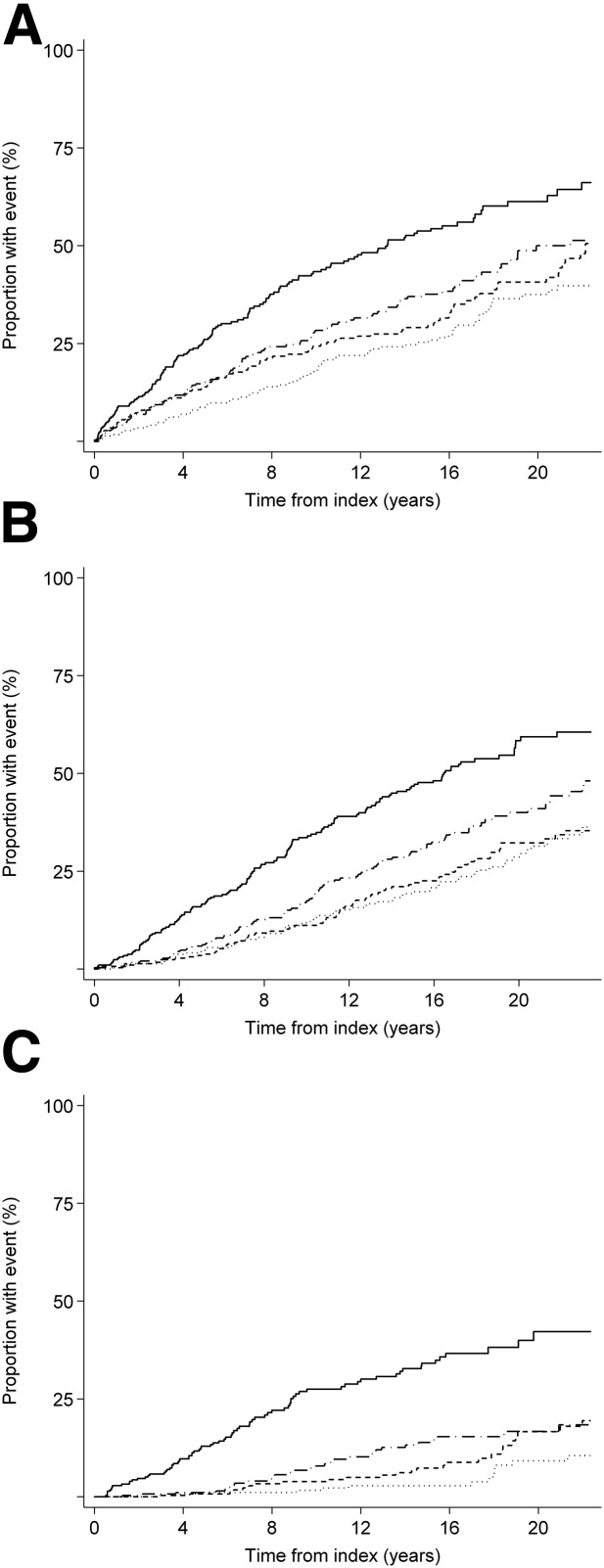
In unadjusted models and after adjustment for age, sex, diabetes duration, HbA1c, systolic blood pressure, total plasma cholesterol, smoking status, and UAER, higher plasma TMAO was associated with a higher risk of all-cause and cardiovascular mortality (Table 2). After additional adjustment for eGFR, significance was lost (HR 1.00 [95% CI 0.90–1.12] and 1.00 [0.83–1.20], respectively). Analyses restricted to the 484 subjects classified with macroalbuminuria showed similar results (data not shown). TMAO was not a predictor of mortality in the subgroup of 151 subjects classified with normoalbuminuria (P ≥ 0.36 in unadjusted and adjusted models). The Kaplan-Meier survival function estimates for all-cause mortality in quartiles of TMAO are illustrated in Fig. 2A (P < 0.001 by log-rank statistics).
Figure 2.
Association of plasma TMAO concentrations and all-cause mortality (A), the combined cardiovascular end point (B), and ESRD (C). For all outcomes, individuals with concentrations of TMAO in the lowest quartile (first) had the lowest risk, and individuals with measurements of TMAO in the highest quartile (fourth) had the highest risk (log-rank P < 0.001 for all). Dotted line, first quartile; short-dash line, second quartile; long-dash line, third quartile; solid line, fourth quartile.
Fatal and Nonfatal CVD Events
In the unadjusted model, higher plasma TMAO concentration was a predictor of the combined CVD end point as well as coronary events, fatal and nonfatal stroke, and heart failure. With adjustments applied as above, higher TMAO remained a predictor of the combined CVD end point (HR 1.17 [95% CI 1.07–1.27]; P < 0.001) and coronary events (1.21 [1.07–1.37]; P = 0.003) in model 2, but significance was lost after further adjustment for eGFR (Table 2). Analyses restricted to the subjects classified with macroalbuminuria showed similar results. TMAO was not a predictor of CVD events in the subjects classified with normoalbuminuria. The Kaplan-Meier survival function estimates for the combined CVD end point in quartiles of TMAO are illustrated in Fig. 2B (P < 0.001 by log-rank statistics).
Renal Events
In the unadjusted model, higher TMAO was a predictor of ESRD (HR 1.86 [95% CI 1.68–2.06]) (Table 2). After adjustment (model 2), plasma TMAO remained a predictor of ESRD (1.67 [1.47–1.96]), but after further adjustment for eGFR (model 3), the association was lost both in the total population and in analyses restricted to the subjects classified with macroalbuminuria. Only one event of ESRD occurred in the subjects classified with normoalbuminuria; analyses were therefore not performed.
rIDI
Addition of TMAO to model 2 (including age, sex, diabetes duration, HbA1c, systolic blood pressure, total cholesterol, smoking status, and UAER) increased the rIDI, with 4.0% (P < 0.001) for all-cause mortality, 5.6% (P < 0.001) for the combined CVD end point, and 6.5% (P = 0.012) for ESRD.
Progression of Nephropathy
In the subgroup with 51Cr-EDTA GFR measured at baseline and a decline in renal function estimated by annual change in 51Cr-EDTA GFR, the GFR at baseline was negatively correlated with plasma TMAO (R2 = 0.33; P < 0.001). Moreover, the annual change in GFR was negatively correlated with TMAO in unadjusted analyses (P = 0.002) and after adjustment for age, sex, diabetes duration, HbA1c, systolic blood pressure, total plasma cholesterol, smoking status, and UAER (P = 0.017); however, significance was lost after further adjustment for baseline GFR (P = 0.59).
Annual change on the basis of eGFR (n = 1,013) was not correlated with TMAO in unadjusted analyses (P = 0.59). After adjustment for age, sex, diabetes duration, HbA1c, systolic blood pressure, total plasma cholesterol, smoking status, and UAER, there was a negative correlation (P = 0.028); however, significance was lost after further adjustment for baseline eGFR (P = 0.068).
Subjects with an accelerated decline in eGFR (≥3 mL/min/year) (n = 192) did not have significantly different TMAO (P = 0.12) compared with slow decliners (<3 mL/min/year) (n = 821). This difference remained insignificant after adjustment for age, sex, diabetes duration, HbA1c, systolic blood pressure, total plasma cholesterol, smoking status, and UAER (P = 0.17) and after further adjustment for eGFR at baseline (P = 0.18).
Sensitivity Analyses
In sensitivity analyses, we included subjects classified with microalbuminuria in the stratified analyses of those classified with normoalbuminuria, where our results remained consistent. Results were overall similar in analyses excluding UAER from the adjustment (data not shown). Moreover, we performed sensitivity analyses to assess the possible influence of the assumption that all deaths were classified as CVD related unless an unequivocal noncardiovascular cause was reported. In these analyses of cardiovascular mortality and the combined CVD end point, excluding the 20 deaths of unknown cause, all results were consistent.
Conclusions
In this study of individuals with type 1 diabetes, higher plasma concentrations of TMAO were predictive of mortality and CVD and renal outcomes. After adjustment for classical CVD risk factors as well as UAER, TMAO remained a significant predictor of incident event risks. Moreover, TMAO was able to improve risk discrimination for all-cause mortality, combined CVD events, and ESRD, as evaluated with rIDI statistics. However, after inclusion of eGFR, TMAO was no longer an independent risk marker. To our knowledge, the association between TMAO and cardio-renal outcomes in type 1 diabetes has not been reported previously.
Several recently published studies have demonstrated an association between higher plasma TMAO concentrations and risk of CVD and mortality. These studies included populations with a high risk of CVD (5,8,21), such as individuals with established coronary artery disease (22–24), heart failure (25), advanced left-ventricular diastolic dysfunction (25), type 2 diabetes (11), and CKD (26–28). In some of these studies, the association persisted even after adjustment for eGFR/GFR (5,21,26–28). Furthermore, a meta-analysis examining the prognostic value of TMAO in subjects either with or without CKD concluded that TMAO provides independent clinical prognostic value for incident CVD and all-cause mortality risks (29). This is in contrast with the present study in which all associations between TMAO and end points became insignificant after further adjustments for eGFR. Factors explaining these conflicting findings with the present study may include fundamental clinical differences in the cohorts. Our cohort solely comprised individuals with type 1 diabetes compared with individuals with no reported type 1 diabetes in other TMAO-related studies. Individuals in our cohort had a much lower mean age of 46 years versus a mean age in the 60s in other cohorts (8,21–23), a high median (IQR) TMAO level of 5.7 (3.8–9.9) μmol/L versus 3.7 (2.4–6.2) and 4.3 (2.6–8.0) μmol/L (23,24), and a lower incidence rate for CVD of 32.5 versus 43 (estimated) per 1,000 person-years (21). Another notable difference between our study and other reported studies is the follow-up duration, which is longer for our cohort, at a median 15.0 (IQR 6.7–19.3) years vs. 3–7 years (8,24), and, in particular, different levels of renal function but with predominately preserved renal function (eGFR >60 mL/min/1.73 m2), ranging in other studies from mean ± SD 40 ± 14 (28) to median (IQR) 99 (74–125) mL/min/1.73 m2 (22).
In contrast to these observational studies and meta-analysis demonstrating association of TMAO with CVD, some other smaller studies have suggested that these associations could be explained by confounding factors, including eGFR(30,31). One study in individuals undergoing coronary revascularization concluded that the risk prediction provided by TMAO was confounded by renal function because TMAO concentrations were not associated with incident CVD, and TMAO concentrations were higher with lower eGFR (32). Another study showed that TMAO was not associated with mortality in subjects with normal renal function (33). A recent 10-year follow-up study in a younger (aged 33–45 years) cohort was unable to demonstrate an association between TMAO and progression of atherosclerosis (34). These results, in relatively healthier subjects, may not have been confounded by other risk factors, such as decline in renal function and age-related increase in TMAO, compared with other studies. Thus, it has been speculated that TMAO is a biomarker associated with CVD but not the cause of CVD (35), even though it has been the result from smaller studies.
Other evidence suggests direct mechanistic links between circulating TMAO and cardio-renal disease pathogenesis. Mice fed a high-choline diet or a diet supplemented with TMAO showed increased TMAO with promotion of atherosclerosis and thrombotic vascular disease (5,8) as well as corresponding increases in tubulointerstitial fibrosis and collagen deposition in the kidneys, including elevated cystatin C and kidney injury molecule-1 (26,36). One study observed abnormally high TMAO levels reduced to normal levels after renal transplantation in humans and that TMAO levels correlated with increased systemic inflammation, serving as an independent predictor of mortality in CKD stages 3–5 (28). Thereby, elevated blood TMAO concentrations contribute to CKD through gut-related factors and processes, such as atherosclerosis and vascular inflammation, contributing to the pathogenesis of cardio-renal disease. Other evidence suggests that transplantation of microbe production of TMAO from donor mice to a host alters the CVD and thrombosis risk (37). Interestingly, direct inhibition of the TMAO pathway in animal models with the small-molecule mimetic of choline 3,3-dimethyl-1-butanol (DMB) has been linked to reduction in fibrosis and reduction in functional renal decline, indicating that TMAO has a direct mechanistic link (38). DMB inhibits microbial production of TMA (TMA lyase antagonist), and data have shown reduction in TMA and TMAO by DMB in mice, protecting mice against enhanced atherosclerosis development (39). In this perspective, adjusting the TMAO cardio-renal event associations for eGFR may be an overadjustment since the effect of increased concentrations of TMAO on the outcome may be mediated through impaired renal function. The association between TMAO and the linear decline in measured 51Cr-EDTA GFR indicates TMAO as a risk factor for renal decline.
The primary source of TMAO is unclear but believed to be derived from both dietary and biliary choline (in the form of both lecithin and choline). Some fish can also have a high concentration of TMAO (if fresh) or TMA (if not fresh) generated by surface bacteria, which is then converted to TMAO. Eating a large amount of fish (depending on the type and freshness) could thus contribute to an elevation in TMAO levels in the postprandial state, and if accompanied with impaired renal clearance, would be expected to promote cardiovascular risk. In general, consumption of fish is related to a cardioprotective lifestyle, although the relationship among TMAO, n-3 fatty acid content, or other comorbidities that coassociate with fish consumption has never been unequivocally explored. Another potential dietary source of TMAO is through ingestion of l-carnitine, which is abundant in red meat. Because carnitine feeding has been shown to both elevate TMAO and increase atherosclerosis (8), the TMAO-related risk may be related to diet. However, a German study in healthy adults from the general population did not indicate an effect of general diet on TMAO, but a positive association with dairy food consumption and TMAO as well as TMAO levels and inflammation was found, suggesting a diet purely based on dairy or animal products (red meat) may modulate specific host-microbe interactions and TMAO production (40). A recent review suggests that reduction in red meat consumption (reflected by low TMAO levels) in patients with CKD may reduce cardiovascular events, further reducing the risk of kidney disease progression (41). Thus, identification of potential biomarkers or surrogate markers of cardiovascular disease may improve risk stratification with better treatment strategies for type 1 diabetes.
Strengths and Limitations
One of the major strengths of our study is the large and well-characterized cohort of individuals with type 1 diabetes, although the generalizability of the results to other subjects is unclear. Another strength of the current study is the quality of Danish registries (17), with no subjects lost to follow-up.
This study also has limitations. The first patient was included in August 1993, and the ICD classification changed from ICD-8 to ICD-10 in January 1994. We only have information on ICD-10 codes available. Thus, there are a few months where we do not have information on hospitalization; we consider this of minor influence. As another potential limitation, TMAO was measured using stored plasma samples, some of which were stored for many years; however, TMAO has been shown to be stable both during storage at −80°C for years and during multiple freeze-thaw cycles, and quantification of TMAO is considered reliable in biobank samples stored for a long time (42). Furthermore, all samples were handled and stored at −80°C under uniform conditions. Another limitation is that samples were examined at one time point compared with the varied duration of follow-up in the subjects. TMAO was also only measured at one time in each individual, and TMAO levels have been noted to show relatively high intraindividual variability, which is probably the result of day-to-day changes in diet (43). However, it has been shown that the intraday coefficient of variance is low in TMAO levels above the median level (i.e., increased variation is observed where TMAO levels are low) (44).
In conclusion, higher concentrations of plasma TMAO were associated with higher risk of mortality, CVD events, and renal failure, independent of traditional risk factors in a large Danish (European) cohort comprising individuals with type 1 diabetes. However, the association became insignificant upon adjustment for baseline eGFR. This could reflect TMAO as a renal function marker or a risk factor for micro- and macrovascular complications mediated through impaired renal function.
Supplementary Material
Article Information
Acknowledgments. The authors thank all participants and acknowledge the work of laboratory technicians at Steno Diabetes Center Copenhagen, Denmark.
Funding. Internal funding was provided by Steno Diabetes Center Copenhagen. The authors acknowledge the support from the Novo Nordisk Foundation grant number NNFOC0013659: “PROTON: Personalizing Treatment of Diabetic Nephropathy.” Z.W. and S.L.H. were supported in part by funding from the National Institutes of Health and the Office of Dietary Supplements (HL-103866, HL-126827, and DK-106000) and a grant from the Leducq Foundation.
Duality of Interest. Since the completion of data management, J.C.Ø. has been employed by Novo Nordisk Scandinavia A/B, Region Denmark. Z.W. and S.L.H. report being named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics and as having the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Quest Diagnostics, and Procter & Gamble. S.L.H. reports having been paid as a consultant by Procter & Gamble and having received research funds from Procter & Gamble and Roche. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A.W. drafted the manuscript. S.A.W., J.C.Ø., N.T., L.T., T.S.A., A.J., S.T., T.W.H., and P.R. analyzed and interpreted the data. S.A.W., J.C.Ø., N.T., L.T., A.J., S.T., H.-H.P., T.W.H., O.P., and P.R. conceived and designed the research. S.A.W., T.S.A., and T.W.H. performed the statistical analysis. Z.W. and S.L.H. measured TMAO. O.P. and P.R. supervised the study. P.R. obtained funding. All authors contributed to the interpretation of the results, reviewed and edited the manuscript, and approved the final version of the manuscript. S.A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0048/-/DC1.
References
- 1.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418 [DOI] [PubMed] [Google Scholar]
- 2.Andrésdóttir G, Jensen ML, Carstensen B, et al. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney Int 2015;87:417–426 [DOI] [PubMed] [Google Scholar]
- 3.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369–2379 [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018;16:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Patel NA, Crombie A, Scrivens JH, Murrell JC. Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc Natl Acad Sci U S A 2011;108:17791–17796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 2016;5002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Li XS, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem 2017;63:297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorsal A, Tarnow L, Frystyk J, et al. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int 2008;74:649–654 [DOI] [PubMed] [Google Scholar]
- 13.Theilade S, Lajer M, Hansen TW, et al. 24-hour central aortic systolic pressure and 24-hour central pulse pressure are related to diabetic complications in type 1 diabetes - a cross-sectional study. Cardiovasc Diabetol 2013;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrillo S. Updated Information for IDMS Traceable VITROS® Chemistry Products CREA Slides [Internet], 2008. Rochester, NY, Ortho Clinical Diagnostics. Available from http://clincalc.com/Downloads/OrthoClinicalDiagnostics-IDMS_20080612.pdf. Accessed 16 May 2019
- 15.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bröchner-Mortensen J, Rödbro P. Selection of routine method for determination of glomerular filtration rate in adult patients. Scand J Clin Lab Invest 1976;36:35–43 [DOI] [PubMed] [Google Scholar]
- 17.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(Suppl.):30–33 [DOI] [PubMed] [Google Scholar]
- 18.Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39(Suppl.):26–29 [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P; Heart Outcomes Prevention Evaluation Study Investigators . Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 2000;342:154–160 [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010;48:1703–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senthong V, Wang Z, Li XS, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc 2016;5002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017;38:814–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 2015;21:91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015;116:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee EP, Clish CB, Ghorbani A, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 2013;24:1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016;27:305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–2956 [DOI] [PubMed] [Google Scholar]
- 30.Lever M, George PM, Slow S, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One 2014;9:e114969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Missailidis C, Hällqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 2016;11:e0141738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller DM, Allenspach M, Othman A, et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015;243:638–644 [DOI] [PubMed] [Google Scholar]
- 33.Gruppen EG, Garcia E, Connelly MA, et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep 2017;7:13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer KA, Benton TZ, Bennett BJ, et al. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc 2016;5003970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 2017;37:157–181 [DOI] [PubMed] [Google Scholar]
- 36.Kim RB, Morse BL, Djurdjev O, et al.; CanPREDDICT Investigators . Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int 2016;89:1144–1152 [DOI] [PubMed] [Google Scholar]
- 37.Skye SM, Zhu W, Romano KA, et al. Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ Res 2018;123:1164–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun G, Yin Z, Liu N, et al. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem Biophys Res Commun 2017;493:964–970 [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr 2016;146:283–289 [DOI] [PubMed] [Google Scholar]
- 41.Mafra D, Borges NA, Cardozo LFMF, et al. Red meat intake in chronic kidney disease patients: two sides of the coin. Nutrition 2018;46:26–32 [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 2014;455:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEntyre CJ, Lever M, Chambers ST, et al. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem 2015;52:352–360 [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019;40:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.