Abstract
Length of postnatal hospitalization has decreased and has been shown to be associated with infant nutritional problems and increase in readmissions. We aimed to evaluate if guidelines for breastfeeding counselling in an early discharge hospital setting had an effect on maternal breastfeeding self‐efficacy, infant readmission and breastfeeding duration. A cluster randomized trial was conducted and assigned nine maternity settings in Denmark to intervention or usual care. Women were eligible if they expected a single infant, intended to breastfeed, were able to read Danish, and expected to be discharged within 50 hr postnatally. Between April 2013 and August 2014, 2,065 mothers were recruited at intervention and 1,476 at reference settings. Results show that the intervention did not affect maternal breastfeeding self‐efficacy (primary outcome). However, less infants were readmitted 1 week postnatally in the intervention compared to the reference group (adjusted OR 0.55, 95% CI 0.37, −0.81), and 6 months following birth, more infants were exclusively breastfed in the intervention group (adjusted OR 1.36, 95% CI 1.02, −1.81). Moreover, mothers in the intervention compared to the reference group were breastfeeding more frequently (p < .001), and spend more hours skin to skin with their infants (p < .001). The infants were less often treated for jaundice (p = 0.003) and there was more paternal involvement (p = .037). In an early discharge hospital setting, a focused breastfeeding programme concentrating on increased skin to skin contact, frequent breastfeeding, good positioning of the mother infant dyad, and enhanced involvement of the father improved short‐term and long‐term breastfeeding success.
Keywords: breastfeeding, cluster‐randomized controlled trial, early discharge, postpartum care, readmission, self‐efficacy
1. INTRODUCTION
In western countries, the length of postnatal hospitalization has decreased over the past 50 years. Today the average length of stay is 2–3 days (Bravo, Uribe, & Contreras, 2011) but many new mothers are discharged much sooner and a recent Danish study reports that one third of mothers are discharged within 12 hr following birth (Nilsson, Kronborg, Knight, & Strandberg‐Larsen, 2016). The postnatal period is a time of major physiological, psychological, and social changes for mother and infant with implications for their health and well‐being (WHO, 2013). Breastfeeding represents an important contribution to public health—in high as well as low income countries (Victora et al., 2016). However, establishing breastfeeding is challenging, as the mother and infant have to become familiar with it, while the milk production increases and the infant adapts to the outside world (Wambach & Riordan, 2015). Many mothers who are discharged early are concerned about establishing breastfeeding (Nilsson et al., 2015). A Danish study (Kronborg & Vaeth, 2004) documented that more than half of the mothers, who cease breastfeeding within 4 months following birth, do it in the first 5 weeks. This underscores that the early postnatal period is crucial for breastfeeding success.
Effective breastfeeding satisfies the needs of mother and infant. Skin‐to‐skin contact (Moore, Anderson, Bergman, & Dowswell, 2012), frequent breastfeeding according to the infant's needs (Wambach & Riordan, 2015) and good positioning of mother and infant (Wambach & Riordan, 2015) contribute to the establishment of effective breastfeeding and support early physiological changes including increase in milk production and infant metabolic adaptation (Wambach & Riordan, 2015; Ward & Deshpande, 2005). However, breastfeeding establishment also seems to depend on modifiable psychosocial factors such as favourable breastfeeding self‐efficacy, knowledge of breastfeeding, experience, and support (de Jager, Skouteris, Broadbent, Amir, & Mellor, 2013). Ineffective breastfeeding in the early postnatal period has implications for both the mother and the infant such as delayed milk production (Brownell, Howard, Lawrence, & Dozier, 2012), lack of maternal security and confidence (Larsen & Kronborg, 2012) and increased infant morbidity such as dehydration, jaundice, and excessive weight loss (Wambach & Riordan, 2015). Infant morbidity might result in enhanced use of the health care system including readmission of the newborn. Existing knowledge of the consequences of early discharge on breastfeeding effectiveness and duration are inconclusive (Bravo et al., 2011).
Health care professionals (HCP) provide mothers with breastfeeding knowledge and psycho‐social support (Kronborg, Vaeth, Olsen, & Harder, 2008). However, to our knowledge no literature exists of how to support mothers during the very short hospital stay (Renfrew, McCormick, Wade, Quinn, & Dowswell, 2012; Skouteris et al., 2014). We therefore need more knowledge about how HCP support mothers towards effective breastfeeding during a short postnatal hospital stay. We hypothesized that an HCP‐provided program that focuses on enhancing breastfeeding self‐efficacy and supporting the mothers' milk production and the infants' metabolic adaptation in an early discharge setting would improve the mothers' breastfeeding self‐efficacy, increase breastfeeding duration, and decrease the prevalence of readmission due to nutritional problems compared to a reference group of mothers who were supported as usual.
Key messages.
A parental breastfeeding programme targeted an early discharge setting and focusing on increased skin‐to‐skin contact, frequent breastfeeding, good positioning, and enhanced father involvement decreased 1 week postnatal readmissions and increased exclusive breastfeeding at 6 months of age.
An increased daily infant breastfeeding frequency might have contributed to the decreased readmission prevalence in the intervention group. However, more mothers in the intervention group experienced frequent breastfeeding as a problem. Health professionals should be aware of this parental ambivalence in order to encourage new parents to adapt to frequent breastfeeding.
The Breastfeeding Self‐efficacy Scale developed by Dennis showed a ceiling effect in this study. Breastfeeding self‐efficacy is an important determinant for breastfeeding duration; hence, a reliable instrument is needed to measure breastfeeding self‐efficacy in countries with strong breastfeeding traditions.
This breastfeeding programme consisting of a few low‐cost components might be easy to implement in other settings and even cause an increased effect if implemented in countries where breastfeeding initiation and duration is lower.
2. METHODS
Intervention Mapping (Bartholomew, Parcel, Kok, Gottlieb, & Fernandez, 2011) was used as the systematic method to develop, implement and evaluate this complex intervention. The main intention was to increase the new parents' self‐efficacy (Bandura, 1997). The intervention focused on forwarding fundamental and individual information to establish effective breastfeeding (Kreuter, Bull, Clark, & Oswald, 1999). The intervention program is described in Table 1.
Table 1.
Description of the intervention programme
| Development and implementation
A need assessment and corresponding programme objectives were the fundament of the development of the intervention programme, which consisted of a breastfeeding programme for parents and a related training programme for health professionals. A participatory approach was used to enhance ownership and ensure implementation at the intervention facilities. In March–May 2013, 350 nurses and midwives participated in the 11‐hr interactive training programme, which was supported by a written manual. The health professionals initiated implementation of the programme immediately after the training. |
| Objective of the parental breastfeeding programme
To increase mothers' breastfeeding self‐efficacy during a short postnatal hospitalization and ensure effective breastfeeding that meets both the mother's and the infant's needs while supporting the metabolic adaptation of the infant and the mother's milk production. |
| Content of the parental breastfeeding programme
The program consisted of four core components:
|
| The parents' breastfeeding self‐efficacy and breastfeeding knowledge was supported by communication based on Bandura's theory of self‐efficacy and his four sources to increase self‐efficacy (mastery experiences, vicarious experiences, social persuasion, and emotional arousal) (Bandura, 1997) together with Kreuter's theory of tailoring knowledge to the specific needs of the individual. (Kreuter et al., 1999) |
| All mothers at the intervention facilities were orally introduced to the four core components, which were also highlighted on a postcard, handed out at recruitment. Subsequently, they were supported postnatally according to the manual and a written pamphlet was actively used during breastfeeding counseling. The parents were supposed to adhere with the programme during the first 3 days while the infant went through the metabolic adaptation and the mother's milk production increased or until the first home visit by the health visitor 3–5 days postnatally. The parents received a follow‐up telephone call 24 hr after discharge. |
2.1. Setting and study design
In Denmark, 98%–99% give birth at a public hospital, which involves antenatal care visits to a midwife or a physician and a third of all mother–infant dyads are discharged within 12 hr following birth (Nilsson et al., 2016). Denmark has not been working actively on WHO's Baby‐Friendly Initiative since 2008, and thus no Danish hospitals have a valid designation. According to the national recommendations, HCPs are expected to base their breastfeeding support on the national handbook on breastfeeding, which addresses available evidence‐based knowledge (Sundhedsstyrelsen [The Danish Health Authority], 2013). However, breastfeeding support may vary depending on hospital routines. In Denmark, nurses and midwives are the primary responsible for breastfeeding support of parents at the hospitals.
A cluster‐randomized trial design was used to compare an intervention group of mothers, who were supported according to the new breastfeeding program with a reference group receiving usual care. All 23 hospital birth facilities in Denmark, except one with too few annual deliveries, were invited to participate in the study (Figure 1). Prerequisite for enrolment was willingness to cover the costs of the HCPs' participation in the 2‐day training program. Ten birth facilities agreed to participate.
Figure 1.
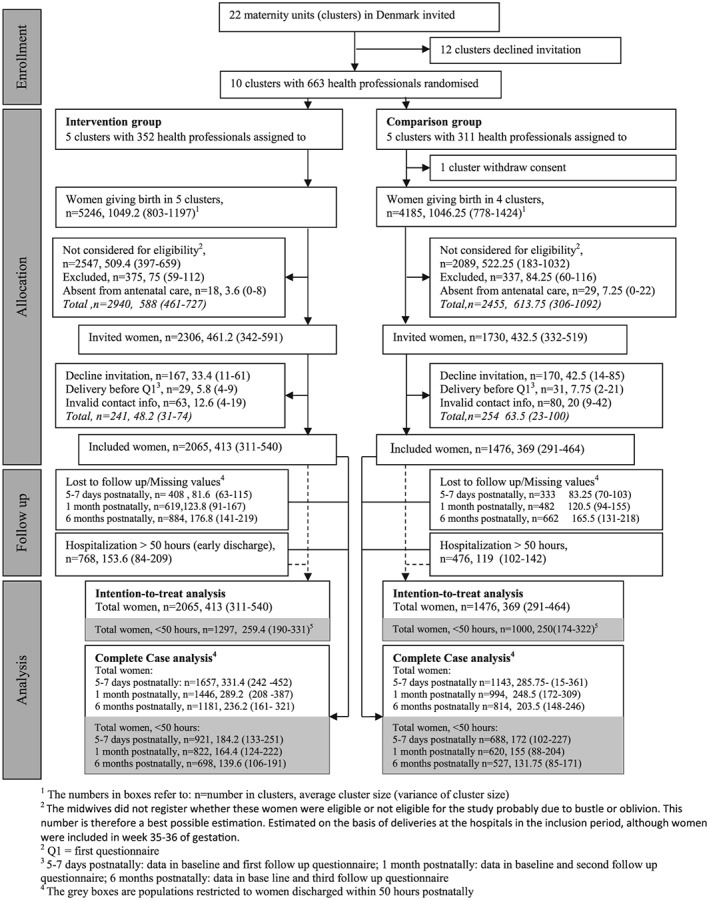
Flow profile for selection of study population
2.2. Randomization and masking
The hospitals were computer randomized to either the intervention or reference group. We stratified the 10 participating birth facilities according to number of births per year (<1,200, 1,200–3,000, >3,000) and the medium size hospitals by geography (mid‐eastern Jutland, other). The birth facilities rather than the mother–infant dyads were randomized to minimize the risk for contamination between groups and to mirror the real‐world implementation of the intervention. The stratification of hospital was to homogenize the two groups according to numbers and characteristics of mothers. The HCPs could not be blinded to the group assignment, because of their involvement in the development of the intervention. Moreover, the mothers could not be blinded due to the nature of the intervention. However, HCPs at the reference facilities were not informed about the content of the intervention, and mothers who agreed to participate were not informed whether their birth facility‐provided breastfeeding support according to the intervention program or the usual practice.
2.3. Participants
Mothers were eligible for the study if they (a) expected a single infant, (b) intended to breastfeed, (c) were able to read information in Danish, and (d) were definitely not expected to be discharged later than 50 hr postnatally due to complications in pregnancy or clinical disease. The target group was mothers discharged within 50 hr postnatally. However, as we did not know length of hospitalization at time of recruitment we had to include all. Mothers were recruited and provided with oral and written information about the study at their regular antenatal care visit with the midwife, scheduled around 35th–36th week of gestation. Informed consent was given by accepting and answering the first questionnaire. The recruitment period lasted from April 2013 to August 2014, where all clusters had recruited the number of mothers to fulfil the power calculation.
2.4. Data collection
Data were collected by four Web‐based self‐administered questionnaires. The baseline questionnaire, completed around time of recruitment included items on socio‐demographic characteristics. Information on breastfeeding experiences, feeding status and related infant morbidity, use of the health care system, and general well‐being in the family were collected at 5–7 days, 1‐month, and 6‐month postpartum. The first questionnaire postnatally also included information on birth and a process assessment of the support offered by the HCPs. The researchers were informed by the birth facilities on time of birth to ensure timely dissemination of the postnatal questionnaires. To reduce the number lost to follow‐up, nonresponders received up to three reminders together with short message service alerts. The questionnaires primarily consisted of instruments, which had either been face‐ and content‐validated or psychometrically tested. The questionnaires were reviewed by three experts and tested for comprehension and acceptability by six mothers. To describe possible systematic selection processes, we used the Danish National Patient Register and the Danish Medical Birth Register to get data on all births in the included birth facilities.
2.5. Measures
Baseline characteristics were socio‐demographic (age, education, marital status, and ethnicity), reproductive experience (parity and expected time of delivery), health (body mass index [BMI] and smoking), and psychosocial breastfeeding factors (breastfeeding experience, self‐efficacy, knowledge, and intention to breastfeed). Length of postnatal hospitalization was calculated from the mothers' reports and defined as completed hours between time of birth and time of discharge. The primary outcome was breastfeeding self‐efficacy at 5–7 days and 1 month postnatally, measured by a breastfeeding self‐efficacy instrument, which has been psychometrically validated in Sweden with a Cronbach's α coefficient of .91 (Gerhardsson et al., 2014). The instrument consists of 14 items and has a maximum score of 70 (Dennis, 2003). Moreover, a global measurement of breastfeeding self‐efficacy was collected by asking mothers how likely they were to carry on breastfeeding until 6 months postnatally, rated on a 5‐point Likert scale from very certain to very uncertain. Secondary outcomes were exclusive breastfeeding at 5–7 days, 1 and 6 months postnatally and readmission due to nutritional problems (breastfeeding problems, dehydration, excessive weight loss, and jaundice) within 5–7 days and 1 month of life. Exclusive breastfeeding was defined as the infant getting nothing other than milk from the mother and was measured as exclusive breastfeeding during the past 24 hr (World Health Organization [WHO], 2008).
2.6. Sample size and statistical analysis
Sample size was calculated for breastfeeding self‐efficacy based on a 2‐sided T‐test by the software program PASS 11. With 5 clusters in each group, an intraclass correlation factor of .03 (Reading, Harvey, & Maclean, 2000), we were required to include minimum 79 mother–infant dyads in each cluster to have 80% power at an alpha‐level of 5% to detect a 5‐point difference in mean breastfeeding self‐efficacy score, with a standard deviation of 12, between the intervention and reference group. The expected difference was based on an intervention study with aims and outcomes measures comparable with this study (Nichols, Schutte, Brown, Dennis, & Price, 2009).
We performed intention‐to‐treat analyses (ITT), including all mother–infant dyads, and complete‐case analyses (CC) restricted to mothers and infants with available information on the specific outcomes (Gupta, 2011). Analyses were performed on mothers irrespective of length of hospitalization and on mothers hospitalized 50 hr or less following birth (early discharge). In order to account for the clustered nature of the data, mixed models (logistic regression models for the binary outcomes, linear regression for modelling breastfeeding self‐efficacy) with random effects for cluster were fitted (Campbell & Walters, 2014), using the GLLAMM routine in STATA 13 (Rabe‐Hesketh & Skrondal, 2008). We adjusted for maternal BMI and mode of delivery, which were imbalanced between the groups (Table 2). For the ITT, missing data were handled by inverse probability weighting (Seaman & White, 2013), with weights generated for each specific outcome using baseline information on maternal socioeconomic status, parity, smoking status and BMI, mode of delivery, and length of admission. Missing baseline information was handled by single imputation (Campbell & Walters, 2014).
Table 2.
Maternal and infant characteristics in the reference and intervention group
| Characteristics | Reference group | Intervention group | p valuea |
|---|---|---|---|
| Total N (%) | 1,476 (100) | 2,065 (100) | |
| Maternal age, years, mean (SD) | 29.7 ( 4.5) | 29.7 (4.8) | .64 |
| Education, n (%) | |||
| No/short (<3 years) | 651 (44.1) | 943 (45.7) | .46 |
| Medium/long (≥3 years) | 717 (48.6) | 986 (47.7) | |
| Missing | 108 (7.3) | 136 (6.6) | |
| Marital status, n (%) | |||
| Married/cohabiting | 1,321 (89.5) | 1,875 (90.8) | .16 |
| Single | 47 (3.2) | 50 (2.4) | |
| Missing | 108 (7.3) | 140 (6.8) | |
| Ethnicity, n (%) | |||
| Both parents Danish | 1,252 (84.8) | 1,738 (84.2) | .26 |
| One or no parents Danish | 116 (7.9) | 185 (9.0) | |
| Missing | 108 (7.3) | 142 (6.9) | |
| Parity, n (%) | |||
| Primiparous | 579 (39.2) | 825 (40.0) | .75 |
| Multiparous | 788 (53.4) | 1,097 (53.1) | |
| Missing | 109 (7.4) | 143(6.9) | |
| Mode of delivery, n (%) | |||
| Vaginal delivery | 1,016 (68.8) | 1,398 (67.7) | .01 |
| Cesarean section | 185 (12.5) | 329 (15.9) | |
| Missing | 275 (18.6) | 338 (16.4) | |
| Gestational age, months, mean (SD) | 39.6 (1.4) | 39.6 (1.5) | .69 |
| Birthweight, gram, mean (SD) | 3,598.5 (484.3) | 3,588.3 (483.3) | .58 |
| BMI, n (%) | |||
| <18.5 | 52 (3.5) | 85 (4.1) | .02 |
| 18.5–24.9 | 827 (56.0) | 1,088 (52.7) | |
| 25–29.9 | 325 (22.0) | 450 (21.8) | |
| 30–34.9 | 101 (6.8) | 199 (9.6) | |
| ≥35 | 62 (4.2) | 100 (4.8) | |
| Missing | 109 (7.4) | 143 (6.9) | |
| Smoking, n (%) | |||
| No | 1,270 (86.0) | 1,766 (85.5) | .25 |
| Yes | 97 (6.6) | 157 (7.6) | |
| Missing | 109 (7.4) | 142 (6.9) | |
p values calculated by χ2 test for categorical values and T‐test for continuous values, excluding missing values
2.7. Ethical approval
This study was registered at The http://clinicaltrials.gov Protocol Registration and Results System prior to the inclusion of the first participant [7111442]. In accordance with Danish legislation, this study was approved by the Danish Data Protection Agency [2012‐41‐1140] and additionally assessed by The Committee for Health Research Ethics, Capital Region, Denmark [1‐2012‐FSP‐34].
3. RESULTS
In total, 2,306 and 1,730 women were invited to the intervention and reference group, respectively, and 2,065 (89%) and 1,476 (85%) accepted the invitation, with cluster size ranges of 291–540 in the ITT (Figure 1). According to the Danish Medical Birth Register, 5,991 women in the intervention group and 4,698 in the reference group were giving birth in the recruitment period, which means that 45% of the women in each group were assessed for eligibility. Women assessed for eligibility were more likely to have vaginal births, were nonsmokers, and had infants with higher birth weight compared to women not assessed for eligibility (Supplementary Table 1). Baseline characteristics did not differ significantly between the women who participated and those who did not, except that more smokers declined invitation (Supplementary Table 2). The frequency of lost to follow‐up or missing were slightly higher in the reference group than in the intervention group (Figure 1) with a follow‐up rate in the intervention and reference group of 80 and 77 after 1 week, 70 and 67 after 1 month, and 57 and 55 after 6 months. However, the final population in the intervention and reference group were comparable in baseline characteristics including length of hospital stay, except that more women in the intervention group delivered by caesarean section and had a lower BMI (Table 2).
Breastfeeding self‐efficacy score, measured by the breastfeeding self‐efficacy instrument showed a ceiling effect (Supplementary Figure 1), and no differences between the intervention and reference group were observed for the global measurement of breastfeeding self‐efficacy at 1‐week and 1‐month postpartum (Table 3). Moreover, we found no differences between the groups for exclusive breastfeeding at 5–7 days and 1‐month postpartum; however, 6 months postnatally, infants in the intervention group had significant higher odds of exclusive breastfeeding compared to the reference group (Table 3). Likewise, infants in the intervention group had lower odds of readmission within the first week, whereas readmissions within 4‐week postpartum did not differ between groups (Table 3). These effects were comparable in ITT and CC (Supplementary Table 3), and the effect of the program on readmission rate within the first week was even more pronounced among women discharged early, OR = 0.39 (95% CI [0.20, 0.74]) than among all women OR = 0.55 (95% CI [0.37, 0.81]). Further, analyses showed that mothers as well as the fathers in the intervention group spent more hours having skin‐to‐skin contact with the infant on the first day postnatally (Table 4). Intervention‐group mothers experienced the most breastfeeding problems, which were mainly problems related to breastfeeding length and frequency, whereas reference‐group mothers experienced more pain and rated pain more severely (Table 4). Intervention‐group infants were breastfed more frequently and were less likely to be tested for hyperbilirubinaemia and treated with phototherapy for jaundice (Table 4). The process assessment found that the intervention‐group mothers compared to the reference‐group mothers more often reported having been instructed by HCPs in skin‐to‐skin contact, frequent breastfeeding, altering breastfeeding position to reduce pain and the father's role (Table 5). Additional exploratory analysis showed that OR for exclusive breastfeeding at 1 week postnatally was 0.14 (95% CI [0.09, 0.21]) if mothers had a very low breastfeeding self‐efficacy, rated as 1 compared to a very high self‐efficacy, rated as 5 on the Likert scale by the global breastfeeding self‐efficacy question (results not shown).
Table 3.
Effects of the program. Comparison of breastfeeding (BF) self‐efficacy and exclusive breastfeeding reported by the mothers in the reference group and intervention
| Reference group | Intervention group | Adjusted odds ratio (95% CI)a | Adjusted mean difference (95% CI)a | p value | ICC | |||
|---|---|---|---|---|---|---|---|---|
| Intention to treat analysis | ||||||||
| 5–7 days postnatally | n/N | % | n/N | % | ||||
| BF self‐efficacyb
mean (SD) |
2.94 (1.29) | 2.92 (1.37) | −0.01 (−0.14–0.12) | .91 | <.0001 | |||
| Exclusive BF | 1,208/1,476 | 82.2 | 1,682/2,065 | 82.0 | 1.01 (0.88–1.16) | .93 | <.0001 | |
| Readmissionc | 55/1,476 | 3.6 | 43/2,065 | 2.2 | 0.55 (0.37–0.81) | <.01 | <.0001 | |
| 1 month postnatally | ||||||||
| BF self‐efficacyb
mean (SD) |
3.01 (1.41) | 3.04 (1.40) | 0.04 (−0.09–0.17) | .52 | <.0001 | |||
| Exclusive BF | 1,129/1,476 | 76.3 | 1,522/2,065 | 74.2 | 0.87 (0.64–1.18) | .38 | .011 | |
| Readmissiond | 40/1,476 | 2.5 | 54/2,065 | 2.8 | 0.96 (0.58–1.59) | .89 | .015 | |
| 6 months postnatally | ||||||||
| Exclusive BF | 73/1,476 | 5.1 | 135/2,065 | 6.6 | 1.36 (1.02–1.81) | .04 | <.0001 | |
| Intention‐to‐treat analysis, early discharge | ||||||||
| 5–7 days postnatally | n/N | % | n/N | % | ||||
| BF self‐efficacy
mean (SD) |
3.05 (1.39) | 3.03 (1.37) | 0.01 (−0.08–0.09) | .91 | <.0001 | |||
| Exclusive BF | 861/1,000 | 86.4 | 1,102/1,297 | 85.9 | 0.95 (0.68–1.31) | .73 | .007 | |
| Readmission | 51/1,000 | 3.8 | 24/1,297 | 1.9 | 0.39 (0.20–0.74) | <.01 | .012 | |
| 1 month postnatally | ||||||||
| BF self‐efficacy
mean (SD) |
3.10 (1.41) | 3.10 (1.39) | 0·01 (−0.10–0.11) | .87 | <.0001 | |||
| Exclusive BF | 811/1,000 | 81.5 | 989/1,297 | 76.8 | 0.80 (0.54–1.17) | .25 | .016 | |
| Readmission | 31/1,000 | 2.3 | 30/1,297 | 2.6 | 0.75 (0.38–1.47) | .40 | .026 | |
| 6 months postnatally | ||||||||
| Exclusive BF | 72/1,000 | 6.1 | 167/1,297 | 7.3 | 1.20 (0.48–2.98) | .70 | .142 | |
Note. CI = confidence interval; ICC = intraclass correlation factor; SD = standard deviation.
Adjusted for maternal body mass index and mode of delivery
Breastfeeding self‐efficacy measured on a Likert scale from 1 = very certain to 5 = very uncertain
Readmission of the infant within 7 days postnatally due to jaundice, dehydration, excessive weight loss, and nutritional problems
Readmission of the infant from 7 to 28 days postnatally due to jaundice, dehydration, excessive weight loss, and nutritional problems
Table 4.
Associations between the reference and intervention group according to potential explanations for the findings at 1 week postnatally
| Potential explanatory variables | Reference group n(%) | Intervention group n(%) | p value |
|---|---|---|---|
| Breastfeeding problems | 546 (46.1) | 867 (50.7) | .014 |
| Experienced difficulties with: | |||
| Breastfeeding pain | 1,020 (86.1) | 1,419 (83.1) | .029 |
| Pain intensity | |||
| No mild | 211 (17.6) | 399 (23.2) | .001 |
| Moderate | 758 (63.4) | 1,034 (60.0) | |
| Severe | 227 (19.0) | 290 (16.8) | |
| Sore nipples | 732 (61.8) | 1,020 (59.7) | .266 |
| Not enough milk | 405 (34.2) | 556 (32.6) | .362 |
| Frequent breastfeeding | 845 (71.3) | 1,284 (75.2) | .020 |
| Long‐lasting breastfeeding | 699 (59.0) | 1,079 (63.2) | .023 |
| Skin‐to‐skin with mothera | |||
| <2 hr | 102 (8.5) | 119 (6.9) | <.001 |
| 2–3 hr | 228 (19.1) | 193 (11.2) | |
| 3–5 hr | 280 (23.4) | 387 (22.4) | |
| 5–8 hr | 275 (23.0) | 432 (25.0) | |
| >8 hr | 266 (22.2) | 541 (31.3) | |
| Do not know | 45 (3.8) | 54 (3.1) | |
| Breastfeeding frequency, times/dayb | |||
| >10 | 227 (19.2) | 462 (27.0) | <.001 |
| 8–10 | 590 (49.8) | 866 (50.7) | |
| 6–7 | 266 (22.5) | 259 (15.2) | |
| 4–5 | 24 (2.0) | 27 (1.6) | |
| <4 | 38 (3.2) | 35 (2.1) | |
| Do not know | 40 (3.4) | 60 (3.5) | |
| Tested for high se‐bilirubine | 213 (18.0) | 170 (10.0) | <.001 |
| Phototherapy | 42 (3.5) | 27 (1.6) | .003 |
| Laid back position | 181 (15.2) | 467 (27.1) | <.001 |
| Supported to skin‐to‐skinc | |||
| Very much–much | 731 (71.4) | 1,193 (75.1) | .037 |
| Neutral–no | 293 (28.6) | 396 (24.9) | |
| Skin‐to‐skin with fatherd | |||
| <1 hr | 536 (45.7) | 536 (31.7) | <.001 |
| 1–3 hr | 465 (39.6) | 837 (49.5) | |
| 3–8 hr | 100 (8.5) | 247 (14.6) | |
| >8hr | 14 (1.2) | 16 (1.0) | |
| Do not know | 58 (4.9) | 55 (3.3) | |
Skin‐to‐skin contact between mother and infant on the first day following birth
Breastfeeding frequency on the day before answering the questionnaire
Mother experienced to be supported to skin‐to‐skin by the father
Skin‐to‐skin contact between father and infant on the first day following birth
Table 5.
Program implementation. Comparison of the received components in the reference and intervention groups reported by all the mothers and by mothers discharged within 50 hr postnatally
| Program components Talked with health professional about: | Reference group n(%) | Intervention group n(%) | p value | Reference group ≤50 h n(%) | Intervention group ≤50 h n(%) | p value |
|---|---|---|---|---|---|---|
| Skin‐to‐skin | ||||||
| Yes | 829 (77.6) | 1,490 (91.2) | <.0001 | 467 (74.2) | 811 (89.2) | <.0001 |
| No | 239 (22.4) | 143 (8.8) | 162 (25.8) | 98 (10.8) | ||
| Total | 1,068 (100) | 1,633 (100) | 629 (100) | 909 (100) | ||
| Breastfeeding ≥8 times/day | ||||||
| Yes | 608 (60.9) | 1,364 (86.8) | <.0001 | 338 (57.0) | 739 (85.2) | <.0001 |
| No | 390 (39.1) | 207 (13.2) | 255 (43.0) | 128 (14.8) | ||
| Total | 998 (100) | 1,571 (100) | 593 (100) | 867 (100) | ||
| Altering positioning if breastfeeding hurts | ||||||
| Yes | 799 (71.5) | 1,250 (77.0) | .001 | 430 (64.6) | 645 (72.4) | .001 |
| No | 319 (28.5) | 373 (23.0) | 236 (35.4) | 246 (27.6) | ||
| Total | 1,118 (100) | 1,623 (100) | 666 (100) | 891 (100) | ||
| How the father can support breastfeeding | ||||||
| Yes | 512 (49.2) | 1,060 (68.7) | <.0001 | 258 (41.7) | 538 (62.9) | <.0001 |
| No | 528 (50.8) | 483 (31.3) | 361 (58.3) | 317 (37.1) | ||
| Total | 1,040 (100) | 1,543 (100) | 619 (100) | 855 (100) | ||
4. DISCUSSION
Overall, our study showed that a hospital‐based intervention program on breastfeeding counselling did not increase breastfeeding self‐efficacy, decreased the odds of infant readmission within the first week of age and increased the odds of exclusive breastfeeding at 6 months for mothers, who were discharged within 50 hr postnatally. Moreover, biological improvements related to lactation such as higher number of daily breastfeeding and more time spent by maternal–infant skin‐to‐skin contact were seen in the intervention group.
In accordance with previous studies, we found a strong correlation between the level of global breastfeeding self‐efficacy and the duration of breastfeeding (Kronborg, Maimburg, & Væth, 2011). However, our study did not succeed in improving maternal breastfeeding self‐efficacy. Other intervention studies aiming to enhance breastfeeding self‐efficacy are inconclusive (Hanne Kronborg et al., 2011; Nichols et al., 2009). The present result might reflect that more mothers in the intervention group experienced breastfeeding problems, which have shown to be associated with lower self‐efficacy (Kingston, Dennis, & Sword, 2007). Another explanation might be the instrument that measured breastfeeding self‐efficacy. Even though previously tested in Denmark, we found a ceiling effect of the instrument. We therefore used a global measurement, which has likewise been tested in a Danish setting, but might be less sensitive for different aspects of breastfeeding self‐efficacy. The results underline the need of a more sensitive measurement for breastfeeding self‐efficacy for countries with strong breastfeeding tradition.
We found a reduction of infants who were readmitted to the hospital in the intervention group and an even more pronounced effect among infants of women who were discharged within 50 hr postnatally. A Cochrane Review from 2012(Renfrew et al., 2012) found only few breastfeeding programs tested in randomized controlled trials and none of these studies found any difference in readmission rates. Subanalysis showed that jaundice was the main indication of readmission in our study. This is supported by previous cohort studies of otherwise healthy newborns (Farhat & Rajab, 2011). Intervention studies with a before–after design have shown that it is possible to reduce readmission for jaundice if the HCP follow clinical guidelines (Waldrop, Anderson, & Brandon, 2013). However, although primarily focusing on jaundice, it followed a guideline that highly weighted screening and blood testing and included support of effective breastfeeding but did not specify how breastfeeding support was offered. Our intervention program emphasized four evidence‐based recommendations on breastfeeding. Although it is not possible to point at a single effective component in a complex intervention, the infants in the intervention group were breastfed significantly more often per day, 1 week postnatally compared to the reference group and subanalysis showed that higher number of daily breastfeeding bouts was strongly correlated with lower frequency tests for hyperbilirubinaemia and treatment with phototherapy. Similar correlation is observed in other studies (Chen, Yeh, & Chen, 2015). Interestingly, although being biologically supportive of lactation (Wambach & Riordan, 2015), frequent breastfeeding was more often experienced as a problem by mothers in the intervention group in the first postnatal week. However, this did not result in a higher number of mothers experiencing pain and sore nipples compared to the reference group. Taking this into account, it might be important to discuss the ambivalence of frequent breastfeeding with the parents and support them in overcoming it.
We found a higher prevalence of breastfeeding at 6 months in the intervention group compared to the reference group. The consistent results of the ITT and CC support the validity of this finding. We based our intervention on support, which was introduced in late pregnancy and mainly offered face‐to‐face within the first 2 days postnatally, and with a follow‐up telephone call 1 day after discharge. Similarly, a review of breastfeeding interventions concluded that postnatal interventions were the most successful interventions to increase breastfeeding duration (Skouteris et al., 2014) and face‐to‐face support is known as the most effective support (Renfrew et al., 2012). Moreover, a Cochrane review stressed that any follow‐up after early discharge was important for preventing adverse effects on breastfeeding (Brown, Small, Faber, Krastev, & Davis, 2002). The higher prevalence of breastfeeding at 6 months in the intervention group might also be explained by the higher frequency of breastfeeding and more hours of skin‐to‐skin contact during the first postnatal days. A Cochrane review among healthy‐term infants supports this finding of a long‐term effect of skin‐to‐skin contact on exclusive breastfeeding (Moore et al., 2012). With these results in mind, we would have expected an increased prevalence of exclusive breastfeeding in the intervention group at 1 week and 1 month postnatally. This might be explained by a ceiling effect of the breastfeeding rates in the early postnatal period, which was also seen in other countries with high initiation rates (Yotebieng et al., 2015). Another explanation might be the effects of extended early skin‐to‐skin contact in this study. A Cochrane review of skin‐to‐skin contact showed the same pattern, which in spite of the positive effect at 4 months, failed to find any positive effect on exclusive breastfeeding at time of postnatal discharge (Moore et al., 2012). Early skin‐to‐skin contact has been found to impact the interaction between the mother and the infant for up to 1 year following birth (Moore et al., 2012), which indicates a long‐term effect of skin‐to‐skin. Skin‐to‐skin contact immediately following birth has been recommended for a number of years in Denmark. In this study, we explicitly recommended extended skin‐to‐skin contact during the first 3 days.
In our study, more father–infant dyads in intervention compared to reference group had skin‐to‐skin contact for more hours in the first 3 days and subanalysis showed that significantly more mothers experienced that the father encouraged them to skin‐to‐skin contact and good positioning and trusted in their ability to produce enough milk (results not shown). These results indicate that the father was more involved in breastfeeding in the intervention group. A review of male‐partner‐focused interventions has shown that fathers' knowledge and encouragement has an impact on breastfeeding duration (Mitchell‐Box & Braun, 2013), and qualitative studies report that fathers find it difficult to support breastfeeding and are not sufficiently supported by the HCPs on how to support their spouse in the breastfeeding process (Sherriff & Hall, 2011). In our study, we trained the health professionals to be specific in their involvement of the father. The enhanced paternal support together with increased duration of skin‐to‐skin contact might have been important relational factors contributing to the higher prevalence of exclusive breastfeeding at 6 months in the intervention group.
A limitation of the study is that only 45% were assessed for eligibility at the hospitals, probably due to oversight among the midwives. A methodological challenge for cluster‐randomized studies is how to prevent biased recruitment, because clusters are recruited and randomized before the recruitment of participants. In our study, an individual random group allocation was not possible because of contamination risk between women giving birth at the same birth facilities and practical issues related to the implementation. However, the women who were assessed for eligibility were comparable in the intervention and reference group and likewise, baseline characteristics of mothers in the two groups were comparable—except for BMI and mode of delivery. These two factors are known to influence breastfeeding duration (Wambach & Riordan, 2015), and we therefore adjusted our results for these factors. It is a great advantage of our study that we have access to register data on all births at the included birth facilities, and based on these data, we do not find selection bias to be a major concern in spite of the considerable number of never‐assessed women.
We had a considerable number of loss to follow‐up and missing values. However, given the comparable CC and inverse probability weighted ITT, we suggest that the results are robust. We aimed to assess the fidelity of the intervention delivery. According to the mothers, the HCPs introduced the four core components of the program, and the parents adhered to them to a great extent. Our data do not successfully capture completeness of the implementation of Bandura's four sources to increase self‐efficacy (mastery experiences, vicarious experiences, social persuasion, and emotional arousal). A comprehensive description might have revealed why the intervention did not succeed in enhancing maternal breastfeeding self‐efficacy. Though, it might be optimistic to think that an 11‐hr interactive training course changes HCPs practice of psychosocial support to the mother. A review of the impact of continuing breastfeeding education of health professionals pointed out that breastfeeding courses with at least 18‐hr education are the most beneficial (Ward & Byrne, 2011). This review did not include how to increase maternal breastfeeding self‐efficacy. Only one study described how an 18‐hr interactive course for HCPs failed to improve breastfeeding self‐efficacy (Kronborg et al., 2011). Improving maternal breastfeeding self‐efficacy might benefit from process‐oriented training for a longer duration.
The intervention was implemented in medium size and small hospitals in Denmark. The large hospitals did not take part in the study, which according to the hospitals, was explained by the requirement of covering the costs of the health professionals' training. However, we do not expect that the size of the hospitals is a barrier for the implementation of this programme at a larger scale, as it consists of only a few components, which might be easy to implement in large hospitals too. Today, early discharge is common for new families with an uncomplicated delivery of a healthy‐term infant. We need to adjust our care to this framework. This study adds evidence on how to deal with breastfeeding counselling in an early discharge setting and finds favourable outcomes related to readmission, increased daily breastfeeding frequency, and breastfeeding duration in the intervention group. The study was performed in Denmark where breastfeeding initiation rates are usually high and breastfeeding duration is long. The higher prevalence of breastfeeding in the intervention group in this study might, therefore, be less than if implemented in countries where breastfeeding initiation and duration is lower.
5. CONCLUSION
In an early postnatal discharge setting, a hospital‐based breastfeeding intervention can decrease readmission of newborns due to nutritional problems within the first week postnatally, support the physiology of lactation and increase the prevalence of exclusive breastfeeding at 6 months. Frequent breastfeeding seemed to contribute to the decreased readmission prevalence in the intervention group; however, more women in this group also experienced the more frequent breastfeeding as problematic. It might be important for HCPs to discuss this parental ambivalence in order to encourage new parents to accommodate frequent breastfeeding.
SOURCE OF FUNDING
All phases of this project were supported by Trygfonden [J.nr. 7‐11‐1442] and The Danish Nurses' Organization [70165].
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
IN, HK, and KSL designed the study. IN and HK developed the intervention and the questionnaires with important input by CHK to the biological part of the intervention and important comments by KSL to all questionnaires. IN recruited the hospitals, trained the healthcare staff, pilot tested the questionnaires, and was responsible for the organization of the dissemination of questionnaires and collection of data. IN suggested the initial strategy for analysis of data, which was further planned in close cooperation with KSL and AVH, and IN and AVH did the practical analysis. Interpretation of data, writing, and designing of tables and figures was suggested by IN in the first draft and developed further by IN in close cooperation with HK, KSL, and CHK. All authors have contributed with substantial revisions to the manuscript and approved the final manuscript as submitted.
Supporting information
Figure S1. Supporting info item
Table S1. Supporting info item
Table S2. Supporting info item
Table S3. Supporting info item
ACKNOWLEDGMENTS
We would like to thank health professionals and mothers at the included hospitals for their contribution to the study.
Nilsson IMS, Strandberg‐Larsen K, Knight CH, Hansen AV, Kronborg H. Focused breastfeeding counselling improves short‐ and long‐term success in an early‐discharge setting: A cluster‐randomized study. Matern Child Nutr. 2017;13:e12432 10.1111/mcn.12432
REFERENCES
- Bandura, A. (1997). Self‐efficacy: The exercise of control (1st ed.). New York: Worth Publishers. [Google Scholar]
- Bartholomew, L. K. , Parcel, G. S. , Kok, G. , Gottlieb, N. H. , & Fernandez, M. E. (2011). Planning health promotion programs. An intervention mapping approach (3rd ed., Vol. 2011). San Fransisco, USA: Jossey‐Bass. [Google Scholar]
- Bravo, P. , Uribe, C. , & Contreras, A. (2011). Early postnatal hospital discharge: The consequences of reducing length of stay for women and newborns. Revista Da Escola de Enfermagem Da USP, 45(3), 758–763. 10.1590/S0080-62342011000300030 [DOI] [PubMed] [Google Scholar]
- Brown, S. , Small, R. , Faber, B. , Krastev, A. , & Davis, P. (2002). Early postnatal discharge from hospital for healthy mothers and term infants. The Cochrane Database of Systematic Reviews, 3, CD002958. 10.1002/14651858.CD002958 [DOI] [PubMed] [Google Scholar]
- Brownell, E. , Howard, C. R. , Lawrence, R. A. , & Dozier, A. M. (2012). Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. The Journal of Pediatrics, 161(4), 608–614. 10.1016/j.jpeds.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, M. J. , & Walters, S. J. (2014). How to design, analyse and report cluster randomised trials in medicine and health related research (1st ed.). Chichester, West Sussex: Wiley‐Blackwell. [Google Scholar]
- Chen, Y.‐J. , Yeh, T.‐F. , & Chen, C.‐M. (2015). Effect of breastfeeding frequency on hyperbilirubinemia in breastfed term neonate. Pediatrics International 10.1111/ped.12667 [DOI] [PubMed]
- Colson, S. (2006). The mechanisms of Biological Nurturing [Ph.d. Thesis]. University of Kent at Canterbury.
- de Jager, E. , Skouteris, H. , Broadbent, J. , Amir, L. , & Mellor, K. (2013). Psychosocial correlates of exclusive breastfeeding: A systematic review. Midwifery 10.1016/j.midw.2012.04.009 [DOI] [PubMed]
- Dennis, C.‐L. (2003). The breastfeeding self‐efficacy scale: Psychometric assessment of the short form. Journal of Obstetric, Gynecologic, and Neonatal Nursing: JOGNN / NAACOG, 32(6), 734–744. [DOI] [PubMed] [Google Scholar]
- Farhat, R. , & Rajab, M. (2011). Length of postnatal hospital stay in healthy newborns and re‐hospitalization following early discharge. North American Journal of Medical Sciences, 146–151. 10.4297/najms.2011.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardsson, E. , Nyqvist, K. H. , Mattsson, E. , Volgsten, H. , Hildingsson, I. , & Funkquist, E.‐L. (2014). The Swedish version of the Breastfeeding Self‐Efficacy Scale–Short Form reliability and validity assessment. Journal of Human Lactation, 890334414523836. [DOI] [PubMed] [Google Scholar]
- Gupta, S. (2011). Intention‐to‐treat concept: A review. Perspectives in Clinical Research, 2(3), 109 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, J. C. , Mitoulas, L. R. , Cregan, M. D. , Ramsay, D. T. , Doherty, D. A. , & Hartmann, P. E. (2006). Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics, 117(3), e387–e395. 10.1542/peds.2005-1417 [DOI] [PubMed] [Google Scholar]
- Kingston, D. , Dennis, C. L. , & Sword, W. (2007). Exploring breast‐feeding self‐efficacy. Journal of Perinatal & Neonatal Nursing, 2007(3), 207–215. [DOI] [PubMed] [Google Scholar]
- Kreuter, M. W. , Bull, F. C. , Clark, E. M. , & Oswald, D. L. (1999). Understanding how people process health information: A comparison of tailored and nontailored weight‐loss materials. Health Psychology, 18(5), 487–494. 10.1037/0278-6133.18.5.487 [DOI] [PubMed] [Google Scholar]
- Kronborg, H. , & Vaeth, M. (2004). The influence of psychosocial factors on the duration of breastfeeding. Scandinavian Journal of Public Health, 32(3), 210–216. 10.1080/14034940310019218 [DOI] [PubMed] [Google Scholar]
- Kronborg, H. , Vaeth, M. , Olsen, J. , & Harder, I. (2008). Health visitors and breastfeeding support: influence of knowledge and self‐efficacy. The European Journal of Public Health, 18(3), 283–288. 10.1093/eurpub/ckm121 [DOI] [PubMed] [Google Scholar]
- Kronborg, H. , Maimburg, R. D. , & Væth, M. (2011). Antenatal training to improve breast feeding: a randomised trial. Midwifery 10.1016/j.midw.2011.08.016 [DOI] [PubMed]
- Larsen, J. S. , & Kronborg, H. (2012). When breastfeeding is unsuccessful – mothers' experiences after giving up breastfeeding. Scandinavian Journal of Caring Sciences. 10.1111/j.1471-6712.2012.01091.x [DOI] [PubMed] [Google Scholar]
- Mitchell‐Box, K. M. , & Braun, K. L. (2013). Impact of male‐partner‐focused interventions on breastfeeding initiation, exclusivity, and continuation. Journal of Human Lactation, 29(4), 473–479. 10.1177/0890334413491833 [DOI] [PubMed] [Google Scholar]
- Moore, E. R. , Anderson, G. C. , Bergman, N. , & Dowswell, T. (2012). Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews (Online), (5), CD003519. 10.1002/14651858.CD003519.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, J. , Schutte, N. S. , Brown, R. F. , Dennis, C.‐L. , & Price, I. (2009). The impact of a self‐efficacy intervention on short‐term breast‐feeding outcomes. Health Education & Behavior, 36(2), 250–258. 10.1177/1090198107303362 [DOI] [PubMed] [Google Scholar]
- Nilsson, I. , Danbjørg, D. B. , Aagaard, H. , Strandberg‐Larsen, K. , Clemensen, J. , & Kronborg, H. (2015). Parental experiences of early postnatal discharge: A meta‐synthesis. Midwifery 10.1016/j.midw.2015.07.004 [DOI] [PubMed]
- Nilsson, I. , Kronborg, H. , Knight, C. H. , Strandberg‐Larsen, K . (2016). Early discharge following birth – what characterises mothers and newborns? Sexual & reproductive health care, 2016. doi 10.1016/j.srhc.2016.10.007 [DOI] [PubMed]
- Rabe‐Hesketh, S. , & Skrondal, A. (2008). Multilevel and longitudinal modeling using Stata. STATA Press, 2008.
- Reading R., Harvey I., Maclean M. (2000). Cluster randomised trials in maternal and child health: implications for power and sample size. Archives of Disease in Childhood, 82(1), 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrew, M. J. , McCormick, F. M. , Wade, A. , Quinn, B. , & Dowswell, T. (2012). Support for healthy breastfeeding mothers with healthy term babies In Cochrane database of systematic reviews John Wiley & Sons, Ltd. Retrieved from http://onlinelibrary.wiley.com.ez.statsbiblioteket.dk:2048/doi/10.1002/14651858.CD001141.pub4/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, S. R. , & White, I. R. (2013). Review of inverse probability weighting for dealing with missing data. Statistical Methods in Medical Research, 22(3), 278–295. 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- Sherriff, N. , & Hall, V. (2011). Engaging and supporting fathers to promote breastfeeding: A new role for health visitors? Scandinavian Journal of Caring Sciences, 25(3), 467–475. 10.1111/j.1471-6712.2010.00850.x [DOI] [PubMed] [Google Scholar]
- Skouteris, H. , Nagle, C. , Fowler, M. , Kent, B. , Sahota, P. , & Morris, H. (2014). Interventions designed to promote exclusive breastfeeding in high‐income countries: A systematic review. Breastfeeding Medicine, 9(3), 113–127. 10.1089/bfm.2013.0081 [DOI] [PubMed] [Google Scholar]
- Sundhedsstyrelsen [The Danish Health Authority] . (2013). Amning—En håndbog for sundhedspersonale [Breastfeeding—A handbook for health professionals].
- Victora, C. G. , Bahl, R. , Barros, A. J. , França, G. V. , Horton, S. , Krasevec, J. , … Lancet Brestfeeding Series Group . (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. [DOI] [PubMed] [Google Scholar]
- Waldrop, J. B. , Anderson, C. K. , & Brandon, D. H. (2013). Guideline‐based educational intervention to decrease the risk for readmission of newborns with severe hyperbilirubinemia. Journal of Pediatric Health Care, 27(1), 41–50. 10.1016/j.pedhc.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Wambach, K. , & Riordan, J. (2015). Breastfeeding and human lactation. (5th Revised edition edition). Burlington, MA: Jones and Bartlett Publishers, Inc. [Google Scholar]
- Ward, K. N. , & Byrne, J. P. (2011). A critical review of the impact of continuing breastfeeding education provided to nurses and midwives. Journal of Human Lactation, 27(4), 381–393. 10.1177/0890334411411052 [DOI] [PubMed] [Google Scholar]
- Ward, P. M. , & Deshpande, S. (2005). Metabolic adaptation at birth. Seminars in Fetal & Neonatal Medicine, 10(4), 341–350. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2008). Indicators for assessing infant and young child feeding practices: conclusions of a consensus meeting held 6-8 November 2007 in Washington D.C., USA. Washington, D.C.: World Health Organization (WHO).
- WHO . (2013). WHO recommendations on postnatal care of the mother and newborn. Retrieved 12 January 2015, from http://www.who.int/maternal_child_adolescent/documents/postnatal-care-recommendations/en/ [PubMed]
- Yotebieng, M. , Labbok, M. , Soeters, H. M. , Chalachala, J. L. , Lapika, B. , Vitta, B. S. , & Behets, F. (2015). Ten steps to successful breastfeeding programme to promote early initiation and exclusive breastfeeding in DR Congo: A cluster‐randomised controlled trial. The Lancet Global Health, 3(9), e546–e555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting info item
Table S1. Supporting info item
Table S2. Supporting info item
Table S3. Supporting info item


