Abstract
Objective
A widely applicable, non-invasive screening method for non-alcoholic fatty liver disease (NAFLD) is needed. We aimed to develop and validate an index combining computed tomography (CT) and routine clinical data for screening for NAFLD in a large cohort of adults with pathologically proven NAFLD.
Materials and Methods
This retrospective study included 2218 living liver donors who had undergone liver biopsy and CT within a span of 3 days. Donors were randomized 2:1 into development and test cohorts. CTL-S was measured by subtracting splenic attenuation from hepatic attenuation on non-enhanced CT. Multivariable logistic regression analysis of the development cohort was utilized to develop a clinical-CT index predicting pathologically proven NAFLD. The diagnostic performance was evaluated by analyzing the areas under the receiver operating characteristic curve (AUC). The cutoffs for the clinical-CT index were determined for 90% sensitivity and 90% specificity in the development cohort, and their diagnostic performance was evaluated in the test cohort.
Results
The clinical-CT index included CTL-S, body mass index, and aspartate transaminase and triglyceride concentrations. In the test cohort, the clinical-CT index (AUC, 0.81) outperformed CTL-S (0.74; p < 0.001) and clinical indices (0.73–0.75; p < 0.001) in diagnosing NAFLD. A cutoff of ≥ 46 had a sensitivity of 89% and a specificity of 41%, whereas a cutoff of ≥ 56.5 had a sensitivity of 57% and a specificity of 89%.
Conclusion
The clinical-CT index is more accurate than CTL-S and clinical indices alone for the diagnosis of NAFLD and may be clinically useful in screening for NAFLD.
Keywords: Non-alcoholic fatty liver disease, Computed tomography, Diagnostic index
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease, affecting up to 40% of the general population in developed countries (1,2). NAFLD may progress to cirrhosis and hepatocellular carcinoma (3,4) and is also associated with metabolic syndrome (5). Due to its high prevalence and asymptomatic presentation, however, NAFLD is often overlooked in healthy controls selected for clinical trials, possibly hampering the validity of study findings (6). Thus, there is a need for a widely applicable, non-invasive screening method allowing for the reliable diagnosis or exclusion of NAFLD.
Liver biopsy is regarded as the gold standard for assessing NAFLD, especially for evaluating inflammation and fibrosis associated with NAFLD. However, the invasiveness of biopsy limits its use in clinical practice and research. Among imaging methods, magnetic resonance (MR) spectroscopy and imaging are the most accurate for quantifying liver fat contents and detecting NAFLD (7,8). However, these techniques are often unavailable in general practice. Although grayscale ultrasonography (US) is commonly used to screen for NAFLD, it may be subject to inter-observer variability and has limited accuracy in detecting NAFLD (7,8).
Hepatic steatosis may be quantitatively assessed by measuring hepatic attenuation on non-enhanced computed tomography (CT). CT is more widely available and less expensive than MR imaging and may provide an objective assessment of NAFLD. Thus, non-enhanced CT has been used to assess NAFLD in candidates for living liver donation (7,9), as well as in cohort studies and clinical trials (10,11,12). Although CT allows for a highly specific diagnosis of moderate to severe fatty liver disease, it is not accurate in detecting a mild degree of fatty liver disease (7,13). Therefore, it may not be reliable for selecting or excluding subjects with NAFLD. Several clinical prediction models based on demographic, anthropometric, and laboratory characteristics may be used to screen for NAFLD (14,15,16) and have been successfully applied to large-scale cohort studies (5,17). Thus, we hypothesized that combinations of clinical parameters and CT results may enable more accurate detection of NAFLD compared with clinical indices or CT alone. A simple index based on CT results and routinely accessible clinical data may be useful for detecting NAFLD in clinical practice and research. Furthermore, this index can be applied to pre-existing CT and clinical data to conduct large-scale retrospective cohort studies. The present study was designed to develop and validate a simple index combining CT and standard clinical data for screening for NAFLD in a large cohort of adults with pathologically proven NAFLD.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of our institution, which waived the requirement for informed consent due to the retrospective nature of this study.
Study Population
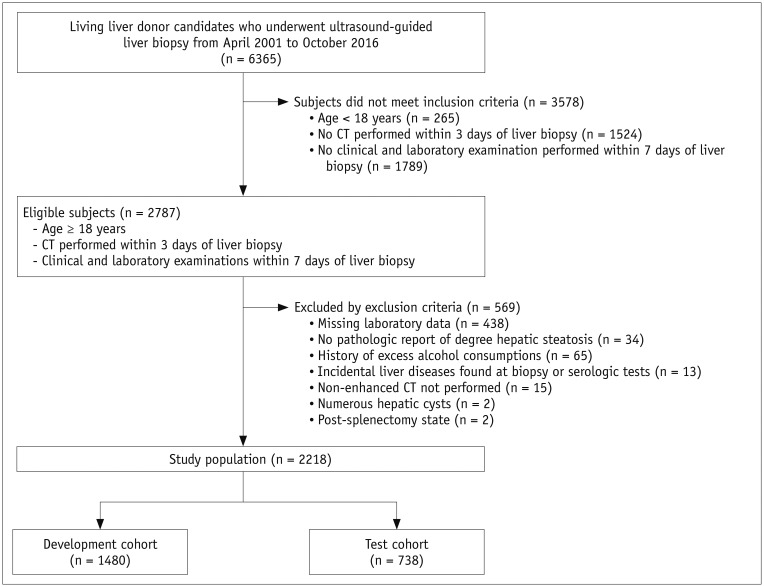
The study population included living liver donor candidates who underwent ultrasound-guided percutaneous liver biopsy as part of a routine donor work-up at our institution between April 2001 and October 2016. Subjects were included if they were aged ≥ 18 years, underwent CT scanning within 3 days of liver biopsy, and underwent clinical and laboratory examinations within 7 days of liver biopsy. Of the 2787 consecutive living liver donor candidates evaluated during the study period, 569 were excluded, including 438 with missing laboratory results (will be discussed later); 34 with pathology reports that did not include the degree of hepatic steatosis; 65 with a history of excess alcohol consumption (i.e., over 20 g of ethanol/day) (18); 13 with liver disease incidentally detected on biopsy or serologic tests; and 19 with conditions that precluded measurement of CT indices, including 15 lacking non-enhanced CT images, two with numerous hepatic cysts, and two with prior splenectomy. The remaining 2218 subjects (1439 men and 779 women; mean age, 31.0 ± 9.0 years; range, 18–62 years) were randomly divided 2:1 into development (n = 1480) and test (n = 738) cohorts. The flow diagram for the study population is shown in Figure 1. The CT data in the study population have been reported previously (13); in that study, the data were used to evaluate the performance of CT indices and determine cutoff values for diagnosing hepatic steatosis.
Fig. 1. Flow diagram for selection of study population.
CT Protocol
Because the CT data in this study were collected over a long period, various CT techniques were used. CT scans were obtained using 4-channel (Lightspeed Qx/i, GE Healthcare, Milwaukee, WI, USA; n = 2), 16-channel (Lightspeed 16, GE Healthcare or Sensation 16, Siemens Healthineers, Erlangen, Germany; n = 1611), 64-channel (Definition AS, Siemens Healthineers; n = 564), and 128-channel (Definition Flash, Siemens Healthineers; n = 41) scanners. Non-enhanced CT images were obtained at beam collimations of 4 × 2.5 mm (Lightspeed Qx/i), 8 × 2.5 mm (Lightspeed 16), 16 × 1.5 mm (Sensation 16), 24 × 1.2 mm (Definition AS), and 64 × 0.6 mm (Definition Flash); at a spiral pitch of 1 to 1.5; at tube voltages of 120 kVp (n = 1672) and 100 kVp (n = 546); and at tube currents of 200 mAs (GE scanners) or variable mAs (Siemens scanners) with an automatic exposure control (Care Dose 4D, Siemens Healthineers; maximum effective dose, 200 mAs). Axial images were reconstructed at section thicknesses of 3 mm (n = 45) and 5 mm (n = 2173), with no gaps. The mean interval between CT and liver biopsy was 0.4 ± 0.7 days (range, 0–3 days), with 1710 (74.8%) subjects undergoing CT scanning and liver biopsy on the same day.
CT Image Analysis
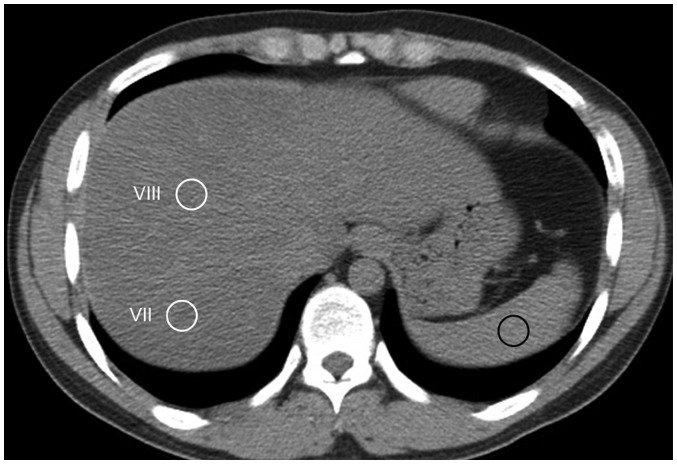
The quantitative CT index used in this study was CTL-S because it was reported to be the most accurate and robust CT index for assessing NAFLD (13). CTL-S was calculated as the mean liver attenuation minus the mean spleen attenuation. Liver and spleen attenuation values on non-enhanced CT images were measured by one of two radiology technicians using in-house software plugged into ImageJ (National Institutes of Health, Bethesda, MD, USA). Liver attenuation was calculated as the average number of Hounsfield units (HU) of eight 1.5 cm2 circular regions of interest (ROIs) of the right hepatic lobe. Splenic attenuation was calculated as the average HU of three 1.5 cm2 circular ROIs of the upper, middle, and lower thirds of the spleen (Fig. 2). The CT images with ROIs were screen-captured and re-evaluated by an abdominal imaging fellow with 2 years of experience in abdominal imaging to reconfirm the adequacy of the ROI locations.
Fig. 2. Measurement of liver and spleen attenuation on non-enhanced axial CT images.
Two 1.5-cm circular ROIs (white circles) were placed on hepatic segments VIII and VII, which were devoid of macroscopic vessels. 1.5-cm circular ROI was positioned on spleen (black circle). ROI = region of interest
Clinical Parameters
Clinical variables included body mass index (BMI), calculated as body weight (kg)/height m2; age; sex; and serum concentrations of aspartate transaminase (AST), alanine aminotransferase (ALT), bilirubin, alkaline phosphatase, triglyceride (TG), cholesterol, high-density lipoprotein (HDL) cholesterol, glucose, and albumin. These parameters were selected based on variables previously included in clinical models for the diagnosis of NAFLD (14,15,16,19). TG, cholesterol, and HDL-cholesterol concentrations were missing from the records of 438 (15.7%) of the 2787 eligible subjects who met the inclusion criteria. Because these variables were frequently included in previous clinical indices and because their rates of absence were too high for reliable data imputation, these subjects were excluded from this study. All laboratory tests were performed after a 12-hour overnight fast. The mean interval between laboratory examination and liver biopsy was 1.4 ± 2.2 days (range, 0–7 days).
Reference Standard
A pathologic diagnosis of NAFLD was defined as the reference standard. All subjects underwent US-guided percutaneous liver biopsy using an 18-gauge needle (Stericut 18G coaxial, TSK Laboratory, Tochigi, Japan), with at least two biopsy specimens measuring approximately 1.5 cm in length each, obtained from different sites in the right hepatic lobe. The biopsy specimens were stained with hematoxylin and eosin, and the degree of parenchymal involvement of macrovesicular steatosis was graded as none (< 5%), mild (5–33%), moderate (34–66%), or severe (> 66%), as defined by the non-alcoholic steatohepatitis Clinical Research Network scoring system (20). NAFLD was defined as the presence of ≥ 5% macrovesicular steatosis (20).
Clinical Indices for Diagnosing NAFLD
Two previously described clinical indices for diagnosing NAFLD, the hepatic steatosis index (HS-I) and fatty liver disease index (FLD-I), were calculated for each subject (14,16). The HS-I was calculated as 8 × (ALT / AST) + BMI (+ 2 if diabetic and + 2 if female), and the FLD-I was calculated as BMI + TG + 3 × (ALT/AST) (+ 2 if hyperglycemic, with hyperglycemia defined as a fasting plasma glucose concentration ≥ 126 mg/dL).
Statistical Analysis
Continuous variables in the development and test cohorts were compared using t tests or Mann–Whitney U-tests, whereas categorical variables were compared using the χ2 test or Fisher's exact test. The normality of continuous variables was tested using the Kolmogorov–Smirnov test. Variables not normally distributed were log-transformed. Variables in subjects in the development cohort with and without NAFLD were compared using univariable logistic regression analysis. Candidate predictors were selected among all variables using multivariable logistic regression analysis with 1000-fold bootstrap resampling; variables selected in more than 50% of bootstrap logistic models were chosen as candidate predictors. Variables independently associated with NAFLD were identified using multivariable logistic regression analysis with backward elimination. To construct a simplified predictive model, logistic models that included an increasing number of variables were sequentially developed by one-by-one addition of independent variables to CTL-S. The diagnostic performance of each logistic model was assessed by calculating the area under the receiver operating characteristic curve (AUC). This procedure was continued until model performance did not improve the AUC by at least 0.005 upon the inclusion of an additional variable. A formula for the clinical-CT index was derived using the variables in the final logistic model and the proportions of the corresponding regression coefficients. The diagnostic performances of the clinical-CT index and CTL-S were evaluated by comparing the AUCs using Delong's method (21). The incremental difference between the clinical-CT index and CTL-S alone was evaluated by calculating the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (22,23). The NRI is used to evaluate the net proportion of subjects reclassified correctly using the new model (i.e., the clinical-CT index) relative to the baseline model (i.e., CTL-S), whereas the IDI measures the improvement in sensitivity of the new model relative to the baseline model without a loss in specificity (22,23). Positive NRI and IDI values indicate the superiority of the new model relative to the baseline model for correct classification. Cutoffs were selected for the clinical-CT index and CTL-S for 90% sensitivity and 90% specificity in diagnosing NAFLD, thus reliably detecting and ruling out NAFLD, and the corresponding sensitivities, specificities, and accuracies were calculated. The diagnostic performance of the clinical-CT index and CTL-S in the test cohort were compared using the AUCs, NRI, and IDI, whereas the diagnostic performances of the clinical-CT index and CTL-S were compared with those of the HS-I and FLD-I using the AUC. The sensitivities, specificities, and accuracies in diagnosing NAFLD were evaluated in the test cohort using the cutoff values for the clinical-CT index and CTL-S determined in the development cohort. A p value less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Characteristics
Table 1 summarizes the characteristics of the study population. NAFLD was present in 620 (41.9%) of the 1480 subjects in the development cohort and in 310 (42.0%) of the 738 in the test cohort. None of the clinical and laboratory characteristics assessed differed significantly between the development and test cohorts.
Table 1. Characteristics of Study Population.
| Characteristics | Developmental Cohort (n = 1480) | Test Cohort (n = 738) | P |
|---|---|---|---|
| Age (years) | 30.9 ± 9.1 (18–62) | 31.3 ± 8.9 (18–58) | 0.40 |
| Sex (female)* | 534 (36.1) | 245 (33.2) | 0.18 |
| Pathologic steatosis grade* | 0.95 | ||
| None (≤ 5%) | 860 (58.1) | 428 (58.0) | |
| Mild (> 5%) | 517 (34.9) | 256 (34.7) | |
| Moderate/severe (≥ 33%) | 103 (7.0) | 54 (7.3) | |
| BMI (kg/m2) | 23.3 ± 3.2 (15.4–41.3) | 23.4 ± 3.0 (15.4–34.9) | 0.69 |
| Laboratory findings | |||
| AST (IU/mL) | 21.3 ± 11.7 (10–365) | 21.4 ± 8.2 (10–129) | 0.77 |
| ALT (IU/mL) | 20.3 ± 13.6 (1–181) | 20.9 ± 13.1 (6–121) | 0.29 |
| Bilirubin (ng/mL) | 0.98 ± 0.37 (0.2–3.5) | 1.00 ± 0.39 (0.3–4.1) | 0.23 |
| ALP (IU/mL) | 62.87 ± 18.22 (20–186) | 62.70 ± 18.30 (10–182) | 0.84 |
| Triglyceride (mg/dL) | 103.81 ± 77.22 (17–935) | 108.02 ± 88.85 (21–1304) | 0.25 |
| Cholesterol (mg/dL) | 173.74 ± 32.83 (68–320) | 174.77 ± 32.51 (98–302) | 0.48 |
| HDL-cholesterol (mg/dL) | 50.70 ± 13.27 (20–106) | 50.40 ± 12.95 (19–94) | 0.61 |
| Glucose (mg/dL) | 94.54 ± 15.02 (58–370) | 95.19 ± 17.05 (63–374) | 0.36 |
| Albumin (g/dL) | 4.37 ± 0.29 (3.2–5.2) | 4.37 ± 0.27 (3.5–5.2) | 0.97 |
| Hepatic steatosis index | 31.45 ± 4.91 (20–54) | 31.62 ± 4.50 (21–47) | 0.43 |
| Fatty liver disease index | 27.28 ± 4.19 (24–30) | 27.45 ± 4.03 (25–30) | 0.25 |
Unless otherwise indicated, data are mean ± standard deviation; data in parentheses are range. *Number (percentages) of patients.
ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate transaminase, BMI = body mass index, HDL = high-density lipoprotein
Development of a Simple Clinical-CT Index in the Development Cohort
Univariable logistic regression analysis showed that all variables analyzed, except for bilirubin, were significantly associated with NAFLD (Table 2). Multivariable logistic regression analysis followed by candidate predictor selection showed that BMI, ALT, TG, cholesterol, HDL-cholesterol, and CTL-S were independently associated with NAFLD (Supplementary Tables 1, 2). These independent variables were utilized to construct logistic models containing different numbers of variables predictive of NAFLD (Supplementary Table 2). The final logistic models included CTL-S, BMI, TG, and ALT (Table 3). The relationship of the regression coefficients of the final logistic models resulted in a clinical-CT index predictive of NAFLD.
Table 2. Univariable and Multivariable Logistic Regression Analysis of Factors Associated with Diagnosis of NAFLD in Development Cohort.
| Variables | Univariable Analysis | Multivariable Analysis† | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Adjusted Odds Ratio (95% CI) | P | |
| Age | 1.03 (1.02–1.04) | < 0.001 | ||
| Sex (female) | 0.44 (0.35–0.55) | < 0.001 | ||
| BMI | 1.34 (1.28–1.40) | < 0.001 | 1.17 (1.12–1.23) | < 0.001 |
| AST* | 5.40 (3.57–8.16) | < 0.001 | ||
| ALT* | 5.57 (4.32–7.17) | < 0.001 | 1.75 (1.31–2.36) | < 0.001 |
| Bilirubin | 1.18 (0.89–1.57) | 0.25 | ||
| ALP | 1.02 (1.01–1.02) | < 0.001 | ||
| Triglyceride* | 3.5 (2.85–4.30) | < 0.001 | 1.58 (1.31–2.08) | < 0.001 |
| Cholesterol | 1.02 (1.01–1.02) | < 0.001 | 1.01 (1.00–1.02) | < 0.001 |
| Glucose* | 8.40 (3.46–20.37) | < 0.001 | ||
| Albumin | 1.68 (1.17–2.40) | 0.005 | ||
| HDL-cholesterol | 0.96 (0.95–0.97) | < 0.001 | 0.98 (0.97–0.99) | < 0.001 |
| CTL-S | 0.84 (0.83–0.86) | < 0.001 | 0.87 (0.85–0.89) | < 0.001 |
Data in parentheses are range. *Analyzed after log transformation, †Multivariable analysis included candidate variables selected in more than 50% of logistic models from 1000-fold bootstrap resampling. CI = confidence interval, CTL-S = mean liver attenuation - mean spleen attenuation on non-enhanced CT, NAFLD = non-alcoholic fatty liver disease
Table 3. Final Logistic Model for Diagnosis of NAFLD.
| Parameters | Coefficient (95% CI) | P |
|---|---|---|
| CTL-S | -0.14 (-0.16 to -0.11) | < 0.001 |
| BMI | 0.18 (0.13–0.23) | < 0.001 |
| Log (triglyceride) | 0.79 (0.56–1.02) | < 0.001 |
| Log (ALT) | 0.64 (0.35–0.93) | < 0.001 |
Clinical-CT index for the prediction of NAFLD = 5 × Loge (ALT × TG) + BMI - CTL-S.
Diagnostic Performance of the Clinical-CT Index and CTL-S in the Development Cohort
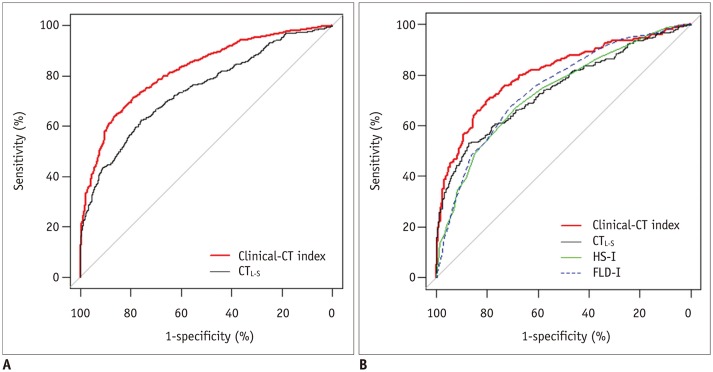
In the development cohort, the AUC for the clinical-CT index was 0.82 (95% confidence interval [CI], 0.80–0.84), which was significantly higher than that of CTL-S (0.74; 95% CI, 0.72–0.76; p < 0.001) (Fig. 3A). The clinical-CT index also showed significant improvement in reclassification (NRI, 0.75; p < 0.001) and discrimination (IDI, 0.12; p < 0.001) over CTL-S. Table 4 summarizes the dual cutoff values for the clinical-CT index and CTL-S for diagnosing NAFLD. A clinical-CT index cutoff of ≥ 46 diagnosed NAFLD with a sensitivity of 90% and specificity of 44%, whereas a CTL-S cutoff of ≤ 12.5 showed a sensitivity of 90% and specificity of 28%. Alternatively, a clinical-CT index cutoff of ≥ 56.5 diagnosed NAFLD with a sensitivity of 57% and specificity of 90%, whereas a CTL-S cutoff of ≤ 3.9 had a sensitivity of 44% and specificity of 90%.
Fig. 3. Receiver operating characteristic curves of performance of clinical-CT index in diagnosing non-alcoholic fatty liver disease, compared with CTL-S in development cohort (A) and compared with CTL-S, HS-I, and FLD-I in test cohort (B).
CTL-S = mean liver attenuation - mean spleen attenuation on non-enhanced CT, FLD-I = fatty liver disease index, HS-I = hepatic steatosis index
Table 4. Cutoff Values for Clinical-CT Index and CTL-S and Their Corresponding Diagnostic Performances in Development and Test Cohorts.
| Indices | Cutoffs | Development Cohort | Test Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | ||
| Clinical-CT index | ≥ 46 | 90.3 (560/620) | 44.2 (380/860) | 53.8 (560/1040) | 86.4 (380/440) | 89.4 (277/310) | 40.7 (174/428) | 52.2 (277/531) | 84.1 (174/207) |
| ≥ 56.5 | 57.4 (356/620) | 90.2 (776/860) | 80.9 (356/440) | 74.6 (776/1040) | 57.1 (177/310) | 88.6 (379/428) | 78.3 (177/226) | 74.0 (379/512) | |
| CTL-S | ≤ 12.5 | 90.2 (559/620) | 27.6 (237/860) | 47.3 (559/1182) | 79.5 (237/298) | 89.0 (276/310) | 26.9 (115/478) | 46.9 (276/589) | 77.2 (115/149) |
| ≤ 3.9 | 44.0 (273/620) | 90.0 (774/860) | 76.0 (273/359) | 69.0 (774/1121) | 46.1 (143/310) | 90.2 (386/478) | 77.3 (143/185) | 69.8 (386/553) | |
Upper cutoff values are those for 90% sensitivity, and lower cutoff values are those for 90% specificity. Results are presented as percentages (number of patients/total number of patients assessed). NPV = negative predictive value, PPV = positive predictive value
Diagnostic Performances of the Clinical-CT Index and CTL-S in the Test Cohort
The AUC for diagnosing NAFLD in the test cohort was significantly higher for the clinical-CT index (0.81; 95% CI, 0.78–0.84) than for CTL-S (0.74; 95% CI, 0.71–0.78; p < 0.001) (Fig. 3B). In addition, the clinical-CT index had significant incremental value in reclassification (NRI, 0.61; p < 0.001) and discrimination (IDI, 0.09; p < 0.001) over CTL-S. Compared with clinical indices, the clinical-CT index significantly outperformed HS-I (AUC, 0.73; 95% CI, 0.70–0.76; p < 0.001) and FLD-I (AUC, 0.75; 95% CI, 0.72–0.78; p < 0.001) for diagnosing NAFLD, whereas the AUC for CTL-S did not differ significantly from the AUCs for HS-I (p = 0.64) and FLD-I (p = 0.19). The cutoff values for the clinical-CT index and CTL-S from the development cohort performed similarly in the test cohort. A clinical-CT index cutoff of ≥ 46 diagnosed NAFLD with a sensitivity of 89.4% and specificity of 40.7%, whereas a cutoff of ≥ 56.5 had a sensitivity of 57.1% and a specificity of 88.6%. By comparison, a CTL-S cutoff of ≤ 12.5 had a sensitivity of 89% and specificity of 26.9%, whereas a cutoff of ≤ 3.9 had a sensitivity of 46.1% and specificity of 90.2%.
DISCUSSION
The present study describes the development of a simple index, combining the CT index and routinely tested blood and anthropometric parameters, to predict NAFLD. The clinical-CT index outperformed CTL-S for diagnosing NAFLD with significantly higher AUCs and significant improvements in reclassification and discrimination in both the development (AUCs, 0.82 vs. 0.74, p < 0.001; NRI, 0.747, p < 0.001; IDI, 0.121, p < 0.001) and test (AUCs, 0.81 vs. 0.74, p < 0.001; NRI, 0.609, p < 0.001; IDI, 0.089, p < 0.001) cohorts. These findings indicate that adding clinical parameters to the CTL-S improved the ability to diagnose NAFLD. The clinical-CT index also performed better than did the clinical indices for diagnosing NAFLD in the test cohort, whereas the AUC for CTL-S did not differ significantly from the AUCs for the clinical indices. These results suggest that using CT alone for the diagnosis or exclusion of NAFLD is not a reasonable approach, as the performance of clinical indices—which can be determined more easily and at lower cost compared with CTL-S—was similar to that of CTL-S. However, the clinical-CT index, incorporating both CT and clinical parameters, may have clinical utility, allowing for better detection of NAFLD compared with the CT index and individual clinical indices.
Dual cutoff values of the clinical-CT index for diagnosing NAFLD were selected. One of these cutoffs was based on 90% sensitivity, which could be used to define a normal control group by excluding most subjects with NAFLD. In the test cohort, cutoffs of ≥ 46 for the clinical-CT index and ≤ 12.5 for CTL-S resulted in the diagnosis of NAFLD with a sensitivity of approximately 90% and specificities of 40.7% and 26.9%, respectively. The other cutoff, based on 90% specificity, could be used to identify a cohort of subjects with NAFLD for clinical research. In the test cohort, cutoffs of ≥ 56.5 for the clinical-CT index and ≤ 3.9 for CTL-S resulted in the diagnosis of NAFLD with a specificity of approximately 90% and sensitivities of 57.1% and 46.1%, respectively.
Several predictive models based on clinical and laboratory parameters have been developed to distinguish subjects with and without NAFLD. Some of these models, however, included parameters not always routinely measured in clinical practice. For example, models have included serum concentrations of insulin (24), uric acid (25), hemoglobin A1C (19), and haptoglobin (26), as well as waist circumference (15,27), which are parameters that may not be easily retrieved from patient databases. This may limit the use of these models in retrospective analyses. Furthermore, many predictive models have been based on the diagnosis of NAFLD using grayscale US (14,15,16,25,27), despite US diagnosis of NAFLD being operator-dependent and having limited accuracy (7). By contrast, the clinical-CT index for diagnosing NAFLD in our study was based on pathologic proof of NAFLD in a large population.
Given the potential radiation hazard of CT and the availability of other imaging methods for the diagnosis of NAFLD, such as MR imaging, MR spectroscopy, quantitative US methods, and the controlled attenuation parameter of transient elastography (8,28,29), our clinical-CT index may not be an optimal method for identifying patients with NAFLD in clinical practice and in prospective research. However, because of the widespread use of CT, the clinical-CT index described in our study, incorporating routinely measured laboratory and anthropometric parameters, may be useful for constructing large cohorts of subjects with NAFLD and normal controls using pre-existing retrospective CT and clinical data; this may further be useful for conducting large-scale retrospective cohort studies to investigate the natural history and outcome of NAFLD.
This study had several limitations. First, the study population was derived from liver donor candidates, most of whom were young and healthy, and therefore may not fully represent the general population. Second, split-sample validation of the diagnostic accuracy of the clinical-CT indices was performed. External validation in a different test population may have been more conclusive. Third, we excluded subjects with excessive alcohol intake in our study to avoid the confounding effects of alcohol on clinical, CT, and pathologic findings. However, in clinical practice and in retrospective research, information regarding alcohol consumption may not be always available, which may potentially affect the performance of the clinical-CT index.
Finally, although percutaneous needle biopsy is a well-accepted reference method for the diagnosis of NAFLD, it may be subject to some degree of sampling error and inter-observer variability.
In conclusion, a clinical-CT index combining clinical parameters and CTL-S was more accurate in the diagnosis of NAFLD compared with CTL-S or clinical indices alone. This clinical-CT index may have utility in screening for NAFLD in clinical practice and research.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, Information and Communications Technology (ICT) and Future Planning (NRF-2017R1A2B4003114), and the Bio and Medical Technology Development Program of the NRF funded by the Ministry of Science and ICT (NRF-2016M3A9A7918706).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2019.0703.
Results of Bootstrap Multivariable Logistic Regression Analysis for Selecting Candidate Predictors
Diagnostic Performance of Logistic Models with Clinical Variables Sequentially Added to CTL-S for Predicting Non-Alcoholic Fatty Liver Disease
References
- 1.Wong VW, Wong GL, Yeung DK, Lau TK, Chan CK, Chim AM, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62:182–189. doi: 10.1016/j.jhep.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 5.Gastaldelli A, Kozakova M, Højlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–1544. doi: 10.1002/hep.22845. [DOI] [PubMed] [Google Scholar]
- 6.Takyar V, Nath A, Beri A, Gharib AM, Rotman Y. How healthy are the “Healthy volunteers”? Penetrance of NAFLD in the biomedical research volunteer pool. Hepatology. 2017;66:825–833. doi: 10.1002/hep.29247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392–7402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogier J, Roullet S, Cornélis F, Biais M, Quinart A, Revel P, et al. Noninvasive assessment of macrovesicular liver steatosis in cadaveric donors based on computed tomography liver-to-spleen attenuation ratio. Liver Transpl. 2015;21:690–695. doi: 10.1002/lt.24105. [DOI] [PubMed] [Google Scholar]
- 10.Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, et al. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol. 2015;63:470–476. doi: 10.1016/j.jhep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickhardt PJ, Hahn L, Muñoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202:752–758. doi: 10.2214/AJR.13.11367. [DOI] [PubMed] [Google Scholar]
- 12.Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, et al. Improvement of nonalcoholic fatty liver disease with carnitine-orotate complex in type 2 diabetes (CORONA): a randomized controlled trial. Diabetes Care. 2015;38:1245–1252. doi: 10.2337/dc14-2852. [DOI] [PubMed] [Google Scholar]
- 13.Byun J, Lee SS, Sung YS, Shin Y, Yun J, Kim HS, et al. CT indices for the diagnosis of hepatic steatosis using non-enhanced CT images: development and validation of diagnostic cut-off values in a large cohort with pathological reference standard. Eur Radiol. 2019;29:4427–4435. doi: 10.1007/s00330-018-5905-1. [DOI] [PubMed] [Google Scholar]
- 14.Fuyan S, Jing L, Wenjun C, Zhijun T, Weijing M, Suzhen W, et al. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig Dis Sci. 2013;58:3326–3334. doi: 10.1007/s10620-013-2774-y. [DOI] [PubMed] [Google Scholar]
- 15.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Choi YJ, Lee DH, Han KD, Yoon H, Shin CM, Park YS, et al. Is nonalcoholic fatty liver disease associated with the development of prostate cancer? A nationwide study with 10,516,985 Korean men. PLoS One. 2018;13:e0201308. doi: 10.1371/journal.pone.0201308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 19.Yip TC, Ma AJ, Wong VW, Tse YK, Chan HL, Yuen PC, et al. Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population. Aliment Pharmacol Ther. 2017;46:447–456. doi: 10.1111/apt.14172. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 22.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 23.Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98:683–690. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 24.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Wang G, Kang K, Wu G, Wang P. Diagnostic accuracy and clinical utility of a new noninvasive index for hepatic steatosis in patients with hepatitis B virus infection. Sci Rep. 2016;6:32875. doi: 10.1038/srep32875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. doi: 10.1186/1476-5926-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;10:98. doi: 10.1186/1471-230X-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol. 2015;13:1337–1345.e6. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol. 2017;23:290–301. doi: 10.3350/cmh.2017.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of Bootstrap Multivariable Logistic Regression Analysis for Selecting Candidate Predictors
Diagnostic Performance of Logistic Models with Clinical Variables Sequentially Added to CTL-S for Predicting Non-Alcoholic Fatty Liver Disease