Abstract
Background:
Gluteal tendinopathy is a common cause of lateral hip pain. Percutaneous ultrasonic tenotomy (PUT) has been used successfully for the treatment of tendinopathy of the elbow, knee, and ankle, but its use in the hip has not been described.
Purpose:
To evaluate the efficacy of PUT in patients who did not respond to nonsurgical management of gluteal tendinopathy.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 29 patients with gluteal tendinopathy (mean age, 62 years) who did not respond to nonsurgical treatment were enrolled in this prospective study and underwent ultrasound-guided PUT in an outpatient setting. Patients with a history of ipsilateral hip surgery were excluded. All patients initially underwent magnetic resonance imaging or a computed tomography arthrogram demonstrating tendinopathy and/or partial tearing of the gluteus minimus or medius tendon or both tendons. Outcomes were assessed with a visual analog scale (VAS) for pain, the Harris Hip Score evaluation, and the 12-Item Short Form Health Survey (SF-12) before the procedure and at subsequent follow-up visits or by telephone interviews at 3 weeks, 3 months, 6 months, and final follow-up (range, 18-30 months).
Results:
The mean final follow-up was at 22 months postoperatively. At final follow-up, VAS scores had improved from a preprocedural mean ± SD of 5.86 ± 1.73 to 2.82 ± 2.22 (P < .01). Harris Hip Scores improved from a preprocedural mean of 60.03 ± 10.86 to 77.47 ± 14.34 (P < .01). Total SF-12 scores improved from a mean of 29.93 ± 5.39 (51% optimal) to 34.41 ± 4.88 (64% optimal) (P < .01). No complications were reported. At final follow-up, when asked whether they would have the procedure again, 15 patients replied “yes definitely,” 3 replied “yes probably,” 3 replied “maybe,” 1 replied “likely not,” and 2 replied “definitely not.” There were 3 patients who eventually had hip abductor tendon repair, and their PUT procedures were considered failures.
Conclusion:
PUT is an effective treatment, with good results for patients with gluteal tendinopathy.
Keywords: tendinitis, ultrasound-guided, minimally invasive, gluteal tendinopathy, greater trochanteric pain syndrome
Gluteal tendinopathy and trochanteric bursitis both fall under the broader term greater trochanteric pain syndrome.17,18,21,26 The problem is most often thought to be related to tendinosis of the gluteus medius or minimus tendon, or both, with or without bursal involvement. The gluteus medius and minimus tendons insert on the greater trochanter of the femur and together act as the hip abductors. A bursa lies between the 2 muscles and beneath the iliotibial band. Over time, friction over the greater trochanter and hip abductor tendons can lead to partial tears of the gluteal tendons and can present as lateral hip pain localized over the greater trochanter.17 The patient’s pain often radiates to the lateral aspect of the thigh and knee. Pain often increases when the patient is going up and down stairs and is often most pronounced while the patient is lying on the affected side. If tendinopathy or tearing of the gluteal tendons is suspected, a magnetic resonance imaging (MRI) scan or ultrasound study can confirm the diagnosis and further detail the pathologic features.13,19
Initially, the treatment regimen for gluteal tendinopathy consists of rest, activity modification, weight reduction, stretching, physical therapy, and nonsteroidal anti-inflammatory medication.26 More invasive techniques include corticosteroid injections, platelet-rich plasma (PRP) injections,10,11 and extracorporeal shock wave therapy.12,22,27 If these measures are not successful, open bursectomy and iliotibial band lengthening5,6,30,33 and arthroscopic bursectomy1 are available treatment options with good success rates.
Advanced ultrasound-guided interventions have been used for tendinopathy.24 Percutaneous ultrasonic tenotomy (PUT) has been used successfully to treat lateral epicondylitis,2,28 Achilles tendinitis,7,20 patellar tendinitis,23 and plantar fasciitis.20 The technique uses high-frequency energy to debride pathologic tissue under continuous irrigation.16 The rationale behind percutaneous tenotomy is that when the pathologic tissue is removed, a chronic degenerative process is converted to an acute process, introducing inflammatory growth factors and promoting tendon healing.17
In 2016, a system modification was made to lengthen the probe (Tenex TX System; Tenex Health) used to deliver ultrasonic energy and irrigation to the diseased tendon. The longer probe allowed this percutaneous technique to be used to treat tendinopathy in the shoulder and the hip (Figure 1).
Figure 1.

Ultrasound-guided percutaneous tenotomy of the hip abductor tendons. GMe, gluteus medius; IT, iliotibial; TFL, tensor fascia lata.
We hypothesized that ultrasound-guided PUT would provide pain relief and functional improvement in patients with gluteal tendinopathy. We report our results in a prospective series of patients in whom the technique was used to treat recalcitrant gluteal tendinopathy after at least 4 months of nonoperative management.
Methods
Study Design
After institutional review board approval was obtained, patients were enrolled in this prospective study if they (1) were 18 years or age or older; (2) had experienced symptoms of gluteal tendinopathy for a minimum of 4 months (ie, pain with gait, pain with activities of daily living, or inability to lie on the affected side at night); (3) had not responded to nonsurgical treatment modalities, including local injection for a minimum of 4 months; and (4) had a noncontrast MRI or computed tomography (CT) arthrogram read by a radiologist that showed either partial tearing or tendinosis of the gluteus medius or minimus tendon, or both. Patients with complete tears were treated with open repair; they were not believed to be candidates for tenotomy. Patients with previous open surgery on the hip were also excluded.
After informed consent was obtained, 29 patients were enrolled in the study and underwent treatment. They completed pretreatment questionnaires for baseline measurement of symptoms. Outcomes were assessed with a visual analog scale (VAS) for pain,25 Harris Hip Score (HHS),14,31 and the 12-Item Short Form Health Survey (SF-12)32 before the procedure and at subsequent follow-up visits or by telephone interviews at 3 weeks, 3 months, 6 months, and a final follow-up after a minimum of 18 months. (The SF-12, a generic measure of patients’ self-reported health status, contains mental and physical domains and provides a total score. A higher score means that the respondent has optimal physical or mental health, or both.) At the final follow-up, a patient satisfaction questionnaire was administered.
The treatment group consisted of 26 females and 3 males, with an average age of 62 years (range, 32-83 years) (Table 1).
Table 1.
Patient Demographics and MRI Results in 29 Patientsa
| Patient | Sex | Age, y | BMI | Length of Symptoms, mo | No. of Injections | MRI or CT Arthrogram Results |
|---|---|---|---|---|---|---|
| 1 | F | 63 | 27.0 | 15 | 1 | Moderate-grade tear of gluteus medius tendon with trochanteric bursitis |
| 2 | F | 66 | 30.7 | 6 | 1 | Moderate gluteal tendinosis with high-grade partial articular-sided tear involving gluteus minimus tendon; mild trochanteric bursitis |
| 3 | F | 83 | 25.1 | 60 | 1 | Gluteal tendinitis |
| 4 | F | 52 | 38.5 | 24 | 1 | Mild abductor tendinosis |
| 5 | F | 71 | 24.5 | 42 | 4 | Mild gluteus minimus tendinosis |
| 6 | F | 61 | 30.5 | 10 | 1 | CT arthrogram; mild gluteus medius tendinosis |
| 7 | F | 53 | 28.6 | 10 | 0 | Mild insertional gluteus medius/minimus tendinosis with mild trochanteric bursitis |
| 8 | F | 70 | 29.6 | 24 | 1 | Mild gluteus minimus tendinitis |
| 9 | F | 76 | 31.1 | 180 | 3 | Insertional tendinosis of gluteus minimus; greater trochanteric bursitis |
| 10 | F | 62 | 24.4 | 6 | 1 | Moderate-grade partial tearing of distal gluteus medius and minimus tendons |
| 11 | F | 65 | 35.0 | 12 | 0 | Moderate-grade partial tearing of distal gluteus medius and minimus tendons |
| 12 | M | 63 | 20.8 | 24 | 4 | Tendinosis and low- to moderate-grade partial tearing of posterior aspect of gluteus minimus and anterior aspect of gluteus medius tendons |
| 13 | F | 71 | 25.0 | 36 | 1 | Gluteus medius tendinitis; greater trochanteric bursitis |
| 14 | F | 69 | 25.2 | 4 | 1 | Gluteus medius and minimus tendinosis with moderate-grade partial-thickness tearing |
| 15 | F | 39 | 21.9 | 24 | 2 | Low-grade partial tearing of the gluteus medius and minimus tendons |
| 16 | F | 37 | 27.0 | 10 | 3 | Moderate gluteal tendinosis without tear; mild trochanteric bursitis |
| 17 | F | 65 | 29.1 | 12 | 1 | Moderate tendinosis with partial-thickness articular-sided tear of the gluteus medius; moderate greater trochanteric bursitis |
| 18 | F | 71 | 34.5 | 60 | 1 | Moderate-grade partial tearing of the gluteus medius tendon |
| 19 | F | 60 | 26.5 | 24 | 1 | Low-grade partial tearing of distal gluteus medius and minimus tendons |
| 20 | F | 64 | 28.7 | 24 | 1 | Gluteus medius tendinosis; trochanteric bursitis |
| 21 | F | 32 | 30.6 | 14 | 1 | Gluteus minimus tendinosis and low-grade partial tearing; mild greater trochanteric bursitis |
| 22 | F | 63 | 23.5 | 6 | 1 | Severe tendinosis of the gluteus minimus tendon |
| 23 | M | 76 | 35.5 | 12 | 1 | CT arthrogram; gluteus minimus tendinopathy |
| 24 | F | 45 | 27.4 | 60 | 1 | Mild abductor tendinosis |
| 25 | F | 66 | 23.8 | 12 | 10 | Gluteus minimus tendinosis with mild- to moderate-grade partial tearing; gluteus medius tendinosis |
| 26 | M | 69 | 31.0 | 24 | 5 | Gluteal tendinitis |
| 27 | F | 59 | 26.4 | 204 | 6 | Gluteus medius tendinitis with small partial-thickness tear of gluteus minimus; mild trochanteric bursitis |
| 28 | F | 63 | 28.8 | 240 | 2 | Moderate gluteal tendinosis with mild trochanteric bursitis |
| 29 | F | 65 | 21.9 | 9 | 1 | Tendinopathy with low-grade partial-thickness tears of the gluteus medius and minimus tendons |
aBMI, body mass index; CT, computed tomography; F, female; M, male; MRI, magnetic resonance imaging.
Technique and Postprocedural Care
For insurance reimbursement of the facility fee, the procedures are typically done in a hospital or surgery center. The patient was placed in the lateral decubitus position with the affected hip up. The point of maximum tenderness either proximal to or over the greater trochanter was identified. Next, a diagnostic ultrasound was performed to localize the area of diseased tissue, which is identified as a hypoechoic area of the insertion of the gluteus medius or minimus tendons over the greater trochanter. The area was then prepared and draped in a sterile fashion (Figure 2). The skin, subcutaneous tissue, and tendon were then anesthetized with a local anesthetic in the trajectory of the intended treatment. A No. 11 blade was used to make an incision through which the ultrasound needle (Tenex TX System) was placed. Under ultrasonic imaging, the needle was advanced to the diseased gluteal tendon insertion (Figure 3). A tenotomy was then performed by use of pulsed ultrasound energy under direct visualization with continuous irrigation and debridement of the pathologic tissue. The procedure, which usually took between 2 and 3 minutes, was complete when all visual evidence of damaged tendon was removed and confirmed by ultrasound visualization. After completion, a sterile dressing was applied, and the patient was allowed to leave the treatment area. Patients were instructed to bear weight as tolerated, perform stretching, and reduce their activities for 7 to 10 days before resuming their normal activities.
Figure 2.
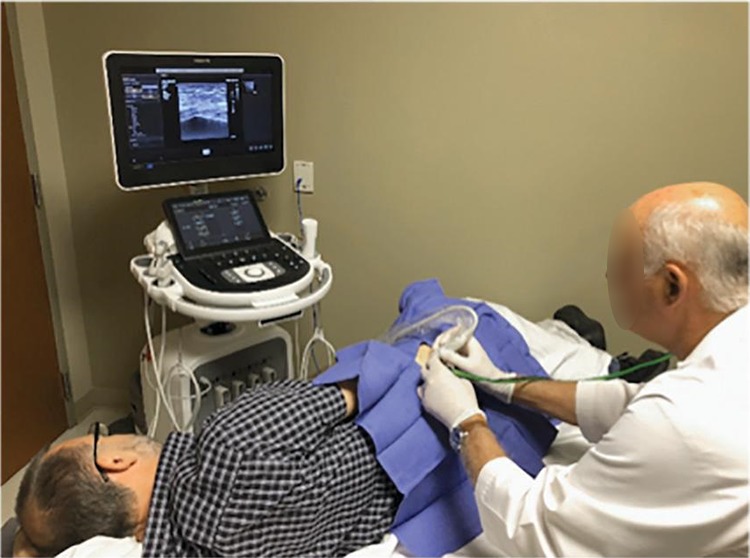
The procedure is performed under ultrasound visualization.
Figure 3.
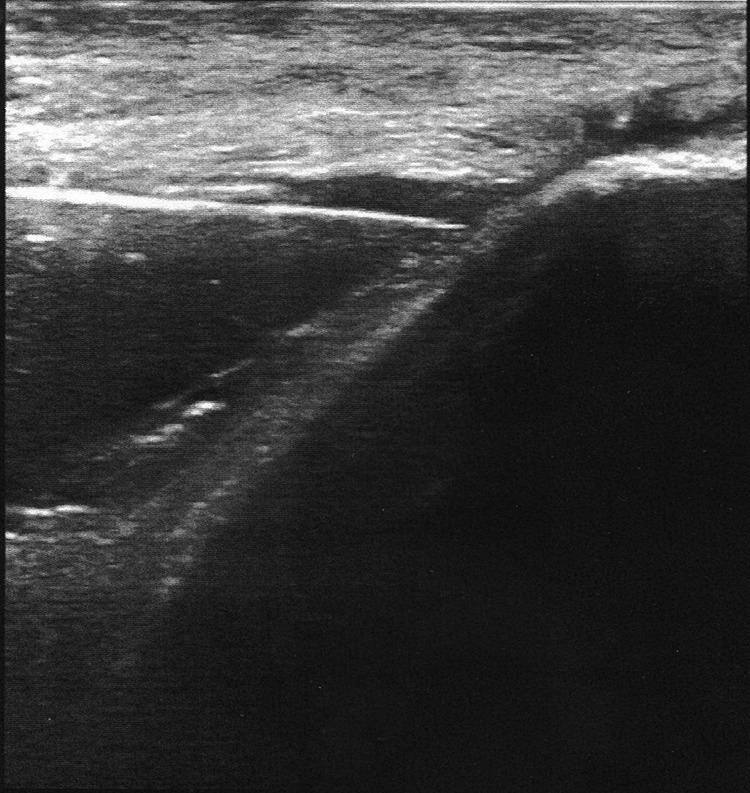
Coronal ultrasound image demonstrating the needle ultrasound probe at the gluteus medius tendon insertion site on the greater trochanter.
Statistical Analysis
We performed repeated-measures analysis of variance (ANOVA) for each of our outcome measures: VAS, HHS, and SF-12. We conducted the Mauchly test of sphericity on each to determine whether adjustments needed to be made to the degree of freedom. Last, we ran Bonferroni post hoc tests to apply the correction to adjust the confidence intervals and P values to take into account multiple comparisons.
Results
Patients were evaluated for an average of 22 months (median, 20 months; range, 18-30 months); 3 patients (10%) had failed results and went on to have open abductor tendon repair.
Visual Analog Scale
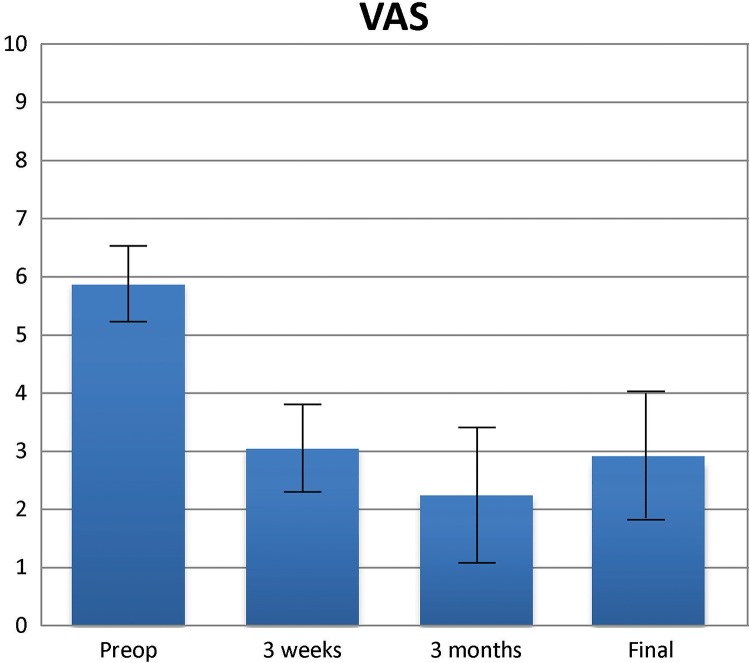
The VAS descriptive analysis showed that pain scores declined gradually over time, with the lowest mean ± SD score at the 3-month interval (2.24 ± 1.97) (Figure 4 and Table 2). A slight increase was noted at 6 months (2.52 ± 2.34) and at 18 months (2.82 ± 2.22); however, mean scores remained below the preprocedural and 3-week postprocedural averages. Overall, pain scores decreased from a preprocedural mean of 5.86 ± 1.73 to 2.82 ± 2.22 at 18 months after the procedure.
Figure 4.
Visual analog scale (VAS) scores (with 95% CI) before and after the procedure.
Table 2.
Visual Analog Scale Descriptive Statistics in 29 Patients
| Time Point | Mean | SD | 95% CI |
|---|---|---|---|
| Before procedure | 5.86 | 1.73 | 5.21 to 6.52 |
| 3 wk | 3.04 | 1.95 | 2.30 to 3.78 |
| 3 mo | 2.24 | 1.97 | 1.49 to 2.99 |
| 6 mo | 2.52 | 2.34 | 1.63 to 3.41 |
| 18 mo | 2.82 | 2.22 | 1.98 to 3.67 |
The repeated-measures ANOVA measured the difference between the VAS mean scores over the course of 18 months after the procedure; the VAS pain score was significantly different at the 4 follow-up time points (F 3.22, 90.08 = 19.78; P < .01; partial η2 = 0.41). The sample effect size based on within-patient factor variability was 0.41 (Table 3).
Table 3.
Visual Analog Scale One-Way Repeated-Measures Analysis of Variancea
| Source | Type 3 Sum of Squares | df | Mean Square | F | Significance | Partial η2 | |
|---|---|---|---|---|---|---|---|
| Time | Greenhouse-Geisser | 249.27 | 3.22 | 77.48 | 19.78 | <.01 | 0.41 |
| Error | Greenhouse-Geisser | 362.87 | 90.08 | 3.92 | — | — | — |
aDashes refer to no value given.
Post hoc analysis with a Bonferroni adjustment showed that the VAS concentration significantly decreased from preprocedural average to each follow-up time point. A significant decrease was seen from preprocedural average to 3 months (mean difference, 2.82; 95% CI, 1.44 to 4.21; P < .01) and from preprocedural average to 18 months (mean difference, 3.04; 95% CI, 1.44 to 4.64; P < .01) (Table 4).
Table 4.
Visual Analog Scale Pairwise Comparisons With Preprocedural Scores (Based on Estimated Marginal Means)
| Time Point | Mean Differencea | SE | Significanceb | 95% CI |
|---|---|---|---|---|
| 3 wk | 2.82 | 0.45 | <.01 | 1.44 to 4.21 |
| 3 mo | 3.63 | 0.45 | <.01 | 2.27 to 4.98 |
| 6 mo | 3.34 | 0.54 | <.01 | 1.71 to 4.97 |
| 18 mo | 3.04 | 0.53 | <.01 | 1.44 to 4.64 |
aMean difference is significant at the .05 level.
bAdjustment for multiple comparisons: Bonferroni correction.
Harris Hip Score
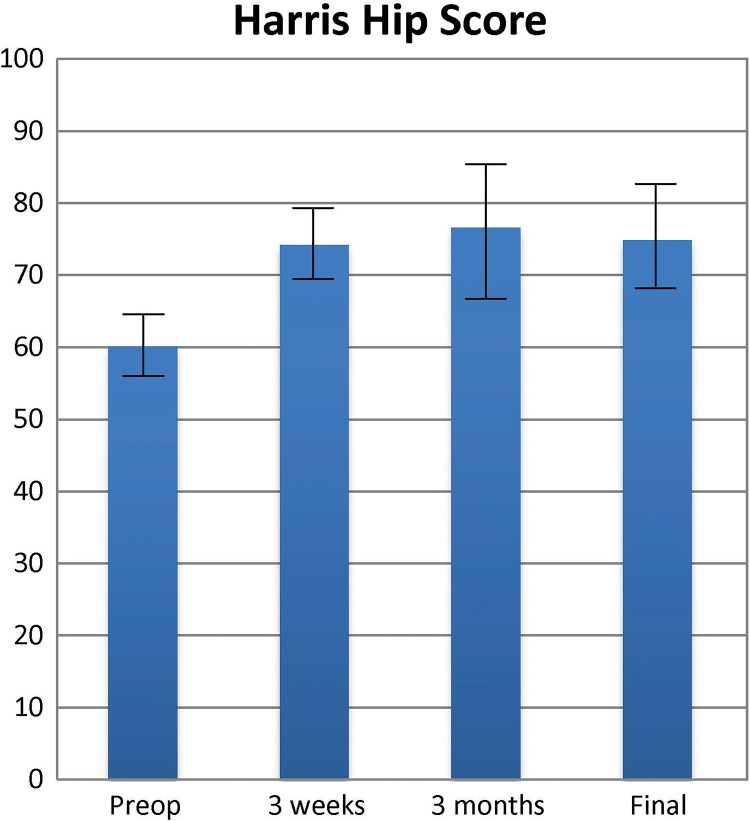
The HHS results improved significantly at the 18-month follow-up (Figure 5). An increase in function was noted from 60.03 ± 10.86 before the procedure to 77.47 ± 14.34 at 18 months (Table 5).
Figure 5.
Harris Hip Scores (with 95% CI) before and after the procedure.
Table 5.
Harris Hip Score Descriptive Statistics in 29 Patients
| Time Point | Mean | SD | 95% CI |
|---|---|---|---|
| Before procedure | 60.03 | 10.86 | 55.90 to 64.17 |
| 3 wk | 74.15 | 12.04 | 69.57 to 78.74 |
| 3 mo | 76.58 | 15.25 | 70.78 to 82.38 |
| 6 mo | 74.83 | 17.15 | 68.30 to 81.35 |
| 18 mo | 77.47 | 14.34 | 72.02 to 82.93 |
The differences in HHS results were statistically significant at each time point (F 2.40, 67.17 = 13.10; P < .01; partial η2 = 0.32). The sample effect size for the within-patient factor variability was 0.32 (Table 6).
Table 6.
Harris Hip Score One-Way Repeated-Measures Analysis of Variancea
| Source | Type 3 Sum of Squares | df | Mean Square | F | Significance | Partial η2 | |
|---|---|---|---|---|---|---|---|
| Time | Greenhouse-Geisser | 5939.66 | 2.40 | 2475.87 | 13.10 | <.01 | 0.32 |
| Error | Greenhouse-Geisser | 362.87 | 90.08 | 3.92 | — | — | — |
aDashes refer to no value given.
A post hoc analysis with Bonferroni adjustment showed that the HHS difference decreased from the preprocedural value to –14.12 (95% CI, –23.01 to –5.23; P < .01) at 3 weeks and –17.44 (95% CI, –25.96 to –8.91; P < .01) at 18 months of follow-up (Table 7).
Table 7.
Harris Hip Score Pairwise Comparisons With Preprocedural Scores (Based on Estimated Marginal Means)
| Time Point | Mean Differencea | SE | Significanceb | 95% CI |
|---|---|---|---|---|
| 3 wk | –14.12 | 2.92 | <.01 | –23.01 to –5.23 |
| 3 mo | –16.54 | 3.54 | <.01 | –27.34 to –5.75 |
| 6 mo | –14.79 | 3.69 | <.01 | –26.02 to –3.56 |
| 18 mo | –17.44 | 2.80 | <.01 | –25.96 to –8.91 |
aMean difference is significant at the .05 level.
bAdjustment for multiple comparisons: Bonferroni correction.
Early and intermediate data showed improvement with no marked deterioration over time.
SF-12 Health Survey
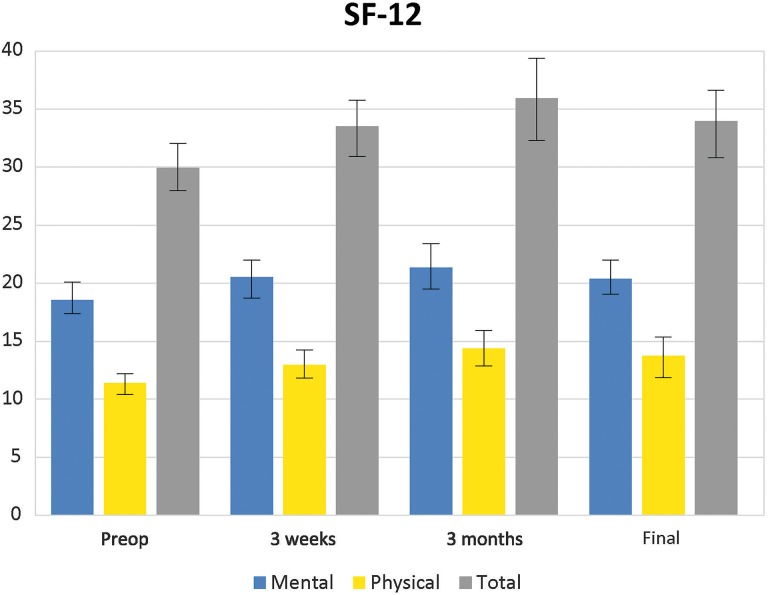
The SF-12 is a quality-of-health survey. Composite measures assessed at follow-up included physical and mental health scores. An increase in total function from before the procedure was noted, with a mean score of 29.93 ± 5.39 to 34.41 ± 4.88 at 18 months after surgery (Figure 6 and Table 8).
Figure 6.
Mean scores for the 12-item Short Form Health Survey (SF-12) (with 95% CI) before and after the procedure.
Table 8.
12-Item Short Form Health Survey Descriptive Statistics in 29 Patients
| Time Point | Mean | SD | 95% CI |
|---|---|---|---|
| Before procedure | 29.93 | 5.39 | 27.88 to 31.98 |
| 3 wk | 33.50 | 5.68 | 31.34 to 35.66 |
| 3 mo | 35.75 | 6.21 | 33.39 to 38.11 |
| 6 mo | 34.09 | 6.47 | 31.62 to 36.55 |
| 18 mo | 34.41 | 4.88 | 35.56 to 36.27 |
Sphericity is assumed; therefore, the 1-way repeated-measures ANOVA made no adjustments (Table 9). A statistically significant difference was noted in composite SF-12 scores at the 5 time points (before the procedure and 3 weeks, 3 months, 6 months, and 18 months after the procedure) (F 4.00, 112.00 = 9.80; P < .01; η2 = 0.26) (Table 9). The sample effect size based on within-patient factor variability was 0.26.
Table 9.
12-Item Short Form Health Survey One-Way Repeated-Measures Analysis of Variancea
| Source | Type 3 Sum of Squares | df | Mean Square | F | Significance | Partial η2 | |
|---|---|---|---|---|---|---|---|
| Time | Greenhouse-Geisser | 550.63 | 4.00 | 137.66 | 9.80 | <.01 | 0.26 |
| Error | Greenhouse-Geisser | 1573.36 | 112.00 | 14.05 | — | — | — |
aDashes refer to no value given.
A post hoc analysis with Bonferroni adjustment indicated that there was a significant mean difference in the SF-12 composite scores at each time point when compared with the preprocedural baseline. The mean difference in SF-12 composite scores at 18 months postoperatively was –4.483 (P < .01) (Table 10).
Table 10.
12-Item Short Form Health Survey Pairwise Comparisons (Based on Estimated Marginal Means)
| Time Point | Mean Differencea | SE | Significanceb | 95% CI |
|---|---|---|---|---|
| 3 wk | –3.571 | 0.89 | <.01 | –6.28 to –0.86 |
| 3 mo | –5.821 | 1.03 | <.01 | –8.96 to –2.68 |
| 6 mo | –4.158 | 1.07 | .01 | –7.42 to –0.90 |
| 18 mo | –4.483 | 1.03 | <.01 | –7.63 to –1.33 |
aMean difference is significant at the .05 level.
bAdjustment for multiple comparisons: Bonferroni correction.
Patient Satisfaction
At final follow-up, when patients were asked whether they would have the procedure again, 15 (52%) responded, “yes definitely,” 3 (10%) responded “yes probably,” 3 (10%) responded “maybe,” 1 (3%) responded “likely not,” and 2 (7%) responded “definitely not.” For 2 patients (7%), 6-month follow-up data were not available. A further 3 patients (10%) in the study group who continued to have pain were considered to have experienced treatment failure and subsequently underwent open debridement and repair of the abductor tendons at 6 weeks, 5 months, and 14 months after their initial procedures. All had resolution of pain after surgery and improved function.
Discussion
The chronic symptoms of greater trochanteric pain syndrome are now recognized to be due to tendon degeneration secondary to repetitive microtrauma rather than an inflammatory bursal condition alone.26 Consequently, measures aimed at minimizing inflammation are unlikely to address the problem of tendinosis.18 Although nonsurgical measures should be considered first for the treatment of gluteal tendinopathy, currently no evidence-based protocol is available for the management of greater trochanteric pain syndrome.26
All of our patients with symptomatic greater trochanteric pain syndrome had confirmed tendinosis or low-grade partial-thickness tears. Although an abnormal finding, this condition can be found in asymptomatic individuals. In a 2004 study,8 investigators performed MRI on patients with and without greater trochanteric pain and demonstrated that 8% of asymptomatic patients had findings consistent with an abductor tear. In 2008, Blankenbaker et al3 reviewed 131 consecutive MRI studies of the pelvis (256 hips). Only 16 of the 256 hips were described as painful. The authors found that 88% of the hips with trochanteric pain had MRI findings of gluteal tendinopathy, whereas 50% of those without symptoms had findings of tendinopathy. All of our patients had symptomatic tendinosis or partial tears confirmed by MRI or CT arthrogram.
Multiple percutaneous techniques have been described in recent literature that are specific to the hip. Jacobson et al15 compared percutaneous tendon fenestration with PRP injection for gluteal tendinosis. They studied 15 patients in each group and found no significant difference in summated pain scores at final follow-up between the 2 groups. At an average final follow-up of 92 days, 71% of patients in the needle fenestration group reported improvement versus 79% of patients in the PRP group. Similarly, Brennan et al4 demonstrated noninferiority of dry needling versus cortisone injection in a noninferiority randomized clinical trial comparing 50 hips in 43 patients evaluated for 6 weeks. Fitzpatrick et al11 published the 2-year results of leucocyte-rich platelet-rich plasma (PRP) treatment for gluteus and medius tendinopathy. Those investigators found that a single injection of PRP performed under ultrasound guidance resulted in greater improvement in pain and function compared with a single corticosteroid injection.
Recently, additional minimally invasive measures have become available to treat tendinopathy. PUT has been studied for chronic elbow tendinopathy.2,28 It offers the benefit of precisely guided removal of tendinopathic tissue; it can be done in a variety of practice settings; and it can be done under local anesthesia. PUT focuses on emulsification and debridement of angiofibroblastic tissues seen within tendinopathy or fasciopathy.
Surgical intervention for greater trochanteric pain syndrome has evolved from open surgery to endoscopic bursectomy and tendon repair. In a 2007 study,1 patients with recalcitrant trochanteric bursitis underwent arthroscopic bursectomy. At final follow-up at an average of 26 months, the authors reported an improvement in mean VAS scores from 7.2 to 3.1 (P = .0001); further, HHS improved from a preoperative mean of 51 to 77 at final follow-up (P = .022). In our study, at final follow-up averaging 23 months (median, 20 months; range, 18-30 months), we saw an improvement in mean VAS scores from 5.86 to 2.82 (P < .01), HHS improved from a mean of 60.03 to 77.47 (P < .01), and total SF-12 scores improved from a mean of 29.93 to 34.41 (P < .01).
In our assessment of clinical outcomes in this study, we used the HHS primarily because it is a validated tool to measure hip symptoms.31 Although the HHS is heavily weighted toward pain, it also measures function, activities, deformity, and range of motion. Our primary goal with this study was pain relief. The patients in this study had a mean score improvement of 17.44 points at final follow-up, which is greater than the minimal clinically important improvements of 16 points as reported by Singh et al.29
We also used a VAS to assess pain and found an overall mean difference of 3.04 points. According to Farrar et al,9 a reduction of approximately 2 points represents a clinically important difference. The most marked difference in pain was seen in the first 12 weeks after treatment. Mean VAS scores slightly increased at final follow-up but were not significant.
Our last outcome measurement, the SF-12, is a subset of the SF-36 Health Survey, which is a generic measure of patients’ self-reported health status; we found a statistically significant difference in pre- and postprocedural total SF-12 scores.
Limitations
This study was a case series, which inherently means there is a lack of a control group. We did not compare ultrasound-guided PUT with open or arthroscopic bursectomy, which is the standard of care for patients who are surgical candidates. Although 19 patients had physical therapy before the procedure at some point for greater trochanteric pain syndrome, none of our patients underwent formal physical therapy after the procedure, which possibly could have improved their results.
Another limitation of this study was the relatively short-term follow-up. We saw a slight increase in pain scores and decrease in HHS scores after 3 months, and we cannot definitively say treatment outcomes will be sustained long term.
Conclusion
We believe that our study is the first prospective evaluation of ultrasound-guided PUT in the hip for the treatment of gluteal tendinopathy using validated outcomes questionnaires. Our patients showed an early improvement in pain and function, and no complications were noted.
This new procedure appears to be an effective and safe option for gluteal tendinopathy and is a valid option for patients who do not want to undergo surgical intervention. The procedure can be done in an outpatient setting under local anesthesia and is well tolerated. It is covered by insurance as a coded procedure, as opposed to PRP injection, which is usually a self-pay treatment, and the risks of open and arthroscopic surgery are avoided.
Acknowledgment
The authors thank Mayra Rodriguez, PhD, MPH, assistant professor and director of preventive medicine and public health, Edward Via College of Osteopathic Medicine at Auburn, for statistical analysis of the data in this study.
Footnotes
Final revision submitted November 25, 2019; accepted December 3, 2019.
One or more of the authors has declared the following potential conflict of interest or source of funding: Partial research funding for the study was provided to the Hughston Foundation by Tenex Health. C.L.B. is on the Medical Advisory Board of Tenex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Hughston Sports Medicine Center Institutional Review Board.
References
- 1. Baker CL, Massie RV, Hurt WG, Savory CG. Arthroscopic bursectomy for recalcitrant trochanteric bursitis. Arthroscopy. 2007;23(8):827–832. [DOI] [PubMed] [Google Scholar]
- 2. Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24(1):67–73. [DOI] [PubMed] [Google Scholar]
- 3. Blankenbaker DG, Ullrick SR, Davis KW, De Smet AA, Haaland B, Fine JP. Correlation of MRI findings with clinical findings of trochanteric pain syndrome. Skeletal Radiol. 2008;37(10):903–909. [DOI] [PubMed] [Google Scholar]
- 4. Brennan KL, Allen BC, Maldonado YM. Dry needling versus cortisone injection in the treatment of greater trochanteric pain syndrome: a noninferiority randomized clinical trial. J Orthop Sports Phys Ther. 2017;47(4):232–239. [DOI] [PubMed] [Google Scholar]
- 5. Brooker AF. The surgical approach for refractory trochanteric bursitis. Johns Hopkins Med J. 1979;145(3):98–100. [PubMed] [Google Scholar]
- 6. Chandrasekaran S, Lodhia P, Gui C, Vemula SP, Martin TJ, Domb BG. Outcomes of open versus endoscopic repair of abductor muscle tears of the hip: a systematic review. Arthroscopy. 2015;31(10):2057–2067. [DOI] [PubMed] [Google Scholar]
- 7. Chimenti RL, Stover DW, Fick BS, Hall MM. Percutaneous ultrasonic tenotomy reduces insertional Achilles tendinopathy pain with high patient satisfaction and a low complication rate. J Ultrasound Med. 2019;38(6):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cvitanic O, Henzie G, Skezas N, Lyons J, Minter J. MRI diagnosis of tears of the hip abductor tendons (gluteus medius and gluteus minimus). AJR Am J Roentgenol. 2004;182(1):137–143. [DOI] [PubMed] [Google Scholar]
- 9. Farrar JT, Young JP, Jr, LaMoreaua L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- 10. Fitzpatrick J, Bulsara MK, O’Donnell J, McCrory PR, Zheng MH. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy: a randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4):933–939. [DOI] [PubMed] [Google Scholar]
- 11. Fitzpatrick J, Bulsara MK, O’Donnell J, McCrory PR, Zheng MH. Leucocyte-rich platelet-rich plasma treatment of gluteus medius and minimus tendinopathy. Am J Sports Med. 2019;47(5):1130–1137. [DOI] [PubMed] [Google Scholar]
- 12. Furia JP, Rompe JD, Maffulli N. Low-energy extracorporeal shock wave therapy as a treatment for greater trochanteric pain syndrome. Am J Sports Med. 2009;37(9):1806–1813. [DOI] [PubMed] [Google Scholar]
- 13. Grimaldi A, Mellor R, Nicolson P, Hodges P, Bennell K, Vicenzino B. Utility of clinical tests to diagnose MRI-confirmed gluteal tendinopathy in patients presenting with lateral hip pain. Br J Sports Med. 2017;51(6):519–524. [DOI] [PubMed] [Google Scholar]
- 14. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737–755. [PubMed] [Google Scholar]
- 15. Jacobson JA, Yablon CM, Henning PT, et al. Greater trochanteric pain syndrome: percutaneous tendon fenestration versus platelet-rich plasma injection for treatment of gluteal tendinosis. J Ultrasound Med. 2016;35(11):2413–2420. [DOI] [PubMed] [Google Scholar]
- 16. Kamineni S, Butterfield T, Sinai A. Percutaneous ultrasonic debridement of tendinopathy: a pilot Achilles rabbit model. J Orthop Surg Res. 2015;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27(6):393–408. [DOI] [PubMed] [Google Scholar]
- 18. Khan KM, Cook JL, Taunton JE, Bonar F. Overuse tendinosis, not tendinitis, part 1: a new paradigm for a difficult clinical problem. Phys Sportsmed. 2000;28(5):38–47. [DOI] [PubMed] [Google Scholar]
- 19. Kong A, Van der Vliet A, Zadow S. MRI and US of gluteal tendinopathy in greater trochanteric pain syndrome. Eur Radiol. 2007;17(7):1772–1783. [DOI] [PubMed] [Google Scholar]
- 20. Langer PR. Two emerging technologies for Achilles tendinopathy and plantar fasciopathy. Clin Podiatr Med Surg. 2015;32(2):183–193. [DOI] [PubMed] [Google Scholar]
- 21. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25(2):279–289. [DOI] [PubMed] [Google Scholar]
- 22. Mani-Babu S, Morrissey D, Waugh C, Screen H, Barton H. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: a systematic review. Am J Sports Med. 2015;43(3):752–761. [DOI] [PubMed] [Google Scholar]
- 23. Nanos KN, Malanga GA. Treatment of patellar tendinopathy refractory to surgical management using percutaneous ultrasonic tenotomy and platelet-rich plasma injection: a case presentation. PM R. 2015;7(12):1300–1305. [DOI] [PubMed] [Google Scholar]
- 24. Peck E, Jelsing E, Onishi K. Advanced ultrasound-guided interventions for tendinopathy. Phys Med Rehabil Clin N Am. 2016;27(3):733–748. [DOI] [PubMed] [Google Scholar]
- 25. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. [DOI] [PubMed] [Google Scholar]
- 26. Reid D. The management of greater trochanteric pain syndrome: a systematic literature review. J Orthop. 2016;13(1):15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rompe JD, Segal NA, Cacchio A, Furia JP, Morral A, Maffulli N. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanteric pain syndrome. Am J Sports Med. 2009;37(10):1981–1990. [DOI] [PubMed] [Google Scholar]
- 28. Seng C, Mohan PC, Koh SB, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy sustainability and sonographic progression at 3 years. Am J Sports Med. 2016;44(2):504–510. [DOI] [PubMed] [Google Scholar]
- 29. Singh JA, Schleck C, Harmsen S, Lewallen D. Clinically important improvement thresholds for Harris Hip Score and its ability to predict revision risk after primary total hip arthroplasty. BMC Musculoskelet Disord. 2016;17:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slawski DP, Howard RF. Surgical management of refractory trochanteric bursitis. Am J Sports Med. 1997;25(1):86–89. [DOI] [PubMed] [Google Scholar]
- 31. Söderman P, Malchau H. Is the Harris Hip Score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. [DOI] [PubMed] [Google Scholar]
- 32. Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 33. Zoltan DJ, Clancy WG, Jr, Keene JS. A new operative approach to snapping hip and refractory trochanteric bursitis in athletes. Am J Sports Med. 1986;14(3):201–204. [DOI] [PubMed] [Google Scholar]