Abstract
Stress is increasingly associated with heart dysfunction and is linked to higher mortality rates in patients with cardiometabolic disease. Glucocorticoids are primary stress hormones that regulate homeostasis through two nuclear receptors, the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR), both of which are present in cardiomyocytes. To examine the specific and coordinated roles that these receptors play in mediating the direct effects of stress on the heart, we generated mice with cardiomyocyte-specific deletion of GR (cardioGRKO), MR (cardioMRKO), or both GR and MR (cardioGRMRdKO). The cardioGRKO mice spontaneously developed cardiac hypertrophy and left ventricular systolic dysfunction and died prematurely from heart failure. In contrast, the cardioMRKO mice exhibited normal heart morphology and function. Surprisingly, despite the presence of myocardial stress, the cardioGRMRdKO mice were resistant to the cardiac remodeling, left ventricular dysfunction, and early death observed in the cardioGRKO mice. Gene expression analysis revealed the loss of gene changes associated with impaired Ca2+ handling, increased oxidative stress, and enhanced cell death and the presence of gene changes that limited the hypertrophic response and promoted cardiomyocyte survival in the double knockout hearts. Re-expression of MR in cardioGRMRdKO hearts reversed many of the cardioprotective gene changes and resulted in cardiac failure. These findings reveal a critical role for balanced cardiomyocyte GR and MR stress signaling in cardiovascular health. Therapies that shift stress signaling in the heart to favor more GR and less MR activity may provide an improved approach for treating heart disease.
Introduction
Stress contributes to numerous human pathologies including heart disease (1, 2). Both persistent psychological stress and work stress have been associated with increased mortality in patients with cardiometabolic disease (3, 4). In response to stress, the hypothalamic-pituitary-adrenal axis is activated resulting in the release of glucocorticoids (cortisol) from the adrenal gland. As the primary stress hormone in humans, glucocorticoids act on nearly every tissue and organ of the body to maintain homeostasis. Despite their prevalent clinical use to suppress inflammation, surprisingly little is known about the role that glucocorticoids play in cardiac physiology and pathology and whether the actions of these hormones on the heart are direct or indirect (5).
The actions of glucocorticoids are mediated classically by the glucocorticoid receptor (GR; encoded by Nr3c1), a member of the nuclear receptor superfamily of ligand-dependent transcription factors. When bound by glucocorticoids, GR regulates the expression of numerous genes by direct binding to DNA and/or by interactions with other chromatin-bound transcription factors. GR is expressed in cardiomyocytes and has an important and direct role for glucocorticoid signaling in the heart (5–8). Adult mice on a mixed genetic background that lack GR specifically in cardiomyocytes spontaneously develop cardiac disease and die prematurely from heart failure, suggesting that glucocorticoid signaling through cardiomyocyte GR is critical for maintaining normal heart morphology and function (6). However, glucocorticoids can also bind the closely related mineralocorticoid receptor (MR; encoded by Nr3c2). Much attention has focused on elucidating the role of cardiac MR because clinical treatment with the MR antagonists eplerenone and spironolactone leads to reduced morbidity and mortality in heart failure patients (9). At a mechanistic level, studies utilizing MR antagonists are limited because they do not discriminate between direct and systemic effects on the heart. Mice with conditional knockout of MR in cardiomyocytes have been generated but exhibit only minimal alterations in heart function at baseline (10–12).
Findings from genetically engineered mice lacking GR or MR are confounded by the presence of an intact signaling pathway for the remaining receptor. Mouse models with concurrent deletion of both GR and MR to eliminate all forms of glucocorticoid signaling are necessary to help elucidate the specific and cooperative or antagonistic activities of these receptors. In the following study, we generated mice lacking both GR and MR specifically in cardiomyocytes and compared the gene expression and functional profiles of the double knockout hearts with their single knockout counterparts. Using this genetic approach, we report that mice lacking both GR and MR in cardiomyocytes were resistant to the cardiac disease that develops in mice lacking cardiomyocyte GR alone. These findings reveal that insufficient GR signaling and unopposed MR signaling are pathogenic in cardiomyocytes and suggest that a balanced stress response through these two receptor signaling pathways is crucial for maintaining a healthy heart.
Results
CardioGRMRdKO mice exhibit improved lifespan compared to cardioGRKO mice
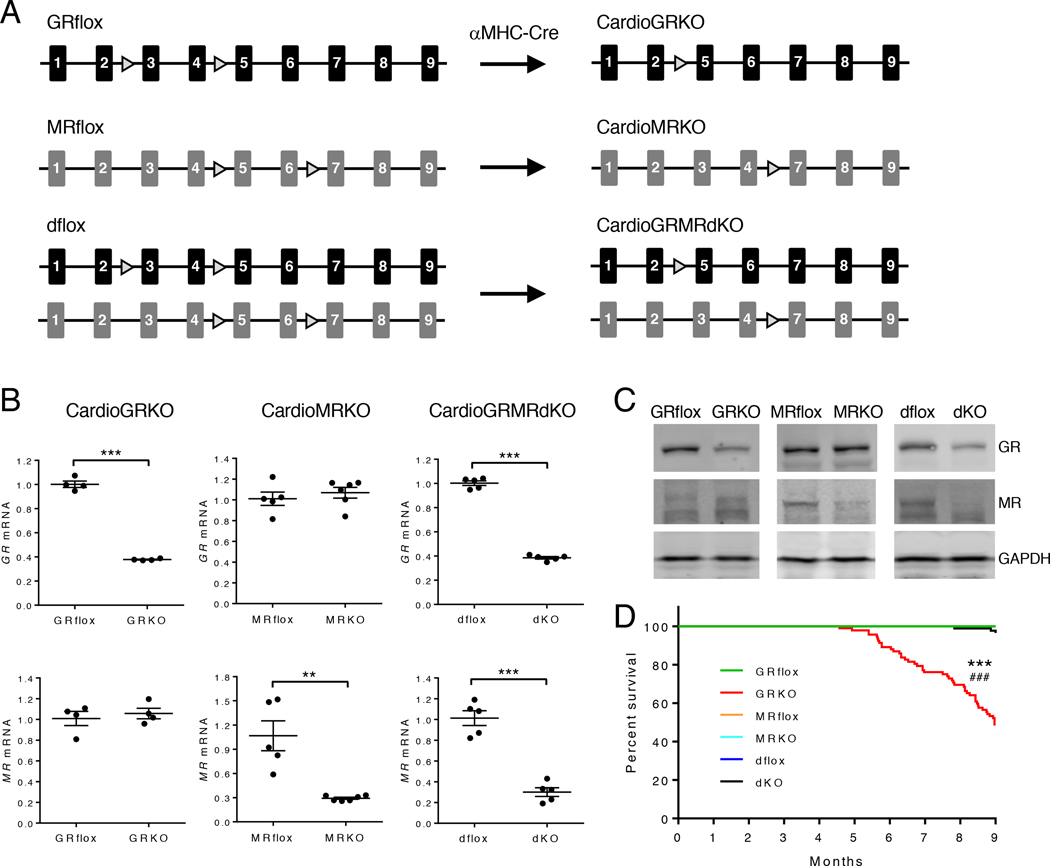
Our laboratory previously generated mice on a mixed FVB/C57BL/6 background with conditional knockout of GR in cardiomyocytes (6). These mice spontaneously develop cardiac hypertrophy, LV systolic dysfunction, and die prematurely from heart failure. Although these studies revealed a critical role for stress signaling in normal heart homeostasis, they did not discriminate between the loss of GR signaling or unabated MR signaling as the molecular culprit for the gene changes and signaling pathways driving the cardiac pathology. To define in vivo the individual and coordinated contributions for these two receptors in mediating the direct actions of glucocorticoids on the heart, we independently generated mice on a C57BL/6 background with conditional knockout of GR in cardiomyocytes (cardioGRKO), MR in cardiomyocytes (cardioMRKO), or both GR and MR in cardiomyocytes (cardioGRMRdKO) (Fig. 1A). The cardioGRKO mice showed reduced expression of GR specific to the heart and no alteration in MR, the cardioMRKO mice showed decreased expression of MR specific to the heart and no change in GR, and the cardioGRMRdKO mice showed reduced expression of both GR and MR specific to the heart (Fig. 1B–C and fig. S1A–C).
Figure 1. Generation and survival of mice with conditional knockout of GR, MR, or both GR and MR in cardiomyocytes.
(A) Mice deficient in cardiomyocyte GR (cardioGRKO), MR (cardioMRKO), or both GR and MR (cardioGRMRdKO) were generated by crossing mice with floxed GR and/or MR alleles with mice expressing Cre recombinase only in cardiomyocytes (αMHC-Cre). (B) RT-PCR of GR and MR mRNA from hearts of 2-month old knockout mice and their littermate controls. Data are mean ± SEM (n = 4–6 mice per group). Student’s t test was performed to determine significance. **P < 0.01 and ***P < 0.001 for GRKO compared to GRflox, for MRKO compared to MRflox, and for dKO compared to dflox. (C) Representative immunoblots of GR and MR protein from hearts of 3-month old knockout mice and their littermate controls (n = 3 independent experiments). (D) Survival curves for GRflox (n = 36), cardioGRKO (n = 92), MRflox (n = 26), cardioMRKO (n = 54), dflox (n = 41), and cardioGRMRdKO (n = 85) mice. Mantel-Cox log-rank test was performed with a Bonferroni corrected threshold to determine significance. ***P < 0.001 for GRKO compared to GRflox. ###P < 0.001 for MRKO compared to GRKO and for dKO compared to GRKO.
The cardioGRKO, cardioMRKO, and cardioGRMRdKO mice were born at the expected Mendelian ratio and appeared normal early in life. By approximately 6 months of age, however, the cardioGRKO mice began to exhibit increased morbidity. Survival curve analysis revealed a reduced lifespan (mean survival time of 8.97 months) for the cardioGRKO mice that is consistent with the early death from heart failure reported for mice on a mixed genetic background that lack cardiomyocyte GR (Fig. 1D) (6). Over the 9-month survival study, the cardioMRKO mice exhibited a normal lifespan, as did the mice lacking both GR and MR in cardiomyocytes. The improved survival of the cardioGRMRdKO mice compared to the cardioGRKO mice suggests a pathogenic role for MR signaling in the cardioGRKO hearts.
CardioGRMRdKO mice are protected from cardiac remodeling
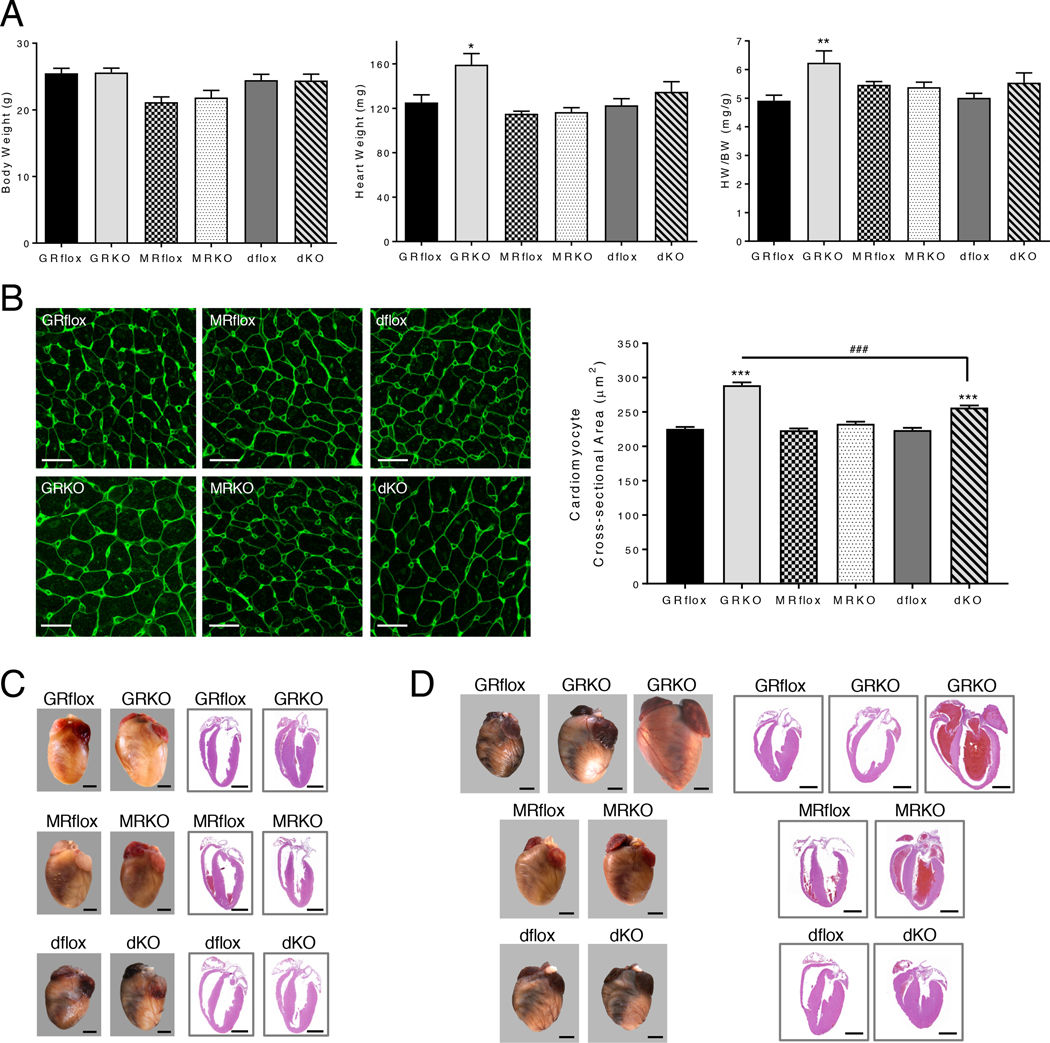
To understand how the loss of cardiomyocyte GR, MR, or both receptors affects cardiac homeostasis and survival in our knockout mice, we initially evaluated heart morphology. Hearts from 2-month old cardioGRKO mice exhibited an increase in heart weight (HW) and heart weight to body weight (HW/BW) ratio (Fig. 2A). In contrast, no change in HW or HW/BW ratio was measured for the cardioMRKO hearts or those depleted of both receptors. The cross-sectional area of cardiomyocytes in the LV of 2-month old cardioGRKO hearts was significantly increased which is indicative of cardiac hypertrophy (Fig. 2B). Cardiomyocytes from the LV of cardioMRKO mice were not altered in size. A small increase in cardiomyocyte cross-sectional area was observed in 2-month old cardioGRMRdKO hearts that was significantly less than the increase measured in the cardioGRKO hearts (Fig. 2B). Gross examination of hearts from 3-month old mice revealed that the cardioGRKO hearts were enlarged compared to controls and H&E-stained sections revealed thickened ventricular walls consistent with a hypertrophic response (Fig. 2C). The cardioMRKO and cardioGRMRdKO retained a normal size and structure (Fig. 2C). These genotype-specific differences in heart morphology could not be attributed to blood pressure alterations because systolic blood pressure did not differ between adult cardioGRKO, cardioMRKO, and cardioGRMRdKO mice (fig. S2).
Figure 2. CardioGRMRdKO mice are protected from LV remodeling.
(A) Body weight (BW), heart weight (HW), and HW/BW ratios were determined for 2-month old control and knockout mice. Data are mean ± SEM (n = 5–9 mice per group). (B) Cross-sectional area of LV cardiomyocytes in 2-month old control and knockout hearts. Left panel shows representative confocal images of heart sections stained with FITC-lectin. Scale bar is 20 μm. Right panel shows quantitation of cardiomyocyte cross-sectional area. Data are mean ± SEM (greater than 400 cardiomyocytes from n = 4–5 mice per group). (C, D) Representative images of intact hearts (left panel) and longitudinal H&E-stained heart sections (right panel) from 3-month (C) and 6-month (D) old control and knockout mice. Scale bar is 2mm. Images are representative of 3–6 mice per genotype. A one-way ANOVA was performed to determine significance. *P < 0.05, **P < 0.01, and ***P < 0.001 for GRKO compared to GRflox and for dKO compared to dflox. ###P < 0.001 for dKO compared to GRKO.
At 6 months of age, cardioGRKO hearts exhibited an even greater increase in size, and a few of the hearts were severely enlarged (Fig. 2D). H&E-stained sections showed pronounced dilation of the LV at this later time point (Fig. 2D). In the greatly enlarged hearts, both the LV and right ventricle (RV) were dilated and a thrombus characterized by a laminar arrangement of fibrin was frequently observed in the left atrium (fig. S3A). In contrast, hearts from 6-month old cardioMRKO and cardioGRMRdKO mice continued to display a normal size (Fig. 2D). The structure of the older cardioMRKO hearts appeared grossly normal. Thickened ventricular walls were occasionally observed in hearts lacking both receptors (Fig. 2D), and analysis of the cross-sectional area of cardiomyocytes in the RV of the cardioGRMRdKO heart revealed hypertrophy (fig. S4A). The RV hypertrophy was not accompanied by evident lung edema in the 6-month old cardioGRMRdKO mice as neither lung weight (LW) nor lung weight to body weight (LW/BW) ratio differed from control dflox littermates (fig. S4B). At a microscopic level, a small number of degenerate cardiomyocytes and a small amount of inflammation were observed in both the 6-month old cardioGRKO and cardioGRMRdKO hearts but not in the cardioMRKO hearts (fig. S3B). The more severely affected cardioGRKO hearts exhibited a greater level of inflammation as well as fibrosis (fig. S3C). These data show that the cardioGRKO hearts exhibit LV remodeling consistent with heart failure whereas the cardioMRKO hearts have a normal morphology and structure. Hearts devoid of both receptors were resistant to the early and extensive LV remodeling observed in the cardioGRKO hearts.
CardioGRMRdKO mice are protected from cardiac dysfunction
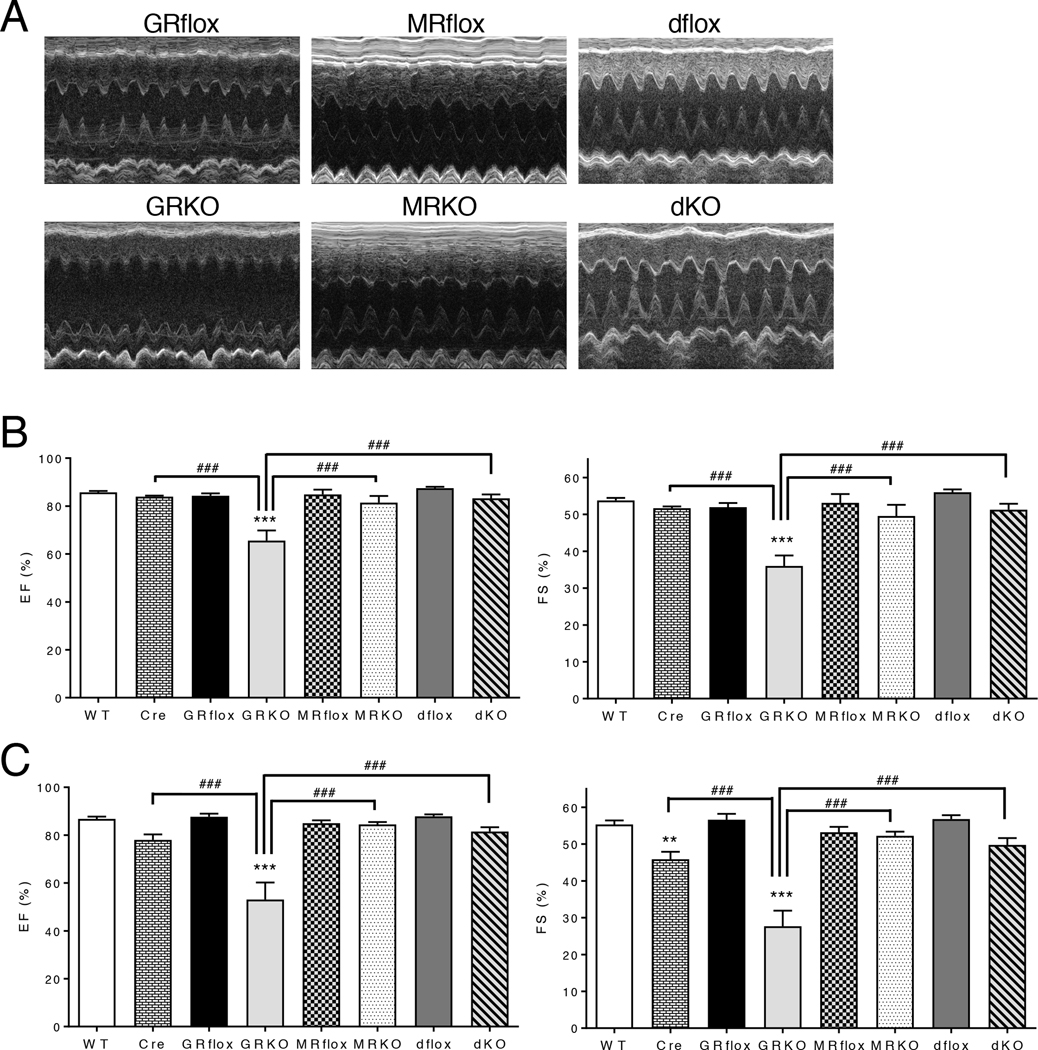
To evaluate the functional consequences of the loss of GR, MR, or both GR and MR from cardiomyocytes, we performed electrocardiography on 3-month old mice to assess the conduction properties of the heart. All parameters were normal in the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts except for the duration of the QRS complex which showed a modest prolongation in the cardioGRKO mice that is consistent with the observed cardiac hypertrophy (table S1). We also evaluated cardiac function by conscious transthoracic echocardiography on 3-month old mice (table S2). Representative motion mode (M-mode) images depicted an enlargement of the LV internal dimension in systole for the cardioGRKO hearts (Fig. 3A). These changes were indicative of LV systolic dysfunction and were accompanied by significant decreases in ejection fraction and fractional shortening (Fig. 3B). Younger 1-month old cardioGRKO mice did not show systolic dysfunction (table S3); however, LV mass and LV mass/BW ratio were significantly increased suggesting the presence of cardiac hypertrophy. Cardiac toxicity due to Cre expression has been described (13); therefore, we also evaluated hearts from 3-month old mice expressing αMHC-Cre alone. Cardiac function was normal in these mice (Fig. 3B), indicating the pathology observed in the cardioGRKO heart is specific to the loss of GR. In contrast to the cardioGRKO heart, deletion of MR alone or concurrent deletion of both GR and MR from cardiomyocytes did not lead to systolic dysfunction at the 3-month time point (Fig. 3A–B).
Figure 3. CardioGRMRdKO mice are protected from LV systolic dysfunction.
(A) Representative M-mode images from 3-month old control and knockout mice. (B, C) Echocardiographic measurements of percent ejection fraction (EF) and percent fractional shortening (FS) were determined from transthoracic M-mode tracings made on control and knockout mice that were 3 months (B) or 6 months (C) old. Data are mean ± SEM (n = 5–11 mice per group). A one-way ANOVA was performed to determine significance. **P < 0.01 and ***P < 0.001 for GRKO compared to GRflox and for Cre compared to WT. ###P < 0.001 for Cre compared to GRKO, for MRKO compared to GRKO, and for dKO compared to GRKO.
By 6 months of age, heart function in the cardioGRKO mice was severely impaired. LV walls were thin in systole and chamber dimensions were enlarged both in diastole and in systole indicative of dilatation (table S4). Large reductions in both ejection fraction and fractional shortening were measured for the cardioGRKO hearts (Fig. 3C). Mice expressing Cre alone exhibited a normal ejection fraction and a small reduction in fractional shortening at the 6-month time point (Fig. 3C). In contrast to cardioGRKO hearts, hearts in 6-month old cardioMRKO and cardioGRMRdKO mice did not show deficits in ejection fraction or fractional shortening (Fig. 3C). These data show that loss of cardiomyocyte GR alone results in LV systolic dysfunction whereas loss of cardiomyocyte MR alone has minimal effects on heart performance. Consistent with their prolonged survival, mice lacking both GR and MR in cardiomyocytes are resistant to the impaired function observed in the cardioGRKO hearts.
CardioGRMRdKO hearts exhibit gene changes associated with cardiac pathology
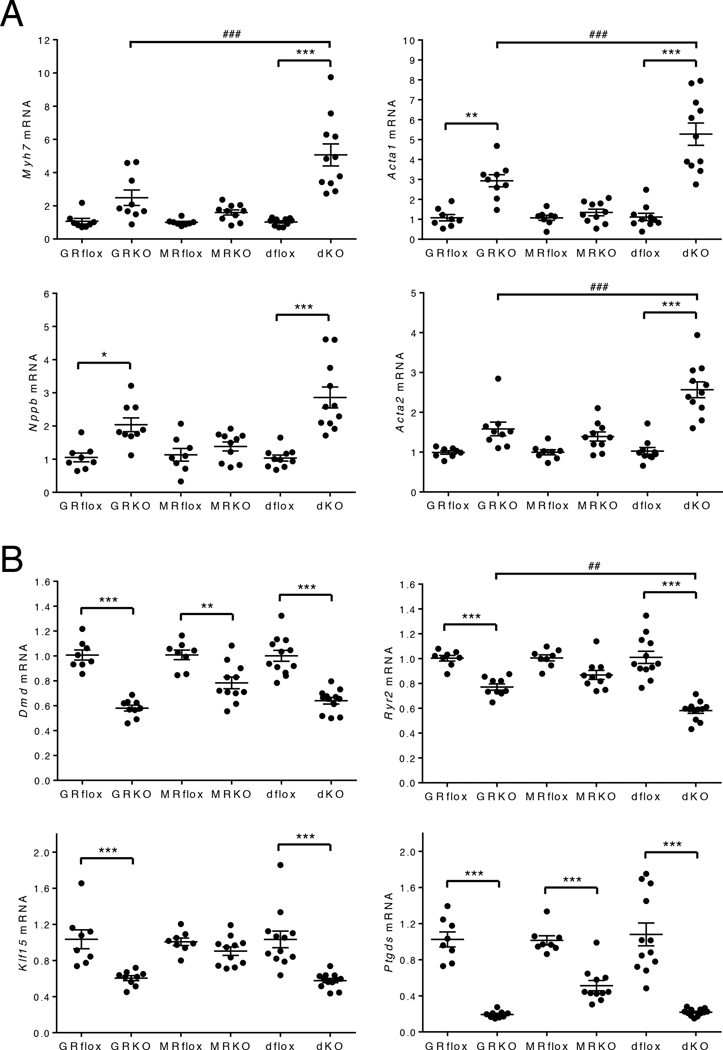
Reactivation of the fetal cardiac gene program is a hallmark of pathological cardiac hypertrophy and heart failure. Although elements of this program may be adaptive initially, these gene changes contribute to pathological remodeling and cardiac dysfunction (14–16). To explore whether differences in the regulation of the fetal cardiac gene program account for the phenotypic differences in the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts, we measured the expression of 4 classic pathological hypertrophic markers: beta-myosin heavy chain (encoded by Myh7), skeletal muscle alpha-actin (encoded by Acta1), brain natriuretic peptide (encoded by Nppb), and smooth muscle alpha-actin (encoded by Acta2). At 2-months of age, the cardioGRKO hearts exhibited significant increases in Acta1 and Nppb expression (Fig. 4A). The cardioMRKO hearts displayed no significant upregulation for any of these genes which is consistent with their normal morphology and function (Fig. 4A). Although the double knockout hearts exhibited only a small increase in cardiomyocyte cross-sectional area and functioned normally through 6 months of age, all 4 fetal cardiac genes were significantly increased in 2-month old hearts (Fig. 4A). In fact, the induction of Myh7, Acta1, and Acta2 expression was significantly greater in magnitude than the induction observed for these genes in the cardioGRKO hearts. Similar results were observed at the 3-month time point for each knockout heart demonstrating that the fetal gene expression profiles were sustained over time (fig. S5A). These data suggest that the hypertrophic response occurring in both the cardioGRKO and cardioGRMRdKO hearts is associated with pathology despite the maintenance of LV function in the double knockout mice.
Figure 4. Genes associated with cardiac pathology are dysregulated in cardioGRMRdKO hearts.
Total RNA was isolated from whole hearts from 2-month old control and knockout mice. (A) Myh7, Acta1, Nppb, and Acta2 mRNA levels were measured by RTPCR. Data are mean ± SEM (n = 8–11 mice per group). (B) Dmd, Ryr2, Klf15, and Ptgds mRNA levels were measured by RTPCR. Data are mean ± SEM (n = 8–12 mice per group). A one-way ANOVA was performed to determine significance. *P < 0.05, **P < 0.01, and ***P < 0.001 for GRKO compared to GRflox, for MRKO compared to MRflox, and for dKO compared to dflox. ##P < 0.01 and ###P < 0.001 for dKO compared to GRKO.
The pathology that develops in hearts deficient in GR signaling alone has been linked to reductions in the abundance of dystrophin (encoded by Dmd), ryanodine receptor 2 (encoded by Ryr2), Kruppel-like factor 15 (encoded by Klf15), and lipocalin-type prostaglandin D synthase (encoded by Ptgds), which act in concert to impair contraction, promote hypertrophy, and inhibit survival of cardiomyocytes (6). We examined the expression of these genes in our cardioGRKO mice and found that each one was significantly decreased in 2-month old hearts (Fig. 4B). The reduced expression profile was sustained over time as all 4 genes were also decreased in hearts from 3-month old cardioGRKO mice (fig. S5B). We next evaluated the expression of these genes in the cardioMRKO and cardioGRMRdKO hearts. Although Dmd was found to be decreased in the cardioMRKO hearts, no changes were observed for Ryr2, Klf15, and Ptgds that persisted across both time points (Fig. 4B and fig. S5B). In contrast, all 4 genes were diminished in expression at each time point in the cardioGRMRdKO hearts even though these hearts maintain normal function through 6 months of age (Fig. 4B and fig. S5B). The reduction in Ryr2, Klf15, and Ptgds expression in both the cardioGRKO and cardioGRMRdKO hearts, but not the cardioMRKO hearts, indicates that the loss of GR signaling is responsible for their dysregulation.
CardioGRMRdKO hearts are protected from alterations in Ca2+ handling, oxidative stress, and cell death
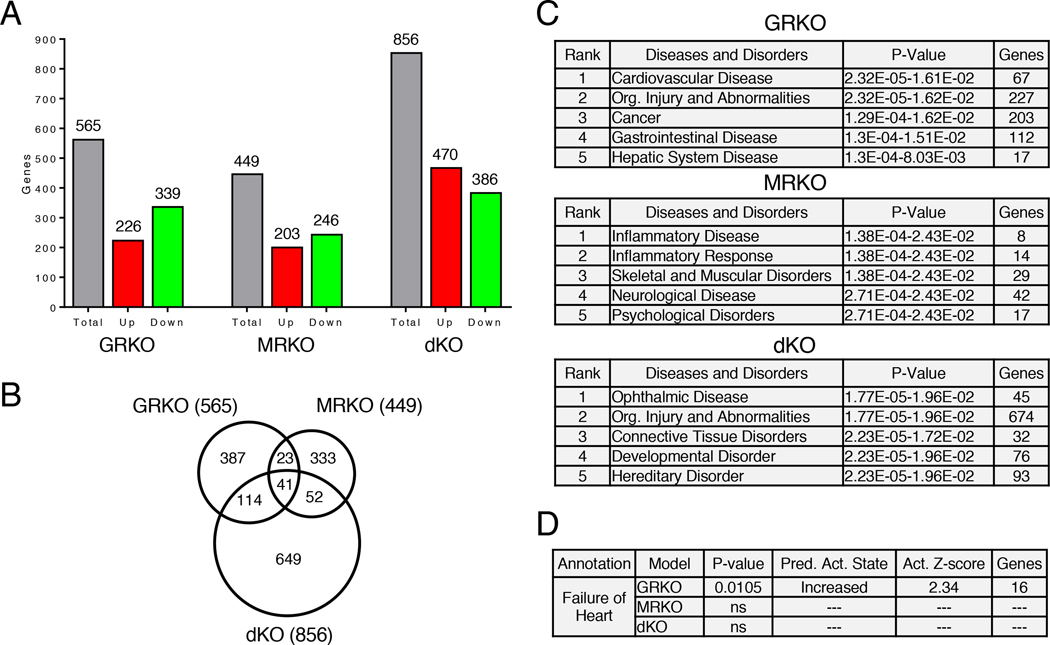
The cardioGRMRdKO hearts showed reactivation of the fetal cardiac gene program and the dysregulation of key genes reported to underlie the pathology in the cardioGRKO hearts. However, unlike the cardioGRKO hearts, the double knockout hearts were resistant to the development of cardiac dysfunction. To identify the genes and signaling pathways that contribute to this cardioprotection, we performed genome-wide microarrays on hearts from 1-month and 2-month old cardioGRKO, cardioMRKO, and cardioGRMRdKO mice and their age-matched littermate controls. Evaluation of the gene expression profiles revealed several features at the early 1-month time point that is prior to cardiac dysfunction. First, a greater number of genes had altered expression in the cardioGRMRdKO hearts compared to the single knockout hearts (Fig. 5A). Furthermore, the majority of dysregulated genes showed increased expression in the double knockout hearts but decreased expression in the cardioGRKO and cardioMRKO hearts (Fig. 5A). Finally, comparison of the 3 sets of dysregulated genes revealed that most of the gene changes were unique to each genotype (Fig. 5B). At the 2-month time point, a greater number of genes were dysregulated in each knockout with the cardioMRKO heart exhibiting the largest number of differentially expressed genes and the greatest increase from the 1-month time point (fig. S6A). Moreover, as observed in the 1-month old hearts, the majority of dysregulated genes at the later time point showed increased expression in the double knockout hearts but decreased expression in the cardioGRKO and cardioMRKO hearts and most of the gene changes were unique to each knockout heart (fig. S6A–B). The complete list of differentially expressed genes is provided for each knockout heart at both the 1- and 2-month time points (Data File S1 and S2), and hierarchical clustering was performed to visualize the differentially expressed genes across all three genotypes (fig. S7A–B).
Figure 5. Global gene expression profile in 1-month old cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Microarrays were performed on RNA isolated from the hearts of 1-month old control and knockout mice. (A) Total number of genes differentially expressed in the hearts of knockout mice compared to their control littermates. (B) Differentially expressed genes in the knockout hearts were compared using a Venn diagram. (C) Diseases and disorders most significantly associated with the dysregulated genes in the knockout hearts as determined by IPA. (D) Gene enrichment comparison analysis of the dysregulated genes associated with “Cardiovascular Disease” in the 3 knockout hearts was performed using IPA. Shown are the disease annotations with a significant activation z-score (absolute value ≥ 2). The retrieved annotation “Failure of Heart” was only significantly associated with the dysregulated genes in the cardioGRKO heart. ns = not significant.
We performed literature-based Ingenuity pathway analysis (IPA) to identify the diseases and disorders most significantly associated with the dysregulated genes from 1-month old knockout hearts (Fig. 5C). “Cardiovascular Disease” was strongly associated with the dysregulated genes in the cardioGRKO heart (1st out of 25 significant annotations) but not the cardioMRKO and cardioGRMRdKO hearts. Gene enrichment comparison analysis of these Cardiovascular Disease-associated genes identified “Failure of Heart” to be significantly associated only with the genes dysregulated in the cardioGRKO heart (Fig. 5D). Moreover, “Failure of Heart” was the only disease annotation with a significant activation z-score for increased activity in the cardioGRKO hearts (Fig. 5D). Similar gene enrichment results were found for the dysregulated genes in 2-month old knockout hearts (fig. S6C–D). These findings demonstrate that the gene expression profiles in hearts at the early 1-month time point predict both the pathology observed later in life in the cardioGRKO hearts and the preserved function observed later in life in the cardioMRKO and cardioGRMRdKO hearts.
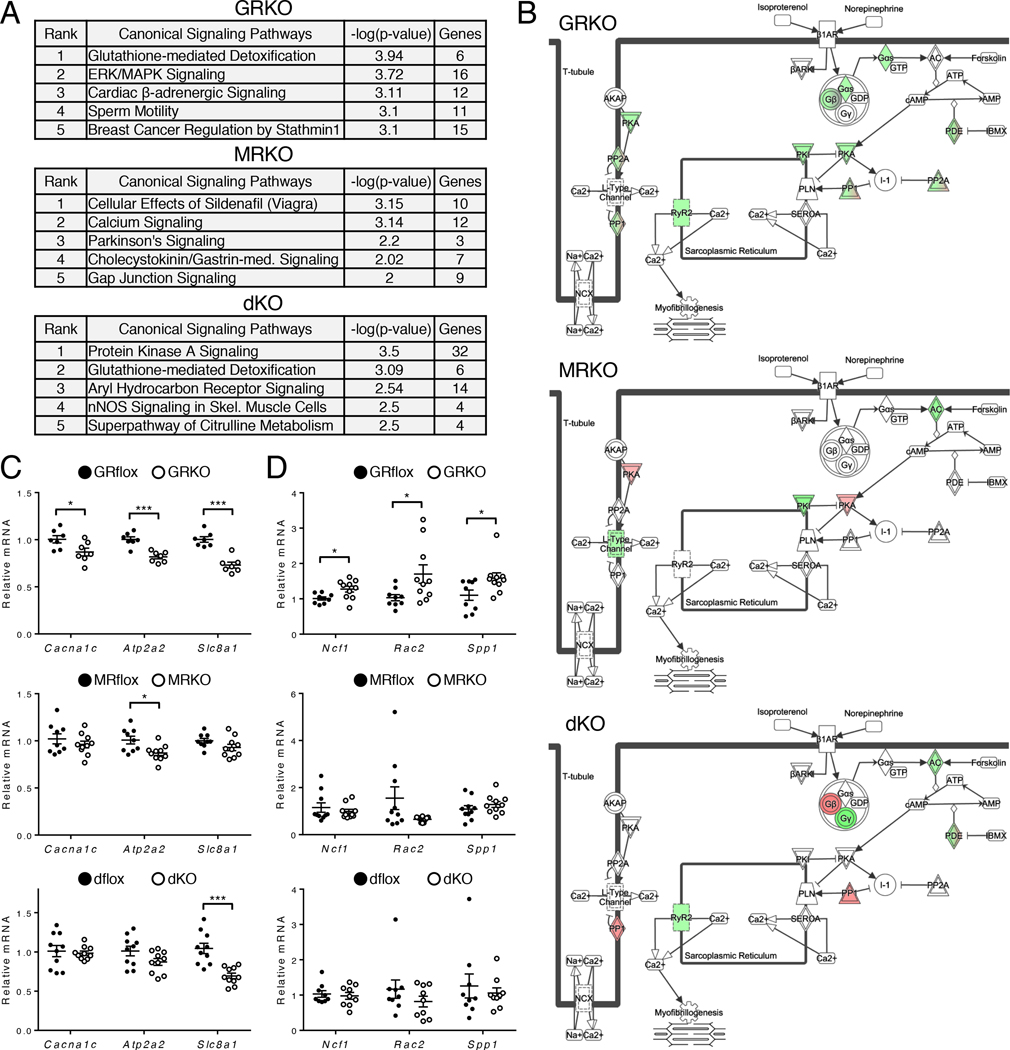
To gain insight into the molecular and cellular mechanisms that contribute to the different heart phenotypes of the knockout mice, we examined the canonical signaling pathways associated with the dysregulated genes. “Cardiac β-Adrenergic Signaling” was strongly associated with the dysregulated genes in the cardioGRKO heart at both the 1-month (3rd of 57 significant annotations) and 2-month (4th out of 67 significant annotations) time points and was not significantly associated with the dysregulated genes in the cardioMRKO and cardioGRMRdKO hearts at either time point (Fig. 6A and fig. S6E). β-Adrenergic receptors modulate cardiac contractility in response to stress. In the 1-month cardioGRKO heart, multiple genes in the β-adrenergic signaling pathway exhibited decreased expression, suggesting Ca2+ handling may be altered in these hearts (Fig. 6B). Consistent with our RTPCR findings in 2- and 3-month old hearts (Fig. 4B and fig. S5B), Ryr2 mRNA showed decreased expression in the cardioGRKO and double knockout heart but not the cardioMRKO heart (Fig. 6B). To determine whether other Ca2+ handling proteins in the β-adrenergic signaling pathway exhibited genotype-specific differences in expression, we evaluated mRNA levels of the voltage-gated L-type calcium channel (LTCC, encoded by Cacna1c), the sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2, encoded by Atp2a2), and the sodium/calcium exchanger 1 (NCX1, encoded by Slc8a1) in 3-month knockout hearts (Fig. 6C). All three genes were significantly decreased in the cardioGRKO heart whereas only Atp2a2 showed a significant reduction in the cardioMRKO heart and only Slc8a1 was repressed in the cardioGRMRdKO heart. “Mitochondrial Dysfunction” was also among the top signaling pathways (5th out of 67 significant annotations) altered in 2-month old cardioGRKO hearts suggesting the presence of oxidative stress (fig. S6E). Alterations in the redox status of the heart not only contribute to the development and progression of heart failure but also modulate the MR signaling profile (17–20). Therefore, we examined the expression of several markers of oxidative stress, specifically the NADPH oxidase subunit p47phox (encoded by Ncf1) and the activator Rac2 (encoded by Rac2), in the hearts of the knockout mice. Additionally, we evaluated the expression of osteopontin (encoded by Spp1), a proinflammatory cytokine that is induced in response to oxidative stress. All three genes were significantly upregulated in the cardioGRKO hearts but not in the cardioMRKO and cardioGRMRdKO hearts (Fig. 6D). These data suggest that the cardioMRKO and cardioGRMRdKO hearts are protected from gene changes occurring in the cardioGRKO hearts that perturb Ca2+ handling and promote oxidative stress.
Figure 6. CardioGRMRdKO hearts are protected from alterations in Ca2+ handling and oxidative stress observed in cardioGRKO hearts.
(A) Signaling pathways most significantly associated with the dysregulated genes in 1-month old knockout hearts as determined by IPA. (B) The “Cardiac β-Adrenergic Signaling” pathway overlaid with dysregulated genes in 1-month old knockout hearts. Red and green colors correspond to up-regulation and down-regulation, respectively. (C, D) mRNA levels for the Ca2+ handling genes Cacna1c, Atp2a2, and Slc8a1 (C) and the oxidative stress genes Ncf1, Rac2, and Spp1 (D) were measured by RTPCR in 3-month old control and knockout hearts. Data are mean ± SEM (n = 7–10 mice per group). *P < 0.05 and ***P < 0.001 for GRKO compared to GRflox, for MRKO compared to MRflox, and for dKO compared to dflox.
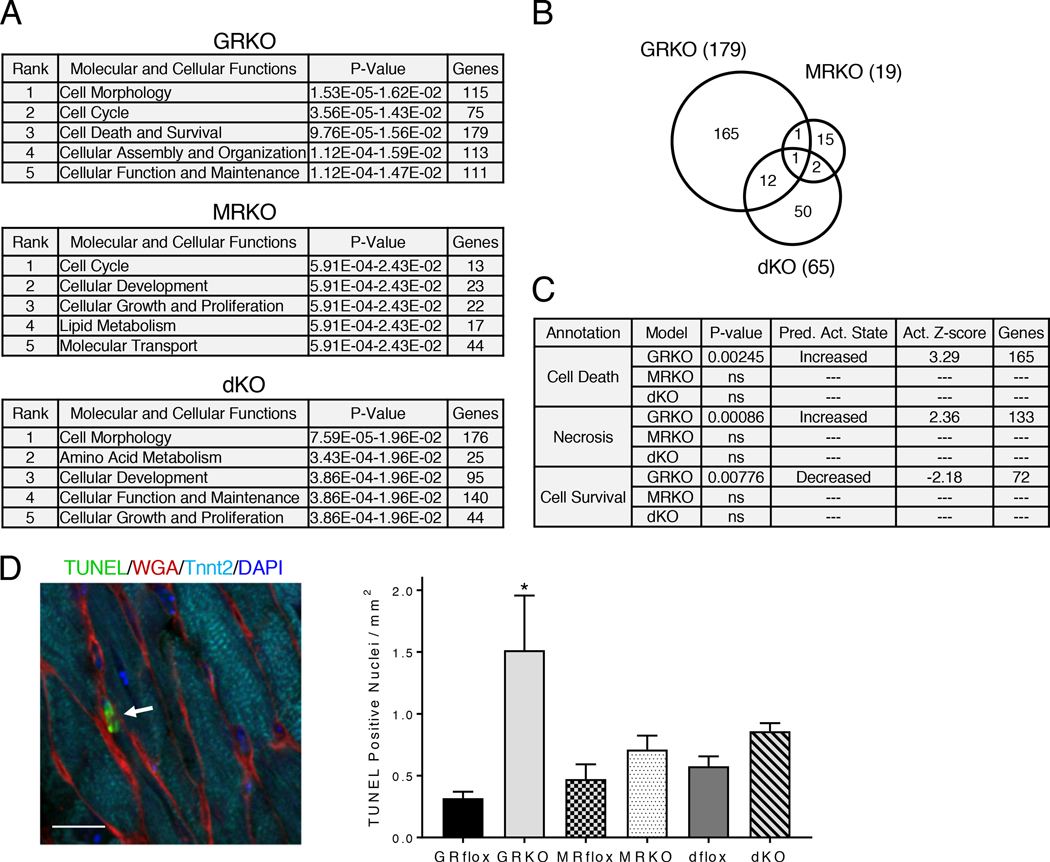
We also evaluated the molecular and cellular functions associated with the differentially expressed genes in the knockout hearts. The annotation “Cell Death and Survival” was strongly associated with the dysregulated genes in the cardioGRKO heart (3rd out of 25 significant annotations) but not the cardioMRKO or cardioGRMRdKO hearts (Fig. 7A). 179 genes were associated with “Cell Death and Survival” in the cardioGRKO heart whereas only 19 and 65 genes were associated with this annotation in the cardioMRKO and cardioGRMRdKO hearts, respectively (fig. S8). Most of these gene changes were unique to each genotype (Fig. 7B). Gene enrichment comparison analysis of the genes in the “Cell Death and Survival” annotation revealed that “Cell Death,” “Necrosis,” and “Cell Survival” were significantly associated only with the genes dysregulated in the cardioGRKO heart (Fig. 7C). “Cell Death” and “Necrosis” were also the only annotations with a significant activation z-score for increased activity in the cardioGRKO hearts and, conversely, “Cell Survival” was the only annotation with a significant activation z-score for decreased activity in the cardioGRKO heart (Fig. 7C). Analysis of the dysregulated genes from the 2-month old knockout hearts yielded similar gene enrichment predictions (fig. S9, A–C). The gene enrichment data suggest that differences in cardiomyocyte cell death may contribute to the differences in morphology and function observed among the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts. To directly test this possibility, we performed TUNEL assays. We measured a 4.8-fold increase in the number of TUNEL-positive nuclei in the cardioGRKO hearts compared to their littermate controls (Fig. 7D). In contrast, no significant differences were detected in the number of TUNEL-positive nuclei in the cardioMRKO and cardioGRMRdKO hearts. Collectively, these data implicate impaired Ca2+ handling, increased oxidative stress, and enhanced cell death in the pathology that develops in the cardioGRKO hearts. The cardioMRKO and cardioGRMRdKO hearts are protected from many of the gene changes that underlie these adverse signaling events.
Figure 7. CardioGRMRdKO hearts are protected from cell death observed in cardioGRKO hearts.
(A) Molecular and cellular functions most significantly associated with the dysregulated genes in 1-month old knockout hearts as determined by IPA. (B) Dysregulated genes associated with “Cell Death and Survival” in the knockout hearts were compared using a Venn diagram. (C) Gene enrichment comparison analysis of the dysregulated genes associated with “Cell Death and Survival” in the 3 knockout hearts was performed using IPA. Shown are the functional annotations with a significant activation z-score (absolute value ≥ 2). The retrieved annotations were only significantly associated with the dysregulated genes in the cardioGRKO heart. ns = not significant. (D) Analysis of cell death in knockout hearts. Left panel shows representative image of TUNEL-positive nuclei (arrow) in LV myocardium of 6-month cardioGRKO heart. Quadruple staining was performed: TUNEL (green), WGA (red), cardiac troponin T (Tnnt2) (cyan), and DAPI (blue). Scale bar is 10 μm. Right panel shows quantitation of TUNEL-positive nuclei in 6-month knockout hearts. Data are mean ± SEM (n = 4–6 mice per group). A one-way ANOVA was performed to determine significance. *P < 0.05 for GRKO compared to GRflox.
CardioGRMRdKO hearts exhibit unique gene changes associated with cardioprotection
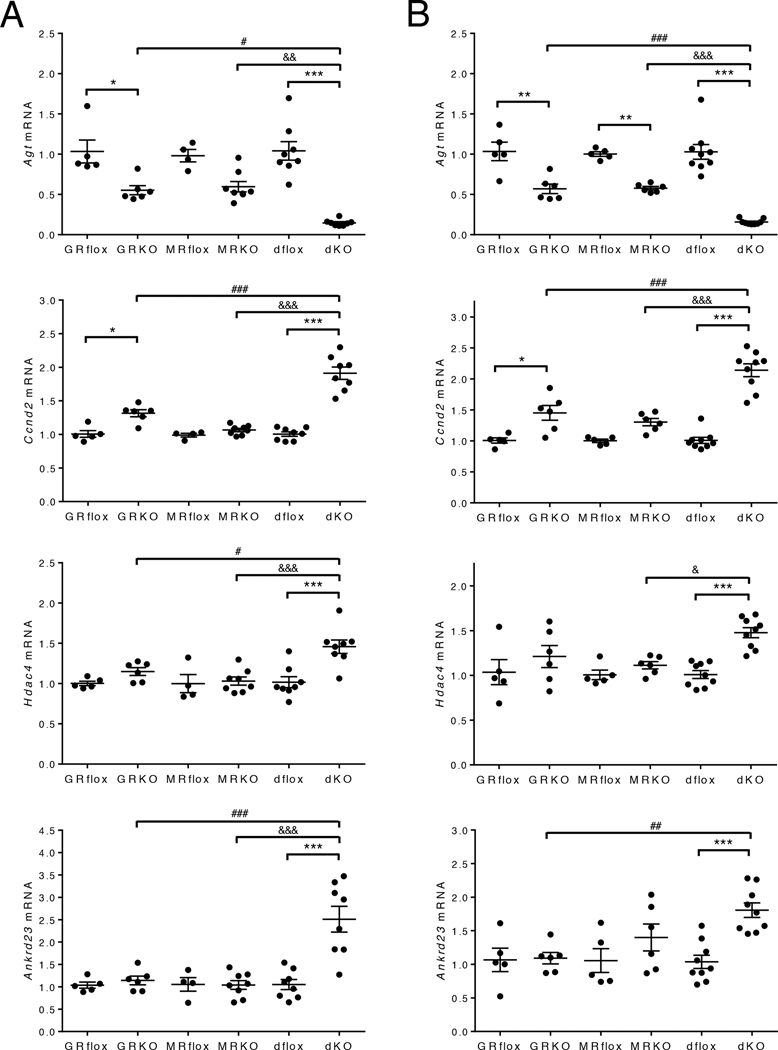
The preserved function of the cardioGRMRdKO hearts suggested a detrimental role for MR signaling in the cardioGRKO hearts. Although the absence of MR signaling in the cardioGRMRdKO heart appeared to prevent the regulation of genes that impair Ca2+ handling, induce oxidative stress, and promote cell death, it may also lead to new gene changes that are cardioprotective. Some of the genes identified by microarray analysis as having altered expression only in 1-month cardioGRMRdKO hearts were Ccnd2 (which encodes cyclin D2; 1.8-fold increase), Hdac4 (which encodes histone deacetylase 4; 1.4-fold increase), and Ankrd23 (which encodes ankyrin repeat domain 23; 1.7-fold increase). Agt (which encodes angiotensinogen) expression was decreased in both the 1-month cardioMRKO and cardioGRMRdKO hearts but the reduction was much greater in the double knockout heart (5.1-fold decrease) compared to the cardioMRKO heart (2.0-fold decrease). Angiotensinogen is an essential component of the cardiac renin-angiotensin system (21), and reduced expression of Agt in the heart inhibits the development of cardiac hypertrophy (22). Cyclin D2 plays a vital role in differentiation and cell-cycle regulation. Increased expression of Ccnd2 in the heart improves cardiac morphology and function by inhibiting cardiac hypertrophy and enhancing cardiomyocyte survival (23–26). Histone deacetylase 4 is a member of the class II histone deacetylases that suppress cardiac hypertrophy by inhibiting pro-hypertrophic transcription factors (27, 28). Ankyrin repeat domain 23 is a member of the muscle ankyrin-repeat protein family involved in muscle gene regulation and myofilament organization, and reduced expression of this gene has been associated with the development of cardiac hypertrophy (29). By limiting cardiac hypertrophy and promoting cardiomyocyte survival, the observed changes in these genes would protect the cardioGRMRdKO heart from disease.
To confirm the changes in expression for these 4 genes, we performed RT-PCR on an independent set of knockout hearts that were 1- and 2-months old. The RT-PCR results were consistent with the microarray data. The expression of Agt was partially reduced in the cardioGRKO hearts as well as in the cardioMRKO hearts (Fig. 8A–B). However, the reduction in Agt expression was much greater in the double knockout hearts at both the 1- and 2-month time points. For Ccnd2, a small upregulation in expression was observed in the cardioGRKO hearts but not in the cardioMRKO hearts (Fig. 8A–B). However, a significantly greater increase in Ccnd2 expression was measured at both time points in the cardioGRMRdKO hearts. Hdac4 and Ankrd23 expression was not altered in either the cardioGRKO or cardioMRKO hearts but was significantly increased in hearts from 1- and 2-month old cardioGRMRdKO mice (Fig. 8A–B). These data indicate that concurrent removal of GR and MR from the heart leads to gene changes distinct from those observed in hearts lacking either receptor alone. The gene changes may contribute to the preserved cardiac function in the cardioGRMRdKO mice by limiting the cardiac hypertrophy, cell death, and maladaptive remodeling that occurs in the cardioGRKO hearts.
Figure 8. Gene changes associated with cardioprotection are uniquely observed in cardioGRMRdKO hearts.
Total RNA was isolated from whole hearts of 1-month (A) and 2-month (B) old control and knockout hearts. Agt, Ccnd2, Hdac4, and Ankrd23 mRNA levels were measured by RTPCR. Data are mean ± SEM (n = 4–9 mice per group). A one-way ANOVA was performed to determine significance. *P < 0.05, **P < 0.01, and ***P < 0.001 for GRKO compared to GRflox, for MRKO compared to MRflox, and for dKO compared to dflox. #P < 0.05, ##P < 0.01, and ###P < 0.001 for dKO compared to GRKO. &P < 0.05, &&P < 0.01, and &&&P < 0.001 for dKO compared to MRKO.
Re-expression of MR in cardioGRMRdKO hearts reverses cardioprotective gene changes and leads to heart disease
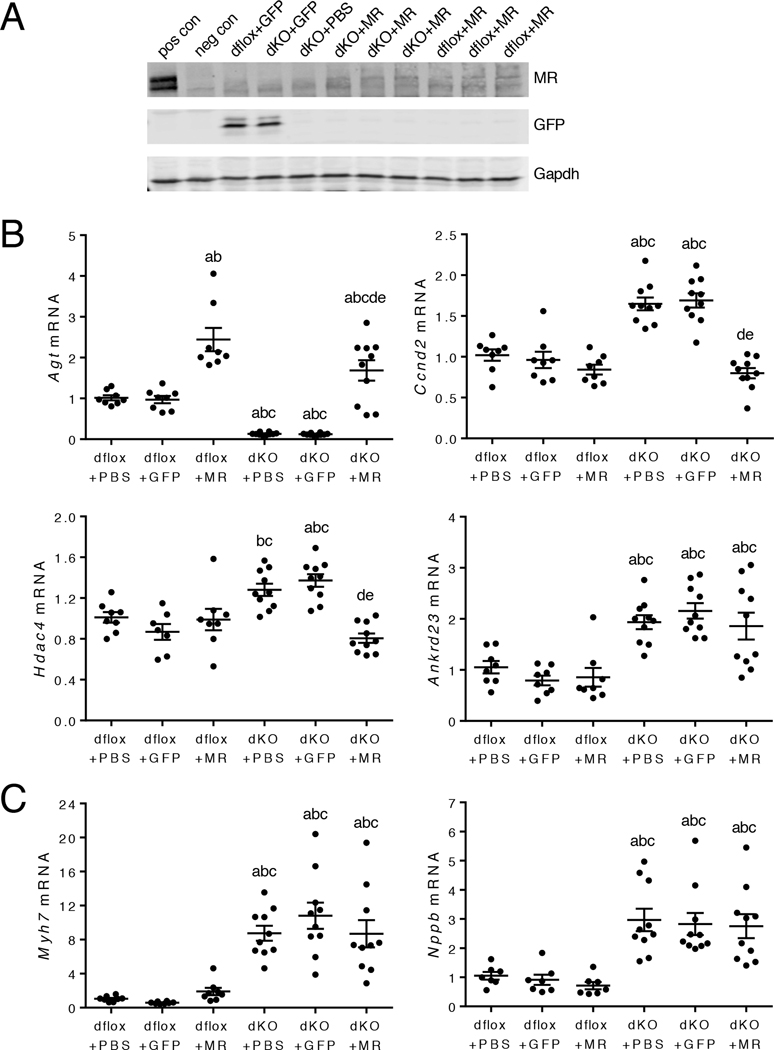
To determine whether MR signaling prevents cardioprotective changes in Agt, Ccnd2, Hdac4, and Ankrd23 expression in the cardioGRKO hearts, we re-expressed MR specifically into cardiomyocytes in the double knockout heart using the adeno-associated virus (AAV) gene delivery system and the cardiac troponin T (Tnnt2) promoter (fig. S10A–B). Intravenous injection of AAV-Tnnt2-MR into 6-month old cardioGRMRdKO mice confirmed successful re-expression of MR in the heart (Fig. 9A). The cardioprotective gene changes discovered in 1-month old double knockout hearts were retained in the older 6-month hearts because the expression of Agt was reduced and the expression of Ccnd2, Hdac4, and Ankrd23 was increased in control double knockout hearts injected with PBS or AAV-Tnnt2-GFP (Fig. 9B). Re-expression of MR in the double knockout hearts completely reversed the cardioprotective changes in Agt, Ccnd2, and Hdac4 but did not significantly impact Ankrd23 expression (Fig. 9B). In contrast to the reversal for many of the cardioprotective gene changes, re-expression of MR in the cardioGRMRdKO hearts did not affect the regulation of Myh7 and Nppb, fetal cardiac genes that have been associated with pathology (Fig. 9C) (14–16).
Figure 9. Re-expression of MR in the cardioGRMRdKO heart reverses cardioprotective gene changes.
CardioGRMRdKO mice and their littermate controls (dflox) were injected intravenously with PBS, AAV-Tnnt2-GFP, or AAV-Tnnt2-MR at 4–6 weeks of age. (A) Representative immunoblot shows MR and GFP expression in hearts isolated from injected mice that were 6 months old (n = 3 independent experiments). Positive (pos) and negative (neg) controls are hippocampal lysates from a wild-type mouse and a littermate mouse with conditional knockout of MR in the hippocampus, respectively. (B) RTPCR analysis of Agt, Ccnd2, Hdac4, and Ankrd23 mRNA levels in hearts from injected dflox and cardioGRMRdKO mice that were 6 months old. Data are mean ± SEM (n = 7–10 mice per group). (C) RTPCR analysis of Myh7 and Nppb mRNA levels in hearts from injected dflox and cardioGRMRdKO mice that were 6 months old. Data are mean ± SEM (n = 7–10 mice per group). A one-way ANOVA was performed to determine significance. aP < 0.05 compared to dflox+PBS. bP < 0.05 compared to dflox+GFP. cP < 0.05 compared to dflox+MR. dP < 0.05 compared to dKO+PBS. eP < 0.05 compared to dKO+GFP.
Echocardiography was performed on 6-month old mice to evaluate whether re-expression of MR in the double knockout heart resulted in cardiac dysfunction (table S5). Hearts from cardioGRMRdKO mice injected with PBS or AAV-Tnnt2-GFP did not differ from control hearts. In contrast, double knockout hearts from mice injected with AAV-Tnnt2-MR exhibited evidence of LV systolic dysfunction because re-expression of MR resulted in significant reductions in both ejection fraction and fractional shortening (table S5). These findings suggest that MR signaling plays a critical role in the cardiac pathology that develops in the cardioGRKO hearts by preventing compensatory gene changes from occurring that would protect the heart from disease.
Discussion
We used a genetic approach to evaluate stress hormone signaling in the heart by generating mice with conditional knockout of GR, MR, or both GR and MR in cardiomyocytes. Mice lacking cardiomyocyte GR alone spontaneously developed cardiac hypertrophy and LV systolic dysfunction and died prematurely from heart failure. In contrast, mice lacking cardiomyocyte MR alone did not exhibit any overt cardiac phenotype at baseline. Mice lacking both GR and MR in the heart were protected from the cardiac disease that develops in the cardioGRKO hearts. Despite the presence of some gene changes associated with myocardial stress, the hearts of cardioGRMRdKO mice functioned normally through 6 months of age and the mice survived longer than the cardioGRKO mice. Gene expression analysis revealed the loss of gene changes associated with impaired Ca2+ handling, increased oxidative stress, and enhanced cell death and the presence of gene changes that limit the hypertrophic response and promote cardiomyocyte survival in the double knockout hearts. Re-expression of MR in cardioGRMRdKO hearts reversed many of these cardioprotective gene changes and resulted in cardiac failure. These findings reveal a critical role for cardiomyocyte MR in the pathology that develops in cardioGRKO hearts and suggest that perturbations in the balance of GR and MR stress signaling in cardiomyocytes regulates heart disease.
The phenotype of the cardioGRKO mice generated in this study on a C57Bl/6 background is similar to that described previously for mice on a mixed genetic background that lack cardiomyocyte GR (6). Mice from both models spontaneously develop cardiac hypertrophy and LV systolic dysfunction and die from heart failure. At a molecular level, both mouse models show reductions in Dmd, Ryr2, Klf15, and Ptgds that occur prior to evidence of dysfunction and may be proximal mediators of the disease. Our current study establishes that the decrease in Ryr2, Klf15, and Ptgds expression depended on the loss of GR signaling because a similar reduction also occurred in cardioGRMRdKO hearts but not in cardioMRKO hearts. Dmd expression, however, was reduced in all three knockout hearts suggesting that GR and MR may function in a redundant manner to control its expression or perhaps the presence of Cre in the cardiomyocytes contributes to the common decrease. Consistent with our findings, both the Klf15 and Ptgds genes are directly regulated by glucocorticoids in a GR-dependent manner in isolated mouse and rat cardiomyocytes (30–32). Ryr2 is also subject to glucocorticoid regulation, because other mouse models with cardiomyocyte depletion of GR exhibit reduced Ryr2 mRNA expression (7), and treatment of isolated mouse cardiomyocytes with the synthetic GR agonist dexamethasone leads to increased Ryr2 mRNA expression (30). Ryr2 is of interest as a proximal mediator of heart failure in the cardioGRKO mice because decreased expression of this gene occurs very early (embryonic day 17) and has been linked to altered Ca2+ responses in isolated GR-deficient cardiomyocytes (6, 7). The cardioGRKO heart also exhibited reductions in the expression of genes encoding the Ca2+ handling proteins LTCC, SERCA2, and NCX1, and each of these genes is induced by dexamethasone treatment of isolated cardiomyocytes (30). Reactivation of the fetal cardiac gene program also occurred in both cardiomyocyte GR knockout mouse models. Moreover, the induction of this program was mediated by the loss of GR because the fetal genes were also increased in cardioGRMRdKO hearts but not in cardioMRKO hearts. These data suggest that a deficiency in GR signaling underlies the initial changes in gene expression that stress the myocardium and promote disease onset in the cardioGRKO heart.
The sustained cardiac function and increased survival of the double knockout mice suggests that MR signaling contributes to the pathology in the cardioGRKO hearts. Evidence that MR signaling is harmful to the failing human heart comes from the benefit conferred by adding the MR antagonists eplerenone and spironolactone to standard drug regimens for heart failure patients (9). However, the specific tissue and cell-types in which the adverse MR signaling occurs remain undefined because MR exhibits a widespread distribution that includes not only cardiomyocytes but also cardiac immune cells, fibroblasts, and endothelial cells as well as epithelial cells of the kidney. Our genetic studies in mice showing that inactivation of the MR gene in cardiomyocytes protected against the pathology that develops in cardioGRKO hearts suggests that the deleterious MR signaling resides within cardiomyocytes. In support of this conclusion, knockout of cardiomyocyte MR also protects the mouse heart in various models of cardiac dysfunction (10–12). The gene regulatory activity of cardiac MR has been reported to change under conditions of oxidative stress and become harmful to the heart (17, 18). For example, glucocorticoids acting through MR increase myocardial infarct size and alter the rate of spontaneous contractions of isolated ventricular myocytes but only in the presence of oxidant stress (19, 20). We found evidence of oxidative stress in the cardioGRKO heart but not in the cardioMRKO and cardioGRMRdKO hearts, suggesting that MR signaling may become pathogenic in the cardioGRKO hearts due to alterations in the redox status of the myocardium. Adverse MR signaling in the GR-deficient cardiomyocytes may also result from MR gaining access to DNA response elements normally occupied by GR and/or potentially by the loss of proposed GR/MR heterodimers.
Little is known about the specific genes and signaling pathways regulated by glucocorticoid-activated MR in healthy or diseased hearts. In our cardioMRKO mice, loss of MR resulted in the dysregulation of 449 genes in 1-month old hearts and 1691 genes in 2-month old hearts. Most of these gene changes were specific to the loss of MR because they were not commonly altered in the cardioGRKO hearts. That MR and GR may regulate many unique genes in the heart is consistent with results from gene expression profiling experiments performed in mice with cardiomyocyte specific overexpression of human MR or human GR that identified few commonly regulated genes (33). Global gene changes have also been examined in hearts from mice with conditional inactivation of cardiomyocyte MR that were generated by the Hein laboratory (11). Compared to our studies, fewer cardiac genes (115) were altered but this reduction likely reflects the application of a fold-change cutoff by their laboratory for identifying differentially expressed genes. Consistent with our findings in the cardioMRKO heart, many gene changes (865) were observed in hearts from mice with cardiomyocyte specific overexpression of human MR (34). Collectively, these studies indicate that alterations in the expression of MR can have a major impact on the gene expression profile in cardiomyocytes.
Results from our microarrays revealed aberrant regulation of Ca2+ handling genes, oxidative stress genes, and cell death genes that occurred primarily in the cardioGRKO hearts. The dysregulation of a large cohort of cell death genes in the cardioGRKO hearts is particularly intriguing as a causative agent of the pathology because glucocorticoids can protect cardiomyocytes from apoptosis (35, 36). The absence of many of these gene changes in the double knockout hearts suggests MR signaling may contribute to their regulation in the cardioGRKO hearts. The microarray analysis also identified genes that were uniquely regulated in the double knockout hearts, such as Agt, Ccnd2, Hdac4, and Ankrd23. Compared to the single knockout hearts, Agt expression was reduced and Ccnd2, Hdac4, and Ankrd23 expression was increased in hearts lacking both receptors. Each of these gene changes has been reported to inhibit the hypertrophic response of cardiomyocytes (22, 23, 28, 29). The cardioGRMRdKO hearts developed pathological cardiac hypertrophy as evidenced by the reactivation of the fetal cardiac gene program; however, LV hypertrophy was smaller in magnitude and slower to develop than observed in the cardioGRKO hearts. These data suggest that the alterations in Agt, Ccnd2, Hdac4, and Ankrd23 limit the extent and progression of the hypertrophic response in the cardioGRMRdKO hearts. By restraining cardiac hypertrophy, these gene changes may prolong the transition from adaptive cardiac hypertrophy to maladaptive remodeling and thereby sustain heart function and improve survival of the double knockout mice. Re-expression of MR into cardiomyocytes of the double knockout mice reversed the gene changes in Agt, Ccnd2, and Hdac4 and resulted in LV systolic dysfunction. This finding not only establishes a critical role for these genes and their signaling pathways in protecting the double knockout heart from disease but also suggests that Agt, Ccnd2, and Hdac4 are targeted by MR under conditions of pathological stress (as occurs in the cardioGRKO hearts) to limit changes in their expression that would otherwise be cardioprotective. Re-expression of MR did not significantly reverse the change in Ankrd23 expression, suggesting additional factors may be involved in its regulation by MR. Clearly, more work is needed to define the genes that are primary targets for MR regulation and to elucidate whether glucocorticoids and/or aldosterone control their regulation through this receptor. Additionally, it will be important to determine the cardiac MR transcriptome both in the absence and presence of myocardial stress to identify the full set of genes regulated by MR that contribute to the pathogenesis of heart failure.
Data from our knockout mice support a model in which a deficiency in cardiomyocyte GR signaling initially stresses the myocardium (Fig. 10). Gene changes that impair Ca2+ handling and limit cell survival likely contribute to this cardiac insult. In the presence of this myocardial stress and consequent alteration in redox status, cardiomyocyte MR signaling becomes deleterious in the cardioGRKO heart and exacerbates the disease both by regulating additional genes in these pathways that promote further pathology and by limiting compensatory gene changes that are cardioprotective. Consequently, the cardioGRKO heart fails by 6 months of age. For the cardioGRMRdKO heart, the loss of MR signaling limits the extent of the myocardial stress and confers protection through gene changes that are cardioprotective. Although the cardioGRMRdKO hearts exhibit normal LV systolic function through 6-months of age, the hearts do show some evidence of pathology such as the reactivation of the fetal gene program, cardiac hypertrophy, and inflammation. This finding suggests that the double knockout hearts may eventually develop LV systolic dysfunction that is similar to that observed in the cardioGRKO hearts but at a later time point. Alternatively, hearts lacking both cardiomyocyte GR and MR may exhibit heart failure with preserved ejection fraction (HFpEF). HFpEF accounts for approximately 50% of all diagnoses of heart failure, is growing in prevalence, and has a poor prognosis (37, 38). The molecular mechanisms responsible for HFpEF are poorly understood which has limited the development of effective therapies to combat this disease. HFpEF is associated with diastolic dysfunction; therefore, it will be important for future studies to evaluate whether the unique gene expression profile in the double knockout heart leads to abnormal diastolic function. It should be noted that lung congestion is a characteristic of HFpEF and we detected no alteration in lung weight or lung weight/body weight ratio at baseline in cardioGRMRdKO mice that were 6-months old. Testing for exercise intolerance, however, may reveal impaired lung function if present in these mice. MR blockade is beneficial in heart failure with reduced ejection fraction but not HFpEF (38).
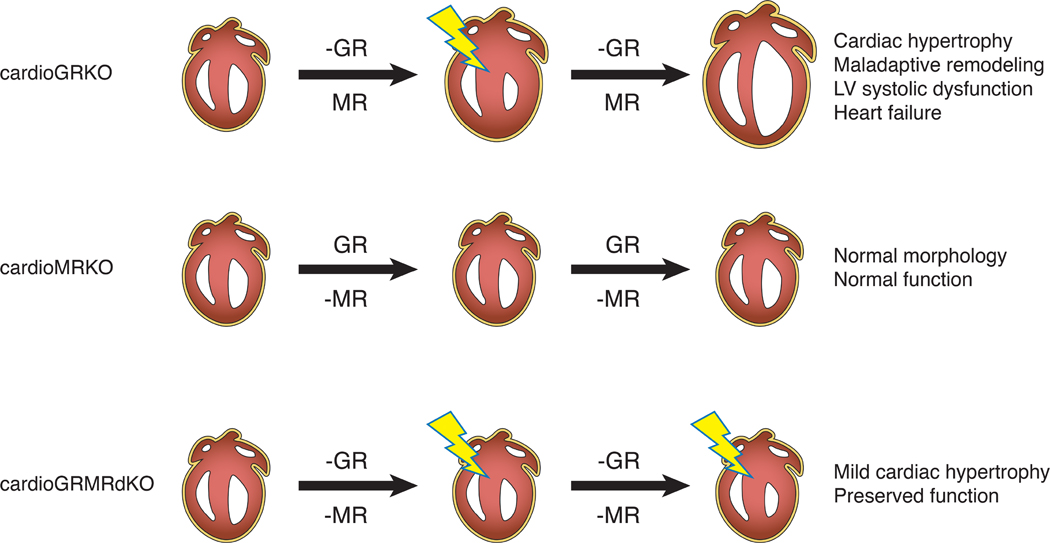
Figure 10. Cardiomyocyte GR and MR signaling and heart disease.
Findings from our genetic mouse models suggest that both insufficient cardiomyocyte GR signaling and deleterious cardiomyocyte MR signaling contribute to heart disease. A deficiency in cardiomyocyte GR signaling alone (-GR) leads to myocardial stress (lightning bolt) and mild hypertrophy in both the cardioGRKO and cardioGRMRdKO hearts. In contrast, a deficiency in cardiomyocyte MR signaling alone (-MR) does not have an overt effect. In the cardioGRKO hearts, cardiomyocyte MR signaling becomes deleterious and exacerbates the hypertrophic response leading to maladaptive remodeling and heart failure. In the cardioGRMRdKO hearts, the mild cardiac hypertrophy triggered by the loss of cardiomyocyte GR signaling is not exacerbated in the absence of cardiomyocyte MR signaling and heart function is preserved for a longer period of time.
Multiple studies have revealed an important role for circulating glucocorticoids in the regulation of heart function though they have not discriminated between direct and systemic actions of these hormones (39, 40). Results from our cardioGRKO, cardioMRKO, and cardioGRMRdKO mouse models suggest that an appropriate amount of cardiomyocyte glucocorticoid signaling through both GR and MR is critical for normal heart homeostasis. Disrupting the balance of these signaling pathways to favor less GR signaling and more MR signaling leads to cardiac hypertrophy, LV systolic dysfunction, and heart failure. A limitation to our studies is that they were performed in male mice. Because sex differences in cardiac physiology and pathology are well recognized (41), an important goal of future studies will be to evaluate hearts from female mice that lack GR, MR, or both GR and MR in cardiomyocytes. In addition, our functional studies focused on the LV whereas gene changes were evaluated in the whole heart. Assessing gene changes and function in a chamber-specific manner will facilitate stronger mechanistic links between deficient glucocorticoid signaling and pathology. Finally, although our current studies were confined to mice, several lines of evidence suggest that alterations in the balance of glucocorticoid signaling contributes to human heart disease. For example, epidemiological studies on a polymorphism of the GR gene (A3669G) that results in increased expression of a dominant negative receptor variant that antagonizes GR signaling and is associated with glucocorticoid resistance have established a link between reduced GR signaling and pathologies of the heart (42–44). Persons with the A3669G polymorphism have an increased risk of coronary artery disease, enlarged hearts, systolic dysfunction, and heart failure. In addition, patients with congestive heart failure have increased levels of cardiac MR but no change in GR expression (45, 46). The expected outcome in both these pathological scenarios would be enhanced MR signaling at the expense of GR signaling.
In summary, we have developed mice lacking both GR and MR in the heart and discovered that these mice are protected from the cardiac hypertrophy, LV systolic dysfunction, and heart failure that spontaneously develop in mice lacking GR alone in the heart. Gene changes resulting from the loss of GR signaling and rise of deleterious MR signaling appear to act in concert to promote the development and progression of cardiac disease in the cardioGRKO hearts. The design of novel compounds that alter the balance of glucocorticoid signaling in cardiomyocytes by selectively activating GR and antagonizing MR may provide an improved therapeutic approach for treating heart failure.
Materials and Methods
Generation of cardioGRKO, cardioMRKO, and cardioGRMRdKO mice
Mice with floxed GR locus (GRfl/fl) have been described previously and were generated by blastocyst (albino B6) injection of recombined C57Bl/6 embryonic stem cells followed by chimera breeding with C57Bl/6 mice (6). The resulting GRfl/+ offspring were backcrossed for 7 generations into C57Bl/6 mice to generate the GRfl/fl mice used in this study. Mice with floxed MR locus (MRfl/fl) on a C57Bl/6 background have been described previously and are available upon request from Pierre Chambon (47). GRfl/fl mice were crossed with MRfl/fl mice to generate double floxed mice (GRfl/flMRfl/fl). GRfl/fl, MRfl/fl, and GRfl/flMRfl/fl mice were each mated with cardiomyocyte-specific αMHCCre/+ mice on a C57Bl/6 background (The Jackson Laboratory, 011038) to generate GRfl/flαMHCCre/+ (cardioGRKO), MRfl/flαMHCCre/+ (cardioMRKO), and GRfl/flMRfl/flαMHCCre/+ (cardioGRMRdKO) mice. Cre negative GRfl/flαMHC+/+ (GRflox), MRfl/flαMHC+/+ (MRflox), and GRfl/flMRfl/flαMHC+/+ (dflox) littermate mice served as controls. All mice were on a C57BL/6NJ background. Data presented are from male mice. Genotypes were determined by PCR using DNA from tail or ear biopsies by standard procedures. Primers for the floxed GR allele were forward primer 5’-GGATTATAGGCATGCACAATTACGGC-3’ and reverse primer 5’-CTTCTCATTCCATGTCAGCATGTTCAC-3’. Primers for the floxed MR allele were forward primer 5’-GGAGATCGTACAAACATACGAACAGC-3’ and reverse primer 5’-CTGTGATGCGCTCGGAAACGG-3’. Primers for Cre were forward primer 5’-ATGACAGACAGATCCCTCCTATCTCC-3’ and reverse primer 5’-CTCATCACTCGTTGCATCGAC-3’. Genotypes were also determined using real time PCR with specific probes designed for each gene (Transnetyx). All experiments on mice were approved and performed according to the guidelines of the Animal Care and Use Committee at the National Institute of Environmental Health Sciences (NIEHS).
Measurement of heart weight/body weight ratio.
Mice were weighed and then sacrificed. Hearts were rapidly removed, trimmed to remove major blood vessels, sectioned, blotted, and then weighed.
Real-time PCR
Total RNA was isolated from whole hearts of mice and individual mRNA abundance was quantified on a 7900HT (Applied Biosystems) or CFX96 (BioRad) sequence detection system. All primer/probe sets were purchased from Applied Biosystems. Relative expression values for each gene were calculated using the double delta Ct analysis method and the housekeeping gene peptidylprolyl isomerase B (Ppib).
Systolic blood pressure
Systolic blood pressure was measured in conscious mice that were approximately 10 weeks old using the non-invasive tail cuff method (4-channel CODA, Kent Scientific). Blood pressure recordings were made for each individual mouse on at least 2 different days. For each session, 5 acclimatization cycles were followed by 20 blood pressure measurement cycles. Reported values for each mouse represent the average of at least 20 measurement cycles.
Immunoblotting
Mouse heart samples were lysed in sodium dodecyl sulfate (SDS) sample buffer. Nitrocellulose membranes with equivalent amounts of protein were analyzed using the Odyssey Infrared Imaging System (LI-COR Biosciences). Primary antibodies were to GR (Cell Signaling, #3660), MR (generously provided by Dr. Celso E. Gomez-Sanchez) (48), green fluorescent protein (GFP) (Cell Signaling, #2956), actin (Millipore, #MAB1501), and Gapdh (Abcam, #ab9485). Goat anti-rabbit Alexa Fluor 680-conjugated (Life Technologies, #A21109) and goat anti-mouse IRDye800-conjugated (LI-COR Biosciences, #926–32210) secondary antibodies were utilized.
Survival Analysis
Control and knockout mice were monitored on a daily basis for morbidity or death until 9 months of age.
Echocardiographic analysis
Transthoracic echocardiography was performed on conscious mice using a Vevo 770 ultrasound biomicroscopy system (VisualSonics) with a 30MHz 707B scan head (6). Two-dimensional guided M-mode analysis of the LV was performed in the parasternal long-axis at the level of the papillary muscle. M-mode measurements from each mouse represent the average of three cardiac cycles.
Electrocardiographic analysis
Electrocardiographs were recorded in conscious mice non-invasively using the ECGenie system (Mouse Specifics) (49–51). Prior to recordings, mice were placed on instrument platform and acclimated for 10 minutes. Data were analyzed using Mouse Specifics software.
Histological analysis
Hearts were removed from mice as a heart-lung pluck and fixed in freshly prepared 4% paraformaldehyde for 48 hours. Following fixation, the hearts were incubated in 70% ethanol for 24 hours, transferred to PBS, trimmed, and processed for paraffin embedding. Serial 5-micron sections were stained with hematoxylin-eosin (H&E) or Masson’s Trichrome using standard protocols. For measurement of LV cardiomyocyte cross-sectional area, heart sections from 2-month old mice were deparaffinized, rehydrated, and processed for antigen retrieval using a citrate-based antigen unmasking solution (Vector Laboratories). Sections were incubated overnight with FITC-labeled lectin (Sigma, #L-4895) and Alexa Fluor 647 mouse anti-cardiac troponin T antibody (BD Biosciences, #565744). Slides were mounted using VECTASHIELD antifade mounting medium with DAPI (Vector Laboratories). Multiple images (10–12 per heart) were captured using a laser confocal microscope (Zeiss LSM 880 with AiryScan) along the LV free wall, apex, and interventricular septum. Cardiomyocytes with clear cardiac troponin T and membrane staining, a central nucleus, and adjacent round capillaries were included in the analysis. The area of selected cardiomyocytes was determined using NIH Image J (FIJI) software. The cross-sectional area of cardiomyocytes in the RV of 6-month old cardioGRMRdKO mice was measured exactly as described above except that multiple images were captured along the RV free wall. The TUNEL assay was performed on heart sections using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Millipore) according to the manufacturer’s instructions. After the incubation with anti-digoxigenin conjugate, sections were stained wheat germ agglutinin (WGA) Alexa Fluor 594 conjugate (Invitrogen, #W11262) and Alexa Fluor 647 mouse anti-cardiac troponin T antibody (BD Biosciences, #565744). Slides were mounted using VECTASHIELD antifade mounting medium with DAPI. TUNEL-positive nuclei were counted throughout the left ventricular free wall, apex, and interventricular septum using a fluorescent microscope, and the number of TUNEL-positive nuclei is presented per millimeter square of LV tissue area. The area of the left ventricular wall which was determined using NIH Image J (FIJI) software. To evaluate specificity of the AAV-Tnnt2-GFP construct for cardiomyocytes, frozen heart sections from mice injected with PBS or AAV-Tnnt2-GFP were stained with Alexa Fluor 647 mouse anti-cardiac troponin T antibody (BD Biosciences, #565744). Slides were mounted using VECTASHIELD antifade mounting medium with DAPI. Images were captured using a laser confocal microscope (Zeiss LSM 880 with AiryScan).
Microarray analysis
Global gene expression analysis was performed on RNA isolated from whole hearts (from 3–4 mice per group) from 1-month old GRflox, cardioGRKO, MRflox, cardioMRKO, dflox, and cardioGRMRdKO mice and from 2-month old GRflox, cardioGRKO, MRflox, cardioMRKO, dflox, and cardioGRMRdKO mice. The Agilent Whole Mouse Genome oligo arrays (014868) (Agilent Technologies) was used following the Agilent 1-color microarray-based gene expression analysis protocol as described previously (52, 53). Data was obtained using the Agilent Feature Extraction software (v9.5), using the 1-color defaults for all parameters. The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. To determine differentially expressed probes, an ANOVA with multiple test correction (FDR q-value) was performed using Partek Genomics Suite software, version 6.6 (Partek). Since only a small number of genes passed the FDR q-value < 0.05 cutoff at the 1-month time point, we expanded the set of differentially expressed genes to include those from the ANOVA analysis with an unadjusted p-value < 0.01. The stringent p < 0.01 cutoff was chosen to minimize the inclusion of potential false positives. Heat maps of differentially expressed genes were generated using hierarchical clustering in Partek Genomics Suit software, version 7.18 (Partek). Significantly regulated genes were analyzed by Ingenuity Pathway Analysis software (Ingenuity Systems). Gene enrichment P values (p-value < 0.05) were determined by IPA using Fisher’s exact test. Validation of differentially expressed genes by RTPCR was performed on RNA isolated from whole hearts from an independent set of mice.
Adeno-associated virus gene delivery
Adeno-associated virus constructs containing GFP (AAV-Tnnt2-GFP) or mouse MR tagged at the amino terminus with FLAG epitope (AAV-Tnnt2-MR) were generated by standard cloning procedures and utilize the cardiac troponin T (Tnnt2) promoter to drive expression of GFP or MR specifically in cardiomyocytes (54, 55). Vector DNA was packaged into AAV9 particles and purified as described previously (56). At approximately 4–6 weeks of age, dflox and cardioGRMRdKO mice were injected intravenously via the retro-orbital venous sinus with 0.1 ml of solution containing PBS, AAV-Tnnt2-GFP (dosage = 5 × 1011 vg), or AAV-Tnnt2-MR (dosage = 5 × 1011 vg). Specificity of the AAV-Tnnt2-GFP for cardiomyocytes was confirmed by immunoblot and histology. Echocardiographic measurements were made on mice at 6 months of age. Following the 6-month echocardiography, mice were sacrificed and hearts removed for isolation of RNA and protein.
Statistical analysis
A student’s t-test (two-tailed) or one-way ANOVA with Tukey’s post hoc analysis was used to evaluate whether differences between groups were statistically significant (defined as p-value < 0.05). The Mantel-Cox log-rank test was used with a Bonferroni corrected threshold for survival curves. The statistical analyses were performed using GraphPad Prism software (version 7.02).
Supplementary Material
Fig. S1. Knockout of GR and/or MR is specific to the heart.
Fig. S2. Systolic blood pressure in cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Fig. S3. Histological analysis of cardioGRKO and cardioGRMRdKO hearts.
Fig. S4. CardioGRMRdKO mice exhibit RV hypertrophy but no lung edema at 6 months of age.
Fig. S5. Genes associated with cardiac pathology are dysregulated in cardioGRMRdKO hearts.
Fig. S6. Global gene expression profile in 2-month old cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Fig. S7. Heat maps of differentially expressed genes in the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Fig. S8. Dysregulation of “Cell Death and Surviva” genes in the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Fig. S9. CardioGRMRdKO hearts are protected from the cell death observed in cardioGRKO hearts.
Fig. S10. AAV constructs utilizing the Tnnt2 promoter are expressed specifically in cardiomyocytes.
Table S1. Electrocardiographic analysis of cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Table S2. Echocardiography on 3-month old αMHC-Cre, cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Table S3. Echocardiography on 1-month old cardioGRKO mice.
Table S4. Echocardiography on 6-month old αMHC-Cre, cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Table S5. Echocardiography on cardioGRMRdKO mice injected with AAV-Tnnt2-MR.
Data File S1. Differentially expressed genes in 1-month cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Data File S2. Differentially expressed genes in 2-month cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Acknowledgments:
We thank Dr. Kevin Gerrish of the Molecular Genomics core laboratory (NIEHS) for assistance with microarray analyses. We thank Jeff Tucker of the Fluorescence Microscopy and Imaging Center (NIEHS) for assistance with confocal microscopy and quantitation of cardiomyocyte cross-sectional area and TUNEL-positive nuclei in the LV. We thank Dr. Tanasa S. Osborne and Dr. David E. Malarkey of the Cellular and Molecular Pathology Branch (NIEHS) for assistance with pathology.
Funding: This research was supported by the Intramural Research Program of the NIH, NIEHS.
Footnotes
Competing interests: The authors have declared that no conflict of interest exists.
Data and materials availability: The microarray data have been deposited in the NCBI Gene Expression Omnibus (GSE95737). All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. The MRflox mice require a material transfer agreement from Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), Illkirch, CU. de Strasbourg, France. The GRflox, dflox, cardioGRKO, cardioMRKO, and cardioGRMRdKO mice require a material transfer agreement from NIEHS/NIH, U.S.A.
References and Notes:
- 1.Brotman DJ, Golden SH, Wittstein IS, The cardiovascular toll of stress. Lancet 370, 1089–1100 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Steptoe A, Kivimaki M, Stress and cardiovascular disease. Nature reviews. Cardiology 9, 360–370 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Kivimaki M, Pentti J, Ferrie JE, Batty GD, Nyberg ST, Jokela M, Virtanen M, Alfredsson L, Dragano N, Fransson EI, Goldberg M, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Luukkonen R, Oksanen T, Rugulies R, Siegrist J, Singh-Manoux A, Suominen S, Theorell T, Vaananen A, Vahtera J, Westerholm PJM, Westerlund H, Zins M, Strandberg T, Steptoe A, Deanfield J, consortium IP-W, Work stress and risk of death in men and women with and without cardiometabolic disease: a multicohort study. Lancet Diabetes Endocrinol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart RAH, Colquhoun DM, Marschner SL, Kirby AC, Simes J, Nestel PJ, Glozier N, O’Neil A, Oldenburg B, White HD, Tonkin AM, Investigators LS, Persistent psychological distress and mortality in patients with stable coronary artery disease. Heart 103, 1860–1866 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Oakley RH, Cidlowski JA, Glucocorticoid signaling in the heart: A cardiomyocyte perspective. J Steroid Biochem Mol Biol 153, 27–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakley RH, Ren R, Cruz-Topete D, Bird GS, Myers PH, Boyle MC, Schneider MD, Willis MS, Cidlowski JA, Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc Natl Acad Sci U S A 110, 17035–17040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, Michailidou Z, Richardson J, Owen E, Watt A, Morrison H, Forrester LM, Bhattacharya S, Holmes MC, Chapman KE, Glucocorticoid receptor is required for foetal heart maturation. Human molecular genetics 22, 3269–3282 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Richardson RV, Batchen EJ, Thomson AJ, Darroch R, Pan X, Rog-Zielinska EA, Wyrzykowska W, Scullion K, Al-Dujaili EA, Diaz ME, Moran CM, Kenyon CJ, Gray GA, Chapman KE, Glucocorticoid receptor alters isovolumetric contraction and restrains cardiac fibrosis. J Endocrinol 232, 437–450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg B, Zannad F, Pitt B, Role of aldosterone blockade for treatment of heart failure and post-acute myocardial infarction. The American journal of cardiology 97, 34F–40F (2006). [DOI] [PubMed] [Google Scholar]
- 10.Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schutz G, Frantz S, Ertl G, Bauersachs J, Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 123, 400–408 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Lother A, Berger S, Gilsbach R, Rosner S, Ecke A, Barreto F, Bauersachs J, Schutz G, Hein L, Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension 57, 746–754 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Rickard AJ, Morgan J, Bienvenu LA, Fletcher EK, Cranston GA, Shen JZ, Reichelt ME, Delbridge LM, Young MJ, Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 60, 1443–1450 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Molkentin JD, Robbins J, With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol 46, 130–136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirkx E, da Costa Martins PA, De Windt LJ, Regulation of fetal gene expression in heart failure. Biochim Biophys Acta 1832, 2414–2424 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara K, Nishikimi T, Nakao K, Transcriptional regulation of the fetal cardiac gene program. Journal of pharmacological sciences 119, 198–203 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Olson EN, A decade of discoveries in cardiac biology. Nature medicine 10, 467–474 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Funder JW, Mineralocorticoid receptor activation and oxidative stress. Hypertension 50, 840–841 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Funder JW, Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integrated blood pressure control 6, 129–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihailidou AS, Loan Le TY, Mardini M, Funder JW, Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension 54, 1306–1312 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Rossier MF, Lenglet S, Vetterli L, Python M, Maturana A, Corticosteroids and redox potential modulate spontaneous contractions in isolated rat ventricular cardiomyocytes. Hypertension 52, 721–728 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Bader M, Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annual review of pharmacology and toxicology 50, 439–465 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Kang N, Walther T, Tian XL, Bohlender J, Fukamizu A, Ganten D, Bader M, Reduced hypertension-induced end-organ damage in mice lacking cardiac and renal angiotensinogen synthesis. Journal of molecular medicine 80, 359–366 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Busk PK, Hinrichsen R, Bartkova J, Hansen AH, Christoffersen TE, Bartek J, Haunso S, Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Experimental cell research 304, 149–161 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Riviere AB, Doevendans PA, Field LJ, Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res 78, 18–25 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ, Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 96, 110–118 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Yamak A, Temsah R, Maharsy W, Caron S, Paradis P, Aries A, Nemer M, Cyclin D2 rescues size and function of GATA4 haplo-insufficient hearts. Am J Physiol Heart Circ Physiol 303, H1057–1066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backs J, Olson EN, Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98, 15–24 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN, Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma C, Ying Y, Zhang T, Zhang W, Peng H, Cheng X, Xu L, Tong H, Establishment of a prediction model of changing trends in cardiac hypertrophy disease based on microarray data screening. Exp Ther Med 11, 1734–1740 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, Smith GL, Chapman KE, Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1alpha. Cell Death Differ, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokudome S, Sano M, Shinmura K, Matsuhashi T, Morizane S, Moriyama H, Tamaki K, Hayashida K, Nakanishi H, Yoshikawa N, Shimizu N, Endo J, Katayama T, Murata M, Yuasa S, Kaneda R, Tomita K, Eguchi N, Urade Y, Asano K, Utsunomiya Y, Suzuki T, Taguchi R, Tanaka H, Fukuda K, Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J Clin Invest 119, 1477–1488 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshikawa N, Nagasaki M, Sano M, Tokudome S, Ueno K, Shimizu N, Imoto S, Miyano S, Suematsu M, Fukuda K, Morimoto C, Tanaka H, Ligand-based gene expression profiling reveals novel roles of glucocorticoid receptor in cardiac metabolism. Am J Physiol Endocrinol Metab 296, E1363–1373 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Latouche C, Sainte-Marie Y, Steenman M, Castro Chaves P, Naray-Fejes-Toth A, Fejes-Toth G, Farman N, Jaisser F, Molecular signature of mineralocorticoid receptor signaling in cardiomyocytes: from cultured cells to mouse heart. Endocrinology 151, 4467–4476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messaoudi S, Gravez B, Tarjus A, Pelloux V, Ouvrard-Pascaud A, Delcayre C, Samuel J, Launay JM, Sierra-Ramos C, Alvarez D. de la Rosa, Clement K, Farman N, Jaisser F, Aldosterone-specific activation of cardiomyocyte mineralocorticoid receptor in vivo. Hypertension 61, 361–367 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Topete D, He B, Xu X, Cidlowski JA, Kruppel-like Factor 13 Is a Major Mediator of Glucocorticoid Receptor Signaling in Cardiomyocytes and Protects These Cells from DNA Damage and Death. J Biol Chem 291, 19374–19386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren R, Oakley RH, Cruz-Topete D, Cidlowski JA, Dual Role for Glucocorticoids in Cardiomyocyte Hypertrophy and Apoptosis. Endocrinology, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM, Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure With Preserved Ejection Fraction. Circulation 138, 198–205 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Plitt GD, Spring JT, Moulton MJ, Agrawal DK, Mechanisms, diagnosis, and treatment of heart failure with preserved ejection fraction and diastolic dysfunction. Expert review of cardiovascular therapy 16, 579–589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz-Topete D, Myers PH, Foley JF, Willis MS, Cidlowski JA, Corticosteroids Are Essential for Maintaining Cardiovascular Function in Male Mice. Endocrinology 157, 2759–2771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Udelsman R, Ramp J, Gallucci WT, Gordon A, Lipford E, Norton JA, Loriaux DL, Chrousos GP, Adaptation during surgical stress. A reevaluation of the role of glucocorticoids. J Clin Invest 77, 1377–1381 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA, The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ Res 118, 1294–1312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geelhoed JJ, van Duijn C, van Osch-Gevers L, Steegers EA, Hofman A, Helbing WA, Jaddoe VW, Glucocorticoid receptor-9beta polymorphism is associated with systolic blood pressure and heart growth during early childhood. The Generation R Study. Early human development 87, 97–102 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Otte C, Wust S, Zhao S, Pawlikowska L, Kwok PY, Whooley MA, Glucocorticoid receptor gene, low-grade inflammation, and heart failure: the Heart and Soul study. J Clin Endocrinol Metab 95, 2885–2891 (2010). [DOI] [PubMed] [Google Scholar]
- 44.van den Akker EL, Koper JW, van Rossum EF, Dekker MJ, Russcher H, de Jong FH, Uitterlinden AG, Hofman A, Pols HA, Witteman JC, Lamberts SW, Glucocorticoid receptor gene and risk of cardiovascular disease. Arch Intern Med 168, 33–39 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Chai W, Hofland J, Jansen PM, Garrelds IM, de Vries R, van den Bogaerdt AJ, Feelders RA, de Jong FH, Danser AH, Steroidogenesis vs. steroid uptake in the heart: do corticosteroids mediate effects via cardiac mineralocorticoid receptors? Journal of hypertension 28, 1044–1053 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Yoshida M, Ma J, Tomita T, Morikawa N, Tanaka N, Masamura K, Kawai Y, Miyamori I, Mineralocorticoid receptor is overexpressed in cardiomyocytes of patients with congestive heart failure. Congestive heart failure 11, 12–16 (2005). [DOI] [PubMed] [Google Scholar]
- 47.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ, Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nature medicine 18, 1429–1433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP, Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147, 1343–1348 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Chu V, Otero JM, Lopez O, Morgan JP, Amende I, Hampton TG, Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol 1, 6 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuldt AJ, Hampton TJ, Chu V, Vogler CA, Galvin N, Lessard MD, Barker JE, Electrocardiographic and other cardiac anomalies in beta-glucuronidase-null mice corrected by nonablative neonatal marrow transplantation. Proc Natl Acad Sci U S A 101, 603–608 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing S, Tsaih SW, Yuan R, Svenson KL, Jorgenson LM, So M, Paigen BJ, Korstanje R, Genetic influence on electrocardiogram time intervals and heart rate in aging mice. Am J Physiol Heart Circ Physiol 296, H1907–1913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duma D, Collins JB, Chou JW, Cidlowski JA, Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal 3, ra74 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galliher-Beckley AJ, Williams JG, Cidlowski JA, Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol 31, 4663–4675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konkalmatt PR, Beyers RJ, O’Connor DM, Xu Y, Seaman ME, French BA, Cardiac-selective expression of extracellular superoxide dismutase after systemic injection of adeno-associated virus 9 protects the heart against post-myocardial infarction left ventricular remodeling. Circulation. Cardiovascular imaging 6, 478–486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prasad KM, Xu Y, Yang Z, Acton ST, French BA, Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene therapy 18, 43–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He B, Cruz-Topete D, Oakley RH, Xiao X, Cidlowski JA, Human Glucocorticoid Receptor beta Regulates Gluconeogenesis and Inflammation in Mouse Liver. Mol Cell Biol 36, 714–730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Knockout of GR and/or MR is specific to the heart.
Fig. S2. Systolic blood pressure in cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Fig. S3. Histological analysis of cardioGRKO and cardioGRMRdKO hearts.
Fig. S4. CardioGRMRdKO mice exhibit RV hypertrophy but no lung edema at 6 months of age.
Fig. S5. Genes associated with cardiac pathology are dysregulated in cardioGRMRdKO hearts.
Fig. S6. Global gene expression profile in 2-month old cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Fig. S7. Heat maps of differentially expressed genes in the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Fig. S8. Dysregulation of “Cell Death and Surviva” genes in the cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Fig. S9. CardioGRMRdKO hearts are protected from the cell death observed in cardioGRKO hearts.
Fig. S10. AAV constructs utilizing the Tnnt2 promoter are expressed specifically in cardiomyocytes.
Table S1. Electrocardiographic analysis of cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Table S2. Echocardiography on 3-month old αMHC-Cre, cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Table S3. Echocardiography on 1-month old cardioGRKO mice.
Table S4. Echocardiography on 6-month old αMHC-Cre, cardioGRKO, cardioMRKO, and cardioGRMRdKO mice.
Table S5. Echocardiography on cardioGRMRdKO mice injected with AAV-Tnnt2-MR.
Data File S1. Differentially expressed genes in 1-month cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.
Data File S2. Differentially expressed genes in 2-month cardioGRKO, cardioMRKO, and cardioGRMRdKO hearts.