Abstract
Objective
To compare the diagnostic performance of contrast-enhanced spectral mammography (CESM) versus ultrasonography (US) in symptomatic patients with dense breasts, while using histology as the gold standard.
Materials and Methods
After obtaining approval from the local ethics board, this prospective study collected data from patients with symptomatic breasts who underwent CESM and US examinations from May 1, 2017 to September 30, 2017. We then selected those with dense breasts and pathological results as our sample population. Both CESM and US results were classified by a radiologist through the Breast Imaging Reporting and Data System, and the results were compared with their corresponding histological results. The chi-square test was conducted to compare the diagnostic performance of CESM and US, and the receiver operating characteristic curves for the two imaging modalities were obtained.
Results
A total of 131 lesions from 115 patients with dense breasts were included in this study. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were 93.8%, 88.1%, 88.2%, 93.7%, and 90.8% for CESM, and 90.6%, 82.1%, 82.9%, 90.2%, and 86.3% for US, respectively. The p values for sensitivity, specificity, PPV, NPV, and accuracy were 0.687, 0.388, 0.370, 0.702, and 0.238, respectively. The area under the curve of CESM (0.917) was comparable with that of US (0.884); however, the differences between CESM and US were not statistically significant (p = 0.225). Eight false-positive cases and 4 false-negative cases for breast cancer were found in CESM, while 12 false-positive cases and 6 false-negative cases were found in US.
Conclusion
The diagnostic performances of CESM and US are comparable in symptomatic women with dense breasts; however, the routine use of additional US imaging is questionable for lesions that can be detected by CESM.
Keywords: Breast neoplasms, Contrast-enhanced spectral mammography, Ultrasonography, Contrast media
INTRODUCTION
Mammography (MG) is an indispensable breast imaging modality for screening and diagnosing breast cancer, and its use has been shown to reduce mortality (1). However, it does have some limitations, which are mainly due to its overlapping effects when reviewing images. Dense fibroglandular tissues can mask lesions, especially iso- and low-density lesions without calcification. Dense breasts are also an independent risk factor for breast cancer, and the proportion of dense breasts in Asian women is higher than that in other ethnic groups (2).
High-resolution ultrasonography (US) is a routine breast imaging examination in clinical practice. US can detect lesions missed by MG in dense breasts, including breast cancers with a diameter less than 1 cm (3). Thus, US is believed to be superior to MG in diagnosing dense breast lesions. However, US has limited ability in detecting breast microcalcifications. US is highly dependent on the operator's experience, and the diagnostic consistency among operators is relatively low (4).
With the development of full-field digital MG, many novel techniques, such as contrast-enhanced spectral mammography (CESM), have been proposed. CESM employs low- and high-energy X-rays after the intravenous injection of iodinated contrast agent. This produces a low-energy image, similar to MG, and also a reconstructed image which is obtained by the subtraction of low- and high-energy images. The reconstructed image can highlight the uptake of iodinated contrast agent throughout the breast by eliminating the influence of dense fibroglandular tissues, which contributes to the detection of hypervascular breast cancer. Many studies (5,6,7,8,9,10) have shown that the diagnostic performance of CESM is superior to that of MG, and comparable with that of magnetic resonance imaging (MRI), especially in patients with dense breasts. MRI is the most accurate imaging method for the diagnosis and preoperative evaluation of breast cancer. However, MG and US are still the most commonly used imaging modalities, as MRI is expensive, time-consuming, and has many contraindications. Since there are few studies that have compared the diagnostic performance of CESM and US, especially in Asian populations, our study aimed to compare the diagnostic performance of CESM with US in symptomatic patients with dense breasts using histology as the gold standard.
MATERIALS AND METHODS
This prospective study was approved by the local ethics board. Written informed consent was obtained from all patients in this study. All study subjects or cohorts have not been previously reported. The diagnostic study was performed at one institution. The examination process and advantages of CESM and US were explained to all eligible patients, in addition to the possibility of an allergic reaction occurring during CESM. All enrolled patients voluntarily participated in the research process and provided written informed consent.
Inclusion and Exclusion Criteria
From May 1, 2017 to September 30, 2017, patients were included based on the following criteria: 1) the patient sought a consultation at our hospital for breast symptoms; 2) the patient could complete both CESM and US examinations within one week.
Patients were excluded based on the following criteria: 1) the patient was pregnant or lactating; 2) the patient had contraindications to iodinated contrast agents, such as allergic reaction to iodinated contrast agents, renal insufficiency, and/or hyperthyroidism; 3) the patient had breast implants; 4) the patient had an American College of Radiology (ACR) breast density classification A or B; and 5) the examinations were performed without histological results, or the histological results were obtained before CESM and US examinations.
CESM Examinations
All CESM examinations were performed using a prototype of full-field digital MG (Senographe Essential, GE Healthcare, Buc, France), which can be used for low- and high-energy intermittent exposures in one position. The patient was seated, and an iodinated contrast agent (iohexol, 35 g/100 mL, Beilu Pharmaceutical Co., Ltd., Beijing, China) was injected into the antecubital vein using a power injector (Vistron Plus, Bayer, Hannover, Germany) at a flow rate of 3 mL/s for a dose of 1.5 mL/kg body weight. After the injection, the patient was disconnected from the power injector, and the venous catheter was secured within the vein to maintain venous access to respond quickly in the event of an allergic reaction. Two minutes after the injection of the contrast agent, dual-energy exposures were performed in bilateral craniocaudal (CC) and mediolateral oblique (MLO) views. The patient was requested to hold her breath during the exposure to reduce motion artifacts. Bilateral CC views were obtained first, beginning with the less suspicious breast. MLO views were then obtained in the same order. Having the same views of the bilateral breasts imaged successively would facilitate the comparison of the bilateral breasts. Each view contained both a low-energy image and a reconstructed image. The reconstructed image was obtained through the specific image processing of the low- and high-energy images, which could suppress fibroglandular tissue and highlight contrast enhancement in the breast. The low-energy image can show microcalcifications, similar to traditional MG.
US Examinations
A radiologist with seven years of experiences in US imaging and diagnosis performed the US examinations for the study. The radiologist was blinded to the results of the patients' other examinations, including CESM, but had knowledge of the chief complaint during the examination, such as “finding a right breast mass for 10 days.” Bilateral breast US examinations were performed using a device (EPIQ 7, Philips Ultrasound, Rotterdam, the Netherlands) equipped with a 5–12 MHz handheld high-resolution linear-array transducer. Both breasts were evaluated in two orthogonal planes (radial and antiradial planes) extending from the nipple to the posterior breast tissue. The bilateral axillae were also routinely scanned. Lesions, if present, were examined with Doppler, and the location and size of the lesions were documented. The Breast Imaging Reporting and Data System (BI-RADS) was assessed for each breast. The scan times were not limited or recorded. In our experience, the examination time of each patient was approximately 10–20 minutes.
Image Analysis
The CESM images were transmitted to a dedicated workstation with a 5 million pixel monitor (HMD5G21, Hisense Medical Equipment Co., Ltd., Qingdao, China) for analysis. A radiologist with 12 years of experience interpreting MG and breast MRI images and 3 days of CESM training from the vendor evaluated the CESM images. The radiologist was blinded to the results of the patients' other examinations, including those of US, but had knowledge of the chief complaint during the interpretation. The breast density was evaluated in the low-energy image. Patients with ACR breast density grade C or D were further analyzed. ACR grade C means that the breasts are heterogeneously dense, which may obscure small masses. ACR grade D refers to extremely dense breasts, which lowers the sensitivity of MG. No specific BI-RADS lexicon for CESM exists. The low-energy image was interpreted similar to MG in terms of morphology, and the reconstructed image was interpreted similar to MRI in terms of contrast enhancement. The radiologist was allowed to review the corresponding enhanced area on the low-energy image. The radiologist classified the BI-RADS based on all available information.
The US examinations in the study were performed by the radiologist, who observed the lesions directly from multiple planes. The radiologist performed real-time analyses and dictated the image description and diagnosis, and a transcriptionist entered the reports in a computer.
Statistical Analysis
For statistical analysis, BI-RADS 1–3 and BI-RADS 4–5 were classified as suspicious benign and suspicious malignant, respectively. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated with histological diagnosis being the gold standard. The chi-square test was used to assess the significance of the differences in sensitivity, specificity, PPV, NPV, and accuracy. The receiver operating characteristic (ROC) curves of CESM and US were plotted, and the significance of the differences in area under the curve was assessed by non-parametric method.
All statistical analyses were performed with SPSS (version 20, IBM Corp., Armonk, NY, USA) and MedCalc (version 11.4.2.0, MedCalc Software, Ostend, Belgium). P < 0.05 was considered statistically significant.
RESULTS
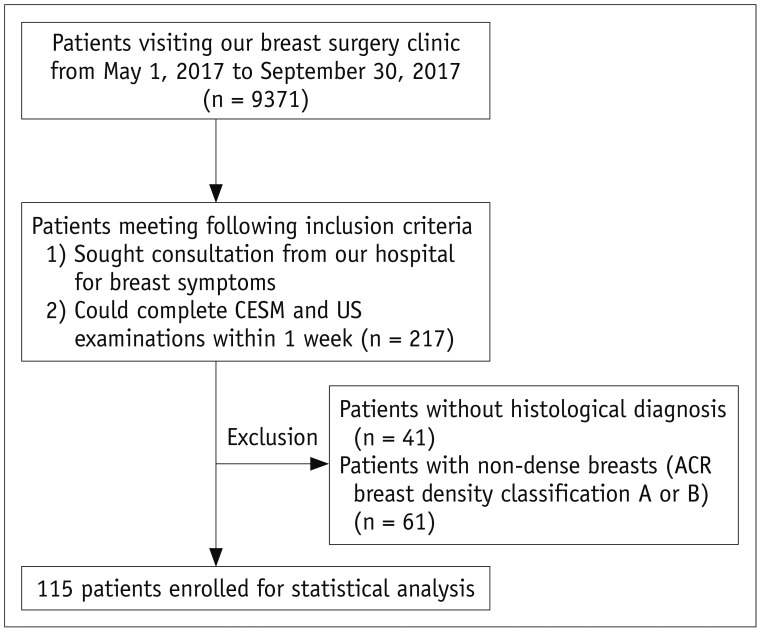
Of the 9371 patients that visited our breast surgery clinic from May 1, 2017 to September 30, 2017, 217 patients met the inclusion criteria and voluntarily participated in the study, of which 102 patients were excluded from further analysis because of non-dense breasts or absence of histological results (Fig. 1). A total of 131 lesions from 115 patients (mean age, 47.4 years; range, 15–75 years) with dense breasts were enrolled in the study for further statistical analysis. Of the 115 patients, 102 patients sought consultation from our hospital for palpable breast mass, 10 patients for nipple discharge, and 3 patients for breast pain (Table 1). Fifteen patients had bilateral breast lesions, and one patient had two lesions in one breast. A total of 66 patients had heterogeneously dense breasts, and 49 patients had extremely dense breasts. None of the patients involved in the study experienced an allergic reaction to the iodinated contrast agent.
Fig. 1. Flow chart of patient inclusion and exclusion criteria.
ACR = American College of Radiology, CESM = contrast-enhanced spectral mammography, US = ultrasonography
Table 1. Clinical Symptoms of 115 Patients and Histological Results of 131 Lesions.
| Main Symptoms | Count | Percentages (%) |
|---|---|---|
| Found palpable breast mass inadvertently | 102 | 88.7 |
| Nipple discharge | 10 | 8.7 |
| Breast pain | 3 | 2.6 |
| Pathology type | ||
| Malignant lesions | 64 | 48.9 |
| IDC (22 with associated DCIS) | 54 | 41.2 |
| ILC (1 with associated LCIS) | 3 | 2.3 |
| DCIS | 2 | 1.5 |
| Intraductal papilloma with DCIS | 2 | 1.5 |
| Others | 3 | 2.3 |
| Benign lesions | 67 | 51.1 |
| Adenosis (18 with fibroadenoma; 4 with intraductal papilloma) | 40 | 30.5 |
| Fibroadenoma | 17 | 13.0 |
| Intraductal papilloma | 7 | 5.3 |
| Others | 3 | 2.3 |
DCIS = ductal carcinoma in situ, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, LCIS = lobular carcinoma in situ
The BI-RADS classification of all lesions as determined by CESM and US imaging along with their corresponding histological results are shown in Table 2. Of the 131 lesions, one borderline phyllodes tumor was classified as a benign lesion for statistical analysis. Finally, 64 lesions were diagnosed as malignant and 67 as benign, through histological diagnosis. Invasive ductal carcinomas (IDCs) were the most common malignant lesions (84.4%, 54/67). Forty cases (59.7%, 40/54) exhibited adenosis, which were the most common benign lesions. Detailed information on the histological results is shown in Table 1.
Table 2. BI-RADS Classification by CESM and US, and Association with Histological Results.
| BI-RADS Classification | Malignant | Benign | Total |
|---|---|---|---|
| CESM | |||
| BI-RADS 1 | 1 | 18 | 19 |
| BI-RADS 2 | 1 | 3 | 4 |
| BI-RADS 3 | 2 | 38 | 40 |
| BI-RADS 4 | 27 | 5 | 32 |
| BI-RADS 5 | 33 | 3 | 36 |
| US | |||
| BI-RADS 1 | 0 | 2 | 2 |
| BI-RADS 2 | 0 | 0 | 0 |
| BI-RADS 3 | 6 | 53 | 59 |
| BI-RADS 4 | 35 | 10 | 45 |
| BI-RADS 5 | 23 | 2 | 25 |
| Total | 64 | 67 | 131 |
BI-RADS = Breast Imaging Reporting and Data System, CESM = contrast-enhanced spectral mammography, US = ultrasonography
The sensitivity, specificity, PPV, NPV, and accuracy of CESM in the diagnosis of breast cancer were 93.8%, 88.1%, 88.2%, 93.7%, and 90.8%, respectively, which were comparable with those of US (90.6%, 82.1%, 82.9%, 90.2%, and 86.3%, respectively) (Table 3). However, chi-square tests showed no significant difference between CESM and US in sensitivity (p = 0.687), specificity (p = 0.388), PPV (p = 0.370), NPV (p = 0.702), and accuracy (0.238). The area under the ROC curves for CESM and US in the diagnosis of breast lesions were 0.917 and 0.884, respectively (p = 0.225), suggesting good diagnostic performance in differentiating between malignant and benign lesions (Fig. 2).
Table 3. Diagnostic Performance of CESM and US.
| Diagnostic Performance | CESM | US | P |
|---|---|---|---|
| Sensitivity (%) | 93.8 (60/64, 87.7–99.8) | 90.6 (58/64, 83.3–98.0) | 0.687 |
| Specificity (%) | 88.1 (59/67, 80.1–96.0) | 82.1 (55/67, 72.7–91.5) | 0.388 |
| PPV (%) | 88.2 (60/68, 80.4–96.1) | 82.9 (58/70, 73.8–91.9) | 0.370 |
| NPV (%) | 93.7 (59/63, 87.5–99.8) | 90.2 (55/61, 82.5–97.9) | 0.702 |
| Accuracy (%) | 90.8 (119/131, 85.8–95.8) | 86.3 (113/131, 80.3–92.2) | 0.238 |
Number of cases used in calculation of diagnostic values and 95% confidence intervals are in parentheses. NPV = negative predictive value, PPV = positive predictive value
Fig. 2. Receiver operating characteristic curves to illustrate diagnostic performances of CESM and US.
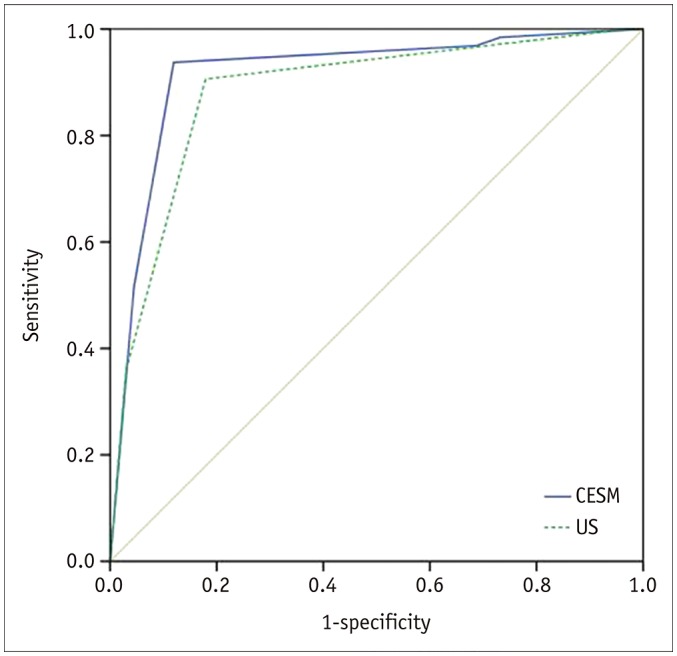
AUC for CESM in diagnosis of breast lesions was 0.917 (95% CI, 0.863–0.970). AUC for US in diagnosis of breast lesions was 0.884 (95% CI, 0.823–0.944). Difference of AUC between CESM and US was not significant (p = 0.225). AUC = area under curve, CI = confidence interval
Nineteen cases were diagnosed as BI-RADS 1 by CESM, versus two by US. Among the 19 cases missed by CESM, one case of IDC presented a slight cottony enhancement, which was misdiagnosed as background enhancement (Fig. 3). The remaining 18 cases were benign lesions, of which 14 cases were adenoses; 2 demonstrated intraductal papillomas; 1 demonstrated fibroadenoma; and 1 demonstrated hyperplasia. Two cases which were missed by US involved adenoses.
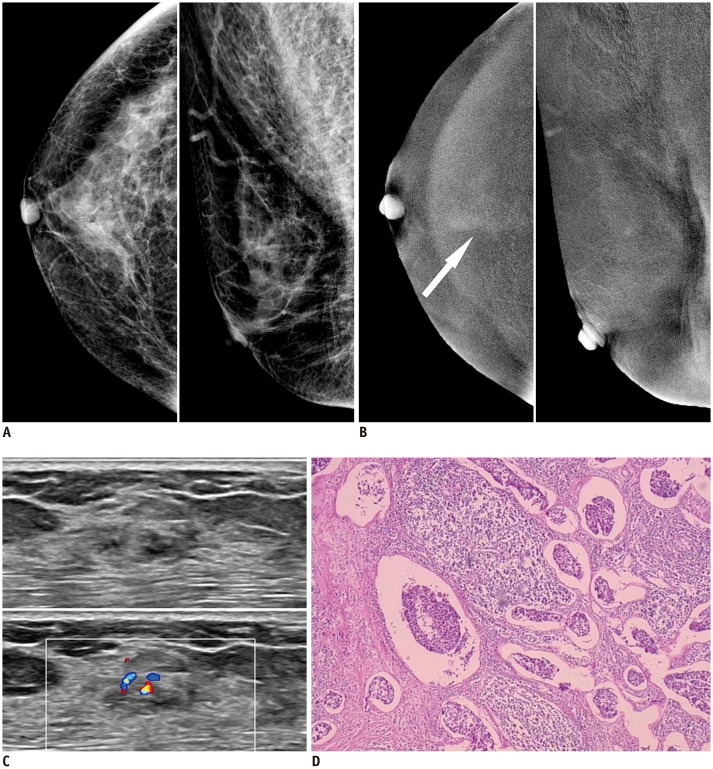
Fig. 3. 59-year-old woman presented to her doctor with dull pain in right breast.
A. Low-energy images of CESM showed heterogeneously dense breast with no abnormal findings. B. Recombined images of CESM showed slight cottony enhancement in craniocaudal view (arrow). C. US showed irregular hypoechoic mass with significant internal blood flow. D. Histological result from surgical specimen: invasive ductal carcinoma, no special type, grade II; nuclear grade II; tumor size: 10 × 5 mm (hematoxylin-eosin staining, original magnification × 40).
CESM had 4 false-negative cases, whereas US had 6 false-negative cases, 2 cases of which were false-negative in both CESM and US. CESM had 8 false-positive cases, and US had 12 cases, 4 of which were false-positive in both CESM and US.
DISCUSSION
The diagnostic performance of MG has some limitations in the detection of breast lesions in patients with dense breasts. Additional breast imaging modalities are needed to validate the diagnosis for those patients (11,12). The low-energy image of CESM is similar to that of MG, and the reconstructed image can eliminate the masking effects of fibroglandular breast tissues. Several studies on CESM have confirmed that CESM's sensitivity is significantly higher than that of MG in detecting breast cancer, especially for patients with dense breasts (13,14,15). The sensitivity of US for breast cancer detection in patients with dense breasts is also higher than that of MG. US has been routinely used in breast cancer screening and diagnosis (16). So, for dense breasts, is it necessary to perform US examination after CESM examination?
Luczyńska et al. (17) included 116 patients with breast symptoms for MG, US, and CESM examinations. A total of 137 lesions were detected, including 90 cases (70%) of breast cancers. The sensitivity, specificity, and accuracy of CESM were 100%, 27%, and 78% respectively, which were 8%, 7%, and 8% higher than those of US, respectively. The sensitivity and accuracy of CESM were significantly different. Klang et al. (18) retrospectively analyzed 953 patients who underwent CESM and US examinations during the same period, among which 87 lesions obtained pathological results through biopsy, as CESM or US could not rule out the possibility of malignancy. These results showed that the sensitivity, specificity, and accuracy of CESM were 97.3%, 40.0%, and 64.4% respectively, which were higher than those of US (i.e., 91.9%, 8.0%, and 44.7%, respectively). The results of our study were similar to those of the above studies. The sensitivity, specificity, PPV, NPV, and accuracy of CESM were comparable with those of US, that is, 93.8% versus 90.6%, 88.1% versus 82.1%, 88.2% versus 82.9%, 93.7% versus 90.2%, and 90.8% versus 86.3%, respectively. The area under the ROC curve of CESM was 0.917, whereas that of US was 0.884. However, no statistical difference was observed, which may be due to the different sample selections. Previous studies have not set a requirement for breast density. In this study, patients with breast-related symptoms and ACR breast density grades of C or D were selected. Thus, the difference in diagnostic performance between CESM and US in patients with dense breasts was smaller than that in fatty breast.
One case of IDC was diagnosed as BI-RADS 1 by CESM and as BI-RADS 3 by US. After learning the histological results, the CESM images were re-reviewed, which indicated a slight cottony enhancement. This observation was considered a background enhancement by the radiologist during the primary diagnosis. US showed an irregular and slightly hypoechoic mass with unclear boundaries, and mixed blood flow was noted on the Doppler US. The histological results showed that the blood vessel density of the IDC was not significantly lower than that of conventional IDC, but the lymphatic vessel density of the IDC was high. These observations were similar to the IDC case reported by Taylor et al. (19), which was also diagnosed as an irregular hypoechoic mass by US, and significant internal blood flow was observed via Doppler US. An US-guided biopsy was performed, and a marker clip was inserted. No obvious abnormal enhancement was observed by CESM at the position of the marker clip. In both cases, abundant blood vessels were observed under a microscope. The lack of CESM enhancement may be related to the maturation and lower permeability of tumor blood vessels. In our case, it may also be related to the increase in lymphatic vessel density (20,21).
Nineteen lesions were missed by CESM in this study. Except for the above mentioned false-negative IDC, the remaining 18 cases were true negatives for the diagnosis of breast cancer. However, the histology results of these 18 cases showed benign lesions which required corresponding treatment. The missed diagnoses from CESM may delay the treatment of benign lesions and aggravate the condition. Only two benign lesions were missed by US, suggesting that the ability of US to detect benign lesions was higher than that of CESM.
This study had some limitations. First, the radiologists had knowledge of the chief complaint during the CESM and US interpretations. This knowledge may affect the detection rate, but it also reflects clinical practice. Second, pre- and postmenopausal women were enrolled and examined during different menstrual periods, which may have resulted in biased results. Third, the sample size of the study was small; thus, the results need to be further confirmed by a multi-center study with a larger sample size.
In conclusion, the diagnostic performances of CESM and US are comparable in symptomatic women with dense breasts. The necessity for additional US imaging is questionable for lesions that can be detected by CESM.
Acknowledgments
The scientific guarantor of this publication is Yan Pan. We are grateful to Tuanzi Jun and San Gongzi for their contributions in statistical analysis. We are grateful to Lurou Sier for her contributions in the English translation of the manuscript.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Løberg M, Lousdal ML, Bretthauer M, Kalager M. Benefits and harms of mammography screening. Breast Cancer Res. 2015;17:63. doi: 10.1186/s13058-015-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, et al. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev. 2003;12:332–338. [PubMed] [Google Scholar]
- 3.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 4.Abdullah N, Mesurolle B, El-Khoury M, Kao E. Breast imaging reporting and data system lexicon for US: interobserver agreement for assessment of breast masses. Radiology. 2009;252:665–672. doi: 10.1148/radiol.2523080670. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol. 2015;59:300–305. doi: 10.1111/1754-9485.12296. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Roth R, Germaine P, Ren S, Lee M, Hunter K, et al. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagn Interv Imaging. 2017;98:113–123. doi: 10.1016/j.diii.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Phillips J, Miller MM, Mehta TS, Fein-Zachary V, Nathanson A, Hori W, et al. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging. 2017;42:193–197. doi: 10.1016/j.clinimag.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol. 2014;24:256–264. doi: 10.1007/s00330-013-3007-7. [DOI] [PubMed] [Google Scholar]
- 9.Fallenberg EM, Schmitzberger FF, Amer H, Ingold-Heppner B, Balleyguier C, Diekmann F, et al. Contrast-enhanced spectral mammography vs. mammography and MRI - clinical performance in a multi-reader evaluation. Eur Radiol. 2017;27:2752–2764. doi: 10.1007/s00330-016-4650-6. [DOI] [PubMed] [Google Scholar]
- 10.Lobbes MB, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer. 2015;6:144–150. doi: 10.7150/jca.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 12.Emaus MJ, Bakker MF, Peeters PH, Loo CE, Mann RM, de Jong MD, et al. MR Imaging as an additional screening modality for the detection of breast cancer in women aged 50-75 years with extremely dense breasts: the DENSE trial study design. Radiology. 2015;277:527–537. doi: 10.1148/radiol.2015141827. [DOI] [PubMed] [Google Scholar]
- 13.Cheung YC, Tsai HP, Lo YF, Ueng SH, Huang PC, Chen SC. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Eur Radiol. 2016;26:1082–1089. doi: 10.1007/s00330-015-3904-z. [DOI] [PubMed] [Google Scholar]
- 14.Lobbes MB, Lalji U, Houwers J, Nijssen EC, Nelemans PJ, van Roozendaal L, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol. 2014;24:1668–1676. doi: 10.1007/s00330-014-3154-5. [DOI] [PubMed] [Google Scholar]
- 15.Fallenberg EM, Dromain C, Diekmann F, Renz DM, Amer H, Ingold-Heppner B, et al. Contrast-enhanced spectral mammography: does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res Treat. 2014;146:371–381. doi: 10.1007/s10549-014-3023-6. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 17.Luczyńska E, Heinze S, Adamczyk A, Rys J, Mitus JW, Hendrick E. Comparison of the mammography, contrast-enhanced spectral mammography and ultrasonography in a group of 116 patients. Anticancer Res. 2016;36:4359–4366. [PubMed] [Google Scholar]
- 18.Klang E, Krosser A, Amitai MM, Sorin V, Halshtok Neiman O, Shalmon A, et al. Utility of routine use of breast ultrasound following contrast-enhanced spectral mammography. Clin Radiol. 2018;73:908.e11–908.e16. doi: 10.1016/j.crad.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Taylor D, O'Hanlon S, Latham B. False-negative contrast-enhanced spectral mammography: use of more than one imaging modality and application of the triple test avoids misdiagnosis. BMJ Case Rep. 2017;2017:bcr2016218556. doi: 10.1136/bcr-2016-218556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dromain C, Balleyguier C, Muller S, Mathieu MC, Rochard F, Opolon P, et al. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. AJR Am J Roentgenol. 2006;187:W528–W537. doi: 10.2214/AJR.05.1944. [DOI] [PubMed] [Google Scholar]
- 21.Luczynska E, Niemiec J, Ambicka A, Adamczyk A, Walasek T, Rys´ J, et al. Correlation between blood and lymphatic vessel density and results of contrast-enhanced spectral mammography. Pol J Pathol. 2015;66:310–322. doi: 10.5114/pjp.2015.54965. [DOI] [PubMed] [Google Scholar]