Abstract
Background:
Proton-pump inhibitors (PPIs) are among the most prescribed medicines worldwide and concern about their long-term use is growing. We used dispensing claims for every person in Australia dispensed publicly subsidized PPIs between 2013 and 2016 to determine the incidence and prevalence of PPI use and to examine the patterns and durations of PPI treatment among individuals continuing treatment beyond the guideline-recommended maximum 12 weeks.
Methods:
We estimated annual prevalence and incidence per 100 people and duration of treatment for every Australian dispensed publicly subsidized PPIs between 2013 and 2016. We examined patterns of PPI treatment in three patient subgroups using PPIs for more than 12 weeks duration; people receiving maintenance, long-term continuous or long-term intermittent treatment. We calculated the proportion in each subgroup stepping down from higher to lower PPI strengths, stepping up from lower to higher PPI strength and discontinuing treatment.
Results:
PPIs were dispensed to 4,388,586 people; 60% were women; median age at initiation was 52 years [interquartile range (IQR): 36–65]. Standard and high strength PPIs accounted for 95% of dispensings. Annual incidence and prevalence were 3.9/100 and 12.5/100, respectively, in 2016 and highest among individuals over 65 years (prevalence range: 33–43/100). Most people (67%) stopped treatment after one dispensing; while 25%, 6% and 10% continued on maintenance, long-term continuous and long-term intermittent treatment, respectively. Median duration of treatment in people continuing treatment was 501 days (IQR: 180–not reached) for maintenance treated individuals and ‘not reached’ for long-term treated individuals. We observed 35%, 20% and 47% of people stepping down from higher to lower treatment strengths on maintenance, long-term continuous and long-term intermittent treatment, respectively.
Conclusions:
Longer-term treatment with higher strength PPIs is common. Targeted regulation of PPI prescribing may improve the uptake of lower strength formulations and reduce both harms and costs associated with long-term PPI treatment.
Keywords: long-term, population-based, proton-pump inhibitor
Introduction
The late 1990s and early 2000s witnessed dramatic increases in the use of proton-pump inhibitors (PPIs) to the point where they now dominate acid suppression treatment and are among the most prescribed medicines worldwide.1–6 PPIs are prescribed to manage gastrointestinal (GI) acid related disorders, such as gastro-oesophageal reflux disease, nonerosive reflux disease, peptic ulcer disease and other ulcerous conditions and Zollinger–Ellison syndrome.5,7,8 Historically, PPIs have been considered well tolerated, effective and generally safe.5,9
While the use of PPIs has grown dramatically, the prevalence the conditions for which they are indicated has remained stable.7,10–12 PPIs are also prescribed to prevent nonsteroidal anti-inflammatory drug (NSAID) related bleeding in which NSAIDs must be continued. However, they are often used for off-label, preventive indications with less clear rationale and no supporting evidence, for example with corticosteroid or anticoagulant therapy, in cancer patients and in intensive care.13 Recent studies using national data have reported increasing annual prevalence of PPI use but constant incidence,2,14 suggesting that people are remaining on PPI treatment for longer than may be necessary.5,15,16 Most indications for acid suppression therapy do not require on-going treatment with high or standard strength PPIs.16–18 While some rare conditions, such as hyper-secretory disorders (e.g. Zollinger–Ellison syndrome, Barrett’s oesophagus, high risks of GI bleed and severe symptoms at baseline),19 may require continual treatment at higher doses,16,17 guidelines recommend people discontinue PPIs following the initial treatment of between 4 and 12 weeks (1–3 months) for most indications.8,9,17 Alternatively, people may continue maintenance treatment by ‘stepping down’ from higher strength PPIs to lower strengths if symptoms persist.9
A growing body of studies have detected possible harms associated with long-term use of PPIs.5,16,20–26 The risk of PPI-related adverse events can be minimized by stepping down the PPI dose or discontinuing treatment altogether.16,27 However, it is not clear what proportion of PPI-treated people are receiving long-term treatment and to what extent long-term treated people are in fact attempting lower treatment strengths or discontinuation during the course of their therapy.
Previous studies have described contemporary real-world patterns of treatment with PPIs and how well this treatment adheres to recommended guidelines,2,14 but questions remain around the typical duration of PPI treatment, the patterns of PPI use over extended periods and the proportion of people who initiate PPIs and continue on maintenance and longer-term therapy. Therefore, in this study we used dispensing claims for every person in Australia dispensed publicly subsidized PPIs between 2013 and 2016 to determine the incidence and prevalence of PPI use and what other medicines people are using concomitant with PPIs. We further examine the patterns and durations of PPI treatment among individuals continuing treatment beyond 12 weeks after initiation.
Data sources and methods
Setting and data
Australia maintains a publicly funded, universal healthcare system entitling all citizens and permanent residents to subsidized prescription medicines through the Pharmaceutical Benefits Scheme (PBS).28 Dispensing claims for medicines subsidized by PBS are processed by the Australian Government Department of Human Services and then provided to the Australian Government Department of Health (DoH) for monitoring and health service planning, as well as to health services and academics for research purposes. The DoH supplied de-identified, individual-level data including demographic information (sex, year of birth) and PBS dispensing records (date of dispensing, dispensed medicine, dispensed medicine strength, quantity dispensed, prescriber type) for every person in Australia dispensed at least one PPI medicine between 1 July 2012 and 30 June 2016 (our data censor date).
Study design and participants
Our population-based cohort study included every person in Australia initiating PBS-subsidized PPI treatment between 1 July 2013 and 30 June 2016. Participants were observed from initiation of PPI treatment until 30 June 2016.
Medicines of interest
We investigated the five PPI medicines available in Australia during the study period: omeprazole (anatomical therapeutic chemical29 code, A02BC01), pantoprazole (A02BC02), lansoprazole (A02BC03), rabeprazole (A02BC04) and esomeprazole (A02BC05). We classified PPI strength as high, standard and low based on tablet strength and guidelines by Therapeutic Guidelines Ltd. and the National Institute for Health and Care Excellence (Table 1).9,17 We excluded PPIs in fixed-dose combination with antibiotics (A02BD), which are generally prescribed short-term for the eradication of Helicobacter pylori infections.
Table 1.
PPI medicines and strengths.
| ATC code | PPI name | Strength classification* | mg | PBS subsidized indications |
|---|---|---|---|---|
| A02BC01 | omeprazole | Standard | 20 | PU, GORD, SO, ZES |
| Low | 10 | GORD, SO, ZES | ||
| A02BC02 | pantoprazole | Standard | 40 | PU, GORD, SO, ZES |
| Low | 20 | GORD, SO, ZES | ||
| A02BC03 | lansoprazole | Standard | 30 | PU, GORD, SO |
| Low | 15 | GORD, SO | ||
| A02BC04 | rabeprazole | Standard | 20 | PU, GORD, SO |
| Low | 10 | GORD, SO | ||
| A02BC05 | esomeprazole | High | 40 | GORD, SO, Pathological hypersecretory conditions including ZES and idiopathic hypersecretion |
| Standard | 20 | GU, GORD, SO, Pathological hypersecretory conditions including ZES and idiopathic hypersecretion |
Strength classification is based on guidelines by Therapeutic Guidelines Ltd. and the National Institute for Health and Care Excellence (NICE 2014): Guideline (CG184).
ATC, anatomical therapeutic chemical; GORD, Gastro-oesophageal reflux disease; GU, Gastric Ulcer; PBS, Pharmaceutical benefits scheme; PPI, proton-pump inhibitors; PU, Peptic Ulcer; SO, Scleroderma oesophagus; ZES, Zoillinger–Ellison syndrome.
Statistical analysis
We used descriptive statistics to summarize the cohort by sex and age. We used dispensing records to identify individuals dispensed NSAIDs and anticoagulant medicines concomitantly with PPI treatment and we applied a validated algorithm (RxRisk), to the dispensing records from up to 1 year prior to initiation of PPI treatment to identify the number of people with treatment for comorbid diseases.30
Prevalence and incidence
We calculated prevalence of PPI use as the number of people with at least one PPI dispensing in an Australian financial year (1 July to 30 June) between July 2013 and July 2016. We calculated the incidence of PPI use as the number of people with a PPI dispensing during a financial year and no PPI dispensing during the preceding 12 months. We used the Australian Bureau of Statistics’ mid-year population estimate for Australia on June of the financial year as the denominator for both measures.31 We stratified prevalence and incidence estimates by sex and age groups (18–34, 35–49, 50–64, 65–74, 75–84 and 85 years and older). We further stratified prevalence and incidence estimates by PPI medicine strength – standard strength, high strength and low strength (Table 1). Estimates are presented per 100 people.
Duration of treatment
We calculated duration of PPI treatment for people initiating treatment between July 2013 and June 2015. PBS dispensing records do not contain information on duration of treatment and, therefore, we estimated duration using dates of PPI dispensing. We examined the time between dispensings of PPI medicines and found a median time-to-refill of 30 days (concordant with typical PBS pack size of 30 tablets). To allow for variation in time-to-refill, we defined treatment discontinuation as a period of time greater than or equal to twice the median time-to-refill (60 days). Therefore, dispensings occurring within 60 days of each other were considered as belonging to a single treatment episode of PPI treatment. We considered dispensing following a break of 60 days as beginning a new treatment episode.
We then estimated duration of treatment for each patient as the time from the first dispensing in a treatment episode to the last dispensing without a discontinuation, plus the median time-to-refill (30 days). In the case when a person had only a single PPI dispensing, we considered the duration of treatment as the dispensing date plus the median time-to-refill (30 days).
Patterns of treatment beyond 12 weeks from PPI initiation
We explored patterns of treatment coverage by organizing each person’s treatment episodes into 30-day months from PPI initiation. We assigned an exposure category to each month from initiation – either high, standard or low strength PPIs or no treatment (Table 1). We considered step-down treatment to have occurred when a month of exposure to higher strength PPIs was followed by a month of exposure to lower strength tablets, and attempts at step-up treatment as a month of exposure to lower strength PPI tablets followed by a month of exposure to higher strength tablets. We defined treatment discontinuation as a period of at least 60 days with no PPI treatment.
We examined patterns of treatment in three groups of people treated for an extended period of time – those on maintenance, long-term continuous and long-term intermittent treatment. We defined individuals receiving maintenance treatment as those with any PPI treatment that continued beyond 90 days (12 weeks of treatment, rounded up to 90 days) from an incident PPI dispensing. People could continuously receive PPI dispensings for greater than 90 days (at least four consecutive dispensings), or they could initiate PPI treatment, stop for a period and then have a second dispensing at any point within 30 days of the date marking 90 days from initiation. We described the demographic characteristics of these individuals, their overall duration of PPI therapy and how they switched (or did not switch) between tablet strengths over the course of use.
We similarly explored the characteristics and patterns of PPI use for individuals receiving long-term PPI treatment. We defined long-term PPI treatment as 12 or more months of PPI use (this group included individuals on maintenance treatment). This threshold was used in a recent UK study1 and is more conservative (longer) than many previous definitions.15 We classified long-term PPI treatment use as either continuous or intermittent. Individuals on continuous long-term treatment were those who initiated treatment and stayed on treatment (no gaps ⩾60 days between dispensings) for at least 12 months. While those on intermittent long-term treatment received at least 12 months of PPI treatment, but with discontinuations in that treatment.
To allow for at least 12 months of follow-up for individuals receiving maintenance or long-term PPI treatment, we restricted these analyses to individuals initiating PPIs between July 2013 and June 2015. We performed all analyses in SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.3.5.
Ethics and data access approvals
The PBS data for our study were provided by the Australian Government Department of Health as a part of a contracted medicines utilization review (DoH Contract number: RG171855). The de-identified PBS unit record level data was approved for release by the DoH Delegate and Data Request Assessment Panel (June 2017). Individual consent for the release of these data has been waived according to the Australian Privacy Act of 1988. The data were stored, analysed and reported in accordance with the Australian Privacy Act of 1988. Access to the datasets analysed during the current study is not permitted without the express permission of the approving human research ethics committees and data custodians.
Results
There were 4,388,586 people dispensed at least one PPI between July 2013 and July 2016. A total of 60% were female and their median age at initiation was 52 years (interquartile range [IQR]: 36–65). Of the 62,202,318 PPI medicines dispensed during the study period, the majority were standard strength (78%), followed by high strength (17%) and low strength (5%).
Prevalence and incidence
The prevalence and incidence of PPI use in 2016 was 12.6 and 3.9 per 100 people, respectively, and similar in previous years (Table 2, Supplemental Table A). Prevalence and incidence were highest among people 65 years and older, particularly older women (Table 2, Supplemental Table A). Most people initiated PPI treatment with standard strength PPIs and these formulations were the most prevalent strength observed during the study period – used by around 9.5 per 100 people in each year (Supplementary Table A).
Table 2.
Prevalence and incidence of PPI use, per 100 people, July 2015–July 2016.
| Prevalence (n) | Incidence (n) | |
|---|---|---|
| All individuals | 12.5 (3,026,652) | 3.9 (943,019) |
| 0–17 | 1.0 (55,733) | 0.8 (42,872) |
| 18–34 | 4.5 (264,389) | 3.0 (173,204) |
| 35–49 | 9.8 (476,517) | 4.5 (217,958) |
| 50–64 | 19.8 (856,727) | 6.0 (261,383) |
| 65–74 | 33.4 (695,782) | 6.9 (143,092) |
| 75–84 | 42.2 (470,173) | 6.7 (74,900) |
| 85+ | 42.8 (207,331) | 6.1 (29,610) |
| Total population | 24,127,159 | |
| Female | 13.8 (1,679,281) | 4.3 (526,199) |
| 0–17 | 1.2 (30,596) | 0.9 (23,861) |
| 18–34 | 5.1 (146,449) | 3.5 (100,258) |
| 35–49 | 10.3 (251,955) | 5.0 (122,127) |
| 50–64 | 21.4 (470,714) | 6.6 (146,224) |
| 65–74 | 35.9 (380,655) | 7.2 (75,818) |
| 75–84 | 44.5 (265,840) | 6.7 (40,054) |
| 85+ | 43.6 (133,072) | 5.9 (17,857) |
| Total female population | 12,135,070 | |
| Male | 11.2 (1,347,371) | 3.5 (416,820) |
| 0–17 | 0.9 (25,137) | 0.7 (19,011) |
| 18–34 | 4.0 (117,940) | 2.5 (72,946) |
| 35–49 | 9.4 (224,562) | 4.0 (95,831) |
| 50–64 | 18.1 (386,013) | 5.4 (115,159) |
| 65–74 | 30.8 (315,127) | 6.6 (67,274) |
| 75–84 | 39.5 (204,333) | 6.7 (34,846) |
| 85+ | 41.3 (74,259) | 6.5 (11,753) |
| Total male population | 11,992,089 |
PPI, proton-pump inhibitor.
Duration of treatment beyond 12 weeks from PPI initiation
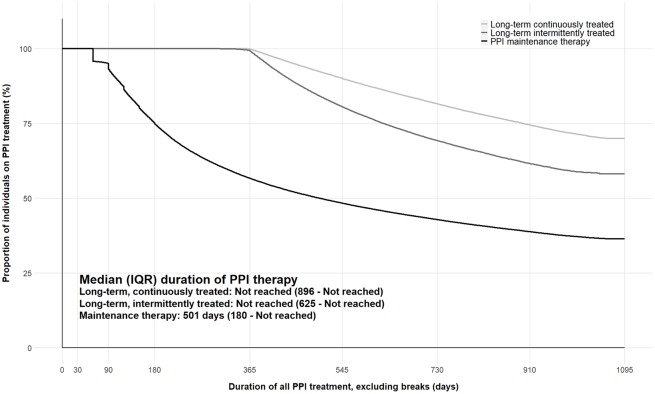
Of the 1,794,133 million people who initiated a PPI between July 2013 and July 2015, 67% filled a PPI prescription once; 25% (455,697) continued on maintenance treatment; 6% (107,993) were treated continuously from initiation for at least 12 months (long-term continuous treatment); and 10% (176,461) were treated for at least 12 months in total but had breaks during treatment of longer than 60 days (long-term intermittent treatment). The median duration of all PPI therapy was 501 days (IQR: 180–not reached [NR]) for individuals continuing on maintenance therapy and was not reached for individuals on long-term PPI therapy (Figure 1).
Figure 1.
Duration of all PPI therapy, excluding breaks, by treatment group.
IQR, interquartile range; PPI, proton-pump inhibitor.
People who continued PPIs beyond 12 weeks from initiation had a higher median age than all people initiating PPIs (Table 3). NSAIDs were dispensed to 37% of those on maintenance treatment and nearly half (48%) of long-term intermittently treated individuals. Over 90% of people in each treatment group were dispensed at least one medicine commonly used to treat comorbidity prior to initiating PPI treatment; and long-term continuously treated individuals had the highest median number of medicines dispensed to treat comorbidity prior to PPI initiation (4 medicines; Table 3).
Table 3.
Characteristics of people initiating PPI treatment between 1 July 2013 and 30 June 2015.
| All people initiating PPI treatment 2013–2015 | Maintenance treatment* | Long-term continuous treatment* | Long-term intermittent treatment* | |
|---|---|---|---|---|
| N (%)† | 1,794,133 (100) | 455,697 (25) | 107,993 (6) | 176,461 (10) |
| Sex (%)‡: | ||||
| Female | 999,266 (56) | 249,164 (55) | 58,497 (54) | 96,874 (55) |
| Male | 794,867 (44) | 206,533 (45) | 49,496 (46) | 79,587 (45) |
| Age at PPI initiation, median (IQR) | 52 (36–65) | 59 (46–71) | 66 (54–77) | 60 (48–70) |
| <18 | 83,331 (5) | 12,775 (3) | 1,179 (1) | 2038 (1) |
| 18–34 | 327,367 (18) | 44,042 (10) | 4,945 (4) | 13419 (8) |
| 35–49 | 413,313 (23) | 83,911 (18) | 13,898 (13) | 31738 (18) |
| 50–64 | 495,860 (28) | 136,322 (30) | 30,028 (28) | 58783 (33) |
| 65–74 | 269,260 (15) | 91,777 (20) | 25,827 (24) | 39277 (22) |
| 75–84 | 145,935 (8) | 57,933 (13) | 20,427 (19) | 22020 (13) |
| 85+ | 59,087 (3) | 28,937 (6) | 11,689 (11) | 9186 (5) |
| Comorbid conditions as identified by the RxRisk algorithm: | ||||
| At least one medicine to treat comorbidity | 1,565,863 (87) | 409,715 (90) | 102,288 (95) | 163,407 (93) |
| Median number of medicines to treat comorbidity (IQR) | 3 (1–4) | 3 (2–5) | 4 (3–6) | 3 (2–5) |
| Select co-administered medicines: | ||||
| Dispensed at least one NSAID during PPI treatment | 516,123 (33) | 166,346 (37) | 35,451 (33) | 83,844 (48) |
| Median (IQR) number of NSAIDs dispensed during PPI treatment | 3 (1–9) | 5 (2–14) | 4 (1–13) | 7 (3–22) |
| Dispensed at least one anticoagulant medicine during PPI treatment | 282,553 (18) | 123,605 (27) | 41,384 (38) | 55,077 (31) |
| Median (IQR) number of anticoagulant medicines dispensed during PPI treatment | 6 (2–22) | 10 (3–30) | 14 (4–27) | 16 (5–49) |
Individuals receiving maintenance treatment may have gone to receive long-term treatment. The groups are not mutually exclusive.
Percentages are out of all individuals initiating PPI treatment 2013–2015.
Percentages from this point to the end of the table are column percentages (by treatment group).
IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton-pump inhibitor.
Patterns of treatment beyond 12 weeks from PPI initiation
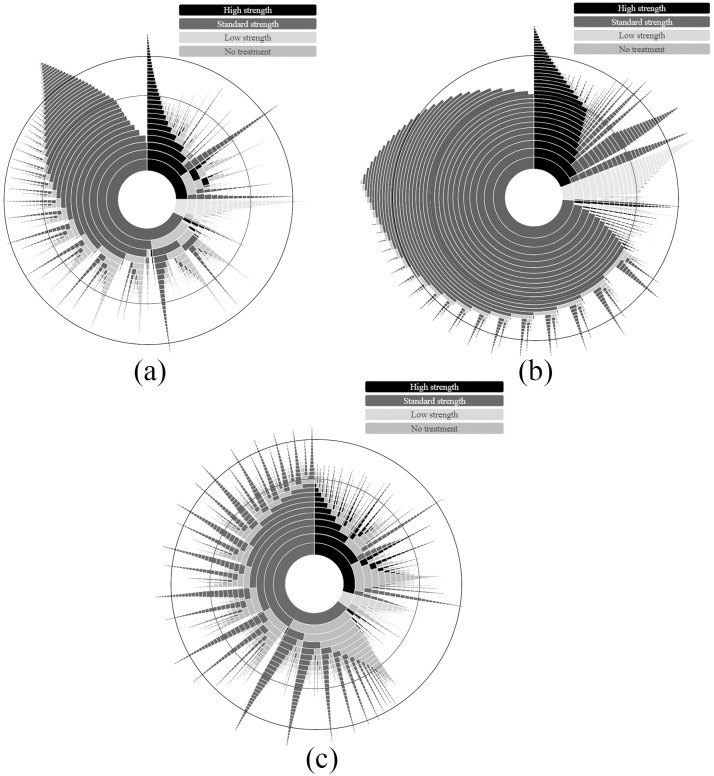
The patterns of treatment for people on maintenance, long-term continuous and long-term intermittent treatment are shown in Figures 2(a–c). These plots show that most people initiated treatment with a standard strength PPI and that a majority of people remained on the initiation treatment strength throughout the course of PPI treatment. Overall, 35%, 20% and 47% of maintenance, long-term continuously and long-term intermittently treated individuals, respectively, stepped down to a lower strength PPI at any time following initiation (37% of all long-term treated people, together). Smaller proportions of individuals in each group increased treatment strengths during the course of their treatment (10% of maintenance, 13% of long-term continuously and 8% of intermittently treated people). Almost no individuals, whether receiving maintenance or long-term treatment, alternated between treatment strengths. Those who stepped down or stepped up their treatment strength remained on the new strength until censored. Over the 3 years observed, 62%, 24% and 82% of maintenance, long-term continuously and long-term intermittently treated individuals discontinued PPI treatment, respectively.
Figure 2.
Sunburst plot showing the monthly patterns of treatment for individuals continuing on (a) maintenance, (b) long-term continuous, and (c) long-term intermittent treatment. Each ring represents a month of treatment, beginning with the innermost ring (treatment initiation) and moving outwards. Months 12 and 24 are indicated by black circles (note, the width of each ring decreases moving outwards from the centre as a feature of the plotting package. The change in size does not denote change in sample size). If a patient had less than 15 days of coverage during a month they were not counted as being on treatment in that month for the purpose of this figure. Coloured segments denote the strength of proton-pump inhibitor (PPI) therapy an individual was receiving in each month from initiation (including no treatment), and the size of each coloured segment within a ring reflects the proportion of individuals receiving that treatment in that month. The figure shows treatment from initiation out to 36 months.
Discussion
In this whole-of-population study we describe the patterns of PPI treatment in one of the largest cohorts in the existing literature to date. There is growing awareness that more people are using PPIs for extended periods. Our study provides useful baseline information on the proportion of people continuing to maintenance or long-term PPI treatment, as well as the patterns of treatment they receive over these extended periods. We found that a substantial proportion of people continued treatment beyond the limits of current recommended guidelines for the most common indications (4–12 weeks); with 16% of initiators treated with PPIs for at least 12 months. While we did not have data for treatment indication, this figure suggests that the number of people continuing PPIs for extended periods exceeds the number of patients with disorders who have no alternatives to ongoing treatment.6,19,32 Our study further highlights that individuals treated with PPIs for extended periods predominantly remained on their initiation treatment strength.
Previous studies have noted the dramatic growth in the overall use of PPIs since the 1990s.1,2,14 In Australia, use of PPIs increased by 1318% between 1995 and 2006, with a pronounced increase in uptake following the removal of prescribing restrictions in 2001.6 Similar increases from 1990 that levelled off between 2012 and 2014 were observed in the UK.1 Our findings suggest that this slowing in the growth of PPI use has continued, but use overall appears to be at unacceptably high levels, with over 12% of the Australian population receiving PPI treatment each year between 2014 and 2016. Previous research from Australia has reported mixed impacts of deprescribing initiatives and it is unclear why the growth in PPI use has slowed.33,34 These prevalence figures are slightly below that reported in the Icelandic population14 and estimated in the UK population,1 but nearly double that reported in the Danish population.2 Similar to these population based studies, we found that more females used and initiated PPIs than males.
These previous European studies also found that while prevalence of PPI use increased dramatically to the early 2010s, incidence did not increase at the same rate. This suggests that people are remaining on PPIs for longer periods of time and our results support this contention. Currently, there is no consistent definition of ‘long-term’ PPI use meaning that characterizing the phenomenon is somewhat problematic. One review noted 11 distinct definitions from 19 different studies.15 We attempted to negotiate this heterogeneity in definitions by examining several groups of people using PPIs beyond the 12 weeks of treatment recommended for the most common indications. The proportion of Australians continuing on long-term PPI treatment was below that reported in a UK study, which also used 12 months of treatment to indicate long-term use,1 but markedly higher than proportions reported in several studies conducted prior to 2005.15
The people on long-term treatment in our study were older than all people initiating PPI treatment in Australia. There are growing concerns around potential harms of long-term PPI use, particularly risk of fracture,35 for which older individuals are already at greater risk. Multiple medicine use is also more widespread among older individuals. The high proportion of longer-term PPI treatment with concomitant NSAIDs or anticoagulants suggests that they may be frequently prescribed for prevention rather than treatment. The evidence for use with NSAIDs was generated for ‘high-risk’ patients, who had previously had GI bleeding.36 The use of PPIs for lower risk patients may increase risks of other NSAID complications.36,37 It has been noted in many other settings that ‘preventative’ use of PPIs with these medicines is a major driver of excessive long-term use.25,37,38 While we observed attempts to step-down treatment in people on maintenance and long-term therapy, low strength PPI dispensing comprises a very small component of overall PPI use in Australia. It is worth noting that the low strength formulation of the most commonly dispensed PPI medicine in Australia, esomeprazole, is not publicly subsidized in Australia (see Table 1), meaning stepping down would require either a change of medication or patients bearing the cost of treatment. Most of the step-down treatment we observed was from high strength to standard strength esomeprazole.
While the use of low strength PPIs has increased since the last major PPI utilization review in Australia,6 in May of 2019 the Australian Government Department of Health reintroduced a number of prescribing restrictions for high and standard strength PPIs to further promote the use of low strength PPIs.39,40 The changes are not as limiting as the pre-2001 restrictions – which required prescribers receive permission from the Australian Government before prescribing and limited the use of PPIs to treat ‘severe refractory ulcerating oesophagitis proven by endoscopy’6 – but they require prescribers to obtain approval to prescribe the highest strength PPIs and that the number of repeat prescriptions for most formulations be reduced from five to one.40
The primary limitation of our study is a lack of information for treatment indication, and we were unable to ascertain whether or not the long-term treatment we observed represented appropriate care. We used a 12-month lookback period to estimate incidence of PPI use and this may have misclassified some individuals using PPIs more sporadically (as-needed as opposed to daily, for example) as incident users, potentially overestimating the incidence of PPI use. We estimated periods of exposure based on dispensing records and assumed that all dispensed medicine was taken.41 This approach may misclassify exposure and step-down status for individuals who used dispensed PPIs on an as-needed/pro re nata basis. PBS data capture PPI dispensed in the community; they do not contain records of PPI dispensed in public hospitals or over the counter.I In this way our estimates of PPI use may be slightly below actual use. The primary strength of our study is the use of longitudinal data from the entire Australian population treated with publicly subsidized PPIs between 2013 and 2016. Our results are highly generalizable to similar, developed nations’ populations.
Conclusion
Our study shows that the proportion of people continuing PPIs beyond the guideline-recommended maximum of 12 weeks is greater than that which might be expected to have conditions indicated for longer-term PPI treatment. Around one third of people continuing on longer-term treatment step-down treatment to a lower strength PPI, however, most individuals do not deviate from their initiation strength. In light of this, prescribers may wish to consider the strength prescribed at initiation, as well as routine medicine reviews to ensure long-term treatment is indeed indicated. Given the many adverse events potentially associated with long-term PPI treatment, increased regulation of these medicines, such as the restrictions recently implemented in Australia, are warranted.
Supplemental Material
Supplemental material, Supplementary_Table for Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013–2016 by Benjamin Daniels, Sallie-Anne Pearson, Nicholas A. Buckley, Claudia Bruno and Helga Zoega in Therapeutic Advances in Gastroenterology
Acknowledgments
We acknowledge the contributions of Dr Louise Bartlett, Ms Prue Twiddle and the Australian Government Department of Health to the present manuscript. We further thank the Australian Government Department of Human Services for providing the data for the study.
Low strength pantoprazole became available for purchase from pharmacies without a prescription in June 2015. Standard strength esomeprazole and low strength rabeprazole and lansoprazole gained over-the-counter marketing approval in Australia in June 2016.
Footnotes
Author contributions: BD, HZ, SAP, NAB and CB conceived of the study concept and design. BD performed all statistical analyses. All authors contributed to the interpretation of the data, drafting of the manuscript and final approval of the manuscript.
Conflict of interest statement: SAP is a member of the Drug Utilisation Sub Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of either committee. The remaining authors have no interests to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Australian Government Department of Health and the NHMRC Centre of Research Excellence in Medicines and Ageing (CREMA; ID: 1060407). HZ is supported by a UNSW Scientia Fellowship. The funders of the study had no role in the data analysis, interpretation or drafting of the final manuscript.
ORCID iDs: Benjamin Daniels  https://orcid.org/0000-0001-8617-6055
https://orcid.org/0000-0001-8617-6055
Claudia Bruno  https://orcid.org/0000-0001-7789-3415
https://orcid.org/0000-0001-7789-3415
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Benjamin Daniels, Medicines Policy Research Unit, Centre for Big Data Research in Health, UNSW Sydney, AGSM Building, Level 2, Kensington, NSW 2052, Australia.
Sallie-Anne Pearson, Medicines Policy Research Unit, Centre for Big Data Research in Health, UNSW, Sydney, NSW, Australia.
Nicholas A. Buckley, Clinical Pharmacology and Toxicology Research Group, Discipline of Pharmacology, University of Sydney, Sydney, NSW, Australia
Claudia Bruno, Medicines Policy Research Unit, Centre for Big Data Research in Health, UNSW, Sydney, NSW, Australia.
Helga Zoega, Medicines Policy Research Unit, Centre for Big Data Research in Health, UNSW, Sydney, NSW, Australia; Centre of Public Health Sciences, Faculty of Medicine, University of Iceland.
References
- 1. Othman F, Card TR, Crooks CJ. Proton-pump inhibitor prescribing patterns in the UK: a primary care database study. Pharmacoepidemiol Drug Saf 2016; 25: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 2. Pottegård A, Broe A, Hallas J, et al. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 2016; 9: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeng W, Finlayson AE, Shankar S, et al. Prescribing efficiency of proton-pump inhibitor in China: influence and future directions. BMC Health Serv Res 2015; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraeyman J, Van Hal G, Godman B, et al. The potential influence of various initiatives to improve rational prescribing for proton-pump inhibitor and statins in Belgium. Expert Rev Pharmacoecon Outcomes Res 2013; 13: 141–151. [DOI] [PubMed] [Google Scholar]
- 5. Strand DS, Kim D, Peura DA. 25 Years of proton-pump inhibitor: a comprehensive review. Gut Liver 2017; 11: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hollingworth S, Duncan EL, Martin JH. Marked increase in proton-pump inhibitor use in Australia. Pharmacoepidemiol Drug Saf 2010; 19: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 7. El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. Epub ahead of print 13 July 2013. DOI: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd; 2018, https://shop.amh.net.au/faq/58. [Google Scholar]
- 9. eTG complete. Australian Therapeutic Guidelines: disorders of the oesophagus. Melbourne, Victoria: Therapeutic Guidelines Limited, 2018. [Google Scholar]
- 10. Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009; 29: 938–946. [DOI] [PubMed] [Google Scholar]
- 11. Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev 2010; 9: A311–A318. [DOI] [PubMed] [Google Scholar]
- 12. Scarpignato C, Pelosini I, Di Mario F. Acid suppression therapy: where do we go from here? Dig Dis 2006; 24: 11–46. [DOI] [PubMed] [Google Scholar]
- 13. Hu X, You X, Sun X, et al. Off label use of proton-pump inhibitor and economic burden in Chinese population: a retrospective analysis using claims database. Value Health 2018; 21: S82–S83. [Google Scholar]
- 14. Halfdanarson OO, Pottegard A, Bjornsson ES, et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol. 2018; 11: 1756284818777943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raghunath AS, O’Morain C, McLoughlin RC. Review article: the long-term use of proton-pump inhibitors. Aliment Pharmacol Ther 2005; 22(Suppl. 1): 55–63. [DOI] [PubMed] [Google Scholar]
- 16. Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton-pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med 2016; 14: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Institute for Health and Care Excellence. (NICE): Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management (Guideline CG184), 2014, https://www.nice.org.uk/guidance/cg184. [PubMed] [Google Scholar]
- 18. Metz DC. Long-term use of proton-pump inhibitor therapy. Gastroenterol Hepatol 2008; 4: 322–325. [PMC free article] [PubMed] [Google Scholar]
- 19. Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep 2009; 11: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol 2009; 104(Suppl. 2): S27–S32. [DOI] [PubMed] [Google Scholar]
- 21. Cao F, Chen CX, Wang M, et al. Updated meta-analysis of controlled observational studies: proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect 2018; 98: 4–13. [DOI] [PubMed] [Google Scholar]
- 22. Nehra AK, Alexander JA, Loftus CG, et al. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc 2018; 93: 240–246. [DOI] [PubMed] [Google Scholar]
- 23. Makunts T, Cohen IV, Awdishu L, et al. Analysis of postmarketing safety data for proton-pump inhibitors reveals increased propensity for renal injury, electrolyte abnormalities, and nephrolithiasis. Sci Rep 2019; 9: 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One 2015; 10: e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301: 937–944. [DOI] [PubMed] [Google Scholar]
- 26. Yadlapati R, Kahrilas PJ. When is proton pump inhibitor use appropriate? BMC Med 2017; 15: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weekes LM. Proton pump inhibitors: too much of a good thing? NPS MedicineWise 2015; 202: 464. [DOI] [PubMed] [Google Scholar]
- 28. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WHO Collaborating Centre for Drug Statistics Methodology. International language for drug utilization research, 2019, https://www.whocc.no/.
- 30. Lu CY, Barratt J, Vitry A, et al. Charlson and Rx-Risk comorbidity indices were predictive of mortality in the Australian health care setting. J Clin Epidemiol 2011; 64: 223–228. [DOI] [PubMed] [Google Scholar]
- 31. Australian Bureau of Statistics: ABS.Stat, 2018, http://stat.data.abs.gov.au/Index.aspx?DataSetCode=ERP_QUARTERLY.
- 32. Pisengna JR, Goyal D, Benhammou J. Zollinger-Ellison syndrome. BMJ Best Pract, 2018, https://bestpractice.bmj.com/topics/en-us/408/epidemiology.
- 33. Pratt NL, Kalisch Ellett LM, Sluggett JK, et al. Use of proton pump inhibitors among older Australians: national quality improvement programmes have led to sustained practice change. Int J Qual Health Care 2016; 29: 75–82. [DOI] [PubMed] [Google Scholar]
- 34. Bruno C, Pearson SA, Daniels B, et al. Passing the acid test? Evaluating the impact of national education initiatives to reduce proton pump inhibitor use in Australia. BMJ Qual Saf. Epub ahead of print 22 October 2019. DOI: 10.1136/bmjqs-2019-009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thong BKS, Ima-Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health 2019; 16: 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American gastroenterological association. Gastroenterology 2017; 152: 706–715. [DOI] [PubMed] [Google Scholar]
- 37. Savarino V, Dulbecco P, de Bortoli N, et al. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med 2017; 37: 19–24. [DOI] [PubMed] [Google Scholar]
- 38. Meadows T. Do proton pump inhibitors reduce the clinical efficacy of clopidogrel? NHS Specialist Pharmacy Service, 2017, https://www.sps.nhs.uk/articles/do-proton-pump-inhibitors-reduce-the-clinical-efficacy-of-clopidogrel-2/.
- 39. Pharmaceutical Benefits Advisory Committee: Recommendations made by the PBAC - July 2018, http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/recommendations-made-by-the-pbac-july-2018 (2018).
- 40. NPS MedicineWise. Proton pump inhibitors: PBS changes May 2019, https://www.nps.org.au/radar/articles/proton-pump-inhibitors-pbs-changes-may-2019 (2019) [Google Scholar]
- 41. Pottegard A, Hallas J. Assigning exposure duration to single prescriptions by use of the waiting time distribution. Pharmacoepidemiol Drug Saf 2013; 22: 803–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table for Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013–2016 by Benjamin Daniels, Sallie-Anne Pearson, Nicholas A. Buckley, Claudia Bruno and Helga Zoega in Therapeutic Advances in Gastroenterology