Abstract
Single cell branching during development in vertebrates is typified by neuronal branching to form neurites and vascular branches formed by sprouting angiogenesis. Neurons and endothelial tip cells possess subcellular protrusions that share many common features from the morphological to the molecular level. Both systems utilize filopodia as their cellular protrusion organelles and depend on specific integrin-mediated adhesions to the local extracellular matrix for guidance in their pathfinding. We discuss the similar molecular machineries involved in these two types of cell branch formation and use their analogy to propose a new mechanism for angiogenic filopodia function, namely as adhesion assembly sites. In support of this model we provide primary data of angiogenesis in zebrafish in vivo showing that the actin assembly factor VASP participates in both filopodia formation and adhesion assembly at the base of the filopodia, enabling forward progress of the tip cell. The use of filopodia and their associated adhesions provide a common mechanism for neuronal and endothelial pathfinding during development in response to extracellular matrix cues.
1. Introduction
Branching morphogenesis is a recurrent theme in the development of multicellular organisms and is critical for the formation of many tissues and organs. There are two basic types of branching morphogenesis that occur in development; branching of multicellular epithelial sheets and tubes, and branching of single cells. The former, which is more widely referred to as branching morphogenesis, involves the development of branched epithelial tubes, as observed in lung, kidney, and salivary gland development in vertebrates (reviewed in (Varner and Nelson, 2014)). Cells within epithelial sheets in these tissues have apical-basal polarity, with their basal surface bound to the extracellular matrix (ECM) and their apical sides facing a lumen. Epithelial sheets can form tubes and tubes can branch either by localized cell division or individual cell shape change. In developing mouse salivary glands, kidneys, and lungs, branches extend from sheets or tubes by out-of-plane asymmetric cell division in response to local growth factor cues that are released by supporting mesenchymal cells (Varner and Nelson, 2014; Bernfield et al., 1972; Qiao et al., 1999; Weaver et al., 2000). In contrast, in mammary and lung morphogenesis, simple changes in the shape or relative positions of groups of cells can drive epithelial sheet bending or puckering to produce a branch (Ewald et al., 2008; Schnatwinkel and Niswander, 2013; Kim et al., 2013).
In contrast to multicellular epithelial sheet branching morphogenesis, in single cell branching morphogenesis, localized subcellular protrusions from the cell body give individual cells a branched architecture. Cells can stably maintain a branched architecture over time as in dendritic cells, neurons and melanocytes (Collin and Milne, 2016; Jan and Jan, 2010; Mort et al., 2015), or the branches can be dynamic and contribute to invasive migratory and pathfinding developmental programs such as elaboration of the nervous and vascular systems in animals or trachea development in Drosophila (Caussinus et al., 2008). In the case of neurons, subcellular branching arises from cone formation and subsequent elaboration of very long cellular processes, i.e. axons and dendrites. In the case of the vascular system, subcellular branches lead the way for the subsequent migration of the trailing cell body and attached cells along the branch pathway to elaborate the arboreal tissue architecture. Branching cells that lead trailing cells in a tissue such as endothelium are known as “tip cells.” Tip cells do not have an apical-basal polarity, but generate protrusive branches at their leading edges and thus have a “front-back” polarity similar to that of a migrating mesenchymal cell. Like migrating mesenchymal cells, productive advance of either growth cones or tip cells during branching migration requires adhesion of the branched protrusions to the surrounding extracellular matrix (ECM), where the ECM serves not only as a source of signal transduction, but also as a physical or haptotactic “road.”
In single cell branching morphogenesis, protrusion of subcellular branches and adhesion of those branches to the ECM are mediated by filopodia and focal adhesions. This review and the primary data presented herein is focused on the functions of filopodia and focal adhesions in single cell branching morphogenesis during neuronal pathfinding and angiogenesis to illustrate common mechanisms regulating these processes.
2. Filopodia fundamentals
To understand the role of filopodia in single cell branching morphogenesis during neuronal pathfinding and angiogenesis, we will first provide a brief overview of the cell biology of filopodia formation and architecture that has been gleaned primarily from studies of mesenchymal cells in tissue culture. Filopodia are thin, rod-like cell protrusions produced by polymerization of unbranched actin filaments arranged in tight parallel bundles with their polymerizing barbed (fast growing) ends at the filopodium tip, and their mechanism of formation and molecular architecture is conserved across large phylogenetic distances (Petersen et al., 2016).
Filopodia formation is mediated by the initiation of very localized actin polymerization at the cell membrane in response to activation of the small GTPase Cdc42 (Nobes and Hall, 1995; Castellano et al., 1999) to produce a small bundle of elongating filaments that generate a cylindrical protrusion of the cell membrane. Downstream of Cdc42, localized actin assembly may either be nucleated de novo, or produced by elongation factors that mediate the assembly of pre-existing leading edge filaments. These two mechanisms are termed “tip nucleation” and “convergent extension” (Yang and Svitkina, 2011). In the tip nucleation model, Cdc42 directly activates formin family proteins such as FMNL2, FMNL3, and mDia2, which nucleate the formation of actin filaments and mediate their subsequent elongation (Skau et al., 2015; Campellone and Welch, 2010; Gardberg et al., 2016; Barzik et al., 2014; Young et al., 2015). In the convergent extension model, Cdc42 activates N-WASP, which in turn activates the Arp2/3 complex, which produces branched actin filament networks (Mullins and Pollard, 1999; Svitkina and Borisy, 1999). Most filaments generated by Arp2/3 are rapidly capped by capping protein, however a subset can be converged at the membrane by clusters of proteins that protect them from capping and mediate their elongation, including either formins or the members of the ena/VASP family (Young et al., 2015; Svitkina et al., 2003; Bear et al., 2002; Bombardier et al., 2015). These two mechanisms of initiation are not mutually exclusive and may be cell type dependent (Yang and Svitkina, 2011; Young et al., 2015).
Once localized actin assembly is initiated at the membrane, filopodia require several other components for their growth and maintenance. Filopodial actin filaments are bundled tightly together by the actin crosslinking protein fascin (Jansen et al., 2011; DeRosier and Edds, 1980; Vignjevic et al., 2006). IRSp53, an I-BAR domain-containing and Cdc42 effector protein, links actin to the membrane and remodels the membrane into curvature consistent with filopodial diameter (Ahmed et al., 2010), and also promotes clustering of the elongation factor VASP (Disanza et al., 2013; Kast et al., 2014). Myosin X, a barbed end-directed, plasma membrane-associated motor protein (Bohil et al., 2006) delivers VASP, growth factor and guidance receptors, and cell-cell and cell-ECM adhesion molecules to the growing filopodia tip (Tokuo and Ikebe, 2004; Zhang et al., 2004; Zhu et al., 2007; Almagro et al., 2010).
In filopodia at steady state, actin is very dynamic, with continuous formin or VASP mediated assembly at the tip and cofilin and/or gelsolin-mediated disassembly at the base leading to treadmilling-driven actin retrograde flow along the filopodia shaft. Additional depolymerization and retraction forces are applied on filopodia actin filaments by non-muscle myosin II, via connections into the lamella and/or cell cortex (Bornschlögl et al., 2013; Medeiros et al., 2006; Craig et al., 2012). Filopodia length is thus governed by regulating the balance between filament assembly at the tip and disassembly at the base (Breitsprecher et al., 2011; Lu et al., 1997).
3. Filopodia in neuronal branching morphogenesis
Both neuritogenesis and growth cone advance depend on filopodia formation (Dent et al., 2007, 2011), and many of the canonical filopodia components discussed above have been shown to be required. For example, Cdc42 activity is required for neurite outgrowth and growth cone filopodia (Brown et al., 2000), and is activated in growth cones in response to the guidance molecule netrin-1 (Rappaz et al., 2016). The Cdc42 effector and membrane remodeling protein IRSp53 is required for neurite formation and dendritic branching in some neurons (Chen et al., 2015), and the actin assembly factors FMN2 and VASP may be involved in neuronal outgrowth and pathfinding (Sahasrabudhe et al., 2016; Lebrand et al., 2004). Although IRSp53 is a well-known upstream activator of Arp2/3, the role of this nucleator of branched actin assembly in growth cone advance is unclear. In some neuronal types, Arp2/3 is inhibitory to growth cone advance and filopodia are straight and unbranched (Strasser et al., 2004), while in others Arp2/3 may play an important role filopodia stability in response to local tyrosine kinase regulation (Robles et al., 2003). Actin bundling by fascin is required for arbor morphology of branching dendrites (Nagel et al., 2012) and growth cone formation in axons (Wei et al., 2014), while myosin X promotes netrin-1-mediated guidance by transporting the DCC receptor to filopodia tips (Zhu et al., 2007).
The data demonstrating that neurite formation and growth cone advance depend on canonical filopodia is substantial, but what exactly are the filopodia needed for? Two main functions have been proposed which are not mutually exclusive. The first is that filopodia function essentially as antennae, with signal receptors at their tips or along their length, and thus enable the neuron to search for cues in their environment (Heckman and Plummer, 2013; Davenport et al., 1993). The second function is simple locomotion of the growth cone. As discussed below, ECM adhesion sites are initiated by β1 integrins that are trafficked to filopodial tips by myosin X where they mediate traction forces along the filopodial axis (Chan and Odde, 2008). Thus, in addition to acting a signal-sensor, neuronal filopodia also provide adhesion formation and locomotion to the neurite.
4. Filopodia in angiogenic branching morphogenesis
The discussion above suggests that neurites and growth cones utilize conserved filopodial machinery, however, the role of filopodia and their molecular components in branching morphogenesis and migration of endothelial tip cells during angiogenesis is less well established. Long thin protrusions at the leading edge of endothelial tip cells have been observed in tissues in situ in several vertebrate species and vessel types (Zhu et al., 2007; Gerhardt et al., 2003; Kurz et al., 2001), and these protrusions are required for angiogenesis and vessel patterning (Carmeliet et al., 2009). However, whether these are canonical filopodia is not clear. On the one hand, there is evidence for filopodial components in angiogenesis. In zebrafish angiogenesis, Cdc42 has been shown to be activated by ArfGEF9b, which is induced by bone morphogenetic protein (BMP) (Wakayama et al., 2015), while in mice, knockout or endothelia-specific deletion of Cdc42 abrogates blood vessel formation during development (Jin et al., 2013; Barry et al., 2015). Furthermore, NRP1, a pro-angiogenic neuropilin, stimulates Cdc4-induced cell protrusions, and NRP1 knockdown or Cdc42 inhibition blocks protrusion formation and angiogenesis in mouse retina and zebrafish models (Fantin et al., 2015), and Cdc42 is required for protrusions and angiogenic sprouting in vitro (Nguyen et al., 2017). Angiogenesis also requires formins, particularly the endothelial-enriched formin FMNL3, which is activated by Cdc42 to induce cell protrusions and tip cell migration and pathfinding (Wakayama et al., 2015; Hetheridge et al., 2012). In addition to Cdc42 activation, BMP (BMP6 in mice) activates myosin X in angiogenesis, where it is required for protrusion formation, retinal angiogenesis, and vascular remodeling (Pi et al., 2007; Heimsath et al., 2017). Finally, like other filopodia, some endothelial tip cell protrusions such as those in retinal angiogenesis are dependent on α5β1 integrin for alignment and adhesion in their branching pattern (Stenzel et al., 2011).
Despite these data, endothelial tip cell protrusions may not be canonical filopodia for several reasons. First, some components of canonical filopodia are not required for angiogenesis. One example is fascin, which is dispensable for angiogenesis (Ma et al., 2013). Second, while canonical filopodia are unbranched and have uniform diameters along their length (Yang and Svitkina, 2011), endothelial tip cell protrusions have bulges along their length, and can be branched (see below). In addition, no role has yet been found for I-BAR proteins such as IRSp53 which is an important component of mesenchymal cell and neuronal filopodia. Finally, while most data indicate that endothelial tip cell protrusions are required for branch pathfinding during angiogenesis and vascular pattern formation, Gerhardt and colleagues showed that low concentrations of Latrunculin B, which inhibits actin polymerization, shifted tip cell protrusion architecture from long and thin to a wide and flat lamellipodia-type morphology, but did not alter the eventual vascular pattern (Phng et al., 2013). This raises the possibility that endothelial tip filopodia are not required for angiogenesis pathfinding per se, but serve to make it more efficient by allowing faster searching for soluble or haptotactic cues.
5. Focal adhesion fundamentals
In migration mediated by single cell branching morphogenesis, filopodial protrusions must be stabilized by adhesion of the protrusion to the ECM to mediate advance of the growth cone of neurons or cell body of endothelial tip cells. Cells adhere to ECM using focal adhesions, which are integrin-based, plasma membrane-associated macromolecular assemblies that physically connect the ECM to the actin cytoskeleton (Case and Waterman, 2015). This indirect coupling of actin to the ECM through integrins enables cells to both apply force to the ECM to migrate and to sense biochemical and mechanical properties of the ECM.
Cell biological studies on various cell types in tissue culture have established that the formation of focal adhesions is fundamentally coupled to actin-based protrusions by the basic mechanism of integrin activation in what has been termed the “molecular clutch” (Case and Waterman, 2015; Mitchison and Kirschner, 1988). While much of the work to elucidate this model has focused on cell migration mediated by lamellipodial protrusions, the underlying principles can be applied to both lamellipodia and filopodia, despite the differences in their actin architecture. At the leading edge of lamellipodial or filopodial protrusions in migrating cells, active actin polymerization and retrograde flow generates force on integrins to drive conformational changes that mediate a 4000-fold increase in their affinity for ECM ligand (Li and Springer, 2017). Mechanistically, this occurs by locally high PIP2 concentrations enabling talin to bind to β integrin cytoplasmic tails (Kalli et al., 2013; Moore et al., 2012) to promote the “extended closed” conformation of the integrin, and at the same time enables talin binding to F-actin, which the integrin dimer cannot do on its own. The force of actin flow transmitted to β integrin through talin induces the “extended open” conformation of integrin, which corresponds to the high-affinity ligand-binding state (Swaminathan et al., 2017). Rearward flowing actin filaments in leading edge protrusions have been likened to the running engine in a car, and the linkage of ECM-bound integrins via talin to the actin has been likened to engagement of the clutch that allows force from the cytoskeleton engine to be transmitted to traction forces on the ECM to drive cell movement (Swaminathan and Waterman, 2016).
As force is applied to the integrin-talin-actin complex and clusters of complexes are formed, a host of hundreds of adapter and signal molecules are recruited (Byron et al., 2011; Kuo et al., 2011) to generate a focal adhesion. This collection of proteins is self-assembled into a conserved nanoscale architecture with three functional layers (Kanchanawong et al., 2010; Case et al., 2015). Integrin cytoplasmic tails and focal adhesion signaling proteins such as focal adhesion kinase (FAK) and paxillin are found in the membrane proximal integrin signaling layer, while actin-associated proteins such as VASP, a-actinin, and zyxin are localized deeper in the cell along cortical actin filaments in the actin regulatory layer. Talin, which binds to both integrin β tails (Bouaouina et al., 2008; Anthis et al., 2010) and actin (Hemmings et al., 1996; Smith and McCann, 2007) spans between the integrin signaling and actin regulatory layers of the focal adhesion (Kanchanawong et al., 2010). Extension of talin under force (del Rio et al., 2009) enables binding of vinculin in an intermediate zone called the force transduction layer (Case et al., 2015), forming an essential part of the molecular clutch (Hu et al., 2007; Thievessen et al., 2013). Vinculin binding to talin and actin in the force transduction layer enables the adhesion to withstand larger pulling forces by myosin II, which further reinforces the strength of the adhesion and induces the recruitment of more proteins such as α-actinin (Stricker et al., 2013; Roca-Cusachs et al., 2013) and VASP (Zaidel-Bar et al., 2003), driving focal adhesion growth along a bundled actin template (Choi et al., 2008; Plotnikov et al., 2012; Atherton et al., 2015). This force-dependent “maturation” process is dependent on actomyosin contractility and RhoA activation (Chrzanowska-Wodnicka and Burridge, 1996), as well as FAK phosphorylation of paxillin (Pasapera et al., 2010).
For a growth cone or cell body to move beyond the site of initial adhesion formation, focal adhesions must disassemble in a regulated manner. While perhaps less well understood than adhesion assembly, disassembly of adhesions has been found to require proteolysis of talin and FAK via calpain cleavage as a rate-limiting step (Franco et al., 2004; Chan et al., 2010), and calpains also act on β-integrin associated proteins including talin (Du et al., 1995; Huttenlocher et al., 1997) and α-actinin (Sprague et al., 2008). In addition to proteolysis, focal adhesion disassembly may also be mediated by clathrin-, dynamin-, and Rab5- dependent endocytosis of focal adhesion components (Ezratty et al., 2009; Mendoza et al., 2013), or by catabolic autophagy programs (Kenific et al., 2016; Sharifi et al., 2016). FAK activity plays a role in both assembly and disassembly of focal adhesions, as FAK null cells accumulate large adhesions with reduced turnover (Ilić et al., 1995; Webb et al., 2004). Phosphorylation of several focal adhesion components by FAK and Src are required for efficient focal adhesion disassembly (Webb et al., 2004). In addition, some of these mechanisms of focal adhesion disassembly may be spatially regulated by microtubule targeting of specific focal adhesions (Kenific et al., 2016; Ezratty et al., 2005; Bhatt et al., 2002).
6. Focal adhesions in neuronal branching morphogenesis
During neuronal branching morphogenesis, for protrusions to result in neurite advance, directional forces must be applied to adhesions to pull the growth cone towards its target in response to chemotactic and haptotactic guidance cues. Neurite extension and axon guidance have long been proposed to depend on the extracellular matrix as a pre-existing path (Bozyczko and Horwitz, 1986; Dodd and Jessell, 1988). Consistent with this, numerous studies in vitro and in vivo have shown that ECM composition and expression of appropriate integrins regulates pathfinding (Myers et al., 2011; Anton et al., 1999; Graus-Porta et al., 2001). However, instead of the focal adhesions found in the lamellipodia of mesenchymal cells, adhesive sites in the advancing growth cone take the form of “point contacts”, and localize to filopodia (Renaudin et al., 1999; Gomez et al., 1996). In spite of their different formation mode, point contacts and focal adhesions share similarities, including the accumulation of talin, paxillin and vinculin, as well as stabilization by RhoA activity (Woo and Gomez, 2006). Like focal adhesions, growth cone point contacts also require calpain-mediated cleavage of talin and FAK activity for their turnover (Robles et al., 2003; Kerstein et al., 2017). Interestingly, the filopodial activator Cdc42 is required for FAK-mediated response to axon guidance cues (Myers et al., 2012), demonstrating the feedback connections between guidance cues, filopodia formation, and adhesion signaling.
Although point contacts in neurons and focal adhesions in mesenchymal cells share many common components, there are some differences in their dynamics. While focal adhesions form in Arp2/3 mediated lamellipodia in mesenchymal cells, work on neurons in tissue culture has shown that growth cone point contacts assemble at filopodia tips. After formation, point contacts often undergo sliding or slippage as they are drawn towards the filopodia base where most point contacts disassemble. A fraction of the adhesions that slide to filopodia bases persist as the growth cone expands between adjacent filopodia to extend beyond the stabilized adhesion sites (Kerstein et al., 2015). Engagement of the point contacts with actin retrograde flow in filopodia enables the forward progress of the growth cone (Lin and Forscher, 1995; Santiago-Medina et al., 2013; Nichol et al., 2016), and the highest traction forces in the growth cone are just behind the filopodia in the lamellar portion, suggesting that the adhesions may mature there (Hyland et al., 2015). However, due to their small size and the depth into tissue at which they exist, high resolution imaging of growth cone point contact dynamics in vivo has been limited (Santiago-Medina et al., 2012).
7. Focal adhesions in endothelial branching
Angiogenesis, like neuronal pathfinding, is well established as an integrin-ECM dependent process (Ingber and Folkman, 1989). Indeed, blocking the angiogenic integrins αvβ3 or α5β1 by antibodies has been a clinically attractive therapeutic strategy to suppress tumor angiogenesis (Reardon and Cheresh, 2011; Hariharan et al., 2007; Saharinen and Ivaska, 2015), although it has had limited long-term success. In addition to integrin-ECM interactions, other focal adhesion proteins are known to be important in endothelial migration and angiogenesis. Loss of FAK, for example, leads to cardiovascular defects in early embryogenesis (Ilić et al., 1995), and FAK is required for angiogenesis events in late embryogenesis and adult animals (Shen et al., 2005; Lechertier and Hodivala-Dilke, 2012) and also plays a role in angiogenic sprouting. Given these data, it is not surprising that FAK, like integrins, has shown promise as an anti-angiogenic therapy target (Stone et al., 2014). Paxillin and its homolog Hic-5, as well as talin have also been shown to play a role in angiogenesis (Dave et al., 2016; Monkley et al., 2011). Our knowledge of many other canonical focal adhesion proteins in angiogenesis such as VASP (Chen et al., 2008), vinculin (Deroanne et al., 1996), and integrin-linked kinase (Xie et al., 2013) has been limited to data from in vitro systems.
Indeed, while much is known about focal adhesion formation and associated actin dynamics in endothelial cells migrating on flat substrates in tissue culture in vitro (e.g., (Lele et al., 2008; Ingber et al., 1995; Kiosses et al., 1999)), little is known about their dynamics and formation in the context of branching morphogenesis during angiogenic sprouting. Unlike neuronal growth cones, which form very similar morphologies in vitro and in vivo (e.g., (Sahasrabudhe et al., 2016; Dwivedy et al., 2007)), endothelial cells can adopt very different morphologies depending on their context both in vitro and in vivo. When plated on 2D ECM-coated substrates in vitro, endothelial cells spread over a large area with a discoid shape and thin lamellae, like their morphology in a patent vessel wall, and as such, have focal adhesion dynamics very similar to those in a mesenchymal migrating cell. In contrast, when cultured in 3D collagen gels, endothelial cells do not form large thin lamellae, but rather form thin branched pseudopodia and filopodia (Gerhardt et al., 2003; Fischer et al., 2009; Nguyen et al., 2013), which much more closely resemble those in tip cells and neuronal protrusions. Indeed, endothelial cells cultured in such 3D contexts closely recapitulate the morphology and dynamics of those undergoing sprouting angiogenesis in vivo (Fischer et al., 2009; Nguyen et al., 2013). While we may presume that focal adhesions are formed in regions of active actin-driven protrusions during sprouting angiogenesis may be similar to the morphologically similar neuronal pathfinding paradigm, neither adhesion formation nor direct actin dynamics have been documented in endothelial tip cell migration in vivo, and so leaves open many questions with mechanistic implications. Do endothelial tip cells use mesenchymal/lamellipodial or growth cone-like/filopodial based mechanism of adhesion generation and subsequent migration? If the latter, this could imply that endothelial tip cells use their filopodia for similar mechanisms of pathfinding as neurons.
8. Primary research: filopodia and focal adhesion dynamics visualized during zebrafish angiogenesis
The above discussion highlights the similarities between neuronal and endothelial branching morphogenesis in terms of the roles for filopodia and integrin-mediated adhesions. However, the function of filopodia and adhesions in neuronal growth cones is more well-established. During guidance, protruding filopodia arrayed along the leading edge of growth cones function for efficient searching for haptotactic guidance cues. Those filopodia tips that encounter conducive ligands serve as adhesion initiation sites to allow traction force generation, and at the same time serve as anchor sites from which the filopodia can further protrude. Forward progress of the growth cone beyond the adhesion sites is mediated by subsequent advance of the growth cone lamellar region spanning between adjacent anchored filopodia. Thus, filopodia and integrin-mediated adhesions act as a dynamic, integrated system that drive directed advance of the growth cone.
Since filopodia and focal adhesions in endothelial cells undergoing branching morphogenesis during sprouting angiogenesis share many morphological and molecular features of neurons undergoing pathfinding, it is possible that these cellular structures serve endothelial tip cells the same guidance function(s) in angiogenesis. This model of filopodia as a focal adhesion initiation site for subsequent advance of a trailing lamellar structure that has been documented in growth cones has been proposed as a more general mechanism for haptotactic mesenchymal cell migration (Johnson et al., 2015). However, as mentioned above, no dynamic observations of endothelial tip cell filopodia and adhesions have been made in vivo, despite evidence that they must occur. Toward this end, we sought to observe tip cell filopodia dynamics and focal adhesions simultaneously in vivo, to determine if there were dynamic spatiotemporal relationships similar to those observed in neuronal growth cones.
One molecule which participates in both filopodia and focal adhesion formation is VASP. VASP, or vasodialator stimulated phosphoprotein, is widely expressed but enriched in endothelial cells (Gambaryan et al., 2001). VASP is an actin filament elongation factor that protects barbed ends from capping (Bear et al., 2002) and collaborates with formins to produce filopodia (Jaiswal et al., 2013; Winkelman et al., 2014; Schirenbeck et al., 2005). Studies of GFP-VASP dynamics in filopodia in cultured cells and neurons has shown that VASP localization to filopodia enhances filopodia lifetime and protrusion persistence (Barzik et al., 2014; Bilancia et al., 2014), in agreement with its role in promoting actin filament assembly. VASP also localizes strongly to focal adhesions and binds directly to several focal adhesion proteins including zyxin and vinculin (Pula and Krause, 2008), however, the role of ena/VASP proteins in adhesion is less clear. On the one hand, selective depletion of ena/VASP proteins from focal adhesions has no effect on fibroblast migration on fibronectin (Bear et al., 2000), however in leukocytes mutation of VASP tyrosine 38, a target for Abl kinase, impairs adhesion to fibronectin (Maruoka et al., 2012). In addition to filopodia and focal adhesions, VASP also localizes to and mediates formation and maintenance of cell-cell junctions (Benz et al., 2008). Consistent with this, VASP has been shown to be critical to blood vessel barrier function (Schmit et al., 2012; Furman et al., 2007). However, the functions of VASP and its role in filopodial and focal adhesion dynamics during sprouting angiogenesis have not been described.
9. Results
To observe localization of VASP during sprouting angiogenesis, we expressed the Danio rerio VASP fused to a danio rerio codon-optimized eGFP in the vasculature of developing embryos, with expression driven by an endothelial specific promoter (Lawson and Weinstein, 2002). GFP-VASP in the developing vasculature was imaged live by spinning disc confocal microscopy, as previously described (Lam et al., 2014). GFP-VASP localized to the tips of filopodia-like protrusions in sprouting endothelial tip cells during intersegmental vessel (ISV) development (Fig. 1A; Movie 1), and at the distal area of lamella-likeprotrusions in larger, elongated, stable plaques (Fig. 1B). As neighboring ISV vessels made contact after rostral-caudal branching to form the dorsal longitudinal anastomotic vessel (DLAV; (Gore et al., 2012)), GFP-VASP was recruited to nascent cell-cell contacts (Fig. 1D, Movie 2). In tip cells of some vessels, transient lamellipodia were observed with GFP-VASP at their leading edges (Fig. 1C, Movie 3). In mature, patent vessels at 48 h post-fertilization, GFP-VASP was enriched at cell-cell junctions (Fig. 1E, Movie 4), consistent with its role in vascular barrier function.
Fig. 1.
GFP-VASP localizes to filipodia-like protrusions and cell-cell junctions in endothelial cells undergoing sprouting angiogenesis in zebrafish in vivo. (A) GFP-VASP localizes to puncta at tips of filopodia (arrowheads) of ISV endothelial cells. Bar equals 5 μm. (B) bases of filopodia and/or distal lamella regions at cell tips show stable, elongated accumulations of GFP-VASP (arrowhead). Bar equals 1 μm. (C) Along dorsal axis, some ISVs exhibit transient lamellipodia-like protrusions (arrowhead) with GFP-VASP at the leading edge. Bar equals 3 μm. (D) As ISVs meet at the dorsal side and connect to form the lumen, lamellipodia form nascent cell-cell junctions where VASP is enriched (arrowhead). Bar equals 3 μm. (E) In dorsal aorta and patent ISVs, GFP-VASP localizes to cell-cell junctions. Bar equals 20 μm. (F) ISV cells with high levels of GFP-VASP expression (asterisk) show numerous small filopodia and fail to properly migrate to the dorsal junction point. Elapsed time shown in minutes, bar equals 100 μm.
Supplementary material related to this article can be found online at doi:10.1016/j.ydbio.2018.08.015.
Since GFP-VASP is known to promote actin filament elongation to induce filopodia formation, we studied GFP-VASP dynamics at endothelial tip cell filopodia-like protrusions in more detail. We observed anecdotally that in ISV tip cells with aberrantly high expression of GFP-VASP, the entire surface of the cell was covered in small filopodia, these cells never advanced to the dorsal side of the neural tube, and they failed to meet their ISV partners (Fig. 1F, Movie 5). In moderately expressing cells, we observed that GFP-VASP was enriched at the tip of filopodia-like protrusions only during extension, but was lost during retraction phases (Fig. 2A–C), consistent with its role in filopodia actin filament elongation. We noted that the tip cell filopodia-like protrusions, unlike canonical filopodia, often had small lamellipodia-like protrusions and branches along their lengths (Movie 6). These results show that GFP-VASP in tip cell protrusions behaves similar to its observed role in canonical filopodia in mesenchymal cells and neurons.
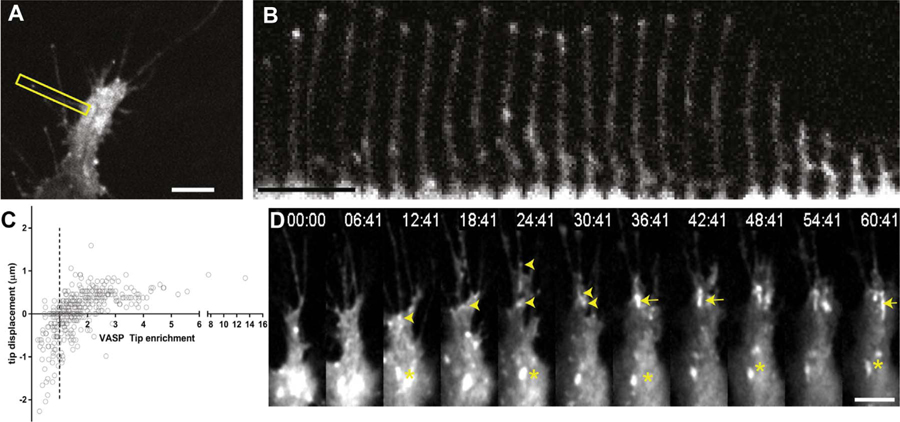
Fig. 2.
GFP-VASP highlights growing filopodia during ISV sprouting in zebrafish. (A) cropped single time point frame of GFP-VASP expressing tip cell with an example filopodium outlined in yellow box. (B) Time-lapse montage of outlined filopodium from (A); total time elapsed in series is 345 s (C) Quantification of filopodia extension as a function of GFP-VASP enrichment at the tip. The mean intensity of GFP-VASP as a ratio to the stalk intensity was quantified over time, and the tip displacement displayed such that positive values indicate extension distance over 30 s, negative values indicate retraction. Dashed vertical line shows tip:stalk intensity ratio of 1. Data shown for 22 individual filopodia. (D) Time-lapse series showing accumulation of GFP-VASP into stable, elongated focal adhesion-like structures at the base of filopodia. Puncta of VASP travel down filopodia, and stabilize at the base of the filopodium (arrowheads). Puncta can accumulate to form large fibrillar plaques (arrows) which are stable over many minutes during advance of the leading edge of the tip cell. Over time, stable plaques accumulate, and eventually begin to disassemble (asterisks), enabling forward progress of the tip cell.
Supplementary material related to this article can be found online at doi:10.1016/j.ydbio.2018.08.015.
We then examined the dynamics of GFP-VASP plaques at the bases of filopodia-like protrusions, regions of tip cells that may be functionally analogous to a growth cone (Fig. 2C, Movie 6). In time-lapse movies, GFP-VASP plaques appeared to form small punctae within filopodia-like protrusions that underwent retrograde flow and subsequently stabilized at the base of the protrusion. These stabilized punctae then grew into elongated plaques up to 3.6 μm in length along the axis of retrograde flow, reminiscent of focal adhesion maturation seen in cells in tissue culture (Thievessen et al., 2013; Choi et al., 2008). Once formed, GFP-VASP plaques were roughly stationary at the base of the cell protrusion. Given that the substrate underneath cell tips was moving in vivo, the GFP-VASP plaques were remarkably stable. Quantitative analysis with automated focal adhesion tracking software (Berginski et al., 2011) demonstrated that plaques of GFP-VASP had an average lifetime of 6.25 +/− 2.1 min, comparable to the lifetime of focal adhesions in cells in tissue culture (Webb et al., 2002; Gupton and Waterman-Storer, 2006). As the endothelial cell tip advanced, GFP-VASP plaques disassembled and areas between leading filopodia-like protrusions filled in, and the cell progressed forward. These results show that GFP-VASP forms stable plaques at the base of protrusions with similar dynamic characteristics as focal adhesions in migrating mesenchymal cells.
10. Model and outlook
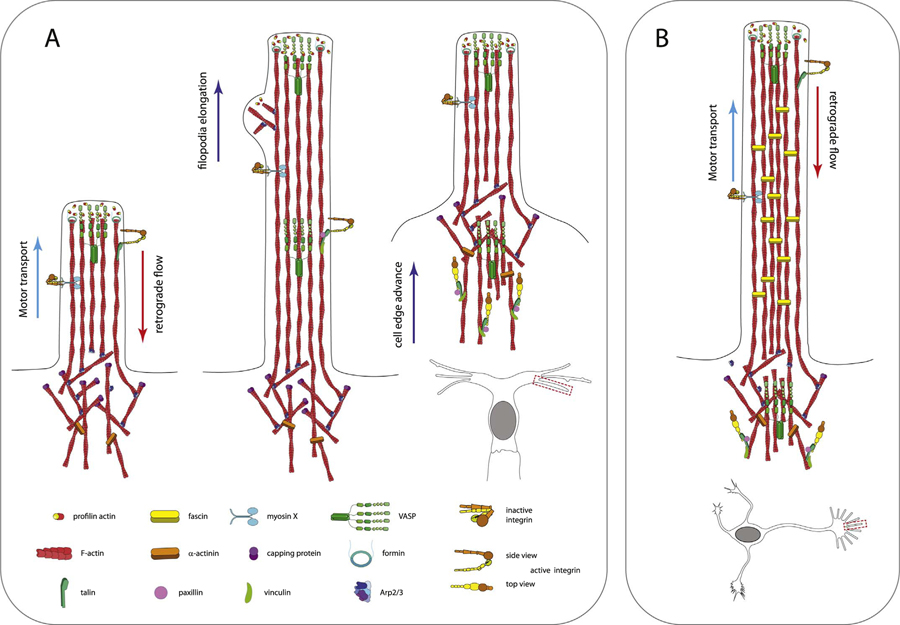
Combining our results with the previous studies reviewed above, we propose a speculative model for filopodia as “adhesion factories” for migration of cells undergoing branching morphogenesis, the main components of which are conserved for both neuronal growth cones and endothelial tip cells (Fig. 3). In filopodia extensions, myosin X transports many components such as VASP (Tokuo and Ikebe, 2004) and inactive integrins (Zhang et al., 2004; He et al., 2017) to the filopodia tip. VASP localizes to the actin filament free barbed ends, where it protects barbed ends from capping and collaborates with formins to enable filament elongation. This rapid polymerization drives both filopodial protrusion and actin filament retrograde flow. VASP is captured by retrograde flow via its actin filament bundling activity. The retrograde flow also drives the activation of integrin clusters which have been “primed” by talin binding, resulting in molecular clutch engagement and traction generation on the ECM. Retrograde flow and further actin bundling results in accumulation of activated integrin clusters, VASP, and other focal adhesion components such as vinculin, paxillin, and α-actinin into maturing elongated adhesions near the base of the filopodia, where the integrins become maximally engaged. The enhanced traction at mature filopodia then enables the cell edge to advance beyond the adhesion point, with further filopodial protrusion and the cycle continues. Some details may differ between neuronal and endothelial filopodia, e.g., neuronal filopodia may rely on fascin cross-links, while endothelial cell filopodia may additionally have Arp2/3 mediated branches and small lamellipodia along their length (Fig. 3). Nevertheless, the main aspects of this model seem likely to be conserved, adding to the growing list of conserved molecular mechanisms in pathfinding between the two branched cell systems. More broadly, the role of filopodia as adhesion-generating organelles may be conserved amongst diverse motile cell types such as fibroblasts and macrophages, where focal adhesion puncta can be observed in these types of protrusive structures (Johnson et al., 2015; Barros-Becker et al., 2017).
Fig. 3.
Conserved mechanism for filopodia generated adhesions in pathfinding mediated by branching morphogenesis in endothelial cells and neurons. (A) In endothelial tip cell filopodia, myosin X transports adhesion molecules including integrins and VASP to the filopodium tip. Along with other tip components, Arp2/3 also is activated and induces small side lamellipodia. At the tip, VASP bundles filaments, protects them from capping, and collaborates with formins to allow rapid F-actin polymerization to advance the tip. Tip advance is partially balanced by retrograde flow of actin, which activates integrins in conjunction with talin binding. Retrograde flow and slippage of active integrins results in accumulation of active focal adhesions at the base of filopodia. These adhesions grow and exert stronger traction allowing forward progress of the cell edge. (B) Key features of the neuronal filopodia architecture are similar to endothelial cells, except that fascin cross-linking generally results in tight parallel bundled actin filaments without branches, although some examples of branched growth cone filopodia exist (see text).
The speculative model presented above will certainly require more intravital imaging of sprouting angiogenesis to observe other focal adhesion components together with F-actin markers in tip cell filopodia for substantiation. Amongst the important molecular components to observe and perturb during imaging of angiogenesis in vivo will be FAK, since it contributes to both focal adhesion formation and turnover and is known to be important in pathfinding in neurons, and thus seems likely to be important in tip cell dynamics during angiogenesis as well. Although the analogy between endothelial tip cell protrusions and neuronal growth cones may not be complete in all details, the common mechanisms of filopodia advance and adhesion dynamics between the two systems make sense given the similar biological requirements of both branching cell systems, namely that they navigate stereotypical paths over long distances and integrate soluble, immobilized, and mechanical cues to do so. Much work over the past several years has shown multiple common molecular guidance cues and mechanisms between angiogenesis and neuronal pathfinding (Quaegebeur et al., 2011), and the use of filopodia-derived adhesions represents another part of this understanding.
11. Methods
Danio rerio husbandry was performed by the Zebrafish Facility at Marine Biological Laboratories, in Woods Hole, MA. D. rerio optimized GFP-VASP was constructed by fusing an eGFP sequence codon-optimized for D. rerio to the D. rerio VASP coding sequence (XM_ 005173679.1) using PCR overlap, and this fusion construct was cloned into a vector with fli1 promoter and enhancer regions to drive expression. All constructs were confirmed by sequence analysis. Transient expression of this construct in D. rerio embryos was performed as previously described (Lam et al., 2014). Briefly Tol2 transposase mRNA was produced in vitro (mMessage mMachine kit, Ambion) and mRNA purified (RNeasy kit, Qiagen). One-cell-stage embryos were injected with 25 ng of plasmid DNA and 50 ng of Tol2 mRNA, and cultivated in E3 water as previously described (Lam et al., 2014). At 20–36 h post fertilization, embryos were dechorionated and anesthetized with tricaine for several minutes, and placed onto BSA coated glass bottom dishes (MatTek), and covered with low melt agarose. After gelation, embryos were imaged on an inverted Nikon Ti microscope equipped with a Yokagawa X-1 confocal scanhead, a water-immersion 60X lens, an Agilent laser combiner, and a Hammamatsu cooled CCD camera. 300–600 msec exposures were taken every 3 s to 5 min, depending on the experiment. For quantification of GFP-VASP tip enrichment, individual filopodia were tracked using a custom ImageJ script. The mean intensity of each filopodium tip and stalk was determined and the fold enrichment was calculated as the ratio (after background substraction) of tip mean intensity/stalk mean intensity. These values for each tip position over time were plotted against the displacement from the previous frame, with positive values indicating extension, and negative values retraction. Focal adhesion lifetime analysis was performed on image series of GFP-VASP using the Focal Adhesion Analysis Server (http://faas.bme.unc.edu/) described previously (Berginski and Gomez, 2013). Focal adhesions were thresholded to find GFP-VASP puncta over local background, and only puncta greater than 40 pixels in size (roughly 4.3 μm2 in area) were kept for analysis.
Supplementary Material
Acknowledgments
Funding
RSF and CMW are supported by the NHLBI Intramural Research program. AH is supported by NIH Grant# NIH R35 GM1 18027 01.
References
- Ahmed S, Goh WI, Bu W, 2010. I-BAR domains, IRSp53 and filopodium formation. Semin. Cell Dev. Biol 21, 350–356. [DOI] [PubMed] [Google Scholar]
- Almagro S, et al. , 2010. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Mol. Cell. Biol 30, 1703–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthis NJ, Wegener KL, Critchley DR, Campbell ID, 2010. Structural diversity in integrin/talin interactions. Structure 18, 1654–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Kreidberg JA, Rakic P, 1999. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron 22, 277–289. [DOI] [PubMed] [Google Scholar]
- Atherton P, et al. , 2015. Vinculin controls talin engagement with the actomyosin machinery. Nat. Commun 6, 10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Becker F, Lam P-Y, Fisher R, Huttenlocher A, 2017. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. J. Cell Sci 130, 3801–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DM, et al. , 2015. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development 142, 3058–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzik M, McClain LM, Gupton SL, Gertler FB, 2014. Ena/VASP regulates mDia2-initiated filopodial length, dynamics, and function. Mol. Biol. Cell 25, 2604–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, et al. , 2000. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101, 717–728. [DOI] [PubMed] [Google Scholar]
- Bear JE, et al. , 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521. [DOI] [PubMed] [Google Scholar]
- Benz PM, et al. , 2008. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J. Cell Biol 180, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berginski ME, Gomez SM, 2013. The focal adhesion analysis server: a web tool for analyzing focal adhesion dynamics. F1000Res 2, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berginski ME, Vitriol EA, Hahn KM, Gomez SM, 2011. High-resolution quantification of focal adhesion spatiotemporal dynamics in living cells. PLoS One 6, e22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield MR, Banerjee SD, Cohn RH, 1972. Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. J. Cell Biol 52, 674–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A, Kaverina I, Otey C, Huttenlocher A, 2002. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell Sci 115, 3415–3425. [DOI] [PubMed] [Google Scholar]
- Bilancia CG, et al. , 2014. Enabled negatively regulates diaphanous-driven actin dynamics in vitro and in vivo. Dev. Cell 28, 394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohil AB, Robertson BW, Cheney RE, 2006. Myosin-X is a molecular motor that functions in filopodia formation. 103, 12411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier JP, et al. , 2015. Single-molecule visualization of a formin-capping protein ‘decision complex’ at the actin filament barbed end. Nat. Commun 6, 8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornschlögl T, et al. , 2013. Filopodial retraction force is generated by cortical actin dynamics and controlled by reversible tethering at the tip. Proc. Natl. Acad. Sci. USA 110, 18928–18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaouina M, Lad Y, Calderwood DA, 2008. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate beta1 and beta3 integrins. J. Biol. Chem 283, 6118–6125. [DOI] [PubMed] [Google Scholar]
- Bozyczko D, Horwitz AF, 1986. The participation of a putative cell surface receptor for laminin and fibronectin in peripheral neurite extension. J. Neurosci 6, 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, et al. , 2011. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J. Cell Sci 124, 3305–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Cornejo BJ, Kuhn TB, Bamburg JR, 2000. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J. Neurobiol 43, 352–364. [DOI] [PubMed] [Google Scholar]
- Byron A, Humphries JD, Bass MD, Knight D, Humphries MJ, 2011. Proteomic analysis of integrin adhesion complexes. Sci. Signal 4, (pt2–pt2). [DOI] [PubMed] [Google Scholar]
- Campellone KG, Welch MD, 2010. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol 11, 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, De Smet F, Loges S, Mazzone M, 2009. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat. Rev. Clin. Oncol 6, 315–326. [DOI] [PubMed] [Google Scholar]
- Case LB, et al. , 2015. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat. Cell Biol 17, 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Waterman CM, 2015. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol 17, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano F, et al. , 1999. Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr. Biol 9, 351–360. [DOI] [PubMed] [Google Scholar]
- Caussinus E, Colombelli J, Affolter M, 2008. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. 18, 1727–1734. [DOI] [PubMed] [Google Scholar]
- Chan CE, Odde DJ, 2008. Traction dynamics of filopodia on compliant substrates. 322, 1687–1691. [DOI] [PubMed] [Google Scholar]
- Chan KT, Bennin DA, Huttenlocher A, 2010. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK). J. Biol. Chem 285, 11418–11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-J, et al. , 2015. SH2B1 and IRSp53 proteins promote the formation of dendrites and dendritic branches. J. Biol. Chem 290, 6010–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Levine YC, Golan DE, Michel T, Lin AJ, 2008. Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J. Biol. Chem 283, 4439–4447. [DOI] [PubMed] [Google Scholar]
- Choi Colin K., Vicente-Manzanares Miguel, Zareno Jessica, Whitmore Leanna A., Mogilner Alex, Horwitz Alan Rick, 2008. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol 10 (9), 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K, 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol 133, 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Milne P, 2016. Langerhans cell origin and regulation. Curr. Opin. Hematol 23, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EM, Van Goor D, Forscher P, Mogilner A, 2012. Membrane tension, myosin force, and actin turnover maintain actin treadmill in the nerve growth cone. Biophys. J 102, 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave JM, et al. , 2016. Hic-5 mediates the initiation of endothelial sprouting by regulating a key surface metalloproteinase. J. Cell Sci 129, 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RW, Dou P, Rehder V, Kater SB, 1993. A sensory role for neuronal growth cone filopodia. Nature 361, 721–724. [DOI] [PubMed] [Google Scholar]
- del Rio A, et al. , 2009. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, et al. , 2007. Filopodia are required for cortical neurite initiation. Nat. Cell Biol 9, 1347–1359. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB, 2011. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol 3, (a001800–a001800). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroanne CF, Colige AC, Nusgens BV, Lapiere CM, 1996. Modulation of expression and assembly of vinculin during in vitro fibrillar collagen-induced angiogenesis and its reversal. Exp. Cell Res 224, 215–223. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Edds KT, 1980. Evidence for fascin cross-links between the actin filaments in coelomocyte filopodia. Exp. Cell Res 126, 490–494. [DOI] [PubMed] [Google Scholar]
- Disanza A, et al. , 2013. CDC42 switches IRSp53 from inhibition of actin growth to elongation by clustering of VASP. EMBO J 32, 2735–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Jessell TM, 1988. Axon guidance and the patterning of neuronal projections in vertebrates. Science 242, 692–699. [DOI] [PubMed] [Google Scholar]
- Du X, et al. , 1995. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J. Biol. Chem 270, 26146–26151. [DOI] [PubMed] [Google Scholar]
- Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C, 2007. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development 134, 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z, 2008. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG, 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol 7, 581–590. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG, 2009. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol 187, 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, et al. , 2015. NRP1 regulates CDC42 activation to promote filopodia formation in endothelial tip cells. Cell Rep 11, 1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM, 2009. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr. Biol 19, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, et al. , 2004. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol 6, 977–983. [DOI] [PubMed] [Google Scholar]
- Furman C, et al. , 2007. Ena/VASP is required for endothelial barrier function in vivo. J. Cell Biol 179, 761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaryan S, Hauser W, Kobsar A, Glazova M, Walter U, 2001. Distribution, cellular localization, and postnatal development of VASP and Mena expression in mouse tissues. Histochem. Cell Biol 116, 535–543. [DOI] [PubMed] [Google Scholar]
- Gardberg M, Heuser VD, Koskivuo I, Koivisto M, Carpén O, 2016. FMNL2/FMNL3 formins are linked with oncogenic pathways and predict melanoma outcome. J. Pathol. Clin. Res 2, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, et al. , 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Roche FK, Letourneau PC, 1996. Chick sensory neuronal growth cones distinguish fibronectin from laminin by making substratum contacts that resemble focal contacts. J. Neurobiol 29, 18–34. [DOI] [PubMed] [Google Scholar]
- Gore AV, Monzo K, Cha YR, Pan W, Weinstein BM, 2012. Vascular development in theZebrafish. Cold Spring Harb. Perspect. Med 2, (a006684–a006684). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, et al. , 2001. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31, 367–379. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM, 2006. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 125, 1361–1374. [DOI] [PubMed] [Google Scholar]
- Hariharan S, et al. , 2007. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann. Oncol 18, 1400–1407. [DOI] [PubMed] [Google Scholar]
- He K, Sakai T, Tsukasaki Y, Watanabe TM, Ikebe M, 2017. Myosin X is recruited to nascent focal adhesions at the leading edge and induces multi-cycle filopodial elongation. Sci. Rep 7, 13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CA, Plummer HK, 2013. Filopodia as sensors. Cell. Signal 25, 2298–2311. [DOI] [PubMed] [Google Scholar]
- Heimsath EG, Yim Y-I, Mustapha M, Hammer JA, Cheney RE, 2017. Myosin-X knockout is semi-lethal and demonstrates that myosin-X functions in neural tube closure, pigmentation, hyaloid vasculature regression, and filopodia formation. Sci. Rep 7, 17354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings L, et al. , 1996. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J. Cell Sci 109 (Pt 11), 2715–2726. [DOI] [PubMed] [Google Scholar]
- Hetheridge C, et al. , 2012. The formin FMNL3 is a cytoskeletal regulator of angiogenesis. J. Cell Sci 125, 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM, 2007. Differential transmission of actin motion within focal adhesions. Science 315, 111–115. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, et al. , 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem 272, 32719–32722. [DOI] [PubMed] [Google Scholar]
- Hyland C, Mertz AF, Forscher P, Dufresne E, 2015. Dynamic peripheral traction forces balance stable neurite tension in regenerating Aplysia bag cell neurons. Sci. Rep 4, 4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić D, et al. , 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J, 1989. How does extracellular matrix control capillary morphogenesis? Cell 58, 803–805. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Sun Z, Betensky H, Wang N, 1995. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J. Biomech 28, 1471–1484. [DOI] [PubMed] [Google Scholar]
- Jaiswal R, et al. , 2013. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Curr. Biol 23, 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y-N, Jan LY, 2010. Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci 11, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, et al. , 2011. Mechanism of actin filament bundling by fascin. J. Biol. Chem 286, 30087–30096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, et al. , 2013. Deletion of Cdc42 enhances ADAM17-mediated vascular endothelial growth factor receptor 2 shedding and impairs vascular endothelial cell survival and vasculogenesis. Mol. Cell. Biol 33, 4181–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HE, et al. , 2015. F-actin bundles direct the initiation and orientation of lamellipodia through adhesion-based signaling. J. Cell Biol 208, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli AC, Campbell ID, Sansom MSP, 2013. Conformational changes in talin on binding to anionic phospholipid membranes facilitate signaling by integrin transmembrane helices. PLoS Comput. Biol 9, e1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P, et al. , 2010. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast DJ, et al. , 2014. Mechanism of IRSp53 inhibition and combinatorial activation by Cdc42 and downstream effectors. Nat. Struct. Mol. Biol 21, 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific CM, et al. , 2016. NBR1 enables autophagy-dependent focal adhesion turnover. J. Cell Biol 212, 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstein PC, Nichol RH, Gomez TM, 2015. Mechanochemical regulation of growth cone motility. Front. Cell. Neurosci 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstein PC, Patel KM, Gomez TM, 2017. Calpain-mediated proteolysis of talin and FAK regulates adhesion dynamics necessary for axon guidance. J. Neurosci 37, 1568–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Varner VD, Nelson CM, 2013. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development 140, 3146–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA, 1999. A role for p21-activated kinase in endothelial cell migration. J. Cell Biol 147, 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J-CC, et al. , 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol 13, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz H, Korn J, Eggli PS, Huang R, Christ B, 2001. Embryonic central nervous system angiogenesis does not involve blood-borne endothelial progenitors. J. Comp. Neurol 436, 263–274. [PubMed] [Google Scholar]
- Lam P, Fischer RS, Shin WD, Waterman CM, Huttenlocher A, 2014. Spinning Disk Confocal Imaging of Neutrophil Migration in Zebrafish. in 219–233. 〈 10.1007/978-1-62703-845-4_14〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM, 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol 248, 307–318. [DOI] [PubMed] [Google Scholar]
- Lebrand C, et al. , 2004. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 42, 37–49. [DOI] [PubMed] [Google Scholar]
- Lechertier T, Hodivala-Dilke K, 2012. Focal adhesion kinase and tumour angiogenesis. J. Pathol 226, 404–412. [DOI] [PubMed] [Google Scholar]
- Lele TP, Thodeti CK, Pendse J, Ingber DE, 2008. Investigating complexity of protein-protein interactions in focal adhesions. Biochem. Biophys. Res. Commun 369, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Springer TA, 2017. Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl. Acad. Sci. USA 114, 4685–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Forscher P, 1995. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron 14, 763–771. [DOI] [PubMed] [Google Scholar]
- Lu M, Witke W, Kwiatkowski DJ, Kosik KS, 1997. Delayed retraction of filopodia in gelsolin null mice. J. Cell Biol 138, 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, et al. , 2013. Fascin 1 is dispensable for developmental and tumour angiogenesis. Biol. Open 2, 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruoka M, et al. , 2012. Abl-1-bridged tyrosine phosphorylation of VASP by Abelson kinase impairs association of VASP to focal adhesions and regulates leukaemic cell adhesion. Biochem. J 441, 889–899. [DOI] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P, 2006. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol 8, 215–226. [DOI] [PubMed] [Google Scholar]
- Mendoza P, et al. , 2013. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J. Cell Sci 126, 3835–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M, 1988. Cytoskeletal dynamics and nerve growth. Neuron 1, 761–772. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, et al. , 2011. Endothelial cell talin1 is essential for embryonic angiogenesis. Dev. Biol 349, 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DT, et al. , 2012. Affinity of talin-1 for the β3-integrin cytosolic domain is modulated by its phospholipid bilayer environment. Proc. Natl. Acad. Sci. USA 109, 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Jackson IJ, Patton EE, 2015. The melanocyte lineage in development and disease. Development 142, 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Pollard TD, 1999. Structure and function of the Arp2/3 complex. Curr. Opin. Struct. Biol 9, 244–249. [DOI] [PubMed] [Google Scholar]
- Myers JP, Santiago-Medina M, Gomez TM, 2011. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev. Neurobiol 71, 901–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Robles E, Ducharme-Smith A, Gomez TM, 2012. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J. Cell Sci 125, 2918–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J, et al. , 2012. Fascin controls neuronal class-specific dendrite arbor morphology. Development 139, 2999–3009. [DOI] [PubMed] [Google Scholar]
- Nguyen D-HT, et al. , 2013. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. USA 110, 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D-HT, Gao L, Wong A, Chen CS, 2017. Cdc42 regulates branching in angiogenic sprouting in vitro. Microcirculation 24, e12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol RH, Hagen KM, Lumbard DC, Dent EW, Gómez TM, 2016. Guidance of axons by local coupling of retrograde flow to point contact adhesions. J. Neurosci 36, 2267–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A, 1995. Rho, Rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM, 2010. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol 188, 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KJ, et al. , 2016. MyTH4-FERM myosins have an ancient and conserved role in filopod formation. Proc. Natl. Acad. Sci. USA 113, E8059–E8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Stanchi F, Gerhardt H, 2013. Filopodia are dispensable for endothelial tip cell guidance. Development 140, 4031–4040. [DOI] [PubMed] [Google Scholar]
- Pi X, et al. , 2007. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J. Cell Biol 179, 1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov SV, Pasapera AM, Sabass B, Waterman CM, 2012. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pula G, Krause M, 2008. Role of Ena/VASP proteins in homeostasis and disease. Handb. Exp. Pharmacol, 39–65. 10.1007/978-3-540-72843-6_3. [DOI] [PubMed] [Google Scholar]
- Qiao J, Sakurai H, Nigam SK, 1999. Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc. Natl. Acad. Sci. USA 96, 7330–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P, 2011. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron 71, 406–424. [DOI] [PubMed] [Google Scholar]
- Rappaz B, Lai Wing Sun K, Correia JP, Wiseman PW, Kennedy TE, 2016. FLIM FRET visualization of Cdc42 activation by netrin-1 in embryonic spinal commissural neuron growth cones. PLoS One 11, e0159405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Cheresh D, 2011. Cilengitide: a prototypic integrin inhibitor for the treatment of glioblastoma and other malignancies. Genes Cancer 2, 1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin A, Lehmann M, Girault J, McKerracher L, 1999. Organization of point contacts in neuronal growth cones. J. Neurosci. Res 55, 458–471. [DOI] [PubMed] [Google Scholar]
- Robles E, Huttenlocher A, Gomez TM, 2003. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron 38, 597–609. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, et al. , 2013. Integrin-dependent force transmission to the extracellular matrix by -actinin triggers adhesion maturation. Proc. Natl. Acad. Sci. USA 110, E1361–E1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Ivaska J, 2015. Blocking integrin inactivation as an anti-angiogenic therapy. EMBO J 34, 1293–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabudhe A, Ghate K, Mutalik S, Jacob A, Ghose A, 2016. Formin 2 regulates the stabilization of filopodial tip adhesions in growth cones and affects neuronal outgrowth and pathfinding in vivo. 143, 449–60. [DOI] [PubMed] [Google Scholar]
- Santiago-Medina M, Myers JP, Gomez TM, 2012. Imaging adhesion and signaling dynamics in Xenopus laevis growth cones. Dev. Neurobiol 72, 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Medina M, Gregus KA, Gomez TM, 2013. PAK-PIX interactions regulate adhesion dynamics and membrane protrusion to control neurite outgrowth. J. Cell Sci 126, 1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirenbeck A, Arasada R, Bretschneider T, Schleicher M, Faix J, 2005. Formins and VASPs may co-operate in the formation of filopodia. Biochem. Soc. Trans 33, 1256–1259. [DOI] [PubMed] [Google Scholar]
- Schmit MA, et al. , 2012. Vasodilator phosphostimulated protein (VASP) protects endothelial barrier function during hypoxia. Inflammation 35, 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnatwinkel C, Niswander L, 2013. Multiparametric image analysis of lung-branching morphogenesis. Dev. Dyn 242, 622–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi MN, et al. , 2016. Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep 15, 1660–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T-L, et al. , 2005. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol 169, 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau CT, Waterman CM, Skau CT, 2015. Specification of architecture and function of actin structures by actin nucleation factors. Annu. Rev. Biophys 44, (null). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, McCann RO, 2007. A C-terminal dimerization motif is required for focal adhesion targeting of Talin1 and the interaction of the Talin1 I/LWEQ module with F-actin. Biochemistry 46, 10886–10898. [DOI] [PubMed] [Google Scholar]
- Sprague CR, Fraley TS, Jang HS, Lal S, Greenwood JA, 2008. Phosphoinositide binding to the substrate regulates susceptibility to proteolysis by calpain. J. Biol. Chem 283, 9217–9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel D, et al. , 2011. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development 138, 4451–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RL, et al. , 2014. Focal adhesion kinase: an alternative focus for anti-angiogenesis therapy in ovarian cancer. Cancer Biol. Ther 15, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM, 2004. Arp2/3 is a negative regulator of growth cone translocation. Neuron 43, 81–94. [DOI] [PubMed] [Google Scholar]
- Stricker J, Beckham Y, Davidson MW, Gardel ML, 2013. Myosin II-mediated focal adhesion maturation is tension insensitive. PLoS One 8, e70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, et al. , 2003. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol 160, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG, 1999. Progress in protrusion: the tell-tale scar. Trends Biochem. Sci 24, 432–436. [DOI] [PubMed] [Google Scholar]
- Swaminathan V, Waterman CM, 2016. The molecular clutch model for mechanotransduction evolves. Nat. Cell. Biol 18 (5), 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan V, Kalappurakkal JM, Mehta SB, Nordenfelt P, Moore TL, Koga N, Baker DA, Oldenbourg R, Tani T, Mayor S, Springer TA, Waterman CM, 2017. Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proc. Natl. Acad. Sci 114 (40), 10648–10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thievessen I, et al. , 2013. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J. Cell Biol 202, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuo H, Ikebe M, 2004. Myosin X transports Mena/VASP to the tip of filopodia. Biochem. Biophys. Res. Commun 319, 214–220. [DOI] [PubMed] [Google Scholar]
- Varner VD, Nelson CM, 2014. Cellular and physical mechanisms of branching morphogenesis. Development 141, 2750–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic D, et al. , 2006. Role of fascin in filopodial protrusion. 174, 863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama Y, Fukuhara S, Ando K, Matsuda M, Mochizuki N, 2015. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev. Cell 32, 109–122. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL, 2000. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127, 2695–2704. [DOI] [PubMed] [Google Scholar]
- Webb DJ, et al. , 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol 6, 154–161. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF, 2002. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat. Cell Biol 4, E97–E100. [DOI] [PubMed] [Google Scholar]
- Wei Z, Sun M, Liu X, Zhang J, Jin Y, 2014. Rufy3, a protein specifically expressed in neurons, interacts with actin-bundling protein Fascin to control the growth of axons. J. Neurochem 130, 678–692. [DOI] [PubMed] [Google Scholar]
- Winkelman JD, Bilancia CG, Peifer M, Kovar DR, 2014. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. USA 111, 4121–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Gomez TM, 2006. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J. Neurosci 26, 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, et al. , 2013. Targeting of integrin-linked kinase with small interfering RNA inhibits VEGF-induced angiogenesis in retinal endothelial cells. Ophthalmic Res 49, 139–149. [DOI] [PubMed] [Google Scholar]
- Yang C, Svitkina T, 2011. Filopodia initiation. Cell Adhes. Migr 5, 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LE, Heimsath EG, Higgs HN, 2015. Cell type-dependent mechanisms for formin-mediated assembly of filopodia. Mol. Biol. Cell 26, 4646–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B, 2003. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci 116, 4605–4613. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. , 2004. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol 6, 523–531. [DOI] [PubMed] [Google Scholar]
- Zhu X-J, et al. , 2007. Myosin X regulates netrin receptors and functions in axonal pathfinding. Nat. Cell Biol 9, 184–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.