Abstract
We investigated whether a history of mild traumatic brain injury (mTBI), or concussion, has any effect on visual working memory (WM) performance. In most cases, cognitive performance is thought to return to premorbid levels soon after injury without further medical intervention. We tested this assumption in undergraduates, among whom a history of mTBI is prevalent. Notably, participants with a history of mTBI performed worse than their colleagues with no such history. Experiment 1 employed a change detection paradigm that manipulated visual WM set size from 1–3 items and revealed a significant deficit at set size 3. Experiment 2 investigated whether feedback could rescue WM performance in the mTBI group and found it failed. Experiment 3 manipulated WM maintenance duration (set size 3; 500–1500 ms) to investigate a maintenance-related deficit. Across all durations the mTBI group was impaired. Experiment 4 tested whether retrieval demands contributed to WM deficits showing a consistent deficit across recognition and recall probes. In short, even years after an mTBI, undergraduates perform differently on visual WM tasks compared to their peers with no such history. Given the prevalence of mTBI, these data may benefit other researchers seeing high variability in their data. Clearly, further studies are needed to determine the breadth of cognitive deficits in those with a history of mTBI, to identify relevant factors contributing to positive cognitive outcomes.
Keywords: visual working memory, chronic mTBI, mild TBI, concussion
Introduction
For some years we have puzzled over low working memory (WM) performance in some undergraduate participants. In search of an underlying cause we noted frequent anecdotal reports of ski accidents, horseback falls, cheerleading catastrophes and other stories of concussion, or mild traumatic brain injury (mTBI). In the USA, TBI causes 235,000 hospitalizations annually (Cameron, Marshall, Sturdivant, & Lincoln, 2012; Corrigan, Selassie, & Orman, 2010; Larrabee, 2012; Taylor, Greenspan, Xu, & Kresnow, 2015), with > 85% considered mild (Bazarian et al., 2005; Faul, Xu, Wald, & Coronado, 2010). Barring the development of post concussive syndrome, recovery is assumed to occur within a few months (Cooper et al., 2015). Early recovery reveals deficits in processing speed (De Monte & Geffen, 2005; Shumskaya, Andriessen, Norris, & Vos, 2012), attention (Catale, Marique, Closset, & Meulemans, 2008; Konrad et al., 2011; Mayer et al., 2012), and episodic memory (Wammes, Good, & Fernandes, 2017) and few assess cognitive performance in the chronic mTBI population who are more than 3 months post-injury. In the chronic mTBI population behavioral deficits are reported in a majority of participants (McInnes, Friesen, MacKenzie, Westwood, & Boe, 2017) accompanied by neural differences (Eierud et al., 2014; Ham et al., 2014; Pan et al., 2016; Sharp & Ham, 2011; Sharp, Scott, & Leech, 2014; Shenton et al., 2012; Tate, Shenton, & Bigler, 2012) detectible even years after an mTBI (Bajaj, Dailey, Rosso, Rauch, & Killgore, 2018; Dall’Acqua et al., 2017).
Why might we see effects of a history of mTBI on WM? WM engages broad frontoparietal networks vulnerable to heterogeneous impacts. Thus, across individuals, diverse injuries could lead to behavioral deficits affecting WM. Yet others investigating this issue report mixed results. For instance, one earlier meta-analysis reported no residual deficits in WM after the acute stage of mTBI (>7 days post-injury) (Belanger & Vanderploeg, 2005), others find WM deficits exclusively in those with post-concussive syndrome but not in the chronic mTBI sample (Dean & Sterr, 2013), whereas others report low WM accuracy accompanied by ERP differences (Gosselin et al., 2012; Hudac, Cortesa, Ledwidge, & Molfese, 2017), or slightly greater proactive interference (Vanderploeg, Curtiss, & Belanger, 2005). Thus, in addition to the heterogeneity of injuries, there were data reporting heterogeneous behavioral effects.
To be clear, the students we tested fell into the chronic mTBI category because their injuries were >3 months before their participation (e.g., Fino et al., 2018; Goetzl et al., 2019; Lotan et al., 2018; McNerney et al., 2019). Furthermore, these undergraduates reported no sustained symptoms (e.g., headache, mental fog, etc.) that could be attributed to the mTBI itself. In other words, the only factor that distinguished the groups we tested was that one group reported having had an mTBI, thus we use the term ‘history of mTBI’ to characterize our participants. With regard to our questions, it is important to note that a history of mTBI is high in our undergraduates. In one 400-level course, 31% reported an mTBI, as did 37% of our Disability Resource Center clients. We conducted these experiments to test whether undergraduates with/without a history of mTBI were impaired on simple change detection WM tasks, and consequently, whether we should continue to include them in our ongoing studies of ‘neurotypical’ participants. Across four behavioral experiments there was a consistent pattern: participants with a history of mTBI performed worse at the group level than those without a history of mTBI. The data are available at https://wolfweb.unr.edu/~mberryhill/ and we note that none of the following experiments were preregistered.
Experiment 1
Materials and Methods
Participants
Undergraduates participated as a self-reported mTBI or control (see Table 1 for demographics). All participants were right-handed throughout all experiments. In this and all experiments, participants reported in written format: whether they had a history of mTBI, how many, and when the injury or injuries took place. UNR’s IRB approved all protocols. Participants provided written consent and received $15/hour or bonus credit, their choice.
Table 1.
Demographics across experiments. The top row of each experiment includes data from those with a history of mTBI, the second row reflects the control population data. The mean number of mTBIs and time (in years) since last mTBI per experiment are compiled.
| Exp | Age (SD) | # (#F) | # TBI (SD) | Range # | Time (SD) | Range Time |
|---|---|---|---|---|---|---|
| 1 | 23.83 (4.0) | 18(6) | 1.61 (1.46) | 1–6 | 5.60 (4.80) | 9 mo–17 y |
| 22.80 (3.5) | 20(8) | |||||
| 2 | 23.44 (3.5) | 25(8) | 1.84 (1.17) | 1–5 | 5.61 (4.11) | 1 y – 15 y |
| 23.52 (2.5) | 25(15) | |||||
| 3 | 21.71 (3.8) | 21(10) | 2.14 (1.62) | 1–7 | 3.79 (4.09) | 4 mo – 9.5 y |
| 21.80 (4.2) | 21(14) | |||||
| 4 | 20.77 (2.1) | 22(15) | 2.32 (2.01) | 1–10 | 4.33 (3.08) | 5 mo – 12.8 y |
| 22.40 (3.5) | 22(15) |
Abbreviations: #: number, Exp: experiment, F: female, mo: months, SD: standard deviation, y: years.
Apparatus
The task was presented on a 19” NEC MultiSync CRT monitor (75 Hz, 1024 × 768) in MATLAB (Mathworks, Natick, MA) Psychophysics Toolbox 3.0 extension (Brainard, 1997; Pelli, 1997) using a Mac mini 2.5 GHz dual-core Intel Core i5.
Stimulus and procedure
The change detection visual WM task (Fukuda, Awh, & Vogel, 2010; Ikkai, McCollough, & Vogel, 2010; Luck & Vogel, 2013; Luria, Balaban, Awh, & Vogel, 2016; McCollough, Machizawa, & Vogel, 2007; Edward K Vogel & Machizawa, 2004; Edward K. Vogel & Machlzawa, 2004) presented 1–3 colored squares (0.7° × 0.7°) chosen from a set of seven colors (cyan, white, red, blue, yellow, green, magenta); see Figure 1A. Trials began with a fixation cross (0.4°x 0.4°, 300 ms), followed by a left or right white arrowhead (2.1°x 0.4°, 200 ms) cueing the hemifield to covertly attend. After a delay (300 – 400 ms) stimuli were presented (100 ms) in 2 rectangular areas (7.1° × 12.2°) 4.6° from fixation. After a delay (900 ms) the probe appeared (3 s). Participants indicated whether the stimulus and probe item matched (“o” key, 50%) or not (“n” key). Self-paced trials included 3 breaks. Before testing, participants completed 24 practice trials. There were 576 trials;192 trials per set size (1–3 items). Participants were instructed to maintain fixation and eye movements were monitored by HD-EEG. The primary performance measure was WM capacity: K = Set size*(Hit rate – False alarm rate) (Cowan, 2001; Pashler, 1988). Median correct reaction time was also recorded. Four controls performed 2+ standard deviations below the mean and were removed.
Figure 1. Task paradigm, stimulus configurations, and behavioral results for experiment 1–3.
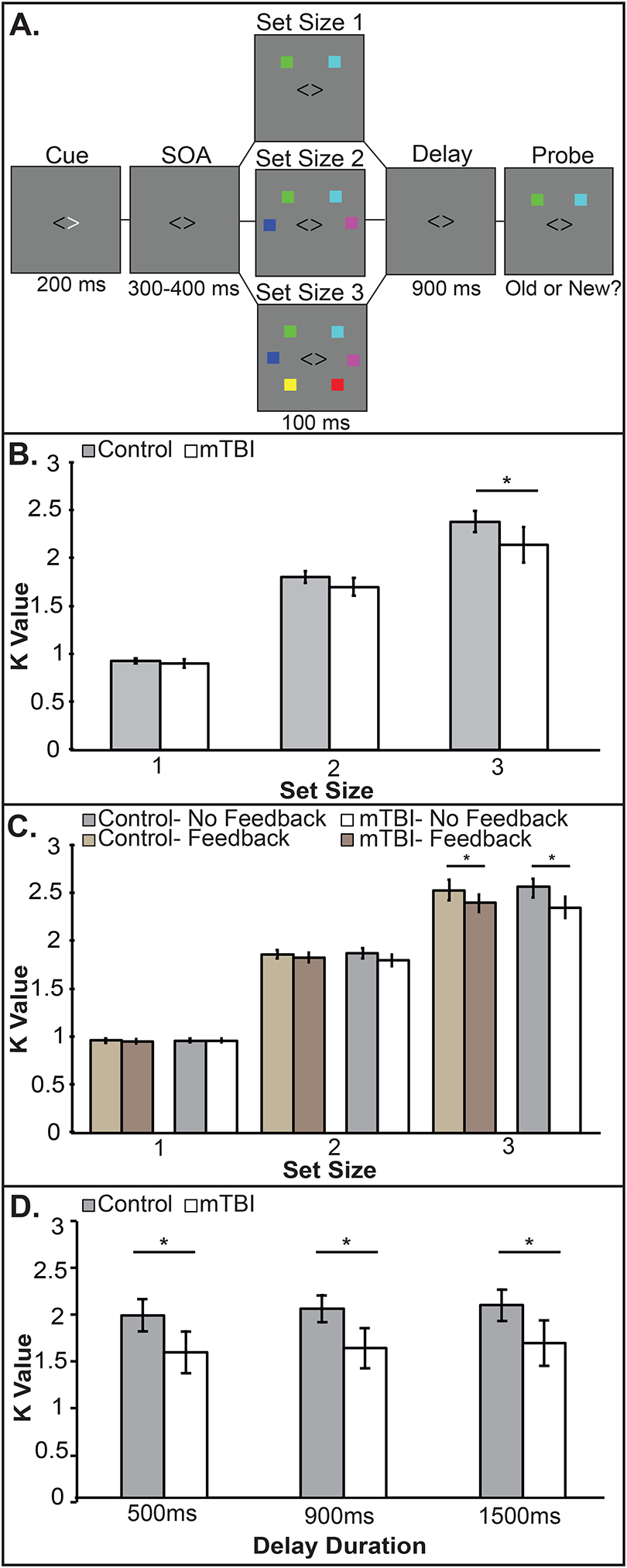
A) Trial sequence B.) Experiment 1. The group x set size interaction emerges at set size 3. C.) Experiment 2. A replication of the set size x group interaction, but feedback made no difference. D.) Experiment 3. The history of mTBI group is impaired across maintenance delays. Error bars indicate 95% confidence intervals.
Results
K values were subjected to a mixed-model ANOVA with the factors of set size (1–3) and group (control, mTBI). Violations of sphericity were Greenhouse-Geisser corrected throughout. There were significant main effects of set size (F2, 64 = 582.61, p < .001, partial η2 = .95) and group (F1, 32 = 4.47, p = .042, partial η 2 = .12) and a significant interaction of set size x group (F2, 64 = 3.74, p = .03, partial η 2 = .1). Pairwise tests revealed mTBI impairment emerged at set size 3 (p < .04) but not at set size 1 (p = .3) or 2 (p = .07); see Figure 1B. Reaction times slowed as set size increased (F1.5,47.94 = 55.2, p < .00001, partial η2 = .64), but comparably across groups (F1,31 = 3.2, p = .08, partial η2 = .1).
Experiment 2
Having observed a significant deficit in the mTBI group at set size 3, we considered the possibility of motivation differences across group (Ransom et al., 2016). We replicated Experiment 1 with the addition of feedback. Our rationale was that feedback might improve motivation (Miranda & Palmer, 2014) and decrease distractibility (Adam & Vogel, 2017) in the mTBI group.
Methods
Participants
Again, mTBI participants provided written self-reports indicating whether they had a history of mTBI, how many and when the injury or injuries took place. New undergraduates participated (see Table 1).
Protocol
Trials were presented in two counterbalanced blocks. One half (N=288) were identical to Experiment 1. The other half included visually presented feedback after each trial indicating “correct” or “incorrect”; see Figure 1A.
Results
Behavioral Results
K measurements were subjected to a mixed-model ANOVA with factors of set size (1–3), feedback (feedback, no feedback), and group (control, mTBI). K values increased with set size (F2, 71.17 = 1901.82, p = .00001, partial η2 = .975), and the mTBI group was significantly impaired (F1, 48 = 6.4, p = .015, partial η2 = .12). Again, a significant set size x group interaction (F1.23, 71.17 = 6.28, p = .01, partial η2 = .116) emerged at set size 3 regardless of feedback (with feedback: p= .01; without feedback: p=.02); see Figure 1C. Feedback had no effect (F < 1, p = ns). No other interactions reached significance (all ps > .5). Reaction time data showed no group differences (F1, 48 = .3, p = .6, partial η2 = .5) but revealed slowing with increased set size (F1.2, 60.5 = 50.62, p < .0001, partial η2 = .5).
Experiment 3
Feedback failed to rescue mTBI performance, suggesting overall motivation level could not fully account for the set size 3 deficit. Given that performance was not significantly different at set sizes of 1–2, we suspected encoding was relatively well preserved. We next varied maintenance delay durations to test whether it was disturbed after a history of mTBI, which would lead to greater effects at longer delays.
Materials and Methods
Participants
We tested 42 participants (see Table 1). Five participants with a history of mTBI who participated in Experiment 2 participated in Experiment 3. Here, we included data collection regarding mTBI etiology and whether they experienced any loss of consciousness (LOC). In addition, 11 mTBI participants reported no LOC. Ten reported LOC (M: 5.47 minutes, SD: 9.04, range: 1–30 minutes). All mTBIs were closed-head injuries: 12 sports-related, 3 falls/accidents, 1 blast, 1 hit by a car, 2 participants did not answer.
Procedure
There were three changes: only set size 3 was included, delay durations of 500, 900, and 1500 ms were pseudorandomized, and participants completed 3 sessions.
Results
K values were subjected to a mixed-model ANOVA including factors of delay (500, 900, 1500 ms) and group (control, mTBI). There was a main effect of delay (F1.48, 59.43= 10.31, p = .001, partial η2 = .205) with lower capacity at 500 ms than at 900 ms (p = .003) or 1500 ms (p = .002). Again, the mTBI group was impaired (F1, 40= 8.78, p = .005, partial η2 = .18); see Figure 1D. However, no interactions approached significance (all ps > .77). Reaction times showed no significant main effect of group (F1,40 = 3.5, p = .07, partial η2 = .08). Response times slowed with increased delays (F1.6,64.2 = 20.51, p < .000001, partial η2 = .34).
Across Experiment 1–3 Analyses
To begin to parse the main effect of group we ran a multiple regression across Experiments 1–3 inputting K-values for the condition included in each study (set size 3, 900 ms delay condition) to test whether number of mTBIs, and time since last mTBI could predict WM performance (K value). However, no significant relationship was identified (F2, 63 = 1.04, p = .36, R2= .03) and neither the number of TBIs nor the time since injury significantly predicted WM performance (ps > .77).
Experiment 4
Increasing maintenance demands revealed no disproportionate deficit in the mTBI group, suggesting WM maintenance cannot fully account for the group differences. We turned to retrieval demands to test retrieval processing accounted for the mTBI deficit. We included recognition and recall probes to evaluate retrieval processing and used new stimuli.
Materials and Methods
Participants
New participants were recruited (see Table 1). In addition, 12 mTBI participants reported no loss of consciousness (LOC), there were 10 who reported LOC (M: 6.41 minutes, SD: 10.52, range: 1 second-30 minutes). The mTBIs were all closed-head injuries: 12 sports-related, 6 falls/accidents, 1 fight, 3 participants elected not to answer.
Procedure
Stimuli were displayed on a 15-inch MacBookPro. This task was previously described in detail(Gozenman, Tanoue, Metoyer, & Berryhill, 2014). Several differences in stimuli, set size, and task demands were implemented. Four oriented line segments (7.5° × 1.5°) appeared 6.5° from fixation. Trials began with fixation (1500ms), followed by encoding (1000ms) and maintenance (900ms). Recognition probes contained one item and participants reported (‘O’ match, ‘N’ non-match, 50%) whether it matched the original orientation. Recall probes required rotation of the probe item (arrow keys, 1°/keypress). Participants completed 200 trials per task in counterbalanced blocks; See Figure 2A. Performance on recall trials was degree error. Participants performed an auditory suppression task throughout to minimize verbal strategies.
Figure 2. Task paradigm, stimulus configurations, and behavioral results for experiment 4.
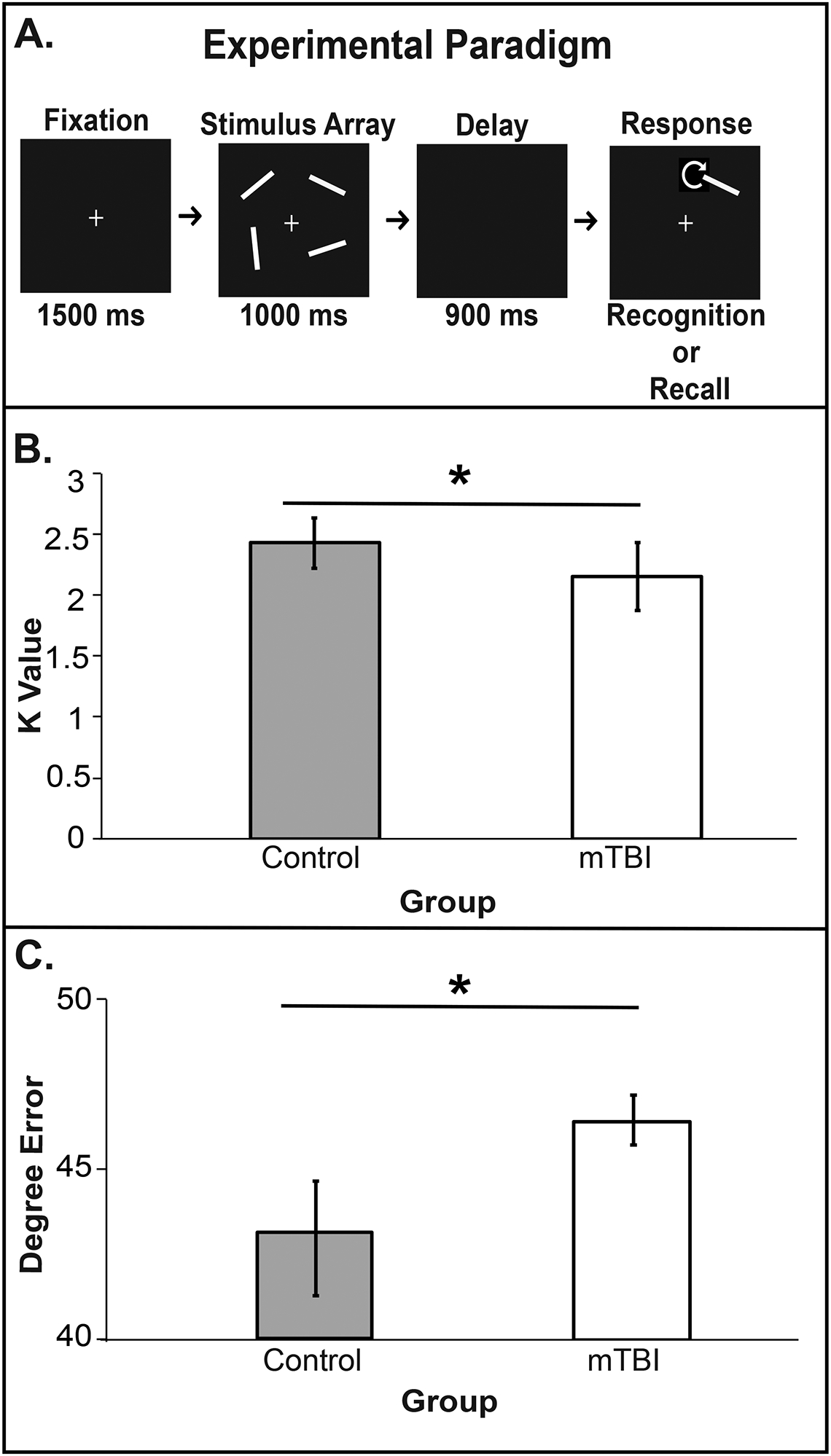
A) Trial sequence for recognition and recall trials. B) Experiment 4 recognition results and C) recall results. Both show impairment in the history of mTBI group. All error bars reflect 95% confidence intervals.
Results
Significant group differences for each retrieval task revealed mTBI impairment (recognition K: t(21) =2.21, p=.03; recognition reaction time: t(21) = −1.96, p=.05; recall error rates: t(21) =−2.84, p=.006); see Figure 2B, C. Recall reaction times were not measured because multiple button presses were required.
To evaluate the interaction across probes using different performance metrics, we converted recognition and recall performance of the mTBI group into z-scores and subtracted them (zRecall - zRecognition). A one-sample t-test using a test value of 0 found no significant difference (t21 =.30, p=.78), indicating the absence of an interaction across retrieval tasks.
Discussion
Few studies investigate the chronic mTBI population, despite its large size and evidence that consequences of mTBI can be long-lasting (Hou et al., 2012; Kenzie et al., 2017; Moser & Schatz, 2002; Stockbridge, 2019). The current participants report no residual symptoms of mTBI, despite having a history of mTBI. In this population, our data revealed that four different samples of undergraduates with a history of mTBI had a WM impairment. Notably, the mean time since injury was measured in years, not weeks or months. Thus, even in active, otherwise healthy undergraduates traces of mTBI remain.
A history of mTBI appears to be detrimental to WM. Specifically, the WM deficit reliably emerged at a set size of 3, suggesting encoding for 1–2 items was adequate. Feedback did not rescue mTBI performance although a financial incentive may have been more compelling (Jones, Gozenman, & Berryhill, 2015)). WM maintenance failures cannot account for the mTBI deficit because lengthening maintenance did not increase WM deficits. Manipulating the retrieval demands did not identify a disproportionate WM deficit in the mTBI group. It also identified a deficit in the mTBI group using an encoding duration ten times longer than that used in Experiments 1–3. The nature of this deficit remains unclear, likely due to the heterogeneity of mTBI. We are left without a clear attribution to a particular stage of WM (e.g., encoding, maintenance, retrieval). Thus, despite a reliable performance difference in such a heterogeneous population the nature of the WM deficits emerges across multiple stages of WM and potentially differently across participants. Having identified a reliable difference in our undergraduate population we may now probe these questions more carefully going forward.
Limitations
We wanted to understand if some of the low WM performance in our laboratory studies could be due to a history of mTBI, as a high number of our participants report having had one or more of them. It seems unlikely that a participant would deliberately mislead us, given the option of participating as a control. We made no attempt to restrict participation and we relied on self-reports of mTBI. We also recruited multiple cohorts of mTBI participants rather than more comprehensively probing a single cohort. We argue that recruiting multiple cohorts would increase the between-subjects noise and work against finding a consistent mTBI specific deficit at the group level. Another limitation is that we only tested WM and did not test cognitive performance more broadly, or measure personality traits to see if high risk taking or impulsivity accounted for some of the difference. It seems quite unlikely that we stumbled on the only task that would reveal impairment. The current data indicate that there are unaddressed long-term consequences of mTBI persisting in an otherwise successful population: enrolled undergraduates. Future efforts must carefully measure and weigh multivariate factors, including etiology, quality and compliance with medical advice, social support surrounding injury, and so forth (Kenzie et al., 2017). We would benefit by more comprehensively testing one cohort to characterize the boundary between intact and impaired function in a within-participants fashion. This information reveals the need for asking participants regarding their history of mTBI and suggests that it will be useful to develop restorative protocols across the mTBI survivor population, regardless of how highly functioning they are or how long since their injury.
Acknowledgments and Funding
Funding was provided from the UNR Office of Undergraduate Research (AKG, JM), NSF OIA 1632849 (MB), NSF OIA 1632738 (MB), NIGMS P20GM103650 (MB), and the Tahoe Institute for Rural Health Research (MB). The content is solely the responsibility of the authors and does not represent the official views of any funding agency. The authors declare they have no conflicts of interest.
References
- Adam KCS, & Vogel EK (2017). Confident failures: Lapses of working memory reveal a metacognitive blind spot. Attention, Perception, & Psychophysics, 79(5), 1506–1523. doi: 10.3758/s13414-017-1331-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj S, Dailey NS, Rosso IM, Rauch SL, & Killgore WDS (2018). Time-dependent differences in cortical measures and their associations with behavioral measures following mild traumatic brain injury. Hum Brain Mapp. doi: 10.1002/hbm.23951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, McClung J, Shah MN, Cheng YT, Flesher W, & Kraus J (2005). Mild traumatic brain injury in the United States, 1998–2000. Brain Injury, 19(2), 85–91. doi: 10.1080/02699050410001720158 [DOI] [PubMed] [Google Scholar]
- Belanger HG, & Vanderploeg RD (2005). The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc, 11(4), 345–357. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial vision, 10, 433–436. [PubMed] [Google Scholar]
- Cameron KL, Marshall SW, Sturdivant RX, & Lincoln AE (2012). Trends in the incidence of physician-diagnosed mild traumatic brain injury among active duty U.S. military personnel between 1997 and 2007. Journal of Neurotrauma, 29(7), 1313–1321. doi: 10.1089/neu.2011.2168 [DOI] [PubMed] [Google Scholar]
- Catale C, Marique P, Closset A, & Meulemans T (2008). Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. Journal of Clinical and Experimental Neuropsychology, 31(3), 331–338. doi: 10.1080/13803390802134616 [DOI] [PubMed] [Google Scholar]
- Cooper DB, Bunner AE, Kennedy JE, Balldin V, Tate DF, Eapen BC, & Jaramillo CA (2015). Treatment of persistent post-concussive symptoms after mild traumatic brain injury: A systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging and Behavior, 9(3), 403–420. doi: 10.1007/s11682-015-9440-2 [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Selassie AW, & Orman JAL (2010). The epidemiology of traumatic brain injury. The Journal of Head Trauma Rehabilitation, 25(2), 72–80. doi: 10.1097/HTR.0b013e3181ccc8b4 [DOI] [PubMed] [Google Scholar]
- Cowan N (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24(1), 87–185. doi: 10.1017/S0140525X01003922 [DOI] [PubMed] [Google Scholar]
- Dall’Acqua P, Johannes S, Mica L, Simmen HP, Glaab R, Fandino J, … Hanggi J (2017). Prefrontal Cortical Thickening after Mild Traumatic Brain Injury: A One-Year Magnetic Resonance Imaging Study. J Neurotrauma, 34(23), 3270–3279. doi: 10.1089/neu.2017.5124 [DOI] [PubMed] [Google Scholar]
- De Monte VE, & Geffen GM (2005). Effects of Mild Traumatic Brain Injury: Comparison of Direct and Indirect Injury Groups. Brain Impairment, 6(2), 109–116. doi: 10.1375/brim.2005.6.2.109 [DOI] [Google Scholar]
- Dean PJ, & Sterr A (2013). Long-term effects of mild traumatic brain injury on cognitive performance. Front Hum Neurosci, 7, 30. doi: 10.3389/fnhum.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, & LaConte SM (2014). Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin, 4, 283–294. doi: 10.1016/j.nicl.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, & Coronado VG (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Retrieved from Atlanta, GA: [Google Scholar]
- Fino PC, Parrington L, Walls M, Sippel E, Hullar TE, Chesnutt JC, & King LA (2018). Abnormal Turning and Its Association with Self-Reported Symptoms in Chronic Mild Traumatic Brain Injury. J Neurotrauma, 35(10), 1167–1177. doi: 10.1089/neu.2017.5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Awh E, & Vogel EK (2010). Discrete capacity limits in visual working memory. Current Opinion in Neurobiology, 20(2), 177–182. doi: 10.1016/j.conb.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Elahi FM, Mustapic M, Kapogiannis D, Pryhoda M, Gilmore A, … Ledreux A (2019). Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J, 33(4), 5082–5088. doi: 10.1096/fj.201802319R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N, Bottari C, Chen JK, Huntgeburth SC, De Beaumont L, Petrides M, … Ptito A (2012). Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg Focus, 33(6), E7: 1–7. doi: 10.3171/2012.10.FOCUS12253 [DOI] [PubMed] [Google Scholar]
- Gozenman F, Tanoue RT, Metoyer T, & Berryhill ME (2014). Invalid retro-cues can eliminate the retro-cue benefit: Evidence for a hybridized account. J Exp Psychol Hum Percept Perform, 40(5), 1748–1754. doi: 10.1037/a0037474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham TE, Bonnelle V, Hellyer P, Jilka S, Robertson IH, Leech R, & Sharp DJ (2014). The neural basis of impaired self-awareness after traumatic brain injury. Brain, 137(Pt 2), 586–597. doi: 10.1093/brain/awt350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, & Belli A (2012). When a minor head injury results in enduring symptoms: A prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. Journal of Neurology, Neurosurgery & Psychiatry, 83(2), 217–223. doi: 10.1136/jnnp-2011-300767 [DOI] [PubMed] [Google Scholar]
- Hudac CM, Cortesa CS, Ledwidge PS, & Molfese DL (2017). History of concussion impacts electrophysiological correlates of working memory. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai A, McCollough AW, & Vogel EK (2010). Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of Neurophysiology, 103(4), 1963–1968. doi: 10.1152/jn.00978.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Gozenman F, & Berryhill ME (2015). The strategy and motivational influences on the beneficial effect of neurostimulation: a tDCS and fNIRS study. Neuroimage, 105, 238–247. doi: 10.1016/j.neuroimage.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzie ES, Parks EL, Bigler ED, Lim MM, Chesnutt JC, & Wakeland W (2017). Concussion As a Multi-Scale Complex System: An Interdisciplinary Synthesis of Current Knowledge. Frontiers in neurology, 8, 513–513. doi: 10.3389/fneur.2017.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Geburek AJ, Rist F, Blumenroth H, Fischer B, Husstedt I, … Lohmann H (2011). Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychological Medicine, 41(6), 1197–1211. doi: 10.1017/S0033291710001728 [DOI] [PubMed] [Google Scholar]
- Larrabee GJ (2012). Mild traumatic brain injury In Larrabee GJ (Ed.), Forensic neuropsychology: A scientific approach. (pp. 231–259). New York, NY, US: Oxford University Press. [Google Scholar]
- Lotan E, Morley C, Newman J, Qian M, Abu-Amara D, Marmar C, & Lui YW (2018). Prevalence of Cerebral Microhemorrhage following Chronic Blast-Related Mild Traumatic Brain Injury in Military Service Members Using Susceptibility-Weighted MRI. AJNR. American journal of neuroradiology, 39(7), 1222–1225. doi: 10.3174/ajnr.A5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, & Vogel EK (2013). Visual working memory capacity: From psychophysics and neurobiology to individual differences. Trends in Cognitive Sciences, 17(8), 391–400. doi: 10.1016/j.tics.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria R, Balaban H, Awh E, & Vogel EK (2016). The contralateral delay activity as a neural measure of visual working memory. Neuroscience and Biobehavioral Reviews, 62, 100–108. doi: 10.1016/j.neubiorev.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Yang Z, Yeo RA, Pena A, Ling JM, Mannell MV, … Mojtahed K (2012). A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain Imaging and Behavior, 6(2), 343–354. doi: 10.1007/s11682-012-9178-z [DOI] [PubMed] [Google Scholar]
- McCollough AW, Machizawa MG, & Vogel EK (2007). Electrophysiological measures of maintaining representations in visual working memory. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 43(1), 77–94. doi: 10.1016/S0010-9452(08)70447-7 [DOI] [PubMed] [Google Scholar]
- McInnes K, Friesen CL, MacKenzie DE, Westwood DA, & Boe SG (2017). Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE, 12(4), e0174847. doi: 10.1371/journal.pone.0174847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNerney MW, Hobday T, Cole B, Ganong R, Winans N, Matthews D, … Lane S (2019). Objective Classification of mTBI Using Machine Learning on a Combination of Frontopolar Electroencephalography Measurements and Self-reported Symptoms. Sports Med Open, 5(1), 14. doi: 10.1186/s40798-019-0187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda AT, & Palmer EM (2014). Intrinsic motivation and attentional capture from gamelike features in a visual search task. Behavior Research Methods, 46(1), 159–172. doi: 10.3758/s13428-013-0357-7 [DOI] [PubMed] [Google Scholar]
- Moser RS, & Schatz P (2002). Enduring effects of concussion in youth athletes. Archives of Clinical Neuropsychology, 17(1), 91–100. doi: 10.1016/S0887-6177(01)00108-1 [DOI] [PubMed] [Google Scholar]
- Pan J, Connolly ID, Dangelmajer S, Kintzing J, Ho AL, & Grant G (2016). Sports-related brain injuries: connecting pathology to diagnosis. Neurosurg Focus, 40(4), E14. doi: 10.3171/2016.1.FOCUS15607 [DOI] [PubMed] [Google Scholar]
- Pashler H (1988). Familiarity and visual change detection. Perception & psychophysics, 44(4), 369–378. [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial vision, 10(4), 437–442. [PubMed] [Google Scholar]
- Ransom DM, Burns AR, Youngstrom EA, Vaughan CG, Sady MD, & Gioia GA (2016). Applying an evidence-based assessment model to identify students at risk for perceived academic problems following concussion. Journal of the International Neuropsychological Society, 22(10), 1038–1049. doi: 10.1017/S1355617716000916 [DOI] [PubMed] [Google Scholar]
- Sharp DJ, & Ham TE (2011). Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol, 24(6), 558–563. doi: 10.1097/WCO.0b013e32834cd523 [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, & Leech R (2014). Network dysfunction after traumatic brain injury. Nat Rev Neurol, 10(3), 156–166. doi: 10.1038/nrneurol.2014.15 [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, … Zafonte R (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav, 6(2), 137–192. doi: 10.1007/s11682-012-9156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumskaya E, Andriessen TMJC, Norris DG, & Vos PE (2012). Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology, 79(2), 175–182. doi: 10.1212/WNL.0b013e31825f04fb [DOI] [PubMed] [Google Scholar]
- Stockbridge MD (2019). The role of personality and cognitive-linguistic deficits in teens and adults with concussion. (80), ProQuest Information & Learning, Retrieved from http://unr.idm.oclc.org/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2018-52505-143&site=ehost-live&scope=site Available from EBSCOhost psyh database. [Google Scholar]
- Tate DF, Shenton ME, & Bigler ED (2012). Introduction to the brain imaging and behavior special issue on neuroimaging findings in mild traumatic brain injury. Brain Imaging Behav, 6(2), 103–107. doi: 10.1007/s11682-012-9185-0 [DOI] [PubMed] [Google Scholar]
- Taylor CA, Greenspan AI, Xu L, & Kresnow M. j. (2015). Comparability of national estimates for traumatic brain injury-related medical encounters. The Journal of Head Trauma Rehabilitation, 30(3), 150–159. doi: 10.1097/HTR.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, & Belanger HG (2005). Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc, 11(3), 228–236. doi: 10.1017/S1355617705050289 [DOI] [PubMed] [Google Scholar]
- Vogel EK, & Machizawa MG (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428(6984), 748–751. [DOI] [PubMed] [Google Scholar]
- Vogel EK, & Machlzawa MG (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428(6984), 748–751. doi: 10.1038/nature02447 [DOI] [PubMed] [Google Scholar]
- Wammes JD, Good TJ, & Fernandes MA (2017). Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain and Cognition, 111, 112–126. doi: 10.1016/j.bandc.2016.11.004 [DOI] [PubMed] [Google Scholar]


