Abstract
Male and female C57BL/6J mice were tested on the predator odor response task, where they needed to cross through a chamber of scented bedding to reach a sucrose reward. Following the behavioral testing, mouse brains were immunohistochemically labeled for expression of the immediate early gene c-fos. In the presence of the novel odorant methyl valerate (MV), both males and females exhibited increased exploration behaviors and delayed rewards compared to control bedding. However, in the presence of the predator odor phenylethylamine (PEA), males exhibited increased exploration that strongly resembled their behavior in MV (a non-predator odor) while females behaved very similarly to the clean bedding controls, quickly traversing the chamber to achieve the reward. Expression of c-fos exhibited significant sex by odor condition interactions overall across brain regions and in the anterior piriform cortex, cingulate cortex, and dorsomedial hypothalamus specifically. In all three regions we observed the general pattern that PEA exposure evoked elevated c-fos expression in females but suppressed c-fos expression in males. Taken together these data suggest that males and females may adopt different behavioral strategies in the presence of predator threat.
Keywords: predator odor, sexual dimorphism, approach-avoidance
1. Introduction
Goal-directed behavior requires a cost-benefit analysis that balances the value of a positive outcome against any risks of consequent harm. In prey species this usually takes the form of approach-avoidance conflicts, where the animal must endure some risk to physically obtain a desired reward. Dent and colleagues [1] introduced a “predator odour risk-taking task” (PORT), in which mice have the opportunity to obtain a desired liquid reward but must traverse a predator odor-scented arena to do so. This naturalistic tension between foraging and predation risk should strongly engage neural circuits that determine behavioral response towards threat-related and reward-related stimuli.
The interpretation of approach-avoidance tasks like the PORT task can be complicated if subjects adopt different strategies. A mouse that seeks to minimize threat-exposure while seeking a reward might dart across the predator-scented open space to reduce the time spent in the “danger zone” or alternatively circumnavigate the perimeter of that space to minimize its physical vulnerability at the cost of a longer time in danger. It is also critical to consider the nature of the risks perceived by the mouse – is the predator odor threatening only because it is from a predator or also because it is novel? To address these questions we have adapted the PORT task to compare behavior between mice presented with a novel neutral odor or a novel predator scent odor and between those adopting one behavioral strategy or another. We then analyzed c-fos expression patterns in the brains of these mice to assess possible correlations between activity-dependent gene expression and PORT behavior.
In threat-related tasks, sex can be a strong predictor of behavioral strategy. Typically, female rodents exhibit more defensive behaviors and are more prone to avoid areas scented with a predator odor than males [2–4]. Sometimes these differences are circumstance-dependent, such as females exhibiting fewer risk assessment behaviors than males (e.g. head extensions out of a hide box, stretch-attend posture) without notable differences in explicit defensive behaviors like freezing [5]. When faced with an explicit approach-avoidance challenge like a foraging task in an arena that delivers unpredictable footshocks, females reduce their time foraging (thus trading off food intake for safety) while males enlarge their meals [6]. We thus hypothesized that males and females would exhibit different patterns of behavior and potentially c-fos expression patterns in the PORT task.
2. Materials and methods
2.1. Animals
A total of 119 C57BL/6 mice (ages ranging from 8 – 35 weeks) of both sexes (female: n = 59, male: n = 60) were used for behavioral analysis [CB: n = 41 (19 males); MV: n = 38 (21 males); PEA: n = 40 (19 males)]. A subset of these mice (n = 58) were used for subsequent Fos quantification [CB: n = 18 (10 males); MV: n = 18 (11 males); PEA: n = 22 (10 males)]. The CB, MV and PEA experimental groups included 10, 9, and 10 mice six months or older, respectively. Note that all mice used are within the age range considered mature adult mice [7–9], and that follow-up analyses found no significant correlation between mouse age and any of the dependent measures (with the exception of one nominally significant correlation driven by a single outlier datapoint in one brain region in one experimental group).
Mice were water restricted to 90% of ad libitum weight and maintained on a 12:12 hour light/dark cycle, with behavioral testing occurring during the light cycle. Each day, mice were given access to a limited volume of water after the behavioral task. The amount of water given was determined by the animal’s weight that day, with the average range being 2–3 mL of water each day. Mice were run in cohorts of 5, meaning that five mice were run through the single apparatus each day with both male and female mice running on the same day. After each cohort completed all phases of the behavioral task, another cohort of 5 mice began training. All experiments were performed in accordance with protocols approved by the Rutgers University Institutional Animal Care and Use Committee.
2.2. Predator odor risk-taking task.
The PORT task apparatus was adapted from Dent et al. [1]. The apparatus was a white, plexiglass arena divided into three equal, distinct chambers (each 30 × 30 × 40 cm, length × width × height) with doorways (5 × 5 cm, width × height) allowing the middle chamber to be entered from either end chamber (Fig. 1). Mice were randomly assigned either the leftmost or rightmost chamber as their “start” chamber. The opposite end chamber was designated as the “reward” chamber and baited with 100 μL of 10% sucrose solution in a cosmetics sample cup placed in the center.
Fig. 1. Predator Odor Risk-Taking (PORT) apparatus.
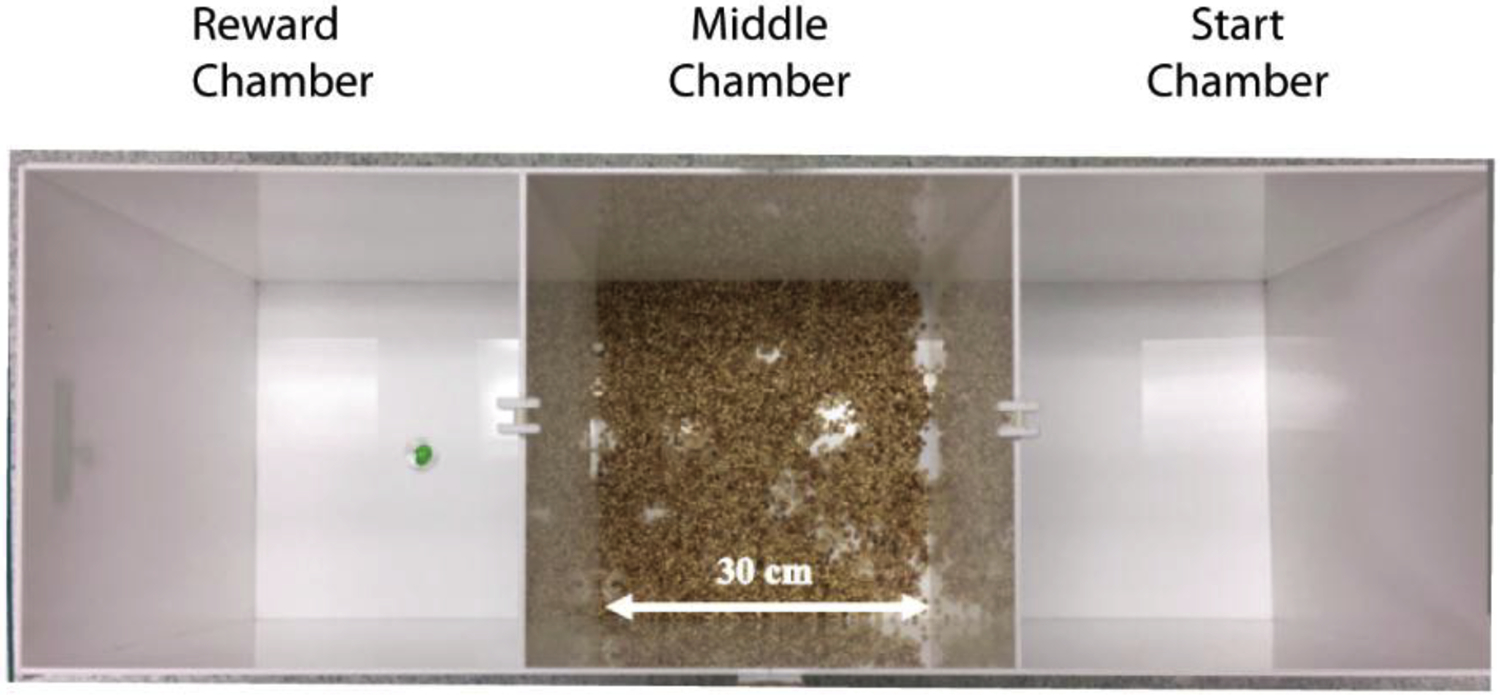
Mice traverse a scented middle chamber in order to retrieve a sucrose reward at the end. The apparatus is a white, plexiglass arena divided into three equal, distinct chambers (each 30 × 30 × 40 cm, length × width × height) with doorways (5 × 5 cm, width × height) allowing the middle chamber to be entered from either end chamber. Mice were randomly assigned either the leftmost or rightmost chamber as their “start” chamber. The opposite end chamber was designated as the “reward” chamber and baited with 100 μL of 10% sucrose solution in a cup placed in the center.
2.3. Odorants
500 mL of standard corn cob bedding (Bed-o’cobs laboratory animal bedding from The Andersons) was mixed with 300 μL of either 2-phenylethylamine (PEA; Sigma-Aldrich; CAS #624-24-8), which served as the predator odor [10, 11], or methyl valerate (MV; Sigma-Aldrich; CAS #64-04-0), as a novel control odor. The clean bedding control condition had no odorants added. All bedding was prepared two days prior to use and stored in sealed plastic bags.
2.4. Behavioral Paradigm
The behavioral paradigm was adapted from Dent et al. [1]. The experimental timeline is depicted in Fig. 2. Mice were single-housed for 6 days prior to beginning water restriction. On the fourth, fifth, and sixth days of water restriction mice underwent daily sucrose preference testing. During this phase, mice were placed within standard cages containing clean bedding and two ceramic dishes (6.65 × 6.65 × 3.81 cm, length × width × height) placed side by side at one end of the cage. One dish contained 3 mL of a 10% sucrose solution, while the other contained 3 mL of water. The left/right placement of the dishes was alternated each day. Mice were given 10 minutes to freely sample and consume the liquid from either of the dishes. The total volume of liquid consumed from each of the dishes was recorded each day to quantify the sucrose preference for each animal. The use of ceramic dishes as opposed to water bottles for sucrose preference testing, enabled animals to become accustomed to this method of liquid delivery, which was used in subsequent testing.
Fig. 2. Timeline of experiment.
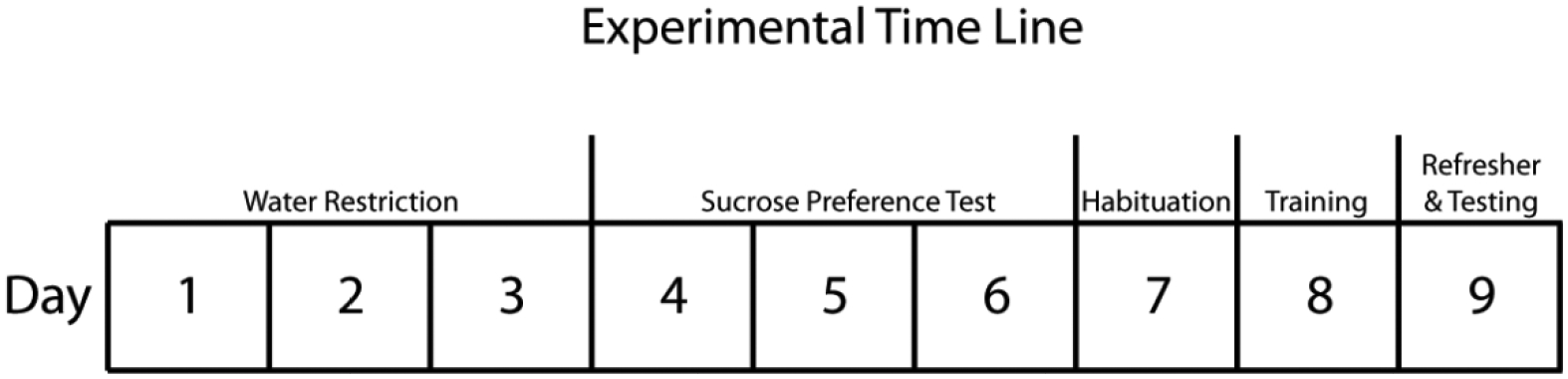
First, mice were single-housed for 6 days prior to water restriction (not depicted in this timeline). Mice were water restricted for three days (corresponding to days 1 −3 on the timeline) prior to beginning the daily sucrose preference testing, also spanning the course of three days (corresponding to days 4 – 6). On the seventh day after beginning water restriction, animals were habituated to the testing apparatus in one 20-minute session. On day 8, animals underwent 5 trials of reward training, in which mice were given unlimited time to traverse the middle chamber (with clean bedding) to obtain the sucrose reward in the end chamber. On the ninth and final day, animals completed a total of 6 trials. Three of these trials occurred in the morning (termed: “refresher” in the timeline) with clean bedding in the middle chamber (as on day 8). The last three trials occurred in the afternoon (termed: “testing”) approximately 3 hours after the AM session. Prior to the testing phase, mice were randomly assigned to have the middle chamber scented with PEA, MV, or clean, unscented bedding (CB) for all three of these trials. CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine.
On the seventh day after beginning water restriction, animals were habituated to the testing apparatus in one 20-minute session in which the middle chamber contained 500 mL of clean bedding and no reward was present. On the morning of day 8, animals underwent 5 trials of reward training, in which mice were placed within the start chamber and given unlimited time to traverse the middle chamber (with clean bedding) to obtain the sucrose reward in the end chamber. After each trial, animals were removed from the apparatus and placed in their home cage for a 1 minute intertrial interval in which the apparatus was cleaned with 1% Tergazyme solution followed by 70% ethanol solution. The bedding remained within the center chamber, so for that chamber only the walls were cleaned between trials. The apparatus was then dried with paper towels to remove any residues of the cleaning agents. For each animal, the bedding was re-used for all five trials. After each animal’s session, the bedding was removed and the apparatus was cleaned before setting up for the next animal’s session. This cleaning procedure was also used for sessions conducted on day 9.
On the ninth and final day, animals completed a total of 6 trials. After the manner of Dent et al. [1], three of these trials occurred in the morning (AM session) with clean bedding in the middle chamber (as on day 8). The last three trials occurred in the afternoon (PM session) approximately 3 hours after the AM session. Prior to the PM session, mice were randomly assigned to have the middle chamber scented with PEA, MV, or clean, unscented bedding (CB) for all three trials. Because PEA can be detected by mice at concentrations that are subthreshold for humans [12], and below the sensitivity of our photoionization detectors, each cohort of five mice received the same odorant to minimize the risk of undetectable contamination of the ambient air with PEA. The order in which cohorts were run was randomly varied. Mice were videorecorded and behavior was analyzed using a combination of Noldus EthoVision (Wageningen, the Netherlands) video tracking systems and manual scoring by a blind experimenter. Behaviors quantified included: latency to reward, time spent in each chamber, time spent immobile, velocity, latency to exit the start chamber, path length in the middle chamber, time in periphery of middle chamber relative to total time in middle chamber (thigmotaxis), and entries into each chamber.
2.5. Immunohistochemistry
2.5.1. Tissue processing
Forty-five minutes after the PM session, mice were deeply anesthetized with pentobarbital and transcardially perfused with 40 mL of chilled 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Whole brains were dissected out, and postfixed in 4% paraformaldehyde in PBS for 24 hours, then submerged in a 10% sucrose, 0.1% sodium azide solution in PBS until sectioning. 40 μm thick coronal brain sections were cut on a cryostat and submerged and stored in 24-well plates containing 30% sucrose and 0.1% sodium azide solution in PBS.
2.5.2. Fos immunohistochemistry
Free-floating sections were permeabilized in 0.1% Triton X-100 in PBS for five minutes, then washed with PBS for another five minutes. The sections were then submerged in a citrate buffer (Sigma Aldrich #C9999) for 30 minutes, then blocked with 10% Normal Goat Serum (NGS) and 0.1% Triton X-100 in PBS for one hour. Sections were incubated overnight at 4 °C with primary antibody (c-Fos-rabbit, Cell Signaling # 2250S), 2% NGS, and 0.3% Triton X-100 in PBS. After 18–22 hours, sections were washed in PBS three times for five minutes each and then incubated with secondary antibody (goat anti-rabbit IgG conjugated to AlexaFluor 488 (ThermoFisher Scientific # A-11008), 2% NGS and 0.3% Triton X-100 in PBS for two hours at room temperature. Sections were kept in the dark for the remainder of the protocol. After the incubation, the sections were washed with PBS three times for five minutes each. The sections were then mounted onto slides and coverslipped with ProLong Gold with DAPI (Invitrogen).
2.5.3. Imaging
Slides were imaged with a 10x, 0.30 NA Evos brand objective on an Evos FL Auto 2 Imaging System (Invitrogen). UV (4’,6-diamidino-2-phylindole (DAPI)-compatible) and blue (AlexaFluor 488- compatible) fluorescence channels were utilized for the collection of images. Slides were autofocused on the AlexaFluor488 channel at 50% light intensity, 0.151 second exposure, and 1.0 gain (see Fig. 2 for an example of Fos staining and a no primary control).
2.5.4. Fos quantification
Sections containing target brain regions were selected and the target regions were outlined in ImageJ based on the DAPI stained image to localize a region of interest (ROI) based on the mouse brain atlas (Franklin & Paxinos, 2008). Two sections containing each region were collected per animal and averaged whenever possible, but sections containing obvious artifacts or tears were discarded. ROI borders were then superimposed onto the Fos-stained images for manual quantification. Before counting, Fos images were converted to 16-bit grayscale, slightly sharpened with the standard ImageJ despeckle and sharpen options, and contrast was set to saturate 0.01% of pixels. Cells within the delineated ROI were manually counted using the multipoint tool. The number of cells counted within each ROI was divided by the ROI’s area to calculate the number of cells per mm2. These measurements were not generated based on stereological sampling methods and should not be extrapolated to the population of all cells in a given region, but any sampling inconsistencies should be similar across experimental groups because quantification was performed by blind experimenters.
2.6. Statistical Methods
A Kolmogorov-Smirnov test indicated that the data violated the assumption of normality, thus a generalized linear model was used for statistical analysis of both behavioral and Fos data, with sex, condition, and the sex*condition interaction as fixed factors using SPSS (IBM). A gamma probability distribution and log link function were selected, and a robust estimator was used. Significant interactions within the behavioral dataset were followed up by post-hoc pairwise comparisons with Bonferroni correction to reduce the false discovery rate from multiple comparisons. Pearson’s correlation coefficients were used for sucrose preference correlation tests.
Analysis of Fos activity as a function of brain region, sex, and odor was performed in two parts. First, we used an omnibus GLM analysis to test the hypothesis that each brain region exhibited a significant sex*condition interaction, mirroring the behavioral data. Second, because we did not have specific hypotheses about Fos expression in each individual brain region we considered the regional data in a more exploratory manner. For instance, because of the very large number of potential pairwise comparisons (15 per brain region) and the much smaller statistical power when comparing individual groups than when considering the overall dataset, we considered overall patterns across and within brain regions by sex and by condition instead of performing many post-hoc tests. Within each group, Spearman’s non-parametric correlation coefficients were used to test for relationships between Fos activity in each brain region and each of the behavioral measures, but no significant relationships were found (perhaps because of the a limited sample size within a single group).
3. Results
3.1. Trial 1 behavior
Trial 1 of the PM session was the first time that the mice in the PEA and MV groups encountered an odorant during the PORT task. The latency from the placement of the mouse in the start chamber to the time of reward retrieval was compared across PEA, MV, and CB groups as an overall metric of the impact of the odor exposure. As shown in Fig. 4A, on the first test trial there were significant differences in the latency to reward across the three odor groups [χ2 (2) = 15.13, p = 0.00]. Compared to CB, there were increased latencies in both the MV and PEA conditions [χ2 (2) = 14.48, p = 0.00; 0.03 (MV, PEA respectively)]. There also was a significant sex*condition interaction [χ2 (2) = 7.87 p = 0.02], such that within the PEA condition females were much quicker than males to obtain the sucrose reward (Fig. 4A).
Fig. 4. Trial 1 behavioral results.
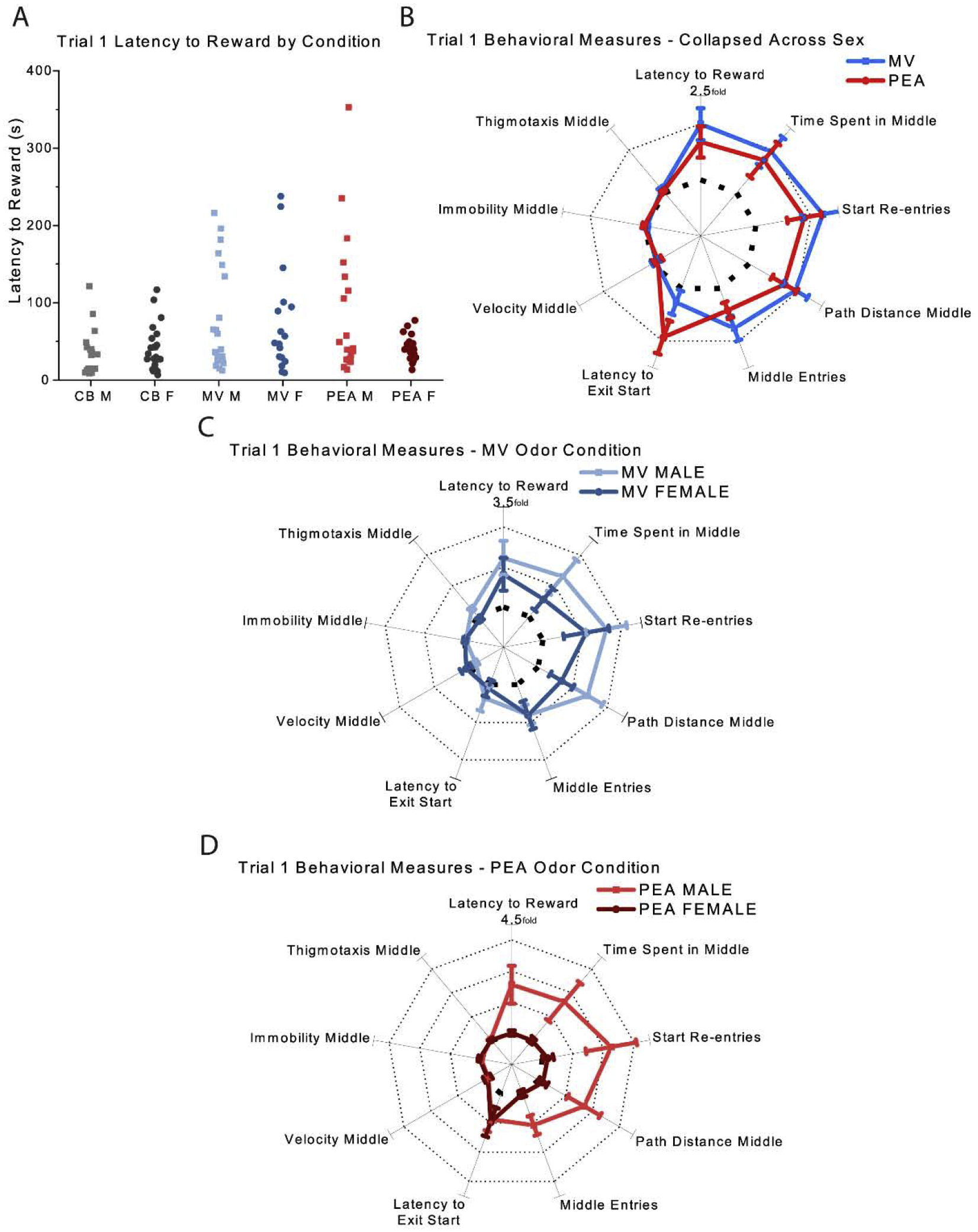
(A) Measures of latency to reward by odor condition and sex (B) Normalized behavioral results collapsed across sex (C&D) Normalized behavioral results grouped by sex for animals in the MV (C) and PEA (D) conditions. Bolded dashed line indicates the unit circle of a one-fold increase, and dotted lines indicate subsequent increases (e.g., two-fold increase, three-fold increase, etc., compared to CB controls) in B, C, and D. Error bars show mean ± SEM. CB: n = 41 (19 males); MV: n = 38 (21 males); PEA: n = 40 (19 males). CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine; M, male; F, female.
These differences in latency could reflect any number of behavioral strategies, from odor avoidance to odor exploration, which could differ across odors but produce similar latencies. To better capture the overall behavior of the mice performing this task, we thus quantified the behavior of each group along nine different axes (Fig. 4B–D) measuring their behavior in the start chamber (e.g. latency to exit), behavior in the middle chamber (e.g. time spent, path length, velocity, immobility, thigmotaxis), and transitions from one chamber to another (e.g. start re-entries, middle entries). We then normalized these measurements to those of the CB group, producing a “behavioral fingerprint” that can be compared across groups. Fig. 3B shows that despite the significant interaction, when collapsing across sex the behavior of the MV (blue) and PEA (red) groups differed markedly from that of the CB group (dotted unit circle) for path distance (p = 0.00 for MV, p = 0.01 for PEA), while the MV group spent significantly more time in the middle chamber (p = 0.03 for MV; p = 0.12 for PEA). Surprisingly, there were no differences on the aversion-related measures of immobility, thigmotaxis, or velocity across any of the three groups (p > 0.16 all comparisons): with the exception that mice in the PEA group took slightly but significantly longer (3.88 sec vs 2.01 sec) to exit the start chamber (p = 0.01) than the CB controls. These analyses suggest that the mice were not afraid of either odorant in this familiar context, even on this first encounter. Collapsing across sex, the mice in the MV and PEA groups behaved quite similarly (Fig. 4B) despite PEA being a predator odor component [10], and despite the very different pattern of c-fos expression each odor evoked (see below).
Fig. 3. Fos images.
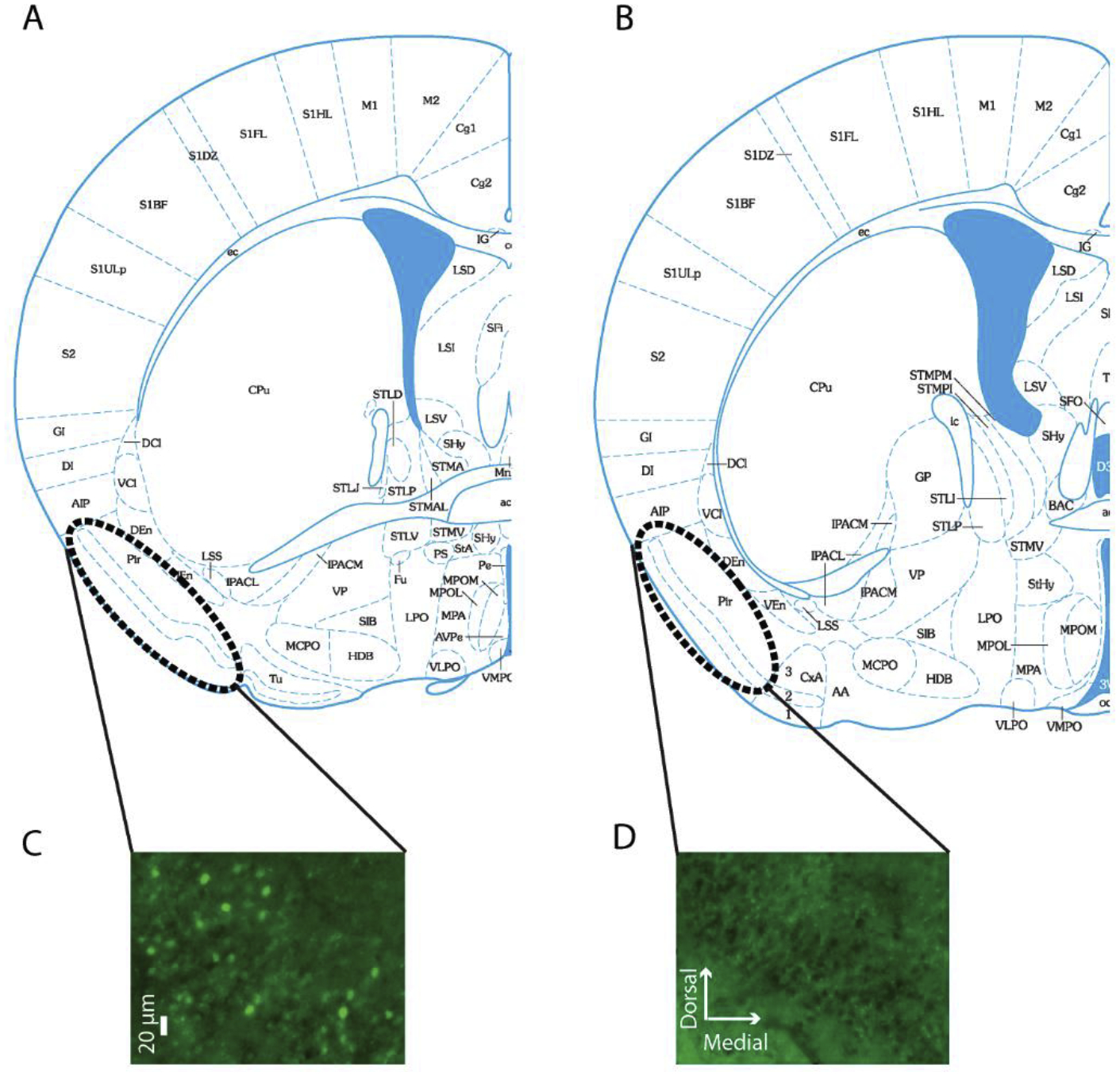
(A&B) Schematic drawings of coronal sections used for quantifying Fos within the Piriform cortex (drawings derived from Paxinos mouse brain atlas, bregma + 0.14 (A) and – 0.10 (B). (C&D) Images of Fos staining (C) and no primary control staining (D) within the piriform cortex of the same female mouse exposed to the methyl valerate odorant.
Mice in the MV group exhibited no significant differences in the latency to reward between the sexes (see above). This was recapitulated in their behavioral fingerprint, now normalized separately to compare MV males to CB males and MV females to CB females (Fig. 4C). The males tended to exhibit more exploratory behaviors (e.g. time in the middle chamber, path distance, start-box re-entries) than females but none of these were significantly different between the sexes. By comparison, mice in the PEA group exhibited large differences between males and females (Fig. 4D), with the females behaving virtually identically to the females in the CB group (dotted line) and the males exhibiting significantly more exploration behaviors (Fig. 4D). There was no difference between males and females on aversion-related behaviors.
One possible explanation for the differences in behavior between males and females is a difference in the motivational value of the sucrose reward. To test this possibility, we compared the sucrose preference of each individual mouse to their subsequent behavior on the PORT task. Overall, females exhibited a slightly but significantly weaker preference for sucrose over water than males [t(115) = 3.82, p = 0.00](Fig. 5 inset), which contradicts the idea that their more rapid approaches to the sucrose were driven by stronger reward motivation. Female mice on average consumed 1.37 mL of sucrose (± 0.33 mL) which was significantly less than the male average of 1.63 mL (± 0.58 mL), [t(112) = 3.00, p = 0.00], and slightly greater total consumption of water than males (female: 0.51 mL ± 0.42 mL; male: 0.38 mL ± 0.27 mL), although this was not significant [t(111) = 1.94, p = 0.06]. Moreover, there was no significant correlation (Fig. 5) between sucrose preference and latency to achieve the reward across individual mice [r = 0.14, p = 0.13]. Similarly, there were no significant correlations between mouse age and latency to reward or any of the other behavioral outcomes (see Fig. 6 for age and latency to reward correlation).
Fig. 5. Sucrose preference and latency to reward.
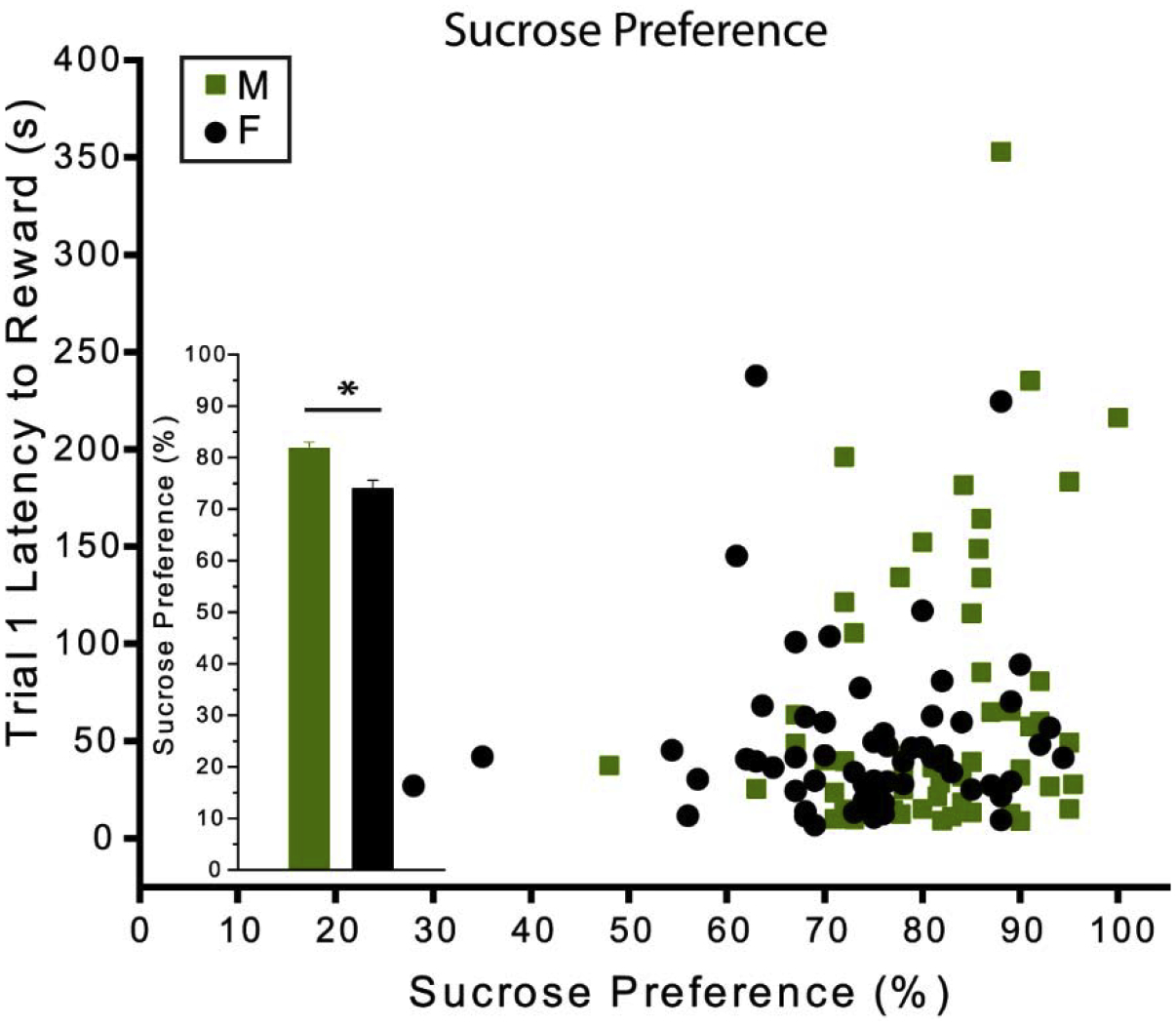
Correlation between sucrose preference and latency to reward in the first trial. The bar graph inset reflects the difference in the mean sucrose preference scores between males and females. There was no significant correlation between sucrose preference and latency to retrieve the reward across individual mice [r = 0.14, p = 0.13]. Females exhibited a slightly but significantly weaker preference for sucrose over water than males [t(115) = 3.82, p = 0.00]. Error bars show mean ± SEM. CB: n = 41 (19 males); MV: n = 36 (20 males); PEA: n = 40 (19 males). Asterisk indicates P < 0.01. CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine; M, male; F, female.
Fig. 6. Mouse age and latency to reward.
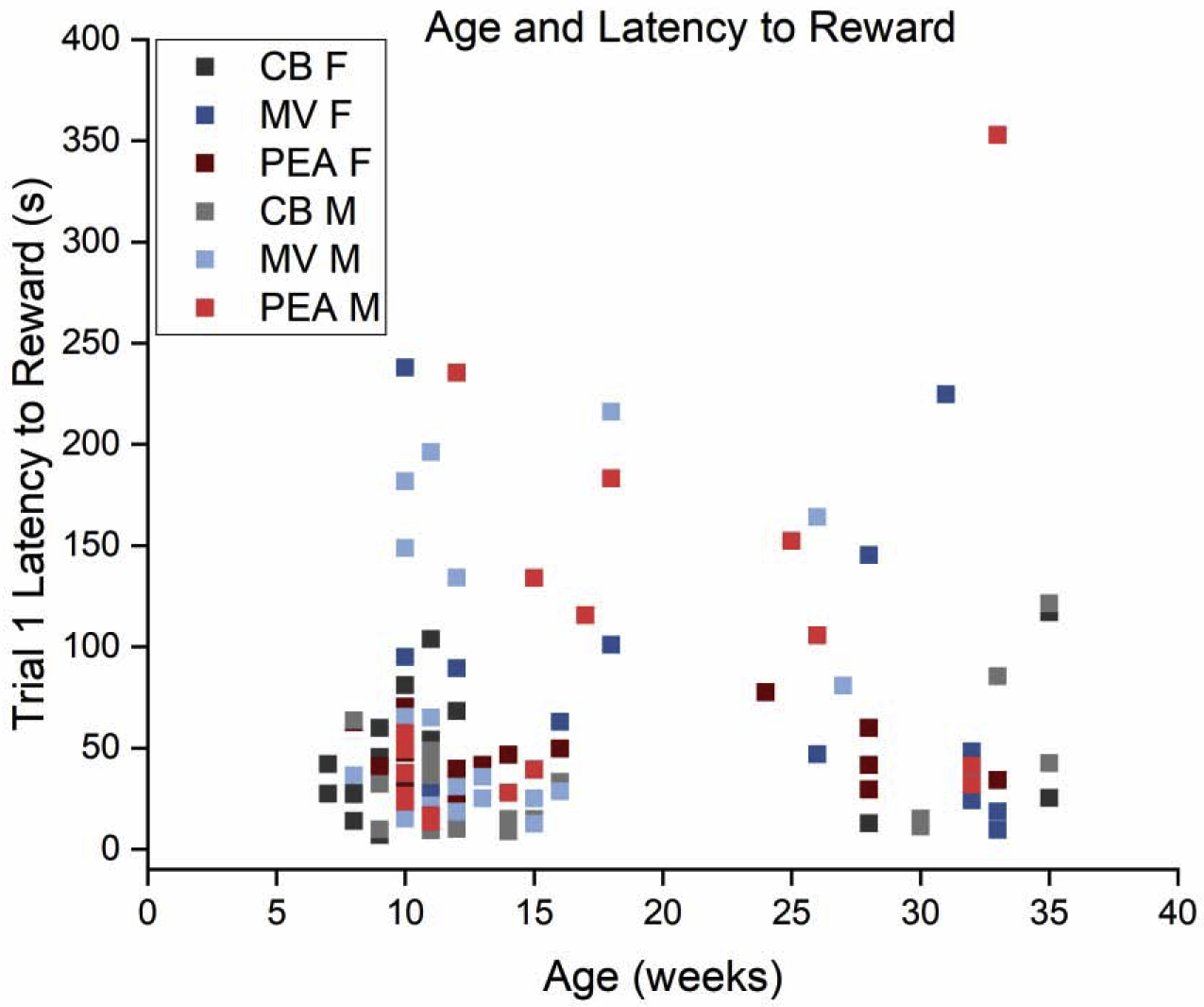
Scatterplot showing latency to reward relative to mouse age. While there are two clear age cohorts (all within the category of mature adults), there is no apparent relationship between age and reward latency. Color code denotes experimental group assignment. CB: n = 41 (19 males); MV: n = 38 (21 males); PEA: n = 40 (19 males). CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine; M, male; F, female.
3.2. Trial 3 behavior
By trial 3, the average latency to reward significantly decreased for all groups compared to trial 1 [χ2 (1) = 26.14, p = 0.00]. Mice in the MV and PEA groups exhibited very similar behavior to those in the CB control group (Fig. 8). Unlike on trial 1, there were no significant effects of odor condition [χ2 (2) = 5.48, p = 0.07], sex [χ2 (1) = 0.00, p = 0.99], or their interaction [χ2 (2) = 0.46, p = 0.80] on overall latency to reward. Examining the behavioral fingerprints (Fig. 7B), mice in the MV condition consistently scored slightly higher than the CB mice on the investigatory behavioral axes, but none of these differences were individually significant (except for some modest effects on the number of re-entries to the start chamber). The males in the PEA group became extremely similar to their counterparts in the CB control group and to the females in the PEA group by trial 3 (Fig. 7D), in contrast to their earlier behavior (Fig. 4D). Unlike trial 1, the latency to exit the start chamber and the path length in the middle chamber were both unaffected by odor condition [latency χ2 (2) = 4.48, p = 0.11; path length χ2 (2) = 3.23, p = 0.20], sex [latency: χ2 (1) = 0.33, p = 0.57; path length: χ2 (1) = 0.73, p = 0.39], or their interaction [latency: χ2 (2) = .78, p = 0.68; path length: χ2 (2) = 1.47, p = 0.48].
Fig. 8. Latencies to reward across the behavioral trials.
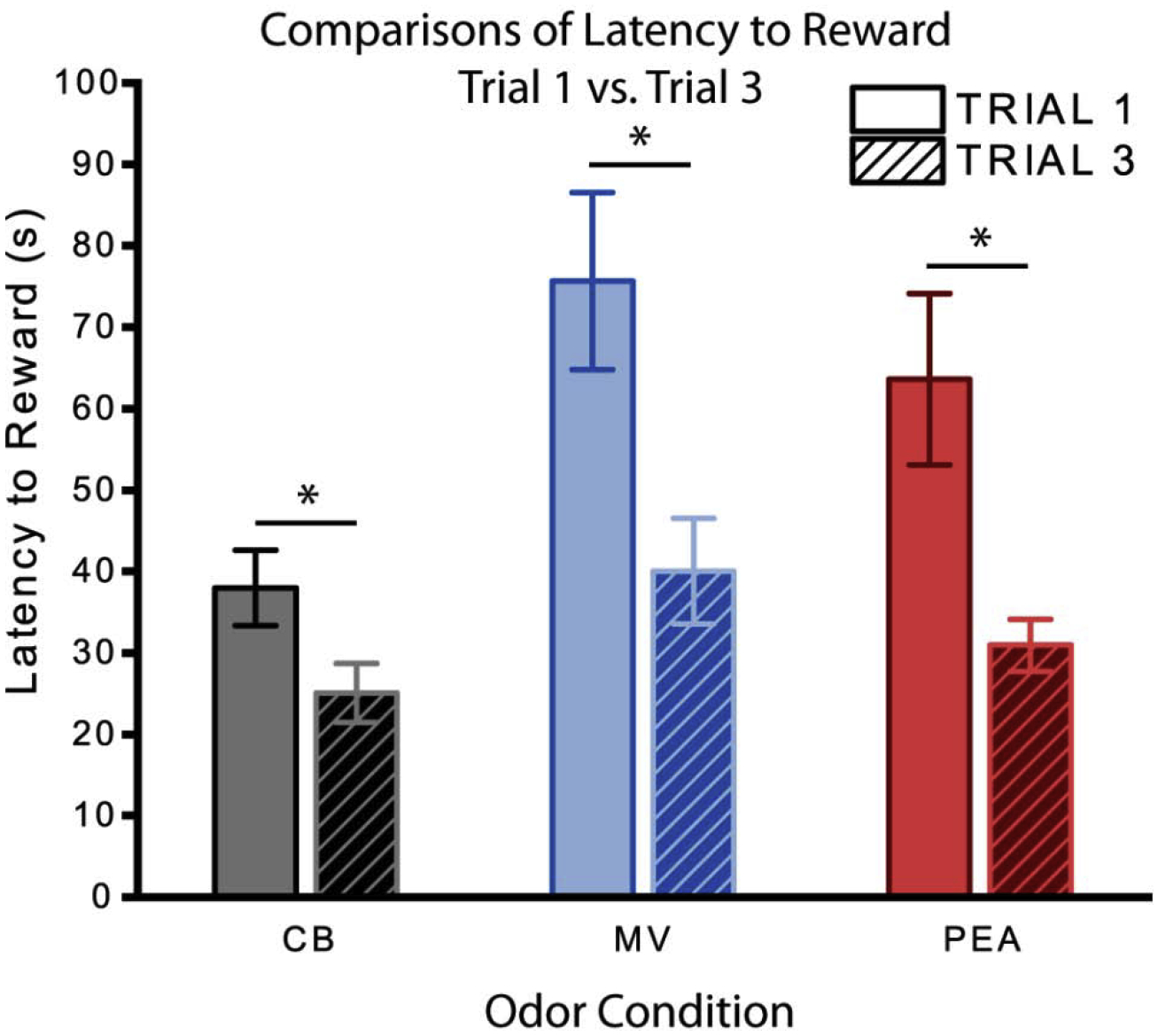
Comparisons of overall latency to obtain reward between trials 1 and 3. By trial 3, the average latency to reward significantly decreased for all groups compared to trial 1 [χ2 (1) = 26.14, p = 0.00]. Error bars show mean ± SEM. Asterisk indicates P < 0.01. CB: n = 41 (19 males); MV: n = 38 (21 males); PEA: n = 40 (19 males). CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine.
Fig. 7. Trial 3 behavioral results.
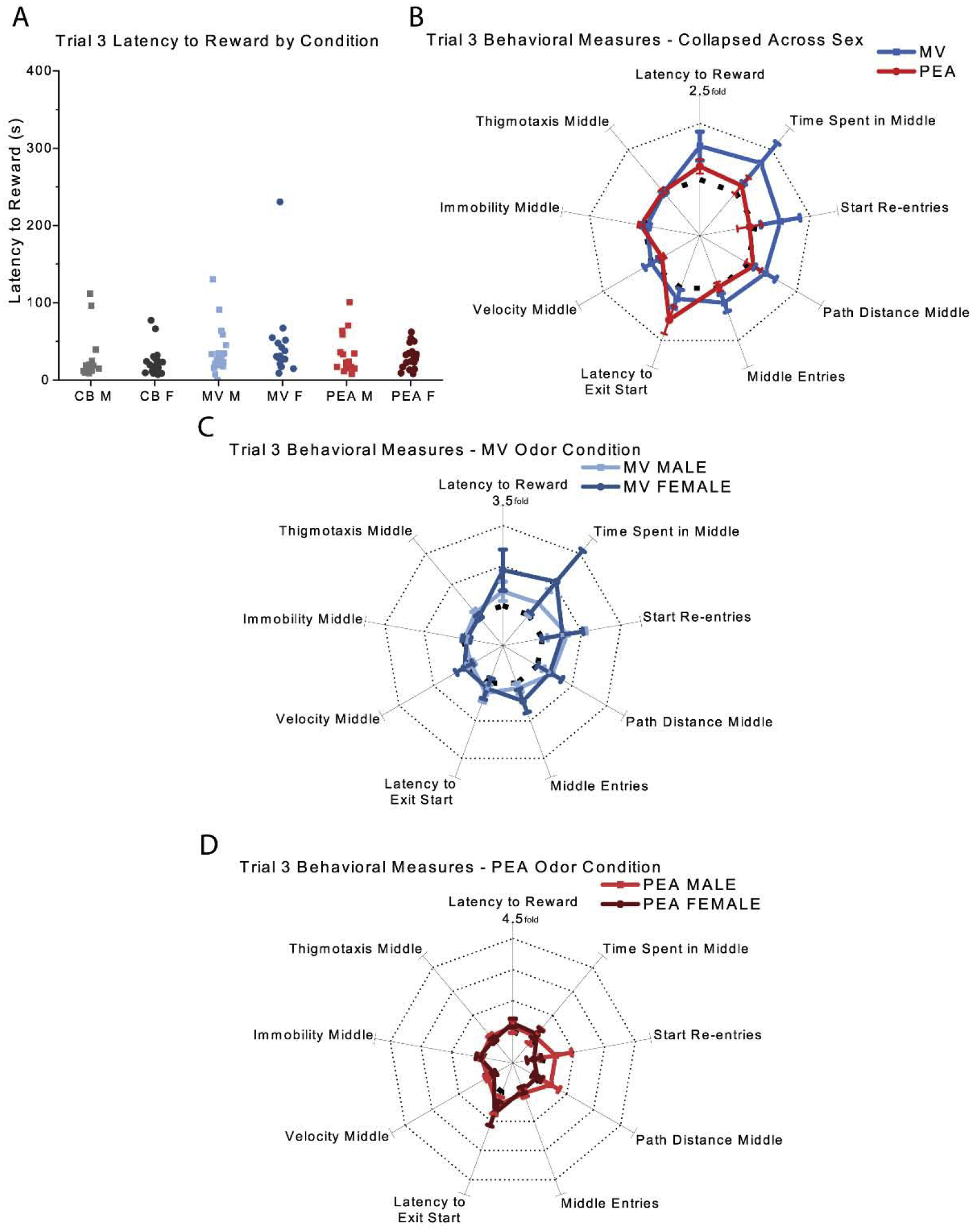
(A) Measures of latency to reward by odor condition and sex (B) Normalized behavioral results collapsed across sex (C&D) Normalized behavioral results grouped by sex for animals in the MV (C) and PEA (D) conditions. Bolded dashed line indicates the unit circle of a one-fold increase, and dotted lines indicate subsequent increases (e.g., two-fold increase, three-fold increase, etc., compared to CB controls) in B, C, and D. Error bars show mean ± SEM. CB: n = 41 (19 males); MV: n = 38 (21 males); PEA: n = 40 (19 males).CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine; M, male; F, female.
3.3. Global patterns of c-fos expression
Collapsing across all brain regions, there was no significant main effect of odor condition [χ2 (2) = 2.84, p = 0.24] or sex [χ2 (1) = 1.10, p = 0.30] on total c-fos expression. To explore the effect of MV and PEA exposure across regions, we normalized the MV and PEA group data to the clean bedding results for each region as shown in Fig. 9A, where the dotted line denotes the unit circle of clean bedding data. Mice in the MV group (blue) exhibited similar levels of c-fos expression to the unscented CB control group (dotted line), with the exception of notably higher Fos levels in paraventricular hypothalamus and modest increases in cortical and basomedial amygdala (both olfactory-driven structures). By contrast, mice in the PEA group exhibited higher Fos levels than the unscented CB control group on average in 12 of the 14 regions assayed, including olfactory regions, hypothalamic regions, and aversion-associated amygdala regions. Absolute values are presented in Table 2 and supplemental Fig. 1.
Fig. 9. Fos results across all brain regions.
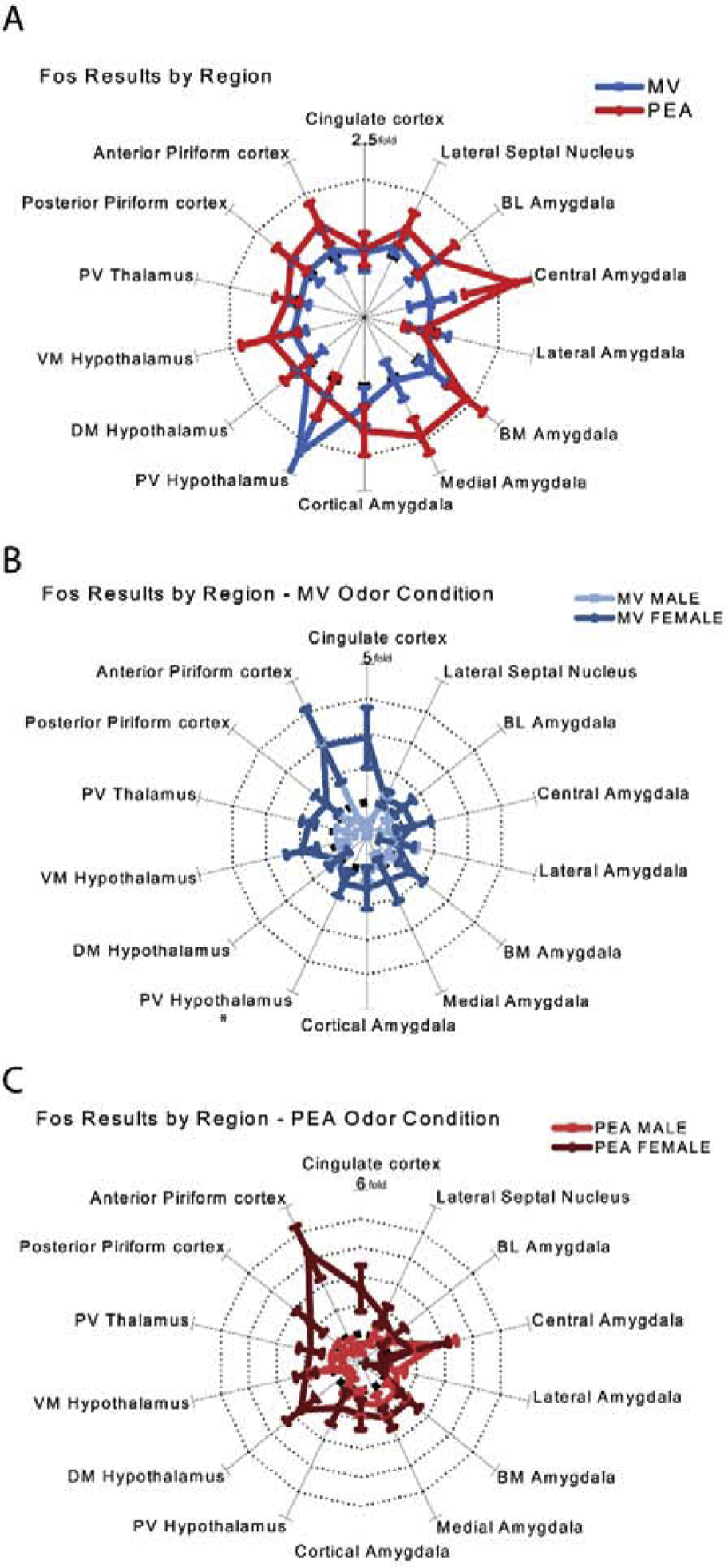
(A) Normalized Fos results collapsed across sex (B&C) Absolute Fos-immunoreactive cell counts per square millimeter for each brain region in males (B) and females (C). (D&E) Normalized Fos results grouped by sex for animals in the MV (D) and PEA (E) conditions. Bolded dashed line indicates the unit circle of a one-fold increase, and dotted lines indicate subsequent increases (e.g., two-fold increase, three-fold increase, etc., compared to CB controls in B, C, and D. Error bars show mean ± SEM. CB: n = 18 (10 males); MV: n = 18 (11 males); PEA: n = 22 (10 males). The data for paraventricular hypothalamus from males within the methyl valerate condition (denoted with an asterisk) were excluded due to a small sample size (n = 3). CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine; BL Amygdala, basolateral amygdala; BM Amygdala, basomedial amygdala, PV Hypothalamus, paraventricular hypothalamus; DM Hypothalamus, dorsomedial hypothalamus; VM Hypothalamus, ventromedial hypothalamus; PV Thalamus, paraventricular thalamus.
Table 2. Fos results across all brain regions.
Absolute Fos-immunoreactive cell counts per square millimeter for each brain region (mean±S.E.M).
| Fos Results | ||||||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Region | CB | MV | PEA | CB | MV | PEA |
| Olfactory related regions | ||||||
| Anterior piriform cortex | 14.9 ± 3.7‡ | 43.5 ± 17.7 | 62.9 ± 15.0† | 44.8 ± 11.7 | 23.9 ± 9.3 | 27.6 ± 11.6 |
| Posterior piriform cortex | 32.5 ± 8.1 | 48.7 ± 14.0 | 73.1 ± 20.0 | 55.9 ± 13.6 | 44.9 ± 16.3 | 44.8 ± 13.4 |
| Cortical amygdala | 31.1 ± 9.0 | 46.8 ± 18.9 | 56.1 ± 16.4 | 25.6 ± 7.6 | 29.3 ± 3.5 | 36.3 ± 11.6 |
| Medial amygdala | 23.0 ± 7.7 | 32.3 ± 16.0 | 49.5 ± 8.7 | 22.3 ± 5.3 | 16.7 ± 4.6 | 36.8 ± 8.9 |
| Amygdala regions | ||||||
| Basomedial amygdala | 23.9 ± 7.2 | 39.3 ± 11.2 | 51.6 ± 10.6 | 22.3 ± 5.0 | 22.3 ± 5.5 | 35.4 ± 9.9 |
| Basolateral amygdala | 18.1 ± 5.2 | 24.7 ± 6.3 | 25.7 ± 9.6 | 11.0 ± 2.4 | 9.2 ± 1.8 | 12.7 ± 1.6 |
| Lateral amygdala | 19.8 ± 5.3 | 17.4 ± 10.3 | 10.8 ± 7.2 | 12.4 ± 4.5 | 13.8 ± 4.8 | 15.7 ± 4.0 |
| Central amygdala | 11.0 ± 4.0 | 15.1 ± 5.8 | 21.1 ± 13.2 | 7.2 ± 2.7 | 4.8 ± 2.0 | 19.1 ± 5.3 |
| Hypothalamic regions | ||||||
| Paraventricular hypothalamus | 79.8 ± 23.5 | 121.7 ± 32.1 | 139.6 ± 51.1 | 82.0 ± 19.0 | 269.0 ± 209.0 | 76.2 ± 28.2 |
| Dorsomedial hypothalamus | 34.3 ± 7.5 | 36.0 ± 12.9‡ | 91.2 ± 22.4 | 84.1 ± 19.4 | 86.4 ± 25.9 | 44.4 ± 9.3 |
| Ventromedial hypothalamus | 15.0 ± 3.5 | 29.1 ± 7.0 | 29.5 ± 5.5 | 32.3 ± 7.6 | 22.9 ± 6.6 | 38.7 ± 20.5 |
| Forebrain regions | ||||||
| Lateral septal nucleus | 66.7 ± 20.1 | 80.8 ± 16.3 | 120.2 ± 24.7 | 70.3 ± 18.3 | 78.2 ± 13.2 | 71.0 ± 23.8 |
| Cingulate cortex | 32.5 ± 7.3‡ | 92.8 ± 28.5 | 83.4 ± 26.0 | 88.1 ± 17.9‡ | 28.7 ± 10.9† | 42.0 ± 15.0 |
| Thalamic region | ||||||
| Paraventricular thalamus | 41.5 ± 9.3 | 71.9 ± 8.9 | 74.2 ± 17.6 | 90.7 ± 25.6 | 56.5 ± 14.2 | 77.3 ± 17.0 |
P<0.05 relative to same sex CB counterparts;
P<0.05 relative to the opposite sex in the same odor condition.
CB: n = 18 (10 males); MV: n = 18 (11 males); PEA: n = 22 (10 males). CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine
Although odor condition and sex by themselves had no main effect, based on the behavioral outcomes we hypothesized that sex and odor condition might interact. Overall c-fos expression across all brain regions did indeed exhibit a significant sex*condition interaction [χ2 (2) = 11.17, p = 0.00] (Fig. 9B&C, 10A). Since we had no a priori hypotheses about the nature of this interaction (beyond the belief that different brain regions should be considered individually) and many possibilities to consider, we performed exploratory comparisons across brain regions. When the MV group was normalized by sex (e.g. MV males normalized to CB males and MV females normalized to CB females) as in Fig. 9D, females exhibited greater average c-fos expression than CB controls in 12 out of 14 brain regions. Males, by contrast, exhibited similar c-fos expression as the CB controls (Fig. 9D). When the PEA group was normalized the same way, females exhibited greater average c-fos expression in 13 out of 14 regions (all but lateral amygdala), while PEA-exposed males exhibited generally similar results to CB males except for an increase in Fos in 5 regions, all of them amygdala subnuclei (Fig. 9E).
Fig. 10. Fos results for selected regions.

There were significant sex*condition interactions in Fos-immunoreactive cells when collapsed across all brain regions [χ2 (2) = 11.17, p = 0.00] (A), and in the anterior piriform cortex [χ2 (2) = 14.54, p = 0.00] (B), dorsomedial hypothalamus [χ2 (2) = 17.58, p = 0.00] (C), and cingulate cortex [χ2 (2) = 21.29, p = 0.00] (D). Error bars show mean ± SEM. CB: n = 18 (10 males); MV: n = 18 (11 males); PEA: n = 22 (10 males).CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine; M, male; F, female.
This interaction seemed especially notable in several brain regions. In the anterior piriform cortex, which is strongly driven by olfactory stimulation, there was a significant [sex*condition interaction [χ2 (2) = 14.54, p = 0.00]. Males surprisingly exhibited similar, even slightly reduced c-fos expression in the MV- and PEA-exposed groups compared to the CB controls despite the increased olfactory stimulation in these groups. By contrast, the females exhibited increased c-fos expression in the PEA group and modestly increased Fos in the MV group compared to CB controls (Fig. 10B). A significant sex*condition interaction was observed in the dorsomedial hypothalamus [χ2 (2) = 17.58, p = 0.00]. PEA exposure evoked significant changes in c-fos expression but in different directions (Fig. 10C) – in females PEA exposure increased c-fos expression relative to clean bedding, while in males it decreased it. In the cingulate cortex, there was a significant sex*condition interaction [χ2 (2) = 21.29, p = 0.00]. Exposure to either odorant increased c-fos expression in females but decreased it in males (Fig. 10D). Interestingly, all three of these regions and only these regions exhibited both significant sex*condition interaction and higher c-fos expression in males than females in the CB control group (Fig. 10A–D). Note that the sample size (N=58 mice) was too small to permit pairwise post-hoc testing of these experimental groups given the need to correct for the large numbers of implicit comparisons (see Methods). We thus limit our claims to the significance of the sex*condition interaction and consider the apparent mean differences among groups to be suggestive.
4. Discussion
In the current study, we demonstrate that in an approach-avoidance paradigm, the first encounter with both a novel neutral odor and a novel predator odor evoked delays in obtaining the reward. Though mice encountering the predator odor exhibited a modest delay before entering the scented chamber compared to neutral odor and no odor controls, they did not otherwise exhibit evidence of fear or aversion compared to the mice exposed to the neutral novel odorant. However, the first predator odor presentation evoked a notable sex difference, where female mice all rapidly traversed the scented chamber while many male mice exhibited exploratory or investigatory behaviors. By the third odor exposure session, all groups transited the scented chamber equally quickly. Patterns of c-fos expression assessed after the behavioral session revealed that the neutral odor and the predator odor evoked different regional expression patterns despite the similar behaviors they evoked. Paralleling the behavioral results, male and female mice exposed to the predator odor exhibited different patterns of c-fos expression.
The predator odor employed was 2-phenylethylamine (PEA), a biogenic amine found in urine of bobcats and other predators that selectively activates trace amine-associated receptors (TAARs) expressed in the nasal epithelium [10, 13]. Rodents have the ability to detect trace quantities of PEA because of the high sensitivity of the TAAR4 receptor [11, 14]. This low detection threshold for amines found in predator urine may be an adaptive mechanism to detect the presence of predators even at long distances in order to ensure survival [11], and PEA does elicit innate avoidance behavior [10, 11]. The special status of PEA was supported in our data by its ability to drive c-fos expression in most amygdalar nuclei, notably including the central amygdala (which regulates the expression of fear and anxiety when faced with a diffuse threat [15–17] as well as in hypothalamic and thalamic regions. Furthermore, the predator odor evoked greater activity than the non-predator odor in almost all of the brain regions examined, which demonstrates that once detected by the sensory systems, information regarding PEA is broadcast widely throughout the brain. It was thus surprising that PEA-exposed mice did not spend any less time in the odorized chamber than MV-exposed mice. Previous studies of PEA’s aversiveness to rodents have not included any specific reason for the animal to approach the PEA, so the present results demonstrate that the aversiveness of PEA can be overcome within the framework of an approach-avoidance conflict. Similarly, the evolution of the behavior across three trials suggests that mice may learn that the predator odor did not represent an immediate threat within the familiar context of the behavioral apparatus.
Interestingly, females and males differed in their response to the PEA odor. In the presence of the predator cue, all females quickly transited the box to obtain the reward, while males exhibited a highly variable, possibly bi-modal distribution where half the males transited quickly but about half took longer than any female. The females’ response to PEA in the current study is reminiscent of previous reports of female “darting” behavior evoked by a shock-predictive auditory cue [18]. Fos expression exhibited an analogous pattern as the behavioral results, with significant sex*condition interactions observed within the anterior piriform cortex, cingulate cortex, and dorsomedial hypothalamus. In all three of these regions, females exposed to the PEA odorant exhibited increased levels of c-fos expression compared to the CB condition. In contrast, males exposed to PEA displayed similar levels of c-fos expression as the CB condition in anterior piriform cortex, and reduced Fos levels in both cingulate cortex and dorsomedial hypothalamus. The results from the dorsomedial hypothalamus are particularly interesting because of its involvement in regulating emotional responses such as arousal, attention, motivation, and the innate defensive response [19, 20] as well as the representation of thirst and fear [21]. The apparent differential activation of the dorsomedial hypothalamus in females and males in the PEA group could be considered a correlate of the animal’s decision to “ignore the threat and go for water.” The anterior cingulate cortex is thought to serve a “gating” function, regulating information flow from sensory cortices to the amygdala [22]. The opposing patterns of anterior cingulate cortex (ACC) activity between PEA-exposed males and females is consistent with previous human literature where acute stressors have been found to enhance ACC activity in females, but not males [23]. Differential Fos activity was also demonstrated in the piriform cortex which not only responds to odor stimuli, but also to stress [24]. Altogether, heightened levels of Fos activity within the anterior cingulate and anterior piriform cortices of PEA-exposed females, but not males suggests sex differences in response to a predator odor stressor. Note that the increased PEA-evoked c-fos expression in females is despite their briefer exposure to the odor than males received because they crossed the arena more quickly. These results suggest that the innate response to predator odor may differ between the sexes, at least in the context of an approach-avoidance conflict.
The PORT task was originally designed to explicitly place the subject in conflict between the desire to obtain the reward and the desire to avoid the predator odor [1]. The present study, which used more than four times as many subjects as the original study, reports a more complex dataset that challenges this interpretation. First, our inclusion of a novel non-predator odor revealed similar behavioral results to the predator odor, at least in terms of the latency metrics employed by Dent et al. [1], suggesting that much of the behavioral delay is a response to novelty, not threat per se. Second, the Dent et al. [1] study found no significant effect of test trial number (p = 0.10, mice from two very different strains pooled) and thus averaged all their data across trials. We observed diverse behavior on test trial 1, but by trial 3 almost all mice quickly transited the middle chamber for a reward and the effects of odor type and sex had vanished. Third, like the Dent et al. [1] study, we observed that mice took significantly longer to enter the PEA-scented middle chamber compared to the MV-scented or unscented chamber on trial 1. However, this accounted for only a few seconds before the mice entered the scented middle arena, where mice spent most of their time. For both the PEA and MV groups, mice spent more time in the middle chamber than in the clean bedding condition. This seemingly contradicts the expected result that animals would seek to avoid PEA by minimizing their exposure to it, even though the seemingly elevated central amygdalar c-fos expression suggests that PEA exposure was more anxiogenic than MV or clean bedding. It may be that in this experimental context PEA evokes exploratory behavior as a form of threat assessment [2] that is similar to the novel odor investigation presumably evoked by MV. Alternatively, it may be that in the absence of a localized odor source (because the odorant was distributed homogeneously through the middle chamber), the mice may not have had appropriate spatial cues to avoid the odorant. Finally, Dent et al. [1] only included male mice in their study, whereas we included both sexes and found significant behavioral and neural differences between males and females.
The results reported here are unlikely to be due to females’ inability to detect PEA, since females exhibited enhanced anterior piriform cortex activity despite their reduced time spent with the odor stimulus. However, given our previous report of differences in peripheral sensory responses to odors in male and female mice [25], it is conceivable that detection thresholds for the PEA odor may differ between males and females, though this has not been observed in previous studies [12] and is not supported by the latency to enter the middle data. Similarly, one conceivable mechanism for the behavioral sex difference would be if the PEA were intense enough to activate the nasal trigeminal system, which responds to potentially noxious chemical stimuli and evokes a feeling of nasal irritation or discomfort. The 300 μL PEA used in this experiment was used undiluted in liquid form but was physically distributed over 500 mL of absorbent bedding, so it is difficult to know whether it would have reached a high enough concentration in the nose to activate trigeminal fibers. This chemosensory system is more sensitive in females than in males [26], and thus has the potential to induce sex-dependent responses.
In the present study, male and female mice exhibited different risk-taking behavior under conditions of potential predator threat, with the females minimizing their exposure to the predator odorant, while some males exhibited notable risk-taking behavior. This is consistent with a broad literature showing sex differences in risk-taking across species, including mice, rats [27, 28], zokors [29], crickets [30], and humans [31, 32]. The comparatively more risk-prone behavior of males has been proposed to correspond to differences in reproductive goals, where the males risking exposure to predators might increase mating opportunities, while females are more risk-averse to facilitate the survival of their offspring [27]. The neuroendocrine basis of these differences may include direct effects of circulating sex hormones on risk taking decision-making [33], or the interaction of sex hormones and stress hormones [34], with evidence accumulating for other modulators like oxytocin and vasopressin [35]. The neural circuits involved in risk taking decision making are complex (e.g. Fig. 9) and likely depend on the behavioral circumstances in addition to the risk-reward balance. Recent evidence suggests that risky decision-making in rodents is even influenced by early life stress, where female mice raised in a stressful environment exhibit slower response times on the PORT task and fewer risk assessment behaviors in the elevated plus maze [36], changes found to have some epigenetic basis. Understanding sexually differentiated risk-taking behavior will thus require disentangling sex differences in sensory processing, epigenetic factors, neuroendocrine states, and neural circuits. However, given the role dysfunctional decision-making plays in mental disorders with sex differences in prevalence, including gambling disorders [37], eating disorders [38], and drug abuse [39], any insights gained could have far-reaching effects on public health.
Supplementary Material
Table 1. Behavioral results for trials 1 and 3.
Results from the 9 behavioral measures scored for trials 1 and 3 (mean±S.E.M).
| Behavioral Results | |||||||
|---|---|---|---|---|---|---|---|
| Female | Male | ||||||
| CB | MV | PEA | CB | MV | PEA | ||
| Trial 1 | Latency to reward (s) | 41.1±6.3 | 74.9±16.8 | 41.6±3.5 | 34.2±6.8 | 76.2±14.7 | 87.8±20.7 |
| Time spent middle (s) | 22.9±5.1 | 35.7±8.1 | 23.1±2.3 | 21.9±6.1 | 50.6±11.8 | 57.7±17.5 | |
| Number of start re-entries | 1.09±0.25 | 2.29±0.63 | 1.28±0.20 | 0.63±0.17 | 1.65±0.3 | 2.05±0.5 | |
| Path distance middle (cm) | 181±23 | 304±60† | 209±30 | 123±19 | 300±54† | 331±75 | |
| Number of middle entries | 2.86±0.44 | 5.17±1.07 | 2.85±0.34 | 2.05±0.29 | 3.70±0.51 | 4.26±0.7† | |
| Latency to exit start (s) | 1.8±0.3 | 2.0±0.4 | 3.6±0.9 | 2.1±0.3 | 2.9±0.8 | 4.1±0.9 | |
| Velocity middle (cm/s) | 11.55±1.11 | 12.43±1.68 | 10.23±1.51 | 10.08±2.07 | 8.07±0.6 | 8.80±1.00 | |
| Immobility middle (s) | 0.6±0.0 | 0.6±0.0 | 0.7±0.0 | 0.7±0.0 | 0.7±0.0 | 0.7±0.0 | |
| Thigmotaxis middle (s) | 0.9±0.1 | 0.8±0.1 | 0.9±0.0 | 0.8±0.0 | 1.0±0.1† | 0.9±0.0 | |
| Trial 3 | Latency to reward (s) | 23.6±3.7 | 44.7±12.2 | 30.4±3.4 | 26.6±6.6 | 36.2±6.4 | 31.4±5.7 |
| Time spent middle (s) | 9.4±2.1 | 19.6±9.6 | 11.3±1.8 | 13.4±5.2 | 18.6±5.6 | 15.0±4.2 | |
| Number of start re-entries | 0.54±0.19 | 0.82±0.29 | 0.38±0.13 | 0.26±0.10 | 0.42±0.13 | 0.36±0.14 | |
| Path distance middle (cm) | 121±22 | 164±43 | 109±20 | 88±15 | 125±23 | 124±28 | |
| Number of middle entries | 1.63±0.25 | 2.41±0.51 | 1.52±0.19 | 1.47±0.22 | 1.66±0.20 | 1.52±0.19 | |
| Latency to exit start (s) | 6.1±1.3 | 6.9±1.7 | 10.5±2.6 | 5.8±1.2 | 7.3±1.4 | 8.5±1.8 | |
| Velocity middle (cm/s) | 17.90±3.38 | 19.86±4.31 | 12.36±1.34 | 12.46±1.45 | 11.45±1.26 | 11.44±1.16 | |
| Immobility middle (s) | 0.5±0.0 | 0.5±0.1 | 0.6±0.1 | 0.6±0.0 | 0.6±0.1 | 0.7±0.0 | |
| Thigmotaxis middle (s) | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | 0.9±0.1 | |
P<0.05 relative to same sex CB counterparts.
CB: n = 41 (19 males); MV: n = 38 (21 males); PEA: n = 40 (19 males). CB, clean bedding; MV, methyl valerate; PEA, phenylethylamine.
Acknowledgements
This work was supported by the National Institute of Mental Health (R01 MH101293 to JPM) and the National Institute on Deafness and Other Communication Disorders (R0I DC013090 to JPM). We would like to thank Christine N. Yohn and Mark M. Gergues for their c-fos immunohistochemistry protocol. We also thank the members of the McGann lab for their helpful discussion on the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None.
References
- 1.Dent CL, Isles AR, and Humby T, Measuring risk-taking in mice: balancing the risk between seeking reward and danger. Eur J Neurosci, 2014. 39(4): p. 520–30. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard RJ, et al. , The characterization and modelling of antipredator defensive behavior. Neurosci Biobehav Rev, 1990. 14(4): p. 463–72. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd JK, et al. , The anxiety/defense test battery: influence of gender and ritanserin treatment on antipredator defensive behavior. Physiol Behav, 1992. 51(2): p. 277–85. [DOI] [PubMed] [Google Scholar]
- 4.Buron G, et al. , Comparative behavioral effects between synthetic 2,4,5-trimethylthiazoline (TMT) and the odor of natural fox (Vulpes vulpes) feces in mice. Behav Neurosci, 2007. 121(5): p. 1063–72. [DOI] [PubMed] [Google Scholar]
- 5.Perrot-Sinal TS, et al. , Sexually dimorphic aspects of spontaneous activity in meadow voles (Microtus pennsylvanicus): effects of exposure to fox odor. Behav Neurosci, 1996. 110(5): p. 1126–32. [DOI] [PubMed] [Google Scholar]
- 6.Pellman BA, et al. , Sexually Dimorphic Risk Mitigation Strategies in Rats. eNeuro, 2017. 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flurkey K, C. J, Harrison DE, The Mouse in Aging Research American College of Laboratory Animal Medicine, ed. Fox JG. 2007, Amsterdam; Boston: Elsevier. [Google Scholar]
- 8.Laboratory H Life Span as a Biomarker. n.d. [cited 2019 December]; Available from: www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker.
- 9.Hagan C When Are Mice Considered Old? 2017. [cited 2019 December]; Available from: www.jax.org/news-and-insights/jax-blog/2017/november/when-are-mice-considered-old.
- 10.Ferrero DM, et al. , Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A, 2011. 108(27): p. 11235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewan A, et al. , Non-redundant coding of aversive odours in the main olfactory pathway. Nature, 2013. 497(7450): p. 486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewan A, et al. , Single olfactory receptors set odor detection thresholds. Nat Commun, 2018. 9(1): p. 2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi LK, Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci, 2014. 8: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, et al. , Ultrasensitive detection of amines by a trace amine-associated receptor. J Neurosci, 2013. 33(7): p. 3228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shackman AJ, et al. , The neurobiology of dispositional negativity and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. J Exp Psychopathol, 2016. 7(3): p. 311–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapp BS, et al. , Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav, 1979. 23(6): p. 1109–17. [DOI] [PubMed] [Google Scholar]
- 17.Davis M, Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol, 2006. 61(8): p. 741–756. [DOI] [PubMed] [Google Scholar]
- 18.Gruene TM, et al. , Sexually divergent expression of active and passive conditioned fear responses in rats. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staubli U, Schottler F, and Nejat-Bina D, Role of dorsomedial thalamic nucleus and piriform cortex in processing olfactory information. Behav Brain Res, 1987. 25(2): p. 117–29. [DOI] [PubMed] [Google Scholar]
- 20.Freitas RL, et al. , GABA(A) receptor blockade in dorsomedial and ventromedial nuclei of the hypothalamus evokes panic-like elaborated defensive behaviour followed by innate fear-induced antinociception. Brain Res, 2009. 1305: p. 118–31. [DOI] [PubMed] [Google Scholar]
- 21.Sewards TV and Sewards MA, Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull, 2003. 61(1): p. 25–49. [DOI] [PubMed] [Google Scholar]
- 22.Hamner MB, Lorberbaum JP, and George MS, Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety, 1999. 9(1): p. 1–14. [PubMed] [Google Scholar]
- 23.Wang J, et al. , Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci, 2007. 2(3): p. 227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham IM and Kovacs KJ, Postnatal handling alters the activation of stress-related neuronal circuitries. Eur J Neurosci, 2000. 12(8): p. 3003–14. [DOI] [PubMed] [Google Scholar]
- 25.Kass MD, et al. , Changes in Olfactory Sensory Neuron Physiology and Olfactory Perceptual Learning After Odorant Exposure in Adult Mice. Chem Senses, 2016. 41(2): p. 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundstrom JN, et al. , Sex differentiated responses to intranasal trigeminal stimuli. Int J Psychophysiol, 2005. 57(3): p. 181–6. [DOI] [PubMed] [Google Scholar]
- 27.Jolles JW, Boogert NJ, and van den Bos R, Sex differences in risk-taking and associative learning in rats. R Soc Open Sci, 2015. 2(11): p. 150485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini CA, et al. , Sex differences in a rat model of risky decision making. Behav Neurosci, 2016. 130(1): p. 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegab IM, Qian Z, Pu Q, Wang Z, Cai Z, Guo H, … & Su J, Gender difference in unconditioned and conditioned predator fear responses in Smith’s zokors (Eospalax smithii). Global ecology and conservation, 2018. 16. [Google Scholar]
- 30.Tanis BP, Bott B, and Gaston BJ, Sex-based differences in anti-predator response of crickets to chemical cues of a mammalian predator. PeerJ, 2018. 6: p. e4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bos R, Homberg J, and de Visser L, A critical review of sex differences in decision-making tasks: focus on the Iowa Gambling Task. Behav Brain Res, 2013. 238: p. 95–108. [DOI] [PubMed] [Google Scholar]
- 32.Cobey KD, et al. , Sex differences in risk taking behavior among Dutch cyclists. Evol Psychol, 2013. 11(2): p. 350–64. [PubMed] [Google Scholar]
- 33.Wallin-Miller KG, et al. , Sex differences and hormonal modulation of ethanol-enhanced risk taking in rats. Drug Alcohol Depend, 2017. 174: p. 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barel E, Shahrabani S, and Tzischinsky O, Sex Hormone/Cortisol Ratios Differentially Modulate Risk-Taking in Men and Women. Evol Psychol, 2017. 15(1): p. 1474704917697333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel N, et al. , Oxytocin and vasopressin modulate risk-taking. Physiol Behav, 2015. 139: p. 254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viola TW, et al. , Postnatal impoverished housing impairs adolescent risk-assessment and increases risk-taking: A sex-specific effect associated with histone epigenetic regulation of Crfr1 in the medial prefrontal cortex. Psychoneuroendocrinology, 2019. 99: p. 8–19. [DOI] [PubMed] [Google Scholar]
- 37.van den Bos R, et al. , Cross-species approaches to pathological gambling: a review targeting sex differences, adolescent vulnerability and ecological validity of research tools. Neurosci Biobehav Rev, 2013. 37(10 Pt 2): p. 2454–71. [DOI] [PubMed] [Google Scholar]
- 38.Steward T, et al. , Enduring Changes in Decision Making in Patients with Full Remission from Anorexia Nervosa. Eur Eat Disord Rev, 2016. 24(6): p. 523–527. [DOI] [PubMed] [Google Scholar]
- 39.Kluwe-Schiavon B, et al. , Similarities between adult female crack cocaine users and adolescents in risky decision-making scenarios. J Clin Exp Neuropsychol, 2016. 38(7): p. 795–810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


