Abstract
Stimulator of interferon genes (STING) is an integral ER membrane protein that can be activated by 2’3’-cGAMP synthesized by cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) upon binding of double-stranded DNA and activates interferon (IFN) and inflammatory cytokine response to defend against microorganism infection. Pharmacologic activation of STING has been demonstrated to induce an antiviral state and boost antitumor immunity. We previously reported a cell-based high throughput screening assay that allowed for identification of small molecule cGAS-STING pathway agonists. We report herein a compound, 6-bromo-N-(naphthalen-1-yl)benzo[d][1,3] dioxole-5-carboxamide (BNBC), that induces proinflammatory cytokine response in a human STING-dependent manner. Specifically, we showed that BNBC induced type I and III IFN dominant cytokine responses in primary human fibroblasts and peripheral blood mononuclear cells (PBMCs). BNBC also induced cytokine response in PBMC-derived myeloid dendritic cells and promoted their maturation, suggesting that STING agonist treatment could potentially regulate the activation of CD4+ and CD8+ T lymphocytes. As anticipated, treatment of primary human fibroblast cells with BNBC induced an antiviral state that inhibited the infection of several members of flaviviruses. Taken together, our results indicate that BNBC is a human STING agonist that not only induces innate antiviral immunity against a broad spectrum of viruses but may also stimulate the activation of adaptive immune response, which is important for treatment of chronic viral infections and tumors.
Keywords: high throughput assay, STING, Innate immune, antiviral
Graphical Abstract
Pattern recognition receptors (PRRs) are host cellular proteins that recognize pathogen associated molecular patterns, such as viral nucleic acids, capsids, bacterial peptidoglycans, lipopolysaccharides or other metabolites1, and subsequently activate a proinflammatory cytokine response and cell death pathways2–3. These innate immune responses not only limit the proliferation and spread of microorganisms in the early phase of infection, but also facilitate the induction of a more powerfully adaptive immune response that can ultimately resolve the infections4–5. In addition, PRR-mediated innate immune response also plays an important role in surveillance of tumor genesis and invasion6–8. Stimulator of interferon genes (STING) is an endoplasmic reticulum (ER) membrane protein that plays a central role in the activation of innate immune response by multiple cellular DNA sensors9–10. Particularly, recognition of cytoplasmic double-stranded DNA by cyclic GMP-AMP synthase (cGAS), the primary cytoplasmic DNA sensor11–13, catalyzes the synthesis of a cyclic dinucleotide c[G(2’,5’)pA(3’,5’)p], or 2’3’-cGAMP for short14, which binds to STING and induces its dimerization and translocation from the ER membrane to perinuclear vesicles and activates downstream signaling components, such as NFkB and TBK-1/IRF315–17, leading to the expression of interferons and other proinflammatory cytokines and chemokines17–18. STING is expressed in professional innate and adaptive immune cells and plays important roles in innate and adaptive immune response to various pathogens and tumors6, 19–21. It is also well documented that STING responds to mitochondrial damages22–25, DNA double-strand breaks26–27, and its over-activation may contribute to the onset of autoimmune diseases such as systemic lupus erythematosus28–29.
Due to its critical role in host immune responses, pharmacological modulation of STING activity has been considered as a viable immunotherapeutic approach for treatment of pathogen infections, tumors and autoimmune disorders. Indeed, recent studies showed that intra-tumor administration of 2’3’-cGAMP induced profound regression of established tumors in mice and generated substantial systemic immune responses capable of rejecting distant metastases and providing long-lived immunologic memory30–31. STING agonists had also been demonstrated to potentiate the efficacy of immune checkpoint blockade therapy32–33 and enhance the immunogenicity of vaccines34–35. In addition, we and others have demonstrated that STING agonist therapy was able to induce a host immune response to control the infection of influenza A virus36, hepatitis B virus18, 37, herpes simplex virus38 and human immunodeficiency virus39. These studies prove the concept that pharmacologic activation of STING is an attractive immunotherapeutic approach to treat viral infection and cancers. Moreover, recent discovery of a small molecular inhibitor of STING paves the way for treatment of systemic lupus erythematosus and other autoimmune diseases40.
Although several analogs of cyclic dinucleotide (CDN)-based STING agonists have been tested in tumor models in mice and demonstrated antitumor activity through activation of anticipated immune response34, their medical application has been significantly limited by the low cell membrane permeability41. Accordingly, extensive efforts have been made to discover non-cyclic dinucleotide small molecule human STING agonists. One approach is to modify the mouse-specific STING agonist 5’,6’-dimethylxanthenone-4-acetic acid (DMXAA), which has not yet resulted in identification of novel derivatives capable of binding human STING42. However, α-mangostin, a dietary xanthone with antitumor and antiviral activities43–44, was demonstrated to bind and activate both mouse and human STING45. Another approach is through biochemistry- or cell-based high throughput screening (HTS). Screening of small molecules that can compete the binding of a radio-labeled cyclic dinucleotide STING agonist to the C-terminal domain of human STING identified a aminobenzimidazole compound and its dimer derivatives as potent human STING agonists46. Alternatively, taking cell-based HTS approaches, three chemotypes of compounds have been identified to activate a cytokine response in a human STING-dependent manner47–49. We report herein another novel human STING specific agonist from a cell-based HTS campaign and its pharmacological properties. Our preliminary medicinal chemistry studies indicate that this carboxamide STING agonist is a good candidate for further lead optimization and development toward a therapeutic agent for treatment of chronic viral infections and tumors.
Results
Discovery of a carboxamide compound that activates cGAS-STING pathway
We previously established a HepG2 cell-derived cell line with reconstituted human cGAS-STING pathway and ISG54 promotor-driven luciferase for high throughput screening of human cGAS-STING pathway agonists49. Screening of the NCI Diversity Set V collection of 1,594 small molecule compounds identified a compound, 6-bromo-N-(naphthalen-1-yl)benzo[d][1,3]dioxole-5-carboxamide (BNBC) (Fig. 1A), that significantly induced ISG54-driven luciferase expression in HepAD38/cGAS-STING/ISG54 cells. As shown in Fig 1B, treatment of HepAD38/cGAS-STING/ISG54Luc cells with BNBC for 4 h induced luciferase expression in a concentration-dependent manner. On the contrary, BNBC did not induce luciferase expression in HepAD38/cGAS-STINGΔC/ISG54Luc cells that express human STING with truncation of carboxyl-terminal domain (CTD). Because the CTD of STING is essential for the activation of IRF3 and subsequent induction of cytokines, the cGAS-STINGΔC reporter cell line serves as a negative control18. While significant ISG54 promotor-driven luciferase activity was detected at low micromolar concentration in cells expressing human STING, no cytotoxicity was detected at up to 200 μM concentration. (Fig. 1C). Furthermore, BNBC also concentration-dependently induced ISG54 driven luciferase expression in HepG2/STING/ISG54 reporter cells lacking cGAS (Fig. 1B). These results thus suggest that BNBC is a cGAS/STING pathway activator and most likely targets a cellular component down-stream of cGAS, but at or up-stream of STING.
Figure 1. Carboxamide compound BNBC activates cGAS-STING pathway in a functional human STING-dependent manner.
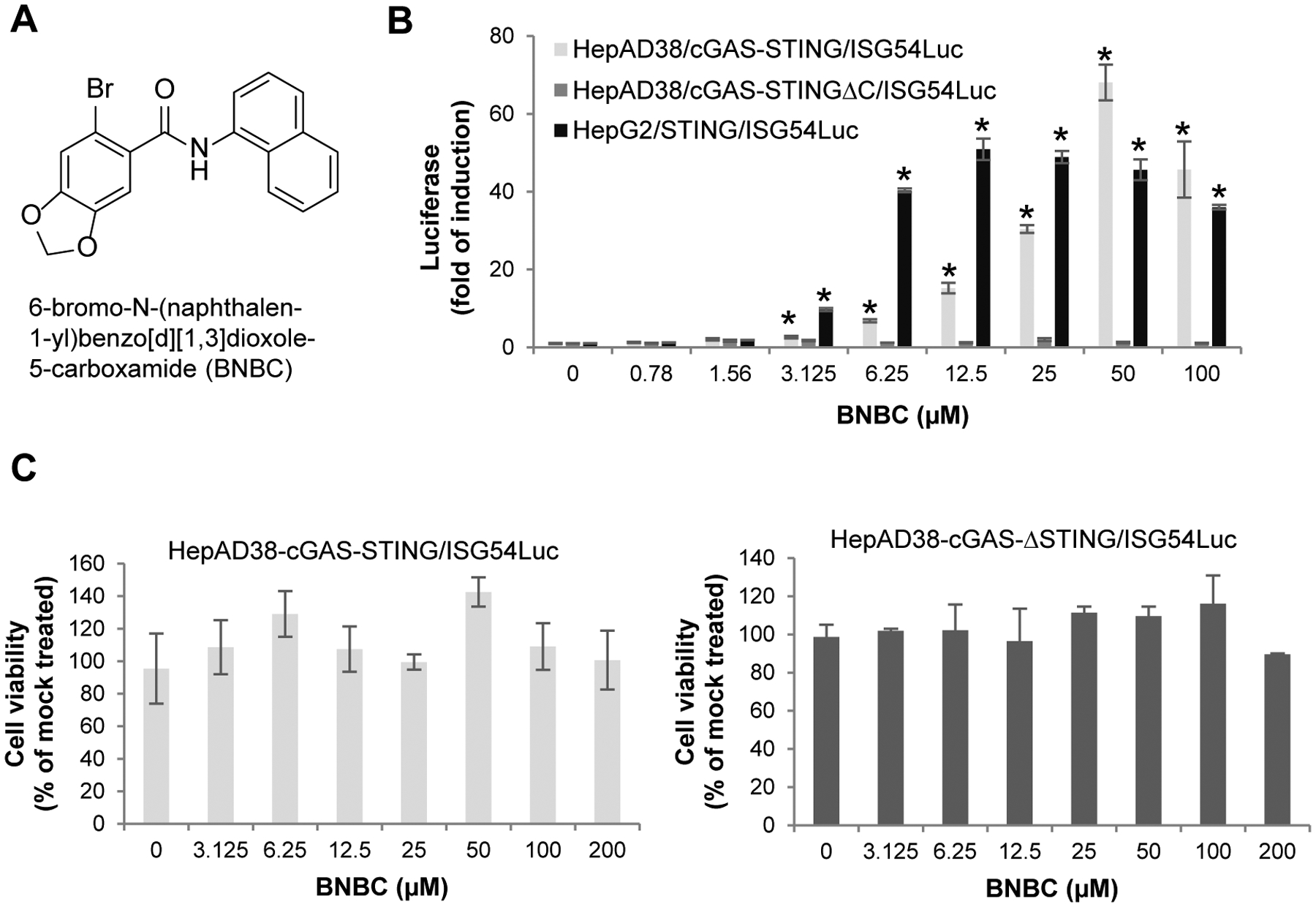
(A) Chemical structure of BNBC. (B) Activation of ISG54 promoter activities by BNBC were determined in HepAD38/cGAS-STING/ISG54Luc, HepAD38/cGAS-STINGΔC/ISG54Luc cells and HepG2/STING/ISG54Luc cells. Luciferase activity was measured at 4 h post treatment and expressed as fold of induction (mean ± standard deviation, n=3). * indicates p<0.05 compared to mock treated control. (C) Cytotoxicity was determined in HepAD38/cGAS-STING/ISG54Luc and HepAD38/cGAS-STINGΔC/ISG54Luc cells after 4 h treatment and expressed as percent of mock treated control (mean ± standard deviation, n=3).
BNBC is a human STING specific agonist
In order to precisely determine the cellular target of BNBC, we first examined the effects of BNBC on proinflammatory cytokine gene expression in HepG2 cells reconstituted with human or mouse STING, respectively, using mouse STING specific agonist DMXAA as a control. As shown in Fig. 2A and B, consistent with results obtained with ISG54 reporter assays, BNBC concentration-dependently induced IFN-β, IL29 (IFN-λ1) and TNF-α mRNA expression in HepG2/STING cells (Fig. 2A), but not in parental HepG2 cells, HepG2/STINGΔC cells and HepG2-derived cell line expressing mouse STING (HepG2/mSTING) (Fig. 2B). As expected, DMXAA only induced IFN-β mRNA expression in HepG2/mSTING cells, but not in HepG2/STING cells expressing human STING. Because the only difference between HepG2/hSTING and HepG2/mSTING cell lines is the expression of STING from human or mice, the results thus indicate that as DMXAA is a specific agonist of mouse STING, BNBC should be a specific agonist of human STING. In agreement with this notion, while DMXAA treatment only induced the peri-nuclear translocation of mSTING and nuclear translocation of IRF3, BNBC treatment induced the peri-nuclear translocation of HepG2 cells expressing human STING, but not mouse STING (Fig. 2C). Interestingly, although BNBC treatment induced peri-nuclear translocation of both full-length and C-terminally truncated hSTING, it only induced IRF3 phosphorylation and nuclear translocation in HepG2/STING cells, but not in HepG2/STINGΔC cells (Fig. 2D). These results imply that although BNBC might specifically bind to human STING and induce its subcellular translocation in a CTD-independent manner, its activation of IRF3 phosphorylation and nuclear translocation is CTD-dependent, which is consistent with the essential role of STING CTD in recruitment of TBK1 and phosphorylation of IRF317. Taken together, our results presented above indicate that BNBC is a human STING specific agonist.
Figure 2. BNBC is a human STING specific agonist.
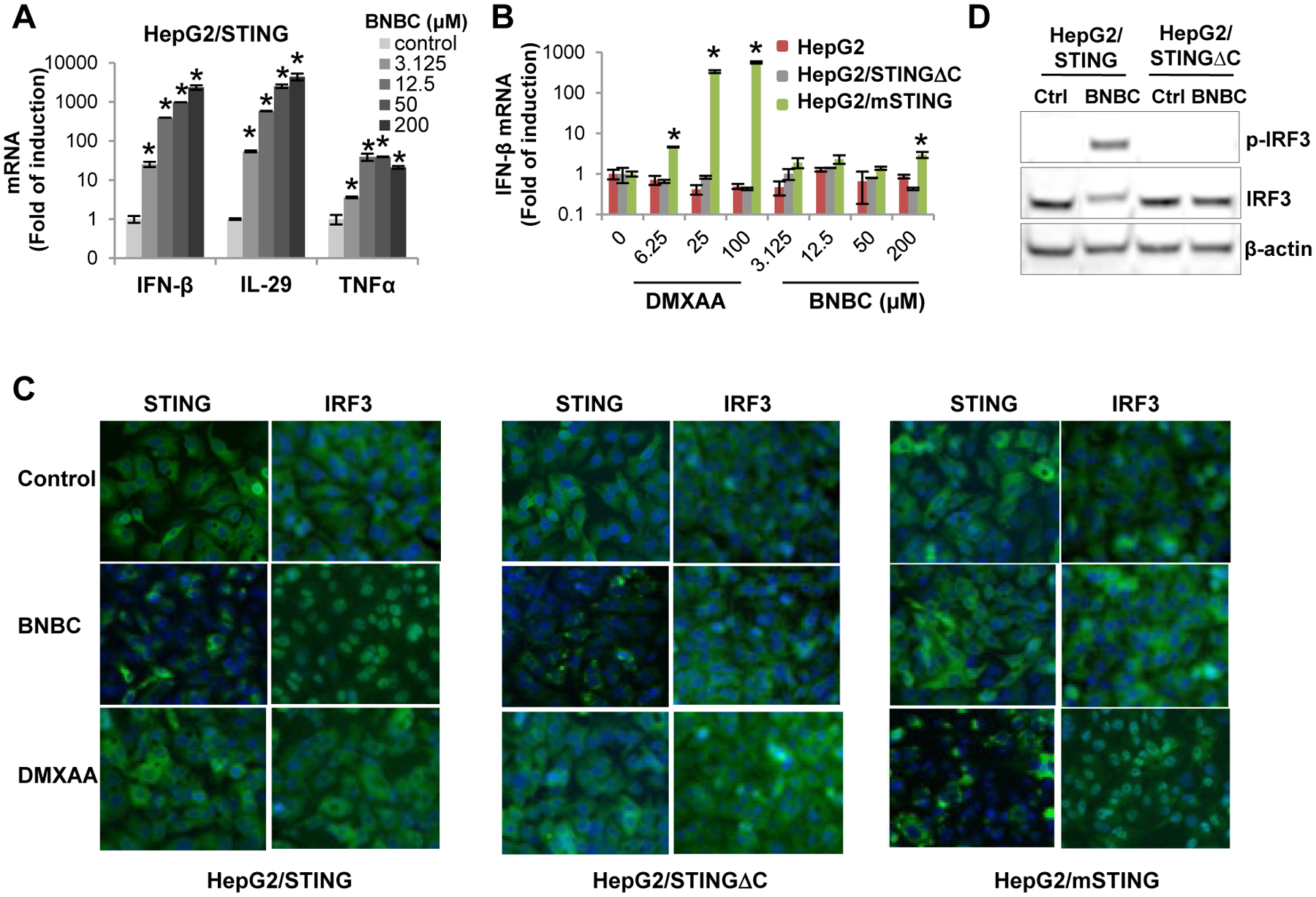
(A) HepG2/STING cells were treated with indicated concentrations of BNBC for 6 h. The cytokine mRNAs were measured by qRT-PCR and expressed as fold of induction (mean ± standard deviation, n=4). (B) HepG2, HepG2/STINGΔC and HepG2/mSTING cells were treated with indicated concentrations of BNBC or DMXAA for 6 h. Induction of IFN-β mRNAs were determined by qRT-PCR and expressed as fold of induction (mean ± standard deviation, n=4). * indicates p<0.05 compared to mock treated control. (C) HepG2/STING, HepG2/STINGΔC and HepG2/mSTING were mock treated or treated with 100 μM of BNBC or 50 μM of DMXAA for 2 h. The expression and subcellular localization of STING and IRF3 were determined by immunofluorescent staining (green). Cell nuclei were stained with DAPI (blue). (D) HepG2/STING and HepG2/STINGΔC cells were treated with 100 μM BNBC for 2 h. Total and phosphorylated IRF3 were determined by Western blot assay. β-actin served as a loading control.
BNBC induces a proinflammatory cytokine response and establishment of antiviral state in human foreskin fibroblast cells (THF) in a STING-dependent manner.
THF is a cell line derived from primary human diploid foreskin fibroblasts (HFF)50 and expresses physiologically relevant levels of STING48–49. As observed in HepG2-derived cell line ectopically expressing functional human STING, BNBC concentration-dependently induced IFN-β, IL-28A and IL-6 mRNA expression as well as secretion of IFN-β into culture media in THF cells (Fig. 3A and B) in the absence of cytotoxicity (Fig. 3C). Also consistent with its role as a STING agonist, knocking out the expression of STING, but not TRIF or IPS-1 (MAVS), in THF cells completely abolished BNBC-induced IFN-β mRNA expression (Fig. 3D).
Figure 3. BNBC activation of inflammatory cytokine response in THF cells is STING-dependent.
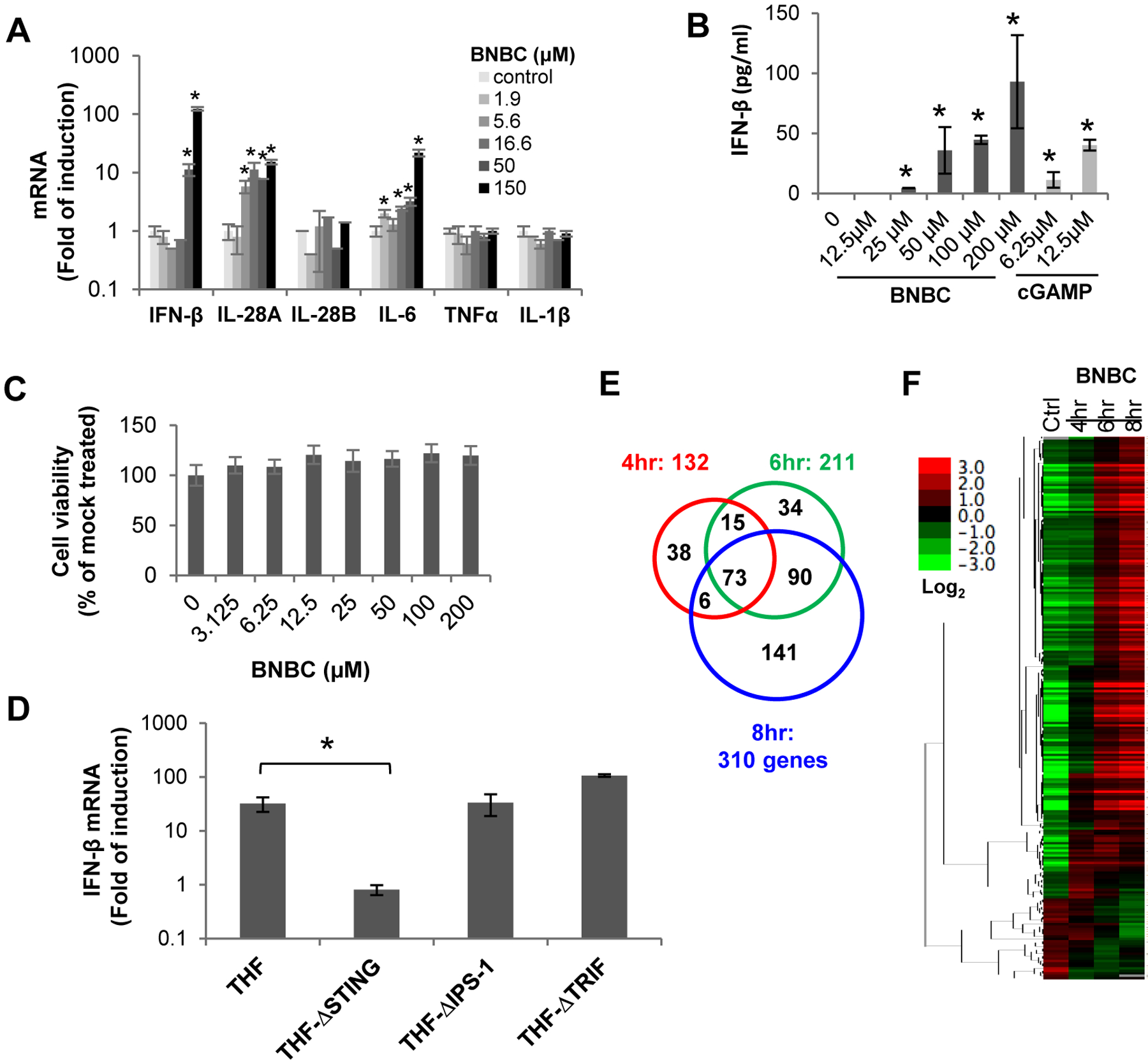
(A) THF cells were treated with indicated concentrations of BNBC for 6 h, followed by quantification of cytokine mRNAs by qRT-PCR assays. The levels of cytokine mRNA were expressed as fold of induction (mean ± standard deviation, n=4). (B) THF cells were treated with indicated concentrations of BNBC or cGAMP for 18 h, followed by detection of IFN-β in culture medium with ELISA assay (mean ± standard deviation, n=4). (C) Cytotoxicity was determined in THF cells and expressed as percent of mock treated control (mean ± standard deviation, n=3). (D) Parental THF cells as well as THF cells with knockout of STING, IPS-1 or TRIF were treated with 50 μM of BNBC for 6 h. IFN-β mRNA was detected by qRT-PCR and expressed as fold of induction (mean ± standard deviation, n=4). * indicates p<0.05 relative to mock treated control. (E and F) RNAseq analysis was performed using RNA samples extracted from THF cells treated with 50 μM of BNBC for indicated time periods. Venn diagram (E) illustration of numbers of up- or downregulated genes with fold change greater than 2 and FDR <20%, relative to mock treated control. The heat map (F) is expressed as the log2 fold difference.
To determine the BNBC-induced gene expression profile, RNAseq analysis was performed with RNA extracted from THF cells harvested at 4, 6 and 8 h post BNBC treatment. As illustrated in Fig. 3E and F, BNBC altered the expression of 132, 211 and 310 genes at 4, 6 and 8 h of treatment, respectively. Ingenuity canonical pathway analysis indicated that many components of PRR and interferon signaling pathways, including downstream factors of IRF3 and IRF7, STAT1 and STAT3, were up-regulated (Table 1). In addition, several top regulator effect networks being altered after BNBC treatment are related to the activation of antigen presenting cells, myeloid cells and leukocytes. The RNAseq analyses thus revealed that BNBC treatment induced a typical innate proinflammatory cytokine response gene expression profile consistent with the activation of STING pathway.
Table 1.
Analysis of gene alterations using Ingenuity analysis
| Ingenuity canonical pathway | p value | Overlap |
|---|---|---|
| Interferon signaling | 4.8E-13 | 27.8% (10/36 |
| Activation of IRF by cytosolic PRR | 2.0-E10 | 15.9% (10/63) |
| Role of PRR in recognition of bacteria and viruses | 3.9E-08 | 8.0% (11/137) |
| Upstream regulator | p value | Predicted activation |
| IRF7 | 2.5E-57 | Activated |
| STAT1 | 6.1-E46 | Activated |
| IRF3 | 5.7E-43 | Activated |
| STAT3 | 8.4E-36 | Activated |
| NKX2–3 | 4.6E-34 | Inhibited |
| Top regulator effect networks | Consistency score | Diseases & functions |
| CNOT7, EF1, IFI16, IRF1,3,5,7, NFATC2, NKX2–3… | 76.42 | Activation of APCs |
| CNOT7, EBF1, ESR1, HIF1A, IFI16, IRF1,3,4,5… | 48.91 | Accumulation of myeloid cells |
| HIF1A, IFI16, IRF5,7, KLF2, STAT4… | 28.74 | Activation of leukocytes |
In agreement with its induced gene expression profile that indicates the induction of antiviral state, BNBC treatment efficiently protected Dengue virus (DENV), Yellow fever virus (YFV) and Zika virus (ZIKV) infection of THF cells. As shown in Fig. 4, pre-treatment of THF cells with BNBC for 8 h concentration-dependently reduced the intracellular DENV, YFV and ZIKV RNA levels by up to 90, 150 and 800-fold, respectively. Similarly, pre-treatment of THF cells with another small molecule human STING agonist, G1048, reduced virial RNAs in similar potencies against the three flaviviruses.
Figure 4. Pretreatment of THF cells with BNBC inhibited the replication of Dengue, Yellow fever, and Zika viruses.
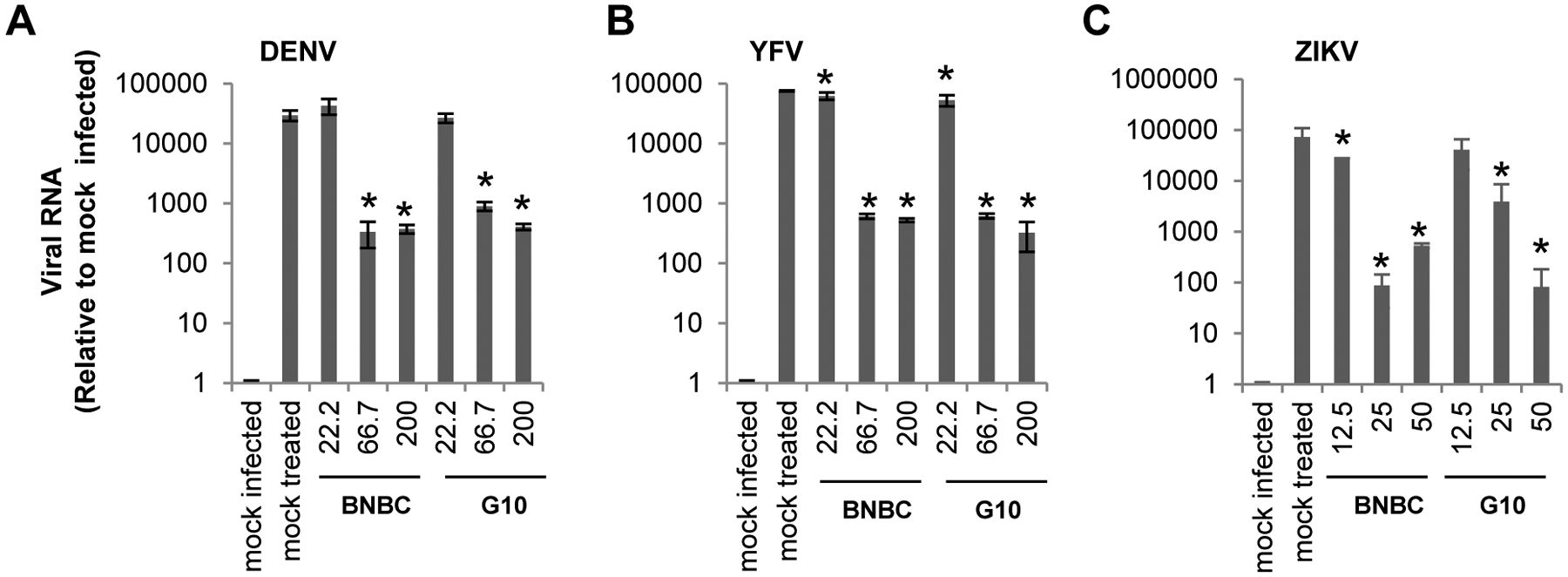
THF cells were treated with indicated concentrations of BNBC or control compound G10 for 8 h. Cells were then infected with DENV (A), YFV (B), or ZIKV (C) at MOI of 0.1 for 1 h and cultured for additional 48 h. Viral RNAs were determined by qRT-PCR, and expressed as values relative to mock infected control (mean ±standard derivations, n=3). * indicates p<0.05 compared to mock treated control.
BNBC induced inflammatory cytokine response in human peripheral blood mononuclear cells (PBMCs) and promoted PBMC-derived dendritic cell maturation
STING is expressed in professional innate and adaptive immune cells and plays important roles in innate and adaptive immune response to various pathogens and tumors6, 51. In order to investigate the effects of BNBC on the function of professional immune cells, we first examined BNBC-induced cytokine response in human PBMCs and demonstrated that BNBC efficiently induced expression of type I and type III IFN, but to a lesser extent, TNF-α (Fig. 5A). Interestingly, while cGAMP significantly induced the expression of IFN-β, IL-28A and IL-29 in PBMCs derived from all three donors (Fig. 5B), BNBC and G10 treatment only significantly enhanced type I and III IFN mRNA expression in PBMCs derived from 2 out of 3 donors (Fig. 5B). It remains to be further investigated whether the unresponsiveness of PBMC-derived from donor 1 is associated with STING gene variation that may impede the binding and activation by BNBC and G10 or other physiological factors that affects the metabolism of the non-cyclic dinucleotide STING agonists.
Figure 5. Activity of BNBC in human PBMCs.
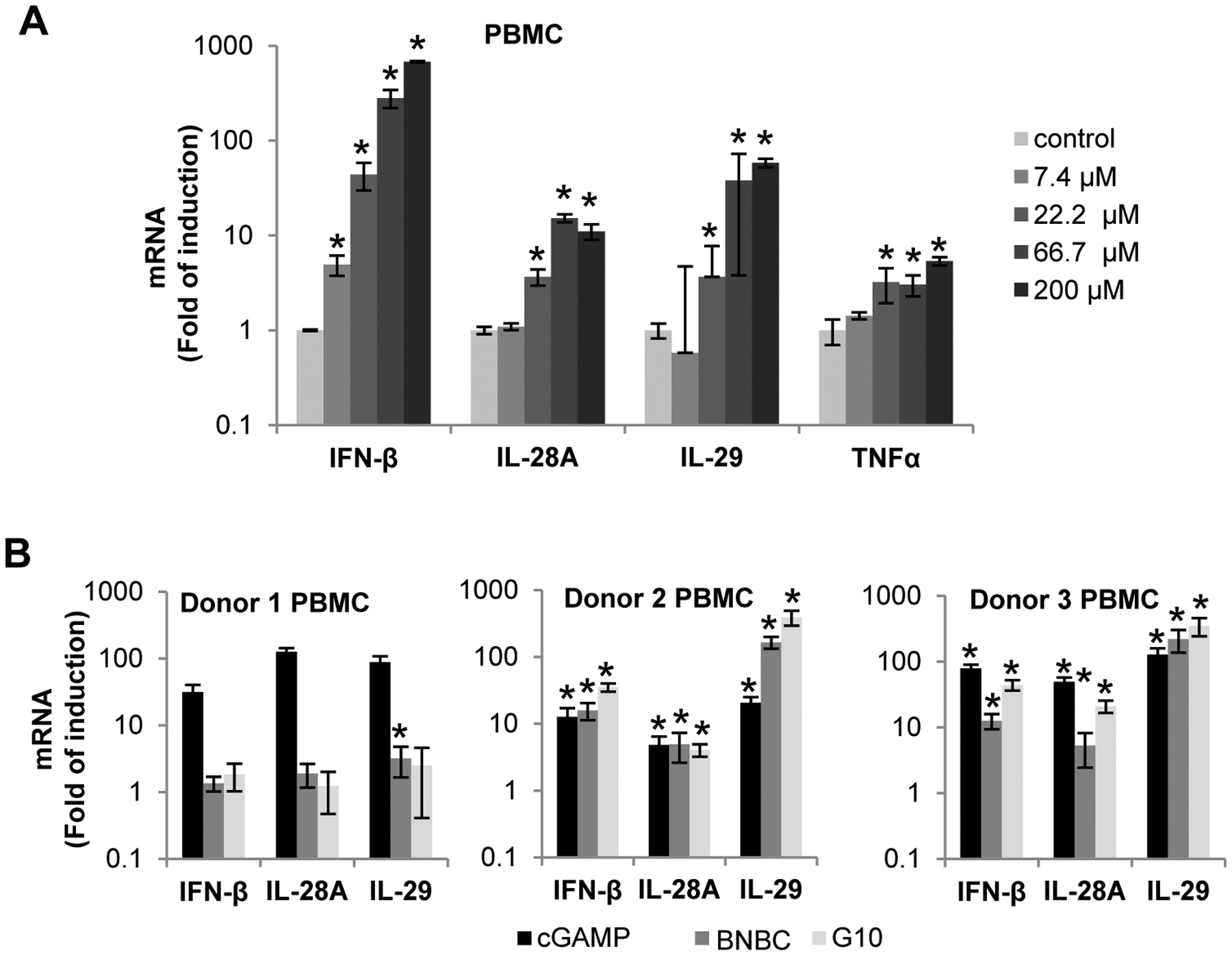
(A) PBMCs freshly isolated from human blood were treated with indicated concentration of BNBC for 6 h and followed by detection of cytokine mRNAs by qRT-PCR. The levels of cytokine mRNA were expressed as fold of induction (mean ± standard deviation, n=4). (B) PBMCs freshly isolated from human blood of additional three healthy donors were treated with 15 μM of 2’3’-cGAMP, 60 μM of BNBC or G10 for 6 h. The mRNAs of IFN-β, IL-28A, IL-29 and TNFα were detected by qRT-PCR and expressed as fold of induction (mean ± standard deviation, n=3). * indicates p<0.05 compared to mock treated control.
Dendritic cells are professional antigen presentation cells that function at the interface of innate and adaptive immune responses and play a critical role in immune control of virus infection. It has been shown that activation of pattern recognition receptors (PRRs) by pathogen-associated molecular patterns (PAMPs) in dendritic cells initiates essential innate immune signaling cascades resulting in maturation of dendritic cells and up-regulation of their antigen presenting machinery as well as costimulatory molecules such as CD80. As shown in Fig. 6A, treatment of PBMC-derived dendritic cells with BNBC (100 μM) or LPS (10 μg/ml) for 6 h efficiently induced the expression of IFN-β and IL-29 mRNAs and to a lesser extent, TNF-α and IL-6 mRNAs. Moreover, treatment of PBMC-derived dendritic cells with BNBC (50 μM) or LPS (10 μg/ml) for 48 h significantly induced expression of CD80 (Fig. 6B and C). These results suggest that activation of STING is indeed able to induce the maturation of dendritic cells.
Figure 6. BNBC activated type I and III interferon dominated inflammatory cytokine response in PBMC-derived dendritic cells, and promoted dendritic cell maturation.
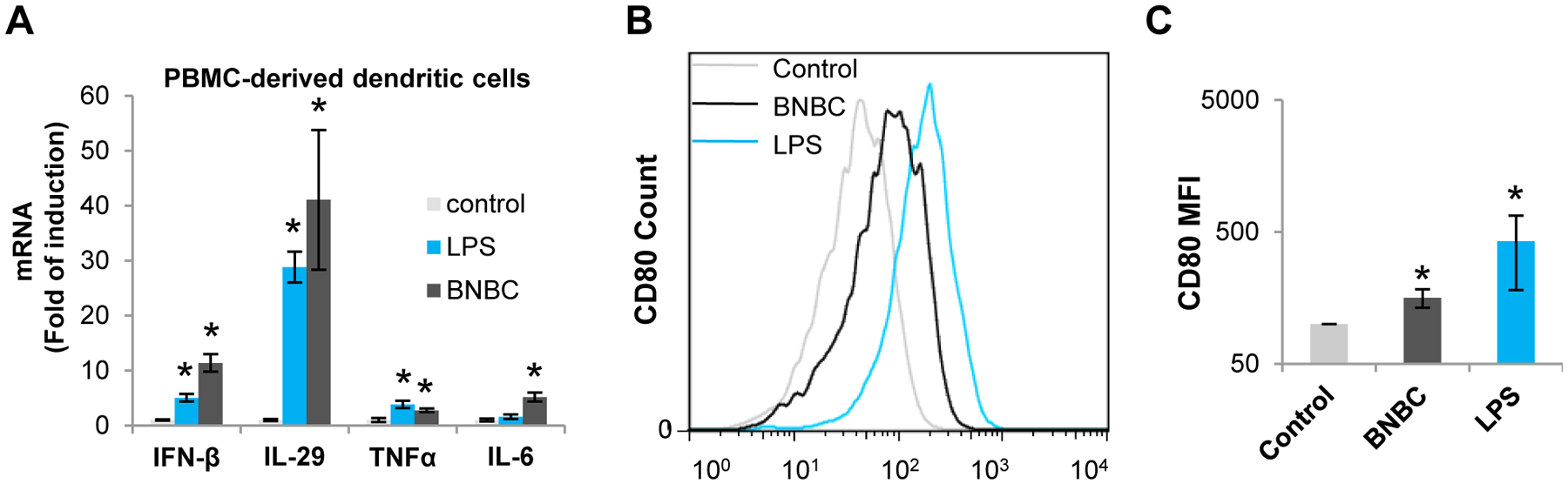
(A) Human PBMC-derived dendritic cells were treated with 10 μg/ml LPS or 100 μM of BNBC for 6 h. Cytokine mRNAs were detected by qRT-PCR and expressed as fold of induction relative to mock treated control (mean ± standard deviation, n=3). (B and C) Human PBMC-derived dendritic cells were treated with 10 μg/ml LPS or 50 μM of BNBC for 48 h. CD80 expression was gated from CD11c+ cells (B). Fluorescent intensity of CD80 from 4 independent experiments using PBMCs from 2 donors were expressed as mean ± standard deviation (MFI) (C). * indicates p< 0.05 compared to mock treated control.
Preliminary structure-activity-relationship studies identified active pharmacophores of BNBC
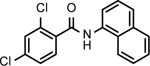
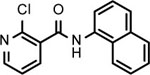
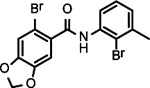
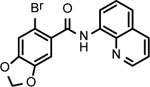
BNBC is an amide with two fused rings on each side of the acid and amine with a MW of 370 and calculated logP of 4.0. The necessary pharmacophores of BNBC were determined through evaluating the analogs with structural alterations around B ring (57093Z, 57057Z), C ring (57071Z, 57076, and 59011Z), or both B and C rings (59016Z) (Table 2). BNBC analogs were prepared in house and tested for their activities to induce ISG54 promoter-driven luciferase expression in HepG2/hSTING cells and ranked by the Minimum Effective Concentration to induce 5-fold luciferase activity (MinEC5X) relative to the mock-treated controls. As shown in Table 2, introduction of a more electronegative nitrogen atom to either B ring (57057Z) or D ring (57076) will make the resulting products more polar and thus more likely improve water solubility. However, both compounds lost activities to induce the ISG54 promotor-driven luciferase expression. Similar inactivation was also observed when a polar and water solubilizing group, morpholine, was added to the 2-position of the C ring (59011Z). These results suggested that the binding site of BNBC might be highly hydrophobic. In contrast, two analogs of BNBC with either opened A ring (57093Z) or D ring (57071Z) maintained low MinEC5X values of 2.5 and 2.6 μM, respectively, similar to that of BNBC (2.2 μM). However, compound 59016Z, with both opened A and D rings, has a much higher MinEC5X of 50 μM, suggesting a reduced activity in activating STING. Interestingly, the compound 57093Z has significantly increased maximum fold of induction of ISG54 promoter activity indicating that it might be a more potent STING activator. These results suggested that either A ring or D ring can be opened and substituted with alkyl, halogen, or other groups to maintain or improve the STING activation activity and thus established a base for further chemical modification.
Table 2.
Structure-activity relationship study of BNBC
| Cmpd* | BNBC | 57093Z | 57057Z | 57071Z | 57076 | 59011Z | 59016Z |
|---|---|---|---|---|---|---|---|
| Structure | 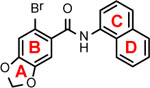 |
 |
 |
 |
 |
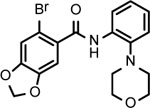 |
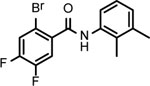 |
|
1MinEC5X (μM) |
2.2 | 2.5 | > 200 | 2.6 | > 200 | > 200 | 50 |
| 1Maximum induction (fold) | 20.5 | 28.0 | - | 17.3 | - | - | 7.8 |
| 2CC50 (μM) | > 200 | > 200 | > 200 | > 200 | > 200 | > 200 | > 200 |
compound.
Luciferase activity was determined in HepG2/STING/ISG54Luc cells and expressed as Minimum Effective Concentration to induce 5-fold luciferase activity or maximum fold of luciferase induction.
Cytotoxicity was determined in HepG2/STING/ISG54Luc cells by MTT assay.
To evaluate the pharmacological properties of BNBC and its active analogs, 57071Z and 57093Z, an in vitro ADME profiling studies were performed. As summarized in Table 3, BNBC has good caco-2 permeability and acceptable plasma protein bindings, which predict a favorable oral absorption rate if the molecule can be made in solution. A panel of 9 cytochrome P450 (Cyp) enzymes (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, CYP2E1 and 3A4 with two different substrates) were analyzed in 10 reactions. While BNBC and 57071Z each inhibits 5 out of 10 Cyps/reactions, including relatively more common enzymes CYP3A4 (determined with Midazolam and Testosterone) and/or CYP2D6, 57093Z, a more potent BNBC analog with an opened A ring, exhibits an improved Cyp inhibition profile and inhibits 2 out of 10 Cyp enzymes, CYP1A2 and CYP2C9, which are not considered to be as critical as CYP3A4 and 2D6. One major issue of BNBC and its analogs is the poor aqueous solubility in both pH 4 and pH 7.4 at sub- or low-micromolar. Further chemical modification is necessary to improve the solubility, since an extremely low aqueous solubility is hard to overcome even through proper formulation and dosing as a suspension. As shown in Table 3, BNBC and its two analogs apparently have very rapid metabolic rate in both human and mouse liver microsomes, predicting a low metabolic stability in vivo and raising concern for insufficient overall drug exposure. However, since high systematic exposure is the major concern for STING agonist to cause systematic inflammatory side effect, the metabolic property of BNBC predicts a low systematic exposure after oral dosing and favors intrahepatic STING activation, which may reduce systematic toxicity and is thus beneficial for treatment of chronic HBV infection.
Table 3.
ADME profile of BNBC and its analogs
| Cmpd* | Aqueous solubility (μM) | Metabolic stability T1/2 (min) | Caco-2 | Cyp# | PPB (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PH = 4 | PH =7.4 | Human | Mouse | Papp (A-B) (10−6, cm/s) | Papp (B-A) (10−6, cm/s) | Efflux Ratio | IC50 < 10 μM | Human | Mouse | |
| BNBC | 3.57 | 2.62 | 19.96 | 2.21 | 14.46 | 5.98 | 0.41 | 5/10 | 97.34 | 97.88 |
| 57093Z | 0.28 | 0.26 | 9.50 | 2.70 | ND | ND | ND | 2/10 | ND | ND |
| 57071Z | 4.14 | 3.94 | 15.39 | 1.81 | ND | ND | ND | 5/10 | ND | ND |
compound.
Cyps inhibited by BNBC are 1A2, 2C19, 2D6, 3A4 (Midazolam) and 3A4 (Testosterone); Cyps inhibited by 57093Z are 1A2 and 2C19; Cyps inhibited by 57071Z are 1A2, 2B6, 2C19, 3A4 (Midazolam) and 3A4 (Testosterone);
Discussion
BNBC is a carboxamide compound from NCI Diversity Set V compound library and was identified to induce the expression of interferon and other proinflammatory cytokines in HepG2 cells expressing functional human, but not mouse STING, suggesting that BNBC is a human STING specific agonist (Fig. 2). In agreement with this notion, BNBC induced STING peri-nuclear translocation as well as phosphorylation and nuclear translocation of IRF3 (Fig. 2). Moreover, BNBC induced cytokine response in THF and PBMCs. Its activity to induce cytokine response in THF cells was dependent on the expression of STING, but not MAVS and TRIF, adaptor for RIG-I-like receptors and TLR3/TLR4, respectively (Figs. 3 and 5). Importantly, BNBC was able to induce a gene expression profile that is consistent with STING activation (Fig. 3) and established an antiviral state in treated cells to restrict the infection of three different flaviviruses (Fig. 4). Although our biological data presented herein suggest that STING is likely the target of BNBC, it remains to be determined whether BNBC can directly bind to STING.
Due to its critical role in innate and adaptive immunity, STING is an important therapeutic target for diseases with dysfunctional immune response, particularly, cancers and chronic viral infections. Preclinical and clinical studies with different formulations of CDN-based STING agonists demonstrated promising efficacy to various tumors and adjuvant activity to induce more robust cellular and humoral immune responses. However, due to its poor cell permeability and metabolic stability, the CNDs were administrated via intra-tumor injection, which limited their application, particularly for treatment of viral infections and non-solid tumors. In fact, studies with DMXAA in mice clearly demonstrated that systematic administration of STING agonists is efficacious to tumors and viral infections52–53. However, all the non-CDN STING agonists discovered through cell-based HTS thus far only specifically activate human, but not mouse STING48–49. This species specificity impeded the in vivo evaluation of their therapeutic efficacy in mice models. Interestingly, α-mangostin and amidobenzimidazole derivative appear to activate both human and mouse STING, indicating that the discovery and development of human and mouse dual active non-CDN STING agonists is possible45–46. Further investigation into the mechanism of different agonists to activate STING should provide clues for the development of dual active STING agonists.
Considering the mode of action on STING agonist therapy of tumors, the current experimental evidence indicates that the activation of STING in tumor cells can induce a cytokine response and cell death31, 34, which will enhance cross-presentation of tumor antigens by dendritic cells and activate T cell immune response against tumors. In addition, activation of STING in tumor stromal cells and infiltrated immune cells may also contribute to the induction of efficient anti-tumor immune response. In the case of chronic viral infection, such as chronic hepatitis B, while activation of the cytokine response in either virus infected cells or non-infected cells may inhibit viral replication, restoration of host adaptive antiviral response is essential to control the persistent viral infection and achieve clinical cure54–56. Therefore, in the course of developing STING agonist, it is important to not only evaluate its activity on cytokine induction but should also assess its ability to enhance adaptive antiviral immunity in relevant cell culture and in vivo models. Our studies reported herein showed that BNBC not only activates an inflammatory cytokine response, but also induces dendritic cell maturation (Fig. 6). With this encouraging observation, we will further investigate immune modulating activity of BNBC and its derivatives (Table 2) in vivo in humanized mice models with human hepatocytes and an adaptive immune system57–58.
Conclusion
BNBC represents a new chemotype human STING agonist that can activate innate and adaptive immune response for treatment of viral infections and tumors.
Materials and Methods
Cells and viruses
HepG2 is a human hepatoblastoma cell line obtained from ATCC. HepG2-derived cell lines with reconstituted human cGAS and/or STING, with/without firefly luciferase under the control of an ISG54 promoter (HepAD38/cGAS-STING/ISG54Luc, HepG2/STING/ISG54Luc and HepG2/STING), with reconstituted human STING with 39 amino acid deletion on CTD required for downstream signaling, but not ligand binding (HepAD38/cGAS-STINGΔC/ISG54Luc, HepG2/STINGΔC), and with reconstituted mouse STING (HepG2/mSTING) were described previously18, 49. THF cells are primary human diploid foreskin fibroblasts (HFF) with reconstitution of the catalytic subunit of human telomerase50. THF with STING knockout (THF-ΔSTING), IPS-1 knockout (THF-ΔIPS-1) or TRIF knockout (THF-ΔTRIF) were described and characterized previously59. Human peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood of four healthy donors (Biological Specialty) using Ficoll-Paque density gradient centrifugation (Miltenyi Biotech). Freshly isolated PBMCs were cultured in RPMI-1640 medium (Corning) containing L-Glutamine and 10% FBS. To isolate PBMC-derived dendritic cells, the suspended cells were removed from freshly isolated PBMCs after overnight culture. The remaining adherent cells were stimulated with human recombination GM-CSF (50 ng/ml, Gibco) and IL-4 (50 ng/ml, Gibco) for 7 days to induce the production of dendritic cells.
Zika virus (PRVABC59 strain) was purchased from ATCC. Yellow fever virus (17D strain) and dengue virus (serotype 2, New Guinea C strain) stocks were produced by electroporation of Huh7.5 cells with in vitro transcribed RNAs from the corresponding cDNA constructs, as described previously49, 60–61.
Chemicals
Diversity Set V compound library containing 1,594 structure diversified small molecule compounds were acquired from the National Cancer Institute (NCI). 6-bromo-N-(naphthalen-1-yl)benzo[d][1,3]dioxole-5-carboxamide (BNBC) and its analogs were synthesized in house with > 95% purity. Synthesis and characterization data of BNBC and its analogs are shown in Supplemental Materials. BNBC and its analogs were dissolved in DMSO at a stock concentration of 50 mM. 2’3’-cGAMP and LPS were purchased from InvivoGen. DMXAA was purchased from AdooQ BioScience. G10, a previously reported human STING agonist, was purchased from Aobious48.
ISG54 luciferase reporter assay
HepAD38/cGAS-STING/ISG54Luc cells, HepAD38/cGAS STINGΔC/ISG54Luc cells or HepG2/STING/ISG54Luc cells were seeded in black wall/clear bottom 96-well plates at a density of 4×104/well in 0.1 mL of medium for overnight. HTS and hit identification were as described previously49. To determine the activity of BNBC, the cells were treated with 1% DMSO (mock treated control) or indicated concentrations of BNBC (or its analogs) for 4 h. The firefly luciferase activities were measured by using Steady-Glo substrate (Promega) with TopCount (Perkin Elmer)49.
Cytotoxicity assay
Cell viability was measured using MTT assay (Sigma).
Analysis of cytokine expression
IFN-β mRNA, TNF-α mRNA, IL-28A, IL-28B, IL-29 mRNA, IL-1β and IL-6 mRNA were measured using total cellular RNA, by quantitative RT-PCR (qRT-PCR) assays18, 49. β-actin mRNA served as internal. Cytokine secreted into culture medium was detected by ELISA assay (RD Systems).
Western blot assay
Cells were lysed with NuPAGE® LDS sample buffer (Thermo Fisher Scientific) supplemented with 2.5% 2-Mercaptoethanol (Sigma) followed by electrophoresis in NuPAGE 4–12% Bis-Tris Gel (Thermo Fischer Scientific) and transfer onto a PVDF membrane (Thermo Fischer Scientific). Primary antibodies against total and phosphorylated IRF3 or β-actin were from Cell Signaling. Secondary antibodies were from LI-COR. Bolts were imaged with LI-COR Odyssey system (LI-COR Biotechnology).
Immunofluorescent assay
HepG2/STING, HepG2/STINGΔC and HepG2/mSTING cells were treated with either BNBC or DMXAA for 2 h. The cells were fixed with PBS containing 4% paraformaldehyde followed by incubation with 0.1% Triton X-100 for 20 min. Antibodies against IRF3 or STING were from Cell Signaling. Alexa Fluor 488-conjugated secondary antibody was from Invitrogen49. Cell nuclei were stained with DAPI.
RNAseq and data analysis
mRNA sequencing (mRNA-Seq) assays were performed in the Genomics Facility of Fox Chase Cancer Center. RNA sample quantity was measured by UV absorbance at 260, 280, and 230 nm with a NanoDrop 1000 spectrophotometer (ThermoScientific). RNA integrity was determined by bioAnalyzer using an RNA Nano chip instrument (Agilent Technologies). 1000 ng total RNAs from each sample were used to make mRNA-seq library using the Truseq stranded mRNA library kit. Specifically, mRNAs were enriched twice via poly-T based RNA purification beads, and fragmentated at 94 °C for 8 min. The first strand cDNA was synthesized by Superscript II and random primers at 42 °C for 15 min, followed by second strand synthesis at 16 °C for 1 h. Adapters with illumine P5, P7 sequences as well as indices were ligated to the cDNA fragment at 30 °C for 10 min. After Ampure bead (BD) purification, a 15-cycle of PCR reaction was used to enrich the fragments. Sample libraries were subsequently pooled and loaded to the Illumina Hiseq2500. Raw sequence reads were aligned to the mouse genome (mm10) using the Tophat algorithm62. Cufflinks algorithm63 was implemented to assemble transcripts and estimate their abundance. Cuffdiff64 was used to statistically assess expression changes in quantified genes in different conditions. Genes with false discovery rate <0.2 and fold change >2 were considered significant.
YFV, DENV and ZIKV infection and antiviral assays
THF cells were treated with indicated concentrations of STING agonists for 8 h followed by infection with DENV, YFV or ZIKV at a multiplicity of infection (MOI) of 0.1 for 1 h. Two days post infection, the viral RNAs were quantified by qRT-PCR assays using LightCycler 480II (Roche). β-actin mRNA was quantified to normalize the levels of viral RNA. Primers used for amplification of viral RNA were reported previously60, 65.
Flowcytometry analysis of PBMC-derived dendritic cells
PBMC-derived dendritic cells were washed twice with PBS containing 2% FBS and 1 mM EDTA, and stained using anti-CD11c FITC and anti-CD80 PE antibodies (eBioscience) for 30 min at room temperature. Data were acquired by GUAVA (Millipore) and analyzed with FlowJo.
In vitro adsorption, distribution, metabolism and elimination (ADME) profiling of BNBC and its analogs
In vitro ADME profiling studies were performed by Pharmaron. Aqueous solubility in PBS at pH 4.0 and 7.4 was determined at up to 300 μM. Metabolic stability in human and mouse liver microsomes was determined at 0, 15, 30, 45 and 60 min post incubation and expressed as time of 50% reduction compared to 0 min (T1/2). Permeability in human epithelial colorectal adenocarcinoma cells Caco-2 was assessed and expressed as transportation rate in both directions (apical to basolateral (A-B) and basolateral to apical (B-A)) across the cell monolayer, as well as efflux ratio, an indicator of a compound’s active efflux. Plasma protein binding (PPB) in samples of human or mouse origins was determined using equilibrium dialysis method and expressed as percentage of binding. Inhibition of each of the 10 cytochrome P450 (CYP) isozymes (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4) was determined as greater than 50% inhibition at 10 μM concentration.
Statistics
P values of unpaired t test were calculated using GraphPad QuickCalcs.
Supplementary Material
Acknowledgements
We thank Wade Bresnahan at University of Minnesota for providing THF cells. A cDNA clone of serotype 2 dengue virus, pACYC177-NGC-DENV-2, was a gift from Pei-Yong Shi at University of Texas Medical Branch. A cDNA clone of Yellow fever virus 17D strain, pACNR/FLYF-17Dx, was a gift from Charles Rice at Rockefeller University.
Funding
This work was supported by grants from the National Institutes of Health, USA (AI134732 and AI113267), Arbutus Biopharma Inc. and the Commonwealth of Pennsylvania through the Hepatitis B Foundation.
Abbreviations
- BNBC
6-bromo-N-(naphthalen-1-yl)benzo[d][1,3]dioxole-5-carboxamide
- cGAS
cyclic guanosine monophosphate-adenosine monophosphate synthase
- DMXAA
5,6-Dimethylxanthenone-4-acetic acid
- HBV
hepatitis B virus
- HFF
human foreskin fibroblast
- IFN
interferon
- LPS
Lipopolysaccharides
- mDCs
Myeloid dendritic cells
- PAMPs
pathogen-associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
- PRRs
pattern recognition receptors
- STING
stimulator of interferon genes
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-alpha
Footnotes
Supporting Information. Methods for the synthesis and characterization of BNBC and its analogs.
REFERENCES
- 1.Zhou P; She Y; Dong N; Li P; He H; Borio A; Wu Q; Lu S; Ding X; Cao Y; Xu Y; Gao W; Dong M; Ding J; Wang DC; Zamyatina A; Shao F, Alpha-kinase 1 is a cytosolic innate immune receptor for bacterial ADP-heptose. Nature 2018, 561 (7721), 122–126. [DOI] [PubMed] [Google Scholar]
- 2.Akira S; Uematsu S; Takeuchi O, Pathogen recognition and innate immunity. Cell 2006, 124 (4), 783–801 DOI: 10.1038/s41586-018-0433-3. [DOI] [PubMed] [Google Scholar]
- 3.Shi J; Gao W; Shao F, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 2017, 42 (4), 245–254 DOI: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A; Medzhitov R, Control of adaptive immunity by the innate immune system. Nat Immunol 2015, 16 (4), 343–53 DOI: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang J; Block TM; Guo JT, The innate immune response to hepatitis B virus infection: implications for pathogenesis and therapy. Antiviral Res 2012, 96 (3), 405–13 DOI: 10.1016/j.antiviral.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Corrales L; McWhirter SM; Dubensky TW Jr.; Gajewski TF, The host STING pathway at the interface of cancer and immunity. J Clin Invest 2016, 126 (7), 2404–11 DOI: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiba S; Baghdadi M; Akiba H; Yoshiyama H; Kinoshita I; Dosaka-Akita H; Fujioka Y; Ohba Y; Gorman JV; Colgan JD; Hirashima M; Uede T; Takaoka A; Yagita H; Jinushi M, Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 2012, 13 (9), 832–42 DOI: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cen X; Liu S; Cheng K, The Role of Toll-Like Receptor in Inflammation and Tumor Immunity. Frontiers in pharmacology 2018, 9, 878 DOI: 10.3389/fphar.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo T; Kobayashi J; Saitoh T; Maruyama K; Ishii KJ; Barber GN; Komatsu K; Akira S; Kawai T, DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A 2013, 110 (8), 2969–74 DOI: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q; Sun L; Chen ZJ, Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 2016, 17 (10), 1142–9 DOI: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 11.Wu J; Sun L; Chen X; Du F; Shi H; Chen C; Chen ZJ, Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339 (6121), 826–30 DOI: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L; Wu J; Du F; Chen X; Chen ZJ, Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339 (6121), 786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai X; Chiu YH; Chen ZJ, The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 2014, 54 (2), 289–96 DOI: 10.1126/science.1232458. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X; Shi H; Wu J; Zhang X; Sun L; Chen C; Chen ZJ, Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 2013, 51 (2), 226–35 DOI: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdette DL; Monroe KM; Sotelo-Troha K; Iwig JS; Eckert B; Hyodo M; Hayakawa Y; Vance RE, STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478 (7370), 515–8 DOI: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Q; Tian Y; Kabaleeswaran V; Jiang X; Tu D; Eck MJ; Chen ZJ; Wu H, Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol Cell 2012, 46 (6), 735–45 DOI: 10.1016/j.molcel.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka Y; Chen ZJ, STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Science signaling 2012, 5 (214), ra20 DOI: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo F; Tang L; Shu S; Sehgal M; Sheraz M; Liu B; Zhao Q; Cheng J; Zhao X; Zhou T; Chang J; Guo JT, Activation of STING in hepatocytes suppresses the replication of hepatitis B virus. Antimicrob Agents Chemother 2017, 61 (10), e00771–17 DOI: 10.1128/AAC.00771-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XD; Wu J; Gao D; Wang H; Sun L; Chen ZJ, Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341 (6152), 1390–4 DOI: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z; Damania B, The cGAS-STING Defense Pathway and Its Counteraction by Viruses. Cell Host Microbe 2016, 19 (2), 150–8 DOI: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao D; Wu J; Wu YT; Du F; Aroh C; Yan N; Sun L; Chen ZJ, Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 2013, 341 (6148), 903–6 DOI: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rongvaux A; Jackson R; Harman CC; Li T; West AP; de Zoete MR; Wu Y; Yordy B; Lakhani SA; Kuan CY; Taniguchi T; Shadel GS; Chen ZJ; Iwasaki A; Flavell RA, Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 2014, 159 (7), 1563–77 DOI: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre S; Fernandez-Sesma A, Collateral Damage during Dengue Virus Infection: Making Sense of DNA by cGAS. J Virol 2017, 91 (14) DOI: 10.1128/JVI.01081-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreeva L; Hiller B; Kostrewa D; Lassig C; de Oliveira Mann CC; Jan Drexler D; Maiser A; Gaidt M; Leonhardt H; Hornung V; Hopfner KP, cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 2017, 549 (7672), 394–398 DOI: 10.1038/nature23890. [DOI] [PubMed] [Google Scholar]
- 25.McArthur K; Whitehead LW; Heddleston JM; Li L; Padman BS; Oorschot V; Geoghegan ND; Chappaz S; Davidson S; San Chin H; Lane RM; Dramicanin M; Saunders TL; Sugiana C; Lessene R; Osellame LD; Chew TL; Dewson G; Lazarou M; Ramm G; Lessene G; Ryan MT; Rogers KL; van Delft MF; Kile BT, BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359 (6378) DOI: 10.1126/science.aao6047. [DOI] [PubMed] [Google Scholar]
- 26.Deng L; Liang H; Xu M; Yang X; Burnette B; Arina A; Li XD; Mauceri H; Beckett M; Darga T; Huang X; Gajewski TF; Chen ZJ; Fu YX; Weichselbaum RR, STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41 (5), 843–52 DOI: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding SM; Benci JL; Irianto J; Discher DE; Minn AJ; Greenberg RA, Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548 (7668), 466–470 DOI: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan N, Immune Diseases Associated with TREX1 and STING Dysfunction. J Interferon Cytokine Res 2017, 37 (5), 198–206 DOI: 10.1089/jir.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowl JT; Gray EE; Pestal K; Volkman HE; Stetson DB, Intracellular Nucleic Acid Detection in Autoimmunity. Annu Rev Immunol 2017, 35, 313–336 DOI: 10.1146/annurev-immunol-051116-052331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrales L; Glickman LH; McWhirter SM; Kanne DB; Sivick KE; Katibah GE; Woo SR; Lemmens E; Banda T; Leong JJ; Metchette K; Dubensky TW Jr.; Gajewski TF, Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell reports 2015, 11 (7), 1018–30 DOI: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iurescia S; Fioretti D; Rinaldi M, Targeting Cytosolic Nucleic Acid-Sensing Pathways for Cancer Immunotherapies. Frontiers in immunology 2018, 9, 711 DOI: 10.3389/fimmu.2018.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H; Hu S; Chen X; Shi H; Chen C; Sun L; Chen ZJ, cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci U S A 2017, 114 (7), 1637–1642 DOI: 10.1073/pnas.1621363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghaffari A; Peterson N; Khalaj K; Vitkin N; Robinson A; Francis JA; Koti M, STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. British journal of cancer 2018. DOI: 10.1038/s41416-018-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu J; Kanne DB; Leong M; Glickman LH; McWhirter SM; Lemmens E; Mechette K; Leong JJ; Lauer P; Liu W; Sivick KE; Zeng Q; Soares KC; Zheng L; Portnoy DA; Woodward JJ; Pardoll DM; Dubensky TW Jr.; Kim Y, STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 2015, 7 (283), 283ra52 DOI: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson MC; Crespo MP; Abraham W; Moynihan KD; Szeto GL; Chen SH; Melo MB; Mueller S; Irvine DJ, Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest 2015, 125 (6), 2532–46 DOI: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirey KA; Nhu QM; Yim KC; Roberts ZJ; Teijaro JR; Farber DL; Blanco JC; Vogel SN, The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), induces IFN-beta-mediated antiviral activity in vitro and in vivo. J Leukoc Biol 2011, 89 (3), 351–7 DOI: 10.1189/jlb.0410216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo F; Han Y; Zhao X; Wang J; Liu F; Xu C; Wei L; Jiang JD; Block TM; Guo JT; Chang J, STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob Agents Chemother 2015, 59 (2), 1273–81 DOI: 10.1128/AAC.04321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skouboe MK; Knudsen A; Reinert LS; Boularan C; Lioux T; Perouzel E; Thomsen MK; Paludan SR, STING agonists enable antiviral cross-talk between human cells and confer protection against genital herpes in mice. PLoS Pathog 2018, 14 (4), e1006976 DOI: 10.1371/journal.ppat.1006976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aroh C; Wang Z; Dobbs N; Luo M; Chen Z; Gao J; Yan N, Innate Immune Activation by cGMP-AMP Nanoparticles Leads to Potent and Long-Acting Antiretroviral Response against HIV-1. J Immunol 2017, 199 (11), 3840–3848 DOI: 10.4049/jimmunol.1700972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haag SM; Gulen MF; Reymond L; Gibelin A; Abrami L; Decout A; Heymann M; van der Goot FG; Turcatti G; Behrendt R; Ablasser A, Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559 (7713), 269–273 DOI: 10.1038/s41586-018-0287-8. [DOI] [PubMed] [Google Scholar]
- 41.Li L; Yin Q; Kuss P; Maliga Z; Millan JL; Wu H; Mitchison TJ, Hydrolysis of 2’3’-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol 2014, 10 (12), 1043–8 DOI: 10.1038/nchembio.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang J; Kang T; Lee J; Choi BS; Han S, Design, synthesis, and biological evaluation of C7-functionalized DMXAA derivatives as potential human-STING agonists. Organic & biomolecular chemistry 2018. DOI: 10.1039/c8ob01798k. [DOI] [PubMed] [Google Scholar]
- 43.Zheng S; Liu J; Faried A; Richard SA; Gao X, Novel Chemically Synthesized, Alpha-Mangostin-Loaded Nano-Particles, Enhanced Cell Death Through Multiple Pathways Against Malignant Glioma. Journal of biomedical nanotechnology 2018, 14 (11), 1866–1882 DOI: 10.1166/jbn.2018.2627. [DOI] [PubMed] [Google Scholar]
- 44.Phan TKT; Shahbazzadeh F; Pham TTH; Kihara T, Alpha-mangostin inhibits the migration and invasion of A549 lung cancer cells. PeerJ 2018, 6, e5027 DOI: 10.7717/peerj.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y; Sun Z; Pei J; Luo Q; Zeng X; Li Q; Yang Z; Quan J, Identification of alpha-Mangostin as an Agonist of Human STING. ChemMedChem 2018. DOI: 10.1002/cmdc.201800481. [DOI] [PubMed] [Google Scholar]
- 46.Ramanjulu JM; Pesiridis GS; Yang J; Concha N; Singhaus R; Zhang SY; Tran JL; Moore P; Lehmann S; Eberl HC; Muelbaier M; Schneck JL; Clemens J; Adam M; Mehlmann J; Romano J; Morales A; Kang J; Leister L; Graybill TL; Charnley AK; Ye G; Nevins N; Behnia K; Wolf AI; Kasparcova V; Nurse K; Wang L; Li Y; Klein M; Hopson CB; Guss J; Bantscheff M; Bergamini G; Reilly MA; Lian Y; Duffy KJ; Adams J; Foley KP; Gough PJ; Marquis RW; Smothers J; Hoos A; Bertin J, Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018, 564 (7736), 439–443 DOI: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 47.Pryke KM; Abraham J; Sali TM; Gall BJ; Archer I; Liu A; Bambina S; Baird J; Gough M; Chakhtoura M; Haddad EK; Kirby IT; Nilsen A; Streblow DN; Hirsch AJ; Smith JL; DeFilippis VR, A Novel Agonist of the TRIF Pathway Induces a Cellular State Refractory to Replication of Zika, Chikungunya, and Dengue Viruses. mBio 2017, 8 (3) DOI: 10.1128/mBio.00452-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sali TM; Pryke KM; Abraham J; Liu A; Archer I; Broeckel R; Staverosky JA; Smith JL; Al-Shammari A; Amsler L; Sheridan K; Nilsen A; Streblow DN; DeFilippis VR, Characterization of a Novel Human-Specific STING Agonist that Elicits Antiviral Activity Against Emerging Alphaviruses. PLoS Pathog 2015, 11 (12), e1005324 DOI: 10.1371/journal.ppat.1005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B; Tang L; Zhang X; Ma J; Sehgal M; Cheng J; Zhang X; Zhou Y; Du Y; Kulp J; Guo JT; Chang J, A cell-based high throughput screening assay for the discovery of cGAS-STING pathway agonists. Antiviral Res 2017, 147, 37–46 DOI: 10.1016/j.antiviral.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bresnahan WA; Hultman GE; Shenk T, Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J Virol 2000, 74 (22), 10816–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larkin B; Ilyukha V; Sorokin M; Buzdin A; Vannier E; Poltorak A, Cutting Edge: Activation of STING in T Cells Induces Type I IFN Responses and Cell Death. J Immunol 2017, 199 (2), 397–402 DOI: 10.4049/jimmunol.1601999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baguley BC, Small-molecule cytokine inducers causing tumor necrosis. Curr Opin Investig Drugs 2001, 2 (7), 967–75. [PubMed] [Google Scholar]
- 53.Ching LM; Young HA; Eberly K; Yu CR, Induction of STAT and NFkappaB activation by the antitumor agents 5,6-dimethylxanthenone-4-acetic acid and flavone acetic acid in a murine macrophage cell line. Biochem Pharmacol 1999, 58 (7), 1173–81. [DOI] [PubMed] [Google Scholar]
- 54.Guo JT; Guo H, Metabolism and function of hepatitis B virus cccDNA: Implications for the development of cccDNA-targeting antiviral therapeutics. Antiviral Res 2015, 122, 91–100 DOI: 10.1016/j.antiviral.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang L; Zhao Q; Wu S; Cheng J; Chang J; Guo JT, The current status and future directions of hepatitis B antiviral drug discovery. Expert opinion on drug discovery 2017, 12 (1), 5–15 DOI: 10.1080/17460441.2017.1255195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Penna A; Artini M; Cavalli A; Levrero M; Bertoletti A; Pilli M; Chisari FV; Rehermann B; Del Prete G; Fiaccadori F; Ferrari C, Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest 1996, 98 (5), 1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu D; Liu L; Yang D; Fu S; Bian Y; Sun Z; He J; Su L; Zhang L; Peng H; Fu YX, Clearing Persistent Extracellular Antigen of Hepatitis B Virus: An Immunomodulatory Strategy To Reverse Tolerance for an Effective Therapeutic Vaccination. J Immunol 2016, 196 (7), 3079–87 DOI: 10.4049/jimmunol.1502061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bility MT; Cheng L; Zhang Z; Luan Y; Li F; Chi L; Zhang L; Tu Z; Gao Y; Fu Y; Niu J; Wang F; Su L, Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog 2014, 10 (3), e1004032 DOI: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gall B; Pryke K; Abraham J; Mizuno N; Botto S; Sali TM; Broeckel R; Haese N; Nilsen A; Placzek A; Morrison T; Heise M; Streblow D; DeFilippis V, Emerging Alphaviruses Are Sensitive to Cellular States Induced by a Novel Small-Molecule Agonist of the STING Pathway. J Virol 2018, 92 (6) DOI: 10.1128/JVI.01913-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo F; Wu S; Julander J; Ma J; Zhang X; Kulp J; Cuconati A; Block TM; Du Y; Guo JT; Chang J, A Novel Benzodiazepine Compound Inhibits Yellow Fever Virus Infection by Specifically Targeting NS4B Protein. J Virol 2016. DOI: 10.1128/JVI.01253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma J; Zhang X; Soloveva V; Warren T; Guo F; Wu S; Lu H; Guo J; Su Q; Shen H; Solon E; Comunale MA; Mehta A; Guo JT; Bavari S; Du Y; Block TM; Chang J, Enhancing the antiviral potency of ER alpha-glucosidase inhibitor IHVR-19029 against hemorrhagic fever viruses in vitro and in vivo. Antiviral Res 2017. DOI: 10.1016/j.antiviral.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trapnell C; Pachter L; Salzberg SL, TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25 (9), 1105–11 DOI: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trapnell C; Williams BA; Pertea G; Mortazavi A; Kwan G; van Baren MJ; Salzberg SL; Wold BJ; Pachter L, Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010, 28 (5), 511–5 DOI: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trapnell C; Hendrickson DG; Sauvageau M; Goff L; Rinn JL; Pachter L, Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 2013, 31 (1), 46–53 DOI: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma J; Zhang X; Soloveva V; Warren T; Guo F; Wu S; Lu H; Guo J; Su Q; Shen H; Solon E; Comunale MA; Mehta A; Guo JT; Bavari S; Du Y; Block TM; Chang J, Enhancing the antiviral potency of ER alpha-glucosidase inhibitor IHVR-19029 against hemorrhagic fever viruses in vitro and in vivo. Antiviral Res 2018, 150, 112–122 DOI: 10.1016/j.antiviral.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


