Abstract
Background:
Observational studies suggest that exposure to wood smoke is associated with a variety of adverse health effects in humans.
Objective:
We aimed to summarise evidence from sub-Saharan Africa on levels of exposure to pollutants in wood smoke and the association between such exposures and adverse health outcomes.
Methods:
PubMed and Google scholar databases were searched for original articles reporting personal exposure levels to pollutants or health outcomes associated with wood smoke exposure in Sub-Saharan African population.
Results:
Mean personal PM2.5 and carbon monoxide levels in the studies ranged from 26.3 ± 1.48 μg/m3 to 1574 ± 287μg/m3 and from 0.64 ± 2.12 ppm to 22 ± 2.4 ppm, respectively. All the reported personal PM2.5 exposure levels were higher than the World Health Organization’s Air Quality Guideline (AQG) for 24-hour mean exposure. Use of wood fuels in domestic cooking is the major source of wood smoke exposure in this population. Occupational exposure to wood smoke included the use of wood fuels in bakery, fish drying, cassava processing and charcoal production. Females were exposed to higher levels of these pollutants than males of the same age range. Major determinants for higher exposure to wood smoke in SSA included use of unprocessed firewood, female gender and occupational exposure. We recorded strong and consistent associations between exposure to wood smoke and respiratory diseases including acute respiratory illness and impaired lung function. Positive associations were reported for increased blood pressure, low birth weight, oesophageal cancer, sick building syndrome, non-syndromic cleft lip and/or cleft palate and under-five mortality.
Conclusion:
There is high level of exposure to wood smoke in SSA and this exposure is associated with a number of adverse health effects. There is urgent need for aggressive programs to reduce wood smoke exposure in this population.
Introduction
Wood smoke is a complex mixture of gases, liquids and solid particles (aerosol) produced by incomplete combustion or pyrolysis of wood and other wood products such as charcoal, wood pellets, sawdust, and so on, at elevated temperatures and reduced oxygen [1]. While complete combustion of wood requires adequate supply of oxygen and produces carbon dioxide and water with no visible smoke, incomplete combustion results in the production of smoke. Besides the major combustion products (carbon dioxide and water), wood smoke consists of more than 200 distinct organic compounds [2], many of which have been shown to induce acute or chronic health effects in exposed humans. Of these, particulate matter, especially the fine particulate matter (PM2.5), is of most concern. Other hazardous components of wood smoke are carbon monoxide, nitrous oxides, formaldehyde, and polycyclic aromatic hydrocarbons (PAHs), including carcinogens such as benzo(a)pyrene [2,3].
Human exposure to wood smoke is as old as mankind. In prehistoric times, man used wood as the primary fuel for heating in the cold, lighting in the dark, and for cooking food [3] and for thousands of years, wood served as the sole source of energy for humankind [4]. Although increasing modernisation has led to the supplementation of wood by fossil fuels (such as coal and petroleum products) and electricity, it is still a major source of energy for the population in developing countries accounting for 50 to 90% of the fuel used for cooking and heating purposes in this population [4,5]. The demand and use of wood fuels has also increased (especially among the poor) in many developed nations due to scarcity of fossil fuels coupled with an increasing interest in sustainable energy production [4,6].
Although there has been a decline in the proportion of the world’s households relying mainly on solid fuels for cooking, with about 60 percent of the world’s population currently using modern fuels [7], the population in sub-Saharan Africa has not kept up with this global trend as reports show that this population still has the most widespread use of solid fuels [8]. About 83% of the population in WHO African region were estimated to be primarily reliant on polluting cooking options [9]. Of all the solid fuels (wood, coal, charcoal, dung, crop residues), wood fuels (firewood, charcoal and other crop residues) are predominantly used among the population in SSA, accounting for more than 90% of residential energy consumption in rural areas [10]. It has been reported that per capita consumption of wood fuel in SSA is 2–3 times higher than that in any other region [11]. Of the 2.770 billion people in developing countries projected to depend on wood fuel by 2030, sub-Saharan Africa alone accounts for 33.14% (918 million people) of this population [12].
Indoor air pollution from inefficient traditional wood burning stoves and open fires remains the major source of wood smoke exposure to humans, especially households in rural areas of developing countries who rely exclusively on woods for their cooking and heating needs [2]. Women, who do most of the household cooking, children under the age of five and the elderly who spend more time in the household are more exposed [13,14]. Higher level of exposure may be seen in individuals with some occupations. These include wild land fire fighters [15], charcoal producers [16,17,18], farmers involved in agricultural burnings [2] and individuals involved in commercial cooking and food processing using wood fuel [19,20,21]. Other significant sources of exposure may include bushfires, consumption of food items preserved or processed with wood smoke [19,22,23] and the ambient air [24,25].
Wood fuel is cheap, has widespread availability and potential renewability [11] and as compared to fossil fuels, might help to reduce impacts of long-term carbon emissions on climate change [26]. However, exposure to smoke from its combustion has been of significant public health concern especially in developing countries. Household air pollution (HAP) from solid fuels globally accounted for 2.576 (2.216–2.969) million deaths and 77.16135 (66.08637–88.04887) million disability-adjusted life years (DALYs) in the year 2016 [27], amounting to 7.87% and 7.14% of total deaths and DALYs, respectively, attributable to all risk factors in 2016. With these figures, HAP was ranked as the 8th leading mortality risk factor and the 10th leading risk factor for disability-adjusted life-years (DALYs), globally in 2016 [27]. Low- and middle-income countries in SSA had the highest number (134) of age-standardized deaths (globally) per 100,000 capita from HAP in 2016 [9]. In 2017, 24% of global deaths and 34% of global DALYs attributable to household air pollution occurred in sub-Saharan Africa [28]. HAP from solid fuels accounted for about 35.64 % and 36.33% of total deaths caused by lower respiratory tract infections in sub-Saharan African children <5 years and women between aged 15–49 years, respectively [29].
Over the past decade, there has been a number of reviews focussing on household solid fuel use and its effect on human health [30,31,32]. However, there remains a lack of solid evidence on wood smoke exposure and the associated health effects in sub-Saharan African population. Hence, this review aims to systematically assess available evidence on exposure to pollutants in wood smoke and the health effects associated with this exposure in sub-Saharan African population. The findings of this review may help prioritize methods to control emissions from wood burning and reduce its associated diseases across sub-Saharan Africa.
Methods
Search strategy
A systematic literature search was done using PubMed and Google scholar databases for papers published in peer-reviewed journals from inception to December 31, 2018. To ensure the widest possible coverage of papers, we searched the databases using broad search terms, after which irrelevant papers were excluded. The following search terms were used: (‘wood smoke’ OR ‘woodsmoke’ OR ‘firewood’ OR ‘charcoal’ OR ‘solid fuel’ OR ‘indoor air pollution’ OR ‘household pollutants’ OR ‘biomass fuel’ OR ‘wildfire’ OR ‘open fire’ OR ‘domestic fuel’ OR ‘traditional stove’ OR ‘particulate matter’ OR ‘PM10’ OR ‘PM2.5’ OR ‘fire place’ OR ‘wood burning’ OR ‘cooking’ OR ‘heating’) AND (‘Names of each country in Sub-Saharan Africa’). The same terms were used for the two databases and papers were limited to those published in English language. No limitations were set for participants’ age and sex. The search was done in December, 2018 and updated in January, 2019. Reference lists of relevant reviews and included studies were also assessed for additional relevant studies.
Study Selection
Epidemiological studies that measured personal exposure to pollutants in wood smoke, examined health effects associated with wood smoke exposure or identified wood smoke as a risk factor to any measured health effect in humans living in sub-Saharan Africa were included.
Inclusion criteria
The population of interest were those living in SSA irrespective of age, sex, country. We considered environmental exposure to wood smoke in household cooking and heating as well as occupational exposure to wood smoke as seen in charcoal production, commercial food processing and preparation, agricultural burning and wildfire fighting. Only studies that reported specific exposure to smoke from wood fuels (firewood, charcoal) were included. Different comparators were considered in this review. Studies that compared wood fuel users and users of liquefied petroleum gas or electricity, those that compared exposure between firewood and charcoal users or between traditional open fire and improved wood stove users, or made comparison between individuals occupationally exposed and those not exposed to wood smoke were all included in the study. Two outcomes of interest were assessed: exposure levels and health outcomes. For the exposure levels, studies that measured personal exposure levels to pollutants in wood smoke (such as personal PM10, PM2.5 or CO measurements) or any biomarker of wood smoke exposure were included. For the health outcomes, papers that reported associations between exposure to wood smoke and any health outcome in any population group living in sub-Saharan Africa were included. The study designs considered were both observational (cohort, case-control, cross-sectional studies) and experimental (randomized controlled trials) studies.
Exclusion criteria
Studies were excluded if they gave reports from countries other than those in SSA, reported exposure to other solid fuels or biomass fuels (such as coal or animal dung) than wood, reported exposure to biomass or solid fuel without specifying exposure to wood smoke. Studies conducted in multiple countries including those in SSA were excluded except those that gave specific data for the SSA Country. Studies without proper comparators, those that compared “clean fuels” to “dirty fuels” and those that compared two types of improved wood stoves were excluded. Studies that measured air quality as proxy to individual exposure to PM2.5 PM10 or CO as well as animal studies were excluded.
Data Extraction
All titles, abstracts and full texts were screened according to the inclusion and exclusion criteria and data extraction forms was used to extract relevant data from the included papers. Paper eligibility was assessed independently by two reviewers and disagreements resolved by consensus. Generally, data extracted included information on: author, year of publication, country of study, study setting, study design, population of study, sample size, exposure assessment, outcome and outcome assessment method, and health effect (with the effect estimate and the associated 95% CI, where available).
Quality Assessment
The articles with evidence on health outcomes that met the inclusion criteria were assessed for quality using a 7-point score designed by the authors, specifically for this review. The following study characteristics were used for the quality assessment and a score of 0 or 1 was given depending on whether a study met a particular characteristic or not: description of study design (papers were scored 1 or 0, depending on whether the study design was adequately described or not), sampling strategy (studies with random selection were considered better and scored 1, as randomisation is an effective safeguard against selection bias), representativeness of study population (the description of the study population was assessed and papers were scored 1 or 0 respectively, depending on whether the study subjects were representative of the population or not), ascertainment of exposure to wood smoke (papers were scored 1 or 0, depending on whether exposure was adequately measured or assessed through questionnaire report), ascertainment of health outcome (papers were scored 1 if the outcome was based on clinical diagnosis or assessed through standard protocol; and 0 if assessed through questionnaire report), selection of non-exposed controls (papers were scored 1 or 0, depending on whether or not wood smoke exposure was ascertained in these individuals) and appropriate method to control for confounding (whether adjustments were made for variable such as age, smoking status and exposure to environmental or second-hand smoke etc.).
Results
The search and selection process for papers included in this review is summarized in Figure 1. From the 1506 abstracts identified from the two databases searched, 195 relevant abstracts were identified. Of these, 44 (forty-four) studies met the inclusion criteria and were included in this review. The characteristics of the included studies are presented in Tables 1 and 2. Sixteen papers reported on levels of exposure to specific pollutants in wood smoke (Table 1), while thirty-three studies reported on health effects associated with wood smoke exposure (Table 2). Two papers [33,34] used the same data but analysed the findings from different viewpoints and so, were merged as one. Five studies [33,34,35,36,37,38] reported both personal exposure and health outcomes of wood smoke exposure and these were reported separately in different tables of this review. The included studies were carried out in fifteen out of the forty-nine countries in sub-Saharan Africa: Nigeria had the highest number of studies (n = 11); followed by Ghana (n = 5); and Kenya (n = 5). Ethiopia, Malawi, Tanzania and Uganda had three studies each; Cameroon and Gambia had two studies each while Burkina-Faso, Congo, Mozambique, South Africa, Sierra Leone and Zambia had one study each. One study [39] combined data from demographic health studies conducted in 23 countries in sub-Saharan Africa. The population groups studied were mainly women (20 studies) and children under five years of age (7 studies). Most of the studies (n = 13) were carried out in rural areas. The study design used in the reviewed papers were mostly cross-sectional design (n = 32). Case-control designs (n = 7), longitudinal cohort (n = 2) and randomized controlled trial (n = 3), designs were also used. Studies made comparisons between wood fuels (firewood and charcoal) and LPG/electricity, biolite, ethanol. However, some studies made comparison between firewood and charcoal users, firewood/charcoal users and non-solid fuel users. Two studies compared level of exposure to wood smoke pollutants between traditional wood stoves and improved wood stoves [40,41].
Figure 1.
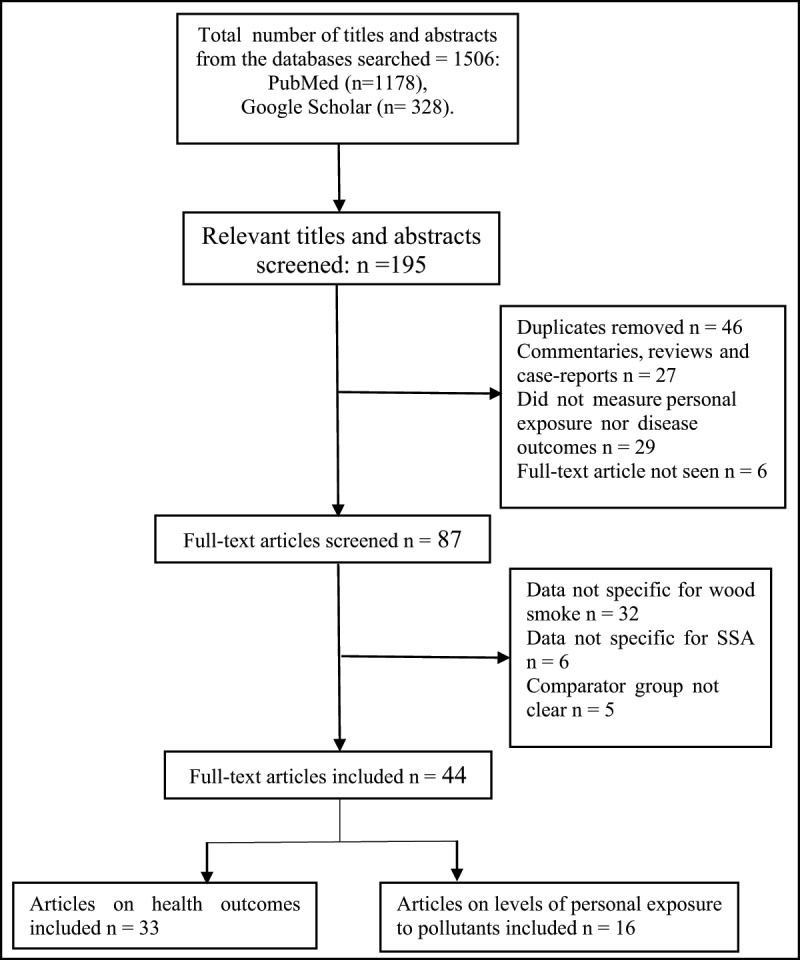
Flow chart of study search and selection process.
Table 1.
Summary of epidemiologic studies on levels of exposures to wood smoke pollutants in sub-Saharan Africa.
| Reference (Country) | Study design (Setting) | Study Population (Sample size) | Mean ± SD (Range) | Determinants for higher exposure |
|---|---|---|---|---|
| Personal PM10 Exposure | ||||
|
Ellegard [35] (Maputo, Mozambique) |
CSS (suburb) |
Women who are household cooks (Wood users = 114 Charcoal users = 78 Electricity users = 8 LPG users = 3) |
Wood: 1200 ± 131 μg/m3 Charcoal: 540 ± 80 μg/m3 Electricity: 380 ± 94 μg/m3 LPG: 200 ± 110 μg/m3 |
Wood users were exposed to higher PM10 than charcoal and electricity/LPG users |
|
Ezzati et al. [33,34]* (Kenya) |
LCS (Rural). |
55 households, 345 Individuals divided into groups by age and sex: |
Females within the age of 6–15 and 16–50 had significantly higher exposure than their male counterparts. | |
| 0–5 years Females (n = 52) Males (n = 41) |
0–5 years Females: 1317 (1188)a Males: 1449 (1067)a |
|||
| 6–15 years: Females (n = 61) Males (n = 48) |
6–15 years: Females: 2795 (2069)a Males: 1128 (638)a |
|||
| 16–50 years: Females (n = 65) Males (n = 55) |
16–50 years Females: 4898 (3663)a Males: 1018 (984)a |
|||
| >50 years: Females (n = 15) Males (n = 8) |
>50 years Females: 2639 (2501)a Males: 2169 (977)a |
|||
| Personal PM2.5 Exposure | ||||
|
Titcombe and Simcik [43] (Tanzania) |
CSS | Women Open firewood users (n = 3) charcoal users (n = 3) |
Open firewood users: 1574 ± 287 μg/m3 Charcoal users: 588 ± 347 μg/m3 LPG users: 14 ng/m3 |
Open firewood users were exposed to higher PM2.5 than charcoal users. |
|
Dionisio et al. [49] (Gambia). |
CSS (urban, peri-urban and rural areas) |
Children under 5 years of age (n = 31) | 65 ± 41 μg/m3 | NR |
|
Van Vliet et al. [45] (Ghana) |
CSS. (Rural) |
29 households cooks (95% females) | 24-hour average PM2.5 Real time 208 (30–618) μg/m3 |
Personal (Integrated) PM2.5 and Black carbon were higher for firewood users [(141.9 (112.5, 171.3)b; and (9.7 (8.4, 11.1)b, respectively], than Charcoal users [(44.6 (1.8, 87.4)b and (3.2 (0.6, 5.8)b, respectively]. |
| PM2.5 Integrated 128.5 (16.6–364.6) μg/m3 |
||||
| Black carbon 8.8 (1.9–18.2) μg/m3 |
||||
|
Downward et al. [44] (Ethiopia) |
CCSS (Urban) |
Workers in biomass and electricity using bakeries (N = 15 workers per group) |
PM2.5: Biomass bakers: 430 (2.0)c μg/m3 Electric bakers: 216 (2.2)c μg/m3 |
Occupational exposure to biomass smoke in bakery; Number of stoves in use; Additional biomass usage in coffee brewing |
| Black Carbon: Biomass bakers: 67 (1.9)c μg/m3 Electric bakers: 15 (1.8)c μg/m3 |
||||
|
Okello et al. [14] (Uganda) |
CSS (Rural) |
General Population (N = 102) divided into 6 age groups. Infants: (n = 17) Young Males: (n = 16) Young females: (n = 17) Adult males: (n = 17) Adult females: (n = 19) Elders: (n = 18) |
Infants: 80.2 (1.34)c μg/m3 Young males: 26.3 (1.48)c μg/m3 Young females: 117.6 (1.49)c μg/m3 Adult males: 32.3 (1.97)c μg/m3 Adult females: 177.2 (1.61)c μg/m3 Elders: 63.9 (2.03)c μg/m3. |
Women and girls had higher exposure to wood smoke than men and boys. |
| Personal CO Exposure | ||||
|
Dionisio et al. [47] (Gambia). |
CSS (urban, peri-urban and rural areas) |
Children Under 5 years of age (N = 1181) |
1.04 ± 1.46 ppm | Rainy season Use of charcoal |
|
Ochieng et al. [40] (Kenya) |
CSS (Rural) |
Women Traditional wood stove users: (n = 50) Improved wood stove users: (n = 50) |
Traditional wood stove users: 5.12 ± 3.89 ppm Improved wood stove users: 3.72 ± 3.74 ppm |
NR |
| Yamamoto et al. [48] (Burkina Faso) | CSS (semi-urban) |
Women aged 15–45 years and children ≤9 years (N = 148) |
Wood users: 3.3 (2.8-3.8)b ppm Charcoal users: 3.3 (2.8–3.7)b ppm |
Cooking outdoors was negatively associated with personal CO levels (>2.5 ppm) |
|
Quinn et al. [36] (Ghana) |
CSS (Rural) |
Pregnant women enrolled in GRAPHS who were primary cooks in households (n = 1183) |
1.6 (1.31) ppm (0.039–15.4 ppm) |
NR |
|
Quinn et al. [37] (Ghana) |
RCT (Rural) |
Pregnant women enrolled in GRAPHS who were primary cooks in households (N = 35). Randomized to: firewood (n =18) LPG (n =13) Biolite (n = 4) |
Pre-intervention CO levels Firewood: 1.04 ppm LPG: 1.74 ppm Biolite: 1.43 ppm Post-intervention CO levels Firewood: 1.55 ppm LPG: 0.63 ppm Biolite: 1.45 |
Only the LPG group showed a significant reduction in mean CO level following the intervention |
|
Downward et al. [44] (Ethiopia) |
CCSS | Workers in biomass and electricity using bakeries (N = 15 workers per group) |
CO: Biomass bakers: 22 (2.4)d ppm Electric bakers: 1 (5.0)d ppm |
Occupational exposure to biomass smoke in bakery; Number of stove in use; Additional biomass usage in coffee brewing were associated with higher exposure. |
|
Yip et al. [41] (Kenya) |
Cross-over study (Rural) |
Women (N = 237) and children aged <5 years (n = 239) | All women: 1.3 (1.3, 1.4)d ppm TCS Women: 2.2 (1.7, 2.8)d ppm Children: 0.8 (0.7, 0.9)d ppm ICS Women:1.1 (1.0, 1.3)d ppm Children: 0.8 (0.7, 0.8)d ppm |
There was a 44.9% reduction in mean personal CO level among women who crossed over to ICS |
|
Okello et al. [14] (Uganda) |
CSS (Rural) |
General Population (N = 102) divided into 6 age groups. Infants: (n = 17) Young Males: (n = 16) Young females: (n = 17) Adult males: (n = 17) Adult females: (n = 19) Elders: (n = 18) |
CO: Infants: 0.64 (2.12)d ppm Young males: 0.02 (3.67)d ppm Young females: 0.81 (3.83)d ppm Adult males: 0.17 (4.34)d ppm Adult females: 0.95 (3.26)d ppm Elders: 0.54 (3.07)d ppm |
Women and girls had higher exposure to wood smoke than men and boys. |
| Carboxy- hemoglobin (COHb) | ||||
|
Olujimi et al. [17] (Nigeria) |
CCSS (Occupational exposure in a rural setting) |
Charcoal workers and Non-Charcoal workers (n = 298 per group) |
Carboxyhemoglobin: Charcoal workers: 13.28 ± 3.91 % (5.00–20.00%). Non-charcoal workers: 8.50 ± 3.68% (1.00–18.00%). |
Working in a charcoal production site was associated with higher COHb level. |
| Polycyclic aromatic hydrocarbons (PAHs) | ||||
|
Titcombe and Simcik [43] (Tanzania) |
CSS | Women Open firewood users (n = 3) charcoal users (n = 3) |
Total PAH Open firewood users: 5040 ± 909 ng/m3 Charcoal users: 334 ± 57 ng/m3 LPG: <1 ng/m3 Benzo(a)pyrene Open firewood users: 767 ng/m3 Charcoal users: 44 ng/m3 LPG users: 0 ng/m3 |
Open firewood users were exposed to higher PM2.5, PAH and benzo(a)pyrene than charcoal. |
|
Awopeju et al. [38] (Ile-Ife, Nigeria) |
CCSS (NS) |
Women >20 years. Those who worked as street cooks for more than 6 months (n = 188); those who have never been street cooks (n = 197) | Benzene concentration in passive samplers worn by the women. Street cooks: 119.3 (82.7–343.7)e μg/m3 Non-street cooks: 0.0 (0.0–51.2)e μg/m3, p < 0.001). |
Benzene concentration in passive samplers worn by the women street cooks was significantly higher that worn by the controls. |
|
Olujimi et al. [18] (Nigeria) |
CCSS (Occupational exposure in a rural setting) |
Charcoal workers at two locations (Igbo-Ora: n = 25; Alabata: n = 20) and Non-Charcoal workers (n = 23) |
Urinary1-Hydroxypyrene Charcoal workers: Igbo-Ora: 2.22 ± 1.27 μmol/mol creatinine Alabata:1.32 ± 0.65 μmol/mol creatinine Non-charcoal workers: 0.32 ± 0.26 μmol/mol creatinine |
Working in a charcoal production site was associated with higher levels (>0.49 μmol/mol creatinine) of Urinary1-Hydroxypyrene. (RR: 3.14, 95% CI: 1.7–5.8, P < 0.01) |
a = Mean (Variance); b = Mean (95% CI); c = Geometric mean (geometric standard deviation); d = Geometric mean (95% CI); e = median (inter-quartile range); CCSS = Comparative Cross-Sectional Study; CSS = Cross-sectional study; RCT = Randomized controlled trial; NR = Not Reported; GRAPHS = Ghana Randomized Air Pollution and Health Study; CO = Carbon monoxide; * = Personal PM10 exposure estimated from a 210, 14-hour days of continuous real-time monitoring of PM10 concentration and time-activity budget of the household members.
Table 2.
Summary of epidemiological studies on health effects of wood smoke in sub-Saharan Africa.
| Reference (Country) | Design (Exposure setting) | Population (sample size) | Source of wood smoke (Comparison) | Method of Exposure assessment | Outcome(s) (Method of Assessment) | Health effect (Risk estimate) | Quality score |
|---|---|---|---|---|---|---|---|
| Respiratory outcomes | |||||||
|
Ellegard [35] (Maputo Mozambique) |
CSS (suburban) |
Women who are household cooks (n = 1200) | Firewood and charcoal for domestic cooking (Electricity/LPG users) | Interview: principal fuel, defined as the fuel used most often to cook the main meal; measurement of personal PM10. | Cough, Dyspnea, Wheezing, Inhalation and exhalation difficulties (questionnaire); PEFR (mini-wright peak flow meter |
Wood users had a significantly higher cough index (2.42 ± 0.104) and lower PEF rates (365 ± 3.4 L/min) than charcoal users (cough index: 1.77 ± 0.108; PEF rates: 382 ± 4.0 L/min) and users of modern fuels (cough index: 1.75 ± 0.198; PEF rates: 379 ± 7.4 L/min). There were no significant differences between the fuel user groups with respect to the non-cough respiratory symptom index. | 6 |
|
Ezziati and Kammen [33,34] (Laikipia, Kenya) |
LCS (Rural) |
55 households made up of Infants (n = 93), Individuals aged 5–49 years (n = 229), Individuals aged >50 years (n = 23). |
Firewood and charcoal for domestic cooking (PM10 ≤ 1000 vs. PM10 > 1000 μg/m3); (PM10 ≤ 200 vs. PM10 > 200 μg/m3). |
Personal exposures to PM10 calculated from Indoor PM10 measurement data and time activity budget. | ARI, ALRI (WHO protocols for clinical diagnosis of ARI). |
Risk of ARI and ALRI increased with higher PM10 exposure. For example, with reference to PM10 of <200, the adjusted odds ratio for ARI was 2·42 (1·53–3·83) in children <5 years with PM10 200–500. |
7 |
|
Ibhazehiebo et al. [74] (Edo, Nigeria) |
CSS (Rural and urban) |
Women aged 20–70 years. Active wood users (n = 350); non-wood users (n = 300) |
Wood use for cooking. (non-wood users) |
Questionnaire: number of years of use of wood as cooking fuel; number of times of such cooking per day. | PEFR (mini-wright peak flow meter), Respiratory symptoms (cough with sputum production, dyspnea, wheezing, chest tightness and chest pain) | Respiratory symptoms were markedly elevated in the subjects compared to controls. Mean PEFR value for the wood users (289 ± 19.6 L/min) was significantly lower than non-wood users (364 ± 17.2 L/min), P < 0.05. PEFR decreased with increase in years of exposure to wood smoke. | 3 |
|
Kilabuko et al. [51] (Bagamoyo, Tanzania) |
CSS (Rural) |
100 households | Wood use for cooking. (Regular women cooks and children under age 5 Vs unexposed men and non-regular women cooks) |
Observation; Kitchen, living room and outdoor measurement of PM10, CO and NO2. | ARI (questionnaire) |
The risk of having ARI was higher for cooks and children under age 5 (exposed group) than the unexposed group (OR: 5.5; 95% CI: 3.6–8.5) | 3 |
|
Fullerton et al. [57] Malawi |
CSS (Rural and urban areas) |
Adults (n = 374) | Firewood vs. Charcoal users |
Questionnaire: type of biomass fuel used for cooking | Lung function (spirometry). |
Wood users had significantly worse lung function than charcoal users {FEV1, ml: 2430 (670) Vs 2780 (680) P < 0.001; Percent predicted FEV1: 99 Vs 106, P < 0.008; FVC, ml: 3190 (830) Vs 3490 (870); FEV1/FVC ratio: 76.54 (9.11) Vs 79.80 (7.48) P = 0.001} for firewood and charcoal users, respectively. | 5 |
|
Ekaru et al. [52] (Moi, Kenya) |
Hospital-based CSS (NS) |
Children Aged 0–5 years (n = 181) |
Use of firewood/charcoal for domestic cooking (Use vs. non-use of firewood/charcoal) |
Care-giver reported use of firewood and charcoal for cooking | Pneumonia (clinical diagnosis) |
Use of firewood/charcoal were risk factors for mild pneumonia (OR 4.23, CI 3.9–4.6) and severe pneumonia (OR 1.1, CI 1.02–1.26). | 4 |
|
Taylor and Nakai (Sierra Leone) |
CSS (rural and peri-urban) |
Women aged 15–45 years (n = 520); children under 5 years of age (n = 520). |
Use of firewood/charcoal for domestic cooking (Wood vs. Charcoal use) |
Questionnaire: Type of biomass fuel normally used for cooking | ARI (interview: cough and rapid breath in the last 2 weeks preceding study as proxy for ARI) |
Relative to charcoal use, wood use was associated with ARI in children but not in women (adjusted OR = 2.03, 95%CI: 1.31–3.13) and (adjusted OR = 1.14, 95%CI: 0.71–1.82), respectively. ARI prevalence was higher for children in homes with wood stoves (64%) compared with homes with charcoal stoves (44%). | 4 |
|
Oloyede et al. [58] (Nigeria) |
Comparative CSS (NS) |
Children aged 6–16 years. Those living in fishing port (n = 358) and those living in farm settlements (n = 400) | Firewood use in fish drying (Residence vs. Non-residence in fishing port) |
Observation: Living in fishing port | Lung function (spirometry). |
Children living in fishing port had reduced lung function (FVC: 1.32 ± 0.67; FEV1: 1.22 ± 0.62) compared with those living in farm settlements (FVC: 1.45 ± 0.43; FEV1: 1.41 ± 0.41. Decline in lung function was associated with increase in duration of exposure to fish drying. | 5 |
|
Ibhafidon et al. [59] (Ile-Ife, Nigeria) |
CSS (NS) |
Male and female Individuals including firewood users (n = 35), kerosene users (n = 34) and LPG users (n = 21). | Firewood for cooking (firewood vs. LPG users) |
Modified BMRC questionnaire: predominant cooking fuel | Respiratory symptoms (Modified BMRC questionnaire); lung function (spirometry). | Firewood users reported more respiratory symptoms, compared with LPG users. 95.2% of LPG users had normal lung function (FEV1/FVC > 0.7 and predicted FEV1 of at least 80%), 71.4% of firewood users, respectively. | 4 |
|
Sanya et al. [56] (Kampala, Uganda) |
Case. Control study (NS) |
Asthma patients aged >13 years. Those with exacerbations (n = 43); Those without exacerbations (n = 43) | Wood and charcoal use for cooking (in-house vs. no in-house wood/charcoal stoves) |
Questionnaire: use of in-house wood/charcoal stoves | Asthma exacerbations (clinical assessment) | In-house wood/charcoal use was not associated with increased risk of asthma exacerbations (OR = 0.882, 95% CI: 0.329–2.36, P = 0.802) | 4 |
|
Umoh and Peters [20] (Akwa-Ibom, Nigeria) |
CCSS (Rural) |
Women involved in fish- smoking activities (n = 324) and women involved in fishing but not fish smoking (n = 346) | Wood use in fish smoking (Fish-smokers vs. non fish-smokers) |
BMRC Respiratory disease Questionnaire: based report of involvement Fish-smoking |
Lung function (spirometry) |
Fish smokers had lower lung function (Mean FEV1/FVC = 68.8 ± 15.3 %) than non-fish smokers (FEV1/FVC = 78.3 ± 9.6 %), P < 0.001. | 5 |
|
Ngahane et al. [60] (Bafoussam, Cameroon) |
CSS (Semi-rural) |
Women >40 years. Those using wood (n = 145) and those using other fuel sources (n = 155) for cooking. | Wood for domestic cooking (wood vs. other fuel [charcoal, gas, electricity] users) |
Questionnaire-based report of cooking fuel. | Lung function (spirometry) Respiratory symptoms (questionnaire) | Use of wood as a cooking fuel was associated with impairment of lung function (Adjusted mean difference in FEV1= –120; 95% CI: –205, –35; P = 0.005). Prevalence of chronic bronchitis was higher (7.6%) in wood smoke group than the other fuels (0.6%). |
3 |
|
Dienye et al. [50] (Rivers State, Nigeria) |
CCSS (rural) |
Women age ≥15 years. Those involved in fish smoking (n = 210) and those not involved in fish smoking (n = 210) | Wood use for fish smoking (involvement vs. non-involvement in fish smoking) |
Self-reported involvement in fish smoking | Respiratory symptoms (questionnaire). PEFR (mini-wright peak flow meter). |
Fish smoking was associated with increased risk of sneezing (OR = 2.49, 95% CI: 1.62–3.82; P < 0.001), catarrh (OR = 3.77; 95% CI: 2.44–5.85, P < 0.001), cough (OR = 3.38, 95% CI: 2.22–5.15, P < 0.001) and chest pain (OR = 6.45, 95% CI: 3.22–13.15, P < 0.001). The mean PEFR of 321 ± 58.93 L/min among the fish smokers was significantly lower than 400 ± 42.92 L/min among the controls (p = 0.0001). | 4 |
|
PrayGod et al. [54] (Mwanza, Tanzania) |
Case-control (NS) |
Children. Under 5 years Cases (n = 45), controls (n = 72). |
Firewood and charcoal (Firewood/charcoal users vs. Gas/Electricity users); (indoor vs outdoor cooking). |
Questionnaire: Source of cooking fuel; location of cook stove. | Clinical/Laboratory diagnosis of pneumonia | Firewood and charcoal use were not associated with increased risk of pneumonia (Unadjusted OR = 2.1; 95% CI: 0.2–27 and OR = 1.9 95% CI: 0.2–18, respectively). An increased risk of severe pneumonia was associated with cooking indoors (OR = 5.5, 95% CI: 1.4–22.1) |
5 |
|
Awopeju et al. [38] (Ile-Ife, Nigeria) |
CCSS (NS) |
Women >20 years. Those who worked as street cooks for more than 6 months (n = 188); those who have never been street cooks (n = 197) | Firewood and charcoal for street cooking (street cooks vs. Non-street cooks. |
Questionnaire: number of hour-years of exposure to street cooking; hours spent cooking daily with biomass fuel at home. Quantification of volatile organic compounds (VOCs) in a sub-sample of the women. |
Respiratory symptoms (Questionnaire); pulmonary function (spirometry) |
The odds of reported cough (adjusted OR: 4.4, 95% CI: 2.2–8.5), phlegm (AOR: 3.9, 95% CI: 1.5–7.3) and airway obstruction, FEV1/FVC < 0.7 (adjusted OR of 3.3 (95% CI 1.3 to 8.7) were significantly higher among the street cooks than controls. | 5 |
|
North et al. [61] (Rural Uganda) |
Prospective cohort study (Rural) |
HIV-infected adults aged >18 years (N = 734; 223 males 511 females) |
Use of Firewood/charcoal for domestic cooking. (Firewood Vs Charcoal) |
Interview: main cooking fuel | cough of ≥4 weeks duration (self-report). | Cooking with firewood was associated with increased risk of chronic cough among females (adjusted OR = 1.41, 95% CI: 1.00–1.99; p = 0.047). | 3 |
|
Okwor et al. [21] (Ogun, Nigeria) |
CCSS (Rural) |
Females aged 13–60 years in two occupations: garri processors (n = 264) and petty traders (n = 264). |
Firewood use in garri processing (occupational exposure vs. non-exposure to wood smoke; ≥10years vs <10 years of occupational exposure. |
Observation: Working in garri production site | Respiratory symptoms (self-report); pulmonary function (spirometry). | Prevalence of obstructive pulmonary defect (FEV1/FVC < 70%) among the cassava processors was 21.3% compared to 6.4% among petty traders (P < 0.001). Occupational biomass (wood) fuel use (OR = 6.101, 95% CI 3.212–11.590) and working as a cassava processor for ≥ 10 years (OR 14.916, CI 5.077–43.820) were associated with increased risk of obstructive pulmonary defect). | 4 |
|
Tazinya et al. [55] (Bameda, Cameroon) |
CSS (NS) |
Children under 5 years of age (n = 512) | Exposure to firewood smoke >30 minutes/day. non-exposure to firewood smoke |
Questionnaire: Exposure to firewood smoke | ARI (WHO guideline for diagnosis management of pneumonia in children |
Exposure to wood smoke was associated with ARI (adjusted OR: 1.85, 95% CI: 1.22–2.78). | 5 |
| Cardiovascular outcomes | |||||||
|
Alexander et al. [62]a. (Ibadan, Nigeria) |
RCT (Peri-urban) |
Pregnant women (N = 108; randomized to firewood (n = 51); randomized to ethanol (n = 58) | Firewood use in domestic cooking (Firewood vs. Ethanol). |
Interview: primary cooking fuel | Blood pressure (standard method). | There was no statistical difference in DBP (model 1: χ25 = 5.24; P = 0.39; model 2: χ23 = 2:09; P = 0.55) and SBP pressure (model 1: χ25 = 4.69; P = 0.45; model 2: χ23 = 3.44; P = 0.33) between the women randomized to firewood and those randomized to ethanol. | 4 |
|
Quinn et al. [36] (Ghana) |
CSS (rural) |
Pregnant women enrolled in GRAPHS (n = 817) | Firewood and charcoal use in domestic cooking (1 ppm increase in CO exposure) |
72-hour personal CO monitoring | Blood pressure (watch automatic BP monitor). | CO exposure was significantly associated with diastolic blood pressure (each 1ppm increase in exposure to CO was associated with 0.43 mmHg higher DBP [95% CI: 0.01, 0.86] and 0.39 mmHg higher SBP (95% CI: –0.12, 0.90). | 7 |
|
Quinn et al. [37] (Ghana) |
RCT (rural) |
Non- Pregnant women enrolled in GRAPHS (N = 44). Randomized to firewood (n =23), Improved biomass stove (n = 5) and LPG (n =16) |
Firewood for domestic cooking. (Firewood vs. Improved biomass stove and LPG) |
Observation: use of firewood, improved biomass stoves or LPG stoves for cooking; Personal 72-hour CO exposure monitoring |
Ambulatory blood pressure measurement (ABPM) | ABPM revealed that peak CO exposure (defined as ≥4.1 ppm) in the 2 hours prior to BP measurement was associated with elevations in hourly systolic BP (4.3 mmHg [95% CI: 1.1, 7.4]) and diastolic BP (4.5 mmHg [95% CI: 1.9, 7.2]), as compared to BP following lower CO exposures. Use of improved cookstoves was not significantly associated with lower post-intervention SBP and DBP (within-subject change in SBP and DBP of –2.1 mmHg, 95% CI: -6.6, 2.4 and –0.1, 95% CI: –3.2, 3.0, respectively). |
7 |
|
Ofori et al. [63] (Rivers State, Nigeria) |
CSS (rural) |
Women aged 18 and above (n = 389) |
Firewood and charcoal (BMF vs. non-BMF) |
Questionnaire: predominant cooking fuel | Blood pressure, lipid profile, carotid intima media thickness (all were measured using standard protocols). | Use of BMF was significantly associated with 2.7mmHg higher systolic blood pressure (p = 0.04), 0.04mm higher CIMT (P = 0.048), increased odds of pre-hypertension (OR:1.67, 95% CI: 1.56, 4.99, P = 0.035), but not hypertension OR: 1.23, 95% CI: 0.73, 2.07, P = 0.440) | 5 |
| Reproductive outcomes | |||||||
|
Amegah et al. [64] Ghana |
CSS (urban) |
Mothers and their new born | Charcoal use in domestic cooking. (Charcoal vs. LPG) |
Questionnaire: type of cooking fuel used | Birth weight (from hospital records); low birth weight (birth weight ≤2500g) | Exposure to charcoal was associated with reduction in birth weight (adjusted β: –381, 95% CI: –523, –239 and –278, 95% CI: –425, –131 p = 0.000) for all births and term births, respectively. Exposure to charcoal smoke was also associated with increased risk of low birth weight in all births (adjusted RR: 2.41, 95% CI: 1.34, 4.35, P = 0.003), but not term births (adjusted RR: 1.79, 95% CI: 0.81, 3.94, P = 0.112) |
6 |
|
Demelash et al. [66] (Ethiopia) |
CCS (rural and urban) |
Mothers with singleton live birth. Cases: those who gave live births weighing less than 2500g, n =136) controls (those who gave live births weighed more than 2500g (n = 272). | Firewood use in domestic cooking. (Firewood vs. Electricity) |
Questionnaire: type of energy used for cooking | Low birth weight (birth weight ≤2500g). | Use of firewood for cooking was associated with low birth weight (Adjusted OR: 2.7; (95% CI:1.00–7.17) | 5 |
|
Whitworth et al. [67] (South Africa) |
CSS (rural) |
Women (n = 420 | Firewood use in domestic cooking. (Open wood fires vs. Electricity) |
Questionnaire: type of cooking fuel mainly used. | Plasma concentrations of anti-Müllerian hormone (ELISA Method) | Compared with women who used an electric stove, no association was observed among women who cooked indoors over open wood fires (Adjusted β: –3, 95% CI: –22, 21 and –9, 95% CI: –24, 9 for outdoor wood users and indoor wood users, respectively). | 5 |
|
Amegah et al. [65] Ghana |
CSS (urban and rural) |
Women aged 15–49 years (n = 7183) | Use of Biomass fuel (charcoal, firewood and straw/shrubs/grass) for domestic cooking. (biomass fuel vs. Non-biomass fuel (electricity, LPG and natural gas) |
Questionnaire: type of fuel used by households for cooking. |
Lifetime experience of stillbirth (questionnaire). | Biomass fuel use mediated 17.7% of the observed effects of low maternal educational attainment on lifetime stillbirth risk | 3 |
| Cancer outcomes | |||||||
|
Patel et al. [68] (Kenya) |
CCS (NS) |
Cases: patients with oesophageal squamous cell carcinoma (n = 159); Controls: Healthy individuals without oesophageal squamous cell carcinoma (n = 159). | Firewood and charcoal use in domestic cooking. [Other fuels (kerosene electricity LPG)] |
Questionnaire: type of cooking fuel | Oesophageal squamous cell carcinoma (histological diagnosis) | Cooking with firewood or charcoal was independently associated with Oesophageal cancer (OR: 2.32, 95% CI: 1.41–3.84) | 4 |
|
Kayamba et al. [69] (Lusaka, Zambia) |
CCS (Rural and urban) |
Cases: patients with oesophageal squamous cell carcinoma (n = 77); Controls: Healthy individuals without oesophageal squamous cell carcinoma (n = 145). | Firewood and charcoal use in domestic cooking. (non-use of Firewood/charcoal) |
Questionnaire: type of cooking fuel | Oesophageal squamous cell carcinoma (histological diagnosis) | Cooking with firewood or charcoal increased the risk of developing Oesophageal squamous cell carcinoma (adjusted odds ratio: 3.5, 95% CI: 1.4–9.3) | 5 |
|
Mlombe et al. [70] Malawi |
CCS (NS) |
Cases: Adult patients with oesophageal squamous cell carcinoma (n = 96); Controls: Healthy individuals without oesophageal squamous cell carcinoma (n = 180). | Firewood/charcoal use in domestic cooking. (Firewood vs. Charcoal) |
Questionnaire: type of cooking fuel | Oesophageal squamous cell carcinoma (histological diagnosis) | The odds of oesophageal cancer were 12.6 times higher among those who used firewood compared to those who used charcoal (Adjusted OR: 12.6, 95% CI: 4.2–37.7). | 5 |
| Other health outcomes | |||||||
| Das et al. [73] (Malawi) | CSS (Rural and urban) |
Household cooks (n = 655) | Use of firewood, charcoal and crop residue for domestic cooking. (Firewood or crop residue vs. Charcoal) |
Interview: cooking technologies and fuels used. | Five categories of health outcomes: cardiopulmonary, respiratory, neurologic, eye health, and burns. (questionnaire). |
Use of low quality firewood or crop residue was associated with significantly higher odds of shortness of breath at rest (Adjusted OR: 1.02; 95% CI: 0.24–4.32 and 6.22; 95% CI: 1.13–34.17, respectively), chest pains (Adjusted OR: 3.00; 95% CI: 0.91–9.86 and 4.46; 95% CI: 0.92–21.66, respectively), night phlegm (Adjusted OR: 5.39; 95% CI: 1.06–27.45 and 11.59; 95% CI: 1.38–97.31, respectively), forgetfulness (Adjusted OR: 4.48; 95% CI: 1.42–14.19 and 9.13; 95% CI: 1.86–44.95, respectively) and dry irritated eyes (Adjusted OR: 1.64; 95% CI: 0.54–4.99 and 3.75; 95% CI: 0.81–36, respectively). | 4 |
|
Owili et al. [39] (23 Countries in SSA) |
CSS | Children under 5 years of age (n = 783,691) | Use of Charcoal as cooking fuel. (Charcoal vs. Clean fuels [electricity, natural gas, biogas or liquefied petroleum gas]). |
Questionnaire: type of cooking fuel | All-cause under-five mortality | Use of charcoal as cooking fuel was significantly associated with the risk of under-five mortality in SSA before and after controlling for other indicators. (Crude HR: 1.97, 95% CI: 1.80, 2.16; Adjusted HR: 1.21, 95% CI: 1.10, 1.34). | 3 |
|
Belachew et al. [72] Ethiopia |
CSS (NS) |
The entire population mate (N = 3405); male: 1567 Female: 1838 |
Use of charcoal for domestic cooking. (Charcoal use vs. No charcoal use) |
Questionnaire/observation: charcoal use for cooking | Sick-building syndrome (questionnaire) |
Sick-building syndrome was significantly associated with charcoal use as cooking energy source (Adjusted OR: 1.4, 95% CI: 1.02–1.91) | 3 |
|
Mbuyi-Musanzayi et al. [71] (Lubumbashi, Congo) |
CCS (NS) |
Cases: New born with Non-syndromic cleft lip and/or cleft palate (n = 162) Controls: clinically normal newborn (n = 162) |
Use of Charcoal as cooking fuel. (Indoor Vs No indoor cooking with charcoal) |
Questionnaire: inhouse cooking with charcoal (yes or no) | Non-syndromic cleft lip and/or cleft palate (clinical diagnosis) | Indoor cooking with charcoal was significantly associated with non-syndromic cleft lip and/or cleft palate (OR = 6.536, 95% CI: 1.229, 34.48. p = 0.0001) | 3 |
CSS = Cross-sectional study; CCSS = Comparative cross-sectional study; LCS = Longitudinal cohort study; CCS = Case-control study; RCT = Randomized controlled trial; NS = Not stated; ISAAC = International Study of Asthma and Allergies in Childhood; BMRD = British Medical Research Council; PEFR = Peak Expiratory Flow Rate; GRAPHS = Ghana Randomized Air Pollution and Health Study; a = only data for baseline firewood users were included in this review.
The outcomes reported in the studies were classified into two: personal exposure levels (Table 1) and health outcomes (Table 2). We identified 16 studies that reported either personal exposure levels to specific pollutants in wood smoke using personal monitors attached to individual clothing, or concentrations of biomarkers of wood smoke in blood or urine. These included personal exposure to PM10, PM2.5, CO, black carbon, levels of carboxy-hemoglobin, total PAH, benzene, benzo(a)pyrene and urinary1-Hydroxypyrene. Health outcomes reported in the reviewed studies included respiratory illness, cardiovascular, reproductive/pregnancy outcomes, cancer, mortality, non-syndromic cleft lip and/or cleft palate and sick building syndrome.
Personal Exposure Levels to Pollutants in Wood Smoke in SSA Population
Exposure to particulate matter
Personal PM10 exposure from household use of wood fuels was reported in two studies [33,34,35]. Higher levels of PM10 exposure was recorded for wood (1200 ± 131 μg/m3) than charcoal (540 ± 80 μg/m3), electricity (380 ± 94 μg/m3) and LPG users (200 ± 110 μg/m3), respectively. The mean personal PM10 exposure levels reported for all categories of fuel users were above the WHO [42] Air Quality Guideline of 50 μg/m3 for 24-hour mean PM10 exposure [35]. Ezzati and Kammen [33,34], estimated personal exposure to PM10 from data obtained from a 210, 14-hour days continuous real-time monitoring of PM10 and time-activity budget of household members. In their report, only 20 (6.2%) of the study population had PM10 level <200 μg/m3 and the mean PM10 levels for all the sub-groups were above the WHO AQG.
Personal exposure to PM2.5 was measured in five studies. Four of these studies [14,43,45,49] reported exposure from household use of wood fuels. The reported mean personal PM2.5 ranged from 26.3 ± 1.48 μg/m3 among young males [14] to 1574 ± 287 μg/m3 among women firewood users [43]. All the reported mean personal PM2.5 levels in the studies were higher than the WHO AQG of 25 μg/m3 [42]. Two of the studies [43,45] compared personal PM2.5 exposure between firewood and charcoal users. Both studies reported higher level of personal PM2.5 in firewood (1574 ± 287 μg/m3 and 141.9 μg/m3, respectively) than charcoal users (588 ± 347 μg/m3 and 44.6, respectively). Mean PM2.5 exposure was higher in women (177.2 ± 1.61 μg/m3) and girls (177.6 ± 1.49 μg/m3) compared to other age groups [14].
Downward et al. [44] recorded high level of PM2.5 exposure among bakers exposed to wood smoke in bakery (430 ± 2 μg/m3), compared to those using electric cookstoves (216 ± 2.2 μg/m3).
Black carbon levels were measured in two studies [44,45]. Van Vliet et al. [45] reported higher mean black carbon level in firewood users (9.7 μg/m3) than charcoal user (3.2 μg/m3). Compared with individuals working in bakeries that use electric cookstoves (15 ± 1.8 μg/m3), those that worked in wood-using bakeries were exposed to higher levels (67 ± 1.9 μg/m3) of black carbon [44].
Exposure to Carbon Monoxide
Eight studies reported average 24-hour or 48-hour personal carbon monoxide (CO) exposure levels. In all but one study [44], the reported personal CO exposures were below the WHO [46] AQG of 7 μg/m3 (6.11 ppm). The reported levels of CO exposure from household use of wood fuels ranged from 0.02 ± 3.67 ppm in young males [14] to 5.12 ± 3.89 ppm among women using traditional cook stoves [40]. Five of the studies [14,36,37,40,41] gave report on CO exposure in women. The reported mean personal CO levels ranged from 0.81 ± 3.83 ppm in young females [14] to 5.12 ± 3.89 ppm in women using traditional wood stove [40]. Three studies [14,41,47] measured CO exposure among children <5 years of age. The reported levels of CO in these three studies were similar and ranged from 0.64 ± 2.12ppm [14] to 1.04 ± 1.46ppm [47]. Two studies [41,48] reported CO levels in both women and children. Okello et al. [14] reported 24-hour CO exposure in six sub-groups of the study population. In their report, women and girls had higher exposure to CO (0.95 ± 3.26 and 0.81 ± 3.83 ppm, respectively) than men and boys (0.17 ± 4.34 and 0.02 ± 3.67, respectively). Downward et al. [44] measured personal CO exposures in bakery workers in Ethiopia and compared CO exposures between bakers who used electric and biomass cookstoves. In their report, bakers who used biomass cookstoves were exposed to higher level of CO (22 ± 2.4 ppm) compared to those that used electric cookstoves (1 ± 5.0 ppm). Yamamoto et al. [48] reported no significant difference in 24-hour personal CO exposure between women and children living in households where firewood (3.3 ppm) and charcoal (3.3) were used for domestic cooking. Personal CO exposure between traditional and improved woodstoves users were compared in two studies [40,41]. Ochieng et al. [40] reported no significant difference in mean personal CO level among traditional (5.12 ± 3.89 ppm) and improved (3.72 ± 3.74 ppm) woodstoves users, but observed a 24.0% reduction in personal CO concentrations associated with stove replacement. In a cross-over study to identify ICS for use in Kenya, Yip et al. [41] reported significant difference in CO exposure among women and children randomized to the two stove types.
Individuals occupationally exposed to wood smoke in bakery [44] had higher level of CO exposure. Olujimi et al. [17] compared COHb levels between charcoal producers and non-charcoal workers. In their report, subjects exposed to wood smoke in charcoal production had higher level of COHb (13.28 ± 3.91 %) than non-charcoal workers (8.50 ± 3.68%). The reported mean COHb levels in both groups were above the WHO guideline of 2% for COHb [46].
Exposure to polycyclic aromatic hydrocarbons (PAHs)
Concentrations of PAHs were measured in three studies [18,38,43]. Titcombe et al. [43] reported mean total PAH and benzo(a)pyrene levels of 5040 ± 909 ng/m3 and 767 ng/m3, 334 ± 57 ng/m3 and 44 ng/m3, and <1 ng/m3 and 0 ng/m3 in open firewood, charcoal and LPG users, respectively. Charcoal production was associated with higher levels (>0.49 μmol/mol creatinine) of urinary1-hydroxypyrene (RR: 3.14, 95% CI: 1.7–5.8, P < 0.01) [18]. Awopeju et al. [38] reported median (interquartile range) benzene concentration of 119.3 (82.7–343.7) and 0.0 (0.0–51.2) in passive samplers worn by street cooks and non-street cooks, respectively.
Sources of wood smoke exposure in SSA
In all but one study [17], the reported sources of exposure to wood smoke were from the use of wood fuels (firewood and charcoal) for domestic or commercial food preparation or processing. Occupational exposure to wood smoke were reported in seven studies. These include charcoal production [17–18], bakery [44], fish drying [20,50], garri (cassava) processing [21] and street cooking [38].
Risk factors for higher exposure to wood smoke pollutants in SSA
Twelve studies gave reports on some determinants for higher exposure to wood smoke in SSA (Table 1) and these factors varied between studies. Users of unprocessed firewood had higher exposure to PM10 [35] PM2.5 [43,45], total PAH and benzo(a)pyrene [43] than charcoal users. Use of charcoal stove was associated with higher level of exposure to CO [47]. Female gender was associated with higher exposure to wood smoke pollutants [14,33,34]. Occupational exposure was associated with higher levels of exposure to pollutants [17,18,38]. Other risk factors identified include rainy season [47], cooking indoors [48], use of multiple wood stoves [44] and use of traditional cook stoves [41].
Health effects associated with wood smoke exposure in SSA
A total of 33 studies gave report on health effects associated with wood smoke exposure. Of these, 18 studies evaluated effect on respiratory outcomes, 4 studies on cardiovascular outcomes, 3 each on cancer and reproductive outcomes and one each on mortality, sick-building syndrome and non-syndromic cleft lip and/or cleft palate, while one study measured five categories of health outcomes.
Respiratory outcomes
The respiratory symptoms evaluated differed in the studies. These symptoms included ARI/ALRI, lung function, asthma, cough, wheezing and dyspnea. Six studies evaluated the impact of wood smoke exposure on acute respiratory infections/acute lower respiratory infections [33,34,51,52,53,54,55]. All of these studies recorded positive associations between exposure to wood smoke and ARI. Ezzati and Kammen [33] reported a positive exposure-response relationship for indoor air pollution and ARI in rural Kenya. Females above five years were reported to be at higher risk of ARI or ALRI than males [34]. Kilabuko et al. [51] recorded higher risk of having ARI among cooks and children under age five compared to men (the unexposed group). With reference to children living in homes with charcoal stoves, Taylor and Nakai [53] reported higher odds of having suffered from ARI in children living in homes with wood stoves. Cooking indoors was associated with increased risk of severe pneumonia [54] but not with asthma exacerbations [56].
All the seven studies on lung function [20,21,38,57,58,59,60] reported positive association between wood smoke exposure and reduced lung function. Three of these studies [20,21,38] reported reduced lung function [20] or higher risk of obstructive pulmonary defect [21,38] in women occupationally exposed to wood smoke. With reference to those who have worked as cassava processors for less than 10 years, working as cassava processor for more than 10 years was associated with higher risk (OR: 14.916, 95% CI: 5.077–43.820) of obstructive pulmonary defect [21].
All the three studies [35,50,61] that examined association between wood smoke exposure and cough reported positive associations. North et al. [61] reported higher risk of chronic cough among HIV-infected females using firewood with reference to those using charcoal as cooking fuel.
Cardiovascular Outcomes
Association between wood smoke exposure and cardiovascular outcomes were investigated in four studies [36,37,62,63]. These four studies examined the effect of wood smoke exposure on blood pressure in women. Three of these studies were carried out among pregnant women [36,37,62]. Quinn et al. [36,37] recorded positive associations between wood smoke exposure and increase in blood pressure. In another study [63], use of biomass fuel (firewood and charcoal) was significantly associated with 2.7 mmHg higher systolic blood pressure, 0.04 mm carotid intima media thickness (CIMT) and increased odds of pre-hypertension (OR: 1.67, 95% CI: 1.57–4.99, P = 0.035) but not hypertension (OR:1.23, 95% CI: 0.73–2.07, P = 0.44).
Reproductive/pregnancy outcomes
Four studies [64,65,66,67] examined the effect of wood smoke exposure on reproductive/pregnancy outcomes. Compared to users of clean fuels (LPG or electricity) exposure to wood smoke in charcoal and firewood was significantly associated with reduced birth weight [64] and low birth weight [64,66]. Use of biomass fuel (charcoal, firewood and shrubs) was reported to mediate 17.7% of the observed effect of low maternal education on lifetime experience of stillbirth among Ghanaian mothers [65]. Whitworth et al. [67] investigated the effect of wood smoke exposure in open wood fires on plasma concentrations of anti-mullerian hormone. In their report, cooking indoors over open wood fires (with reference to cooking with electricity) was not associated with lower plasma anti-mullerian hormone.
Cancer
Three studies investigated the association between wood smoke exposure and oesophageal cancer [68,69,70]. In all the studies, exposure to wood smoke was associated with increased risk of oesophageal cancer.
Other health outcomes
Owili et al. [39] reported significant association between charcoal use and all-cause under-five mortality in 23 countries in SSA. The effect of wood smoke exposure on non-syndrome cleft lip/cleft palate and sick-building syndrome were investigated by one study each. Indoor cooking with charcoal was associated with cleft lip or cleft palate [71]. Use of charcoal (with reference to non-use of charcoal) was also associated with sick-building syndrome [72]. Das et al. [73] investigated the effect of firewood (with reference to charcoal) on five categories of health outcomes (cardiopulmonary, respiratory, neurologic, eye health and burns). Compared to charcoal use, use of firewood was associated with higher odds of shortness of breath, difficulty in breathing, chest pains, night phlegm, forgetfulness, dizziness and dry irritated eyes.
With the exception of one study [67], other studies that made comparisons between wood fuels and electricity/LPG users [35,59,64,66], reported positive association between wood fuel use and adverse health effects.
All of the five studies that made comparison between firewood and charcoal use [53,57,61,70,73] reported positive associations between firewood use and health outcomes studied. The effect of indoor woods fuel use (Vs outdoor use) was evaluated in three studies [54,56,71]. While Sanya et al. [56] observed no association between in-house use of wood/charcoal and increased risk of asthma exacerbations, indoor cooking with wood fuels was associated with severe pneumonia [54] and non-syndromic cleft lip/cleft palate [71].
The effect of fuel switch on blood pressure was investigated in two studies [37,62]. In these studies, switch to clean fuels was not associated with significant changes in blood pressure.
Discussion
This systematic review summarized the reports of published studies assessing levels of exposure to pollutants in wood smoke and the disease outcomes associated with this exposure in population living in sub-Saharan Africa. We identified 16 studies that examined personal exposures to specific wood smoke pollutants or measured biomarkers of wood smoke exposure. However, only three of these studies [17,18,43] measured wood smoke biomarkers in body fluid. We also identified 33 studies that examined associations between exposure to wood smoke and health outcomes in this population. Given the high degree of variability in study designs, type of wood fuel used in households (firewood or charcoal, open fire or improved cook stoves), comparator fuels used (electricity, liquefied petroleum gas, charcoal), population groups examined, exposure settings (rural or urban; occupational or environmental) we did not carry out a meta-analysis of the results. The reported levels of exposure to some pollutants (PM10, PM2.5 and CO and benzo[a]pyrene) were compared with the World Health Organization’s Air Quality Guidelines (AQGs) for the specific pollutants. The overall assessment of evidence of causality between exposure to wood smoke and disease outcomes reported was based on the strength, consistency and plausibility of the associations.
Personal exposure levels to wood smoke
The levels of exposure to particulate matter (PM10 and PM2.5) from wood smoke as reported in reviewed studies indicate that the population in sub-Saharan Africa are exposed to very high levels of this pollutants in wood smoke. The reported personal PM10 and PM2.5 levels for wood users were all above the World Health Organisation’s Air Quality Guideline of 50 μg/m3 and 25 μg/m3 for 24-hour mean PM10 and PM2.5, respectively. Although wood users had higher levels of exposure to PM10 and PM2.5, the reported PM10 levels of 380 ± 94 μg/m3 and 200 ± 110 μg/m3 among electricity and LPG users, respectively35 and PM2.5 levels of 216 (2.2) μg/m3 among electricity users [44] were of concern, given that these fuels do not emit visible smoke. Although the mean carboxy-hemoglobin concentration of 8.5 ± 3.68% reported among non-charcoal producers in Nigeria [17] was significantly lower than that reported in charcoal producers, the value was still 400% higher than WHO [46], reference level of 2% for carboxy-hemoglobin. This could be expected, given that the comparator group in that study was drawn from the same community as the case group [17]. These reports generally suggest that the general population in sub-Saharan African may be significantly exposed to high background levels of ambient particulate matter and other pollutants in wood smoke or other emission sources. There may not be an “unexposed group” in this population, especially among the rural dwellers. However, because exposure to these pollutants lacks unique clinical symptoms, the magnitude and effects of life-long exposure to wood smoke pollutants through use of wood fuels in SSA may go unrecognised and under-reported.
From the reviewed studies, the reported sources of wood smoke exposure in sub-Saharan Africa were mostly through use of wood fuels in domestic cooking. Occupational exposure to wood smoke was reported in seven studies and included the use of wood fuels in bakery [44] commercial fish drying [20,50], cassava (garri) processing [21], street cooking [38] and charcoal production [17,18]. Generally, in SSA, more than 90% of households (especially those living in rural areas) rely on wood fuels as the only source of energy for all their cooking needs [10]. These fuels are burnt on characteristic unvented open fire stoves, emitting significant quantity of smoke containing health damaging pollutants into the immediate environment. Besides these reported sources, the population in SSA could be exposed to wood smoke through other sources [19,75,76,77]. However, the extent and health effects of exposure to wood smoke from these other sources remains to be studied in this population.
Health effects of wood smoke in sub-Saharan Africa
The health effects associated with wood smoke exposure in the reviewed studies included respiratory and non-respiratory diseases. We recorded strong and consistent associations between exposure to wood smoke and respiratory diseases such as ARI [33,34,51,53], ALRI [33,34,52,54], reduced lung function [20,21,38,57,58,59,60] and cough [35,50,61]. Wood smoke consists of more than 200 distinct organic compounds [2], many of which have been shown to induce acute or chronic health effects in exposed humans. Of these, fine particles are thought to be the best single indicator of the health impacts [2]. Although studies on health effects of respirable particles have focussed on those derived from fossil fuels, wood smoke particles are usually within the size range (0.02–2.5 μm), thought to be most damaging to human health [2]. PM2.5 from wood smoke can penetrate into the deep lung, producing a variety of morphological and biochemical changes [24] and resulting in a range of respiratory diseases. The inflammatory potential of particulate matter has been linked to chronic pulmonary diseases [78]. Based on in vivo toxicological studies, wood smoke may also affect pulmonary immune defence mechanisms, with the lung macrophages as a likely target [2].
Strong and consistent associations were recorded between wood smoke exposure and blood pressure [36,37,63]. In line with this observation, Dutta et al. [79] reported higher prevalence of hypertension and pre-hypertension among rural Indian women chronically exposed to biomass fuel during cooking. A number of observational studies have linked exposure biomass fuels to other cardiovascular disease outcomes, including stroke [80] and atherosclerosis [81]. In a group of Chinese women, use of biomass fuel for cooking resulted in a median indoor concentration of PM2.5 of 52 μg/m3 in summer and 105 μg/m3 during the winter and were associated with increases in systolic and diastolic blood pressure [82]. Although not fully understood, the mechanisms proposed to explain the cardiovascular effects of biomass exposure include systemic inflammation, particle-induced oxidative stress, endothelial damage, pro-coagulation and autonomic stimulation [83]. During the past four decades, the highest worldwide blood pressure levels have shifted from high-income countries to low-income countries in south Asia and sub-Saharan Africa [84]. Women in a few countries in sub-Saharan Africa (Niger, Guinea, Malawi, and Mozambique) had the highest levels of mean systolic blood pressure, surpassing 132 mm Hg [84]. However, the contribution of wood smoke exposure to the increasing incidence of high blood pressure and other cardiovascular diseases is yet to be investigated in this population.
Consistent association was also observed between wood smoke exposure and oesophageal cancer [68,69,70]. Reports from SSA [85,86] indicate increasing incidence of oesophageal cancer in this region. Most recently, there has been an increasing attention on the high incidence of oesophageal squamous cell carcinoma throughout the eastern corridor of Africa, extending from Ethiopia to South Africa [87] and a call for multisite investigations into its etiology and to identify targets for primary prevention.
Wood smoke contains several known carcinogens including benzo[a]pyrene and benzene [2]. These PAHs have been widely used as markers for carcinogenic risk levels in epidemiological studies [46,88]. The corresponding concentrations for lifetime exposure to B[a]P producing excess lifetime cancer risks of 1/10,000, 1/100,000 and 1/1,000,000 are approximately 1.2, 0.12 and 0.012 ng/m3, respectively [46]. Similarly, the concentrations of airborne benzene associated with an excess lifetime risk of 1/10,000, 1/100,000 and 1/1,000,000 are 17, 1.7 and 0.17 μg/m3, respectively [46]. The reported concentrations of Benzo(a)pyrene [43] and benzene [38] among wood fuel users may therefore have serious implications for cancer in this population. Our finding therefore, necessitates a closer look at exposure to wood smoke as an important etiologic factor, not only for oesophageal cancer, but for other prevalent cancers in this population.
Exposure to wood smoke was strongly associated with poor pregnancy outcomes [64,65,66]. This observation is in line with that of previous systematic reviews on solid fuel use [30], ambient air pollution [89] and secondhand smoke [90] and risk of adverse pregnancy outcomes. Contrary to these findings, exposure to wood smoke was not associated with elevated risk of intrauterine growth retardation (IUGR) in a population-based birth cohort in British Columbia, Canada [91]. It has been suggested that CO in wood smoke may exert its effects on fetal growth indirectly by reducing the oxygen-carrying capacity of maternal hemoglobin, which could adversely affect oxygen delivery to fetal circulation [92]. Because CO also crosses the placental barrier, the resultant fetal tissue hypoxia has the potential to reduce fetal growth [30]. Although highest in Asia, the burden of IUGR in Africa has been estimated at 20%, with the burden in developing countries rated six times higher than that in developed countries [93]. More studies are needed to understand the role of exposure to wood smoke in these and other adverse reproductive outcomes experienced by this population.
Positive associations were reported for sick building syndrome [72], and non-syndromic cleft lip and/or cleft palate [71]. In a large study involving 783,691 children living in 23 countries in SSA, use of charcoal as cooking fuel was significantly associated with the risk of under-five mortality [39]. However, the number of relevant studies on these outcomes is limited, and more information is needed to confirm these observations.
Determinants for higher exposure
Several risk factors for higher exposure to wood smoke were identified across the studies. It was demonstrated that young and adult women had higher exposures to wood smoke pollutants than their male counterparts. Although the study population in most of the studies were women and children under five years of age, the reports of the two studies [14,33,34] that examined personal exposure levels to pollutants in different population groups showed that women and girls are exposed to higher levels of these pollutants than men and boys of the same age range. Also, infants in the examined households were exposed to higher levels of these pollutants than men and boys and these exposures were linked to their mothers’ 24-h CO exposure in one study [14]. Household cooking is nearly universally done by women and girls, who often also are responsible for the care of young children. Many women in SSA carry their infants on their back while cooking, exposing the infant to smoke from cooking activities. These two groups generally have the highest exposure levels because they are always nearer the stove during combustion and spend longer time at home than the other age groups. Although exposure-response relationship was examined in only one study [33,34], women and infants who represent the population sub-group with higher exposure in households were reported to have higher risk of ARI compared to men [33,34,51].
Over the last two decades, use of improved wood and charcoal stoves have been introduced in many sub-Saharan African countries and there have been significant improvements in stove designs [94]. Although these are viewed as cost-effective strategy to reduce household air pollution through more efficient combustion of wood fuels and ventilation, the evidence on the effect of these improvements on exposure to pollutants has been mixed [95,96,97,98]. While none of the reviewed studies measured personal particulate matter exposure between traditional and improved wood stove users, the reports of two studies comparing personal CO exposure levels between traditional open firewood stove and improved stove users, are inconsistent [40,41]. While Yamamoto et al. [48] reported no significant difference in personal CO exposure between firewood and charcoal users, the levels of personal exposure to PM10 and PM2.5 in charcoal users [35,43] were lower than that reported for open firewood users, although this remained many folds higher than the WHO AQG for these pollutants. Comparing pollutant emissions in three improved stove types used in sub-Saharan Africa, Mitchell et al. [98] reported that charcoal stoves emitted up to 4 times less PM, but 6 times higher CO than dry wood stoves. These findings suggest that use of these improved wood stoves is still associated with significant exposure to these health damaging pollutants. However, studies that compared health effects between charcoal and unprocessed firewood users [53,57,61,70,73] suggest that use of charcoal (with reference to firewood) may be associated with lower risk of wood smoke-associated diseases.
The impact of fuel switch on personal CO exposure and blood pressure was investigated in two RCT studies [37,62]. The switched from firewood use to LPG use was associated with significant (more than 100%) reduction in personal CO levels among the women [37]. This is in line with the reports of other studies [99]. However, change in CO level did not translate to lowered blood pressure [37] and there was no significant change in blood pressure following switch from firewood use to ethanol [62].
It has been demonstrated that total switch from firewood to biogas as cooking fuel can reduce airborne emissions of PM2.5 and CO to levels below the WHO AQG [100], which is likely to produce health benefits associated with reduced exposure to particulate matter and CO in the long run. One major reason that clean fuel interventions have not recorded consistent positive impact on health in this population is that most households only partially convert to the use of these clean fuels and continue to use wood fuels for part of their cooking needs [100]. Also, because particles in ambient air readily penetrate inside residences, individuals that do not use wood fuels can be exposed to smoke from neighbouring households that use wood fuels. More studies are however, needed to ascertain the effect of fuel switch on exposure to wood smoke pollutants in SSA population.
Limitations of Study
Some limitations, however, were observed across the studies. One major limitation is the use of different comparators in the different studies which made adequate comparison of the results impossible. Another limitation observed in these studies was the use of questionnaire and interview approach for exposure assessment. Only four of the studies examined association between disease and measured exposure to wood smoke [33,34,35,36,37]. For the other studies, the approach used to characterize exposure to wood smoke was mainly questionnaires and interviews on household’s primary cooking fuel. This method of exposure assessment does not provide reliable estimates of individual exposure to wood smoke, as it does not give information on magnitude of exposure to wood smoke [101] and so may affect the estimated relationship between exposure and disease outcomes investigated, because the ‘unexposed’ may still be significantly exposed to wood smoke. However, the approach is simple; use of a particular fuel may be more stable over a year than a single measurement of personal exposure or area concentration [7]. Active personal monitors are effective in accurately monitoring personal exposures, but implementing personal monitoring on a large scale is expensive and almost impracticable in resource-poor settings such as those in SSA [101]. Measurements of wood smoke biomarkers (which represent the absorbed dose of pollutants, accounting for interindividual differences in absorption, ventilation, and personal behaviours that modify exposure) has several advantages over other exposure assessment techniques [101]. However, these biomarkers were measured in only three of the reviewed studies and these studies did not investigate health effects of these exposures. Furthermore, only a few studies had randomized controlled trial (n = 3) or case-control (n = 7) designs. The majority of the studies had cross-sectional designs in which it was not possible to ascertain a causal and temporal relationship between wood smoke exposure and the health outcomes. These major methodological drawbacks had obvious implications for the quality of the reported evidence. However, the generally consistent results from the different study designs indicate that exposure to wood smoke adversely affects the overall health of this population.
Recommendations
The findings of this review have important public health implications for people living in SSA. It should be noted that there is considerably high level of awareness of the health implications from the use of wood fuels among SSA women [102,103]. Despite this, these fuels have continued to be in use due to high cost of the alternatives, scarcity of refilling points for alternatives (especially in rural areas) and lack of capital [103]. Given these hindrances, it is likely that domestic use of wood fuels will become even more widespread in this population [12]. As a result, the magnitude of exposure to these wood smoke pollutants may remain the same at least in the near future, and more health effects are likely to be associated with wood smoke exposure. Strategies and interventions aimed at reducing smoke emissions should, therefore, be upregulated. There is need for more vigorous campaigns on the dangers of wood smoke and the need for switching to clean fuels. There is also need for adequate education on ways to reduce exposures to these emissions especially among women and those with occupational exposure to wood smoke. Furthermore, government and international agencies should help by subsidizing the cost of clean fuels and making them available in rural areas. These evidences are mostly from studies conducted in women and children. Further research on the effect of exposure to wood smoke pollutants in other population groups, especially the elderly, is urgently needed. Large epidemiological studies, especially prospective cohort and case-control studies with improved measures of exposure such as active personal monitors and specific wood smoke biomarkers are needed to adequately quantify exposures and estimate their effects on health in this population.
Conclusion
Available evidence suggests that the general population in sub-Saharan Africa are exposed to very high levels of pollutants in wood smoke from use of wood fuels such as firewood and charcoal for domestic cooking needs and this exposure is associated with a number of disease outcomes, especially respiratory diseases in women and children under five years of age. There is urgent need for effective strategies to reduce exposure in other to control the morbidity and mortality rate attributable to wood smoke exposure in this population.
Competing Interests
The authors have no competing interests to declare.
References
- 1.Toledo RT. Wood Smoke Components and Functional Properties. In: Kramer DE, Brown L (eds.), International Smoked Seafood Conference Proceedings Fairbanks: Alaska Sea Grant College Program; 2008: 55–61. DOI: 10.4027/isscp.2008.12 [DOI] [Google Scholar]
- 2.Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke Health Effects: A Review. Inhalation Toxicology. 2007; 19: 67–106. DOI: 10.1080/08958370600985875 [DOI] [PubMed] [Google Scholar]
- 3.Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bulletin of the World Health Organization. 2000; 78(9): 1078–1092. [PMC free article] [PubMed] [Google Scholar]
- 4.Sepp S. Multiple-Household Fuel Use – A balanced choice between firewood, charcoal and LPG Federal ministry of economic cooperation and development technical report. Deutsche Gasellschaft fur Internationale Zusammenrbeit (GIZ). GmbH; 2014: 49. [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations (FAO). Criteria and indicators for sustainable woodfuels Rome: FAO Forestry paper; 2010: 103 Available from: www.fao.org. [Google Scholar]
- 6.Nielsen E, Dybdahl M, Larsen PB. Health effects assessment of exposure to particles from wood smoke. Miljostyrelsen. Environmental project. 2008; No. 1235 Available from: http://orbit.dtu.dk.
- 7.Smith KR, Pillarisetti A. Household Air Pollution from Solid Cookfuels and Its Effects on Health In: Mock CN, Nugent R, Kobusingye O, Smith KR (eds.), Injury Prevention and Environmental Health. 3rd edition. Washington DC: The International Bank for Reconstruction and Development/The World Bank; 2017; 133–152. DOI: 10.1596/978-1-4648-0522-6_ch7 [DOI] [PubMed] [Google Scholar]
- 8.Smith KR, Bruce N, Balakrishnan K, et al. Millions Dead: How Do We Know and What Does It Mean? Methods Used in the Comparative Risk Assessment of Household Air Pollution. Annual Review of Public Health. 2014; 35: 185–206. DOI: 10.1146/annurev-publhealth-032013-182356 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). Burden of disease from household air pollution for 2016 – Summary Geneva: World Health Organization; 2018. Available from: www.who.int>data. [Google Scholar]
- 10.Falcao MP. Charcoal production and use in Mozambique, Malawi, Tanzania and Zambia: Historical overview, present situation and outlook. In: Kwaschik R (Ed). Proceedings on the conference on charcoal and communities in Africa Maputo; 2008. DOI: 10.13140/RG.2.1.1677.8729 [DOI] [Google Scholar]
- 11.Food and Agricultural Organisation. Incentivizing Sustainable Wood Energy in Sub-Saharan Africa: A Way Forward for Policy Food and Agriculture Organization of the United Nations; Viale Delle Terme Di Caracalla 00153 Rome, Italy: 2017; Available from: www.fao.org. [Google Scholar]
- 12.International Energy Agency (IEA). World energy outlook 2006 France; 2006: 431 Available from: http://www.iea.org. [Google Scholar]
- 13.Aboubacar B, Deyi X, Razak MYA, Leyla BH. The Effect of PM2.5 from Household Combustion on Life Expectancy in Sub-Saharan Africa. Int J Environ Res Public Health. 2018; 15(4): E748 DOI: 10.3390/ijerph15040748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okello G, Devereux G, Semple S. Women and girls in resource-poor countries experience much greater exposure to household air pollutants than men: Results from Uganda and Ethiopia. Environ Int. 2018; 119: 429–437. DOI: 10.1016/j.envint.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neitzel R, Naeher LP, Paulsen M, Dunn K, Stock A, Simpson CD. Biological monitoring of smoke exposure among wildland firefighters: A pilot study comparing urinary methoxyphenols with personal exposures to carbon monoxide, particular matter, and levoglucosan. Journal of Exposure Science and Environmental Epidemiology. 2009; 19: 349–358. DOI: 10.1038/jes.2008.21 [DOI] [PubMed] [Google Scholar]
- 16.Kato M, Loomis D, Brooks LM, et al. Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiol Biomarkers Prev. 2004; 13(6): 1005–1012. [PubMed] [Google Scholar]
- 17.Olujimi OO, Ana GR, Ogunseye OO, Fabunmi VT. Air quality index from charcoal production sites, carboxyheamoglobin and lung function among occupationally exposed charcoal workers in South Western Nigeria. Springerplus. 2016; 5(1): 1546 DOI: 10.1186/s40064-016-3227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olujimi OO, Ogunseye OO, Oladiran KO, Ajakore SD. Preliminary Investigation into Urinary 1-Hydroxypyrene as a Biomarker for Polycyclic Aromatic Hydrocarbons exposure among Charcoal Workers in Ogun and Oyo States, Nigeria. Saf Health Work. 2018; 9(4): 416–420. DOI: 10.1016/j.shaw.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adewole OO, Desalu OO, Nwogu KC, Adewole TO, Erhabor GE. Respiratory symptoms and lung function patterns in workers exposed to wood smoke and cooking oil fumes (mai suya) in Nigeria. Ann Med Health Sci Res. 2013; 3(1): 38–42. DOI: 10.4103/2141-9248.109475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umoh V, Peters E. The relationship between lung function and indoor air pollution among rural women in the Niger Delta region of Nigeria. Lung India. 2015; 32(2): 199 DOI: 10.4103/0970-2113.152672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okwor TJ, Ozoh OB, Okonkwo IJ, Osibogun A. Occupational exposure to particulate matter from biomass smoke and its relationship to respiratory symptoms and pulmonary function among rural women involved in cassava processing in Nigeria. Open Journal of Preventive Medicine. 2017; 7: 41–54. DOI: 10.4236/ojpm.2017.73004 [DOI] [Google Scholar]
- 22.Jira W. Polycyclic aromatic hydrocarbons in German smoked meat products. European Food Research and Technology. 2010; 230(3): 447–455. DOI: 10.1007/s00217-009-1187-9 [DOI] [Google Scholar]
- 23.Silva BO, Adetunde OT, Oluseyi TO, Olayinka KO, Alo BI. Effects of the methods of smoking on the levels of polycyclic aromatic hydrocarbons PAHs in some locally consumed fishes in Nigeria. African Journal of Food Science. 2011; 5(7): 384–391. [Google Scholar]
- 24.Zelikoff JT, Chen LC, Cohen MD, Schlesinger RB. The toxicology of inhaled woodsmoke. Journal of Toxicology and Environmental Health. 2002; 5: 269–282. DOI: 10.1080/10937400290070062 [DOI] [PubMed] [Google Scholar]
- 25.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Hohani H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012; 380(9859): 2224–2260. DOI: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miner RA, Abt RC, Bowyer JL, et al. Forest carbon accounting considerations in US bioenergy policy. Journal of Forestry. 2014; 112(6): 591–606. [Google Scholar]
- 27.GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390: 1345–422. DOI: 10.1016/S0140-6736(17)32366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health Effect Institute. State of Global Air/2019: A special report on global exposure to air pollution and its disease burden Boston, MA: Health Effect Institute; 2019. Available online at: https://www.stateofglobalair.org/sites/default/files/soga_2019_report.pdf. [Google Scholar]
- 29.Institute for Health Metrics and Evaluation (IHME). GBD Compare Seattle, WA: IHME; University of Washington; 2018. Available online at: http://vizhub.healthdata.org/gbd-compare. [Google Scholar]
- 30.Amegah AK, Quansah R, Jaakkola JJK. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: A systematic review and meta-analysis of the empirical evidence. PLoS ONE. 2014; 9(12): e113920 DOI: 10.1371/journal.pone.0113920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayamba V, Heimburger DC, Morgan DR, Atadzhanov M, Kelly P. Exposure to biomass smoke as a risk factor for oesophageal and gastric cancer in low-income populations: A systematic review. Malawi Med J. 2017; 29(2): 212–217. DOI: 10.4314/mmj.v29i2.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sana A, Somda SMA, Meda N, Bouland C. Chronic obstructive pulmonary disease associated with biomass fuel use in women: A systematic review and meta-analysis. BMJ Open Resp Res. 2018; 5: e000246 DOI: 10.1136/bmjresp-2017-000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: An exposure-response study. Lancet. 2001; 358: 619–624. DOI: 10.1016/S0140-6736(01)05777-4 [DOI] [PubMed] [Google Scholar]
- 34.Ezzati M, Kammen DM. Indoor air pollution from biomass stoves as a risk factor for acute respiratory infections in Kenya. Proceedings: Indoor Air. 2002: 970–975. [Google Scholar]
- 35.Ellegård A. Cooking fuel smoke and respiratory symptoms among women in low-income areas in Maputo. Environ Health Perspect. 1996; 104(9): 980–985. DOI: 10.1289/ehp.96104980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn AK, Ae-Ngibise KA, Kinney PL, Kaali S, Wylie BJ, Boamah E, Shimbo D, Agyei O, Chillrud SN, Mujtaba M, Schwartz JE, Abdalla M, Owusu-Agyei S, Jack DW, Asante KP. Ambulatory monitoring demonstrates an acute association between cookstove-related carbon monoxide and blood pressure in a Ghanaian cohort. Environ Health. 2017; 16(1): 76 DOI: 10.1186/s12940-017-0282-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn AK, Ae-Ngibise KA, Jack DW, et al. Association of Carbon Monoxide exposure with blood pressure among pregnant women in rural Ghana: Evidence from GRAPHS. Int J Hyg Environ Health. 2016; 219(2): 176–83. DOI: 10.1016/j.ijheh.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awopeju OF, Nemery B, Afolabi OT, et al. Biomass smoke exposure as an occupational risk: Cross-sectional study of respiratory health of women working as street cooks in Nigeria. Occup Environ Med. 2017; 0: 1–8. DOI: 10.1136/oemed-2016-104107 [DOI] [PubMed] [Google Scholar]
- 39.Owili PO, Muga MA, Pan WC, Kuo HW. Cooking fuel and risk of under-five mortality in 23 sub-Saharan African countries: A population-based study. Int J Environ Health Res. 2017; 27(3): 191–204. DOI: 10.1080/09603123.2017.1332347 [DOI] [PubMed] [Google Scholar]
- 40.Ochieng CA, Vardoulakis S, Tonne C. Are rocket mud stoves associated with lower indoor carbon monoxide and personal exposure in rural Kenya? Indoor Air. 2013; 23(1): 14–24. DOI: 10.1111/j.1600-0668.2012.00786.x [DOI] [PubMed] [Google Scholar]
- 41.Yip F, Christensen B, Sircar K, et al. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ Int. 2017; 99: 185–191. DOI: 10.1016/j.envint.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization (WHO). WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide – Global update 2005 Summary of risk assessment Geneva: WHO Press; 2006: 9 Available from: http://apps.who.int>bitstream. [Google Scholar]
- 43.Titcombe ME, Simcik M. Personal and indoor exposure to PM2.5 and polycyclic aromatic hydrocarbons in the southern highlands of Tanzania: a pilot-scale study. Environ Monit Assess. 2011; 180(1–4): 461–76. DOI: 10.1007/s10661-010-1799-3 [DOI] [PubMed] [Google Scholar]
- 44.Downward GS, van der Zwaag HP, Simons L, et al. Occupational exposure to indoor air pollution among bakery workers in Ethiopia: A comparison of electric and biomass cookstoves. Environ Pollut. 2018; 233; 690–697. DOI: 10.1016/j.envpol.2017.10.094 [DOI] [PubMed] [Google Scholar]
- 45.Van Vliet ED, Asante K, Jack DW, et al. Personal exposures to fine particulate matter and black carbon in households cooking with biomass fuels in rural Ghana. Environ Res. 2013; 127: 40–48. DOI: 10.1016/j.envres.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization (WHO). WHO guidelines for indoor air quality: Selected pollutants. WHO Press, Copenhagen, Denmark. 2010: 38(6): 323 Available from: www.euro.who.int [PubMed] [Google Scholar]
- 47.Dionisio KL, Howie SR, Dominici F, et al. The exposure of infants and children to carbon monoxide from biomass fuels in The Gambia: a measurement and modelling study. J Expo Sci Environ Epidemiol. 2012; 22(2): 173–81. DOI: 10.1038/jes.2011.47 [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto SS, Louis VR, Sié A, Sauerborn R. Biomass smoke in Burkina Faso: What is the relationship between particulate matter, carbon monoxide, and kitchen characteristics? Environ Sci Pollut Res Int. 2014; 21(4): 2581–2591. DOI: 10.1007/s11356-013-2062-6 [DOI] [PubMed] [Google Scholar]
- 49.Dionisio KL, Howie SR, Dominici F, et al. Household concentrations and exposure of children to particulate matter from biomass fuels in The Gambia. Environ Sci Technol. 2012; 46(6): 3519–3527. DOI: 10.1021/es203047e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dienye P, Akani A, Okokon I. Respiratory effects of biomass fuel combustion on rural fish smokers in a Nigerian fishing settlement: A case-control study. Afr Health Sci. 2016; 16(2): 516–523. DOI: 10.4314/ahs.v16i2.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilabuko JH, Matsuki H, Nakai S. Air quality and acute respiratory illness in biomass fuel using homes in Bagamoyo, Tanzania. Int J Environ Res Public Health. 2007; 4(1): 39–44. DOI: 10.3390/ijerph2007010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ekaru H, Mbarak N, Shurie S, Kosgei E, Oyungu E, Kwena A. Community acquired pneumonia among children admitted in a tertiary hospital: The burden and related factors. East Afr Med J. 2012; 89(9): 301–305. [PubMed] [Google Scholar]
- 53.Taylor ET, Nakai S. Prevalence of acute respiratory infections in women and children in Western Sierra Leone due to smoke from wood and charcoal stoves. Int J Environ Res Public Health. 2012; 9(6): 2252–2265. DOI: 10.3390/ijerph9062252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.PrayGod G, Mukerebe C, Magawa R, Jeremiah K, Torok ME. Indoor air pollution and delayed measles vaccination increase the risk of severe pneumonia in children: Results from a case-control study in Mwanza, Tanzania. PLoS One. 2016; 11(8): e0160804 DOI: 10.1371/journal.pone.0160804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tazinya AA, Halle-Ekane GE, Mbuagbaw LT, Abanda M, Atashili J, Obama MT. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm Med. 2018; 18(1): 7 DOI: 10.1186/s12890-018-0579-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanya RE, Kirenga BJ, Worodria W, Okot-Nwang M. Risk factors for asthma exacerbation in patients presenting to an emergency unit of a national referral hospital in Kampala, Uganda. Afr Health Sci. 2014; 14(3): 707–15. DOI: 10.4314/ahs.v14i3.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fullerton DG, Suseno A, Semple S, et al. Wood smoke exposure, poverty and impaired lung function in Malawian adults. Int J Tuberc Lung Dis. 2011; 15(3): 391–398. [PubMed] [Google Scholar]
- 58.Oloyede IP, Ekrikpo UE, Ekanem EE. Lung function indices of children exposed to wood smoke in a fishing port in South-South Nigeria. J Trop Pediatr. 2013; 59(5): 399–402. DOI: 10.1093/tropej/fmt033 [DOI] [PubMed] [Google Scholar]
- 59.Ibhafidon LI, Obaseki DO, Erhabor GE, Akor AA, Irabor I, Obioh I. Respiratory symptoms, lung function and particulate matter pollution in residential indoor environment in Ile-Ife, Nigeria. Niger Med J. 2014; 55(1): 48–53. DOI: 10.4103/0300-1652.128164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mbatchou Ngahane BH, Afane Ze E, Chebu C, et al. Effects of cooking fuel smoke on respiratory symptoms and lung function in semi-rural women in Cameroon. Int J Occup Environ Health. 2015; 21(1): 61–65. DOI: 10.1179/2049396714Y.0000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.North CM, Valeri L, Hunt PW, et al. Cooking fuel and respiratory symptoms among people living with HIV in rural Uganda. ERJ Open Res. 2017; 3(2): 00094-2016. DOI: 10.1183/23120541.00094-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexander D, Northcross A, Wilson N, et al. Randomized controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. American Journal of Respiratory and Critical Care Medicine. 2017; 195(12): 1629–1639. DOI: 10.1164/rccm.201606-1177OC [DOI] [PubMed] [Google Scholar]
- 63.Ofori SN, Fobil JN, Odia OJ. Household biomass fuel use, blood pressure and carotid intima media thickness: A cross-sectional study of rural dwelling women in Southern Nigeria. Environ Pollut. 2018; 242: 390–397. DOI: 10.1016/j.envpol.2018.06.102 [DOI] [PubMed] [Google Scholar]
- 64.Amegah AK, Jaakkola JJ, Quansah R, Norgbe GK, Dzodzomenyo M. Cooking fuel choices and garbage burning practices as determinants of birthweight: A cross-sectional study in Accra, Ghana. Environ Health. 2012; 11: 78 DOI: 10.1186/1476-069X-11-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amegah AK, Näyhä S, Jaakkola JJ. Do biomass fuel use and consumption of unsafe water mediate educational inequalities in stillbirth risk? An analysis of the 2007 Ghana Maternal Health Survey. BMJ Open. 2017; 7(2): e012348 DOI: 10.1136/bmjopen-2016-012348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demelash H, Motbainor A, Nigatu D, Gashaw K, Melese A. Risk factors for low birth weight in Bale zone hospitals, South-East Ethiopia: A case-control study. BMC Pregnancy Childbirth. 2015; 15: 264 DOI: 10.1186/s12884-015-0677-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitworth KW, Baird DD, Steiner AZ, et al. Anti-Müllerian hormone and lifestyle, reproductive, and environmental factors among women in rural South Africa. Epidemiology. 2015; 26(3): 429–35. DOI: 10.1097/EDE.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal Cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol. 2013; 2013: 503249 DOI: 10.1155/2013/503249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kayamba V, Bateman AC, Asombang AW, et al. HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: A case-control study. Cancer Med. 2015; 4(4): 588–95. DOI: 10.1002/cam4.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mlombe YB, Rosenberg NE, Wolf LL, et al. Environmental risk factors for oesophageal cancer in Malawi: A case-control study. Malawi Med J. 2015; 27(3): 88–92. DOI: 10.4314/mmj.v27i3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mbuyi-Musanzayi S, Kayembe TJ, Kashal MK, et al. Non-syndromic cleft lip and/or cleft palate: Epidemiology and risk factors in Lubumbashi DR Congo, a case-control study. J Craniomaxillofac Surg. 2018; 46(7): 1051–1058. DOI: 10.1016/j.jcms.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 72.Belachew H, Assefa Y, Guyasa G, et al. Sick building syndrome and associated risk factors among the population of Gondar town, northwest Ethiopia. Environ Health Prev Med. 2018; 23(1): 54 DOI: 10.1186/s12199-018-0745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das I, Jagger P, Yeatts K. Biomass cooking fuels and health outcomes for women in Malawi. Ecohealth. 2017; 14(1): 7–19. DOI: 10.1007/s10393-016-1190-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibhazehiebo K, Dimkpa U, Uche OK, Iyawe VI. Peak expiratory flow rate and respiratory symptoms following chronic domestic wood smoke exposure in women in Edo, Nigeria. African Journal of Biomedical Research. 2007; 10: 33–39. DOI: 10.4314/ajbr.v10i1.48920 [DOI] [Google Scholar]
- 75.Isichei AO, Muoghalu JI, Akeredolu FA, Afolabi OA. Fuel characteristics and emissions from biomass burning and land-use change in Nigeria. Environ Monit Assess. 1995; 38(2–3): 279–289. DOI: 10.1007/BF00546769 [DOI] [PubMed] [Google Scholar]
- 76.Igwe JC, Odo EO, Okereke SE, Asuqou EE, Nnorom IC, Okpareke OC. Levels of polycyclic aromatic hydrocarbons (PAHs) in some fish samples from Mushin Area of Lagos, Nigeria: Effects of smoking. Terrestrial and Aquatic Environmental Toxicology. 2012; 6(1): 30–35. [Google Scholar]
- 77.Tongo I, Ogbeide O, Ezemonye L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicology Reports. 2016; 4: 55–61. DOI: 10.1016/j.toxrep.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolling AK, Pagels J, Yttri KE, et al. Health effects of residential wood smoke particles: The importance of combustion conditions and physicochemical particle properties. Particle and Fibre Toxicology. 2009; 6: 29 DOI: 10.1186/1743-8977-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dutta A, Mukherjee B, Das D, Banerjee A, Ray MR. Hypertension with elevated levels of oxidized low-density lipoprotein and anticardiolipin antibody in the circulation of premenopausal Indian women chronically exposed to biomass smoke during cooking. Indoor Air. 2011; 21(2): 165e176 DOI: 10.1111/j.1600-0668.2010.00694.x [DOI] [PubMed] [Google Scholar]
- 80.Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: A cross-sectional analysis of the Shanghai Putuo study. Environ Health. 2012; 11: 18 DOI: 10.1186/1476-069X-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Painschab MS, Davila-Roman VG, Gilman RH, et al. Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaques. Heart. 2013; 99: 984–991. DOI: 10.1136/heartjnl-2012-303440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baumgartner J, Schauer JJ, Ezzati M, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011; 119: 1390–1395. DOI: 10.1289/ehp.1003371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brook RD, Rajagopalan S, Pope CA, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, Council on Nutrition and Physical Activity and Metabolism. Circulation. 2010; 121: 2331–2378. DOI: 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- 84.Risk Factor NCD Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017; 389: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tettey M, Edwin F, Aniteye E, et al. The changing epidemiology of esophageal cancer in sub-Saharan Africa – The case of Ghana. Pan African Medical Journal. 2012; 13(6). [PMC free article] [PubMed] [Google Scholar]
- 86.Sewram V, Sitas F, O’Connell D, Myers J. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol. 2016; 41: 13–121. DOI: 10.1016/j.canep.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 87.Loon KV, Mwachiro MM, Abnet CC, et al. The African esophageal cancer consortium: A call to action. Journal of Global Oncology. 2018; 4: 1–9. DOI: 10.1200/JGO.17.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding J, Zhong J, Yang Y, et al. Occurrence and exposure to polycyclic aromatic hydrocarbons and their derivatives in a rural Chinese home through biomass fuelled cooking. Environmental Pollution. 2012; 169: 160–166. DOI: 10.1016/j.envpol.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012; 117: 100–111. DOI: 10.1016/j.envres.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 90.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: A meta-analysis. Pediatr. 2011; 127(4): 734–741. DOI: 10.1542/peds.2010-3041 [DOI] [PubMed] [Google Scholar]
- 91.Brauer M, Lencar C, Tamburic L, et al. The impact of woodsmoke, point sources and traffic-related air pollution on intrauterine growth retardation (IUGR). Epidemiology. 2007; 18(5): s197 DOI: 10.1097/01.ede.0000289016.50964.35 [DOI] [Google Scholar]
- 92.Salam MT, Millstein J, Li YF, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: Results from the Children’s Health Study. Environ Health Perspect. 2005; 113(11): 1638–1644. DOI: 10.1289/ehp.8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saleem T, Sajjad N, Fatima S, Habib N, Ali SR, Qadir M. Intrauterine growth retardation – Small events, big consequences. Italian Journal of Pediatrics. 2011; 37: 41 DOI: 10.1186/1824-7288-37-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ayo SA. Design, construction and testing of an improved wood stove. Assumption University Journal of Technology. 2009; 13(1): 12–18. [Google Scholar]
- 95.Thomas E, Wickramasinghe K, Mendis S, Roberts N, Foster C. Improved stove interventions to reduce household air pollution in low- and middle-income countries: a descriptive systematic review. BMC Public Health. 2015; 15(1): 650 DOI: 10.1186/s12889-015-2024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chartier R, Phillips M, Mosquin P, et al. A comparative study of human exposures to household air pollution from commonly used cookstoves in Sri Lanka. Indoor Air. 2017; 27: 147–159. DOI: 10.1111/ina.12281 [DOI] [PubMed] [Google Scholar]
- 97.Mortimer K, Ndamala CB, Naunje AW, et al. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): A cluster randomised controlled trial. The Lancet. 2017; 389: 167–175. DOI: 10.1016/S0140-6736(16)32507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell EJS, Ting Y, Allan J, et al. Pollutant emissions from improved cookstoves of the type used in sub-Saharan Africa. Combustion Science and Technology. 2019; 0: 1–22. DOI: 10.1080/00102202.2019.1614922 [DOI] [Google Scholar]
- 99.Fandiño-Del-Rio M, Goodman D, Kephart JL, et al. Cardiopulmonary outcomes and Household Air Pollution trial (CHAP) Trial Investigators. Effects of a liquefied petroleum gas stove intervention on pollutant exposure and adult cardiopulmonary outcomes (CHAP): Study protocol for a randomized controlled trial. Trials. 2017; 18: 518 DOI: 10.1186/s13063-017-2179-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tumwesige V, Okello G, Semple S, Smith J. Impact of partial fuel switch on household air pollutants in sub-Saharan Africa. Environ Pollut. 2017; 231: 1021–1029. DOI: 10.1016/j.envpol.2017.08.118 [DOI] [PubMed] [Google Scholar]
- 101.Simpson CD, Naeher LP. Biological monitoring of wood-smoke exposure. Inhalation Toxicology. 2010; 22(02): 99–103. DOI: 10.3109/08958370903008862 [DOI] [PubMed] [Google Scholar]
- 102.Afolabi OT, Awopeju OF, Aluko OO, et al. Awareness of indoor air pollution and prevalence of respiratory symptoms in an urban community in South West Nigeria. Nigeria Journal of Health Sciences. 2016; 16: 33–38. DOI: 10.4103/1596-4078.190036 [DOI] [Google Scholar]
- 103.Orifah MO, Ijeoma MC, Ehien AE, Nasiru A, Fadairo OS. Awareness of the health implications of use of biomass energy sources among women in rural households of Jigawa State, Nigeria. Agricultura Tropica Et Subtropica. 2018; 51(2): 93–101. DOI: 10.1515/ats-2018-0010 [DOI] [Google Scholar]


