Key Points
Question
What is the incidence of nongeographic atrophy (NGA) in eyes treated with anti–vascular endothelial growth factor for neovascular age-related macular degeneration, and how often does it progress to geographic atrophy (GA)?
Findings
In this longitudinal study, the cumulative risk of NGA was 35%, 59%, and 81% at 1-year, 2-year, and 5-year follow-ups, respectively. The cumulative risk of progression from incident NGA in years 1 and 2 to GA was 29%, 43%, and 50% at 1, 3, and 4 years, respectively.
Meaning
Nongeographic atrophy, as defined in CATT, is common and progresses to GA in about 50% of participants by 4 years after onset.
This post hoc analysis of a cohort study within the Comparison of Age-Related Treatments Trials (CATT) clinical trial seeks to determine incidence and progression of and risk factors for nongeographic atrophy in eyes treated with anti–vascular endothelial growth factor for neovascular age-related macular degeneration.
Abstract
Importance
Retinal hypopigmentation and hyperpigmentation are precursors of geographic atrophy (GA). Incidence and progression to GA in eyes treated with anti–vascular endothelial growth factor for neovascular age-related macular degeneration (nAMD) have not been investigated.
Objective
To determine the incidence and progression of non-GA (NGA) and associated risk factors.
Design, Setting, and Participants
This study is a post hoc analysis of a cohort study within the Comparison of Age-Related Treatments Trials (CATT) clinical trial. Participants were recruited February 20, 2008, through December 9, 2009; released from protocol follow-up and treatment after 2 years; and recalled from March 14, 2014, through March 31, 2015. Data analyses were conducted from January 11, 2019, through November 27, 2019.
Interventions
Participants were randomized to ranibizumab or bevacizumab for (1) 2 years of monthly or as-needed injections or (2) monthly injections for 1 year and as-needed injections the following year. Participants were treated according to best medical judgement thereafter.
Main Outcomes and Measures
Incidence of nAMD-associated NGA (hypopigmentation and hyperpigmentation in color images) and progression; adjusted risk ratios (aRR) for baseline characteristics.
Results
Among 1107 participants, risk of NGA was 35% (391 eyes), 59% (246 eyes), and 81% (122 eyes) at 1, 2, and 5 years, respectively. Risk factors for NGA included worse visual acuity (20/200-20/320: aRR, 1.74 [95% CI, 1.24-2.43], compared with ≤20/40; P = .006), larger neovascularization area (>4 disc areas: aRR, 1.31 [95% CI, 1.01-1.71], compared with ≤1 disc areas; P = .007), switched drug regimen (aRR, 1.28 [95% CI, 1.06-1.54], compared with as-needed injections; P = .02), and single-nucleotide variants Age-Related Maculopathy Susceptibility 2 (ARMS2) (TT variant: relative risk [RR], 1.53 [95% CI, 1.22-1.93]; P = .001) and HtrA Serine Peptidase 1 (HTRA1) (AG variant: RR, 1.23 [95% CI, 1.01-1.48]; AA variant: RR, 1.51 [95% CI, 1.20-1.91]; P = .002). Sub–retinal pigment epithelium thickness was protective (>275 μm: aRR, 0.59 [95% CI, 0.46-0.75], compared with ≤75 μm; P < .001). Among 389 eyes with NGA by 2 years and subsequent color images, risk of progression to GA was 29%, 43%, and 50% at 1, 3, and 4 years, respectively. Risk factors for progression to GA included worse visual acuity (20/200-20/320: aRR, 2.75 [95% CI, 1.54-4.93], compared with ≤20/40; P < .001), worse fellow-eye visual acuity (<20/40: aRR, 1.77 [95% CI, 1.12-2.79], compared with ≥20/40; P = .01), fellow-eye GA (aRR, 1.71 [95% CI, 1.06-2.75]; P = .03), and pseudodrusen in either eye (aRR, 1.65 [95% CI, 1.17-2.34]; P = .005). Subretinal fluid was associated with a decreased risk of progression (aRR, 0.42 [95% CI, 0.28-0.63]; P < .001).
Conclusions and Relevance
In this study, after 2 years of protocol-guided anti–vascular endothelial growth factor treatment for nAMD, more than half of the eyes in the study developed NGA in the location of nAMD. After 3 additional years of regular care, half of them progressed to GA.
Trial Registration
ClinicalTrials.gov Identifier: NCT00593450
Introduction
The neovascular form of age-related macular degeneration (nAMD) is responsible for nearly 90% of the severe central visual acuity (VA) loss (20/200 or worse) associated with AMD. Intravitreal injections of anti–vascular endothelial factor (anti-VEGF) are highly effective during the first 2 years of treatment for nAMD, but long-term results in the absence of protocol-guided management have shown deterioration of mean VA over time.1,2,3,4 The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) and other trials of anti-VEGF use for nAMD treatment have reported on the incidence of, growth of, and vision loss associated with geographic atrophy (GA) in eyes receiving anti-VEGF treatment.5,6,7,8,9,10 Geographic atrophy associated with nAMD is also known as macular atrophy; in this article, we use GA to be consistent with previous reports from our study group.11 There is a pressing need to investigate and better understand precursors to GA development, to develop methods to prevent or delay the occurrence of GA and associated vision loss.
Focal hypopigmentation and hyperpigmentation have been recognized as risk factors in the formation of drusen-associated or nascent GA.11,12,13,14 With minor differences in their definitions, these focal areas of hypopigmentation and hyperpigmentation have been described in previously published literature as incipient GA, retinal pigment epithelium (RPE) atrophy, stippled RPE, atrophic scar, and nongeographic atrophy (NGA).11,14,15,16 However, only a few studies17,18,19,20 have investigated their role in the evolution of nAMD-associated GA (macular atrophy). Eyes with nAMD treated with anti-VEGF often develop fibrotic scars, nonfibrotic scars, NGA, or GA, as observed on stereo color digital images and fluorescein angiograms (FA).21,22,23 Absence of FA or color fundus photographic sequelae in the area of the original choroidal neovascularization (CNV) is rare. The evolution of different types of morphological features in the area of CNV in eyes with nAMD treated with anti-VEGF injections influences VA outcomes, especially if they occur in the foveal region. Visual acuity is reduced considerably when subfoveal fibrotic scarring or GA develops, whereas new NGA has a minimal association with VA.24,25,26 However, there is little information about whether NGA that develops within or overlaps the initial CNV lesion converts to GA and has an adverse effect on VA. This report aims to assess the incidence of and risk factors for NGA and the progression from NGA to GA in the CATT study.
Methods
This is a secondary analysis of the data from the CATT study and the CATT Follow-up Study (CATT-FS). The methods used have been described previously.2,3,4,24,27,28
Enrollment and Follow-up of Participants
Study eyes had untreated active CNV associated with AMD. Either CNV or fluid needed to be at the foveal center. The CATT participants enrolled from 43 clinical centers in the United States between February 2008 and December 2009 were assigned randomly to treatment with intravitreal injections of ranibizumab or bevacizumab and 1 of 3 dosing regimens for the 2 years of the clinical trial. Institutional review boards associated with each center approved the study. The trial adhered to the tenets of the Declaration of Helsinki and complied with Health Insurance Portability and Accountability Act regulations.
Color fundus photographs (CFPs), FA images, optical coherence tomography (OCT) images, and VA measurements were obtained at baseline and 1, 2, and 5 years. At 2 years, participants were released from the clinical trial protocol and continued to receive regular care but were no longer managed by protocol-defined visit schedules.
Assessment of Images, NGA, and GA
Images were graded applying the same methods for all visits.27,28 Independent dual-reader grading was performed by certified CATT readers masked to demographic and treatment assignment. Color fundus photographs and FA images were graded at the University of Pennsylvania, where 2 readers independently graded the images, discrepancies were adjudicated between the readers and the director of the reading center (E.D.), and unresolved discrepancies were reviewed by the principal investigator (J.E.G.). Grading for CFPs and FA features included fluorescein leakage, fibrotic scars, nonfibrotic scars, NGA, and GA. Morphologic features at and away from the foveal center were identified. Optical coherence tomography features were evaluated at the Duke University Reading Center, where 2 readers independently graded the images and discrepancies were arbitrated by a third, independent senior reader. Grading for OCT features included the location of fluid, retinal thickness, subretinal tissue complex, subretinal hyperreflective material, RPE elevation, epiretinal membrane, and vitreomacular attachment.
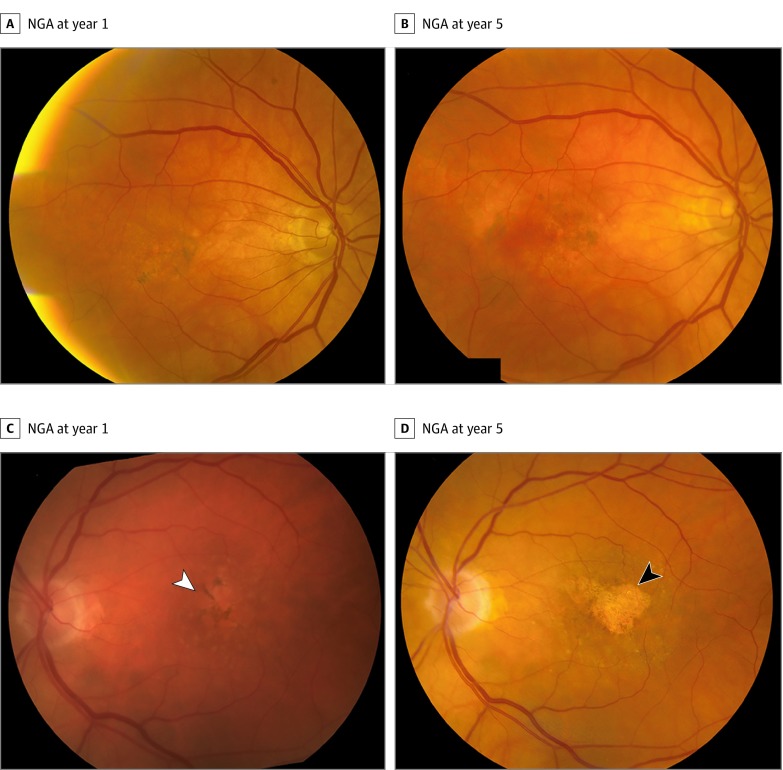
Nongeographic atrophy was defined as retinal pigment disturbances including hypopigmentation and hyperpigmentation in CFPs that typically corresponded to hyperfluorescence and hypofluorescence in FA images within or overlapping areas previously occupied by CNV. In comparison with GA, areas of NGA are less well defined and less regular in shape, with less severe hypopigmentation. When FA showed areas of hyperfluorescence that did not correspond to changes observed in CFPs, the area was considered to be NGA (Figure 1 and eFigure 1 in the Supplement).
Figure 1. Examples of Nongeographic Atrophy (NGA) Evolution in Color Fundus Photographs in the Area of Baseline Choroidal Neovascularization.
A and B, NGA present at year 1 remained present at year 5 without progressing to geographic atrophy (GA). C and D, NGA present at year 1 (C; white arrowhead) progressed to GA at year 5 (D; black arrowhead).
Images at 2 and 5 years or eyes with NGA at year 1 were assessed for scars and GA within or overlapping the area of NGA at year 1. Scars were identified as small or large (occupying >25% of the NGA area at year 1). Geographic atrophy within the macular vascular arcades was defined as 1 or more patches with 25 μm or more in the longest linear dimension of partial or complete depigmentation that had at least 1 of the following: sharply demarcated borders, visibility of underlying choroidal vessels, an excavated or punched-out appearance on stereoscopy, or uniform hyperfluorescence bounded by sharp borders on late FA.5,6,8 The grade-regrade agreement after adjudication for CATT year 2 follow-up visit grading of study eyes (n = 32 patients) with the original grading period between March 31, 2010, to September 30, 2010, and the regrading period between March 16, 2011, to March 18, 2011, showed a 75% agreement (κ, 0.52) for the presence of NGA.
Statistical Analysis
To account for deaths and losses to follow-up, we used the Kaplan-Meier method to estimate cumulative incidence of NGA and progression from first observed NGA to GA. We used univariate and multivariate Cox proportional hazard models to determine the risk factors for incident NGA and the progression from NGA to GA. The risk factors associated with P < .20 from univariate analysis were included in the initial multivariate model, which went through backward variable selection to only keep the statistically significant risk factors in the final multivariate model. From multivariate Cox models, we calculated the adjusted risk ratios (aRR) and their 95% CIs. We also evaluated the association of genetic single-nucleotide variants with NGA through univariate analysis. Multivariate analyses adjusted by age, sex, and smoking status were performed for each of the significant single-nucleotide variants in univariate analysis. All the statistical analyses were performed in SAS version 9.4 (SAS Institute Inc), and 2-sided P < .05 was considered significant.
Results
A total of 1185 participants were initially enrolled in CATT. Of the 914 patients alive approximately 5.5 years after the initiation of CATT, 647 (70.8%) participated in the CATT-FS. At baseline, nonparticipants (compared with participants) were older (mean [SD], 79.8 [7.8] years vs 77.5 [7.3] years; P < .001) and had worse VA (mean [SD] VA, 59.1 [13.6] letters vs 62.2 [13.1] letters; P = .002). At 2 years, nonparticipants had worse VA (mean [SD] VA, 64.3 [19.5] letters vs 69.7 [16.6] letters; P < .001) and fewer injections (in the group receiving as-needed injections) than participants (mean [SD] injections, 11.5 ]7.1] vs 13.3 [6.8]; P = .01).4
Incidence and Risk Factors for NGA
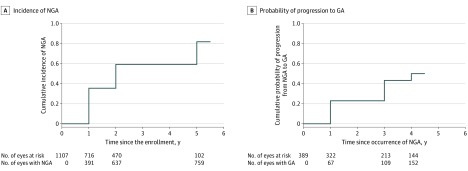
Among 1107 study eyes with gradable photographs available to determine NGA, new NGA was observed in 391 eyes at year 1, 246 eyes at year 2, and 122 eyes at year 5, with cumulative incidence of NGA of 35%, 59%, and 81%, respectively (Figure 2A). In univariate analysis, the risk factors of NGA incidence were increasing age (compared with ages 50-69 years; risk ratio [RR], ≥90 years, 1.60 [95% CI, 1.15-2.24]; P = .03), switched regimen–group status (compared with the as-needed group; RR, 1.28 [95% CI, 1.08-1.53]; P = .01), worse baseline VA in the study eye (compared with 20/25-20/40; RR, 20/200-20/300, 1.68 [95% CI, 1.25-2.26]; P = .007), larger areas of the CNV lesion (compared with ≤1 disc areas; RRs: >1-≤2, 0.86 [95% CI, 0.71-1.06]; <2-≤4 disc areas, 1.13 [95% CI, 0.93-1.36]; >4 disc areas, 1.03 [95% CI, 0.83-1.27]; P = .03), presence of pseudodrusen in the fellow eye (RR, 1.22 [95% CI, 1.03-1.45]; P = .02), and genetic single-nucleotide variants Age-Related Maculopathy Susceptibility 2 (ARMS2) (TT variant: RR, 1.53 [95% CI, 1.22-1.93]; P = .001) and HtrA Serine Peptidase 1 (HTRA1) (compared with GG variant; AG variant: RR, 1.23 [95% CI, 1.01-1.48]; AA variant: RR, 1.51 [95% CI, 1.20-1.91]; P = .002). Increasing subretinal pigment epithelial (RPE) thickness (compared with ≤75 μm; >275 μm: RR, 0.72 [95% CI, 0.58-0.88]; P < .001), any retinal fluid in the foveal center (compared with no fluid; RR, 1.20 [95% CI, 1.00-1.43]; P = .004), and sub-RPE fluid in the foveal center (RR, 0.76 [95% CI, 0.64-0.91]; P = .002) were associated with decreased risk of developing NGA (eTable 1 in the Supplement).
Figure 2. Kaplan-Meier Curves for the Incidence of Nongeographic Atrophy and Probability of Progression From Nongeographic Atrophy (NGA) to Geographic Atrophy (GA).
A, Kaplan-Meier curve for the cumulative incidence of NGA. B, Kaplan-Meier curve for the cumulative probability of progression from to geographic atrophy.
In multivariate analysis (Table 1), the baseline factors associated with increased risk of developing NGA were worse VA in the study eye (compared with a VA of ≤20/40; 20/100-20/160: aRR, 1.28 [95% CI, 1.03-1.60]; 20/200-20/320: aRR, 1.74 [95% CI, 1.24-2.43]; P = .006); larger total areas of baseline CNV (compared with <1 disc area; 2-4 disc areas: aRR, 1.28 [95% CI, 1.05-1.57]; >4 disc areas: aRR, 1.31 [95% CI, 1.01-1.71]; P = .007); and a switched drug regimen (compared with as-needed injections; aRR, 1.28 [95% CI, 1.06-1.54]; P = .02). Increasing sub-RPE thickness (compared with a thickness of ≤75 μm; >275 μm: aRR, 0.59 [95% CI, 0.46-0.75]; P < .001) was associated with a decreased risk of NGA.
Table 1. Multivariate Analysis for the Risk Factors of Nongeographic Atrophy (n = 990)a.
| Baseline risk factor | No. of study eyes | Nongeographic atrophy, No. (%) | Adjusted risk ratio (95% CI) | P value |
|---|---|---|---|---|
| Study-eye visual acuity | ||||
| 20/25-40 | 365 | 251 (68.8) | 1 [Reference] | .006 |
| 20/50-80 | 364 | 246 (67.6) | 1.10 (0.92-1.33) | |
| 20/100-160 | 185 | 131 (70.8) | 1.28 (1.03-1.60) | |
| 20/200-320 | 59 | 45 (76.3) | 1.74 (1.24-2.43) | |
| Total area of CNV (disc areas) | ||||
| ≤1 | 444 | 304 (68.5) | 1 [Reference] | .007 |
| >1-≤2 | 219 | 142 (64.8) | 0.91 (0.74-1.12) | |
| >2-≤4 | 206 | 151 (73.3) | 1.28 (1.05-1.57) | |
| >4 | 104 | 76 (73.1) | 1.31 (1.01-1.71) | |
| Sub-RPE thickness, μm | ||||
| >0-≤75 | 244 | 173 (70.9) | 1 [Reference] | <.001 |
| >75-≤160 | 239 | 177 (74.1) | 1.06 (0.85-1.32) | |
| >160-≤275 | 255 | 189 (74.1) | 0.92 (0.74-1.14) | |
| >275 | 235 | 134 (57.0) | 0.59 (0.46-0.75) | |
| Treatment regimen | ||||
| As needed | 490 | 326 (66.5) | 1 [Reference] | .02 |
| Switched | 244 | 183 (75.0) | 1.28 (1.06-1.54) | |
| Monthly | 239 | 164 (68.6) | 0.97 (0.80-1.18) |
Abbreviations: CNV, choroidal neovascularization; RPE, retinal pigment epithelium.
Seventeen patients with missing data in any of these risk factors in the multivariate model were excluded from analysis.
In multivariate analysis adjusted by age, sex, and smoking status, genetic single-nucleotide variant ARMS2 was significantly associated with increased risk of developing NGA, with an aRR of 1.58 (95% CI, 1.25-1.99) for the TT variant and 1.20 (95% CI, 0.99-1.45) for the GT variant (compared with the GG variant; P < .001). A similar association was found for HTRA1, with an aRR of 1.56 (95% CI, 1.23-1.97) for the AA variant and an aRR of 1.33 (95% CI, 1.02-1.50) for the AG variant (compared with the GG variant; P = .001).
Progression From NGA to GA
Of 391 study eyes with NGA at year 1, 72 study eyes already had GA in the area of the CNV lesion, and 27 did not have any follow-up after year 1. Among the remaining 292 study eyes with NGA, 67 (22.9%) progressed from NGA to GA by year 2. Among the 225 eyes that did not progress to GA at year 2, 122 had year 5 CFPs for GA grading, and 43 of these (35.2%) progressed to GA at year 5 (eFigure 2 in the Supplement). Among 246 eyes with NGA first observed at year 2, 110 study eyes were in patients who did not complete year 5 follow-up, and 19 study eyes already had GA in the area of the CNV lesion. Among the remaining 97 study eyes with year 5 CFPs for GA grading, progression from NGA to GA was observed in 42 eyes (43.3%) at year 5 (eFigure 3 in the Supplement).
Among a total of 389 study eyes with NGA at either year 1 or year 2, 152 progressed from NGA to GA, with a cumulative risk of progression to GA of 29%, 43%, and 50% after 1, 3, and 4 years of the NGA, respectively (Figure 2B). Among the 79 eyes with NGA at year 1 that did not progress to GA by year 5, 63 (80%) still had at least 75% of NGA at year 5. Of the remaining 16 eyes (20%) that had less than 75% of the original NGA at 5 years, 14 eyes developed fibrotic scars (eFigure 4 in the Supplement), and 1 eye each developed a pigmented fibrotic scar and a nonfibrotic scar (eFigure 4 in the Supplement).
A total of 272 study eyes with incident NGA at either year 1 or year 2 had a VA measurement at year 5. When the 122 eyes that had progressed from NGA to GA were compared with the 150 that had not progressed, the eyes that progressed had worse mean VA scores at the time of developing NGA (68 letters vs 72 letters; P = .03) and worse mean VA score at 5 years (54 letters vs 63 letters; P = .002) and tended to have more VA loss between the time of development of NGA and year 5 (mean change, −5.2 letters vs −0.7 letters; P = .10).
In univariate analysis, the baseline factors associated with increased risk of progression from NGA to GA were increasing age (compared with ages 50-69 years; ≥90 years: RRs, 3.47 [95% CI, 1.52-7.91]; P < .001), female sex (male patients: RR, 0.66 [95% CI, 0.46-0.94]; P = .02), worse baseline VA in the study eye (compared with a VA 20/20 or better; ≤20/50: RR, 1.97 [95% CI, 1.28-3.06]; P = .005) as well as the fellow eye (compared with VA 20/25-40; 20/200-20/320: RR, 2.66 [95% CI, 1.53-4.62]; P = .001), retinal angiomatous type of neovascularization (RR, 1.70 [95% CI, 1.09-2.67]; P = .02), fellow-eye GA (RR, 1.89 [95% CI, 1.20-2.95]; P = .006), pseudodrusen in either the study eye or fellow eye (RR, 1.59 [95% CI, 1.13-2.22]; P = .007), GA outside the NGA area at the time that the NGA was identified (RR, 2.19 [95% CI, 1.11-4.31]; P = .02), and presence of intraretinal fluid (compared with no fluid; intraretinal fluid in the foveal center: RR, 1.99 [95% CI, 1.29-3.07]; P = .005) (eTable 2 in the Supplement). Subretinal fluid was associated with a decreased risk of progression from NGA to GA (fluid not in fovea center: RR, 0.49 [95% CI, 0.32-0.73]; fluid in fovea center: RR, 0.38 [95% CI, 0.25-0.59]; P < .001). None of the genetic single-nucleotide variants showed any significant association with progression from NGA to GA. The treatment drug and regimen were not associated with progression from NGA to GA. In multivariate analysis (Table 2), the baseline factors associated with increased risk of progression from NGA to GA were a worse study-eye VA (compared with ≤20/40; 20/100-20/160: aRR, 2.12 [95% CI, 1.35-3.35]; 20/200-20/320: aRR, 2.75 [95% CI, 1.54-4.93]; P < .001), a worse fellow-eye VA (compared with a VA of ≥20/20; 20/25-20/40: aRR, 1.79 [95% CI, 1.19-2.72]; ≤20/40: aRR, 1.77 [95% CI, 1.12-2.79]; P = .01), presence of GA in the fellow eye (aRR, 1.71 [95% CI, 1.06-2.75]; P = .03), and presence of pseudodrusen in either eye (aRR, 1.65 [95% CI, 1.17-2.34]; P = .005). Presence of subretinal fluid was associated with decreased risk of progression from NGA to GA (aRR, 0.42 [95% CI, 0.28-0.63]; P < .001).
Table 2. Multivariate Analysis for the Risk Factors of Progression From Nongeographic Atrophy to Geographic Atrophy (n = 372)a.
| Baseline Risk Factors | Eyes with nongeographic atrophy | Progression to geographic atrophy, No. (%) | Adjusted risk ratio (95% CI) | P value |
|---|---|---|---|---|
| Fellow eye visual acuity | ||||
| 20/20 or better | 109 | 46 (42.2) | 1 [Reference] | .01 |
| 20/25-20/40 | 145 | 64 (44.1) | 1.79 (1.19-2.72) | |
| 20/50 or worse | 118 | 36 (30.5) | 1.77 (1.12-2.79) | |
| Study eye visual acuity | ||||
| 20/25-20/40 | 149 | 51 (34.2) | 1 [Reference] | <.001 |
| 20/50-20/80 | 127 | 48 (37.8) | 1.03 (0.69-1.56) | |
| 20/100-20/160 | 69 | 31 (44.9) | 2.12 (1.35-3.35) | |
| 20/200-20/320 | 27 | 16 (59.3) | 2.75 (1.54-4.93) | |
| Geographic atrophy in fellow eye | ||||
| No | 332 | 124 (37.4) | 1 [Reference] | .03 |
| Yes | 40 | 22 (55.0) | 1.71 (1.06-2.75) | |
| Pseudodrusen in either eye | ||||
| No | 259 | 93 (35.9) | 1 [Reference] | .005 |
| Yes | 113 | 53 (46.9) | 1.65 (1.17-2.34) | |
| Subretinal fluid | ||||
| No | 57 | 35 (61.4) | 1 [Reference] | <.001 |
| Yes | 315 | 111 (35.2) | 0.42 (0.28-0.63) |
Seventeen patients with missing data in any of these risk factors in the multivariate model were excluded from analysis.
Discussion
A high percentage of eyes treated for nAMD with anti-VEGF injections develop NGA in the area of CNV, with one-third having NGA at year 1 and four-fifths by year 5. By 4 years after detection, approximately 50% of the eyes with NGA progressed to GA. Although the mean VA at the time of detection of NGA was relatively good (approximately 20/40), eyes that progressed to GA had a mean VA at the 5-year visit approximately 2 lines worse than eyes with NGA that had not progressed.
Poor VA at baseline was a risk factor for developing NGA and was previously reported as a risk factor for GA8; thus, factors responsible for initially poor VA may contribute to the development of both NGA and GA.8 Baseline poor VA was not identified as a risk factor in the development of fibrotic scars.23 While large sub-RPE thicknesses (>275 μm) are associated with an increased risk of scars, they appear to substantially reduce the risk of developing NGA. These risk factors provide the ophthalmologist with some prognostic knowledge of the likely morphological outcomes.
The minor allele (T) in ARMS2 and the minor allele (A) in HTRA1 were associated with increased risk of NGA. Earlier, we reported a weaker association for the risk of GA.8 This association was absent for the risk of developing scars, suggesting that the genetic factors affecting scar and atrophy formation during anti-VEGF treatment are different.23
Follow-up of the NGA yielded a rate of progression to GA of 23% in year 1, 43% in year 3, and 50% in year 4. Pigment alteration is a strong precursor of GA in eyes without nAMD.12,29 In the Age-Related Eye Disease Study (AREDS), hyperpigmentation preceded GA in 96% of eyes and hypopigmentation in 82% of eyes.15 The period from focal hyperpigmentation appearance to GA onset was around 5 years, and for hypopigmentation, it was 2.5 years. Geographic atrophy appearing from pigment disturbances in the location of nAMD treated with anti-VEGF appears to manifest earlier than that of GA in eyes without nAMD. It is possible that the presence of neovascularization, or intravitreal anti-VEGF, or both may contribute to the quicker development of GA from NGA.
In this study, a little fewer than half of the eyes that had NGA at year 1 progressed to GA within 4 years, and about 20% of the remaining eyes with NGA that did not progress to GA by 5 years developed a scar. The specific process underlying the formation of either a scar or GA in the area of NGA is unclear in eyes that are treated with anti-VEGF for nAMD, but several risk factors, both genetic and phenotypic, can provide information about future outcomes. We have shown that the progression from NGA to GA is twice as likely when there is poor baseline VA in either the study eye or fellow eye. Geographic atrophy in the fellow eye increases the risk of conversion to GA from NGA in the study eye. Pseudodrusen confers a greater risk. Fellow eyes with ribbon-dominant pseudodrusen in patients with unilateral exudative AMD are likely to develop late AMD.30 Several studies have recognized pseudodrusen to be a risk factor for GA development in nAMD eyes treated with anti-VEGF therapy.31,32
In drusen-associated GA, the pigment appears to be migrating RPE cells or macrophages that have engulfed pigment overlying drusen or PEDs, as evidenced by hyperreflective foci overlying the druse or pigment epithelial detachments. The pigment in nAMD-associated GA has not been investigated to the extent that the drusen-associated GA has been, and its pathophysiology remains unclear.
The Inhibition of VEGF in Age-Related Choroidal Neovascularisation (IVAN) study investigated the development of intralesional macular atrophy (GA) in nAMD treated with anti-VEGF and reported that the presence of subretinal fluid at the final visit was associated with more than halving of the odds of developing macular atrophy.20 This protective effect of subretinal fluid has been reported in CATT as well as in other studies. Our results show that the presence of subretinal fluid at the baseline visit is a protective factor against the conversion of NGA to GA during treatment.33,34 Subretinal fluid is thought to be a by-product of minimally exudative, type-1 CNV serving as a natural protective mechanism for the retina by providing nutritional support but not producing enough exudation (as in intraretinal fluid) to cause harm.
Our definitions of NGA and GA were based on CFPs and FA images. While detection of GA on these images is relatively straightforward, detection of NGA is more challenging and prone do grading error because of the absence of sharp borders and highly distinct differences in color or fluorescence for NGA compared with areas without lesions in an eye with AMD. The recently proposed OCT definitions of incomplete RPE and outer retinal atrophy (iRORA) and complete RPE and outer retinal atrophy (cRORA) may have an association with NGA, but as evolving concepts, they are yet to be characterized in a large population.11 The CATT trial had only time-domain OCTs at baseline and year 1 in all eyes, but spectral-domain OCT (SD-OCT) images were available in some patients during year 2 and almost all patients at year 5. When the morphologic features on SD-OCT were colocalized with areas of GA and NGA on a subset of CATT eyes (n = 69) with SD-OCT at 2 years, areas of NGA had SD-OCT features that were quite different from areas of GA.35 Retinal pigment epithelium atrophy covered 75% of the area designated as GA but only 22% of the area designated as NGA. Areas of RPE atrophy without overlying lesion (subretinal highly reflective material or serous pigment epithelial detachment) were common in areas of GA and rare in areas of NGA. The percentage of the areas with photoreceptor loss was considerably higher within GA areas than within NGA area (median, 85% vs 42%).
Limitations
Approximately 30% of the elderly participants in CATT (mean age at baseline, 79 years) were lost to follow-up by the 5-year visit, and these patients were had worse VA values at baseline. Because worse baseline VA values were a risk factor for both NGA and GA, the proportion of patients with these outcomes may be underestimated. In addition, the 3-year gap between the end of the clinical trial and the 5-year visit limits estimation of the time course for conversion from NGA to GA.
Conclusions
In summary, we identified risk factors that could help ophthalmologists recognize eyes that are likely to develop NGA and subsequently progress to GA. During follow-up of eyes treated with anti-VEGF for nAMD, when NGA is identified, there is a 50% chance of GA developing in these areas within 4 years.
eFigure 1. Example of non-geographic atrophy (NGA) evolution in color fundus photographs (CFP), fluorescein angiograms (FA) and OCT images in the area of baseline choroidal neovascularization at baseline, year 1, 2 and 5.
eFigure 2. Progression from non-geographic atrophy (NGA) to geographic atrophy (GA) in the Year 1 NGA cohort.
eFigure 3. Progression from non-geographic atrophy (NGA) to geographic atrophy (GA) in the Year 2 Non-geographic atrophy cohort.
eFigure 4. A1, A2 Non-geographic atrophy surrounding a non-fibrotic scar at year 1 developing into geographic atrophy at year 5. B1, B2 Non-geographic atrophy at 1 year with a large fibrotic scar at year 5.
eTable 1. Univariate analysis for the baseline risk factors for incident non-geographic atrophy (NGA).
eTable 2. Univariate analysis for baseline risk factors for progression from non-geographic atrophy (NGA) to geographic atrophy (GA).
References
- 1.American Academy of Ophthalmology Retina/Vitreous Panel Age-related macular degeneration PPP 2019. Published 2015. Accessed February 18, 2020. https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp
- 2.CATT Research Group Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897-1908. doi: 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin DF, Maguire MG, Fine SL, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398. doi: 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maguire MG, Martin DF, Ying GS, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Ophthalmology. 2016;123(8):1751-1761. doi: 10.1016/j.ophtha.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gemenetzi M, Lotery AJ, Patel PJ. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye (Lond). 2017;31(1):1-9. doi: 10.1038/eye.2016.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunwald JE, Pistilli M, Daniel E, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group . Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124(1):97-104. doi: 10.1016/j.ophtha.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122(4):809-816. doi: 10.1016/j.ophtha.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunwald JE, Daniel E, Huang J, et al. ; CATT Research Group . Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150-161. doi: 10.1016/j.ophtha.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Channa R, Sophie R, Bagheri S, et al. Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am J Ophthalmol. 2015;159(1):9-19.e1, 2. doi: 10.1016/j.ajo.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munk MR, Ceklic L, Ebneter A, Huf W, Wolf S, Zinkernagel MS. Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol. 2016;94(8):e757-e764. doi: 10.1111/aos.13157 [DOI] [PubMed] [Google Scholar]
- 11.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ. Incidence and progression of geographic atrophy. Ophthalmology. 2013;120(10):2042-2050. doi: 10.1016/j.ophtha.2013.03.029 [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Jefferys JL, Hawkins BS, Bressler NM, Bressler SB; Submacular Surgery Trials Research Group . Risk factors for second eye progression to advanced age-related macular degeneration: SST report No. 21 Submacular Surgery Trials Research Group. Retina. 2009;29(8):1080-1090. doi: 10.1097/IAE.0b013e3181b1baeb [DOI] [PubMed] [Google Scholar]
- 14.Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group Risk factors for choroidal neovascularization and geographic atrophy in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2008;115(9):1474-1479, 1479.e1-1479.e6. doi: 10.1016/j.ophtha.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 15.Klein ML, Ferris FL III, Armstrong J, et al. ; AREDS Research Group . Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026-1031. doi: 10.1016/j.ophtha.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 16.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond). 1988;2(Pt 5):552-577. doi: 10.1038/eye.1988.106 [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kim JW, Kim CG, Lee DW. Focal retinal pigment epithelium atrophy at the location of type 3 neovascularization lesion. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1661-1669. doi: 10.1007/s00417-019-04373-4 [DOI] [PubMed] [Google Scholar]
- 18.Kuroda Y, Yamashiro K, Tsujikawa A, et al. Retinal pigment epithelial atrophy in neovascular age-related macular degeneration after ranibizumab Treatment. Am J Ophthalmol. 2016;161:94-103.e1. doi: 10.1016/j.ajo.2015.09.032 [DOI] [PubMed] [Google Scholar]
- 19.Schütze C, Wedl M, Baumann B, Pircher M, Hitzenberger CK, Schmidt-Erfurth U. Progression of retinal pigment epithelial atrophy in antiangiogenic therapy of neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(6):1100-1114.e1. doi: 10.1016/j.ajo.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey C, Scott LJ, Rogers CA, et al. ; Writing Committee for the IVAN Study Group . Intralesional macular atrophy in anti-vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN trial. Ophthalmology. 2019;126(1):75-86. doi: 10.1016/j.ophtha.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 21.Daniel E, Ying GS, Kim BJ, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials . Five-year follow-up of nonfibrotic scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2019;126(5):743-751. doi: 10.1016/j.ophtha.2018.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel E, Pan W, Ying GS, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials . Development and course of scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2018;125(7):1037-1046. doi: 10.1016/j.ophtha.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel E, Toth CA, Grunwald JE, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656-666. doi: 10.1016/j.ophtha.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaffe GJ, Ying GS, Toth CA, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2019;126(2):252-260. doi: 10.1016/j.ophtha.2018.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma S, Toth CA, Daniel E, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(4):865-875. doi: 10.1016/j.ophtha.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe GJ, Martin DF, Toth CA, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(9):1860-1870. doi: 10.1016/j.ophtha.2013.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunwald JE, Daniel E, Ying GS, et al. ; CATT Research Group . Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(8):1634-1641. doi: 10.1016/j.ophtha.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCroos FC, Toth CA, Stinnett SS, Heydary CS, Burns R, Jaffe GJ; CATT Research Group . Optical coherence tomography grading reproducibility during the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(12):2549-2557. doi: 10.1016/j.ophtha.2012.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunness JS. Choroidal neovascularisation and atrophy. Br J Ophthalmol. 2006;90(4):398-399. doi: 10.1136/bjo.2005.084830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurada Y, Sugiyama A, Kikushima W, et al. Pseudodrusen pattern and development of late age-related macular degeneration in the fellow eye of the unilateral case. Jpn J Ophthalmol. 2019;63(5):374-381. doi: 10.1007/s10384-019-00680-9 [DOI] [PubMed] [Google Scholar]
- 31.Cho HJ, Yoo SG, Kim HS, et al. Risk factors for geographic atrophy after intravitreal ranibizumab injections for retinal angiomatous proliferation. Am J Ophthalmol. 2015;159(2):285-92.e1. doi: 10.1016/j.ajo.2014.10.035 [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q, Daniel E, Maguire MG, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group . Pseudodrusen and incidence of late age-related macular degeneration in fellow eyes in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(7):1530-1540. doi: 10.1016/j.ophtha.2016.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho AC, Busbee BG, Regillo CD, et al. ; HARBOR Study Group . Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181-2192. doi: 10.1016/j.ophtha.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration. Ophthalmology. 2014;121(1):193-201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 35.Toth CA, Tai V, Pistilli M, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Distribution of OCT features within areas of macular atrophy or scar after 2 years of anti-VEGF treatment for neovascular AMD in CATT. Ophthalmol Retina. 2019;3(4):316-325. doi: 10.1016/j.oret.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Example of non-geographic atrophy (NGA) evolution in color fundus photographs (CFP), fluorescein angiograms (FA) and OCT images in the area of baseline choroidal neovascularization at baseline, year 1, 2 and 5.
eFigure 2. Progression from non-geographic atrophy (NGA) to geographic atrophy (GA) in the Year 1 NGA cohort.
eFigure 3. Progression from non-geographic atrophy (NGA) to geographic atrophy (GA) in the Year 2 Non-geographic atrophy cohort.
eFigure 4. A1, A2 Non-geographic atrophy surrounding a non-fibrotic scar at year 1 developing into geographic atrophy at year 5. B1, B2 Non-geographic atrophy at 1 year with a large fibrotic scar at year 5.
eTable 1. Univariate analysis for the baseline risk factors for incident non-geographic atrophy (NGA).
eTable 2. Univariate analysis for baseline risk factors for progression from non-geographic atrophy (NGA) to geographic atrophy (GA).