Abstract
BACKGROUND
Biological aging pathways accelerated by cancer treatments may be a mechanism for cognitive impairment in cancer survivors. The goal of the present study was to examine whether indicators of biological aging, namely elevated levels of DNA damage, reduced telomerase enzymatic activity, and shorter peripheral blood mononuclear cell (PBMC) telomere length (TL) would be related to cognitive function in a cohort of breast cancer survivors.
METHODS
We evaluated a cross-sectional sample of 94 women aged 36–69 treated for early-stage breast cancer 3–6 years earlier. Leukocyte DNA damage, PBMC telomerase enzymatic activity, PBMC TL, and the inflammatory marker sTNF-RII were determined from blood samples. Cognitive function was assessed by a neuropsychological test battery and self-report. Linear regression models examined the relationship of biological aging predictors with cognitive outcomes.
RESULTS
Both higher DNA damage and lower telomerase were statistically significantly related to lower executive function scores, adjusting for age, BMI, race, years from treatment, and intelligence score (Standardized Coefficients [B] = −0.23 & 0.30, P’s < 0.05). In addition, lower telomerase activity was associated with worse attention and motor speed (B’s = 0.30 & 0.24, P < 0.05). sTNF-RII and TL were unrelated to any of the neurocognitive domains.
CONCLUSIONS
These results suggest a significant association between measures of biological aging and objective measures of cognitive performance in breast cancer survivors. Future prospective studies are needed to confirm a causal role of biological aging as a driver of declines in cognitive function after cancer treatment.
Keywords: breast cancer, survivors, cognition, biological aging, executive function, DNA damage, telomerase
Condensed abstract
In women treated for early-stage breast cancer on average over 4 years earlier, both high DNA damage and low telomerase activity were related to worse executive function, high DNA damage was associated with worse memory, and low telomerase activity was associated with worse attention and motor speed. These findings provide initial evidence for a link between markers of biological aging and cognitive performance in breast cancer survivors.
Introduction
Breast cancer is the most common cancer in women, with over two-hundred and sixty thousand new cases expected in the United States in 2018.1 There are estimated to be more than 3 million breast cancer survivors in the US due to substantial advances in detection and treatment.1,2 However, treatments also increase risk for long-term and late toxicities, including persistent fatigue, pain, and cognitive dysfunction. Further research is needed to better understand the factors that contribute to these adverse secondary health outcomes.3–6
In this paper, we focus on cognitive dysfunction in breast cancer survivors and its association with processes that are part of biological aging, paralleling the aging related phenotype seen in some cancer survivors.7,8 Cancer treatments, particularly radiation and some chemotherapeutic agents, work by damaging the DNA of cancer cells, preventing cell replication and causing cell death. However, these same treatments can induce damage to DNA of normal cells,9,10 causing acute elevations in inflammation,5,11–13 increasing expression of a marker of cellular senescence, p16INK4,14 and having detectable effects on telomerase activity and DNA damage years later;15 all factors that contribute to accelerated biological aging.14,16–24 However, few studies have examined whether biological aging plays a role in cognitive dysfunction in cancer patients and survivors.
Conroy and colleagues reported that among breast cancer survivors 3–10 years since treatment, elevated DNA damage was associated with reduced gray matter density particularly in regions showing compromise, suggesting this marker could relate to cognitive function in cancer survivors many years after completion of treatment.9 More research has focused on associating various markers of inflammation with cognitive dysfunction in cancer survivors.13,25–28 The extant literature supports a role of biological aging and inflammation in cancer-related cognitive difficulties, but substantial gaps remain. Despite some initial investigations of the relationship in aging populations,29–32 no one has yet examined whether cellular markers of biological aging correlate with objective and subjective cognitive function in cancer survivors. The goal of the present study was to test the hypothesis that cellular markers of biological aging and inflammation are related to worse subjective cognitive impairment and objective neuropsychological function in breast cancer survivors.
Methods
Participants
Participants for the current study were recruited from the UCLA Mind Body Study (MBS), a longitudinal, prospective cohort study of women with early stage breast cancer enrolled after the end of primary treatment and prior to initiating adjuvant endocrine therapy if indicated.33–35 Full details of the design, eligibility/exclusion, recruitment and procedures used in the MBS are described elsewhere, 33–37 and are summarized here. The MBS was designed to assess cognitive changes due to breast cancer endocrine therapy. Study eligibility for MBS required that women were between the ages of 18 and 65, had received a diagnosis of stage 0-IIIA breast cancer, and were fluent in English. Women were excluded from participation if they had any immune-related conditions such as autoimmune disease, evidence of uncontrolled depression, or a neurological condition.
MBS participants underwent comprehensive neurocognitive assessments as well as blood specimen collection at study enrollment, and 6 and 12 months later. Immediately after the 12-month MBS study visit, participants were invited to join a long-term follow-up study that included annual questionnaires that assessed cognitive complaints and other behavioral symptoms on an annual basis. After several years of follow-up, these participants were invited for an in-person assessment that occurred between 3–6 years after initial study enrollment, the timepoint of the current analyses. Of the 190 women in the original MBS cohort, 170 agreed to participate in the follow-up study and 94 of these women ultimately attended an in-person visit that replicated the initial neuropsychological assessments and blood specimen collection for inflammatory markers, as well as the assessment of telomerase, DNA damage, and telomere length. English was not the first language of one participant, and we excluded her neuropsychological data from analyses, resulting in a sample of n=93 for models of neuropsychological domains only, otherwise n=94. All procedures were approved by the University of California, Los Angeles institutional review board, and all participants provided informed consent.
Subjective Cognitive Function
Subjective cognitive function was measured with The Functional Assessment of Cancer Therapy: Cognitive Function (FACT-Cog) version 3,38 a valid and reliable self-report instrument, of cancer-related cognitive difficulties. The FACT-Cog subscale measuring Perceived Cognitive Impairment (range 0–72) was selected as the main outcome as is recommended by the scale’s developers, with psychometric properties and scoring guidelines available from the Functional Assessment of Cancer Therapy website: http://www.facit.org/.
Neuropsychological Testing
All participants were administered a comprehensive neuropsychological battery. Raw test scores were population normalized and transformed into z-scores and averaged to produce the following 6 key domain scores: Learning, Memory, Attention, Visuospatial, Executive Function, and Motor Speed. Additional details on specific tests used in the assessments of each domain can be found in Table 1. Analyses examining cognitive function adjust for age and premorbid estimates of IQ determined by Wechsler Test of Adult Reading (WTAR) scores.39
Table 1.
Neuropsychological tests administered to patients in the Mind-Body Study.
| Domain | Test/Measure |
|---|---|
| Learning | CVLT-II List A Total Trials 1–5 WMS-IV LM I BVMT-R Total Trials 1–3 |
| Memory | CVLT-II List A Long Delay Free Recall WMS-IV LM II BVMT-R Delayed Recall ROCFT 3-minute Delayed Recall |
| Attention | WAIS-IV Digit Span, Coding, Letter-Number Sequencing, and Symbol Search TMT A PASAT |
| Visuospatial | ROCFT copy WAIS-IV Block Design |
| Executive Functioning | TMT B Verbal Fluency40,41 |
| Motor Speed | Grooved Pegboard42 |
CVLT-II = California Verbal Learning Test-II43; WMS-IV LM = Wechsler Memory Scale, 4th edition, Logical Memory44; BVMT-R = Brief Visuospatial Memory Test, Revised45; ROCFT= Rey-Osterrieth Complex Figure test46; WAIS-IV = Wechsler Adult Intelligence Scale – 4th edition47; PASAT = Paced Auditory Serial Attention Test48; TMT = Trail Making Test49
Telomerase
To determine telomerase activity the telomere repeat amplification protocol (TRAP) was performed as previously described.15 Values are expressed as total telomerase product generated (TPG) per 10,000 cells.
DNA Damage
DNA damage was determined using the comet assay as reported in Singh et al.50 with minor modifications51, and has been previously described.15 The comet assay is a single cell gel electrophoresis assay that assesses the extent of DNA damage in nucleated white blood cells (WBC) by applying a computerized scoring algorithm. The extent of DNA damage is estimated from approximately 100 comets per sample analyzed by CASP software (CASP, Wroclaw, Poland)52 and values are expressed as a mean score from 0–4 (maximum damage/large tail).53
Telomere Length
Telomere length was determined using real-time quantitative polymerase chain reaction (qPCR) methodology, as described in previously published telomere length protocols,54–56 and in the previous study.15 Genomic DNA was extracted from peripheral blood mononuclear cells (PBMC). The telomere inter- and intra-assay coefficient of variation (CV) were all < 5%. Using the standard curve method value were determined for telomere DNA repeat sequences (T) and the HGB single-copy gene (S); Telomere length values are expressed as the T/S ratio.
Circulating Inflammatory Marker: Soluble TNF receptor II (sTNF-RII)
Previously, we reported sTNF-RII to be elevated after treatment, to be associated with cognitive complaints, and to be higher in those with low telomerase activity and high DNA damage.13,15,34 Thus we sought to determine the association of sTNF-RII with cognitive function in this follow up study several years later. To do this, blood samples were collected at the study visit by venipuncture into EDTA tubes, chilled, and centrifuged for the collection of plasma. Aliquots of plasma were then stored at −80 C until batch testing could be performed on all samples. Soluble tumor necrosis factor (TNF) receptor type II (sTNF-RII) was assessed using enzyme-linked immunosorbent assays (ELISAs; R&D Systems, Minneapolis, MN) per manufacturer’s protocol; lower limits of detection were 234 pg/mL. All samples were run in duplicate with an average intra-assay and inter-assay precision of < 5%.
Statistical Analyses
Analyses were conducted using IBM SPSS software (v.23). Descriptive statistics were calculated using the entire cohort (N=94). Distribution analyses revealed that DNA damage had modest skew and high kurtosis, such that the majority of individuals had low average damage scores. We created an upper quartile cutoff (≥.85) to indicate high damage, and compared this with the lowest 3 quartiles (<.85), indicating low damage. Decile ranking of the telomerase data was performed to address non-normal distribution of the data and these transformed values are used in statistical analyses, while figures display raw telomerase values.
Linear regression models with adjustments for age, BMI, race, and years from last treatment were performed to test the hypothesis that elevated levels of markers of biological aging namely higher DNA damage, reduced telomerase enzymatic activity, shorter peripheral blood mononuclear cell (PBMC) telomere length (TL), and higher soluble Tumor Necrosis Factor Receptor II (sTNF-RII) would be related to worse subjective cognitive impairment and objective neuropsychological function. Models testing predictors of neuropsychological test scores further adjust for WTAR. Linear regression models examined the relationship of predictors DNA damage (highest quartile/lower three quartiles), telomerase enzymatic activity (deciles), telomere length (T/S ratio), and sTNF-RII - with self-reported cognitive functioning and neuropsychological test domains (Betas (β) reflect unstandardized coefficients and standard error (SE), and B reflects standardized coefficients). To minimize the likelihood of false positive observations we corrected for multiple comparisons with the false discovery rate (FDR) procedure,57 setting the FDR rate at .05, and calculating the threshold for tests within each cognitive domain. We report uncorrected p-values, and the FDR-corrected q value threshold.
Results
Demographics and clinical characteristics of the study participants are reported in Table 2. Ages ranged from 36 to 69, with a mean of 56.5, with average time since treatment of 4.4 years. In initial models, as was reported previously15, we examined the association of demographic and clinical characteristics with cellular aging measures. Age was positively associated with DNA damage and inversely associated with telomerase activity, but was not significantly associated with telomere length. Higher BMI was related to shorter telomere length, consistent with previous reports58, but unrelated to DNA damage and telomerase activity.
Table 2.
Demographic characteristics of study participants
| Total N=94 | |
|---|---|
| Age, mean (SD) [range] | 56.5 (8.1) [36.4–69.5] |
| Race | |
| White, non-Hispanic | 75 (80%) |
| Hispanic | 8 (9%) |
| Black | 4 (4%) |
| Asian | 3 (3%) |
| Other | 4 (4%) |
| Body Mass Index, mean (SD) [range] | 25.7(5.1) [18–42.5] |
| Education | |
| Post college | 47 (50%) |
| College | 29 (31%) |
| No college degree | 18 (19%) |
| Years from treatment, mean (SD) [range] | 4.4 (0.6) [3–6.1] |
| Cancer Treatment Received | |
| Chemotherapy Alone | 11 (11.7%) |
| Radiation Alone | 28 (29.8%) |
| Both Chemotherapy and Radiation | 40 (42.6%) |
| Surgery Alone | 15 (16%) |
| Received Endocrine Therapy | 72% |
| Post-menopausal | 80.9% |
Self-Reported Cognitive Function
Neither DNA damage, telomerase activity, nor telomere length were related to FACT-Cog scores of Perceived Cognitive Impairment, adjusting for age, BMI, years from last treatment, and race, all P’s>.21. Likewise, sTNF-RII was not a significant predictor of self-reported cognitive impairments (data not shown).
Neuropsychological Domains
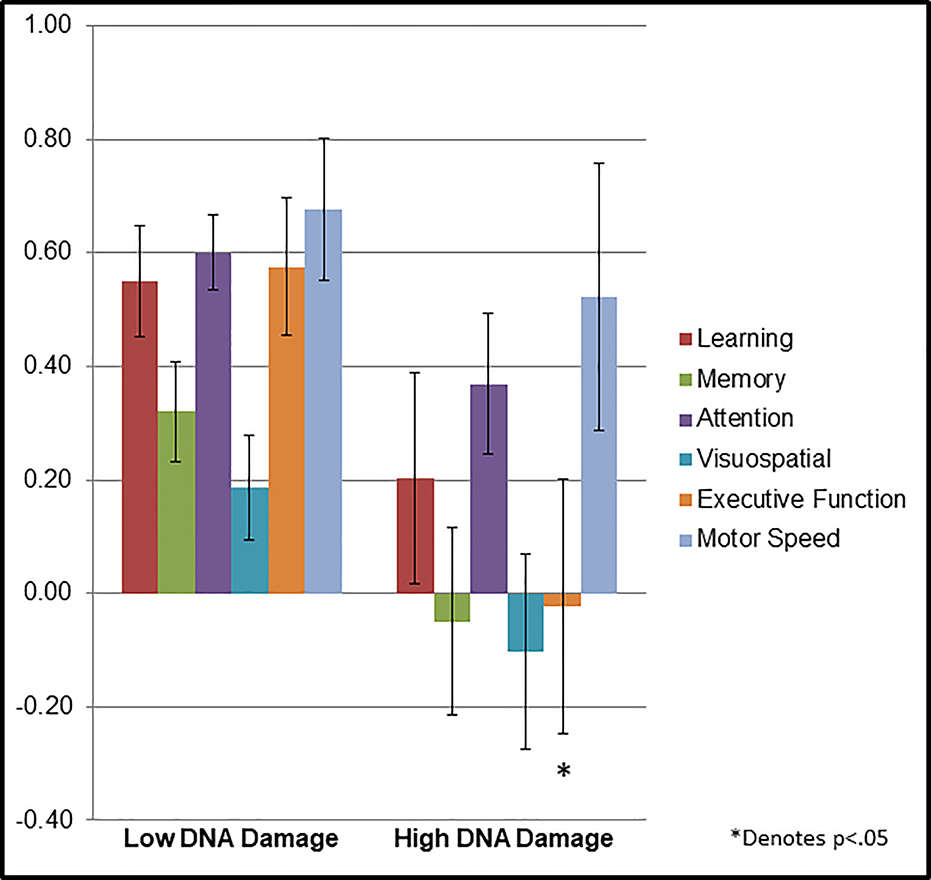
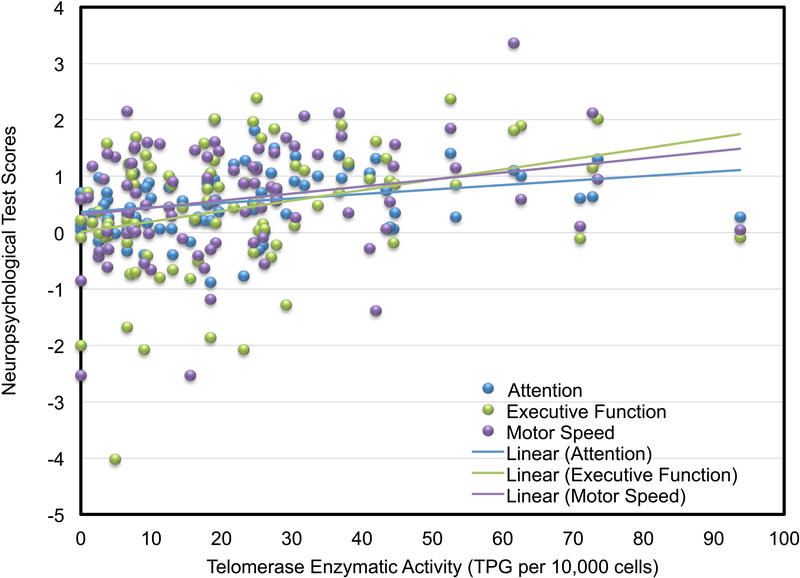
On the whole, participants’ neuropsyschological domain scores were normally distributed, with mean scores consistently above zero, indicating generally intact status in most of the sample: Learning =.46, ±.85; Memory =.43, ±.74; Attention =.54, ±.56; Visuospatial =.11, ±.76; Executive Function =.43, ±1.12; Motor Speed =.64, ±1.03. Likewise, very few participants’ domain scores were < −2 z-score (i.e., in the impaired range) – four or fewer in any domain. High DNA damage was associated with having a .23 lower standardized executive function score (P=0.027; q=0.025) compared to low DNA damage, with a similar trend for a relationship of high DNA damage with lower memory scores (P=.06, q=0.013), in adjusted models (See Table 3; Figure 1). Models testing the association of telomerase activity with cognitive domains showed that with each decile decline in telomerase activity there were also a .3 lower standardized attention (P=0.006; q=0.013) and executive function (P=.002;q=0.013) score, and a .24 lower standardized motor speed (P=0.037; q=0.013 See Table 3; Figure 2). Telomere length and sTNF-RII were unrelated to cognitive domains in adjusted regression models (See Table 3).
Table 3.
Multivariate analyses examining aging biology parameters as predictors of neuropsychological test scores, adjusting for age, BMI, race, years from treatment, and intelligence score (WTAR).
| DNA Damage (High vs. Low) N=93 | Telomerase (Deciles) N=84 | Telomere Length (T/S) N=85 | sTNF-RII (pg/mL) N=91 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropsychological Test Domain | β(SE) | B | P Value | β(SE) | B | P Value | β(SE) | B | P Value | β(SE) | Beta | P Value |
| Learning | −.348(.22) | −.18 | 0.12 | −.001(.03) | −.003 | 0.98 | .092(.34) | .029 | 0.79 | .00(.00) | −.08 | 0.50 |
| Memory | −.371(.20) | −.22 | 0.06 | .019(.03) | .076 | 0.50 | .075(.31) | .027 | 0.81 | .00(.00) | −.05 | 0.70 |
| Attention | −.231(.15) | −.18 | 0.12 | .058(.02) | .301 | 0.006 | −.155(.23) | −.075 | 0.50 | .00(.00) | −.09 | 0.45 |
| Visuospatial | −.289(.21) | −.16 | 0.16 | .044(.03) | .162 | 0.15 | .198(.32) | .069 | 0.54 | .00(.00) | −.03 | 0.78 |
| Executive Function | −.599(.27) | −.23 | 0.027Ŧ | .118(.04) | .300 | 0.002 | .042(.42) | −.010 | 0.92 | .00(.00) | −.12 | 0.28 |
| Motor Speed | −.154(.28) | −.07 | 0.58 | .083(.04) | .239 | 0.037 Ŧ | −.515(.43) | −.134 | 0.24 | .00(.00) | −.11 | 0.38 |
Betas (β), unstandardized coefficients; standard error (SE); standardized coefficients (B)
Denotes criterion for significance not met after correction for multiple testing using False Discovery Rate, q = .025.
Figures 1.
Mean population normalized neuropsychological test score by domain in cancer survivors categorized by low DNA damage and high DNA damage.
Figure 2.
Scatterplot of population normalized attention, executive function, and motor speed scores as a function of telomerase enzymatic activity in breast cancer survivors. Lower scores indicate worse cognitive performance.
Discussion
Cognitive difficulties following cancer treatment are a serious clinical concern and threat to quality of life in cancer survivorship. In the present cross-sectional analyses of a well-characterized cohort of breast cancer survivors several years after completion of treatment, we observed that markers of aging biology including high DNA damage and reduced telomerase activity were associated with worse neuropsychological performance, after covariate adjustments. In particular, higher DNA damage was related to lower executive function scores, and low telomerase activity was related to lower executive function, attention, and motor speed. After adjusting for multiple comparisons, the association of DNA damage with executive function and telomerase with motor speed were no longer considered significant at q=0.025.
Our findings provide evidence of an association of aging biology with cognitive domains commonly affected in both cancer-related cognitive impairment59–61 and normal aging62. The observed relationships are not dramatic; in both the executive function and motor domains the difference between those in the lowest and highest deciles is within one standard deviation. These modest differences in neuropsychological function are consistent with the subtle declines of both age- and cancer-related cognitive changes. Further, even those in the lowest decile are performing in the average range, an unremarkable performance clinically. However, subtle changes in cognitive function are consistent with what is known about cancer-related cognitive difficulties59 and can nonetheless result in noticeable changes in daily functioning and quality of life.
In regards to the role of inflammation, our previous work found that sTNF-RII was elevated early after chemotherapy, and was associated with cognitive difficulties.13,33,34 In the current follow up of 3 to 6 years after treatment, sTNF-RII was unrelated to subjective or objective cognition cross-sectionally; however, we previously reported that both DNA damage and telomerase activity were associated with inflammation.15 Exposure to elevated inflammation immediately following treatment by chemotherapy and/or radiation could be an early indicator of risk for biological aging, with lasting DNA damage and changes in telomerase activity indicating sustained aging effects. In this regard, several other potential markers of aging might be considered for future research. For example, extensive cell replication cycles or cell stress pathways can induce cellular senescence.63,64 Future work might consider whether cellular senescence in the periphery and in the brain is associated with cognitive function after treatment. Further research is needed to disentangle the temporal relationship between treatment - inflammation, DNA damage, cell senescence, and cognitive function. As our study is among the first to begin investigating these mechanisms, substantial additional work is needed to address these hypotheses.
Interestingly, no associations were found between cellular aging marker and subjective cognitive functioning similar to the findings of Conroy and colleagues (2013).9 Subjective cognitive functioning in cancer survivorship is complex and does not consistently tightly correspond to neuropsychological performance,65 but may also be associated with other factors such as mood and stress.66 So despite our observed relationships between aging markers and neuropsychological performance, the multi-factorial nature of subjective cognitive functioning may be less strongly linked to specific biological processes. Given the cross sectional nature of this study, it will be important to continue examining these relationships in longitudinal study designs.
Contrary to our hypothesis, PBMC telomere length was unrelated to cognitive function domain scores. A lack of association suggests that this measure of biological age is not necessarily capturing the specific pathways of aging that occur after cancer treatment. Consistent with this, we did not see an effect of cancer treatment on telomere length in our previous report,15 similar to others.14 Thus, cancer treatments may not accelerate aging by contributing to blood cell telomere length shortening per se, but rather via induction of DNA damage and cell senescence.14 Indeed, telomere length shortening is driven by cell replication, a process often halted during cancer treatments.67 Cellular senescence, on the other hand, is reached through either cell replication cycles (that shorten telomeres) or cell stress pathways (e.g., extensive damage to DNA). It is also possible that a one-time sampling approach fails to capture temporal dynamics. Further research should consider within-individual changes in telomere length from pre to post treatment as an indicator of cellular aging and assess the extent to which this is predictive of cognitive function.
Regardless of pathway to senescence, senescent cells no longer divide, and express very low levels of telomerase activity. Thus, our measure of telomerase activity may reflect the extent of senescence within PBMCs, regardless of whether it is replicative or stress induced senescence.21 Telomerase can also repair DNA damage, and helps resist stress induced growth arrest,20–23 suggesting the enzyme is important for defense against cell aging independent of its role in protecting telomeric ends.
Our analyses were performed in a cross-sectional sample of women after completing treatment for breast cancer, and therefore causal pathways cannot be confirmed. We recognize that an alternative interpretation could also be made, which is that cognitive function may impact telomerase activity and contribute to DNA damage by modifying behavior. Future research should consider additional assessment of these markers earlier after the course of treatment including before, during, and after cancer treatment, which may yield important information about early indicators of vulnerability to developing cognitive difficulties that can signal the need for intervention or prevention. Additional study limitations include a relatively well-educated sample with generally intact cognitive function, limited diversity in terms of racial/ethnic groups, and a relatively young cohort of women, resulting in a possible sampling bias. The lack of representation of higher risk groups, including older women and a wider range of cognitive impairment, may contribute to smaller detected effects. Future studies in more diverse samples of age, race and ethnicity, and socioeconomic backgrounds are needed. Another limitation is the small sample size that limits our ability to measure smaller effects, raising the possibility of a type II error. Larger sample sized studies are needed to determine whether effects that were subthreshold are detectable with more power. Along this line, the reported medium effects for the associations of DNA damage with executive function and of telomerase with motor speed scores did not survive the FDR correction for multiple tests.
In light of our findings, future work is warranted to further investigate the mechanistic role of aging as a key factor contributing to elevated inflammation and poorer cognitive function, a symptom commonly experienced by cancer patients years after completing treatment. Future research might consider examining aging pathways in current interventions that target cognitive function, diet, and physical activity. Such behaviors are known to modify inflammatory signaling68–73, telomerase activity74, and cellular aging pathways,75 but have not yet been tested in the context of cancer and aging. Other possible avenues of research may be manipulating mechanisms of cellular senescence with pharmaceutical agents and employing mouse models76–78 to characterize plausible targets for intervention.
These results add support for an accelerated aging model of cancer-related cognitive impairment, and harmonize with other studies in non-cancer populations pursuing the link between these markers and cognition.29,30 Together our findings point to an aging-like effect of cancer treatments on cellular biology and further connect this to cognitive function. An important next research objective will be examining comprehensive and well-powered models that include repeated assessments of neuropsychological function, cellular aging markers, inflammation, and neuro-inflammatory factors.
Acknowledgments
We would like to thank our participants for their time and contribution to this research, and to the Cousins Center for Psychoneuroimmunology for a long and supportive relationship with the researchers involved in this project.
FUNDING
This work was supported by the National Cancer Institute at the National Institute of Health grant number R01 CA109650, the American Cancer Society (JEC and KVD), the Breast Cancer Research Foundation (PAG), for which Dr. Ganz is a Scientific Advisory Board member, R01 CA160245-01 and R01CA207130-01 (MRI), and the Cousins Center for Psychoneuroimmunology.
Footnotes
Conflict of Interest: Dr. Carroll serves on the Peer Review Committee at the American Cancer Society. Dr. Ganz is a Scientific Advisory Board member for the Breast Cancer Research Foundation. There are no conflict of interest disclosures for LP, RS, KVD, JEB, ZS, MRI.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/jco.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE, Ganz PA. Symptoms : Fatigue and Cognitive Dysfunction In: Ganz PA, ed. Improving Outcomes for Breast Cancer Survivors. Breast Cancer Research Foundation; 2015:53–75. doi:DOI 10.1007/978-3-319-16366-6_5. [DOI] [PubMed] [Google Scholar]
- 7.Vichaya EG, Chiu GS, Krukowski K, et al. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of Illness in Cancer Survivors: Findings From a Population-Based National Sample. JNCI J Natl Cancer Inst. 2004;96(17):1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 9.Conroy SK, McDonald BC, Smith DJ, et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buttiglieri S, Ruella M, Risso A, et al. The aging effect of chemotherapy on cultured human mesenchymal stem cells. Exp Hematol. August 2011. doi: 10.1016/j.exphem.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Pucci M, Bravata V, Forte GI, et al. Caveolin-1, Breast Cancer and Ionizing Radiation. Cancer Genomics Proteomics. 12(3):143–152. http://apps.webofknowledge.com/full_record.do?product=WOS&search_mode=CitingArticles&qid=1&SID=3ATL6s7TnOYWslCTAJC&page=1&doc=10. Accessed March 7, 2016. [PubMed] [Google Scholar]
- 12.Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain Behav Immun. 2013;27(1):155–161. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30 Suppl:S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of Cytotoxic Chemotherapy on Markers of Molecular Age in Patients With Breast Cancer. JNCI J Natl Cancer Inst. 2014;106(4):dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scuric Z, Carroll JE, Bower JE, et al. Biomarkers of aging associated with past treatments in breast cancer survivors. npj Breast Cancer. 2017;3(1):50. doi: 10.1038/s41523-017-0050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 17.Armbrecht JH. A biological perspective of ageing In: Sinclair AJ, Morley JE, Vellas B, eds. Pathy’s Principles and Practice of Geriatric Medicine. 5th ed. West Sussex, UK: John Wiley & Sons, Inc.; 2012:13–22. [Google Scholar]
- 18.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 20.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1–2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorbunova V, Seluanov A, Pereira-Smith OM. Expression of Human Telomerase (hTERT) Does Not Prevent Stress-induced Senescence in Normal Human Fibroblasts but Protects the Cells from Stress-induced Apoptosis and Necrosis. J Biol Chem. 2002;277(41):38540–38549. doi: 10.1074/jbc.M202671200. [DOI] [PubMed] [Google Scholar]
- 23.Gorbunova V, Seluanov A. Telomerase as a Growth-Promoting Factor. Cell Cycle. 2003;2(6):534–537. doi: 10.4161/cc.2.6.515. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z, Jay KA, Smith DL, et al. Early Telomerase Inactivation Accelerates Aging Independently of Telomere Length. Cell. 2015;160(5):928–939. doi: 10.1016/j.cell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study†. Ann Oncol. 2015;26(7):1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74–82. doi: 10.1016/J.JNEUROIM.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung YT, Lim SR, Ho HK, Chan A. Cytokines as Mediators of Chemotherapy-Associated Cognitive Changes: Current Evidence, Limitations and Directions for Future Research. Forloni G, ed. PLoS One. 2013;8(12):e81234. doi: 10.1371/journal.pone.0081234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7(4):511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migliore L, Fontana I, Trippi F, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26(5):567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL. Relationship Between Leukocyte Telomere Length, Telomerase Activity, and Hippocampal Volume in Early Aging. JAMA Neurol. 2014;71(7):921. doi: 10.1001/jamaneurol.2014.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hägg S, Zhan Y, Karlsson R, et al. Short telomere length is associated with impaired cognitive performance in European ancestry cohorts. Transl Psychiatry. 2017;7(4):e1100. doi: 10.1038/tp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris SE, Marioni RE, Martin-Ruiz C, et al. Longitudinal telomere length shortening and cognitive and physical decline in later life: The Lothian Birth Cohorts 1936 and 1921. Mech Ageing Dev. 2016;154. doi: 10.1016/j.mad.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganz PA, Petersen L, Castellon SA, et al. Cognitive Function After the Initiation of Adjuvant Endocrine Therapy in Early-Stage Breast Cancer: An Observational Cohort Study. J Clin Oncol. 2014;32(31):3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosswell AD, Lockwood KG, Ganz PA, Bower JE. Low heart rate variability and cancer-related fatigue in breast cancer survivors. Psychoneuroendocrinology. 2014;45:58–66. doi: 10.1016/j.psyneuen.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study. J Clin Oncol. 2016;34(8):816–824. doi: 10.1200/JCO.2015.64.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner LI, Sweet J, Butt Z, Lai J, Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. J Support Oncol. 2009;7(6):W32–39. [Google Scholar]
- 39.Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- 40.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 41.Heaton R, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for African American and Caucasian adults. In: Psychological Assessment Resources; Lutz, FL; 2004. [Google Scholar]
- 42.KLOVE H. CLINICAL NEUROPSYCHOLOGY. Med Clin North Am. 1963;47:1647–1658. http://www.ncbi.nlm.nih.gov/pubmed/14078168. Accessed July 3, 2018. [PubMed] [Google Scholar]
- 43.Delis D, Kaplan E, Kramer JH. California Verbal Learning Test-II. 2nd ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 44.Wechsler D. Wechsler Memory Scale. 4th ed. San Antonio, TX: Pearson; 2009. [Google Scholar]
- 45.Benedict R. Brief Visuospatial Memory Test. Revised. Odessa, FL: Psychological Assessment Resources, Inc.; 1997. [Google Scholar]
- 46.Shin M-S, Park S-Y, Park S-R, Seol S-H, Kwon JS. Clinical and empirical applications of the Rey–Osterrieth Complex Figure Test. Nat Protoc. 2006;1(2):892–899. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler Adult Intelligence Scale, Forth Edition (WAIS-IV). 4th ed. San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 48.Gronwall DMA. Paced Auditory Serial-Addition Task: A Measure of Recovery from Concussion. Percept Mot Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 49.Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept Mot Skills. 1958;8(3):271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 50.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. http://www.ncbi.nlm.nih.gov/pubmed/3345800. Accessed August 18, 2016. [DOI] [PubMed] [Google Scholar]
- 51.ALAPETITE C. Use of the alkaline comet assay to detect DNA repair deficiencies in human fibroblasts exposed to UVC, UVB, UVA and gamma-rays. Int J Radiat Biol. 1996;69(3):359–369. doi: 10.1080/095530096145922. [DOI] [PubMed] [Google Scholar]
- 52.Końca K, Lankoff A, Banasik A, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res Toxicol Environ Mutagen. 2003;534(1–2):15–20. doi: 10.1016/S1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Xu C, Du LQ, et al. Evaluation of the comet assay for assessing the dose-response relationship of DNA damage induced by ionizing radiation. Int J Mol Sci. 2013;14(11):22449–22461. doi: 10.3390/ijms141122449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robles TF, Carroll JE, Bai S, Reynolds BM, Esquivel S, Repetti RL. Emotions and family interactions in childhood: Associations with leukocyte telomere length. Psychoneuroendocrinology. 2016;63:343–350. doi: 10.1016/j.psyneuen.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carroll JE, Esquivel S, Goldberg A, et al. Insomnia and Telomere Length in Older Adults. Sleep. 2016;39(3):559–564. http://www.ncbi.nlm.nih.gov/pubmed/26715231. Accessed January 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):47e–47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benjamini Y, Hochberg Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing With Independent Statistics. J Educ Behav Stat Spring. 2000;25(1):60–83. [Google Scholar]
- 58.Lee J-W, Park KD, Im J-A, Kim MY, Lee D-C. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta. 2010;411(7–8):592–596. doi: 10.1016/j.cca.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 59.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–138. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jim HSL, Phillips KM, Chait S, et al. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson RJ, Ahles TA, Saykin AJ, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freund A, Orjalo AV, Desprez P-Y, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treat Rev. 2012;38(7):926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Pullens MJJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 67.Dy GK, Adjei AA. Systemic cancer therapy: Evolution over the last 60 years. Cancer. 2008;113(S7):1857–1887. doi: 10.1002/cncr.23651. [DOI] [PubMed] [Google Scholar]
- 68.Irwin MR, Olmstead R, Breen EC, et al. Tai Chi, Cellular Inflammation, and Transcriptome Dynamics in Breast Cancer Survivors With Insomnia: A Randomized Controlled Trial. JNCI Monogr. 2014;2014(50):295–301. doi: 10.1093/jncimonographs/lgu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. September 2014:n/a–n/a. doi: 10.1002/cncr.29063. [DOI] [PubMed] [Google Scholar]
- 70.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanton AL, Bower JE. Psychological Adjustment in Breast Cancer Survivors. Adv Exp Med Biol. 2015;862:231–242. doi: 10.1007/978-3-319-16366-6_15. [DOI] [PubMed] [Google Scholar]
- 72.Bower JE, Irwin MR. Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lengacher CA, Reich RR, Kip KE, et al. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438–447. doi: 10.1177/1099800413519495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015;18(1):57–89. doi: 10.1089/rej.2014.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2015;22(1):78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demaria M, O’Leary MN, Chang J, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017;7(2):165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baar MP, Brandt RMC, Putavet DA, et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169(1):132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]