Abstract
Association of maternal obesity with shorter breastfeeding duration may involve different factors and might be modified by parity. In a national birth cohort, we aimed to estimate the association between prepregnancy body mass index (pBMI) and breastfeeding duration after adjustment for sociodemographic, pregnancy, and other characteristics and assess the effect modification of parity in such associations. In 2012, 3,368 mother–infant dyads were randomly included at birth in the French Epifane cohort. Breastfeeding information was collected in maternity wards and by phone interview at 1, 4, 8, and 12 months postpartum. Poisson regression analyses estimated the association of pBMI with the number of days of “any breastfeeding” (ABF) and “exclusive breastfeeding” (EBF) in unadjusted and adjusted models. Interactions between parity and pBMI were tested. Obesity before pregnancy was independently associated with shorter ABF duration (incidence rate ratio [IRR] = 0.86, 95%CI [0.74, 0.99]) compared to normal‐weight status. Parity showed an effect modification only with EBF duration. Among primiparae, no association was found for obesity, but overweight was significantly associated with shorter EBF duration independently of all covariates (IRR = 0.74 [0.58, 0.95]). Among multiparas, obesity was associated with shorter EBF duration after controlling for sociodemographic factors (IRR = 0.71 [0.53, 0.95]). This association was no longer statistically significant after controlling for other covariates. Obesity appears to be a strong risk factor in shorter ABF duration. Furthermore, parity is a key factor in the relationship of pBMI to shorter EBF duration. Overweight primiparous and obese multiparous women need additional support to prolong breastfeeding duration.
Keywords: birth cohort, BMI, breastfeeding, infant, maternal obesity, obstetrics
1. INTRODUCTION
Because maternal obesity is associated with adverse health outcomes for mother and offspring, the worldwide increase in obesity among childbearing women has become an important global public health issue (Godfrey et al., 2017; Poston et al., 2016). In addition to numerous documented benefits for infants (Victora et al., 2016), breastfeeding has a preventive impact on adverse health consequences related to maternal obesity in mothers. Indeed, breastfeeding for at least 3 months (Aune, Norat, Romundstad, & Vatten, 2014) and 5 months (Scoccianti et al., 2015) might decrease the risk of diabetes and breast cancer, both associated with obesity (Kessous, Davidson, Meirovitz, Sergienko, & Sheiner, 2017; Poston et al., 2016).
A reduction in breastfeeding duration has been observed in obese women in different countries (Baker, Michaelsen, Sørensen, & Rasmussen, 2007; Castillo, Santos, & Matijasevich, 2016; Donath & Amir, 2008; Guelinckx, Devlieger, Bogaerts, Pauwels, & Vansant, 2012; Hauff, Leonard, & Rasmussen, 2014; Winkvist et al., 2015). This negative relationship may involve various factors. Maternal obesity has been shown to be associated with a set of sociodemographic factors (Kim, Dietz, England, Morrow, & Callaghan, 2007), themselves associated with shorter breastfeeding duration (Bartok, Schaefer, Beiler, & Paul, 2012). Maternal obesity is a risk factor in adverse maternal and neonatal outcome, especially Caesarean delivery (Poston et al., 2016). Caesarean section has been associated with difficulty in initiating lactation by delaying the onset of lactogenesis, involving biological factors (Bever Babendure, Reifsnider, Mendias, Moramarco, & Davila, 2015) and postoperative care after Caesarean section (Moore, Bergman, Anderson, & Medley, 2016). Psychosocial factors and poor social support have also been shown to influence maternal confidence in breastfeeding and to reduce breastfeeding duration (Hauff et al., 2014). However, that study showed that, after controlling for such psychosocial factors, women with high prepregnancy body mass index (pBMI) remained at greater risk of not breastfeeding and/or of shorter breastfeeding. This suggests that biological factors also mediate this association via a lower prolactin response (Rasmussen & Kjolhede, 2004) and delayed onset of lactogenesis (Rasmussen, 2007).
Furthermore, primiparity has also been shown to be a risk factor in delayed onset of lactogenesis (Dewey, Nommsen‐Rivers, Heinig, & Cohen, 2003), especially in obese women (Nommsen‐Rivers, Chantry, Peerson, Cohen, & Dewey, 2010). In a U.S. cohort of primiparous women, 40% reported concern about milk quantity in the first week after birth (Wagner, Chantry, Dewey, & Nommsen‐Rivers, 2013). On the basis of a comprehensive questionnaire concerning breastfeeding difficulties, obese women were more likely to report insufficient milk production in the first 2 weeks postpartum than normal‐weight women (O'Sullivan, Perrine, & Rasmussen, 2015). These concerns may have led to stopping exclusive breastfeeding (EBF) in the first weeks (Wagner et al., 2013). In previous analyses conducted in the Epifane cohort, we pointed out that parity had an effect modification on the association of maternal obesity with sociodemographic factors and pregnancy outcomes (data not published). These considerations suggest that high pBMI may influence breastfeeding differentially according to whether the mother is primiparous or multiparous.
Identification of subgroups at risk of early breastfeeding cessation in a national population appears essential, given its benefits. Using a nationwide birth cohort of pregnant women followed for 1 year after delivery (the Epifane cohort), our objectives were (a) to estimate the association between pBMI and breastfeeding duration, after adjustment for sociodemographic, pregnancy, and other characteristics and (b) to assess the effect modification of parity in such associations.
Key messages.
Parity plays an effect modification in the association of prepregnancy BMI with exclusive breastfeeding duration, but not with any breastfeeding duration.
Overweight primiparous women, but not obese primiparous women, were more likely to exclusively breastfeed for shorter duration than normal‐weight women.
Among multiparous women, obesity was not significantly associated with shorter EBF duration after taking into account maternal outcomes, early breastfeeding within the first 30 min, and psychosocial factors.
Duration of any breastfeeding was statically shorter in obese women than in normal‐weight women.
Further research is needed to assess the effectiveness of breastfeeding supportive actions, targeting obese and overweight women.
2. PARTICIPANTS AND METHODS
2.1. Study sample and follow‐up
Mother–infant dyads included in these analyses were participants in the French nationwide Epifane birth cohort. We previously provided a description of the inclusion process (Boudet‐Berquier, Salanave, De Launay, & Castetbon, 2016). In brief, this was a prospective cohort using two‐stage random sampling: 136 maternity wards were selected in mainland France, in which 25 dyads were included 1 or 2 days after delivery. The rate of mothers refusing to participate in the Epifane study was 19.3%. In total, 3,368 dyads were included between January and April 2012. To be included in the cohort, mothers had to be aged 18 years or over, not institutionalized, and had to speak French or to obtain help from someone who did. Eligibility criteria of newborns included gestational age at birth equal to or above 33 amenorrhea weeks (AWs) and birth without severe pathology requiring hospitalization. Mothers filled in a questionnaire at the maternity ward. They were then interviewed by phone and completed an Internet or paper self‐questionnaire at 1, 4, 8, and 12 months after birth. At 12 months, 2,806 dyads among the 3,368 included (83%) were still followed up.
2.2. Ethics
The Epifane cohort protocol was approved by the Committee for Data Processing in Health Research (registration no. 11.335) and the French Data Protection Authority (CNIL, authorization no. 911 299).
2.3. Maternal prepregnancy BMI
Mothers self‐reported prepregnancy weight and height after delivery. Maternal pBMI was calculated as weight before pregnancy in kilogrammes, divided by height in square metres. pBMI was grouped into four classes following the World Health Organization (WHO, 2000) classification: underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obesity (≥30.0).
2.4. Breastfeeding duration
At the maternity ward, mothers self‐reported current feeding mode, categorized as exclusive formula feeding, mixed feeding (formula and breastfeeding), or EBF. During phone interviews at 1, 4, 8, and 12 months, mothers were asked about current infant feeding mode and infant age on days when breastfeeding (on the breast and pumped breastmilk) was stopped. Mothers also filled in infant age upon introduction of liquids other than breastmilk (water, sugary water, infusions, and fruit juice) and solid foods. This retrospective information was used to confirm the mother's statement on feeding mode at previous interviews. It also enabled identifying changes in infant feeding mode during the first year. On the basis of this information, we defined EBF duration according to WHO (2007) standards for the period when the baby received only breastmilk and no other liquid or solid. (Vitamins and medication were allowed.) Any breastfeeding (ABF) duration corresponded to the total period during which the mother breastfed. It included women who chose mixed feeding at birth, women who breastfed exclusively at birth and switched to mixed feeding after, and mothers who breastfed exclusively without a period of mixed breastfeeding before stopping breastfeeding.
2.5. Covariates
Sociodemographic characteristics were self‐reported by the mother at the maternity ward. We defined gestational weight gain (GWG) as the difference in kilogrammes between self‐reported maternal weight in the last month of pregnancy and prepregnancy weight. GWG was computed into three classes according to the 2009 Institute of Medicine (IOM) recommendations (GWG below the IOM recommendations, GWG within the IOM recommendations, and GWG above the IOM recommendations), which take into account pBMI (IOM [US] and National Research Council [US] Committee to Reexamine IOM Pregnancy Weight Guidelines, 2009). Midwives obtained maternal and neonatal information from medical records. For the present analyses, maternal outcome included type of pregnancy (multiple or single), gestational diabetes mellitus (yes or no), hypertensive complications (hypertension and/or pre‐eclampsia) during pregnancy (yes or no) and mode of delivery (vaginal or Caesarean). Neonatal outcome included prematurity (33 to <37 AW and ≥37 AW), infant birthweight (<2.5 kg, ≥2.5 to <4.0 kg, and ≥4.0 kg) and Apgar score 5 min after birth (≤7, 8–9, and ≥10).
We assumed that the negative association between obesity and breastfeeding duration might be mediated through other factors. On the basis of previous research (Hauff et al., 2014; Kronborg, Vaeth, & Rasmussen, 2013), we retained self‐reported skin‐to‐skin contact after birth (skin‐to‐skin contact immediately after birth or not); early breastfeeding within 30 min after birth (yes or no); mother's report of concern about newborn feeding (yes or no); partner's perception of the breastfeeding woman before pregnancy (self‐reported by the mother after delivery and coded as “positive,” “negative,” or “no opinion or unknown”); partner's perception of the breastfeeding woman at 1 month after birth (also self‐reported by the mother and coded as previously mentioned); and whether the mother herself had been breastfed (yes or no or unknown).
2.6. Statistical analyses
For approximately 5% of the 3,205 women who had indicated their feeding mode at birth (Figure S1), the exact date of breastfeeding cessation was missing (n = 176); we were aware only of their minimal breastfeeding period, for example, over 1 month. In this case, for these women, the date of breastfeeding cessation was imputed by the median date in days of breastfeeding cessation observed in the group of women who had breastfed for more than 1 month and who had provided their exact date of breastfeeding cessation.
Sociodemographic characteristics with a rate of missing data above 5% (maternal education, occupation, birthplace, and parity) were imputed using logistic regression models, including, when appropriate, maternal age, marital status, parity, education, occupation, and partner's birthplace and education. In addition, missing smoking status (n = 59) was imputed by the mode “no smoking before and during pregnancy.”
The inverse probability method was used to provide statistical estimates representative of the source population: Inclusion probabilities were first calculated, and marginal calibration was next performed on maternal age, marital status, education, and type of pregnancy. We used as a reference percentages observed in the French National Perinatal Survey 2010 (Blondel & Kermarrec, 2011). Random two‐stage sampling design and final weights were taken into account in all analyses using the “svyset” command (Stata® V12).
In each parity stratum, rates of infant feeding mode at birth were compared between pBMI categories using adjusted Wald tests. Kaplan–Meier curves were used to assess probabilities of EBF duration during the first year according to pBMI. The rate of women still exclusively breastfeeding at 1, 3, and 5 months after birth was estimated using Kaplan–Meier functions.
Because the assumption of hazard proportionality was not met, we used Poisson regression models to estimate the association of pBMI with the number of ABF days and the number of EBF days during the first year of life. Incidence rate ratios (IRRs) were estimated with their 95% confidence interval. IRR was interpreted as the number by which the number of EBF or ABF days was multiplied in a pBMI group compared to a normal‐weight group, used as a reference (Falissard, 2005).
Covariates associated with a p‐value <.20 for ABF or EBF duration in univariate analyses were included in multivariate models. To take into account the hierarchical structure of covariates, adjusted models were progressively built (Figure S2). The first model (called Model 1) was adjusted for sociodemographic characteristics, selected using a back stepwise procedure, and associated with the number of days of ABF or EBF with a p‐value <.05. However, we retained a covariate if its removal led to a change in the IRRs >10%. Then, maternal and neonatal outcomes were added one by one to Model 1 using a bottom‐up procedure and were retained when they were associated with the number of days of EBF or ABF with a p‐value <.05, or if adding them changed the IRRs by more than 10% (Model 2). Next, complementary factors (skin‐to‐skin contact, early breastfeeding, and psychosocial factors) were added one by one to Model 2, also using a bottom‐up procedure, and with the same criteria for retaining a covariate (Model 3).
Interactions between parity and pBMI with the number of ABF or EBF days were then investigated. Interaction in the model addressing EBF duration was p < .10 (p = .07); therefore, unadjusted and adjusted Poisson regression models were stratified on parity. ABF duration was analysed for both primiparous and multiparous women together.
Lastly, sensitivity analyses were performed in a sample of women with available information concerning their parity status.
3. RESULTS
3.1. Sample description
From the 3,368 dyads included at birth, 2,316 mothers who initiated breastfeeding were included in the study of the association between pBMI and ABF duration. Analyses of EBF duration were performed on a sample of 1,868 mothers ( Figure S1 ).
Sociodemographic characteristics, maternal and neonatal outcomes, and complementary factors by pBMI within parity classes are presented in Table 1. Median durations of ABF and EBF according to sociodemographic characteristics, maternal and neonatal outcomes, and complementary factors are presented in Table S1 .
Table 1.
Factors associated with pBMI, within parity groups, among women who initiated exclusive breastfeeding (n = 1,868)
| Primiparous (n = 804) | Multiparous (n = 1,064) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pBMI category | pBMI category | |||||||||
| <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30.0 | p a | <18.5 | 18.5‐24.9 | 25.0–29.9 | ≥30.0 | p a | |
| N = 53 | N = 562 | N = 128 | N = 61 | N = 71 | N = 707 | N = 206 | N = 80 | |||
| pBMI (kg/m2) | ||||||||||
| Medianb (25th–75th) | 18.0 (17.3–18.3) | 21.5 (20.1–22.9) | 26.6 (25.6–27.9) | 32.8 (30.6–34.9) | <10−3 | 17.6 (17.1–18.1) | 21.5 (20.3–23.1) | 26.7 (26.0–28.2) | 33.6 (31.3–35.9) | <10−3 |
| Sociodemographic characteristics | ||||||||||
| Maternal age (years) | ||||||||||
| 18–24 | 30.9 | 20.5 | 22.8 | 26.4 | .05 | 15.0 | 7.5 | 6.7 | 13.6 | .01 |
| 25–29 | 47.4 | 42.6 | 37.4 | 53.0 | 31.8 | 23.7 | 32.9 | 24.0 | ||
| 30–34 | 13.5 | 26.7 | 32.0 | 10.0 | 36.7 | 40.9 | 36.3 | 25.2 | ||
| ≥35 | 8.1 | 10.2 | 7.8 | 10.6 | 16.5 | 27.9 | 24.1 | 37.2 | ||
| Maternal birthplace | ||||||||||
| France | 74.8 | 83.5 | 84.6 | 88.1 | .56 | 78.4 | 80.0 | 77.2 | 67.6 | <10−3 |
| Africa | 8.8 | 6.9 | 8.2 | 9.3 | 10.1 | 12.1 | 18.4 | 8.3 | ||
| Europe, Asia, America, and Oceania | 16.4 | 9.6 | 7.2 | 2.6 | 11.5 | 7.9 | 4.4 | 24.1 | ||
| Marital status | ||||||||||
| Married | 44.4 | 39.1 | 38.8 | 35.4 | .83 | 48.9 | 60.1 | 63.0 | 65.1 | .20 |
| Not married | 55.6 | 60.9 | 61.2 | 64.6 | 51.1 | 39.9 | 37.0 | 34.9 | ||
| Maternal education | ||||||||||
| Primary school | 3.5 | 0.7 | 2.6 | 0.0 | .01 | 9.9 | 0.4 | 5.4 | 6.4 | <10−3 |
| Junior high school | 15.7 | 14.0 | 17.2 | 26.0 | 15.4 | 16.0 | 18.3 | 23.7 | ||
| High school | 17.3 | 19.2 | 26.5 | 31.3 | 16.7 | 21.8 | 23.9 | 29.5 | ||
| University | 63.4 | 66.1 | 53.7 | 42.7 | 58.0 | 61.8 | 52.3 | 40.5 | ||
| Maternal occupation | ||||||||||
| Farmer, craftswoman, and merchant | 3.1 | 3.1 | 1.2 | 1.3 | .05 | 2.1 | 3.2 | 2.2 | 4.5 | .05 |
| Management | 26.0 | 25.3 | 13.4 | 13.8 | 20.4 | 25.3 | 16.7 | 9.5 | ||
| Intermediate profession | 56.7 | 57.1 | 68.4 | 60.0 | 62.3 | 54.5 | 62.6 | 61.3 | ||
| Manual worker | 1.2 | 7.2 | 6.6 | 7.8 | 6.5 | 9.8 | 8.1 | 9.3 | ||
| Unemployed | 13.1 | 7.3 | 10.4 | 17.1 | 8.7 | 7.2 | 10.5 | 15.4 | ||
| Time of return to work | ||||||||||
| <4 months | 42.1 | 46.6 | 36.6 | 37.9 | .05 | 19.8 | 24.9 | 23.8 | 23.5 | .02 |
| 4–6 months | 14.0 | 13.3 | 7.9 | 21.4 | 22.6 | 14.1 | 9.4 | 8.1 | ||
| >6–12 months | 19.6 | 21.0 | 31.6 | 14.1 | 27.2 | 27.5 | 22.1 | 18.9 | ||
| Did not go back at 12 months | 24.3 | 19.1 | 23.9 | 26.6 | 30.4 | 33.5 | 44.7 | 49.5 | ||
| Health behaviour | ||||||||||
| Antenatal classes | ||||||||||
| Attended | 74.8 | 84.2 | 82.6 | 78.4 | .36 | 43.0 | 44.1 | 37.2 | 27.1 | .02 |
| Did not attend | 25.2 | 15.8 | 17.4 | 21.6 | 57.0 | 55.9 | 62.8 | 72.9 | ||
| Smoked before or during pregnancy | ||||||||||
| Did not smoke before or during | 60.6 | 66.2 | 66.9 | 71.2 | .94 | 69.2 | 76.0 | 80.5 | 75.8 | .41 |
| Smoked before, but not during | 23.4 | 19.8 | 20.9 | 17.2 | 10.9 | 12.4 | 8.9 | 12.1 | ||
| Smoked before and during | 16.0 | 14.0 | 12.2 | 11.6 | 19.9 | 11.6 | 10.6 | 12.1 | ||
| Alcohol in pregnancy | ||||||||||
| No consumption | 92.9 | 93.6 | 95.5 | 98.3 | .44 | 95.0 | 93.6 | 94.9 | 90.6 | .58 |
| Consumption | 7.1 | 6.4 | 4.5 | 1.7 | 5.0 | 6.4 | 5.1 | 9.4 | ||
| Maternal and fetal outcomes | ||||||||||
| Gestational weight gain (kg) | ||||||||||
| Medianb (25th–75th) | 15.0 (12.0–17.0) | 14.0 (11.0–17.0) | 14.0 (10.0–18.0) | 9.0 (4.0–14.0) | <10−3 | 14.0 (11.0–16.8) | 13.0 (10.7–16.0) | 12.0 (8.0–15.0) | 9.8 (6.0–12.0) | <10−3 |
| Within IOM | 59.4 | 37.5 | 21.1 | 26.5 | <10−3 | 49.7 | 38.6 | 33.4 | 29.1 | <10−3 |
| Below IOM | 30.4 | 29.6 | 9.6 | 29.5 | 33.1 | 33.5 | 13.4 | 20.4 | ||
| Above IOM | 10.2 | 32.9 | 69.3 | 44.0 | 17.2 | 27.9 | 53.2 | 50.5 | ||
| Type of pregnancy | ||||||||||
| Multiple | 0.0 | 0.2 | 0.0 | 0.0 | .94 | 1.6 | 0.5 | 1.0 | 1.1 | .75 |
| Single | 100.0 | 99.8 | 100.0 | 100.0 | 98.4 | 99.5 | 99.0 | 98.9 | ||
| GDM | ||||||||||
| No | 98.2 | 92.5 | 91.9 | 87.5 | .20 | 98.8 | 94.8 | 88.0 | 79.3 | <10−3 |
| Yes | 1.8 | 7.5 | 8.1 | 12.5 | 1.2 | 5.2 | 12.0 | 20.7 | ||
| Hypertensive complications | ||||||||||
| No | 97.7 | 97.0 | 93.3 | 84.8 | <10−3 | 100.0 | 99.0 | 98.3 | 93.4 | <10−3 |
| Yes | 2.3 | 3.0 | 6.7 | 15.2 | 0.0 | 1.0 | 1.7 | 6.6 | ||
| Delivery mode | ||||||||||
| Vaginal | 90.0 | 81.5 | 75.4 | 75.2 | .08 | 93.8 | 86.9 | 85.3 | 68.7 | <10−3 |
| Caesarean | 10.0 | 18.5 | 24.6 | 24.8 | 6.2 | 13.1 | 14.7 | 31.3 | ||
| Gestation duration | ||||||||||
| ≥37 weeks | 98.1 | 97.6 | 98.0 | 97.4 | .98 | 92.4 | 97.8 | 96.6 | 99.0 | .05 |
| 33–36 weeks | 1.9 | 2.4 | 2.0 | 2.6 | 7.6 | 2.2 | 3.4 | 1.0 | ||
| Infant's sex | ||||||||||
| Male | 56.6 | 50.3 | 47.2 | 54.2 | .68 | 43.8 | 49.3 | 51.5 | 53.0 | .71 |
| Female | 43.4 | 49.7 | 52.8 | 45.8 | 56.2 | 50.7 | 48.5 | 47.0 | ||
| Infant's birthweight (kg) | ||||||||||
| <2.5 | 7.7 | 2.6 | 1.3 | 4.4 | 5.4 | 1.6 | 1.2 | 2.3 | .12 | |
| 2.5–4 | 89.2 | 92.8 | 90.6 | 92.1 | .21 | 90.2 | 88.6 | 89.2 | 81.8 | |
| ≥4 | 3.1 | 4.6 | 8.1 | 3.5 | 4.4 | 9.8 | 9.6 | 15.9 | ||
| Apgar score at 5 min | ||||||||||
| 10 | 95.2 | 91.9 | 91.1 | 94.7 | .90 | 95.5 | 97.6 | 96.6 | 95.4 | .56 |
| 8–9 | 4.8 | 7.6 | 8.9 | 5.3 | 3.3 | 2.2 | 3.1 | 4.6 | ||
| ≤7 | 0.0 | 0.5 | 0.0 | 0.0 | 1.2 | 0.2 | 0.3 | 0.0 | ||
| Complementary factors | ||||||||||
| STS contact after birth | ||||||||||
| STS directly after birth | 89.3 | 85.0 | 76.0 | 72.9 | .01 | 91.5 | 87.2 | 89.7 | 71.7 | <10−3 |
| No direct STS | 10.7 | 15.0 | 24.0 | 27.1 | 8.5 | 12.8 | 10.3 | 28.3 | ||
| Mother had been breastfed | ||||||||||
| Yes | 46.0 | 50.3 | 52.7 | 45.3 | .27 | 41.2 | 47.0 | 37.1 | 40.4 | .01 |
| No | 30.1 | 31.5 | 35.8 | 27.3 | 19.7 | 31.7 | 34.6 | 37.1 | ||
| Not known | 23.9 | 18.2 | 11.5 | 27.4 | 39.1 | 21.3 | 28.3 | 22.5 | ||
| Mother had concerns about baby's feeding | ||||||||||
| Yes | 41.1 | 51.9 | 48.8 | 59.1 | .29 | 32.0 | 26.4 | 25.6 | 43.5 | .02 |
| No | 58.9 | 48.1 | 51.2 | 40.9 | 68.0 | 73.6 | 74.4 | 56.5 | ||
| Partner's perception of breastfeeding woman before delivery | ||||||||||
| Positive | 85.8 | 87.0 | 86.8 | 84.5 | .93 | 81.5 | 90.4 | 92.8 | 90.7 | .19 |
| Negative | 1.6 | 0.8 | 1.5 | 0.0 | 0.0 | 0.5 | 0.4 | 0.0 | ||
| No opinion | 12.6 | 12.2 | 11.7 | 15.5 | 18.5 | 9.1 | 6.8 | 9.3 | ||
| Partner's perception of breastfeeding woman at 1 month | ||||||||||
| Positive | 76.9 | 77.8 | 80.2 | 79.6 | .55 | 62.2 | 76.2 | 72.4 | 70.4 | .25 |
| Negative | 1.7 | 0.1 | 0.6 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | ||
| No opinion | 21.4 | 22.1 | 19.2 | 20.4 | 37.8 | 23.4 | 27.6 | 29.6 | ||
| Early breastfeeding | ||||||||||
| Yes | 66.7 | 62.7 | 58.4 | 58.8 | .70 | 72.5 | 76.0 | 76.0 | 62.2 | .08 |
| No | 33.3 | 37.3 | 41.6 | 41.2 | 27.5 | 24.0 | 24.0 | 37.8 | ||
Note. pBMI = prepregnancy body mass index; IOM = Institute of Medicine; GDM = gestational diabetes mellitus; STS = skin to skin.
Significance was determined using adjusted Wald tests.
For these variables, we used Kruskal–Wallis test to determine significance.
3.2. Infant feeding at birth according to pBMI class
Among primiparas, obese women were more likely to initiate mixed feeding and less likely to exclusively breastfeed compared to normal‐weight or overweight women; they were less likely to exclusively use formula than underweight women (Table 2). Multiparous obese women were more likely to use mixed feeding and less likely to exclusively breastfeed compared to all other multiparous women. Like thin mothers, they were more likely to use only formula than normal‐weight or overweight women.
Table 2.
Mode of infant feeding at birth according to prepregnancy body mass index (BMI) class, among primiparous and multiparous women (n = 3,205)
| Maternal prepregnancy BMI class | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Underweight < 8.5 kg/m2 | Normal weight 18.5–24.9 kg/m2 | Overweight 25.0–29.9 kg/m2 | Obese ≥30.0 kg/m2 | ||||||
| % | n | % | n | % | n | % | n | p | |
| Primiparous | |||||||||
| Exclusive formula feeding | 33.7 | 35 | 24.6 | 233 | 23.0 | 49 | 25.7 | 32 | 0.03 |
| Formula + breastfeeding | 17.0 | 18 | 13.6 | 122 | 19.2 | 39 | 23.2 | 26 | |
| Exclusive breastfeeding | 49.3 | 53 | 61.8 | 562 | 57.8 | 128 | 51.1 | 61 | |
| Multiparous | |||||||||
| Exclusive formula feeding | 30.2 | 43 | 25.7 | 311 | 27.3 | 108 | 31.9 | 78 | <10−3 |
| Formula + breastfeeding | 14.5 | 19 | 11.4 | 130 | 16.2 | 53 | 24.8 | 41 | |
| Exclusive breastfeeding | 55.3 | 71 | 62.9 | 707 | 56.5 | 206 | 43.3 | 80 | |
3.3. Association between pBMI class and ABF duration
The median duration of ABF of overweight women and obese women was equal to 92 days, 2 weeks shorter than that of normal‐weight women (105 days) and 3 weeks shorter than underweight women (112 days) (Figure S3). However, the overall differences were not statistically significant (p = .68).
In all models, underweight and overweight women breastfed for a period similar to that of normal‐weight women (Table 3). In the unadjusted model, the number of ABF days in obese women was not significantly different from that in normal‐weight women. However, after adjusting for sociodemographic factors, the number of ABF days in obese women decreased by about 16% compared to normal‐weight women. This association remained statistically significant (p < .05) after additional adjustment for GWG and type of pregnancy; the other maternal and neonatal outcomes were not significantly associated with ABF duration in the bottom‐up procedure. Final adjustment in Model 3 did not change IRRs for obese mothers compared to Model 2, and the association remained significant.
Table 3.
Unadjusted and adjusted IRRs (95% CI) of Poisson regression, estimating the association of the number of any breastfeeding days with prepregnancy BMI classes
| Maternal prepregnancy BMI classes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Underweight <18.5 kg/m2 | Normal weight 18.5–24.9 kg/m2 | Overweight 25.0–29.9 kg/m2 | Obese ≥30.0 kg/m2 | |||||
| IRR [95% CI] | n | IRR [95% CI] | n | IRR [95% CI] | n | IRR [95% CI] | n | |
| Unadjusted model | 0.97 [0.81, 1.15] | 161 | 1 | 1,521 | 1.01 [0.90, 1.13] | 426 | 0.90 [0.76, 1.08] | 208 |
| Model 1: model adjusted for sociodemographic factorsa | 1.02 [0.87, 1.19] | 159 | 1 | 1,500 | 0.98 [0.89, 1.09] | 416 | 0.84* [0.72, 0.97] | 204 |
| Model 2: Model 1 + maternal outcomesb | 1.01 [0.86, 1.18] | 159 | 1 | 1,500 | 1.00 [0.90, 1.11] | 416 | 0.85* [0.73, 0.98] | 204 |
| Model 3: Model 2 + complementary factorsc | 1.06 [0.91, 1.23] | 156 | 1 | 1,462 | 1.01 [0.91, 1.12] | 398 | 0.86* [0.74, 0.99] | 195 |
Note. IRR = incidence rate ratio; CI = confidence interval; BMI = body mass index; IOM = Institute of Medicine.
Maternal age, birthplace, education level, occupation, timing of return to work, and smoking before and during pregnancy.
Gestational weight gain according to IOM recommendations and type of pregnancy.
Concerns about infant feeding, mother having been breastfed herself, partner's perception of breastfeeding woman before pregnancy, and partner's perception of breastfeeding woman 1 month after birth.
p<0.05.
3.4. EBF duration according to pBMI class
Compared to median duration of EBF of underweight and normal‐weight women (30 days), median duration of EBF was 2 weeks shorter for obese women (17 days) and 1 week shorter for overweight women (24 days). These differences were statistically significant (p = .02). However, an effect modification of parity in the relationship of pBMI with EBF duration was identified. Analyses were then carried out in primiparous and multiparous women separately.
Among primiparous women (Figure 1a), rates of EBF were lower in overweight women than in other pBMI groups at 1 month. However, 3 months after birth, rates of EBF were similar between underweight, overweight, and obese women and were lower than observed in the group of normal‐weight women. Then, rates of EBF were all below 3% at 5 months. Among multiparas (Figure 1b), rates of EBF were lower in obese women compared with other pBMI groups at 1, 3, and 5 months.
Figure 1.
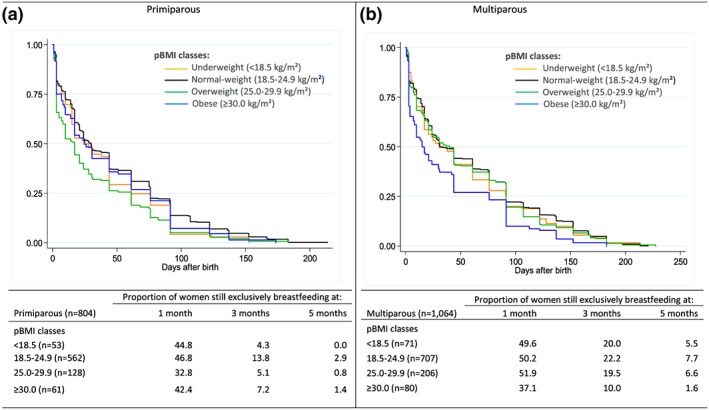
Unadjusted Kaplan–Meier curves estimating the proportion of women continuing exclusive breastfeeding during the first year of life, according to maternal prepregnancy body mass index (pBMI), among (a) primipara (n = 804) and (b) multipara (n = 1,064) who initiated exclusive breastfeeding
3.5. Association between pBMI class and EBF duration
Among primiparas (Table 4), the number of EBF days in overweight women decreased by about 30% compared to normal‐weight women after controlling for sociodemographic factors (Model 1) and by about 25% in the other two models.
Table 4.
Unadjusted and adjusted IRRs (Poisson regression models) measuring the association of the number of exclusive breastfeeding days with prepregnancy BMI classes, stratified on parity groups
| Unadjusted model | Model 1: adjusted for sociodemographic factorsa | Model 2: Model 1 + maternal outcomesb | Model 3: Model 2 + complementary factorsc | |||||
|---|---|---|---|---|---|---|---|---|
| IRR [95% CI] | n | IRR [95% CI] | n | IRR [95% CI] | n | IRR [95% CI] | n | |
| Primiparous | ||||||||
| Underweight (<18.5 kg/m2) | 0.82 [0.60, 1.12] | 53 | 0.86 [0.62, 1.19] | 51 | 0.83 [0.60, 1.14] | 51 | 0.86 [0.62, 1.17] | 50 |
| Normal weight (18.5–24.9 kg/m2) | 1 | 562 | 1 | 549 | 1 | 549 | 1 | 536 |
| Overweight (25.0–29.9 kg/m2) | 0.70* [0.56, 0.88] | 128 | 0.69* [0.54, 0.87] | 123 | 0.74* [0.58, 0.94] | 123 | 0.74* [0.58, 0.95] | 120 |
| Obese (≥30.0 kg/m2) | 0.87 [0.64, 1.19] | 61 | 0.94 [0.70, 1.26] | 60 | 0.96 [0.71, 1.30] | 60 | 0.96 [0.72, 1.30] | 58 |
| Multiparous | ||||||||
| Underweight (<18.5 kg/m2) | 0.89 [0.68, 1.17] | 71 | 0.98 [0.76, 1.25] | 71 | 0.95 [0.74, 1.22] | 71 | 1.04 [0.82, 1.32] | 69 |
| Normal weight (18.5–24.9 kg/m2) | 1 | 707 | 1 | 687 | 1 | 687 | 1 | 669 |
| Overweight (25.0–29.9 kg/m2) | 0.95 [0.80, 1.12] | 206 | 0.98 [0.83, 1.15] | 199 | 1.03 [0.87, 1.22] | 199 | 1.05 [0.88, 1.24] | 190 |
| Obese (≥30.0 kg/m2) | 0.67* [0.50, 0.89] | 80 | 0.71* [0.53, 0.95] | 77 | 0.75 [0.56, 1.00] | 77 | 0.80 [0.60, 1.06] | 74 |
Note. IRR = incidence rate ratio; BMI = body mass index; CI = confidence interval; IOM = Institute of Medicine.
Maternal age, educational level, timing of return to work, and smoking before and during pregnancy.
Gestational weight gain according to IOM recommendations and delivery mode.
Concerns about infant feeding, mother having been breastfed herself, and partner's perception of breastfeeding woman at 1 month after birth and early breastfeeding after birth.
p<0.05.
Among multiparas (Table 4), the number of EBF days for obese women decreased by 30% compared to normal‐weight women in the nonadjusted model and after adjusting for sociodemographic covariates (p < .05). After controlling for GWG according to IOM recommendations and mode of delivery, the association between obesity and number of EBF days was no longer statistically significant (p = .05). Adding complementary factors additionally reduced the statistical difference between obese and normal‐weight women (p = .12).
3.6. Sensitivity analyses
Sensitivity analyses were performed in the sample of women with complete information about parity. Concerning the association between pBMI and ABF duration, the same results as in analyses with imputed values of parity were found (data not shown). Concerning EBF duration, we observed the same findings as in analyses with imputed values; except that, among multiparous women, obesity was still significantly associated with shorter EBF duration in the model adjusted for sociodemographic factors and pregnancy outcomes (IRR = 0.73 [0.55–0.98], p = .03). After adding complementary factors to the model, the association between obesity before pregnancy and number of EBF days was no longer statistically significant (IRR = 0.79 [0.59–1.04], p = .09).
4. DISCUSSION
Findings from the Epifane study showed that, in France, obese women were less likely to exclusively breastfeed at birth and continued breastfeeding for a shorter duration compared to normal‐weight women. We also documented that parity may have a modification effect in the association of pBMI with duration of EBF. Among primiparas, overweight but not obesity before pregnancy was associated with shorter EBF duration independently of all covariates. In contrast, among multiparous women, obesity was associated with shorter EBF duration, after controlling for sociodemographic factors. However, this association was no longer statistically significant after taking into account maternal outcome and complementary factors.
In our study, obese women were more likely to initiate mixed feeding and less likely to exclusively breastfeed at birth. Mixed feeding could be purposely chosen by the parents, but it could also reflect difficulties in successfully initiating EBF. Studies concerning intention to breastfeed according to pBMI show mixed findings. Indeed, some authors observed that obese women were less likely to intend to breastfeed than normal‐weight women (Guelinckx et al., 2012; Visram et al., 2013). In other studies, no association was found between pBMI and breastfeeding intention (Hauff & Demerath, 2012; Hauff et al., 2014). Furthermore, when they intended to breastfeed, obese women were less likely than normal‐weight women to exclusively breastfeed at maternity discharge (Perrine, Scanlon, Li, Odom, & Grummer‐Strawn, 2012) or at 1 week postpartum (Donath & Amir, 2008). This may be explained by a lower prolactin response to suckling (Rasmussen & Kjolhede, 2004) and delayed onset of lactogenesis (after 72 hr postpartum; Rasmussen, 2007) among obese mothers, which is associated with greater risk of stopping breastfeeding in the early postpartum period.
Median duration of breastfeeding was 3 months for obese and overweight women in our study. In a retrospective study at a university hospital in Belgium between 2006 and 2007 (Guelinckx et al., 2012), obese mothers breastfed for a median of less than 2 months and overweight mothers for 3 months. Such durations are substantially lower than those observed in the U.S. (Hauff et al., 2014), Australian (Donath & Amir, 2008), Norwegian (Winkvist et al., 2015), and Danish (Baker et al., 2007) studies. These wide differences between countries in breastfeeding duration in obese women may reflect differences in the social environment, especially social norms concerning EBF and public financial support (e.g., duration of paid maternity leave).
In line with numerous studies (Baker et al., 2007; Castillo et al., 2016; Donath & Amir, 2008; Guelinckx et al., 2012; Hauff et al., 2014; Li, Jewell, & Grummer‐Strawn, 2003; Winkvist et al., 2015), we observed that obesity before pregnancy was associated with shorter ABF duration compared to normal‐weight women. In contrast, in two studies performed during the 2000s in the United States (Bartok et al., 2012; O'Sullivan et al., 2015), obesity was no longer associated with ABF duration after controlling for sociodemographic and perinatal characteristics and breastfeeding intention. In our study, the negative association between maternal obesity and ABF duration was independent of sociodemographic factors, pregnancy outcomes, and psychosocial factors, suggesting the involvement of other factors. We did not collect information on breastfeeding intention, because women were included after delivery. Qualitative research is needed to identify specific problems encountered by obese women at different periods of postpartum. The wide differences observed in breastfeeding duration among obese women between France and Nordic and Anglo‐Saxon countries suggest that extensive efforts to promote breastfeeding in France could positively influence ABF duration in the general population, as well as among obese women. Thus, we assumed that supportive breastfeeding policies might help obese women to partially overcome some breastfeeding difficulties. Such a conclusion may also be relevant for EBF.
In the Epifane cohort, obese and overweight women exclusively breastfed only during an average of 17 and 24 days, respectively. As was the case for ABF, median durations of EBF among overweight and obese women were close to those observed in Belgium (Guelinckx et al., 2012) but markedly lower than those observed in the United States (Hauff et al., 2014), Brazil (Castillo et al., 2016), Norway (Winkvist et al., 2015), or Denmark (Baker et al., 2007). However, all of those studies (except for that performed in the US; Hauff et al., 2014) used a less restrictive definition of EBF that may also have contributed to the observed difference.
In line with other authors (Kronborg et al., 2013; O'Sullivan et al., 2015), we found an interaction between parity and pBMI in the number of EBF days. Among primiparas, overweight before pregnancy was independently associated with EBF duration even after taking into account a set of confounding and complementary factors. This suggests that overweight among primiparas could negatively impact the likelihood of exclusively breastfeeding via a mechanism unrelated to sociodemographic factors, pregnancy outcome, or psychosocial factors. Surprisingly, however, obesity was not associated with EBF duration in any models among primiparous women. The absence of a significant association between obesity and EBF duration among primiparas could be due to a lack of statistical power. However, we also assumed that obese primiparous women, considered as a group at risk, might have received special attention, with intensive follow‐up at antenatal care, especially considering GWG and glucose tolerance impairment. Only women who initiated EBF at the maternity ward were included in analyses concerning EBF. We thus presume that, when obese primiparous women could overcome breastfeeding difficulties in the first hours or days after birth and then start exclusively breastfeeding in the maternity ward, they will continue to exclusively breastfed as long as normal‐weight women do. Identifying characteristics and strategies used by these obese primiparous women to overcome early difficulties is a promising avenue of research.
Among multiparous women, the association between maternal obesity and shorter EBF duration was no longer statistically significant after controlling for mode of delivery and GWG, but the result was at the boundary of the significance level. It is now well established that obesity before pregnancy is associated with increased risk of dysfunctional labour and Caesarean birth (Poston et al., 2016). The negative association between Caesarean delivery and breastfeeding may be due to the elevated leptine levels observed among obese mothers (Moynihan, Hehir, Glavey, Smith, & Morrison, 2006). Leptine levels may be associated with dysfunctional labour (Moynihan et al., 2006) and also, potentially, with lactation (Bever Babendure et al., 2015). In addition, in relation to postoperative care, Caesarean section might delay timing of the first feeding, which is associated with successful breastfeeding (Moore et al., 2016). In a recent meta‐analysis, however, no association between mode of delivery and breastfeeding at 6 months was found (Prior et al., 2012).
In our analyses, we found that the association between obesity and EBF duration may be partially mediated by complementary factors, but only among multiparous women. Breastfeeding at birth has been observed to be an intermediate factor in the relationship between prepregnancy obesity and breastfeeding duration. Her partner's perception of breastfeeding, along with having been breastfed herself, may reflect two dimensions: support of the home environment towards breastfeeding (which we assume is important in overcoming breastfeeding difficulties) and the image of the breastfeeding mother in a private or public environment. Hauff et al. (2014) pointed out that self‐confidence and social influence concerning breastfeeding were associated with both pBMI and breastfeeding duration but only partially explained the negative association of obesity with breastfeeding duration. Further research is needed to clarify why such factors did not impact the association between body weight status and breastfeeding duration among primiparous women.
Limits and strengths of our study need to be pointed out. Because the interaction test between parity and pBMI in the association with EBF duration was significant, we stratified our models on parity. Because stratified analyses lead to decreased statistical power, our results should be interpreted with caution. However, our findings are supported by biological explanations. We imputed parity in order to limit lack of statistical power and bias selection due to nonrandom missing values. Sensitivity analyses without imputation values for parity showed the same results, except in the association between maternal obesity and EBF among multiparous women after adjustment for maternal outcomes. This reinforces our assumption that women with missing values for parity had specific characteristics. Furthermore, maternal height and weight were self‐reported. Previous studies reported that women with high pBMI were more likely to underreport their prepregnancy weight (Holland, Moore Simas, Doyle Curiale, Liao, & Waring, 2013; Yu & Nagey, 1992). This may have led to misclassification of obese women into the overweight BMI group and of overweight women into the normal‐weight BMI group. Unfortunately, to our knowledge, the prevalence of prepregnancy obesity based on anthropometric measures in a French nationally representative survey is not available. We were thus unable to assess the level of misclassification and the magnitude of error, but we presume that these misclassifications may have potentially biased our results towards the null. As a major strength, the design of the Epifane cohort led us to provide precise definitions of duration of EBF and ABF in accordance with the WHO (2007) classification. At each interview, to limit recall bias, interviewers helped mothers to remember infant feeding declared at the previous encounter. Data collected in this nationally representative cohort provided a comprehensive set of confounding and complementary factors. Nonetheless, a potential residual confounding bias cannot be ruled out. For example, we were unable to take into account the perception of a previous positive breastfeeding experience, which was shown to be associated with the decision to breastfeed subsequent children among multiparous women (Schafer, Campo, Colaizy, Mulder, & Ashida, 2017). Breastfeeding is a complex form of behaviour involving a mother and child dyad; it is influenced by many different factors that are difficult to entirely capture in epidemiologic studies. Further research is needed to evaluate the impact of different types of mechanisms (biological, sociocultural, medical, and psychosocial) affecting breastfeeding duration and exclusivity at different times during the postpartum period.
To our knowledge, this is the first study performed in France in a nationally representative birth cohort aimed at estimating the association of obesity before pregnancy with breastfeeding duration and taking into account a large set of confounding and intermediate factors. Regarding practical implications of our findings, health care providers should be aware that obese women, but also primiparous overweight women, may benefit from extra support during pregnancy, after birth, and during the first few months of life so as to initiate and continue breastfeeding. The familial environment should also be considered, and partners and family should be involved in implementing efforts to promote breastfeeding. Several studies documented the effectiveness of supportive interventions in breastfeeding among obese and overweight women (Carlsen et al., 2013; Chapman et al., 2013; Rasmussen, Dieterich, Zelek, Altabet, & Kjolhede, 2011). Nevertheless, such actions should respect the woman's choice to breastfeed or not and should help her to attain her own breastfeeding goals.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
KC and BS designed the research. KC, BS, and CDL conducted the research. JBB analysed the data and wrote the initial manuscript. JBB had primary responsibility for the final content. All authors read and approved the final manuscript.
Supporting information
Figure S1: Flow chart for selection of dyads included in this study, from the Epifane cohort
Figure S2: Conceptual framework of the relationship between pre‐pregnancy BMI (pBMI) and any breastfeeding (ABF) and exclusive breastfeeding (EBF) durations.
Table S1: Any breastfeeding (ABF) and exclusive breastfeeding (EBF) durations according to the sociodemographic factors, pregnancy conditions, and behavioral and psychosocial factors
Figure S3: Unadjusted Kaplan Meir curves estimating the proportion of women continuing breastfeeding during the first year of life, according to maternal pre‐pregnancy BMI, among women who initiated breastfeeding (n = 2,316)
ACKNOWLEDGMENTS
The authors are grateful to the midwives who contributed to data collection in the maternity wards and to parents who participated in the survey. They are also grateful to Catherine de Launay and Caroline Guerrisi, who contributed to data monitoring and descriptive analyses, and to Jerri Bram, who edited the manuscript.
Boudet‐Berquier J, Salanave B, Desenclos J‐C, Castetbon K. Association between maternal prepregnancy obesity and breastfeeding duration: Data from a nationwide prospective birth cohort. Matern Child Nutr. 2018;14:e12507 10.1111/mcn.12507
REFERENCES
- Aune, D. , Norat, T. , Romundstad, P. , & Vatten, L. J. (2014). Breastfeeding and the maternal risk of type 2 diabetes: A systematic review and dose–response meta‐analysis of cohort studies. Nutrition, Metabolism, and Cardiovascular Diseases, 24(2), 107–115. [DOI] [PubMed] [Google Scholar]
- Baker, J. L. , Michaelsen, K. F. , Sørensen, T. I. A. , & Rasmussen, K. M. (2007). High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. The American Journal of Clinical Nutrition, 86(2), 404–411. [DOI] [PubMed] [Google Scholar]
- Bartok, C. J. , Schaefer, E. W. , Beiler, J. S. , & Paul, I. M. (2012). Role of body mass index and gestational weight gain in breastfeeding outcomes. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine, 7(6), 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever Babendure, J. , Reifsnider, E. , Mendias, E. , Moramarco, M. W. , & Davila, Y. R. (2015). Reduced breastfeeding rates among obese mothers: A review of contributing factors, clinical considerations and future directions. International Breastfeeding Journal, 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel, B. , & Kermarrec, M. (2011). French National Perinatal Survey. [Births in 2010 and their trends since 2003]. Paris: Inserm; Retrieved from http://www.sante.gouv.fr/IMG/pdf/Les_naissances_en_2010_et_leur_evolution_depuis_2003.pdf [Google Scholar]
- Boudet‐Berquier, J. , Salanave, B. , De Launay, C. , & Castetbon, K. (2016). Introduction of complementary foods with respect to French guidelines: Description and associated socio‐economic factors in a nation‐wide birth cohort (Epifane survey). Maternal & Child Nutrition. (Epub ahead of print); 10.1111/mcn.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen, E. M. , Kyhnaeb, A. , Renault, K. M. , Cortes, D. , Michaelsen, K. F. , & Pryds, O. (2013). Telephone‐based support prolongs breastfeeding duration in obese women: A randomized trial. The American Journal of Clinical Nutrition, 98(5), 1226–1232. [DOI] [PubMed] [Google Scholar]
- Castillo, H. , Santos, I. S. , & Matijasevich, A. (2016). Maternal pre‐pregnancy BMI, gestational weight gain and breastfeeding. European Journal of Clinical Nutrition, 70(4), 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, D. J. , Morel, K. , Bermúdez‐Millán, A. , Young, S. , Damio, G. , & Pérez‐Escamilla, R. (2013). Breastfeeding education and support trial for overweight and obese women: A randomized trial. Pediatrics, 131(1), e162–e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , Nommsen‐Rivers, L. A. , Heinig, M. J. , & Cohen, R. J. (2003). Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics, 112(3 Pt 1), 607–619. [DOI] [PubMed] [Google Scholar]
- Donath, S. M. , & Amir, L. H. (2008). Maternal obesity and initiation and duration of breastfeeding: Data from the longitudinal study of Australian children. Maternal & Child Nutrition, 4(3), 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falissard, B. (2005). La régression de Poisson In Comprendre et utiliser les statistiques dans les sciences de la vie (3rd ed). (pp. 175–176). Paris, France: Masson. [Google Scholar]
- Godfrey, K. M. , Reynolds, R. M. , Prescott, S. L. , Nyirenda, M. , Jaddoe, V. W. V. , Eriksson, J. G. , … Broekman, B. F. P. (2017). Influence of maternal obesity on the long‐term health of offspring. The Lancet Diabetes & Endocrinology, 5(1), 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelinckx, I. , Devlieger, R. , Bogaerts, A. , Pauwels, S. , & Vansant, G. (2012). The effect of pre‐pregnancy BMI on intention, initiation and duration of breast‐feeding. Public Health Nutrition, 15(5), 840–848. [DOI] [PubMed] [Google Scholar]
- Hauff, L. E. , & Demerath, E. W. (2012). Body image concerns and reduced breastfeeding duration in primiparous overweight and obese women. American Journal of Human Biology: The Official Journal of the Human Biology Council, 24(3), 339–349. [DOI] [PubMed] [Google Scholar]
- Hauff, L. E. , Leonard, S. A. , & Rasmussen, K. M. (2014). Associations of maternal obesity and psychosocial factors with breastfeeding intention, initiation, and duration. The American Journal of Clinical Nutrition, 99(3), 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, E. , Moore Simas, T. A. , Doyle Curiale, D. K. , Liao, X. , & Waring, M. E. (2013). Self‐reported pre‐pregnancy weight versus weight measured at first prenatal visit: Effects on categorization of pre‐pregnancy body mass index. Maternal and Child Health Journal, 17(10), 1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines . (2009). In Rasmussen K. M., & Yaktine A. L. (Eds.), Weight gain during pregnancy: Reexamining the guidelines. Washington (DC): National Academies Press (US) Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK32813 [PubMed] [Google Scholar]
- Kessous, R. , Davidson, E. , Meirovitz, M. , Sergienko, R. , & Sheiner, E. (2017). Prepregnancy obesity: A risk factor for future development of ovarian and breast cancer. European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP), 26(2), 151–155. [DOI] [PubMed] [Google Scholar]
- Kim, S. Y. , Dietz, P. M. , England, L. , Morrow, B. , & Callaghan, W. M. (2007). Trends in pre‐pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring, Md.), 15(4), 986–993. [DOI] [PubMed] [Google Scholar]
- Kronborg, H. , Vaeth, M. , & Rasmussen, K. M. (2013). Obesity and early cessation of breastfeeding in Denmark. European Journal of Public Health, 23(2), 316–322. [DOI] [PubMed] [Google Scholar]
- Li, R. , Jewell, S. , & Grummer‐Strawn, L. (2003). Maternal obesity and breast‐feeding practices. The American Journal of Clinical Nutrition, 77(4), 931–936. [DOI] [PubMed] [Google Scholar]
- Moore, E. R. , Bergman, N. , Anderson, G. C. , & Medley, N. (2016). Early skin‐to‐skin contact for mothers and their healthy newborn infants. The Cochrane Database of Systematic Reviews, 11, CD003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan, A. T. , Hehir, M. P. , Glavey, S. V. , Smith, T. J. , & Morrison, J. J. (2006). Inhibitory effect of leptin on human uterine contractility in vitro. American Journal of Obstetrics and Gynecology, 195(2), 504–509. [DOI] [PubMed] [Google Scholar]
- Nommsen‐Rivers, L. A. , Chantry, C. J. , Peerson, J. M. , Cohen, R. J. , & Dewey, K. G. (2010). Delayed onset of lactogenesis among first‐time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. The American Journal of Clinical Nutrition, 92(3), 574–584. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, E. J. , Perrine, C. G. , & Rasmussen, K. M. (2015). Early breastfeeding problems mediate the negative association between maternal obesity and exclusive breastfeeding at 1 and 2 months postpartum. The Journal of Nutrition, 145(10), 2369–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine, C. G. , Scanlon, K. S. , Li, R. , Odom, E. , & Grummer‐Strawn, L. M. (2012). Baby‐friendly hospital practices and meeting exclusive breastfeeding intention. Pediatrics, 130(1), 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston, L. , Caleyachetty, R. , Cnattingius, S. , Corvalán, C. , Uauy, R. , Herring, S. , … Gillman, M. W. (2016). Preconceptional and maternal obesity: Epidemiology and health consequences. The Lancet Diabetes & Endocrinology, 4(12), 1025–1036. [DOI] [PubMed] [Google Scholar]
- Prior, E. , Santhakumaran, S. , Gale, C. , Philipps, L. H. , Modi, N. , & Hyde, M. J. (2012). Breastfeeding after cesarean delivery: A systematic review and meta‐analysis of world literature. American Journal of Clinical Nutrition, 95(5), 1113–1135. [DOI] [PubMed] [Google Scholar]
- Rasmussen, K. M. (2007). Association of maternal obesity before conception with poor lactation performance. Annual Review of Nutrition, 27, 103–121. [DOI] [PubMed] [Google Scholar]
- Rasmussen, K. M. , Dieterich, C. M. , Zelek, S. T. , Altabet, J. D. , & Kjolhede, C. L. (2011). Interventions to increase the duration of breastfeeding in obese mothers: The Bassett Improving Breastfeeding Study. Breastfeeding Medicine, 6(2), 69–75. [DOI] [PubMed] [Google Scholar]
- Rasmussen, K. M. , & Kjolhede, C. L. (2004). Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics, 113(5), e465–e471. [DOI] [PubMed] [Google Scholar]
- Schafer, E. J. , Campo, S. , Colaizy, T. T. , Mulder, P. J. , & Ashida, S. (2017). Influence of experiences and perceptions related to breastfeeding one's first child on breastfeeding initiation of second child. Maternal and Child Health Journal. 10.1007/s10995-016-2228-1 [DOI] [PubMed] [Google Scholar]
- Scoccianti, C. , Key, T. J. , Anderson, A. S. , Armaroli, P. , Berrino, F. , Cecchini, M. , … Romieu, I. (2015). European Code against Cancer 4th Edition: Breastfeeding and cancer. Cancer Epidemiology, 39(Suppl 1), S101–S106. [DOI] [PubMed] [Google Scholar]
- Victora, C. G. , Bahl, R. , Barros, A. J. D. , França, G. V. A. , Horton, S. , Krasevec, J. , … Lancet Breastfeeding Series Group . (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet, 387(10017), 475–490. [DOI] [PubMed] [Google Scholar]
- Visram, H. , Finkelstein, S. A. , Feig, D. , Walker, M. , Yasseen, A. , Tu, X. , & Keely, E. (2013). Breastfeeding intention and early post‐partum practices among overweight and obese women in Ontario: A selective population‐based cohort study. Journal of Maternal‐Fetal and Neonatal Medicine, 26(6), 611–615. [DOI] [PubMed] [Google Scholar]
- Wagner, E. A. , Chantry, C. J. , Dewey, K. G. , & Nommsen‐Rivers, L. A. (2013). Breastfeeding concerns at 3 and 7 days postpartum and feeding status at 2 months. Pediatrics, 132(4), e865–e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkvist, A. , Brantsæter, A. L. , Brandhagen, M. , Haugen, M. , Meltzer, H. M. , & Lissner, L. (2015). Maternal prepregnant body mass index and gestational weight gain are associated with initiation and duration of breastfeeding among Norwegian mothers. The Journal of Nutrition, 145(6), 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2000). Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series, 894, i–xii. 1‐253 [PubMed] [Google Scholar]
- World Health Organization . (2007). Indicators for assessing infant and young child feeding practices. Part 1: Definition. Conclusions of a consensus meeting held 6–8 November 2007 in Washington, DC, USA. (USAID, AED, UCDavis, IFPRI, Unicef, WHO). Retrieved from http://www.who.int/maternal_child_adolescent/documents/9789241596664/en/. Accessed 4 April 2014.
- Yu, S. M. , & Nagey, D. A. (1992). Validity of self‐reported pregravid weight. Annals of Epidemiology, 2(5), 715–721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flow chart for selection of dyads included in this study, from the Epifane cohort
Figure S2: Conceptual framework of the relationship between pre‐pregnancy BMI (pBMI) and any breastfeeding (ABF) and exclusive breastfeeding (EBF) durations.
Table S1: Any breastfeeding (ABF) and exclusive breastfeeding (EBF) durations according to the sociodemographic factors, pregnancy conditions, and behavioral and psychosocial factors
Figure S3: Unadjusted Kaplan Meir curves estimating the proportion of women continuing breastfeeding during the first year of life, according to maternal pre‐pregnancy BMI, among women who initiated breastfeeding (n = 2,316)


