Abstract
Objective
To evaluate the performance of predicting early recurrence using preoperative factors only in comparison with using both pre-/postoperative factors.
Materials and Methods
We retrospectively reviewed 549 patients who had undergone curative resection for single hepatcellular carcinoma (HCC) within Milan criteria. Multivariable analysis was performed to identify pre-/postoperative high-risk factors of early recurrence after hepatic resection for HCC. Two prediction models for early HCC recurrence determined by stepwise variable selection methods based on Akaike information criterion were built, either based on preoperative factors alone or both pre-/postoperative factors. Area under the curve (AUC) for each receiver operating characteristic curve of the two models was calculated, and the two curves were compared for non-inferiority testing. The predictive models of early HCC recurrence were internally validated by bootstrap resampling method.
Results
Multivariable analysis on preoperative factors alone identified aspartate aminotransferase/platelet ratio index (OR, 1.632; 95% CI, 1.056–2.522; p = 0.027), tumor size (OR, 1.025; 95% CI, 0.002–1.049; p = 0.031), arterial rim enhancement of the tumor (OR, 2.350; 95% CI, 1.297–4.260; p = 0.005), and presence of nonhypervascular hepatobiliary hypointense nodules (OR, 1.983; 95% CI, 1.049–3.750; p = 0.035) on gadoxetic acid-enhanced magnetic resonance imaging as significant factors. After adding postoperative histopathologic factors, presence of microvascular invasion (OR, 1.868; 95% CI, 1.155–3.022; p = 0.011) became an additional significant factor, while tumor size became insignificant (p = 0.119). Comparison of the AUCs of the two models showed that the prediction model built on preoperative factors alone was not inferior to that including both pre-/postoperative factors {AUC for preoperative factors only, 0.673 (95% confidence interval [CI], 0.623–0.723) vs. AUC after adding postoperative factors, 0.691 (95% CI, 0.639–0.744); p = 0.0013}. Bootstrap resampling method showed that both the models were valid.
Conclusion
Risk stratification solely based on preoperative imaging and laboratory factors was not inferior to that based on postoperative histopathologic risk factors in predicting early recurrence after curative resection in within Milan criteria single HCC patients.
Keywords: Prediction model, Laboratory factors, Imaging factors, Pathologic factors
INTRODUCTION
Surgical resection of hepatocellular carcinoma (HCC) is the first-line treatment option with curative intent in patients with well-preserved liver function. Unfortunately, the recurrence rate within five years after curative resection has been reported to be as high as 50%, and more than 80% of tumor recurrences occur in the liver (1,2,3). Among them, intrahepatic metastasis usually occurs within the first 2 years after curative resection and is associated with a poorer prognosis (4,5). Therefore, it is particularly important to individually assess the risk of postoperative tumor recurrence to predict the prognosis. However, the most widely accepted risk factors associated with increased risk of tumor recurrence are mostly histopathologic factors, such as microvascular invasion (MVI), worse tumor cell grade, and microsatellite nodules, which are available only after surgery (6,7,8). Extensive efforts have been made to identify high-risk factors of early HCC recurrence, which can be extracted from preoperative laboratory and/or imaging studies (9,10,11,12). Recently, a few studies have reported that certain findings of gadoxetic acid-enhanced magnetic resonance imaging (MRI) reflect increased risk of early HCC recurrence (10,12). However, to the best of our knowledge, previous literature fails to describe how well such preoperative factors can recapitulate the actual risk of early HCC recurrence, in comparison with postoperative histopathological factors.
In the current study, we searched for risk factors of early tumor recurrence in within Milan criteria single HCC patients who underwent curative resection, first by evaluating preoperative findings only, and then reassessed them by considering preoperative factors and postoperative histopathologic factors altogether. Next, two prediction models for early HCC recurrence either based on preoperative factors alone or both pre-/postoperative factors were built, and a non-inferiority test was performed to compare their performance for risk stratification. The purpose of this study was to evaluate the performance of predicting early recurrence using preoperative factors only in comparison with using both pre-/postoperative factors.
MATERIALS AND METHODS
Study Population
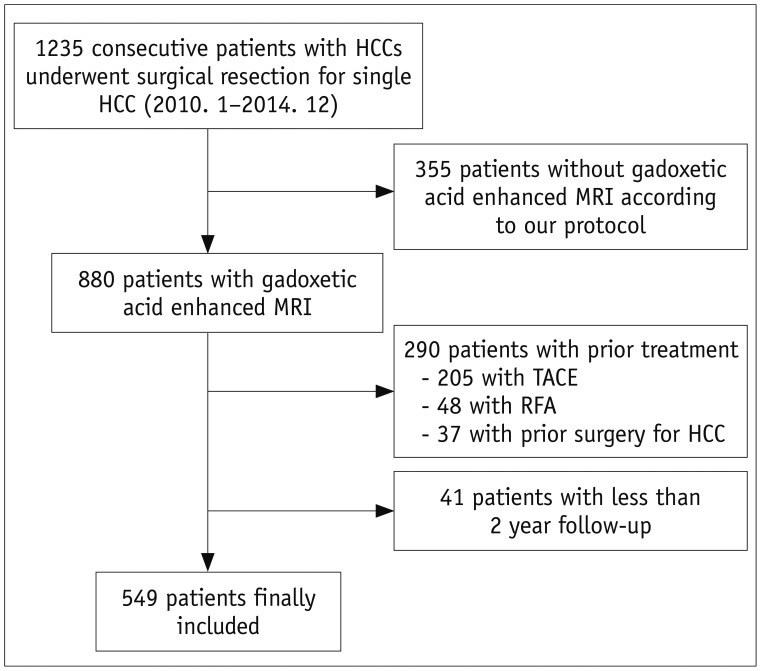
This was a retrospective study, conducted at a single, tertiary academic center. The Institutional Review Board approved this study and waived the requirement for informed consent. A study coordinator, with 14 years of experience in abdominal MRI, retrospectively searched our institution's surgicopathologic, medical, and radiologic database between January 2010 and December 2014, to identify patients who met the following inclusion criteria: 1) high risk for HCC, with conditions such as chronic hepatitis, and liver cirrhosis (LC), according to American Association for the Study of Liver Diseases guidelines; 2) contrast-enhanced liver computed tomography (CT), and gadoxetic acid-enhanced liver diffusion-weighted MRI, according to our standard protocol performed within two months prior to curative resection; 3) no prior treatment for HCC; 4) single HCC within the Milan criteria; and 5) at least two years of follow-up after surgery (13). A total of 549 patients met the inclusion criteria (Fig. 1), and their characteristics are shown in Table 1. The criteria for surgical resection of HCC are as follows: 1) Child's classification of liver function of A or B with an indocyanine green (ICG) 15-minute retention rate < 30%; 2) tumors that involved no more than three Healey's segments, without portal vein main trunk involvement or distant metastasis; and 3) absence of other major diseases that could complicate the surgery. The mean time interval between acquisition of MRI and surgery was 18 days (range, 0–58 days).
Fig. 1. Flow diagram of patient selection for study.
HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, RFA = radiofrequency ablation, TACE = transarterial chemoembolization
Table 1. Characteristics of Included 549 Patients and Univariable Analysis.
| Factors | Total (n = 549) | Early HCC Recurrence (n = 109) | No Early HCC Recurrence (n = 440) | Odds Ratio (95% CI) | P |
|---|---|---|---|---|---|
| Clinical factors | |||||
| Age (year)* | 55 (26–84) | 55 (31–81) | 56 (26–84) | 0.99 (0.96–1.01) | 0.388 |
| Sex (male) | 442 (80.5) | 88 (80.7) | 354 (80.5) | 1.01 (0.60–1.73) | 0.947 |
| Cause of liver disease | |||||
| Hepatitis B virus | 465 (84.7) | 94 (86.2) | 371 (84.3) | 1 (reference) | |
| Hepatitis C virus | 32 (5.8) | 9 (8.3) | 23 (5.2) | 1.54 (0.69–3.44) | 0.288 |
| Other | 52 (9.5) | 6 (5.5) | 46 (10.5) | 0.51 (0.21–1.20) | 0.139 |
| Liver cirrhosis | 247 (45.0) | 60 (55.0) | 187 (42.5) | 1.65 (1.08–2.52) | 0.019 |
| Laboratory factors | |||||
| AFP (ng/mL)* | 17.5 (1.3–47524.4) | 44.4 (1.3–8275.0) | 13.8 (1.3–47524.4) | 1.00 (1.00–1.00) | 0.546 |
| PIVKA-II* | 37 (3–35940) | 63 (13–7466) | 35 (3–35940) | 1.00 (1.00–1.00) | 0.732 |
| GGT* | 35 (8–600) | 44 (14–257) | 3335 (8–600) | 1.00 (0.99–1.00) | 0.834 |
| NLR* | 1.559 (0.539–10.750) | 1.481 (0.557–5.603) | 1.577 (0.539–10.750) | 0.80 (0.61–1.06) | 0.129 |
| APRI* | 0.438 (0–3.953) | 0.531 (0–3.953) | 0.425 (0–3.196) | 2.02 (1.36–3.06) | < 0.001 |
| NA‡ | 32 (5.8) | 6 (5.5) | 26 (5.9) | - | - |
| ≥ 0.413 | 291 (56.3) | 72 (69.9) | 219 (52.9) | 2.068 (1.301–3.286) | 0.002 |
| < 0.413 | 226 (43.7) | 31 (30.1) | 195 (47.1) | 1 (reference) | |
| ALBI score* | -3.12 (-3.79–1.82) | -3.11 (-3.71–1.82) | -3.12 (-3.79–1.99) | 1.47 (0.74–2.90) | 0.262 |
| Imaging factors | |||||
| Tumor size (mm)† | 2.81 ± 1.03 | 3.02 ± 1.00 | 2.76 ± 1.03 | 1.24 (1.02–1.51) | 0.018 |
| ≥ 35 | 177 (32.2) | 51 (28.8) | 126 (71.2) | 1.018 (1.007–1.028) | 0.001 |
| < 35 | 372 (67.8) | 58 (15.6) | 314 (84.4) | 1 (reference) | - |
| Irregualr tumor contour | 272 (49.5) | 63 (57.8) | 209 (47.5) | 1.51 (0.99–2.31) | 0.055 |
| Typical HCC enhancement pattern | 472 (86.0) | 89 (81.7) | 383 (87.0) | 0.66 (0.37–1.15) | 0.148 |
| Capsule | 374 (68.1) | 77 (70.6) | 297 (67.5) | 1.15 (0.73–1.83) | 0.528 |
| Intratumoral fat | 112 (20.4) | 25 (22.9) | 87 (19.8) | 1.20 (0.72–2.00) | 0.463 |
| Satellite nodule | 10 (1.8) | 1 (0.9) | 9 (2.0) | 0.44 (0.05–3.53) | 0.442 |
| Arterial rim enhancement | 75 (13.7) | 24 (22.0) | 51 (11.6) | 2.15 (1.25–3.69) | 0.005 |
| Peritumoral enhancement | 63 (11.5) | 19 (17.4) | 44 (10.0) | 1.90 (1.05–3.40) | 0.031 |
| Pertumoral hypointensity | 87 (15.8) | 15 (13.8) | 72 (16.4) | 0.81 (0.44–1.48) | 0.506 |
| NHHN | 64 (11.7) | 21 (19.3) | 43 (9.8) | 2.20 (1.24–3.89) | 0.006 |
| ADC value | 1023 (270–2791) | 997 (643–1896) | 1030 (270–2791) | 1.00 (0.99–1.00) | 0.508 |
| Pathologic factors | 66 (12.0) | 12 (11.0) | 54 (12.3) | 0.88 (0.45–1.71) | 0.716 |
| Anatomic resection | |||||
| Serosal invasion | 2 (0.3) | 1 (0.9) | 1 (0.2) | 4.06 (0.25–65.50) | 0.322 |
| Safety margin | 289 (52.6) | 62 (56.9) | 227 (51.6) | 1.26 (0.82–1.93) | 0.278 |
| Satellite nodule | 12 (2.2) | 6 (5.6) | 6 (1.4) | 4.21 (1.33–13.33) | 0.014 |
| Gross vascular invasion | 10 (1.8) | 3 (2.8) | 7 (1.6) | 1.75 (0.44–6.88) | 0.422 |
| MVI | 196 (35.7) | 57 (52.3) | 139 (31.6) | 2.37 (1.55–3.63) | < 0.001 |
| Edmonson grade | - | - | - | - | 0.215 |
| Grade 1 (reference) | 50 (9.1) | 4 (3.7) | 46 (10.5) | 1 (reference) | - |
| Grade 2 | 460 (83.8) | 97 (89.0) | 363 (82.5) | 3.07 (1.08–8.74) | 0.035 |
| Grade 3 | 37 (6.7) | 8 (7.3) | 29 (6.6) | 3.17 (0.87–11.49) | 0.078 |
| Grade 4 | 2 (0.3) | 0 (0) | 2 (0.5) | < 0.001 (< 0.001 to > 999.99) | 0.986 |
Unless otherwise indicated, data are numbers with percentage in parentheses. *Data are medians with range in parentheses, †Data are means ± standard deviations, ‡NA (missing) cases. Percentages were calculated after excluding NA (missing) cases. ADC = apparent diffusion coefficient, AFP = alpha-fetoprotein, ALBI = albumin-bilirubin, APRI = AST/platelet ratio index, AST = aspartate aminotransferase, CI = confidence interval, GGT = gamma-glutamyl transferase, HCC = hepatocellular carcinoma, MVI = microvascular invasion, NA = non-assessable, NHHN = nonhypervascular hepatobiliary hypointense nodule, NLR = neutrophil/lymphocyte ratio, PIVKA-II = protein induced by vitamin K absence-II
Liver CT and MR Technique
Multiphase liver CT composed of unenhanced, hepatic arterial, portal venous, and equilibrium (delayed) phases, was conducted with 64-multidetector CT scanners (Aquilion 64, Canon Medical Systems, Ottawara, Japan and LightSpeed VCT, GE Healthcare, Waukesha, WI, USA). MRI was performed using a 3T whole-body MR system (Intera Achieva 3.0T, Philips Healthcare, Best, The Netherlands), with a 32-channel phased-array coil used as a receiver coil. Gadoxetic acid (Primovist, Schering AG, Berlin, Germany) was used as a contrast agent. Details of imaging techniques of liver CT and MRI are provided in the Supplementary Materials.
Assessed Preoperative Factors
The included clinical, laboratory, and imaging factors for this study are listed in Table 1. Among the laboratory factors that have been reported to be related with HCC recurrence in previous studies, those that were evaluated within one month before surgery were recorded (9,14,15,16,17). The formulae of the calculated factors including neutrophil/lymphocyte ratio (NLR), aspartate aminotransferase (AST)/platelet ratio index (APRI), and albumin-bilirubin (ALBI) score in this study were as follows:
NLR = neutrophil count / lymphocyte count;
APRI = (AST [IU/L] / upper limit normal of AST / platelet [× 109/L]) × 100; and
ALBI score = (log 10 bilirubin [µmoL/L] × 0.66) + (albumin [g/L] × −0.085).
Imaging factors evaluated in this study were selected based on previous literature (10,12,18,19,20,21,22,23,24,25,26). For imaging analysis, two abdominal radiologists (each with 20 and 17 years of liver MRI interpretation experience) who were blinded to the clinicopathological data of the patients, reviewed the MR images independently on picture archiving, and communication system (Pathspeed, GE Medical Systems Integrated Imaging Solutions, Mount Prospect, IL, USA). Consensus data were used for image analysis. Details for assessed imaging factors and interobserver agreements are provided in Supplementary Materials. One reviewer measured the apparent diffusion coefficient (ADC) values of the tumors, using a manually drawn round region of interest (ROI) on the ADC map. ROI was drawn three times for each tumor, and the average value was used as the ADC value. The ROI was drawn on the axial slice at the largest cross-sectional area of the tumor, for uniformity of ROI placement at homogeneous solid components. Cystic, necrotic, or hemorrhagic areas, if any, were avoided as much as possible.
Pathologic Factors
The study coordinator reviewed the pathologic reports of the resected HCCs and recorded the following: 1) anatomic resection vs. nonanatomic resection, 2) serosal invasion, 3) safety margin (≤ 1 cm vs. > 1 cm), 4) presence of satellite nodule, 5) presence of gross vascular invasion, 6) presence of MVI, and 7) Edmonson grade of HCC. Details for assessed pathologic factors are provided in Supplementary Materials.
Follow-Up Protocol after Surgical Resection
Multiphasic abdominal CT or MRI was performed every three months after surgery. Physical examination, chest radiography, and laboratory tests including serum alpha-fetoprotein (AFP), and protein induced by vitamin K absence-II (PIVKA-II) were also performed at every visit. Early recurrence was defined as recurrence within two years after resection of HCC.
Statistical Analysis
The characteristics of the patients were summarized using mean with standard deviation, or median with range for continuous data, and number with percentage for categorical data. Univariable logistic regression was used to study the relation between each variable and early HCC recurrence. Factors with p value < 0.15 in univariable analysis were considered for further multivariable analyses. For multivariable analyses, stepwise variable selection method based on Akaike information criterion (AIC) was performed twice, once with preoperative laboratory and imaging factors only, and once more after adding postoperative pathologic factors of early recurrence of HCC. If significant factors from multivariable analysis were different between using preoperative factors only, and using both pre- and postoperative factors, a subgroup analysis was performed to confirm a relationship between the discrepant risk factors.
Two prediction models for early HCC recurrence were then developed, based on the results of multivariable logistic regression models using preoperative factors only, and using both pre- and postoperative factors, respectively. To quantify the prognostic performance, area under the curve (AUC) for each receiver operating characteristic (ROC) curve for the prediction model was then calculated with 95% confidence interval (CI). Afterwards, the two AUCs were compared for non-inferiority testing with the non-inferiority margin of 0.05 (27).
An appropriate cut-off value for continuous factors among selected factors in the prediction models was determined using ROC analysis by maximizing the Youden index. Variance inflation factor was used as an indicator of autocorrelation or collinearity of independent variables included in the model. When the value of variance inflation factor was > 10, the multicollinearity was considered to be high (28).
A p value < 0.05 was considered statistically significant. The predictive models of early HCC recurrence were internally validated by bootstrap resampling method with 1000 replicates (29). The performance of the bootstrap sample applied to the prediction models was estimated, and it was determined that the model was valid if the performance of the bootstrap sample showed a similar level of performance. Statistical analyses were performed using R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria). The kappa (κ) test and 95% CIs were used to determine interobserver agreement of imaging findings (Supplementary Table 1).
RESULTS
The characteristics of the patients and various risk factors for early tumor recurrence are shown in Table 1. Among 549 patients, 109 exhibited early HCC recurrence, and median time of early recurrence after surgery was 10.8 months (range 2.2–24.0 months).
Univariable Analysis of Laboratory, Imaging, and Pathologic Factors for Early Recurrence
Table 1 shows the results of univariable logistic regression analysis. Early recurrence rate of HCC with LC was significantly higher compared to that with noncirrhotic liver (24.3% [60/247] vs. 16.2% [49/302], p = 0.019). Among the laboratory factors, APRI was significantly different between patients with early tumor recurrence and those without (p = 0.0005). Early recurrence rate of HCC with optimal cut off value of APRI ≥ 0.413 was significantly higher compared with that of APRI < 0.413 [13.7% (31/226) vs. 24.7% (72/291), p = 0.002]. Among imaging factors, tumor size with optimal cut off value of ≥ 35 mm (p = 0.018), arterial rim enhancement (Rim) of tumor (p = 0.005), peritumoral parenchymal enhancement (p = 0.031), and presence of nonhypervascular hepatobiliary hypointense nodules (NHHNs) (p = 0.006) were significant factors associated with early HCC recurrence. Among pathologic factors, presence of satellite nodule and MVI (p = 0.014, < 0.001, each) were significant for early HCC recurrence.
Multivariable Analysis of Laboratory, Imaging, and Pathologic Factors for Early Recurrence
Table 2 shows the results of multivariable analyses using selected factors based on AIC. Multivariable analysis using preoperative factors identified APRI, tumor size, Rim of the tumor, and presence of NHHNs as significant factors (p < 0.05) (Figs. 2, 3). Multivariable analysis including postoperative histopathologic factors as well indicated APRI, Rim of the tumor, presence of NHHNs and MVI to be significant factors. There was no evidence of multicollinearity among the variables. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of each significant predictive factor for early recurrence are shown in Table 3. Subgroup analysis to confirm a relationship between tumor size groups (≥ 35 mm vs. < 35 mm) and presence of MVI showed that the frequency of MVI was 28.8% (107/372) in ≥ 35 mm group, and 50.3% (89/177) in < 35 mm group, which was statistically significant (p < 0.001, odds ratio 2.505, 95% CI: 1.729–3.628).
Table 2. Multivariable Analysis Using Stepwise Variable Selection Method Based on Akaike Information Criterion for Early HCC Recurrence.
| Factors | Preoperative Factors Only | Adding Postopertive Pathologic Factors | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Liver cirrhosis | 1.604 (0.999–2.576) | 0.051 | 1.546 (0.959–2.492) | 0.074 |
| NLR | 0.781 (0.585–1.061) | 0.116 | 0.806 (0.597–1.089) | 0.16 |
| APRI | 1.632 (1.056–2.522) | 0.027 | 1.712 (1.103–2.659) | 0.017 |
| Tumor size | 1.025 (1.002–1.049) | 0.031 | 1.019 (0.995–1.043) | 0.119 |
| Arterial rim enhancement | 2.350 (1.297–4.260) | 0.005 | 2.004 (1.091–3.680) | 0.025 |
| Peritumoral enhancement | 1.873 (0.977–3.590) | 0.059 | 1.696 (0.880–3.270) | 0.115 |
| NHHN | 1.983 (1.049–3.750) | 0.035 | 2.003 (1.050–3.822) | 0.035 |
| MVI | NA | NA | 1.868 (1.155–3.022) | 0.011 |
Numbers in parentheses are 95% CIs.
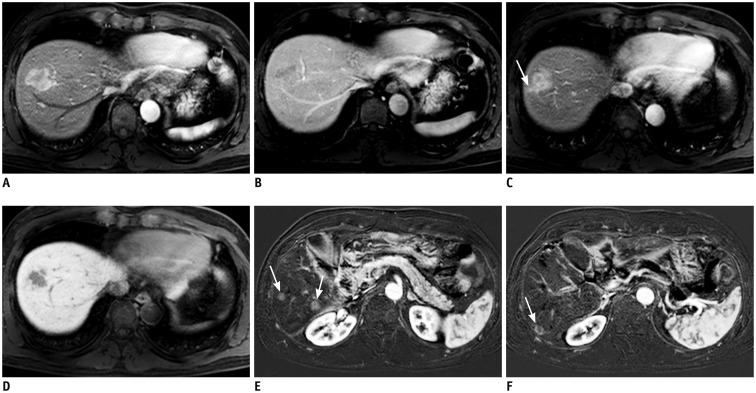
Fig. 2. MRI of 54-year-old male with HCC.
Arterial phase tumor rim enhancement, peritumoral parenchymal enhancement, and high APRI were noted. APRI level was 0.456.
In S8 dome, 3-cm sized lobulated mass showing strong enhancement on arterial phase (A) with subsequent washout on portal phase (B) is present. Of note, arterial phase enhancement is not homogeneous; enhancement is seen mostly in outer or peripheral portion of tumor while inner portion shows poor enhancement. Peritumoral parenchymal enhancement on arterial phase (arrow) (C) was noted beyond tumor margins that can be defined on hepatobiliary phase (D). E, F. Segmentectomy was performed for HCC. Seven months after surgery, subtraction of pre-contrast from arterial phase images demonstrated multiple recurrent HCCs in liver (arrows). APRI = AST/platelet ratio index, AST = aspartate aminotransferase
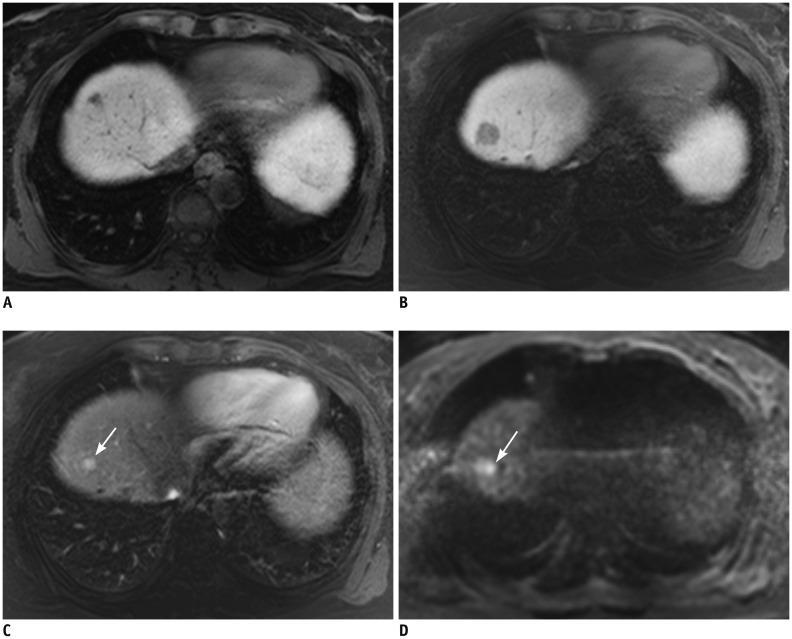
Fig. 3. MRI of 54-year-old female with 1.6-cm sized HCC in S7 of liver (not shown).
A. Preoperative MRI revealed 1-cm sized NHHN at S4 on HBP. Enhancement of this lesion was not perceivable on arterial phase (not shown). B. Gadoxetic acid-enhanced MRI with diffusion-weighted images performed 18 months after right hemihepatectomy showed that NHHN on HBP had increased in size, measuring 1.9 cm. C. On arterial phase, nodular enhancement within lesion had developed (arrow). D. On diffusion-weighted images, enhancing focus showed high signal intensity (arrow) indicating nodule-in-nodule type HCC. HBP = hepatobiliary phase, NHHN = nonhypervascular hepatobiliary hypointense nodules
Table 3. Diagnostic Performance of Preoperative and Postoperative Each Significant Factors in Prediction of Early Recurrence.
| Factors | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| APRI ≥ 0.413 | 69.7 (76/109) | 47.1 (207/440) | 24.6 (77/309) | 86.3 (207/240) | 51.6 (283/549) |
| Tumor size ≥ 35 mm | 46.8 (51/109) | 71.4 (314/440) | 28.8 (51/177) | 84.4 (314/372) | 66.5 (365/549) |
| Arterial rim enhancement | 22.0 (24/109) | 88.4 (389/440) | 32.0 (24/75) | 82.1 (389/474) | 75.2 (413/549) |
| Peritumoral parenchymal enhancement | 17.4 (19/109) | 90.0 (396/440) | 30.2 (19/63) | 81.5 (396/486) | 75.6 (415/549) |
| Presence of NHHNs | 19.3 (21/109) | 90.2 (397/440) | 32.8 (21/64) | 81.9 (397/485) | 76.1 (418/549) |
| MVI | 52.3 (57/109) | 68.4 (301/440) | 29.1 (57/196) | 85.3 (301/353) | 65.2 (358/549) |
NPV = negative predictive value, PPV = positive predictive value
Prediction Models for Early HCC Recurrence
Two prediction models for early HCC recurrence based on multivariable logistic regression models using preoperative factors only and using both pre- and postoperative factors, respectively, were created. Their equations are as follows:
Preoperative factor-only model
log odds = = − 2.576 + 0.472 × LC − 0.239 × NLR + 0.490 × APRI + 0.627 × peritumoral + 0.855 × Rim + 0.685 × NHHN + 0.025 × size
Preoperative and postoperative factor model
log odds = = − 2.673 + 0.436 × LC − 0.215 × NLR + 0.538 × APRI + 0.528 × peritumoral + 0.695 × Rim + 0.695 × NHHN + 0.625 × MVI + 0.019 × size
Peritumoral, peritumoral enhancement.
The ROC curves of the two prediction models made by using only preoperative factors and by adding postoperative pathologic factors are displayed in Figure 4. Comparison of the AUCs of the two models showed that the prediction model based on preoperative factors only was non-inferior to the one that used both pre-/postoperative factors {AUC for preoperative factors only, 0.673 (95% confidence interval [CI], 0.623–0.723) vs. AUC after adding postoperative factors, 0.691 (95% CI, 0.639–0.744); p = 0.0013}. The AUCs of the bootstrap sample were 0.675 (95% CI, 0.614–0.722) and 0.693 (95% CI, 0.634–0.740) for preoperative factors-only model and pre-/postoperative factors altogether-model, respectively, which were both similar to their primary models. Therefore, bootstrap resampling method showed that both the models were valid.
Fig. 4. ROC curve analysis of two prediction models using preoperative laboratory and imaging factors of gadoxetic acid-enhanced MRI only (gray curve) and using both preoperative and postoperative pathologic factors (black curve).
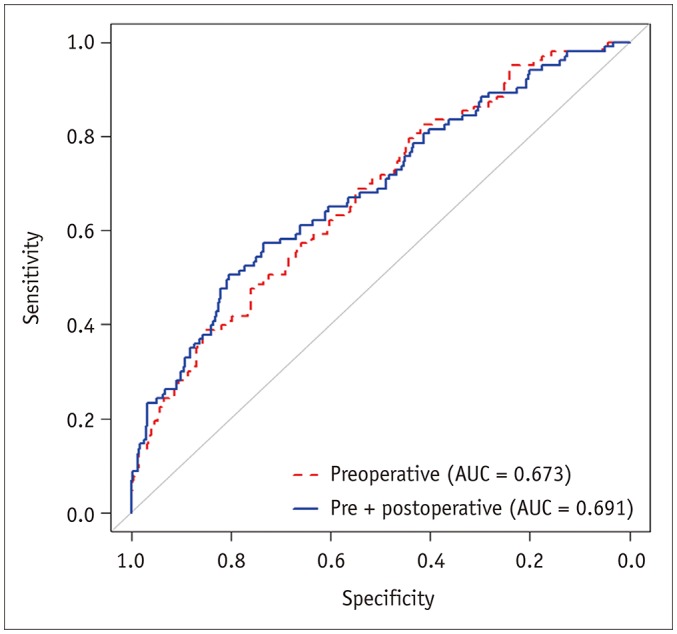
Comparison of area under ROC curves of two models showed that performance of prediction model built on preoperative factors only was not inferior to that of one using both pre-/postoperative factors (preoperative factors only; 0.673 [95% CI: 0.623–0.723] vs. after adding postoperative factors; 0.691 [95% CI: 0.639–0.744]; p = 0.0013). AUC = area under curve, CI = confidence interval, ROC = receiver operating characteristic
DISCUSSION
The results of our study showed that the prediction model built on only preoperative laboratory and imaging factors was not inferior to that using both preoperative factors and postoperative histopathologic factors altogether, as demonstrated by comparing the AUCs. This seemed to have clinical significance, considering that a large number of HCCs are being treated without histologic confirmation.
In our study, we confined our scope to single HCC patients within the Milan criteria because we considered them as optimal candidates for curative resection. We intended to simulate the clinical situation of patients being initially diagnosed with HCC, who have a demand for risk stratification on postoperative tumor recurrence, solely based on the preoperative factors. However, unlike most previous studies that simply focused on identifying meaningful preoperative risk factors, we attempted to directly compare their performance with that of postoperative histopathological factors (which are considered as gold standard for predicting clinical outcome), based a non-inferiority test design.
Previous studies have made considerable progress in elucidating preoperative imaging and postoperative histopathological factors, reflecting high risk of postoperative tumor recurrence in HCC patients after curative resection (9,10,11,12,14,16,30). Histopathological factors, such as MVI, seem to be the most important factors directly linked to treatment outcome (30). However, at least from the practical point of view, these factors have little value during treatment planning as histopathological information is available only after surgery.
In our study, when postoperative factors were added to multivariable analysis in addition to preoperative factors, MVI was an additional significant factor, while tumor size, which was a significant factor when using preoperative factors only, became insignificant. This may indicate the possible association between tumor size and MVI, which seems meaningful, as MVI is known as one of the most important factors associated with early HCC recurrence after surgical resection (30). Subgroup analysis also showed that presence of MVI was significantly different according to tumor size. The importance of MVI for postoperative tumor recurrence and lower survival has also been reported in many studies (31,32). In a multicentric study of 1073 patients by Pawlik et al. (33), the rates of MVI were reported to be 25%, 40%, 55%, and 63% in patients with HCC < 3 cm, 3–5 cm, > 5–6.5 cm, and > 6.5 cm, respectively. Studies by Du et al. (34) and Yamashita et al. (35) reported the rates of MVI of surgically resected small HCC to be 18.1% (n = 458, tumor ≤ 3 cm) and 28.9% (n = 149, tumor ≤ 2 cm), respectively. Taking these studies together, the fact that tumor size becoming insignificant after adding MVI in the multivariable analysis seems meaningful and possibly reflects an association between the tumor size and MVI. It can also be suggested that some difference in the diagnostic performance between the preoperative prediction model and postoperative pathologic factor-added prediction model may have been due to the presence of MVI in some small tumors.
Among the preoperative laboratory factors, we found APRI to be significant both before and after adding postoperative histopathologic factors. APRI was originally developed as a predictive marker for liver fibrosis, and cirrhosis, especially for hepatitis C virus infected patients (36). A few studies have suggested APRI to be associated with tumor recurrence and survival after surgical resection of HCC (9,37,38). Similar to our study, it has been reported that higher levels of APRI are associated with increased risk of HCC recurrence. The exact mechanism of high APRI and poor prognosis remains unclear, but some researchers hypothesize that platelets might have a role in stimulating tumor angiogenesis, and therefore, promote tumor growth (9,36,37). Another potential explanation would be that platelets reduce the cytolytic activity of natural killer cells and thus, might protect tumor cells (39). The optimal cut-off value of APRI differs among studies; the values reported in previous studies range from 0.40 to 1.94, whereas our study showed a value of 0.413 (37,40,41,42). We believe that a prospective multicenter-large population study would be necessary to confirm the value of APRI as a significant risk factor of early HCC recurrence.
The presence of NHHNs on gadoxetic acid-enhanced MRI and Rim of the tumor on CT or MRI were also significant imaging factors before and after adding postoperative histopathologic factors to the multivariable analysis. The features of NHHNs include hypovascular well-differentiated HCCs, dysplastic nodules, and other benign nodules, and it is difficult to specify their precise nature by imaging (43). In a previous study, 75 (35.0%) of 214 NHHNs in 135 patients with LC eventually progressed to hypervascular HCC during a mean follow-up period of 388 days, and similar results have been reported in other studies as well (44,45,46). Meanwhile, the presence of an Rim of the tumor on CT or MRI has been reported to be a feature associated with scirrhous and sarcomatous HCCs as well as early tumor recurrence (11,47,48). Similarly, Rim of HCC on gadoxetic acid-enhanced MRI was described to be a feature associated with cytokeratin 19-positive HCCs, which is a disease entity known to have poorer prognosis and higher rate of recurrence (49,50). Therefore, Rim of HCC could be regarded as a preoperative risk factor, associated with early HCC recurrence after curative resection.
Some studies have reported additional factors such as age, AFP, PIVKA–II, tumor grade, peritumoral parenchymal enhancement on arterial phase, non-smooth tumor margin, and peritumoral hypointensity on hepatobiliary phase, and ADC value as significantly associated with early tumor recurrence (10,51). However, we were unable to find statistical significance in our study.
There were several limitations in our study. First, this was a retrospective study, and therefore, selection bias could exist. Second, this was a single-center study, and the study population mostly consisted of patients with underlying hepatitis B virus (HBV)-related liver disease. Therefore, the results of our study may not be generalizable to populations where HBV is not the dominant cause of liver disease. Third, an external validation study using different set of patients was not performed to verify our results. However, we did perform internal validation by bootstrap resampling, and the AUCs were similar to those of the primary models. Fourth, we only included patients who underwent surgery with single tumor within the Milan criteria. Therefore, our results may not be applicable to patients who are planned for other therapies, such as radiofrequency ablation, or those who have more advanced tumors. Lastly, APRI was missing in 32 (5.8%) patients due to missing AST levels prior to surgery. However, this seems to be a relatively small portion of the patients, and the similar rate of missing data in patients with or without early recurrence (5.5% vs. 5.9%) may suggest that its influence would be limited.
In conclusion, our results indicated larger tumor size, presence of NHHNs, arterial phase tumor rim enhancement, and higher APRI as high-risk factors for early HCC recurrence after curative resection that can be assessed in the preoperative period. The performance of risk stratification based on preoperative factors alone was not inferior to that including postoperative histopathologic factors, as well for predicting early postoperative tumor recurrence in within Milan criteria single HCC patients who underwent curative resection.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2019.0538.
Inter-Observer Agreement of Reviewers for Imaging Findings
References
- 1.Harada T, Shigemura T, Kodama S, Higuchi T, Ikeda S, Okazaki M. Hepatic resection is not enough for hepatocellular carcinoma. A follow-up study of 92 patients. J Clin Gastroenterol. 1992;14:245–250. doi: 10.1097/00004836-199204000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 6.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 7.Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 8.Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. doi: 10.1016/0016-5085(93)90724-q. [DOI] [PubMed] [Google Scholar]
- 9.Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21:3802–3809. doi: 10.1245/s10434-014-3771-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526–534. doi: 10.1016/j.jhep.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 11.An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ. Single hepatocellular carcinoma: preoperative MR imaging to predict early recurrence after curative resection. Radiology. 2015;276:433–443. doi: 10.1148/radiol.15142394. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015;62:1122–1130. doi: 10.1016/j.jhep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 14.Cillo U, Giuliani T, Polacco M, Herrero Manley LM, Crivellari G, Vitale A. Prediction of hepatocellular carcinoma biological behavior in patient selection for liver transplantation. World J Gastroenterol. 2016;22:232–252. doi: 10.3748/wjg.v22.i1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 16.Ji F, Liang Y, Fu SJ, Guo ZY, Shu M, Shen SL, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI) BMC Cancer. 2016;16:137. doi: 10.1186/s12885-016-2189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong ZR, Zou J, Sun D, Shi GM, Ke AW, Cai JB, et al. Preoperative albumin-bilirubin score for postoperative solitary hepatocellular carcinoma within the Milan criteria and Child-Pugh A cirrhosis. J Cancer. 2017;8:3862–3867. doi: 10.7150/jca.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, et al. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:575–585. doi: 10.1007/s00534-010-0369-y. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30–50. doi: 10.1148/radiol.14132362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, et al. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics. 2005;25:321–331. doi: 10.1148/rg.252045083. [DOI] [PubMed] [Google Scholar]
- 21.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55:132–140. doi: 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19:1744–1751. doi: 10.1007/s00330-009-1331-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, et al. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35:629–634. doi: 10.1002/jmri.22876. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Sone Y, et al. Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol. 2013;58:1174–1180. doi: 10.1016/j.jhep.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto D, Yoshimitsu K, Nishie A, Tajima T, Asayama Y, Ishigami K, et al. Enhancement pattern analysis of hypervascular hepatocellular carcinoma on dynamic MR imaging with histopathological correlation: validity of portal phase imaging for predicting tumor grade. Eur J Radiol. 2012;81:1116–1121. doi: 10.1016/j.ejrad.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 27.Liu JP, Ma MC, Wu CY, Tai JY. Tests of equivalence and non-inferiority for diagnostic accuracy based on the paired areas under ROC curves. Stat Med. 2006;25:1219–1238. doi: 10.1002/sim.2358. [DOI] [PubMed] [Google Scholar]
- 28.Neter J, Wasserman W, Kutner MH. Multicollinearity diagnostics—variance inflation factor. In: Kutner MH, Nachtsheim CJ, Neter J, Li W, editors. Applied linear statistical models. 5th ed. Homewood, IL: McGraw-Hill Irwin; 1990. pp. 407–411. [Google Scholar]
- 29.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York, NY: Chapman and Hall; 1993. [Google Scholar]
- 30.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 31.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 32.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 33.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 34.Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. doi: 10.1186/1471-2407-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita Y, Tsuijita E, Takeishi K, Fujiwara M, Kira S, Mori M, et al. Predictors for microinvasion of small hepatocellular carcinoma ≤ 2 cm. Ann Surg Oncol. 2012;19:2027–2034. doi: 10.1245/s10434-011-2195-0. [DOI] [PubMed] [Google Scholar]
- 36.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 37.Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691–699. doi: 10.1007/s12072-010-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hann HW, Wan S, Lai Y, Hann RS, Myers RE, Patel F, et al. Aspartate aminotransferase to platelet ratio index as a prospective predictor of hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2015;30:131–138. doi: 10.1111/jgh.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 40.Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 1985;5:367–375. doi: 10.1002/hep.1840050305. [DOI] [PubMed] [Google Scholar]
- 41.Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, et al. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. 2011;46:536–544. doi: 10.1007/s00535-010-0349-7. [DOI] [PubMed] [Google Scholar]
- 42.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233–1242. doi: 10.1007/s00330-010-2030-1. [DOI] [PubMed] [Google Scholar]
- 44.Kim YK, Lee WJ, Park MJ, Kim SH, Rhim H, Choi D. Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology. 2012;265:104–114. doi: 10.1148/radiol.12112649. [DOI] [PubMed] [Google Scholar]
- 45.Jin S, Zhang B, Zhang L, Li S, Li S, Li P. Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT. Quant Imaging Med Surg. 2018;8:480–490. doi: 10.21037/qims.2018.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang J, Kim YK, Jeong WK, Choi D, Rhim H, Lee WJ. Nonhypervascular hypointense nodules at gadoxetic acid-enhanced MR imaging in chronic liver disease: diffusion-weighted imaging for characterization. Radiology. 2015;276:137–146. doi: 10.1148/radiol.15141350. [DOI] [PubMed] [Google Scholar]
- 47.Kim SH, Lim HK, Lee WJ, Choi D, Park CK. Scirrhous hepatocellular carcinoma: comparison with usual hepatocellular carcinoma based on CT-pathologic features and long-term results after curative resection. Eur J Radiol. 2009;69:123–130. doi: 10.1016/j.ejrad.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Gu KW, Kim YK, Min JH, Ha SY, Jeong WK. Imaging features of hepatic sarcomatous carcinoma on computed tomography and gadoxetic acid-enhanced magnetic resonance imaging. Abdom Radiol (NY) 2017;42:1424–1433. doi: 10.1007/s00261-016-1038-7. [DOI] [PubMed] [Google Scholar]
- 49.Jeong HT, Kim MJ, Kim YE, Park YN, Choi GH, Choi JS. MRI features of hepatocellular carcinoma expressing progenitor cell markers. Liver Int. 2012;32:430–440. doi: 10.1111/j.1478-3231.2011.02640.x. [DOI] [PubMed] [Google Scholar]
- 50.Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, et al. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851–857. doi: 10.1111/j.1349-7006.2003.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33:347–354. doi: 10.1111/jgh.13843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inter-Observer Agreement of Reviewers for Imaging Findings