ABSTRACT
Antimicrobial resistance (AMR) has emerged as one of the most pressing threats to public health. AMR evolution occurs in the clinic but also in the environment, where antibiotics and heavy metals can select and co-select for AMR. While the selective potential of both antibiotics and metals is increasingly well-characterized, experimental studies exploring their combined effects on AMR evolution are rare. It has previously been demonstrated that fluoroquinolone antibiotics such as ciprofloxacin can chelate metal ions. To investigate how ciprofloxacin resistance is affected by the presence of metals, we quantified selection dynamics between a ciprofloxacin-susceptible and a ciprofloxacin-resistant Escherichia coli strain across a gradient of ciprofloxacin concentrations in presence and absence of zinc. The presence of zinc reduced growth of both strains, while ciprofloxacin inhibited exclusively the susceptible one. When present in combination zinc retained its inhibitory effect, while ciprofloxacin inhibition of the susceptible strain was reduced. Consequently, the minimal selective concentration for ciprofloxacin resistance increased up to five-fold in the presence of zinc. Environmental pollution usually comprises complex mixtures of antimicrobial agents. In addition to the usual focus on additive or synergistic interactions in complex selective mixtures, our findings highlight the importance of antagonistic selective interactions when considering resistance evolution.
Keywords: Antimicrobial resistance, Selection dynamics, Heavy metals, Chelation, Fluroquinolone, Antibiotic resistance
Selection for ciprofloxacin resistance in a bacterial strain is reduced when zinc cations are simultaneously present during exposure to the antibiotic ciprofloxacin.
INTRODUCTION
The emergence and spread of antimicrobial resistance (AMR) genes in bacterial pathogens constitutes a major threat to human health (WHO 2014). Although AMR genes are ancient and have evolved as a result of microbial interactions, as for example evidenced by their presence in permafrost samples minimally impacted by anthropogenic activity (D'Costa et al. 2011; Perron et al. 2015), the use and misuse of antibiotics in healthcare and agriculture has caused a rapid and worrying increase in the prevalence of AMR in the human as well as environmental microbiomes (Knapp et al. 2010). The environmental dimensions of AMR evolution are increasingly appreciated (Wellington et al. 2013; Larsson et al. 2018; Smalla et al. 2018), with two major research strands emerging relating to selection for resistance. First, recent studies utilising both single species (Gullberg et al. 2011, 2014; Liu et al. 2011; Klümper et al. 2019b) and complex microbial communities (Lundström et al. 2016; Kraupner et al. 2018; Murray et al. 2018) have demonstrated that selection for AMR can occur at antibiotic concentrations much lower than those preventing the growth of susceptible bacteria. These studies highlight the importance of considering the minimal selective concentration (MSC) in addition to the minimal inhibitory concentration (MIC) for assessing risks associated with antibiotic concentrations in the environment. Second, environmental pollution with non-antibiotic compounds such as biocides and/or metals can contribute to the spread and selection of AMR through processes such as cross-resistance, co-selection and co-regulation (Baker-Austin et al. 2006; Pal et al. 2017; Dickinson et al. 2019) or by altering transfer dynamics of AMR plasmids (Klümper et al. 2017, 2019a). Pollution with metals is especially problematic as metals are highly persistent and toxic even at low concentrations (Gadd and Griffiths 1977). In certain environmental settings, heavy metals such as copper (Cu) and zinc (Zn) may even constitute stronger selective agents for antibiotic resistance than antibiotics (Song et al. 2017).
While the selective potential of antibiotics and heavy metals for AMR has been well-characterized, experimental studies exploring their combined effect on resistance evolution are relatively rare. The presence of a second antibiotic could, for example, either potentiate or decrease antibiotic efficacy (Cao et al. 2012; Churski et al. 2012) and hence cause a decrease or increase in MSC. Metals and antibiotics can similarly have synergistic or antagonistic effects. For example, metal complexation can decrease the hydrolysis potential of β-lactam antibiotics by β-lactamases and hence increase antibiotic potency (Anacona 2001). Additionally, the selective potential of mechanisms conferring co-resistance to metals and antibiotics, such as efflux pumps, can be increased in the presence of metals (Mata, Baquero and Pérez-Díaz 2000; Aendekerk et al. 2002). Lastly, it is also possible that metals could diminish the activity of antibiotics by binding and inactivation (Li, Nix and Schentag 1994). As metal-antibiotic interactions are varied and highly relevant in environmental pollution scenarios, it is important to address how antibiotics and metals jointly affect bacterial resistance spread.
Fluoroquinolones are recognized as critically important antibiotics for human health by the WHO (WHO 2016) and are characterized by a high degree of persistence in the environment (Kümmerer, Al-Ahmad and Mersch-Sundermann 2000). Concentrations in environmental settings range from low ng/L to μg/L, while exceptionally high levels of ciprofloxacin in the mg/L range have been found in effluents from drug manufacturers and in nearby industrially polluted environments (Larsson, de Pedro and Paxeus 2007; Fick et al. 2009; Gothwal and Shashidhar 2015). Interactions between fluoroquinolones and metals have received previous attention but with mixed results. In metal(II)-ciprofloxacin complexes, the drug ligand is coordinated through two carbonyl oxygen atoms (Chohan, Supuran and Scozzafava 2005). Among these, Zn(II)-ciprofloxacin complexes were shown to have better solubility and greater activity against Gram-positive and Gram-negative pathogens compared to uncomplexed ciprofloxacin (Chohan, Supuran and Scozzafava 2005; Imran et al. 2007; Patel, Chhasatia and Parmar 2010). However, a number of studies have also demonstrated that metal-chelated ciprofloxacin, even whilst more readily transported across the bacterial cell membrane, has reduced antimicrobial efficacy (Li, Nix and Schentag 1994; Ma, Chiu and Li 1997; Seedher and Agarwal 2010).
To test whether metal chelation could impact selection for antibiotic resistance, we here quantified growth rates of a ciprofloxacin-susceptible and a ciprofloxacin-resistant Escherichia coli MG1655 strain across a gradient of ciprofloxacin concentrations in the presence and absence of Zn. We also performed a competition experiment between both strains in mine-waste contaminated stream water to test whether complex environmental mixtures of metals could affect selection for ciprofloxacin resistance. Our results shed light on a hitherto unappreciated dimension of environmental AMR selection, namely, that there is a selective window where metal pollution may reduce the selective effects of fluoroquinolone antibiotics.
MATERIAL AND METHODS
Strains
An Escherichia coli MG1655 strain was chromosomally tagged with a Tn7 gene cassette encoding constitutive red fluorescence, expressed by the mCherry gene (Klümper, Dechesne and Smets 2014; Klümper et al. 2015). A strain resistant to ciprofloxacin and a strain resistant to gentamicin were derived from this red fluorescent strain. The ciprofloxacin resistant strain was created through spontaneous chromosomal mutation by evolving the susceptible ancestor by serial inoculation of overnight culture in LB medium (10 g/L Tryptone, 10 g/L NaCl and 5 g/L Yeast Extract) supplemented with incremental concentrations of ciprofloxacin (0.0625, 0.125, 0.25, 0.5 and 1 µg/mL), until a resistant phenotype evolved able to grow at the highest concentration. A gentamicin resistant strain was constructed through electroporation of the susceptible ancestor with the pBAM delivery plasmid containing the mini-Tn5 delivery system (Martínez-García et al. 2011) for gentamicin resistance gene aacC1 encoding a gentamicin 3′-N-acetyltransferase (Kovach et al. 1995). The ciprofloxacin- and gentamicin-resistant strains as well as the ancestral susceptible strain were grown overnight in sterile LB broth (supplemented with 0.5 μg/mL ciprofloxacin, 10 μg/mL gentamicin or no antibiotics, respectively), harvested by centrifugation (3500 × g, 20 min, 4°C), three times washed with sterile 0.9% NaCl solution to remove residual antibiotics with the finally density adjusted to OD600 0.1 (∼107 bacteria/mL) in sterile 0.9% NaCl solution for use in experiments. All chemicals used in this study were supplied by Sigma-Aldrich (St. Louis, MO, USA).
Water sampling
A water sample was collected in May 2019 from the Carnon River near Bissoe, Cornwall, UK (50°13’54.6’ N, 5°07’48.7’ W). The surrounding area has a long history of mining-related heavy metal contamination (Pirrie et al. 2003; Environment Agency UK 2019), resulting in contamination of soils, sediments and water with a complex mixture of metals, including zinc. A sterile 1 L Duran bottle was filled and immediately transported back to the laboratory to use for the growth rate and competition assays on the same day of sampling. The water was supplemented with sterile LB broth powder (25 g/L) and sterilized through 0.2 μm2 pore filters (Whatman). Sterility was confirmed by incubating 10 mL of the liquid medium at 37°C overnight. An aliquot of the water sample was frozen at -20åC and analysed in triplicate for metal content using ICP-MS on an Agilent 7700x (Agilent Technologies, Santa Clara, Ca, USA) at the Camborne School of Mines laboratory at the University of Exeter (Table S1, Supporting Information).
Growth rate assays
Individual strains, originating from a single overnight culture, were inoculated in 96 well plates at ∼105 cells/mL in technical triplicates into 300 μL LB broth supplemented with ZnSO4 (0.0, 0.5 and 1 mM) and either ciprofloxacin (11 two-fold decreasing concentrations starting at 0.4 μg/mL) or gentamicin (11 two-fold decreasing concentrations starting at 10 μg/mL). To account for different batches of LB broth as a complex growth medium potentially containing minimal background levels of Zn as a micronutrient, all experiments were carried out using LB from the same 1 kg batch. After 24 h growth under shaking conditions (120 rpm) OD600 was measured to determine bacterial growth using a Synergy 2 spectrophotometer (Biotek Instruments Inc., VT, USA). Individual growth rates of resistant strains were calculated relative to growth of the susceptible strain in a control treatment unamended with zinc or antibiotics.
Relative fitness of the susceptible strain (ρs) was calculated using the specific growth rate (γ) of the susceptible (s) and the resistant (r) strain at each individual concentration, with bacterial numbers (n) estimated using OD600 measurements at time 0 (T0h) and after 24 h (T24h).
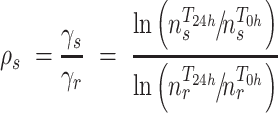 |
The experimental data linking relative fitness to antibiotic exposure at the different metal doses were fitted with a four parameter log-logistic dose-response curve using the ‘drc’ (analysis of dose-response curves) package in R (Knezevic, Streibig and Ritz 2007) with separate curves fitted for each level of zinc, using ‘curveid’ with Zn as factor. To integrate the no antibiotic control (c = 0 μg/mL) in the log-logistic model its concentration was set to a minimum threshold as per the developers’ instructions (i.e. by dividing lowest non-zero value by 10). Using these modelled curves the MSC at which ρs = ρr = 1 was calculated for each scenario, as well as their standard deviation and 95% confidence intervals. To test if Zn alters the dose response relationship, the fitted model was compared to a reduced model where fitness data were pooled across ciprofloxacin concentrations.
Competition assay
The gentamicin-resistant, ciprofloxacin-susceptible strain was competed with the gentamicin-susceptible, ciprofloxacin-resistant strain. This allowed for simple identification of the ciprofloxacin-susceptible and resistant strain on selective plates. The pair was inoculated at a 1:1 ratio and initial density of ∼105 cells/mL into 10 mL of LB broth supplemented with ZnSO4 (0.0 and 0.5 mM) and ciprofloxacin (0.001, 0.01, 0.025 and 0.1 μg/mL) in triplicate 30 mL glass vials. Glass vials were incubated shaken (120 rpm) at 37°C for 24 h, after which 100 μL (1%) was transferred daily to fresh LB broth for a total of seven days. To obtain initial (T0d) and final (T7d) cell densities for each vial, dilution series were prepared in sterile 0.9% NaCl solution and plated on LB agar containing either 10 μg/mL gentamicin (selective for ciprofloxacin-susceptible strain) or 0.5 μg/mL ciprofloxacin (selective for ciprofloxacin-resistant strain). Plating of the respective strains on the counter selecting plates did not lead to any growth of spontaneous mutants. The relative fitness of the susceptible strain was calculated as before using growth rate ratios.
Statistics
For each experimental condition (combination of Zn and ciprofloxacin concentration), we used a two-tailed t-test to test whether the relative fitness of the susceptible strain differed from a scenario with no selection (ρ = 1). We used a similar approach to test whether under a given experimental condition relative growth of either strain significantly differed from that of the susceptible one in the absence of antibiotics and zinc. To compare the relative fitness or growth rates between different experimental conditions ANOVA tests were performed.
RESULTS
Effect of Zinc on selection for ciprofloxacin resistance
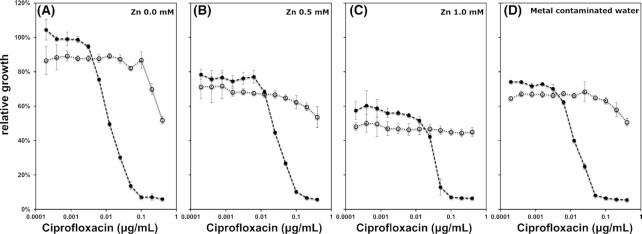
To determine the effect of zinc on selection for ciprofloxacin resistance, ciprofloxacin-resistant and ciprofloxacin-susceptible E. coli strains were individually grown across a gradient of ciprofloxacin and Zn concentrations for 24 h. The growth rate of each strain was plotted relative to that of the ciprofloxacin-susceptible strain grown in control LB (without ciprofloxacin and Zn) (Fig. 1A-C; Table S2, Supporting Information). Growth of both strains significantly decreased with increasing Zn concentrations in the absence of ciprofloxacin to 78.7 ± 4.1% (0.5 mM, P < 0.0001, two-tailed t-test against 1) and 55.4 ± 2.4% (1 mM, P < 0.0001, two-tailed t-test against 1). Ciprofloxacin resistance caused a small but significant reduction in relative growth rate in the absence of antibiotics and metals (82.8 ± 3.4% relative growth, mean ± SD) (P = 0.012, two-tailed t-test against 1) (Fig. 1A). This cost of resistance was also apparent across both Zn concentrations tested (0.5 mM: 90.7 ± 5.3%, P = 0.093; 1 mM: 83.8 ± 3.7%, P = 0.0161, two-tailed t-test against 1) (Fig. 1B and C).
Figure 1.
Growth rate of a ciprofloxacin-susceptible strain (filled circles) and a ciprofloxacin-resistant strain (open circles) in LB medium amended with ciprofloxacin and zinc as well as in metal contaminated water supplemented with LB, relative to the growth rate of the ciprofloxacin-susceptible strain in control LB. Growth was quantified based on OD600 after 24 h of growth at 37åC (n = 3, average ± SD).
A significant growth rate advantage for the ciprofloxacin-resistant strain was detected at 0.00625 μg/mL ciprofloxacin (ρs,0 mM = 0.949 ± 0.001, P = 0.0002, two-tailed t-test against 1) in the absence of Zn (Fig. 2). However, at the same antibiotic concentration in the presence of both Zn concentrations, this advantage disappeared (0.5 mM: ρs = 1.050 ± 0.018, P = 0.0412; 1.0 mM: ρs,1.0 mM = 1.073 ± 0.010, P = 0.0289; two-tailed t-test against 1). In the presence of 0.5 mM Zn, the minimal concentration with a significant selective advantage for the resistant strain increased from 0.00625 μg/mL ciprofloxacin to 0.025 μg/mL (ρs = 0.848 ± 0.008; P = 0.0008, two-tailed t-test against 1) and for 1 mM Zn to 0.05 μg/mL (ρs = 0.422 ± 0.150; P = 0.0218, two-tailed t-test against 1) (Fig. 2).
Figure 2.
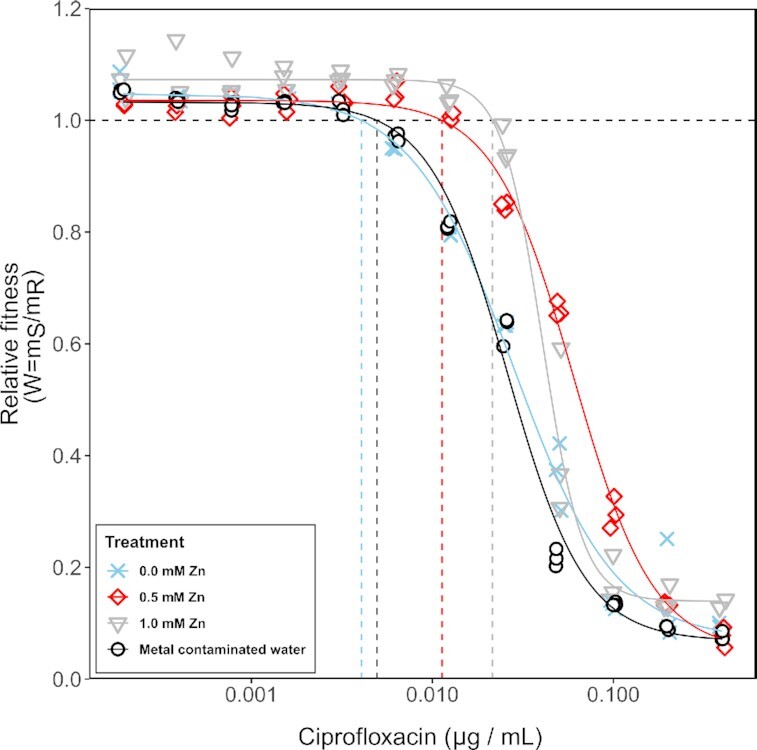
Fitness of a ciprofloxacin susceptible E. coli strain relative to fitness of an isogenic ciprofloxacin resistant strain across a gradient of ciprofloxacin for three LB broth treatments amended with Zn and one metal contaminated water source supplemented with LB. Horizontal dashed line indicates different strains perform equally well (ρs = ρr = 1), and the vertical lines represent the corresponding minimal selective concentration for the different Zn treatments.
We fitted a log-logistic dose response model to the relative fitness values across the antibiotic gradient for different Zn concentrations (Fig. 2). This significantly improved the fit compared to a reduced model (F12,140 = 51.113, P < 0.001, ANOVA), suggesting that increasing Zn concentrations alter the dose response relationship. Based on the full model, MSCs were estimated as the intercept with ρs = 1 (no selection) and significantly increased from 0.0041 ± 0.0005 μg/mL in the absence of Zn to 0.0113 ± 0.0013 μg/mL (0.5 mM Zn) and further to 0.0215 ± 0.0014 μg/mL (1.0 mM Zn) (Table 1).
Table 1.
Minimal selective concentrations (MSC) of ciprofloxacin modelled for the different Zn2+ concentrations and metal contaminated water.
| Medium | MSC | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| 0.0 mM Zn2+ | 0.0041 ± 0.0005 μg/mL | 0.0030 μg/mL | 0.0051 μg/mL |
| 0.5 mM Zn2+ | 0.0113 ± 0.0013 μg/mL | 0.0087 μg/mL | 0.0139 μg/mL |
| 1.0 mM Zn2+ | 0.0215 ± 0.0014 μg/mL | 0.0188 μg/mL | 0.0242 μg/mL |
| Metal contaminated water | 0.0049 ± 0.0006 μg/mL | 0.0037 μg/mL | 0.0062 μg/mL |
Zn also affected the relative growth rate of the ciprofloxacin-resistant strain: growth significantly decreased at 0.2 and 0.4 μg/mL ciprofloxacin (P = 0.002–0.0498, df = 5, ANOVA) for no Zn and 0.5 mM of Zn (Fig. 1A and B). No such decrease in growth was detected when exposed to 1 mM of Zn (P = 0.114–0.194, df = 5, ANOVA) (Fig. 1C). Selection dynamics for gentamicin, a non-fluoroquinolone antibiotic that does not chelate metals, were not similarly affected by Zn (Figs S1 and S2, Supporting Information).
Effect of environmental metal contamination on selection for ciprofloxacin resistance
To explore the relevance of the results under more natural conditions, we performed a competition experiment for ciprofloxacin using sterilized water from the metal-contaminated Carnon River (Cornwall, United Kingdom; Table S1, Supporting Information) amended with LB. The contaminated water significantly decreased relative growth of both the ciprofloxacin-resistant (77.9 ± 1.0%, P = 0.0007, two-tailed t-test against 1) and ciprofloxacin-susceptible strain (74.2 ± 0.6% P = 0.0002, two-tailed t-test against 1) relative to control medium made up with deionized water. In the absence of ciprofloxacin the susceptible strain had greater fitness relative to the resistant one (ρs = 1.052 ± 0.003; P = 0.0012, two-tailed t-test against 1). Growth inhibition (22.1 ± 1.0%) was quantitatively and qualitatively similar to that observed for 0.5 mM Zn (21.3 ± 4.1% P = 0.426, df = 10, ANOVA) (Fig. 1D). However, the MSC estimated from the dose response curve model was at 0.0049 ± 0.0006 μg/mL ciprofloxacin, similar to that observed for non Zn-amended broth (Table 1). Again a significant decrease in growth rate of the resistant strain at high concentrations of 0.4 μg/mL ciprofloxacin was observed in naturally metal contaminated water (P = 0.0005, df = 5, ANOVA) (Fig. 1D).
To test whether differences in growth rate translate to selection dynamics, the ciprofloxacin-susceptible and ciprofloxacin-resistant strains were competed for seven days across four ciprofloxacin concentrations in LB Broth in the presence and absence of 0.5 mM Zn as well as in naturally metal contaminated water (Fig. 3). Again, relative fitness of the susceptible strain did not significantly differ between the unpolluted control and metal contaminated water at any of the tested ciprofloxacin concentrations (all P > 0.05, ANOVA). While metal contaminated water had an effect on the growth rates of both susceptible and resistant strains (Fig. 1A and D), no effect on selection for ciprofloxacin resistance was observed in the environmental sample. This is most likely explained by the fact that the total metal content was high enough to negatively affect growth, but that the concentration of Zn (0.028 mM; Table S1, Supporting Information) and other chelating metals was too low to significantly affect ciprofloxacin. The seven-day selection experiment supported the results of the short-term growth assays in that it showed the competitive advantage of a ciprofloxacin-susceptible strain in the presence of Zn. This is evidenced by the fact that at 0.5 mM Zn, the susceptible strain outcompetes the resistant strain at 0.025 μg/mL ciprofloxacin (ρs,0.5 mM = 1.084 ± 0.017), whereas the reverse is true in the absence of Zn (ρs,0.0 mM = 0.863 ± 0.069; P = 0.0058, df = 5, ANOVA) (Fig. 3). The effect of Zn is apparent at concentrations as low as 0.01 μg/mL ciprofloxacin (ρs,0.5 mM = 1.177 ± 0.078; ρs,0.0 mM = 1.052 ± 0.014; P = 0.053, df = 5, ANOVA) (Fig. 3).
Figure 3.
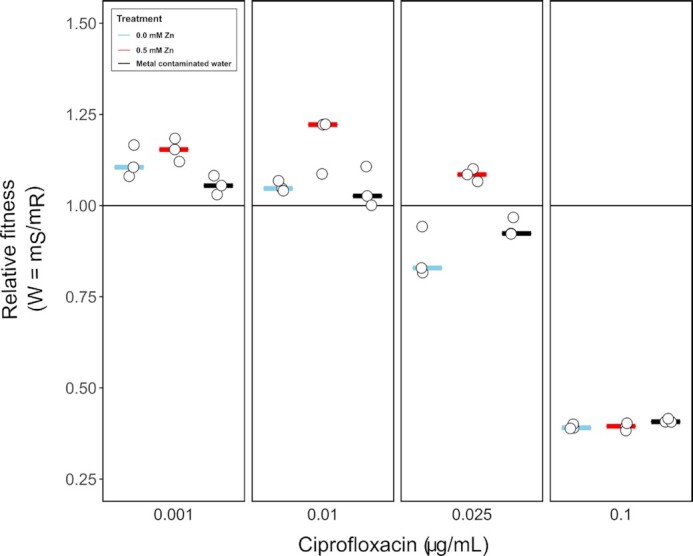
Relative fitness of the ciprofloxacin-susceptible strain based on seven-day competition experiments across a gradient of ciprofloxacin in the absence and presence of 0.5 mM Zn and in naturally metal contaminated water.
DISCUSSION
Here, we present evidence that metals have the potential to reduce selection for ciprofloxacin resistance. The addition of zinc resulted in a five-fold increase in the MSC for ciprofloxacin resistance at a concentration of 1.0 mM Zn. Zinc was found to reduce both absolute growth rate and final density of the ciprofloxacin-resistant strain, hence decreasing the total incidence of resistant phenotypes. The non-fluoroquinolone antibiotic gentamicin, which does not chelate metal, did not exhibit a similar interaction with Zn, which is consistent with a scenario where zinc reduces selection for ciprofloxacin resistance through metal-antibiotic specific formation of chelates.
Fluoroquinolones, such as ciprofloxacin, are hydrophilic and hence possess high mobility in aquatic environments (Felis et al. 2020). In effluents from hospitals, concentrations of ciprofloxacin reach levels as high as 0.026 μg/mL (Verlicchi et al. 2012), well in the range of effect concentrations determined in this study. Despite its relatively low stability with half-life times estimated as approximately 46 hours (Cardoza et al. 2005; Felis et al. 2020), ciprofloxacin has regularly been detected in diverse aquatic environments such as groundwater and drinking water (Hanna et al. 2018; Reis et al. 2019).
Ciprofloxacin resistance in E. coli can be conferred through diverse genetic changes which consequently confer diverse fitness costs/gains on the ciprofloxacin resistant isolates (Marcusson, Frimodt-Møller and Hughes 2009; Baker et al. 2013; Machuca et al. 2014). Most strains unable to develop a favourable set of mutations in the quinolone resistance-determining regions (QRDR) have to rely primarily on the overexpression of efflux pumps which is energetically demanding and impacts fitness (Johnson et al. 2015; Fuzi, Szabo and Csercsik 2017). Only a few E. coli Sequence Types (ST131, ST1193) are capable of evolving multiple energetically favourable QRDR mutations in the gyrase or topoisomerase IV (Fuzi, Szabo and Csercsik 2017), which might have contributed to their widespread dissemination. In our study, ciprofloxacin resistance conferred a major fitness cost on E. coli strain MG1655. This resulted in an increased MSC in the presence of Zn, mainly by decreasing the inhibitory effects of ciprofloxacin on the susceptible strain. For strains with neutral or favourable QRDR mutations selection for resistance is positive or neutral at any concentration, thus no direct effect on the MSC is to be expected. However, the Zn induced decrease in ciprofloxacin inhibition of the susceptible strain remains relevant in these scenarios as it will allow susceptible strains to persist as competitors at an increased range of ciprofloxacin concentrations.
The effect of Zn on selection for ciprofloxacin resistance was apparent at relatively high metal concentrations (0.5–1 mM) in artificial media. Experiments performed using a broth-supplemented metal-contaminated water source did not reveal an effect on selection dynamics. This can be explained by the fact that the environmental Zn concentration was over an order of magnitude lower (0.028 mM, Table S1, Supporting Information) than the lowest defined concentration used (even though the environmental sample contained a range of other metals implicated in fluoroquinolone chelation (Ma, Chiu and Li 1997)). However, it remains possible that other, more heavily polluted sites have metal concentrations high enough to interfere with selection for resistance to low levels of fluoroquinolone. For example, landfill leachates have been shown to contain Zn concentrations reaching up to 3.8 mM (Roy 1994), higher than those concentrations we have demonstrated affecting selection for ciprofloxacin resistance.
Our results could have implications for human or veterinary medicine. For instance, prevalence of ciprofloxacin resistance can reach up to 21.2% of all Enterococci spp. isolates in pig manure samples (Hölzel et al. 2010), making any factors altering selection dynamics highly relevant. Zinc compounds are regularly used as agricultural feed additives and growth promoters in agriculture (Poulsen 1998; Castillo et al. 2008) with concentrations in liquid pig manure reaching up to 4 mmol/kg wet weight (Hölzel et al. 2012), considerably higher than the concentrations used here. In humans, where ciprofloxacin is prescribed for a wide range of infections caused by both Gram-negative and Gram-positive bacteria (Redgrave et al. 2014), the effects observed here could potentially be relevant when patients take zinc supplements, for which daily doses of as high as 40 mg have been reported (Liu et al. 2017). It remains, however, crucial to take speciation and bioavailability rather than total Zn measurements into account.
The focus of this study was exclusively on selection for pre-existing antibiotic resistance mutations in a focal species. Co-occurrence of metal and antibiotic stressors could, however, have additional effects on de novo evolution of antibiotic resistance. Metal stress could for example increase the rate at which antibiotic and metal resistance evolves through increased mutation rate (Lemire, Harrison and Turner 2013). In contrast, effects of metals on the spread of antibiotic resistance might be harder to predict when embedded in a complex microbial community. Selection for resistance has been shown to be further reduced when competing with other community members (Klümper et al. 2019b). However, in complex communities a greater source of resistance genes will be available to the focal species through horizontal gene transfer. While chromosomal resistance determinants could be lost through negative selection, those embedded on self-transmissible plasmids can persist or even increase in abundance, as a consequence of their sometimes extremely broad host ranges and high transfer frequencies (Musovic et al. 2014; Shintani et al. 2014; Klümper et al. 2015; Arias-Andres et al. 2018). Transfer rates of resistance plasmids can consequently be directly impacted by metals, both positively or negatively (Klümper et al. 2017, 2019a).
In summary, we have shown that a selective window exists in which zinc reduces the strength of selection on ciprofloxacin resistance under laboratory conditions. It is highly likely that additional metal-antibiotic interactions affect the efficacy of antibiotics (Li, Nix and Schentag 1994), and hence these deserve future research attention. Our data highlight that co-occurring metals and antibiotic residues add a further level of complexity when assessing the risks of environmental AMR evolution, but it remains to be tested whether these conditions occur in real-world pollution scenarios.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Mr Louis Jolly for support during laboratory experiments.
FUNDING
This work was supported by funding from the European Union's Horizon 2020 research and innovation program under Marie Skłodowska-Curie Grant agreement no. 751699 to UK, through a MRC/BBSRC Grant (MR/N007174/1) to UK and WG, a MRC Grant (MR/S037713/1) to WG and a NERC Innovation People scheme award (NE/S006257/1) to WG.
Conflicts of interest . None declared.
REFERENCES
- Aendekerk S, Ghysels B, Cornelis Pet al.. Characterization of a new efflux pump, MexGHI-OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology. 2002;148:2371–81. [DOI] [PubMed] [Google Scholar]
- Anacona JR. Synthesis and antibacterial activity of some metal complexes of beta-lactamic antibiotics. J Coord Chem. 2001;54:355–65. [Google Scholar]
- Arias-Andres M, Klümper U, Rojas-Jimenez Ket al.. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ Pollut. 2018;237:253–61. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, Wright MS, Stepanauskas Ret al.. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14:176–82. [DOI] [PubMed] [Google Scholar]
- Baker S, Duy PT, Nga TVTet al.. Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. Elife. 2013;2:e01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Kürsten D, Schneider Set al.. Uncovering toxicological complexity by multi-dimensional screenings in microsegmented flow: Modulation of antibiotic interference by nanoparticles. Lab Chip. 2012;12:474–84. [DOI] [PubMed] [Google Scholar]
- Cardoza LA, Knapp CW, Larive CKet al.. Factors affecting the fate of ciprofloxacin in aquatic field systems. Water Air Soil Pollut. 2005;161:383–98. [Google Scholar]
- Castillo M, Martín-Orúe SM, Taylor-Pickard JAet al.. Use of mannan-oligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: Effects on microbiota and gut function. J Anim Sci. 2008;86:94–101. [DOI] [PubMed] [Google Scholar]
- Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enzyme Inhib Med Chem. 2005;20:303–7. [DOI] [PubMed] [Google Scholar]
- Churski K, Kaminski TS, Jakiela Set al.. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip. 2012;12:1629. [DOI] [PubMed] [Google Scholar]
- D'Costa VM, King CE, Kalan Let al.. Antibiotic resistance is ancient. Nature. 2011;477:457–61. [DOI] [PubMed] [Google Scholar]
- Dickinson AW, Power A, Hansen MGet al.. Heavy metal pollution and co-selection for antibiotic resistance: A microbial palaeontology approach. Environ Int. 2019;132:105117. [DOI] [PubMed] [Google Scholar]
- Environment Agency UK. The Catchment Data Explorer (https://environment.data.gov.uk/catchment-planning/WaterBody/GB108048001231). 2019.
- Felis E, Kalka J, Sochacki Aet al.. Antimicrobial pharmaceuticals in the aquatic environment - occurrence and environmental implications. Eur J Pharmacol. 2020;866:172813. [DOI] [PubMed] [Google Scholar]
- Fick J, Söderström H, Lindberg RHet al.. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem. 2009;28:2522–7. [DOI] [PubMed] [Google Scholar]
- Fuzi M, Szabo D, Csercsik R. Double-serine fluoroquinolone resistance mutations advance major international clones and lineages of various multi-drug resistant bacteria. Front Microbiol. 2017;8:2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd GM, Griffiths AJ. Microorganisms and heavy metal toxicity. Microb Ecol. 1977;4:303–17. [DOI] [PubMed] [Google Scholar]
- Gothwal R, Shashidhar T. Antibiotic pollution in the environment: a review. CLEAN - Soil, Air, Water. 2015;43:479–89. [Google Scholar]
- Gullberg E, Albrecht LM, Karlsson Cet al.. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. MBio. 2014;5:e01918–14-e01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg E, Cao S, Berg OGet al.. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7:e1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna N, Sun P, Sun Qet al.. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ Int. 2018;114:131–42. [DOI] [PubMed] [Google Scholar]
- Hölzel CS, Müller C, Harms KSet al.. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ Res. 2012;113:21–7. [DOI] [PubMed] [Google Scholar]
- Hölzel CS, Schwaiger K, Harms Ket al.. Sewage sludge and liquid pig manure as possible sources of antibiotic resistant bacteria. Environ Res. 2010;110:318–26. [DOI] [PubMed] [Google Scholar]
- Imran M, Iqbal J, Iqbal Set al.. In vitro antibacterial studies of ciprofloxacin-imines and their complexes with Cu(II),Ni(II),Co(II), and Zn(II). Turkish J Biol. 2007;31:67–72. [Google Scholar]
- Johnson JR, Johnston B, Kuskowski MAet al.. Intensity and mechanisms of fluoroquinolone resistance within the H30 and H30Rx subclones of Escherichia coli sequence type 131 compared with other fluoroquinolone-resistant E. coli. Antimicrob Agents Chemother. 2015;59:4471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klümper U, Dechesne A, Riber Let al.. Metal stressors consistently modulate bacterial conjugal plasmid uptake potential in a phylogenetically conserved manner. ISME J. 2017;11:152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klümper U, Dechesne A, Smets BF. Protocol for evaluating the permissiveness of bacterial communities toward conjugal plasmids by quantification and isolation of transconjugants. In: McGenity T., Timmis K., Nogales B. (eds) Hydrocarbon and Lipid Microbiology Protocols. Springer Protocols Handbooks. Springer, Berlin, Heidelberg, 2014, 275–88. [Google Scholar]
- Klümper U, Maillard A, Hesse Eet al.. Short-term evolution under copper stress increases probability of plasmid uptake. bioRxiv 2019a:610873.
- Klümper U, Recker M, Zhang Let al.. Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. ISME J. 2019b;13:2927–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klümper U, Riber L, Dechesne Aet al.. Broad host range plasmids can invade an unexpectedly diverse fraction of a soil bacterial community. ISME J. 2015;9:934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CW, Dolfing J, Ehlert PAIet al.. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ Sci Technol. 2010;44:580–7. [DOI] [PubMed] [Google Scholar]
- Knezevic SZ, Streibig JC, Ritz C. Utilizing R software package for dose-response studies: the concept and data analysis. Weed Technol. 2007;21:840–8. [Google Scholar]
- Kovach ME, Elzer PH, Steven Hill Det al.. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–6. [DOI] [PubMed] [Google Scholar]
- Kraupner N, Ebmeyer S, Bengtsson-Palme Jet al.. Selective concentration for ciprofloxacin resistance in Escherichia coli grown in complex aquatic bacterial biofilms. Environ Int. 2018;116:255–68. [DOI] [PubMed] [Google Scholar]
- Kümmerer K, Al-Ahmad A, Mersch-Sundermann V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere. 2000;40:701–10. [DOI] [PubMed] [Google Scholar]
- Larsson DGJ, Andremont A, Bengtsson-Palme Jet al.. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ Int. 2018;117:132–8. [DOI] [PubMed] [Google Scholar]
- Larsson DGJ, de Pedro C, Paxeus N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J Hazard Mater. 2007;148:751–5. [DOI] [PubMed] [Google Scholar]
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371–84. [DOI] [PubMed] [Google Scholar]
- Li RC, Nix DE, Schentag JJ. Interaction between ciprofloxacin and metal cations: Its influence on physicochemical characteristics and antibacterial activity. Pharm Res. 1994;11:917–20. [DOI] [PubMed] [Google Scholar]
- Liu A, Fong A, Becket Eet al.. Selective advantage of resistant strains at trace levels of antibiotics: a simple and altrasensitive color test for detection of antibiotics and genotoxic agents. Antimicrob Agents Chemother. 2011;55:1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Zhang MN, Tong GYet al.. The effectiveness of zinc supplementation in men with isolated hypogonadotropic hypogonadism. Asian J Androl. 2017;19:280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström SV, Östman M, Bengtsson-Palme Jet al.. Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Sci Total Environ. 2016;553:587–95. [DOI] [PubMed] [Google Scholar]
- Ma HHM, Chiu FCK, Li RC. Mechanistic investigation of the reduction in antimicrobial activity of ciprofloxacin by metal cations. Pharm Res. 1997;14:366–70. [DOI] [PubMed] [Google Scholar]
- Machuca J, Briales A, Labrador Get al.. Interplay between plasmid-mediated and chromosomal-mediated fluoroquinolone resistance and bacterial fitness in Escherichia coli. J Antimicrob Chemother. 2014;69:3203–15. [DOI] [PubMed] [Google Scholar]
- Marcusson LL, Frimodt-Møller N, Hughes D. Interplay in the selection of fluoroquinolone resistance and bacterial fitness. PLoS Pathog. 2009;5:e1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García E, Calles B, Arévalo-Rodríguez Met al.. PBAM1: An all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol. 2011;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata MT, Baquero F, Pérez-Díaz JC. A multidrug efflux transporter in Listeria monocytogenes. FEMS Microbiol Lett. 2000;187:185–8. [DOI] [PubMed] [Google Scholar]
- Murray AK, Zhang L, Yin Xet al.. Novel insights into selection for antibiotic resistance in complex microbial communities. MBio. 2018;9:e00969–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musovic S, Klümper U, Dechesne Aet al.. Long-term manure exposure increases soil bacterial community potential for plasmid uptake. Environ Microbiol Rep. 2014;6:125–30. [DOI] [PubMed] [Google Scholar]
- Pal C, Asiani K, Arya Set al.. Metal resistance and its association with antibiotic resistance. Adv Microb Physiol. 2017;70:261–313. [DOI] [PubMed] [Google Scholar]
- Patel M, Chhasatia M, Parmar P. Antibacterial and DNA interaction studies of zinc(II) complexes with quinolone family member, ciprofloxacin. Eur J Med Chem. 2010;45:439–46. [DOI] [PubMed] [Google Scholar]
- Perron GG, Whyte L, Turnbaugh PJet al.. Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics. Brown SP (ed.). PLoS One. 2015;10:e0069533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrie D, Power MR, Rollinson Get al.. The spatial distribution and source of arsenic, copper, tin and zinc within the surface sediments of the Fal Estuary, Cornwall, UK. Sedimentology. 2003;50:579–95. [Google Scholar]
- Poulsen H. Zinc and copper as feed additives, growth factors or unwanted environmental factors. J Anim Feed Sci. 1998;7:135–42. [Google Scholar]
- Redgrave LS, Sutton SB, Webber MAet al.. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22:438–45. [DOI] [PubMed] [Google Scholar]
- Reis EO, Foureaux AFS, Rodrigues JSet al.. Occurrence, removal and seasonal variation of pharmaceuticals in Brasilian drinking water treatment plants. Environ Pollut. 2019;250:773–81. [DOI] [PubMed] [Google Scholar]
- Roy WR. Groundwater contamination from municipal landfills in the USA. Contam Groundwaters. 1994:411–46. [Google Scholar]
- Seedher N, Agarwal P. Effect of metal ions on some pharmacologically relevant interactions involving fluoroquinolone antibiotics. Drug Metabol Drug Interact. 2010;25:17–24. [DOI] [PubMed] [Google Scholar]
- Shintani M, Matsui K, Inoue JIet al.. Single-cell analyses revealed transfer ranges of incP-1, incP-7, and incP-9 plasmids in a soil bacterial community. Appl Environ Microbiol. 2014;80:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalla K, Cook K, Djordjevic SPet al.. Environmental dimensions of antibiotic resistance: assessment of basic science gaps. FEMS Microbiol Ecol. 2018;94, DOI: 10.1093/femsec/fiy195. [DOI] [PubMed] [Google Scholar]
- Song J, Rensing C, Holm PEet al.. Comparison of metals and tetracycline as selective agents for development of tetracycline resistant bacterial communities in agricultural soil. Environ Sci Technol. 2017;51:3040–7. [DOI] [PubMed] [Google Scholar]
- Verlicchi P, Al Aukidy M, Galletti Aet al.. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ. 2012;430:109–18. [DOI] [PubMed] [Google Scholar]
- Wellington EM, Boxall AB, Cross Pet al.. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect Dis. 2013;13:155–65. [DOI] [PubMed] [Google Scholar]
- WHO. Antimicrobial Resistance Global Report on Surveillance, 2014. ISBN: 978 92 4 156474 8, https://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/ [Google Scholar]
- WHO. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals, 2016. ISBN: 978 92 4 156526 4, https://www.who.int/gho/publications/world_health_statistics/2016/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.