Abstract
Purpose
Aromatase inhibitors (AIs) are associated with musculoskeletal symptoms and risk of developing carpal tunnel syndrome (CTS), which can impair quality of life and prompt treatment discontinuation. The incidence of CTS and clinical utility of diagnostic tests such as 2-point discrimination (2-PD) have not been prospectively examined among women receiving AIs.
Methods
Postmenopausal women with stage 0-III hormone receptor-positive breast cancer who were enrolled in a randomized clinical trial investigating adjuvant AIs (Exemestane and Letrozole Pharmacogenetics, ELPh) underwent prospective evaluation of 2-PD with the Disc-criminator™ (sliding aesthesiometer) and completed a CTS questionnaire at baseline, 3, 6, and 12 months, following initiation of AI. Changes in mean 2-PD were analyzed with multivariable mixed effects modelling. A p value < 0.05 was considered statistically significant.
Results
Of 100 women who underwent baseline 2-PD testing, CTS was identified by questionnaire in 11% at baseline prior to AI initiation. Prevalence of CTS at any time in the first year was 26%. A significant increase in worst 2-PD score was observed from baseline to 3 months (3.7 mm to 3.9 mm, respectively, p = 0.03) when adjusted for age, prior chemotherapy, randomized treatment assignment, and diabetes. There were no significant differences in treatment discontinuation due to CTS between the arms.
Conclusion
For women receiving adjuvant AI, 2-PD scores were significantly worse at 3 months compared to baseline. Studies are required to assess whether change in 2-PD is an adequate objective assessment for CTS with AI therapy. Early diagnosis of CTS may expedite management, improve AI adherence, and enhance breast cancer outcomes.
Keywords: Breast cancer survivor, Aromatase inhibitor induced musculoskeletal symptoms, Carpal tunnel syndrome, Endocrine therapy, Early detection
Introduction
Third-generation aromatase inhibitors (AIs) for 5–10 years represent an integral component of endocrine therapy for postmenopausal women with hormone receptor positive (HR +) breast cancer [1]. Despite substantial benefits of adjuvant AIs in reducing risk of recurrence by about 30% and death by 15% compared to tamoxifen, over 30% of women discontinue the medication due to side effects [2–6]. AIs are associated with common musculoskeletal symptoms such as myalgias and arthralgias, which can severely impact quality of life [7–11]. Initial rates of AI-associated musculoskeletal symptoms reported in randomized trials were 19–39%. However, subsequent series report that up to 58% of women report these symptoms, [3], [12], [13] which usually begin within the first few months of initiating these medications [14–17]. In the Exemestane or Letrozole Pharmacogenomics (ELPh) prospective randomized trial, we reported that 24.3% of women discontinued adjuvant AI therapy within 2 years due to musculoskeletal symptoms, with a median time to treatment discontinuation of 6.1 months [2].
AIs may also be associated with an increased risk of specific pathophysiological conditions such as carpal tunnel syndrome (CTS) [18]. CTS is a pressure-induced entrapment neuropathy caused by compression on the median nerve, which is more prevalent in patients with breast cancer receiving AIs than in those treated with tamoxifen, and surgical release might be necessary in some cases [19]. The association of CTS and AI therapy was not initially recognized in large randomized trials but emerged in subsequent analyses. The investigators from the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial reported CTS in 2.6% of participants in the anastrozole arm compared to 1% in the tamoxifen arm (p < 0.0001) [14]. The Intergroup Exemestane Study (IES) investigators reported a similar proportion of CTS (2.8%) to that reported in ATAC (p < 0.0001) [20].
The clinical diagnosis of CTS is made with symptoms of pain, weakness, and paresthesias in the affected hand and digits [21]. Numbness in the distribution of the median nerve, nocturnal symptoms, thenar muscle atrophy, positive Tinel’s sign, and abnormal sensory testing such as finger 2-point discrimination (2-PD) have been standardized as diagnostic criteria [22]. Ultrasonography and electrodiagnostic studies can help confirm the diagnosis in atypical cases. If missed or neglected, CTS may result in irreversible damage to the median nerve [23].
We aimed to prospectively characterize the prevalence of CTS in postmenopausal women receiving adjuvant AI therapy, and to evaluate whether there is a change in 2-PD over one year. We hypothesized that since 2-PD is one of the measures that can be used for diagnosing CTS, we would observe a correlation between change in 2-PD and clinical diagnosis of CTS.
Methods
Study design and oversight
The parent study titled “A multi-center randomized clinical trial correlating the effects of 24 months of AIs (exemestane or letrozole) on surrogate markers of response with aromatase polymorphisms” enrolled participants at three centers, including Indiana University School of Medicine, University of Michigan Health System and the Johns Hopkins School of Medicine (ELPh trial; clinicaltrials.gov identifier # NCT00228956). Previous publications include details regarding design and enrollment [2]. This sub-study was added to the parent study in 2008 and included all patients enrolled from that time forward. The study was approved by each local Institutional Review Board. All participants signed a written informed consent before participating in the trial. Eligible participants included postmenopausal women with histologically proven HR + stage 0–III breast cancer and were candidates for adjuvant AI therapy either upfront or following tamoxifen. Participants were randomized to exemestane or letrozole daily for 2 years.
Assessment of CTS and two-point discrimination
Sub-study participants completed the validated Kamath 9-item CTS survey to determine the presence of CTS at baseline and 3, 6, and 12 months following initiation of the AI [24]. A score of equal to or greater than five (out of a maximum score of 10) is diagnostic of CTS and can be used for referral to a hand surgeon for surgical decompression without the need for confirmatory nerve conduction studies. 2-PD measurements were taken by trained study personnel with the Disc-criminator™ (sliding aesthesiometer) at baseline and 3, 6, and 12 months following AI initiation. Briefly, the sharp ends of the Disc-criminator™ were simultaneously applied at two points to the skin on the volar tip pulp of the index fingers, until the capillary bed in the skin surface beneath the prongs just barely began to blanch. The distance between the two points was varied by the examiner during measurements. The examiner and participant were sitting, the participant with closed eyes and forearm in supination on a desk, and indicated whether she felt one or two points as static measurements were taken. The initial distance between the two points started at 20 mm and was gradually decreased until the participant could not differentiate the two points. The threshold value was determined as the shortest distance between the two points that was differentiated correctly at least seven out of 10 times and was repeated three times at each index finger for both hands. Average 2-PD was the average of the sum of the 3 measurements on each hand divided by 6. The worst 2-PD was the largest value of the two average 2-PD measurements calculated on each hand.
Statistical analyses
Baseline descriptive characteristic were examined. Changes from baseline in the average of the 2-PD for the left and right fingers and worst 2-PD were estimated from mixed effects linear regression models with indicators for the time points (3, 6, or 12 months) and a random intercept for each participant, while adjusting for age, prior taxane chemotherapy, randomized treatment assignment, and history of diabetes. This modelling approach allows for all observed data points to be used in the estimation, regardless of whether patients had missing data during follow-up. To check that patients with missing follow-up data were not different from those who continued through the study, we compared their baseline characteristics.
Changes in the probability of having CTS (score of five or higher) over time were estimated similarly using logistic regression. Differential changes in these outcomes according to subgroups (i.e., age, body mass index [BMI], prior chemotherapy, randomized drug assignment) at each time point were assessed with interaction terms in the mixed effects regression models. Differences between participants with CTS at 3 and 6 months were explored using logistic regression models adjusted for randomized drug assignment. The association between CTS (yes/no) over time and 2-PD scores over time was assessed with mixed effects linear regression models with CTS as the outcome and terms for the 2-PD score, time-point, and their interaction, randomized drug assignment, and a random intercept for each participants. Analyses were completed in R version 3.4.2.
Results
From 2005 to 2009, 503 participants enrolled in the parent ELPh trial. The sub-study was added in 2008 and included patients who received exemestane or letrozole from 2008 to 2009. A total of 100 women underwent baseline 2-PD testing and were included in the analytical cohort (Fig. 1). Patient characteristics are described in Table 1. Mean age was 59 years, the majority (92%) were Caucasian, 39% received adjuvant taxanes, 76% were overweight or obese, 14.1% had diabetes, and 12% reported prior history of CTS. Those with a self-reported history of CTS were more likely to have CTS on baseline survey (odds ratio = 5.8, 95% CI: 1.4, 24.0, p = 0.02) and a higher worst baseline 2-PD (mean difference = 1.3, 95% CI: 0.38, 2.2, p = 0.006) than those without a history of CTS.
Fig. 1.
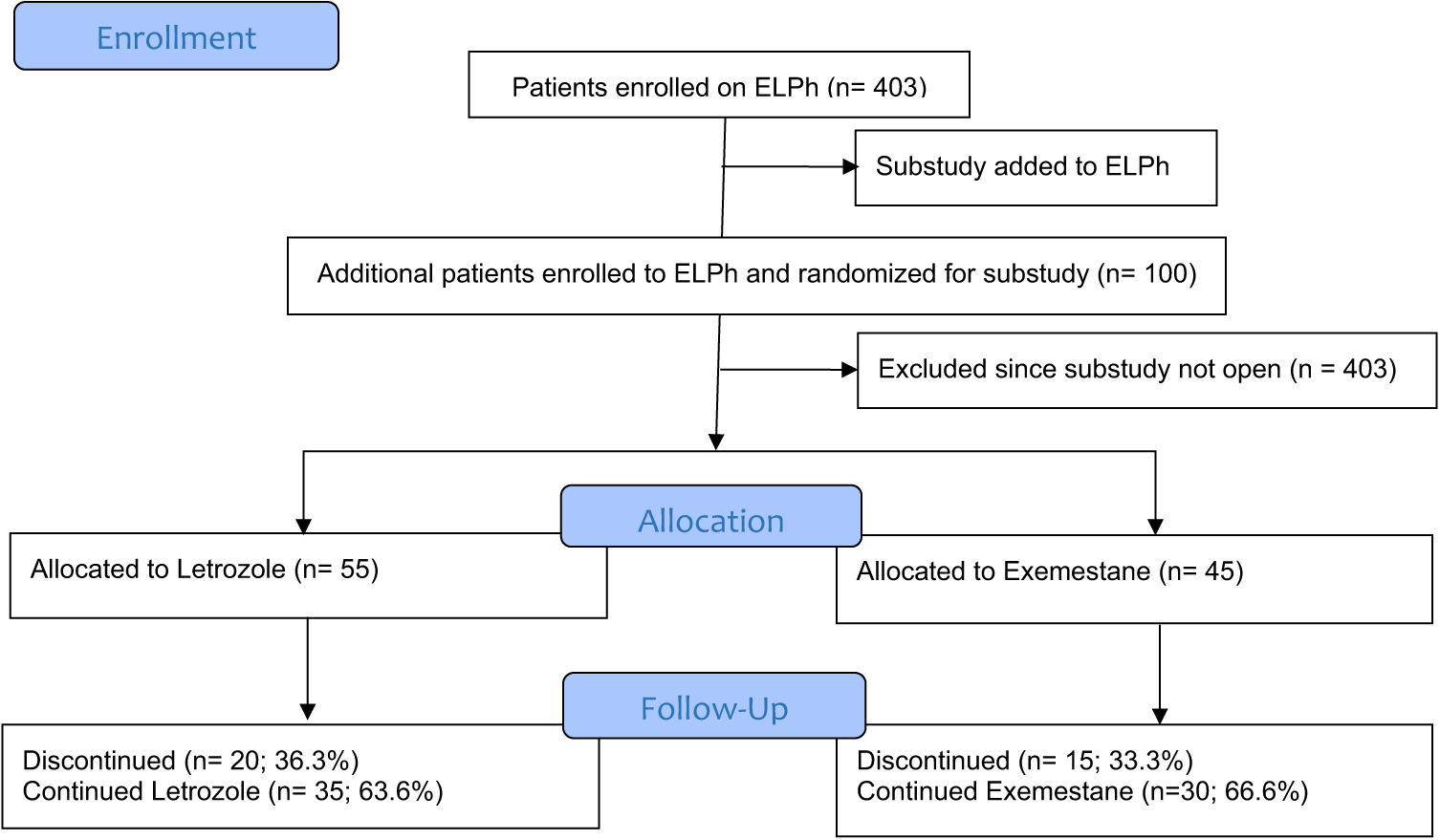
CONSORT Flow Diagram
Table 1.
Baseline patient characteristics (N = 100)
| Age – mean (SD) | 58.52 (9.36) |
| Race – no. (%) | |
| Asian | 3 (3) |
| Black | 5 (5) |
| White | 92 (92) |
| BMI – mean (SD) | 29.76 (5.85) |
| BMI – no. (%) | |
| < 25 | 24 (24) |
| 25–30 | 35 (35) |
| 30 | 41 (41) |
| Tumor size – mean in cm | 1.49 |
| Drug assignment – no. (%) | |
| Exemestane | 45 (45) |
| Letrozole | 55 (55) |
| Stage – no. (%) | |
| 0 | 8 (8) |
| I | 54 (54) |
| II | 27 (27) |
| III | 11 (11) |
| Prior chemotherapy – no. (%) | 44 (44) |
| Taxane – no. (%) | 39 (41.1) |
| Platinum – no. (%) | 3 (3.2) |
| Diabetes – no. (%) | 14 (14.1)a |
| Thyroid disorder – no. (%) | 20 (20.2)a |
| Patient-reported history of CTS – no. (%) | 12 (12) |
| CTS total score – mean (SD) | 3 (1.33, 0 to 8) |
| CTS ≥ 5 at baseline – no. (%) | 11 (11) |
| 2-PD left arm average – mean in mm (SD) | 3.17 (1.02) |
| 2-PD right arm average – mean in mm (SD) | 3.43 (1.55) |
| Average of left and right finger 2-PD Score – mean in mm (SD) | 3.32 (1.15) |
| Worst 2-PD Score – mean in mm (SD) | 3.64 (1.58) |
One patient had missing information regarding diabetes and thyroid history
SD standard deviation; no. number; BMI body mass index; CTS carpal tunnel syndrome; 2-PD 2-point discrimination
Prevalence of CTS based on baseline survey was 11% (11/100), compared to 12% after 3 months of AI (10/84; p = 0.84 relative to baseline), 17% after 6 months of AI (13/78; p = 0.24), and 15% after 12 months of AI (10/65; p = 0.21). The prevalence of CTS at any point in the first year was 26%. Fifteen new cases of CTS were diagnosed with the CTS questionnaire during the study. The AI discontinuation rates in sub-study participants were 19% at 3 months and 53% at 12 months. There was no difference in baseline characteristics between those who continued on study compared to those with missing follow-up data. We observed a statistically significant increase in the worst 2-PD score of 0.3 points at 3 months (p = 0.03) when adjusted for age, prior chemotherapy, randomized drug assignment, diabetes, and history of CTS. We did not observe significant changes in average 2-PD at any time points, incidence of CTS by questionnaire or 2-PD by drug, average of left and right finger 2-PD, or prevalence of CTS at 6 and 12 months (Table 2).
Table 2.
Changes in average (left and right) and worst score for two-point discrimination (2-PD) and carpal tunnel syndrome (CTS) score at 3, 6, and 12 months from baseline
| 3 months | 6 months | 12 months | |
|---|---|---|---|
| 2-PD Average (mm) | |||
| No. in analysis | 81 | 74 | 47 |
| Baseline, mean (SD) | 3.3 (1.2) | 3.3 (1) | 3.1 (0.9) |
| Follow-up, mean (SD) | 3.5 (1.4) | 3.4 (0.9) | 3.2 (0.7) |
| Follow-up, median (range) | 3.1 (1, 12.2) | 3 (1, 5.8) | 3.1 (2, 5.3) |
| Change from baseline, mean (SD) | 0.2 (1) | 0 (0.8) | 0.1 (0.7) |
| P value, change from baselinea | 0.08 | 0.47 | 0.42 |
| Worst 2-PD (mm) | |||
| No. in analysis | 81 | 74 | 47 |
| Baseline, mean (SD) | 3.7 (1.7) | 3.6 (1.2) | 3.3 (1) |
| Follow-up, mean (SD) | 3.9 (2.2) | 3.6 (1) | 3.5 (0.8) |
| Follow-up, median (range) | 3.3 (1, 20) | 3.3 (1, 7) | 3.3 (2, 6) |
| Change from baseline, mean (SD) | 0.3 (1.3) | 0 (0.9) | 0.1 (0.8) |
| P value, change from baselinea | 0.03 | 0.72 | 0.55 |
| CTS Total Score | |||
| No. in analysis | 84 | 78 | 65 |
| Baseline, mean (SD) | 3 (1.3) | 3.1 (1.3) | 3 (1) |
| Follow-up, mean (SD) | 2.9 (1.4) | 3.3 (1.3) | 3.3 (1.2) |
| Follow-up, median (range) | 3 (−1, 7) | 3 (−1, 6) | 3 (1, 6) |
| Change from baseline, mean (SD) | −0.1 (1.3) | 0.2 (1.3) | 0.3 (1.3) |
| P value, change from baselinea | 0.42 | 0.21 | 0.2 |
| CTS Score ≥ 5 | |||
| No. in analysis | 84 | 78 | 65 |
| No. with CTS ≥ 5 (%) | 10 (12) | 13 (17) | 10 (15) |
| P value, change from baselinea | 0.84 | 0.24 | 0.21 |
P values from a single mixed effects linear regression model with a random intercept for each participant. Model-based p values include adjustment for age, prior chemotherapy, randomized drug assignment, and diabetes (2-PD and CTS total score only)
SD standard deviation
In a subgroup analysis, the worst 2-PD following 3 months of AI therapy deteriorated significantly among women with baseline BMI 25 or higher (change = 0.29 [0.03, 0.56], p = 0.03, Table 3), though this change was not significantly different from those with BMI < 25 (change 0.1, interaction p = 0.43). The changes in worst 2-PD from baseline to 3 months of AI therapy also did not differ according to age, randomized drug assignment, prior chemotherapy, or history of CTS.
Table 3.
Mean changes in worst two-point discrimination (worst 2-PD) score at 3, 6, and 12 months from baseline, by patient subgroups
| Mean in mm (SD), BL | 3 m vs. BL | 6 m vs. BL | 12 m vs. BL | IntP, 3 M | IntP, 6 M | IntP, 12 M | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| < 60 | 3.2 (1.2) | 0.23 [−0.09, 0.55], P = 0.16 | 0 [−0.33, 0.33], P = 1 | −0.02 [−0.42, 0.38], P = 0.91 | |||
| ≥ 60 | 3.5 (1) | 0.34 [−0.15, 0.83], P = 0.18 | 0.15 [−0.37, 0.67], P = 0.57 | 0.05 [−0.56, 0.66], P = 0.88 | 0.94 | 0.77 | 0.47 |
| Prior chemo | |||||||
| None | 3.2 (1) | 0.26 [−0.01, 0.52], P = 0.06 | 0.07 [−0.2, 0.34], P = 0.62 | 0.13 [−0.19, 0.45], P = 0.42 | |||
| Yes | 3.4 (1.3) | 0.22 [−0.14, 0.59], P = 0.24 | −0.01 [−0.39, 0.37], P = 0.97 | −0.02 [−0.46, 0.43], P = 0.95 | 0.89 | 0.74 | 0.53 |
| BMI | |||||||
| <25 | 3 (1.2) | 0.1 [−0.27, 0.46], P = 0.6 | 0.09 [−0.28, 0.47], P = 0.63 | 0.22 [−0.2, 0.64], P = 0.31 | |||
| ≥ 25 | 3.4 (1.1) | 0.29 [0.03, 0.56], P = 0.03 | 0.01 [−0.26, 0.28], P = 0.92 | 0.02 [−0.31, 0.34], P = 0.91 | 0.43 | 0.77 | 0.49 |
| Drug assignment | |||||||
| Exemestane | 3.1 (0.9) | 0.33 [0.02, 0.64], P = 0.04 | 0.24 [−0.09, 0.58], P = 0.16 | 0.12 [−0.25, 0.49], P = 0.52 | 0.47 | 0.14 | 0.63 |
| Letrozole | 3.5 (1.3) | 0.17 [−0.12, 0.46], P = 0.26 | −0.11 [−0.4, 0.19], P = 0.49 | 0.03 [−0.34, 0.4], P = 0.88 | |||
| History of CTS | |||||||
| None | 3.2 (1) | 0.14 [−0.03, 0.3], P = 0.11 | 0.08 [−0.09, 0.25], P = 0.36 | 0.12 [−0.08, 0.33], P = 0.24 | |||
| Yes | 4 (1.9) | 0.27 [−0.37, 0.91], P = 0.42 | −0.03 [−0.7, 0.64], P = 0.93 | −0.11 [−0.87, 0.65], P = 0.78 | 0.57 | 0.59 | 0.34 |
P values for within-group changes and interactions for differential changes by time point and subgroup
SD standard deviation; BL baseline; IntP interaction p value, follow-up time points (3, 6, or 12 M) compared to baseline; BMI body mass index
We assessed the association between change in 2-PD and score on the CTS questionnaire (Fig. 2). We found no significant association between the average of the left and right finger 2-PD score and CTS score across all time points, and there was no interaction between individual time points; the same was true for the worst score. While not statistically significant, patients with worsening average 2-PD had a higher prevalence of CTS by questionnaire at 3 months (13.2% vs. 9.8%) and 6 months (24% vs. 12.5%); this was not assessed at 12 months due to diminishing sample size. We found no significant association between mean change in worst 2-PD scores and a CTS questionnaire score ≥ 5 consistent with a clinical diagnosis of CTS at 3 and 6 months (Fig. 3).
Fig. 2.
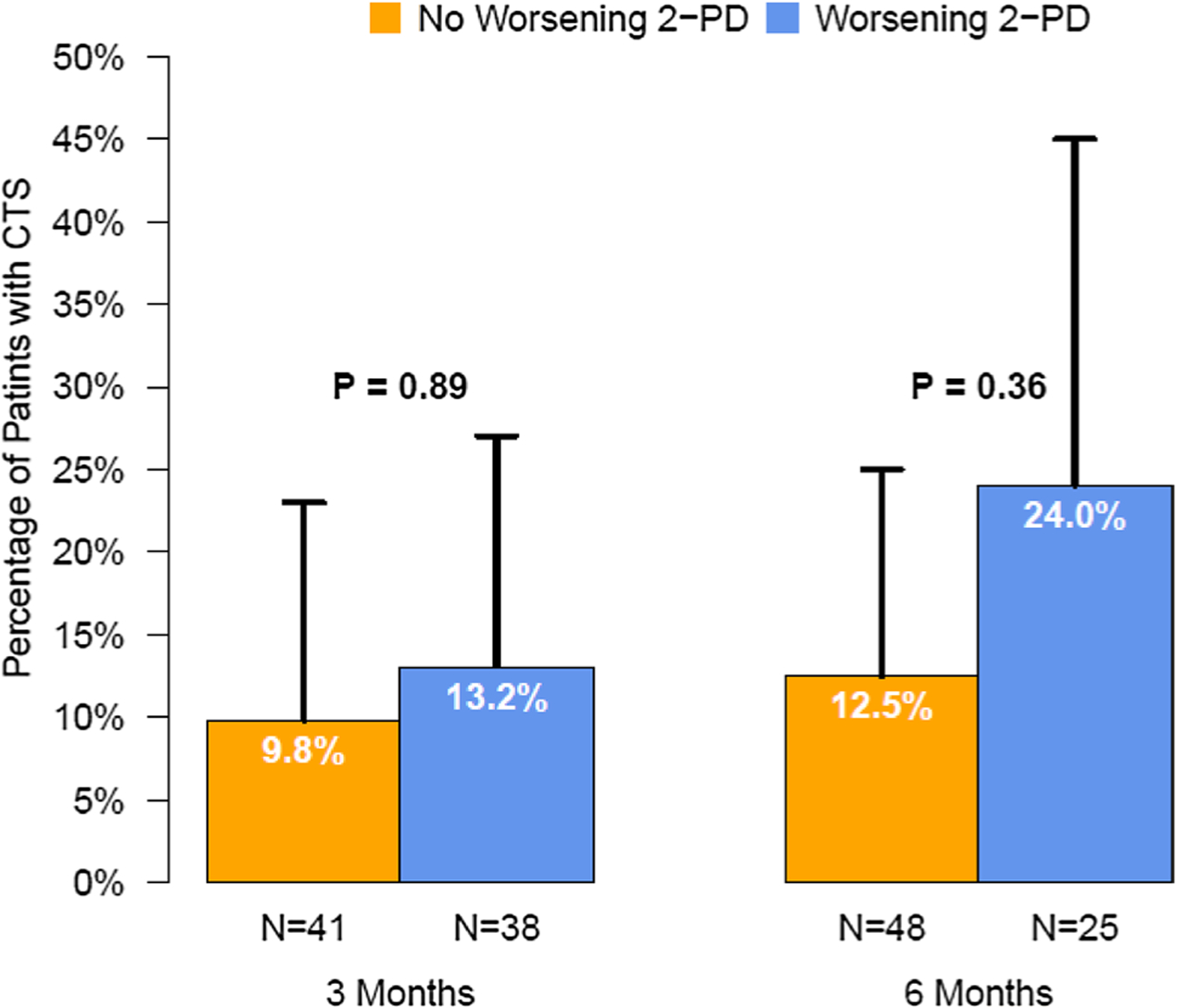
Percentage of patients with a diagnosis of Carpel Tunnel Syndrome (CTS) according to whether the worst 2-PD score worsened (increased) at 3 or 6 months compared to baseline
Fig. 3.
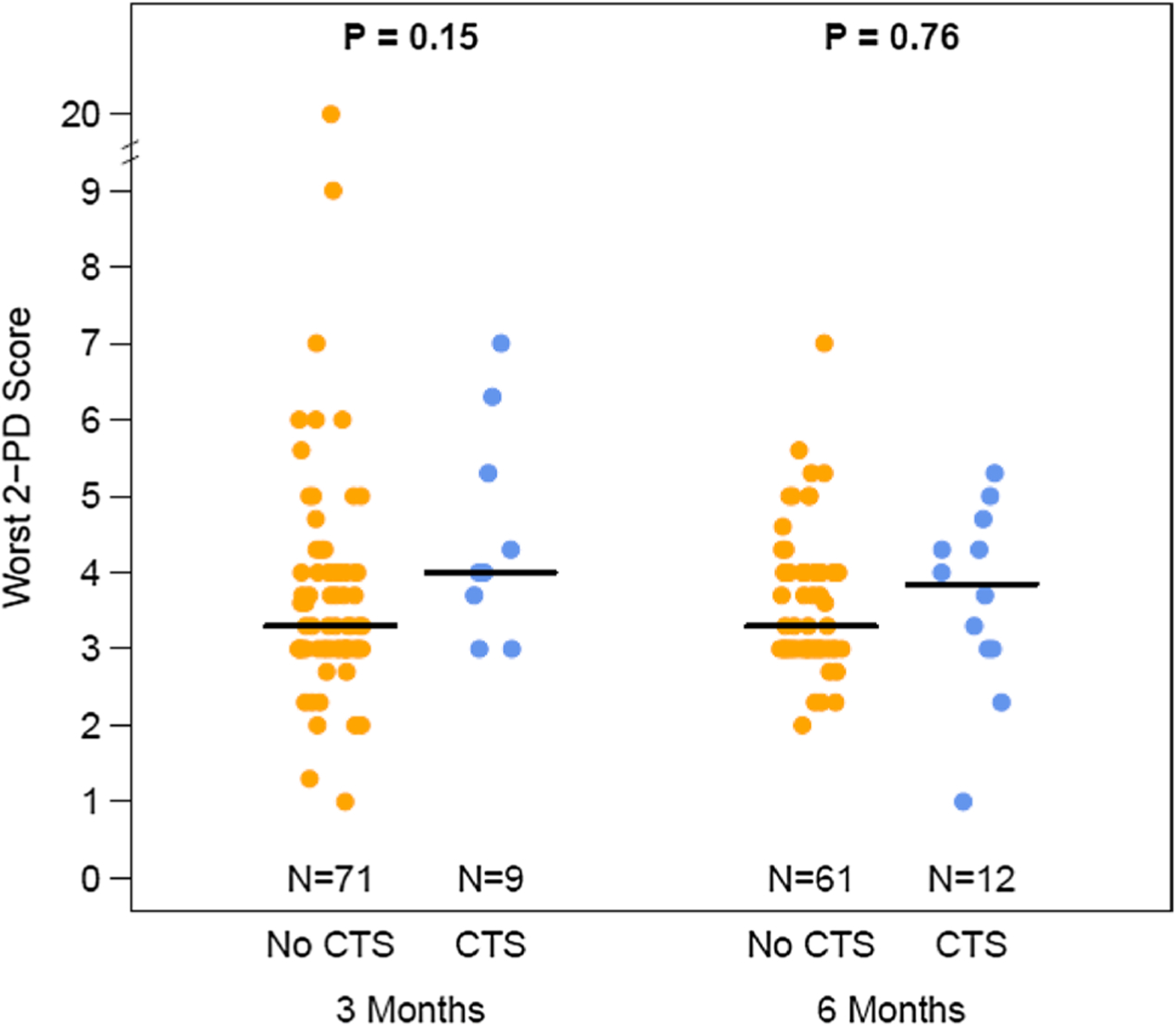
Distribution of the worst two point discrimination (2-PD) for those with and without CTS, at 3 and 6 months, among women receiving adjuvant aromatase inhibitor therapy for breast cancer. The median of the distribution is shown by the horizontal bar
Discussion
Adjuvant endocrine therapy for 5–10 years is associated with substantial survival benefits. Therefore, clinicians should proactively identify and intervene on symptoms, such as CTS, which can impact daily life activities, impede adherence and ultimately lead to increased morbidity. In our prospective study, we observed a high prevalence of CTS within the first year of starting AIs (26%) and a significant worsening of worst 2-PD at 3 months, suggesting that this condition emerges over time. The incidence of CTS while on AIs reported in our study appears to be higher than that reported in previous studies [25], [26].
With an aging population and earlier diagnosis of breast cancer, the breast cancer survivors and users of AI therapy are likely to increase over the next decade [27]. Therefore, conditions associated with AIs may rise, and healthcare professionals should be able to promptly and accurately recognize and manage emerging symptoms. Understanding risk factors for the development of CTS may be critical to prompt detection and early intervention. In a retrospective analysis of data from the ATAC trial, the prevalence of reported CTS in AI users was seven times that of participants receiving tamoxifen therapy and CTS was significantly increased for women who used prior hormone replacement therapy or received prior chemotherapy. Those who were 60 years of age or older at entry were at lower risk of CTS compared with their counterparts [28]. In the ATAC trial, BMI > 30 kg/m2 was associated with increased risk of developing AI-associated musculoskeletal symptoms [28]. Excess body mass markedly increases the risk of CTS by 1.5–2 fold [29]. Although the interaction test for a subgroup effect according to BMI (normal weight v. overweight or obese) was not statistically significant, our data suggest that overweight or obese women may be at higher risk of worsening 2-PD.
Adjuvant AI therapy may be associated with significant change in worst 2-PD as early as 3 months; therefore, early diagnosis and treatment might reduce the need for surgery [30]. The management of CTS is based on severity of symptoms and functional consequences. Earlier stages usually respond to analgesics and/or steroids, while advanced cases may need surgical intervention [31]. In a retrospective analysis of data from IES, CTS greatly affected daily-life activities in 36.2% of participants and 69.0% of those affected underwent surgical release [32]. Clinicians should consider a drug holiday or a change to another endocrine agent and encourage physical activity [33], [34]. Additional research is needed to evaluate whether early detection of CTS and/or 2-PD worsening could lead to reduction in surgical interventions for CTS, or improvement in AI compliance, quality of life, and ultimately survival outcomes.
Several mechanisms by which AI could contribute toward development of CTS have been proposed including local inflammatory response in the tenosynovial structures of the wrist [35–37], profound estrogen deprivation, or mediation of BMI and hand grip strength by growth hormone/insulin like growth factor-I (GH/IGF-I) which is controlled by sex steroids [38]. Single-nucleotide polymorphisms (SNPs) related to cytokines such as the gene (TCL1A) related to cytokine IL-17 may be associated with increased risk of musculoskeletal symptoms from AIs [39].
While our study’s main strength is its prospective assessment, there are a few limitations. A small sample size with short follow-up provides limited power to do subgroup analyses. The reason for discontinuation was not collected in this sub-study, and due to the high AI treatment discontinuation rates in the study, the reported prevalence of CTS at each follow-up is likely an underestimation. Finally, we did not evaluate whether early detection of sub-clinical CTS by 2-PD will ultimately influence adherence to AI or survival outcomes.
In conclusion, compared to a clinical questionnaire, 2-PD may provide a reliable and quantitative measure of sensory loss and lead to greater recognition and assessment of CTS as well as the need for intervention. Further research is needed to define a cutoff for predicting CTS before 2-PD assessment can be routinely incorporated into clinical practice. Ultimately, early diagnosis of CTS may improve the quality of life and survival outcomes in women with early HR + breast cancer.
Acknowledgments
We thank Gary Rosner ScD for assistance in study development, Chenguang Wang PhD in reviewing analysis, David Cornblath in study design and Suzanne Lemler in enrollment.
Funding This study was funded by Pharmacogenetics Research Network Grant U-01 GM61373 (supports the Consortium on Breast Cancer Pharmacogenomics), investigator-initiated grants from Novartis and Pfizer, and Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale ™ (DFH). The study drug was provided by Novartis and Pfizer.
Footnotes
Conflict of interest JS, ALB, RV, GR, JG, SCJ, ZZ, JH, AN, AMS, and KT declare that they have no conflict of interest. AB has received grants from Biothernostics Inc, Immunomedics, Novartis, Genentech/Roche, Merck, Radius Health, Spectrum Pharma, Innocrin, and Mersana Pharmaceuticals; consultant to Immunomedics, Novartis, Genentech/Roche, Merck, Radius Health, Spectrum Pharma, Taiho Pharma, Biothernostics Inc and Sanofi. GR has received grants from AxoGen and Allergan; patents US PCT/US2009/000,887, Europe 09710338.61222, Brazil PI0908087–2, licensed to Aegeria Soft Tissue; patent US 6 + 2/119,047 issued to Sonavex. DFH has received grants from Pfizer, Novartis and Astra Zeneca; Stock in Oncimmune LLC and Inbiomotion; Consultant/advisory panels with Cepheid, Freenome Inc, Artiman Ventures (CellWorks), CVS Caremark, Agendia Inc.; Sponsored Research with Merrimack Pharmaceuticals, Eli Lilly, Menarini Silicon BioSystems, Puma Biotechnology, Pfizer, Astra Zeneca; Patents US 8790, 878 B2 licensed to Menarini Silicon BioSystems. NLH has received grants from NIH, Pfizer, Novartis, AbbVie, H3 Biomedicine, and Innocrin Pharmaceuticals. VS has received grants from Abbvie, Biocept, Pfizer, Novartis, Medimmune, and Puma Biotechnology; data safety monitoring board for Immunomedics; consultant to Iridium Therapeutics, Inc.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Research involving human and animal participants This article does not contain any studies with animals performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burstein HJ et al. (2018) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: aSCO clinical practice guideline focused update. J Clin Oncol 37(5):423–438 [DOI] [PubMed] [Google Scholar]
- 2.Henry NL et al. (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26(4):556–562 [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet 386(10001):1341–1352 [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Kim J, Haque R (2014) Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev. Res 7(4):378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C (2013) Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 108(7):1515–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman RE et al. (2008) Aromatase inhibitor-induced arthralgia: clinical experience and treatment recommendations. Cancer Treat Rev 34(3):275–282 [DOI] [PubMed] [Google Scholar]
- 8.Aiello Bowles EJ et al. (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J. Oncol. Pract 8(6):e149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaillard S, Stearns V (2011) Aromatase inhibitor-associated bone and musculoskeletal effects: new evidence defining etiology and strategies for management. Breast Cancer Res 13(2):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirgwin JH et al. (2016) Treatment adherence and its impact on disease-free survival in the Breast International Group 1–98 trial of Tamoxifen and Letrozole, alone and in sequence. J Clin Oncol 34(21):2452–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry NL et al. (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111(2):365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Din OS, Dodwell D, Wakefield RJ, Coleman RE (2010) Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat 120(3):525–538 [DOI] [PubMed] [Google Scholar]
- 13.Hershman DL et al. (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzdar A, Howell A, Cuzick J (2006) Arimidex, tamoxifen, alone or in combination trialists’ group comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 7:633–643 [DOI] [PubMed] [Google Scholar]
- 15.Crew KD et al. (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25(25):3877–3883 [DOI] [PubMed] [Google Scholar]
- 16.Castel LD et al. (2013) Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer 119(13):2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao JJ et al. (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115(16):3631–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padua L et al. (2016) Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 15(12):1273–1284 [DOI] [PubMed] [Google Scholar]
- 19.Nishihori T et al. (2008) Carpal tunnel syndrome associated with the use of aromatase inhibitors in breast cancer. Clin. Breast Cancer 8(4):362–365 [DOI] [PubMed] [Google Scholar]
- 20.Coombes RC et al. (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369(9561):559–570 [DOI] [PubMed] [Google Scholar]
- 21.Padua L, Padua R, Lo Monaco M, Aprile I, Tonali P (1999) Multiperspective assessment of carpal tunnel syndrome: a multicenter study. Italian CTS Study Group. Neurology 53(8):1654–1659 [DOI] [PubMed] [Google Scholar]
- 22.Wipperman J, Goerl K (2016) Carpal tunnel syndrome: diagnosis and management. Am Fam Physician 94(12):993–999 [PubMed] [Google Scholar]
- 23.Gillig JD, White SD, Rachel JN (2016) Acute carpal tunnel Syndrome: a review of current literature. Orthop Clin North Am 47(3):599–607 [DOI] [PubMed] [Google Scholar]
- 24.Kamath V, Stothard J (2003) A clinical questionnaire for the diagnosis of carpal tunnel syndrome. J. Hand Surg 28(5):455–459 [DOI] [PubMed] [Google Scholar]
- 25.Morales L et al. (2007) Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat 104(1):87–91 [DOI] [PubMed] [Google Scholar]
- 26.Spagnolo F, Sestak I, Howell A, Forbes JF, Cuzick J (2016) Anastrozole-induced carpal tunnel syndrome: results from the International Breast Cancer Intervention Study II prevention trial. J Clin Oncol 34(2):139–143 [DOI] [PubMed] [Google Scholar]
- 27.Rowland JH, Bellizzi KM (2014) Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol 32(24):2662–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sestak I, Sapunar F, Cuzick J (2009) Aromatase inhibitor-induced carpal tunnel syndrome: results from the ATAC trial. J Clin Oncol 27(30):4961–4965 [DOI] [PubMed] [Google Scholar]
- 29.Shiri R, Pourmemari MH, Falah-Hassani K, Viikari-Juntura E (2015) The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev 16(12):1094–1104 [DOI] [PubMed] [Google Scholar]
- 30.Liedke PE, Goss PE (2012) Aromatase inhibitors and musculoskeletal adverse events. Lancet Oncol. 13(4):333–334 [DOI] [PubMed] [Google Scholar]
- 31.Vasiliadis HS, Georgoulas P, Shrier I, Salanti G, Scholten RJPM (2014) Endoscopic release for carpal tunnel syndrome. Rev Cochrane Database Syst. 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mieog JSD, Morden JP, Bliss JM, Coombes RC, van de Velde CJH, IES Steering Committee (2012) Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2–3 years of tamoxifen: a retrospective analysis of the Intergroup Exemestane Study. Lancet Oncol 13(4):420–432 [DOI] [PubMed] [Google Scholar]
- 33.Brown JC, Mao JJ, Stricker C, Hwang W-T, Tan K-S, Schmitz KH (2014) Aromatase inhibitor associated musculoskeletal symptoms are associated with reduced physical activity among breast cancer survivors. Breast J. 20(1):22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeNysschen CA, Burton H, Ademuyiwa F, Levine E, Tetewsky S, O’Connor T (2014) Exercise intervention in breast cancer patients with aromatase inhibitor-associated arthralgia: a pilot study. Eur. J. Cancer Care (Engl.) 23(4):493–501 [DOI] [PubMed] [Google Scholar]
- 35.Morales L et al. (2008) Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol 26(19):3147–3152 [DOI] [PubMed] [Google Scholar]
- 36.Lintermans A et al. (2013) Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Ann Oncol 24(2):350–355 [DOI] [PubMed] [Google Scholar]
- 37.Henry NL et al. (2010) A prospective study of aromatase inhibitor-associated musculoskeletal symptoms and abnormalities on serial high-resolution wrist ultrasonography. Cancer 116(18):4360–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lintermans A, Neven P (2011) Pharmacology of arthralgia with estrogen deprivation. Steroids 76(8):781–785 [DOI] [PubMed] [Google Scholar]
- 39.Ingle JN et al. (2010) Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol 28(31):4674–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]


