Short abstract
Over the decades, many studies have illustrated the critical roles of Wnt signaling pathways in both developmental processes as well as tumorigenesis. Due to the complexity of Wnt signaling regulation, there are still questions to be addressed about ways cells are able to manipulate different types of Wnt pathways in order to fulfill the requirements for normal or cancer development. In this review, we will describe different types of Wnt signaling pathways and their roles in both normal developmental processes and their role in cancer development and progression. Additionally, we will briefly introduce new strategies currently in clinical trials targeting Wnt signaling pathway components for cancer therapy.
Impact statement
Wnt pathway does not only play a critical role in mammalian development but has been hijacked by cancer cells and the tumor microenvironment to promote tumor progression and metastasis. Recent evidence supports further interrogation of the Wnt pathway for bench-to-bedside translation into cancer therapy. This review highlights the role of the Wnt pathway in normal development and tumorigenesis, along with an overview of new therapies currently undergoing clinical trials.
Keywords: Wnt signaling, development, tumorigenesis
Introduction
The Wnt proteins were first identified as Wingless (Wg) protein in Drosophila through genetic screenings and phenotypic analysis.1 Segmentation pattern defects in larva with Wg mutations indicated that these proteins played an important role in early embryonic development.2 Work in mice first identified the oncogenic properties of Wnt proteins through analysis of genomic integration of the mouse mammary tumor virus (MMTV); this study showed that integration of MMTV near the Int1 loci (now known as Wnt1) induced tumorigenesis.3 Further studies determined the mouse proto-oncogene Int1 and the Wg gene in Drosophila to be homologous.4 These studies laid out the significance of Wnt genes in developmental and oncogenic processes. Since, Wnt signaling pathways have been shown to play a role in many other processes such as tissue homeostasis, apoptosis, cell motility, and cancer progression.
Overview of Wnt signaling pathways
Wnt proteins and Frizzled (Fzd) receptors
Wnt proteins
Wnt proteins are cysteine rich molecules ∼40 kDa in size expressed by all metazoan species containing several conserved features, with 19 independent Wnt genes identified in mice and humans.5 Following synthesis and translocation to the endoplasmic reticulum (ER), Wnt proteins go through multiple post-translational modifications (PTMs), which are important for secretion.6,7 The most prominent PTMs in Wnt proteins that occur in the ER are glycosylation and acylation. Variable glycosylation processing allows for differentiation between basolateral and apical secretion.8 It used to be thought that acylation occurred at both cysteine and serine residue of Wnt molecules; however, recent studies showed that mono-palmitoylation occurs only at a conserved serine residue.7,9 An ER locating O-acyltransferase Porcupine (PORCN) is responsible for transferring the palmitoleic acid onto the Wnt molecules.10 PORCN mutations abolish Wnt palmitoylation and lead to embryonically lethal phenotypes in mice knockouts.11
After PTMs in the ER, Wnt molecules are ready for secretion through vesicular trafficking in cells.6 In the ER, palmitoylated Wnt molecules bind to a sorting receptor Wnt ligand secretion (WLS) modulator, which requires palmitoylation on the conserved serine residue of Wnt.12,13 WLS binds to Wnt molecules and accompanies them to the Golgi, where cargo p24 family proteins aid in promoting the exit and re-localization of WLS–Wnt complexes to the cell surface.14,15 V-ATPase-mediated vacuolar acidification allows for release of Wnt molecules from WLS and p24 proteins allowing for subsequent release into the extracellular environment where they bind to and activate transmembrane receptors, initiating a variety of Wnt signaling pathways.16
Frizzled receptors
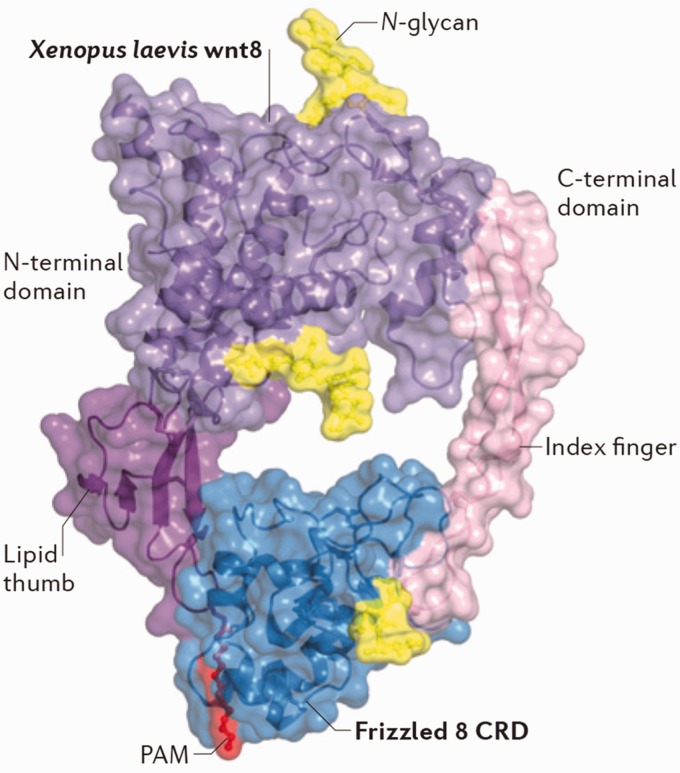
Fzd proteins are seven-pass transmembrane ligand-activated receptors with a conserved extracellular cysteine rich domain (CRD) at the N-terminal and an intracellular C-terminal domain. This protein family consists of 10 members where each Fzd receptor has a preferred Wnt ligand.17,18 Structural studies identified the general hand-like structure of Wnt proteins with thumb and index fingers pinching the extracellular CRD of Fzd receptors (Figure 1).20 The N-terminal CRD of Fzd proteins recognizes the unsaturated palmitoleic acid on the Wnt molecule. The palmitoleic acid moiety on Wnts is suggested to determine the Wnt–Fzd specificity, as it inserts into a hydrophobic crevice in the CRD of the Fzd receptor.20 Binding to Wnt bridges two CRD monomers on Fzd, which leads to the dimerization of the Fzd proteins.21 Additionally, Wnt binding to Fzd induces the formation of dimeric/multimeric structure with Wnts and other co-receptors, such as low density lipoprotein receptor related protein 5/6 (LRP 5/6).22–24 Once activated, the C-terminal domain of Fzd proteins interacts with downstream cellular proteins like the Dishevelled (DVL) protein or non-receptor tyrosine kinases for signal transduction.25,26 Additionally, Wnt binding to Fzd receptors has been shown to be coupled with heterotrimeric G proteins, triggering G protein-mediated signal transduction.27,28 To date, the signaling pathways initiated by Wnt–Fzd binding include the canonical Wnt/β-catenin pathway and other non-canonical pathways.
Figure 1.
Crystal structure of the Wnt–Frizzled binding complex. Overall cryo-EM structure of the interaction between X. laevis Wnt8 and the CRD of Frizzled 8 receptor, shown in a ‘face on’ presentation. Purple: the core of the X. laevis Wnt8. Deep purple: lipid thumb domain on the amino-terminal (N-terminal) of the X. laevis Wnt8. Light pink: The index finger domain on the carboxy-terminal of the X. laevis Wnt8. Red: the palmitoleic acid motif on the X. laevis Wnt8. Yellow: N-glycans motifs on the X. laevis Wnt8. Blue: The CRD of the Frizzle 8 receptor. Source: Figure cited from Niehrs.19
CRD: cysteine rich domain; PAM: palmitoleic acid motif.
Wnt pathways
Wnt pathways are generally classified as either canonical (β-catenin dependent) or non-canonical (β-catenin independent); however, the boundaries between these pathways vary in different cellular context (Figure 2).19 Despite the controversy surrounding Wnt pathway classification, the initiation of Wnt signaling events requires a Wnt molecule binding to a Fzd receptor and/or other co-receptors to initiate signal transduction.
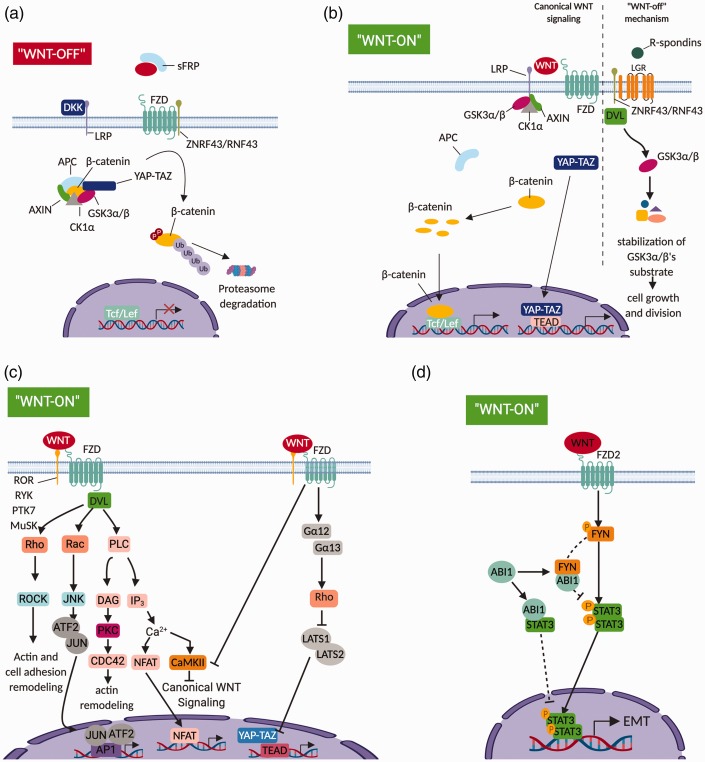
Figure 2.
Wnt signaling pathways. (a) In the absence of Wnt molecules, the canonical Wnt/β-catenin pathway is inactivated due to the existence of the β-catenin destruction complex, which is made of scaffold protein APC, Axin, Hippo pathway proteins YAP–TAZ, and kinases CK1a, GSK3a/b. The destruction complex mediates β-catenin phosphorylation for its following proteasome degradation. The canonical Wnt pathway can be negatively regulated by extracellular Wnt-binding protein like sFRPs, or by proteins like Dkk1 that downregulate membrane co-receptors (LRP5/6) of the canonical pathway. (b) In the presence of canonical Wnt molecules, Wnt binding to Fzd receptors promotes dimerization of the Fzd receptor to its co-receptor LRP5/6. Then, LRP5/6 recruits components of the β-catenin destruction complex to its cellular carboxy-terminal tails, which dissociates the destruction complex and leads to β-catenin stabilization and accumulation in cells to allow β-catenin nuclear translocation for its mediated downstream transcriptional responses. Also, the dissociation of the destruction complex prevents GSK3a/b’s substrates phosphorylation and degradation, which is called Wnt–STOP mechanism. (c) In the presence of non-canonical Wnt molecules, Wnt binding to Fzd receptors promotes dimerization of the Fzd receptor to variable types of co-receptors for recruitment of the adaptor protein DVL. The recruitment of DVL triggers multiple β-catenin independent non-canonical downstream events to mediate cellular actin remodeling processes, variable types of transcriptional responses, or canonical Wnt pathway downregulations. (d) For multiple types of cancers, a novel Wnt5a-mediated non-canonical pathway is found to mediate the EMT. In this pathway, Wnt5A binding to Frizzled-2 receptors activates Src family kinases Fyn by promoting its phosphorylation. Then, Fyn activates the STAT3 to trigger STAT3-mediated EMT processes. This non-canonical pathway is negatively regulated by Abl interactor 1 (ABI1). ABI1 can directly interact with either Fyn or STAT3, although roles of these interactions in regulating the Wnt5a–STAT3 pathway remain unknown. (A color version of this figure is available in the online journal.) Source: Figure modified from Wang et al.29 and Murillo-Garzon and Kypta.30
DVL: Dishevelled; EMT: epithelial–mesenchymal-transition; JNK: JUN-N-terminal kinase; LATS1: larger tumor suppressor 1; LATS2: larger tumor suppressor 2; LGR: leucine-rich repeat-containing G-protein coupled receptor; LRP: lipoprotein receptor related protein; RNF43: ring finger protein 43; ROCK: Rho-associated kinase; sFRP: secreted frizzled related protein; ZNRF3: zinc and ring finger 3.
The canonical Wnt/β-catenin pathway
The Wnt/β-catenin pathway is the most studied Wnt-mediated pathway and has been shown to play critical roles in developmental and oncogenic processes. The hallmark of this pathway is the stabilization and nuclear translocation of β-catenin in the cell (Figure 2(a) and (b)). In the absence of Wnts, β-catenin levels remain low in the cell due to the presence of a ubiquitin-dependent proteasome degradation complex, initially thought to be composed of scaffold proteins Axin and APC with kinases CK1α and GSK3α/β.31 However, a study in 2010 illustrated the involvement of the Hippo signaling pathway components, the Yes-associated protein (YAP) and the transcription co-activator with PDZ-binding motif (TAZ) in stabilizing the destruction complex through binding to Axin.32 Kinase components of the destruction complex phosphorylate conserved N-terminal serine/threonine residues on β-catenin, which leads to recruitment of E3-ubiquitin ligase SCFβ-TRCP for ubiquitination and subsequent proteasome-mediated degradation of β-catenin.33,34
Wnt binding to Fzd-LRP5/6 complex triggers recruitment of the cytoplasmic protein DVL to the intracellular C-terminal domain of Fzd.35 DVL recruits the Axin–GSK3 complex to the membrane by interacting with Axin. After that, the PPPSP motif on the LRP5/6 intracellular tail is phosphorylated by GSK3 and another kinase complex CDK14–Cyclin Y and eventually, the LRP5/6 intracellular tail is further phosphorylated by the membrane-anchored kinase casein kinase 1 γ.36–38 Earlier studies showed that the phosphorylated tail of LRP5/6 has high affinity for Axin, thereby leading to destabilization of the destruction complex and the stabilization of β-catenin.39 More recent studies showed that the phosphorylated tail of LRP5/6 directly blocks GSK3 activity to prevent β-catenin degradation.40 Both of these studies highlight the importance of LRP5/6-mediated β-catenin stabilization but through different mechanisms. More studies need to be conducted to determine if LRP5/6-mediated mechanisms can act simultaneously, or if different cellular states favor one over the other.
Once stabilized and accumulated in the cell, β-catenin is translocated to the nucleus where it serves as a transcriptional co-activator by interacting and modulating the activity of T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) DNA binding proteins.41,42 TCF/LEF proteins are repressors of Wnt responsive genes and their binding to β-catenin allows for transcription of Wnt responsive genes, promoting cell differentiation and proliferation during developmental and oncogenic processes.43,44
Non-canonical Wnt pathways
Non-canonical Wnt pathways or β-catenin independent pathways are those that do not depend on β-catenin/Tcf or β-catenin/Lef binding for modulating downstream signaling. Some non-canonical Wnt pathways elicit transcriptional responses, while others directly regulate cellular actin dynamics and modulate actin-related events such as polarity, migration, and adhesion. Currently, five non-canonical Wnt pathways have been identified: Wnt/PCP pathway, Wnt/Ca2+ pathway, Wnt/ROR pathway, Wnt/YAP–TAZ pathway, and Wnt–FYN–STAT3 pathway (Figure 2(c) and (d)).
Wnt/PCP signaling pathway
Planar cell polarity (PCP) refers to the polarization of epithelial cells along the plane of a tissue, a process highly controlled by Wnt/PCP signaling. PCP formation involves reorganization of actin dynamics as well as transcriptional responses of several downstream genes. During development, Wnt/PCP signaling is maintained by the asymmetric distribution of highly conserved core Wnt/PCP components, which leads to asymmetric actin rearrangements.45–47 In the dorsal sites of cells, Wnt binding to the Fzd receptor recruits DVL to the C-terminal tail of the Fzd receptors, which triggers activation of Rho family GTPases and JUN-N-terminal kinase (JNK).48,49 The downstream output of this pathway is Rho-associated kinase (ROCK) mediated cellular actin remodeling and JNK-mediated transcriptional responses (Figure 2(c)). Negative regulators of Wnt/PCP signaling tetraspanin-like transmembrane scaffold protein Van Gogh (Vangl in mammals) and intracellular scaffold Prickle (Pk) form a complex to antagonize the signal transduction of the Fzd/DVL complex in the proximal sites of the cell.50 Vangl and Pk recruit other proteins to negatively regulate the Wnt/PCP pathway either by mediating DVL ubiquitination and proteasome degradation, or directly altering Rho GTPase-mediated actin cytoskeleton remodeling and JNK-mediated transcriptional responses.51–54 Negative regulatory mechanisms of the Wnt/PCP pathway are still not well understood and require further investigation.
Wnt/Ca2+ pathway
The Wnt/Ca2+pathway (Figure 2(c)) was first noticed with the finding that in some instances Wnt–Fzd binding activated heterotrimeric G proteins and triggered Ca2+ release from the ER through phosphatidylinositol signaling.55 Further work indicated that the binding of Wnt to Fzd receptors recruits DVL to activate heterotrimeric G proteins by GEFs through direct protein–protein interactions. The activated G proteins trigger phosphatidylinositol signaling to release Ca2+ from the ER to the cytosol, which further promotes actin rearrangement in cells, or triggers downstream transcriptional responses.56–58 In addition, the Wnt/Ca2+ was suggested to inhibit the canonical β-catenin signaling, suggesting negative feedback inhibition exerted by activation of non-canonical Wnt pathways over canonical Wnt pathways.59 However, there are few primary studies addressing the function of this pathway and its existence remains controversial, and believed to only play a role under some extreme circumstance.
Wnt/ROR pathway
Receptor tyrosine kinase-like orphan receptors (RORs) are transmembrane receptor tyrosine kinases that contain an extracellular CRD for Wnt recognition and an intracellular tyrosine kinase domain.60 There are two well-known RORs: ROR1 and ROR2. RORs, especially ROR2, can serve as co-receptors for Fzd receptors to trigger non-canonical Wnt signaling.61 Similar to the activation mechanism of canonical Wnt pathway, ROR2 binding to Fzd promotes serine/threonine phosphorylation of the ROR2 intracellular tail for the recruitment of downstream scaffold proteins, thereby initiating signal transduction.61 Meanwhile, ROR itself can also serve as an individual receptor for Wnt molecules to trigger multiple non-canonical signal events, like actin remodeling and JNK-mediated transcriptional responses (Figure 2(c)).62–64
Wnt/Yap–TAZ pathway
Through major Hippo pathway modulators larger tumor suppressor 1 and 2 (LATS1/2) mediated phosphorylation, the YAP–TAZ complex is ubiquitinated and degraded.65 A recent study indicated that the YAP–TAZ can be activated through small GTPase Rho-mediated LATS1/2 inhibition.66 Based on that, a following study showed the novel activation mechanism of the YAP–TAZ pathway through Wnt signaling.67 The Wnt5a/b or Wnt3a binding to the Fzd receptors promotes formation of the Wnt–Fzd–ROR complex, which activates G protein Gα12/13. The activated Gα12/13 turns on Rho’s activity, which inhibits LATS1/2 and stabilizes the YAP–TAZ complex, allowing for activation of downstream transcriptional responses (Figure 2(c)).
Wnt–FYN–STAT3 pathway
The epithelial–mesenchymal-transition (EMT) is a reversible process in which epithelial cells adopt mesenchymal characteristics through alterations in morphology, cellular architecture, adhesion, and migratory capacity.26 During tumor progression, cells undergoing EMT obtain enhanced invasiveness, which allows them to invade surrounding tissue. Through screening multiple cancer cell lines and high-grade tumors, Gujral et al.26 identified overexpression of Wnt5A/B and their receptor Fzd2, and that Wnt5A/B binding to Fzd2 initiates an unrecognized non-canonical Wnt pathway which promotes EMT phenotypes. In this study, Gujral et al. illustrated that Wnt5A/B binding to Fzd2 promotes tyrosine phosphorylation on the C-terminal tail of the Fzd2 receptor, which recruits the Src family kinase FYN through protein–protein interactions. Binding with Fzd2 activates FYN, which then can promote tyrosine phosphorylation of its substrate signal transducer and activator of transcription 3 (STAT3). Through this novel non-canonical Wnt–FYN–STAT3 pathway, STAT3, as a key EMT activator transcriptional factor, is activated to promote the EMT process in several cancer types (Figure 2(c)).26 A recent study identified ABI1, an integral component of WAVE complex, as novel regulator of Wnt–FYN–STAT3 pathway and suppressor of EMT in prostate cancer (PCa) (see also ‘PCa’ section) (Figure 2(c)).68 Further studies are needed to determine whether the ABI1-mediated regulation of Wnt pathway is pertinent to tumors originating from other tissues.
Modulation of Wnt pathways
Due to their important role in development and oncogenic processes, Wnt signaling pathways are tightly regulated. A Wnt ligand has a preference for specific types of Fzd and co-receptors, which provides the first level of regulation based on receptor and ligand availability.19 Direct modulation of Wnt molecules provides further regulation of downstream signal transduction. A typical regulation of Wnt molecules is through a transmembrane protease Tiki-mediated N-terminal cleavage, which oxidizes Wnt molecules and abrogate their ligand activity without inhibiting Wnt secretion.69 Additionally, Wnt could be modified extracellularly by the secreted Wnt deacylase Notum, which removes the palmitoleic acid on Wnt molecules. This leads to oxidized Wnt oligomer formation and removal of its ligand activity.70 Furthermore, Wnt molecules interact with other secreted proteins that contain CRD like Wnt inhibitory factor and secreted Frizzled related proteins (sFRPs), which prevent them from binding to Fzd receptors (Figure 2(a)).71–73
Another important signal modulating mechanism in Wnt signaling is endocytosis. For downstream signal transduction, studies have shown that the signalosome complex of ligand, receptors, and downstream signal adaptors need to be internalized into the cell.74–76 During endocytosis, elements of the β-catenin destruction complex, like Axin and GSK3, are sequestered to prolong the half-life of many GSK3 substrates like β-catenin.19,77 For the Wnt/PCP pathway or other non-canonical pathways, the mechanism of how endocytosis promotes signal transduction in cells is currently unclear, but clathrin-mediated endocytosis of Wnt-receptor complex is suggested to be important for Wnt/PCP signaling transduction.74 Several negative-feedback mechanisms exist to terminate or dampen Wnt signaling at the receptor level, which also involves endocytosis. A famous Wnt antagonist, Dickkopf-1 (Dkk1), is upregulated through the β-catenin pathway, and Dkk1 forms a ternary complex with its transmembrane receptor Kremen and LRP5/6.78 This ternary complex is rapidly endocytosed to stop Wnt signaling cascades (Figure 2(a)).79 Negative-feedback mechanisms have also been reported as β-catenin signaling induces upregulation of transmembrane E3-ubiquitin-ligase ring finger protein 43 (RNF43) and zinc and ring finger 3 (ZNRF3).80,81 RNF43/ZNRF3 promote ubiquitination of Fzd receptors at the plasma membrane, leading to Fzd receptor endocytosis followed by proteasome degradation (Figure 2(a) and (b)).
Recently, an important positive-feedback mechanism has been revealed to promote cellular responses under low ligand availability. R-spondins are secreted proteins that were first identified as Wnt agonist for Wnt/β-catenin pathways.82 R-spondins do not possess intrinsic signaling activity, but they form a ternary complex with RNF43/ZNRF43 and membrane receptor protein leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5).83 This ternary complex is endocytosed rapidly to decrease RNF43/ZNRF3 levels in the plasma membrane, thereby preserving Fzd receptors to amplify both Wnt/β-catenin and Wnt/PCP pathway activity (Figure 2(b)). Interestingly, LGR5, a well-established stem cell marker, is also upregulated by the β-catenin pathway (Figure 2(b)).84,85 This positive-feedback mechanism has been shown to amplify Wnt signaling in early embryonic development, stem cell renewal, and tissue homeostasis. This mechanism has been exploited by cancer cells and is the basis for some Wnt-targeting drugs that will be discussed later in this review.
Wnt signaling pathways in development and tissue homeostasis
After fertilization, the fertilized egg undergoes several rounds of cleavage. On day 3.5 a blastocyst is formed, which contains an inner cell mass (ICM) surrounded by a trophoblast epithelium (trophectoderm). During implantation, the trophoblast attaches and invades through the maternal uterine epithelium and further differentiates into nutrient providing tissues like the placenta. Meanwhile, the embryonic stem cells (ESCs) in the ICM rapidly proliferate to form the epiblast from where the entire embryo will originate. Then, through the process of gastrulation the blastocyst further differentiates into the three-layers of the embryo (endoderm, mesoderm, and ectoderm). Through these processes, the embryo forms major body axis, the primitive streak and somites and stem cells differentiate into tissues and organs. The canonical Wnt/β-catenin and non-canonical Wnt/PCP pathways are major regulators of these developmental processes, which will be reviewed in detailed below.43,86
ESCs’ pluripotency
ESCs are pluripotent and have the potential to further differentiate into all adult cell types; the pluripotency preservation process in proliferating ESCs is called self-renewal which is lost during gastrulation. Mechanisms for ESCs’ self-renewal and differentiation are important for tissue/organ formation in early embryonic development. Maintenance of pluripotency requires the functional interplay between Wnt/β-catenin pathway activation, leukemia inhibitor factor (LIF) signaling pathway activation, and MAPK pathway inhibition.43 The Wnt/β-catenin pathway was initially found to not be required for ESCs’ self-renewal as β-catenin−/− mouse ESCs (mESCs) can fully differentiate into cell types from all three germ layers when injected into a mouse blastocyst.87 However, as LIF signaling is required for the maintenance of pluripotency of mESCs in vitro, amplifying Wnt/β-catenin signaling by using GSK inhibitors or recombinant Wnt3a can bypass this requirement.88 Once injected into a permissive site of a host, normal mESCs form teratoma tumors containing a variety of cell types derived from all three germ layers.89 However, genetically engineered mESCs with homozygous deletion of β-catenin destruction complex scaffold APC fail to form teratomas or form tumors deficient in neuroectoderm differentiation.89,90 Results from these studies suggest that upregulation of Wnt/β-catenin pathway directs the mESCs to the self-renewal track by inhibiting their differentiation.
The primary dorsoventral and anteroposterior axis formation
In vertebrates, the Wnt gene is first shown to regulate primary body axis formation in Xenopus laevis embryos.91 Before gastrulation, the primary body axis or dorsoventral axis coincides with the formation of early embryonic signaling center known as Spemann organizer in amphibians, the shield in zebrafish, and the node in mice.92 During gastrulation, the single-layered blastula is reorganized into a multilayer structure known as the gastrula. In triploblastic organisms, the well-known three germ layer of the gastrula (ectoderm, mesoderm, and endoderm) will further differentiate into specific tissues and organs. The dorsoventral and anteroposterior body axis determines the spatial distribution of tissues and organs.
Dorsoventral body axis formation
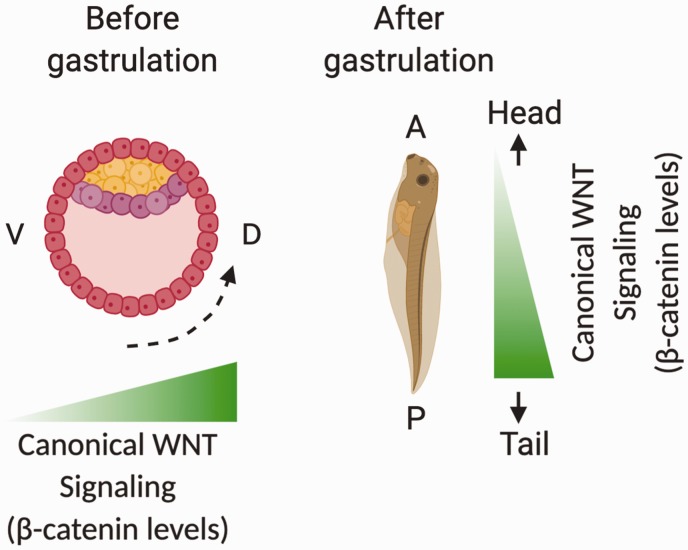
During fertilization, entry of the sperm triggers microtubule-dependent rearrangement of the cortical plasma from the vegetal pole to the future dorsal side in the egg, a process known as cortical rotation.92 An important outcome of cortical rotation is the accumulation of the β-catenin in dorsal nuclei of early blastulae, which can be detected as early as in the 2–4 cell stage.93 The nuclear β-catenin accumulation activates the transcription of TCF genes, many of which are specific for dorsoventral body axis formation.94 Mutations that reduce β-catenin dorsal accumulation cause defects in zebrafish embryo dorsal development.95 Cortical rotation also leads to the dorsal accumulation of downstream Wnt/β-catenin components to stabilize β-catenin for constitutive activation of the β-catenin pathway at the dorsal site96 (Figure 3).
Figure 3.
Roles of Wnt/β-catenin pathways in early X. laevis embryo body axis formation. The post-fertilization cortical rotation (black dotted arrow) leads to early accumulation of maternal β-catenin in the dorsal equatorial region, which activates canonical Wnt pathway genes to generate the Spemann organizer. At the same time, several Wnt antagonists are upregulated at the dorsal site and translocated to the anterior site to inhibit anterior activation of canonical Wnt pathway for anterior site development. During gastrulation, the zygotic activation of the canonical Wnt8 molecule leads to asymmetric β-catenin accumulation, specifically in the ventral and posterior sites of the embryo. The ventral and posterior activation of the canonical Wnt pathway upregulates genes important for ventroposterior mesodermal fate determination and subsequent tail formation. (A color version of this figure is available in the online journal.) Source: Figure modified from McMahon and Moon91 and Hikasa and Sokol.92
Following initiation of β-catenin pathway activation at the early stage of body axis development, maternal β-catenin target genes activate secondary developmental pathways that crosstalk with the canonical Wnt pathway to regulate embryonic development.97,98 The activation of the bone morphogenetic protein signaling pathway triggers zygotic Wnt8 signaling at the ventral and posterior side to initiate ventral/posterior development in the blastula.97 Activation of the canonical Wnt pathway upregulates their antagonists at the dorsal and anterior sides of the embryo, inhibiting their activity.92
Anteroposterior body axis formation
The anterior inhibition of the canonical pathway triggers anteroposterior body axis formation, which is marked by the initiation of head structure development.43 Another maternal β-catenin targets the FGF signaling pathway, which is suggested to be important for dorsal and posterior development.99 The FGF signaling pathway triggers Wnt8 expression at the dorsal and posterior site. The zygotic Wnt8, together with other secreted Wnt molecules, preferably triggers the canonical pathway at the dorsal and posterior sites, which initiates tail development at the posterior end.100
Interestingly, Wnt/β-catenin pathway shows variable activation patterns at different developmental stages of both dorsoventral and anteroposterior axis formation, suggesting a complicated regulatory mechanism of the canonical pathway during early embryonic development. As described, in early body axis formation, Wnt/β-catenin pathway is regulated through secreted antagonists, and signaling integration with other developmental pathways.72,92,101 An important role of β-catenin is to modulate histone methylation through recruitment of arginine methyltransferase to the promoter of target genes, an important epigenetic change involved in regulation of gene transcription at various developmental stages.92,102
Primitive streak formation, somitogenesis, and left–right determination
Early mice embryonic developmental processes are tightly regulated by the Wnt/β-catenin pathway.86 During early embryologic development, fertilized mice oocytes undergo complicated division and differentiation processes before and after gastrulation for primary body axis formation. At the same time, embryos develop the primitive streak at the blastula stage to establish bilateral symmetry. After that, somitogenesis and left–right determination processes further establish the embryos’ spatial morphology for tissue and organ development.
Primitive streak formation
In the early blastocyst developmental stage, an ICM region is formed surrounded by a layer of trophoblast epithelium. The ICM rapidly proliferates to form the epiblast, from where the entire embryo originates. The Wnt/β-catenin signaling pathway triggers the proximal-posterior epiblast axis formation during early body axis development.103 The proximal-posterior epiblast axis is the place where the primitive streak forms and along this axis, the expression of Wnt molecules extends distally in the epiblast, marking the formation of the primitive streak.104
Formation of the primitive streak involves rearrangement of the epiblast polarity. During early body axis formation, the initiation of the Wnt/β-catenin pathway upregulates several Wnt molecules, which trigger the non-canonical PCP pathway to regulate epiblast polarity by modulating actin-dynamics and JNK-mediated transcriptional responses.19 Deletion of negative PCP pathway regulator Prickle (Pk) (mice homolog mpk1) leads to early embryonic lethality with failure of primitive streak formation due to abnormal epiblast polarity formation.105
Somitogenesis
During gastrulation, the Wnt3a secretion along the primitive streak creates a gradient for its target Brachyury (T), a transcription factor. In response to T-mediated gene transcription, epiblast cells enter through the primitive streak to give rise to the mesoderm and definitive endoderm.106 After gastrulation, the primitive streak continues to produce mesoderm that further differentiates into the paraxial presomitic mesoderm (PSM) and lateral plate mesoderm for somitogenesis. In mammals, somites eventually develop into the trunk of the body. In mice embryos, the uneven Wnt3a secretion forms a Wnt3a gradient along the primitive streak, which activates the canonical Wnt pathway in the PSM to form a somite every 2 h.107 In this process, the activated Wnt3a pathway leads to expression of Notch pathway ligand protein Delta-like1 (Dll1) to trigger Notch pathway in the PSM for somite formation.108–110 Gradual reduction of Wnt activity in the anterior PSM arrests Notch activity, which sets boundaries for the somite and activates genes for somite determination (Figure 4).111
Figure 4.
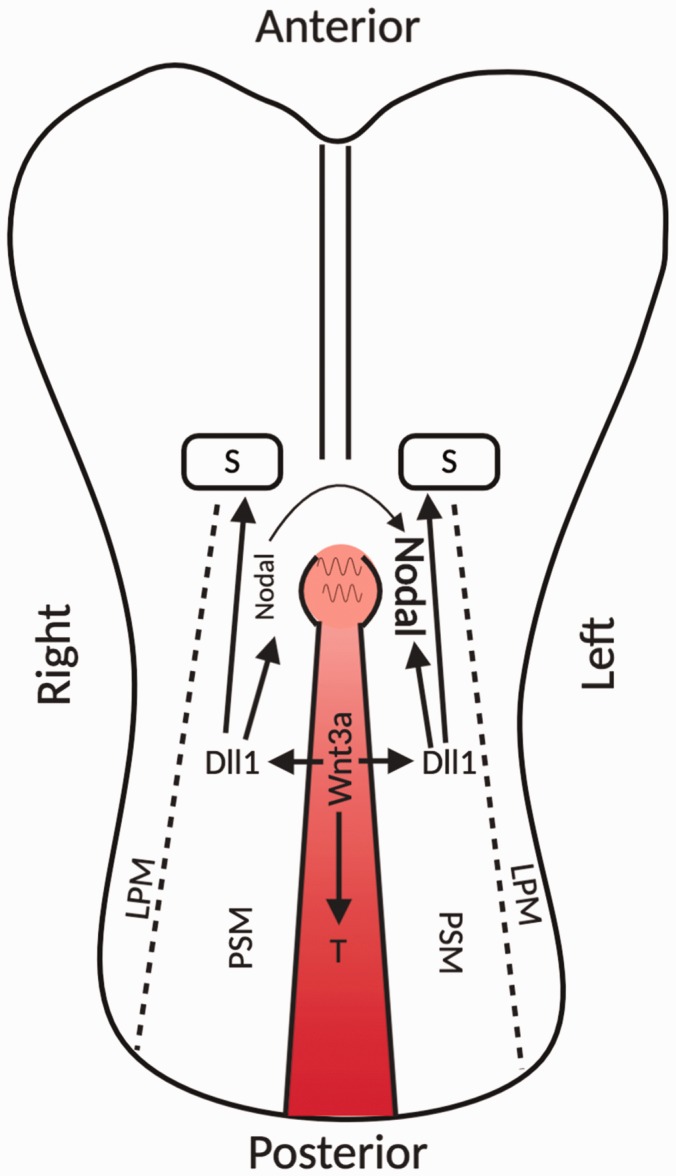
Roles of Wnt/β-catenin pathways in mice embryo somitogenesis and left–right axis determination. The expression of canonical Wnt3a molecules along the primitive streak upregulates the transcription factor T which sustains mesoderm production. In addition, the canonical Wnt3a pathway initiates the expression of the Notch pathway ligand Dll1 to regulate somitogenesis in the adjacent PSM. The expression of Dll1 in the PSM also mediates Nodal expression at the periphery of the node by activating the Notch signaling pathway. The leftward flow generated by rotating nodal cilia leads to left side Nodal accumulation to eventually enhance Nodal signaling on the left side for left–right body axis determination. (A color version of this figure is available in the online journal.) Source: Figure modified from Barker et al.85 and Wang et al.86
LPM: lateral plate mesoderm; PSM: paraxial presomitic mesoderm.
Left–right determination
After gastrulation, a transient midline structure, called ‘node,’ is formed at the rostral end of the primitive streak.112 On the ventral surface of nodal cells, there is a ciliated pit, where the rotational movements of the node cilia generate a leftward fluid flow that eventually leads the left–right axis determination in the embryo. The node cilia is initially positioned centrally and gradually posteriorly tilted, which decides its specific rotation pattern.113 The posteriorly tilted pattern of the node cilia is inhibited in mice embryos with defective Wnt/PCP signaling.114 This illustrates that Wnt/PCP pathway is involved in left–right axis determination by regulating posterior tilt of the node cilia. After that, the canonical Wnt3a pathway finalizes the left–right body axis. Once the position of nodal cilia is determined, the tilted cilia-mediated leftward flow enhances Nodal signaling on the left side. Nodal is a product of previously described Wnt3a–Notch cascade and it activates Smad2/3-mediated transcriptional responses to promote development.115 Nodal is initially symmetrically expressed along both the left and right periphery of the node. The final asymmetrical activation of Nodal signaling pathway breaks the left–right symmetry to finalize the left–right body axis (Figure 4).116
Tissue development and homeostasis
During gastrulation, three germ layers are formed. This process requires inhibition of Wnt/β-catenin pathway to allow ESCs’ differentiation. During specific tissue/organ developmental processes, Wnt/PCP pathway promotes the original germ layer cells to align themselves with certain polarity to ensure proper cell organization for tissue morphogenesis. After development, specific stem cells mediate tissue/organ turn over processes routinely, to maintain their homeostasis. Thus, the canonical and non-canonical Wnt pathways work together to regulate the development and homeostasis of tissues and organs.
Tissue development
In early embryologic development, the asymmetric activation of Wnt/β-catenin pathways along the body axis leads to organ/tissue initiation at the β-catenin pathway inactive sites, where ESCs differentiate. After that, the PCP pathways are important to regulate tissue morphological development. Mice with dysfunctional Wnt/PCP inhibitor Vangl show randomized orientation of the stereocilia, which are the mechanosensing organelles of the inner ear hair cells that regulate hearing and balance.117 Moreover, disruption of PCP signaling in Xenopus, zebrafish, and mice embryos manifests abnormalities in the shapes of many organs and tissues like the cochlea tube, stomach, and intestines.118,119 Xenopus and mice embryos with PCP mutations also show defects in neural tube closure, which is essential for central nervous system formation.120–122 Similar neural tube closure defects are also observed in patients carrying Vangl1/2 mutations.123,124 Some patients with congenital skeletal disorders have been shown to carry Wnt5a or ROR2 mutations, supporting the important role of Wnt5a-mediated non-canonical Wnt pathways in normal limb development.125–127
Tissue homeostasis
Aside from development, the balance between proliferation and differentiation of tissue-specific stem cells maintains adult tissue homeostasis in the body, which, as described, is tightly regulated by the Wnt/β-catenin pathway. For instance, adult intestinal epithelia turn over fully every 4–5 days, which requires rapid intestinal stem cell proliferation and differentiation. Genetic disruption of the Wnt/β-catenin pathway abolishes intestinal epithelium proliferation and turnover, which disrupts intestinal homeostasis.128–130 Similar regulatory mechanisms are found in bone where genetical modulation of Wnt/β-catenin pathway co-receptor LRP5/6 leads to abnormal mesenchymal stem cell differentiation. This process breaks the balance between osteoblast and osteoclast and leads to tremendous decreases or increases in bone mass density.131 In hair follicle development, autocrine β-catenin pathway is required for hair follicle stem cell cycling for hair follicle renewal.132 All these examples illustrate the important role of the Wnt/β-catenin pathway in maintaining adult tissue homeostasis.
Wnt signaling pathways in cancer
During oncogenesis, benign cells undergo de-differentiation processes and obtain enhanced proliferation and anti-apoptotic abilities allowing them to sustain uncontrolled growth. The identification of mice proto-oncogene Int1, now known as Wnt1 gene, suggested that the Wnt signaling pathway may be a key regulator of carcinogenic processes.5 As discussed before, activation of Wnt pathways promotes proliferation, survival, migration, polarity, and specification of cell fate, all of which are important for tumor initiation, progression, and metastasis. Genetic studies and pathological analysis of patients show that Wnt pathways are frequently dysregulated in different cancers, which makes Wnt signaling pathways a potential therapeutic target for cancer therapies. Our current understanding of the role of Wnt pathways in oncogenesis will be reviewed in detail below, along with current anti-Wnt pathway treatments undergoing clinical trials for cancer treatment.
Role of the canonical Wnt/β-catenin signaling pathway in cancer
Wnt/β-catenin pathway promotes cell proliferation by upregulating the transcription of many oncogenes. Meanwhile, activation of canonical Wnt signaling initiates a β-catenin-independent Wnt–STOP mechanism (Figure 2(b)). Wnt binding to Fzd inhibits GSK3β-mediated poly-phosphorylation and poly-ubiquitination of its target proteins which comprise 20% of the proteome.77,133 These target proteins include prominent oncogenes, like c-Myc.134 Mutations in this pathway are found in a variety of different cancers.44 Here, we will review the Wnt/β-catenin pathway in hormone independent gastrointestinal cancers, hormone dependent prostate cancer, and leukemia to illustrate how these pathways have been shown to be dysregulated in various types of cancers.
Gastrointestinal cancers
Abnormal β-catenin stabilization and accumulation by mutations drive gastrointestinal cancer development. Loss of function mutations on APC is commonly found in patients with colorectal carcinoma (CRC) (80% of studied cases).135 APC loss of function mutations found in CRC lead to constant upregulation of Wnt/β-catenin pathway. This carcinogenic process can be model in APC CRISPR knockout human intestinal organoids.136 A study using reversible APC shRNA knock down in mice of CRC models indicates that APC restoration reverses the adenoma phenotypes.137 APC mutations upregulate Wnt–STOP signaling which promotes microtubule dynamics, causing abnormal mitotic processes and triggering chromosomal instability, leading to CRC development.138 Furthermore, upregulation of Fzd receptors is often observed in CRC, pancreatic ductal adenocarcinoma (PDAC), and cholangiocarcinoma, with enhanced R-spondin/Lgr5/RNF43 modulating activity.139 R-spondins’ overexpression or RNF43 loss of function mutations reduce RNF43 activities to preserve Fzd receptors. Thus, tumor cells with dysfunctional RNF43 rely heavily on Wnt secretion to sustain the β-catenin signaling, rendering them highly susceptible to Wnt secretion targeted therapy.
Prostate Cancer (PCa)
In its initial hormone driven state, PCa is highly reliant on androgen receptor (AR) signaling for survival and growth. Unlike in CRC, APC mutations are rarely found in PCa.140 It seems like β-catenin is not a major driver for PCa since in normal mice prostate tissue, upregulation of β-catenin pathway only results in high-grade prostate intraepithelial neoplasia, a non-invasive benign tumor. A secondary event, like Pten loss or upregulated AR signaling, is required for tumorigenesis.141,142 In these mice, overexpression of AR accelerates tumor development and invasion with reduced survival, while castration shrinks tumors.142 In untreated patient tumors, β-catenin is shown to work with AR through direct protein–protein binding to promote oncogenic transcriptional responses.143 AR and β-catenin are enriched in early-onset (diagnosed at ≤ 50 years old) high-grade tumors with enhanced progression potential.144 In fact, AR has been shown to compete with transcriptional regulator Tcf/Lef for β-catenin binding.145 In the presence of Wnt, AR overexpression inhibits β-catenin targeted canonical Wnt pathway antagonists expression, while the Wnt–STOP is still activated to preserve several oncogenic proteins in the cell, thereby promoting oncogene tumor progression.142 In castration-resistant prostate cancer (CRPC) patient samples, β-catenin signaling upregulation is frequently found.146 Also, in human PCa cell line LNCaP, AR antagonist enzalutamide redirects β-catenin from AR to Tcf/Lef, thereby activating Wnt/β-catenin signaling.147 These studies suggest that without AR, the β-catenin signaling pathway is switched on to promote tumor progression in CRPC patients.
Leukemia
Wnt/β-catenin signaling is important for proliferation and self-renewal of stem cells, including hematopoietic stem cells. Wnt activity is increased in most leukemias, which maintains cancer cells in an undifferentiated state and promotes their growth.148 In acute myelogenous leukemia (AML), frequent chromosomal translocations upregulate Wnt/β-catenin signaling by enhancing key components of transcription.149 In AML, leukemia initiating cells (LICs) arise from a pre-LIC stage, which is promoted by Wnt/β-catenin signaling through transcriptional modifications.149,150 LICs for T-cell acute lymphoblastic leukemia (T-cell ALL) usually harbor active Notch signaling mutations.151 However, β-catenin pathway activation in T-cell ALL mouse models inhibits Notch signaling in T-cells.152 Those T-cells still exhibit enhanced proliferation and resistance to apoptosis, suggesting a novel Notch independent T-cell ALL subtype. A subset of chronic lymphocytic leukemia (CLL) has been shown to be highly reliant on canonical Wnt signaling for survival.153 In CLL cases, epigenetic silencing of the Wnt/β-catenin pathway negative regulators is frequently found.154 Meanwhile, 14% of CLL studied cases carry somatic gain-of-function mutations on Wnt/β-catenin pathway components, while knockdown or mutated Wnt/β-catenin pathway components decrease the viability of CLL cells.29
Roles of non-canonical Wnt pathways in cancer
Robust evidence suggests that non-canonical pathways are important for tumor cell growth, survival, invasion, and angiogenesis.30 The non-canonical Wnt5a is highly expressed in 30% of 237 high-grade gastric cancer cases, promoting its invasiveness and migration.49 Similar regulations are also observed in other types of cancer.155 However, Wnt5a has also been reported to sometimes serve as a tumor suppressor in low-grade tumors.156 Here, we will review the role of non-canonical Wnt pathways in hormone independent gastrointestinal cancers, prostate cancer, and leukemia to describe how non-canonical Wnt pathways are dysregulated in a variety of different malignancies.
Gastrointestinal cancers
Interestingly, studies have shown that in non-metastatic HCT 116 cells derived mice CRC xenografts, Wnt5a overexpression impairs tumor growth.157 This suggests Wnt5a is a potential tumor suppressor for CRC initiation, potentially through suppression of the canonical Wnt/β-catenin pathway. However, paradoxical results from another study suggest that Wnt5a promotes high-grade metastatic tumor invasiveness and metastasis through accelerating focal adhesion assembly.158 Also, receptors and co-receptors of Wnt5a pathways have been correlated with a poor prognosis in patients with CRC as they promote tumor metastasis.155,159,160 Taken together, the role of non-canonical Wnt5a pathway might differ depending on the disease stage in CRC. Similar regulations have also been illustrated in other types of gastrointestinal cancers. As described, Wnt5a has been shown to also enhance tumor aggressiveness by upregulating focal adhesion kinase and Rac activity in gastric cancer.49 Also, a recent study illustrates that targeting Wnt5a receptor Fzd5 with anti-Fzd5 antibodies decreases proliferation of PDAC cells and reduces tumor burden in PDAC derived mice xenografts.161
Prostate Cancer (PCa)
A study showed Wnt5a expression is upregulated in 27 out of 98 cases (28%) of PCa and in those cases, Wnt5a overexpression is more frequently found in high-grade invasive tumors with Gleason score 8.162 Wnt5a initiates JNK-mediated transcriptional response for MMP-1 to enhance tumor invasiveness. After androgen deprivation therapy, non-canonical Wnt genes and their receptors are expressed to promote CRPC growth and/or neuroendocrine differentiation.30 Enhanced Wnt5a is associated with AR-dependent tumor growth in mice xenografts studies.163 Another potential role of Wnt5a is promoting EMT through activation of Fzd2-mediated FYN–STAT3 pathway in high grade PCa.68,164 Activation of FYN–STAT3 is inhibited by ABI1, which sequesters FYN and thus controls STAT3 activation and its nuclear localization; as a result EMT is suppressed. Although these studies have shown that ABI1 interacts with key members of this pathway, FYN and STAT3, the mechanism of how ABI1 is able to regulate this pathway is still not completely understood.68
Wnt5a can also serve as tumor suppressor by inhibiting the canonical Wnt pathway in localized low-grade PCa tumors, as 503 patients with localized low-grade tumors show Wnt5a overexpression with higher biochemical recurrence free survival rates and lower bone metastasis.165–167 As in CRC, the role of Wnt5a signaling in PCa also appears to be dependent on disease stage.
Leukemia
In leukemia, non-canonical Wnt pathway components are regularly found to be upregulated, promoting cell migration, invasion, survival, and resistance to chemotherapy.155 In AML, Wnt5a co-receptor PTK7 is often found to be upregulated. PTK7 is an orphan tyrosine kinase and in AML cells, PTK7 promotes cell migration by promoting focal adhesion assembly and vascular endothelial growth factor signaling via phosphorylation.168 PTK7 overexpression reduces chemotherapy-induced apoptosis in AML patients. Furthermore, patients with PTK7 overexpression show a poor disease-free survival rate, suggesting that PTK7 can be used as a biomarker to predict poor clinical outcomes and could also be a potential therapeutic target. In addition, PTK7 is abnormally upregulated in the bone marrow of T-ALL patients with unknown function in promoting T-ALL cell growth.169 For CLL, multiple PCP pathway components are upregulated with some of them accumulating as the disease progresses, enhancing cell migration in response to Wnt5a through activation of Rho family small GTPases.170 It has been shown that targeting PCP component Ror1 in CLL decreases organ localization in immunodeficient mice models.
Targeting Wnt pathways for cancer therapies
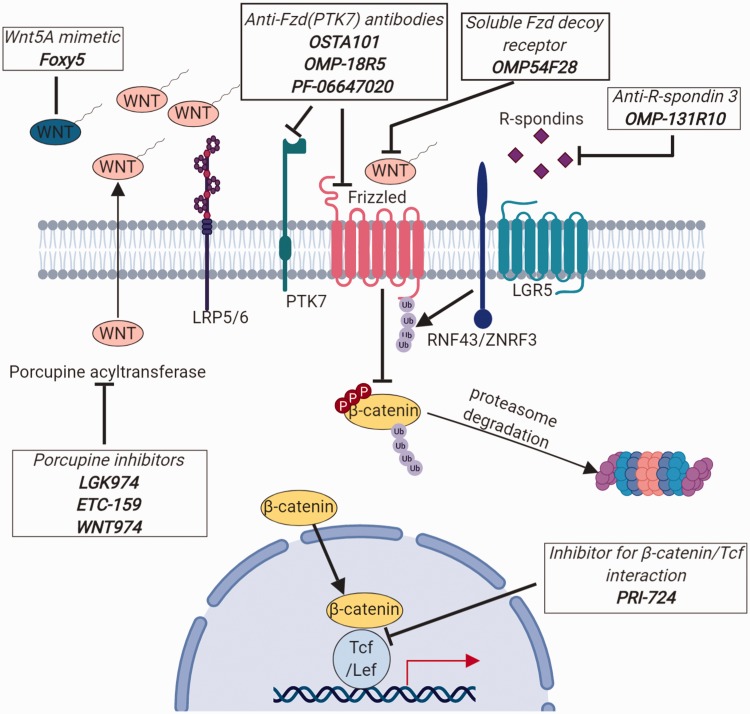
Because of their prominent role in promoting oncogenesis and tumor progression, Wnt pathways have been suggested to be promising therapeutic targets for cancer therapy. In the past decades, many compounds have been used to target Wnt pathways both in vitro and in vivo, some of which are currently undergoing clinical trials44,155,156 (Figure 5 and Table 1). As mentioned, Wnt molecules require Porcupine-mediated palmitoylation for secretion and ligand recognition by its cognate receptors. Small molecules LGK974 and ETC-159 have been shown to significantly inhibit Porcupine’s activity, which impairs tumor growth in variable cancers (Table 1).171,172 Currently, they have been used in clinical trials against metastatic cancers that highly rely on Wnt secretion.173,174 Wnt ligands and their (co)receptors are also a potential target for cancer treatment. Wnt5a mimetic Foxy5 is undergoing clinical trials as it can activate non-canonical Wnt5a pathways to either inhibit tumors that highly rely on canonical Wnt/β-catenin signaling for their growth or to reduce cell motility to inhibit metastasis.175–179 The small molecule OMP54F28 is a Fzd8 containing fusion protein that competes with native membrane Fzd8 receptor for Wnt binding and dampens signal transduction. It decreases tumor size and the number of tumor initiating cells in mice xenografts of multiple cancer types and is currently under clinical trials.180–184 OMP-131R10, a monoclonal antibody, specifically binds to R-spondin3 to release RNF43 allowing it to mediate Fzd ubiquitination and degradation, thereby preventing Wnt pathway activation.44,185 Other compounds like OTSA101, OMP-18R5, and PF-06647020 are monoclonal antibodies that target specific membrane Fzd receptors or co-receptor PTK7 to block signal initiation of the canonical Wnt signaling or non-canonical signaling, respectively.186–188 The small molecule PRI-724 was discovered to inhibit self-renewal transcriptome by blocking the interaction between β-catenin and Tcf, and it was shown to decrease tumor burden in ALL and metastatic CRC.189,190
Figure 5.
Drugs targeting Wnt pathways in cancer treatments. All depicted drugs are currently undergoing phase1/2 clinical trials against various types of cancers. (A color version of this figure is available in the online journal.)
LRP 5/6: lipoprotein receptor related protein 5/6.
Table 1.
Drugs targeting Wnt pathways. All depicted drugs are currently undergoing phase1/2 clinical trials against various types of cancers.
| Target gene | Compound | Cancer |
|---|---|---|
| Wnt-5a | Foxy5 | BCa, CRC, PCa |
| Porcupine | LGK974, ETC-159 | Metastatic CRC, HNSCC, other solid tumors |
| PTK7 | PF-06647020 | Advanced solid tumors |
| R-Spondin 3 | OMP131R10 | Metastatic CRC |
| FRIZZLED-10 | SYNFRIZZ, OTSA101 | Advanced synovial sarcoma |
| FRIZZLED-7 | OMPI8R5 (vantictumab) | NSCLC, metastatic BCa, PDAC |
| FIRZZLED-8 | OMP54F28 | Ovarian cancer, PDAC, HCC |
| TCF/β-catenin interaction | PRI-724 | AML, CML, CRC, PDAC |
AML: acute myeloid leukemia; BCa: breast cancer; CML: chronic myelogenous leukemia; CRC: colorectal cancer; HCC: hepatocellular carcinoma; HNSCC: head and neck squamous cell carcinoma; NSCLC: non-small-cell lung carcinoma; PCa: prostate cancer; PDAC: pancreatic ductal adenocarcinoma.
Conclusions
Decades ago, the mammalian Wnt gene and its Drosophila homolog Wingless gene were found to promote oncogenesis and embryogenic development, respectively.19 Since then, studies of Wnt signaling have elucidated their complex roles in different developmental stages and their intricate role in promoting or inhibiting tumor growth in different malignancies. However, a lot still needs to be determined in order to allow us to continue to generate targeted therapies with high efficacy and low off-target effects. Further studies in this area will strengthen our understanding of both developmental and cancer biology. Also, research in Wnt pathways in the context of cancer helps us understand how tumor cells hijack developmental pathways to sustain their growth when faced with severe survival stresses.
ACKNOWLEDGMENTS
Authors thank Dr Gustavo Miranda Carboni for helpful discussions on Wnt pathway regulation.
Authors’ contributions
LK designed the scope and focus of the review. XL and MAO designed the figures. XL, MAO, and LK wrote the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the grant from the National Cancer Institute (R01CA161018) and Joyce Curry Pancreatic Research Fund to LK.
ORCID iDs
Leszek Kotula https://orcid.org/0000-0002-7977-7124
References
- 1.Sharma RP, Chopra VL. Effect of the wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol 1976; 48:461–5 [DOI] [PubMed] [Google Scholar]
- 2.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287:795–801 [DOI] [PubMed] [Google Scholar]
- 3.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982; 31:99–109 [DOI] [PubMed] [Google Scholar]
- 4.Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 1987; 50:649–57 [DOI] [PubMed] [Google Scholar]
- 5.Papkoff J, Brown AM, Varmus HE. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol 1987; 7:3978–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol 2012; 4:a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 2006; 11:791–801 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Awada C, Hanaki H, Sakane H, Tsujimoto I, Takahashi Y, Takao T, Kikuchi A. The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J Cell Sci 2013; 126:2931–43 [DOI] [PubMed] [Google Scholar]
- 9.MacDonald BT, Hien A, Zhang X, Iranloye O, Virshup DM, Waterman ML, He X. Disulfide bond requirements for active Wnt ligands. J Biol Chem 2014; 289:18122–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in wingless processing. Genes Dev 1996; 10:3116–28 [DOI] [PubMed] [Google Scholar]
- 11.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci Usa 2011; 108:12752–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Chia J, Canning CA, Jones CM, Bard FA, Virshup DM. WLS retrograde transport to the endoplasmic reticulum during Wnt secretion. Dev Cell 2014; 29:277–91 [DOI] [PubMed] [Google Scholar]
- 13.Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol 2012; 361:392–402 [DOI] [PubMed] [Google Scholar]
- 14.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006; 125:509–22 [DOI] [PubMed] [Google Scholar]
- 15.Buechling T, Chaudhary V, Spirohn K, Weiss M, Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO Rep 2011; 12:1265–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, Concepcion GP, Bugni TS, Harper MK, Mihalek I, Jones CM, Ireland CM, Virshup DM. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci 2010; 123:3357–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem 2002; 277:41762–9 [DOI] [PubMed] [Google Scholar]
- 18.Schulte G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev 2010; 62:632–67 [DOI] [PubMed] [Google Scholar]
- 19.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012; 13:767–79 [DOI] [PubMed] [Google Scholar]
- 20.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science 2012; 337:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nile AH, Mukund S, Stanger K, Wang W, Hannoush RN. Unsaturated fatty acyl recognition by frizzled receptors mediates dimerization upon Wnt ligand binding. Proc Natl Acad Sci USA 2017; 114:4147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. Arrow encodes an LDL-receptor-related protein essential for wingless signalling. Nature 2000; 407:527–30 [DOI] [PubMed] [Google Scholar]
- 23.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000; 407:530–5 [DOI] [PubMed] [Google Scholar]
- 24.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 2000; 407:535–8 [DOI] [PubMed] [Google Scholar]
- 25.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a wingless receptor. Nature 1996; 382:225–30 [DOI] [PubMed] [Google Scholar]
- 26.Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 2014; 159:844–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koval A, Purvanov V, Egger-Adam D, Katanaev VL. Yellow submarine of the Wnt/Frizzled signaling: submerging from the G protein harbor to the targets. Biochem Pharmacol 2011; 82:1311–9 [DOI] [PubMed] [Google Scholar]
- 28.Jernigan KK, Cselenyi CS, Thorne CA, Hanson AJ, Tahinci E, Hajicek N, Oldham WM, Lee LA, Hamm HE, Hepler JR, Kozasa T, Linder ME, Lee E. Gbetagamma activates GSK3 to promote LRP6-mediated beta-catenin transcriptional activity. Sci Signal 2010; 3:ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Shalek AK, Lawrence M, Ding R, Gaublomme JT, Pochet N, Stojanov P, Sougnez C, Shukla SA, Stevenson KE, Zhang W, Wong J, Sievers QL, MacDonald BT, Vartanov AR, Goldstein NR, Neuberg D, He X, Lander E, Hacohen N, Regev A, Getz G, Brown JR, Park H, Wu CJ. Somatic mutation as a mechanism of Wnt/beta-catenin pathway activation in CLL. Blood 2014; 124:1089–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murillo-Garzon V, Kypta R. WNT signalling in prostate cancer. Nat Rev Urol 2017; 14:683–96 [DOI] [PubMed] [Google Scholar]
- 31.Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol 2013; 5:a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. Yap/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 2014; 158:157–70 [DOI] [PubMed] [Google Scholar]
- 33.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev 1999; 13:270–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol 1999; 9:207–10 [DOI] [PubMed] [Google Scholar]
- 35.Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA, Noutsou M, Rudiger SG, Schwamborn K, Schambony A, Maurice MM. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci USA 2012; 109:E812–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 2005; 438:867–72 [DOI] [PubMed] [Google Scholar]
- 37.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 2005; 438:873–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of Wnt receptor activation. Dev Cell 2009; 17:788–99 [DOI] [PubMed] [Google Scholar]
- 39.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical Wnt signaling pathway. Mol Cell 2001; 7:801–9 [DOI] [PubMed] [Google Scholar]
- 40.Stamos JL, Chu ML, Enos MD, Shah N, Weis WI. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. eLife 2014; 3:e01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382:638–42 [DOI] [PubMed] [Google Scholar]
- 42.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The intestinal Wnt/TCF signature. Gastroenterology 2007; 132:628–32 [DOI] [PubMed] [Google Scholar]
- 43.Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development 2018; 145:dev146589 [DOI] [PubMed] [Google Scholar]
- 44.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017; 36:1461–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warrington SJ, Strutt H, Fisher KH, Strutt D. A dual function for prickle in regulating frizzled stability during feedback-dependent amplification of planar polarity. Curr Biol 2017; 27:2784–97 e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asad M, Wong MK, Tan TZ, Choolani M, Low J, Mori S, Virshup D, Thiery JP, Huang RY. FZD7 drives in vitro aggressiveness in Stem – a subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis 2014; 5:e1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002; 1:279–88 [DOI] [PubMed] [Google Scholar]
- 48.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 2002; 3:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66:10439–48 [DOI] [PubMed] [Google Scholar]
- 50.Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J 2003; 22:4409–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anastas JN, Biechele TL, Robitaille M, Muster J, Allison KH, Angers S, Moon RT. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene 2012; 31:3696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Luga V, Armitage SK, Musiol M, Won A, Yip CM, Plotnikov SV, Wrana JL. A lateral signalling pathway coordinates shape volatility during cell migration. Nat Commun 2016; 7:11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wald JH, Hatakeyama J, Printsev I, Cuevas A, Fry WHD, Saldana MJ, VanderVorst K, Rowson-Hodel A, Angelastro JM, Sweeney C, Carraway K. Suppression of planar cell polarity signaling and migration in glioblastoma by Nrdp1-mediated Dvl polyubiquitination. Oncogene 2017; 36:5158–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puvirajesinghe TM, Bertucci F, Jain A, Scerbo P, Belotti E, Audebert S, Sebbagh M, Lopez M, Brech A, Finetti P, Charafe-Jauffret E, Chaffanet M, Castellano R, Restouin A, Marchetto S, Collette Y, Goncalves A, Macara I, Birnbaum D, Kodjabachian L, Johansen T, Borg JP. Identification of p62/SQSTM1 as a component of non-canonical Wnt VANGL2-JNK signalling in breast cancer. Nat Commun 2016; 7:10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997; 390:410–3 [DOI] [PubMed] [Google Scholar]
- 56.Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 2001; 413:856–60 [DOI] [PubMed] [Google Scholar]
- 57.Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 2003; 161:769–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-at and promotes ventral cell fate in Xenopus embryos. Nature 2002; 417:295–9 [DOI] [PubMed] [Google Scholar]
- 59.Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 1999; 399:798–802 [DOI] [PubMed] [Google Scholar]
- 60.Saldanha J, Singh J, Mahadevan D. Identification of a frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci 1998; 7:1632–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 2010; 24:2517–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003; 8:645–54 [DOI] [PubMed] [Google Scholar]
- 63.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 2006; 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci USA 2012; 109:4044–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moroishi T, Hansen CG, Guan KL. The emerging roles of Yap and TAZ in cancer. Nat Rev Cancer 2015; 15:73–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150:780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative Wnt signaling activates Yap/TAZ. Cell 2015; 162:780–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nath D, Li X, Mondragon C, Post D, Chen M, White JR, Hryniewicz-Jankowska A, Caza T, Kuznetsov VA, Hehnly H, Jamaspishvili T, Berman DM, Zhang F, Kung SHY, Fazli L, Gleave ME, Bratslavsky G, Pandolfi PP, Kotula L. Abi1 loss drives prostate tumorigenesis through activation of EMT and non-canonical WNT signaling. Cell Commun Signal 2019; 17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Abreu JG, Yokota C, MacDonald BT, Singh S, Coburn KL, Cheong SM, Zhang MM, Ye QZ, Hang HC, Steen H, He X. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell 2012; 149:1565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Cheong SM, Amado NG, Reis AH, MacDonald BT, Zebisch M, Jones EY, Abreu JG, He X. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell 2015; 32:719–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA 1997; 94:2859–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 1997; 88:747–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 1999; 398:431–6 [DOI] [PubMed] [Google Scholar]
- 74.Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell 2011; 20:303–14 [DOI] [PubMed] [Google Scholar]
- 75.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007; 316:1619–22 [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell 2006; 11:213–23 [DOI] [PubMed] [Google Scholar]
- 77.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010; 143:1136–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 2001; 11:951–61 [DOI] [PubMed] [Google Scholar]
- 79.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 2002; 417:664–7 [DOI] [PubMed] [Google Scholar]
- 80.Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012; 485:195–200 [DOI] [PubMed] [Google Scholar]
- 81.Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012; 488:665–9 [DOI] [PubMed] [Google Scholar]
- 82.Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell 2004; 7:525–34 [DOI] [PubMed] [Google Scholar]
- 83.Janda CY, Dang LT, You C, Chang J, de Lau W, Zhong ZA, Yan KS, Marecic O, Siepe D, Li X, Moody JD, Williams BO, Clevers H, Piehler J, Baker D, Kuo CJ, Garcia KC. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 2017; 545:234–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449:1003–7 [DOI] [PubMed] [Google Scholar]
- 85.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010; 6:25–36 [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harb Perspect Biol 2012; 4:a007963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of beta-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol 2011; 13:753–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10:55–63 [DOI] [PubMed] [Google Scholar]
- 89.Merrill BJ. Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb Perspect Biol 2012; 4:a007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet 2002; 32:594–605 [DOI] [PubMed] [Google Scholar]
- 91.McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 1989; 58:1075–84 [DOI] [PubMed] [Google Scholar]
- 92.Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol 2013; 5:a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 1997; 136:1123–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 1997; 11:2359–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, Chu F, Talbot WS, Weinberg ES. Essential and opposing roles of zebrafish beta-catenins in the formation of dorsal axial structures and neurectoderm. Development 2006; 133:1299–309 [DOI] [PubMed] [Google Scholar]
- 96.Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol 1997; 13:611–67 [DOI] [PubMed] [Google Scholar]
- 97.Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev 1998; 71:119–29 [DOI] [PubMed] [Google Scholar]
- 98.Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development 2002; 129:37–52 [DOI] [PubMed] [Google Scholar]
- 99.Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development 1993; 118:477–87 [DOI] [PubMed] [Google Scholar]
- 100.Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 1991; 67:753–65 [DOI] [PubMed] [Google Scholar]
- 101.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998; 391:357–62 [DOI] [PubMed] [Google Scholar]
- 102.Blythe SA, Cha SW, Tadjuidje E, Heasman J, Klein PS. beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell 2010; 19:220–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 2003; 100:3299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohamed OA, Clarke HJ, Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn 2004; 231:416–24 [DOI] [PubMed] [Google Scholar]
- 105.Tao H, Suzuki M, Kiyonari H, Abe T, Sasaoka T, Ueno N. Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proc Natl Acad Sci USA 2009; 106:14426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev 1999; 13:3185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamaguchi TP. Genetics of Wnt signaling during early mammalian development. Methods Mol Biol 2008; 468:287–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galceran J, Sustmann C, Hsu SC, Folberth S, Grosschedl R. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev 2004; 18:2718–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hofmann M, Schuster-Gossler K, Watabe-Rudolph M, Aulehla A, Herrmann BG, Gossler A. WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev 2004; 18:2712–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development 2005; 132:5425–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, Lewandoski M, Pourquie O. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat Cell Biol 2008; 10:186–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell 2006; 125:33–45 [DOI] [PubMed] [Google Scholar]
- 113.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol 2005; 3:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 2010; 466:378–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 2003; 19:589–621 [DOI] [PubMed] [Google Scholar]
- 116.Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev 2002; 16:2339–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003; 423:173–7 [DOI] [PubMed] [Google Scholar]
- 118.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet 2005; 37:980–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matsuyama M, Aizawa S, Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet 2009; 5:e1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wallingford JB, Harland RM. Neural tube closure requires dishevelled-dependent convergent extension of the midline. Development 2002; 129:5815–25 [DOI] [PubMed] [Google Scholar]
- 121.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 2006; 133:1767–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Savory JG, Mansfield M, Rijli FM, Lohnes D. Cdx mediates neural tube closure through transcriptional regulation of the planar cell polarity gene Ptk7. Development 2011; 138:1361–70 [DOI] [PubMed] [Google Scholar]
- 123.Kibar Z, Bosoi CM, Kooistra M, Salem S, Finnell RH, De Marco P, Merello E, Bassuk AG, Capra V, Gros P. Novel mutations in VANGL1 in neural tube defects. Hum Mutat 2009; 30:E706–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kibar Z, Salem S, Bosoi CM, Pauwels E, De Marco P, Merello E, Bassuk AG, Capra V, Gros P. Contribution of VANGL2 mutations to isolated neural tube defects. Clin Genet 2011; 80:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C, DeChiara TM, Kimble RB, Valenzuela DM, Yancopoulos GD, Wilkie AO. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet 2000; 24:275–8 [DOI] [PubMed] [Google Scholar]
- 126.Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, Tuysuz B, Murday VA, Patton MA, Wilkie AO, Jeffery S. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 2000; 25:419–22 [DOI] [PubMed] [Google Scholar]
- 127.Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ, Jr, van Bokhoven H, Hoogeboom JM, Mazzeu JF, Petryk A, Schimmenti LA, Brunner HG, Ekker SC, Lohr JL. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn 2010; 239:327–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking tcf-4. Nat Genet 1998; 19:379–83 [DOI] [PubMed] [Google Scholar]
- 129.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 2007; 27:7551–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003; 17:1709–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML, Osteoporosis-Pseudoglioma Syndrome Collaborative G. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001; 107:513–23 [DOI] [PubMed] [Google Scholar]
- 132.Lim X, Tan SH, Yu KL, Lim SB, Nusse R. Axin2 marks quiescent hair follicle bulge stem cells that are maintained by autocrine Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 2016; 113:E1498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 2009; 8:4032–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Acebron SP, Karaulanov E, Berger BS, Huang YL, Niehrs C. Mitotic Wnt signaling promotes protein stabilization and regulates cell size. Mol Cell 2014; 54:663–74 [DOI] [PubMed] [Google Scholar]
- 135.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997; 275:1784–7 [DOI] [PubMed] [Google Scholar]
- 136.Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 2015; 21:256–62 [DOI] [PubMed] [Google Scholar]
- 137.Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 2015; 161:1539–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ertych N, Stolz A, Stenzinger A, Weichert W, Kaulfuss S, Burfeind P, Aigner A, Wordeman L, Bastians H. Increased microtubule assembly rates influence chromosomal instability in colorectal cancer cells. Nat Cell Biol 2014; 16:779–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giannakis M, Hodis E, Jasmine Mu X, Yamauchi M, Rosenbluh J, Cibulskis K, Saksena G, Lawrence MS, Qian ZR, Nishihara R, Van Allen EM, Hahn WC, Gabriel SB, Lander ES, Getz G, Ogino S, Fuchs CS, Garraway LA. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 2014; 46:1264–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat Rev Urol 2012; 9:418–28 [DOI] [PubMed] [Google Scholar]
- 141.Francis JC, Thomsen MK, Taketo MM, Swain A. Beta-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet 2013; 9:e1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee SH, Luong R, Johnson DT, Cunha GR, Rivina L, Gonzalgo ML, Sun Z. Androgen signaling is a confounding factor for beta-catenin-mediated prostate tumorigenesis. Oncogene 2016; 35:702–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem 2006; 99:402–10 [DOI] [PubMed] [Google Scholar]
- 144.Weischenfeldt J, Simon R, Feuerbach L, Schlangen K, Weichenhan D, Minner S, Wuttig D, Warnatz HJ, Stehr H, Rausch T, Jager N, Gu L, Bogatyrova O, Stutz AM, Claus R, Eils J, Eils R, Gerhauser C, Huang PH, Hutter B, Kabbe R, Lawerenz C, Radomski S, Bartholomae CC, Falth M, Gade S, Schmidt M, Amschler N, Hass T, Galal R, Gjoni J, Kuner R, Baer C, Masser S, von Kalle C, Zichner T, Benes V, Raeder B, Mader M, Amstislavskiy V, Avci M, Lehrach H, Parkhomchuk D, Sultan M, Burkhardt L, Graefen M, Huland H, Kluth M, Krohn A, Sirma H, Stumm L, Steurer S, Grupp K, Sultmann H, Sauter G, Plass C, Brors B, Yaspo ML, Korbel JO, Schlomm T. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell 2013; 23:159–70 [DOI] [PubMed] [Google Scholar]
- 145.Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the tcf signaling axis. Oncogene 2003; 22:5602–13 [DOI] [PubMed] [Google Scholar]
- 146.Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, Jarosz M, Lipson D, Tagawa ST, Nanus DM, Stephens PJ, Mosquera JM, Cronin MT, Rubin MA. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol 2013; 63:920–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee E, Ha S, Logan SK. Divergent androgen receptor and beta-catenin signaling in prostate cancer cells. PLoS One 2015; 10:e0141589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lento W, Congdon K, Voermans C, Kritzik M, Reya T. Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harb Perspect Biol 2013; 5:a008011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, Ferraro F, Shterental S, Lin CP, Gilliland DG, Scadden DT, Armstrong SA, Williams DA. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood 2011; 118:2849–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 2010; 327:1650–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ferrando AA. The role of NOTCH1 signaling in T-ALL. Hematology Am Soc Hematol Educ Program 2009;( 1):353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaveri D, Kastner P, Dembele D, Nerlov C, Chan S, Kirstetter P. beta-Catenin activation synergizes with Pten loss and Myc overexpression in Notch-independent T-ALL. Blood 2013; 122:694–704 [DOI] [PubMed] [Google Scholar]
- 153.Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2004; 101:3118–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moskalev EA, Luckert K, Vorobjev IA, Mastitsky SE, Gladkikh AA, Stephan A, Schrenk M, Kaplanov KD, Kalashnikova OB, Potz O, Joos TO, Hoheisel JD. Concurrent epigenetic silencing of Wnt/beta-catenin pathway inhibitor genes in B cell chronic lymphocytic leukaemia. BMC Cancer 2012; 12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Daulat AM, Borg JP. Wnt/planar cell polarity signaling: new opportunities for cancer treatment. Trends Cancer 2017; 3:113–25 [DOI] [PubMed] [Google Scholar]
- 156.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13:11–26 [DOI] [PubMed] [Google Scholar]
- 157.Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y, Gu Q. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J Cell Physiol 2014; 229:1908–17 [DOI] [PubMed] [Google Scholar]
- 158.Bakker ER, Das AM, Helvensteijn W, Franken PF, Swagemakers S, van der Valk MA, ten Hagen TL, Kuipers EJ, van Veelen W, Smits R. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis 2013; 34:2629–38 [DOI] [PubMed] [Google Scholar]
- 159.Lhoumeau AC, Martinez S, Boher JM, Monges G, Castellano R, Goubard A, Doremus M, Poizat F, Lelong B, de Chaisemartin C, Bardin F, Viens P, Raoul JL, Prebet T, Aurrand-Lions M, Borg JP, Goncalves A. Overexpression of the promigratory and prometastatic PTK7 receptor is associated with an adverse clinical outcome in colorectal cancer. PLoS One 2015; 10:e0123768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mei H, Lian S, Zhang S, Wang W, Mao Q, Wang H. High expression of ROR2 in cancer cell correlates with unfavorable prognosis in colorectal cancer. Biochem Biophys Res Commun 2014; 453:703–9 [DOI] [PubMed] [Google Scholar]
- 161.Steinhart Z, Pavlovic Z, Chandrashekhar M, Hart T, Wang X, Zhang X, Robitaille M, Brown KR, Jaksani S, Overmeer R, Boj SF, Adams J, Pan J, Clevers H, Sidhu S, Moffat J, Angers S. Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat Med 2017; 23:60–8 [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 2010; 29:2036–46 [DOI] [PubMed] [Google Scholar]
- 163.Takahashi S, Watanabe T, Okada M, Inoue K, Ueda T, Takada I, Watabe T, Yamamoto Y, Fukuda T, Nakamura T, Akimoto C, Fujimura T, Hoshino M, Imai Y, Metzger D, Miyazono K, Minami Y, Chambon P, Kitamura T, Matsumoto T, Kato S. Noncanonical Wnt signaling mediates androgen-dependent tumor growth in a mouse model of prostate cancer. Proc Natl Acad Sci USA 2011; 108:4938–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sandsmark E, Hansen AF, Selnaes KM, Bertilsson H, Bofin AM, Wright AJ, Viset T, Richardsen E, Drablos F, Bathen TF, Tessem MB, Rye MB. A novel non-canonical Wnt signature for prostate cancer aggressiveness. Oncotarget 2017; 8:9572–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Syed Khaja AS, Helczynski L, Edsjo A, Ehrnstrom R, Lindgren A, Ulmert D, Andersson T, Bjartell A. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS One 2011; 6:e26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khaja AS, Egevad L, Helczynski L, Wiklund P, Andersson T, Bjartell A. Emphasizing the role of Wnt5a protein expression to predict favorable outcome after radical prostatectomy in patients with low-grade prostate cancer. Cancer Med 2012; 1:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thiele S, Gobel A, Rachner TD, Fuessel S, Froehner M, Muders MH, Baretton GB, Bernhardt R, Jakob F, Gluer CC, Bornhauser M, Rauner M, Hofbauer LC. WNT5A has anti-prostate cancer effects in vitro and reduces tumor growth in the skeleton in vivo. J Bone Miner Res 2015; 30:471–80 [DOI] [PubMed] [Google Scholar]
- 168.Prebet T, Lhoumeau AC, Arnoulet C, Aulas A, Marchetto S, Audebert S, Puppo F, Chabannon C, Sainty D, Santoni MJ, Sebbagh M, Summerour V, Huon Y, Shin WS, Lee ST, Esterni B, Vey N, Borg JP. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood 2010; 116:2315–23 [DOI] [PubMed] [Google Scholar]
- 169.Jiang G, Zhang M, Yue B, Yang M, Carter C, Al-Quran SZ, Li B, Li Y. PTK7: a new biomarker for immunophenotypic characterization of maturing T cells and T cell acute lymphoblastic leukemia. Leuk Res 2012; 36:1347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaucka M, Plevova K, Pavlova S, Janovska P, Mishra A, Verner J, Prochazkova J, Krejci P, Kotaskova J, Ovesna P, Tichy B, Brychtova Y, Doubek M, Kozubik A, Mayer J, Pospisilova S, Bryja V. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res 2013; 73:1491–501 [DOI] [PubMed] [Google Scholar]
- 171.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL. Targeting Wnt-driven cancer through the inhibition of porcupine by LGK974. Proc Natl Acad Sci USA 2013; 110:20224–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, Manoharan V, Ong EH, Sangthongpitag K, Hill J, Petretto E, Keller TH, Lee MA, Matter A, Virshup DM. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene 2016; 35:2197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pharmaceuticals N, Novartis. A study of LGK974 in patients with malignancies dependent on Wnt ligands, https://ClinicalTrials.gov/show/NCT01351103 (2011, accessed 18 December 2019)
- 174.EDDC (Experimental Drug Development Centre), A*STAR Research Entities. A study to evaluate the safety and tolerability of ETC-1922159 in advanced solid tumours, https://ClinicalTrials.gov/show/NCT02521844 (2019, accessed 18 December 2019)
- 175.Safholm A, Tuomela J, Rosenkvist J, Dejmek J, Harkonen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res 2008; 14:6556–63 [DOI] [PubMed] [Google Scholar]
- 176.Osman J, Bellamkonda K, Liu Q, Andersson T, Sjolander A. The WNT5A agonist Foxy5 reduces the number of colonic cancer stem cells in a xenograft mouse model of human colonic cancer. Anticancer Res 2019; 39:1719–28 [DOI] [PubMed] [Google Scholar]
- 177.WntResearch AB. Phase I study to evaluate safety, tolerability, anti-tumour activity and PK profiles of foxy-5 in metastatic breast, colon or prostate cancer, https://ClinicalTrials.gov/show/NCT02020291 (2016, accessed 18 December 2019)
- 178.Courtneidge SA. Cancer: escape from inhibition. Nature 2003; 422:827–8 [DOI] [PubMed] [Google Scholar]
- 179.WntResearch AB. Dose escalating study of Foxy-5 in breast-, colon- or prostate cancer patients, https://ClinicalTrials.gov/show/NCT02655952 (2018, accessed 18 December 2019)
- 180.Yeung P, Beviglia L, Cancilla B, Dee-Hoskins C, Evans JW, Fischer MM, Yen W-C, Gurney A, Lewicki J, Hoey T, Kapoun AM. Abstract 1907: Wnt pathway antagonist OMP-54F28 (FZD8-Fc) inhibits tumor growth and reduces tumor-initiating cell frequency in patient-derived hepatocellular carcinoma and ovarian cancer xenograft models. Cancer Res 2014; 74:1907 [Google Scholar]
- 181.OncoMed Pharmaceuticals, Inc. A dose escalation study of OMP-54F28 in subjects with solid tumors, https://ClinicalTrials.gov/show/NCT01608867 (2017, accessed 18 December 2019)
- 182.Cristofanilli M, Broglio KR, Guarneri V, Jackson S, Fritsche HA, Islam R, Dawood S, Reuben JM, Kau SW, Lara JM, Krishnamurthy S, Ueno NT, Hortobagyi GN, Valero V. Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 2007; 7:471–9 [PubMed] [Google Scholar]
- 183.OncoMed Pharmaceuticals, Inc. Dose escalation study of OMP-54F28 in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer, https://ClinicalTrials.gov/show/NCT02092363 (2018, accessed 18 December 2019) [DOI] [PMC free article] [PubMed]
- 184.OncoMed Pharmaceuticals, Inc. Dose escalation study of OMP-54F28 in combination with nab-paclitaxel and gemcitabine in patients with previously untreated stage IV pancreatic cancer, https://ClinicalTrials.gov/show/NCT02050178 (2018, accessed 18 December 2019)
- 185.Dai Z, Quackenbush RC, Courtney KD, Grove M, Cortez D, Reuther GW, Pendergast AM. Oncogenic Abl and Src tyrosine kinases elicit the ubiquitin-dependent degradation of target proteins through a Ras-independent pathway. Genes Dev 1998; 12:1415–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Giraudet A-L, Badel J-N, Cassier P, Desuzinges C, Kriza D, Perol D, Blay J-Y. SYNFRIZZ-A phase Ia/Ib of a radiolabelled monoclonal AB for the treatment of relapsing synovial sarcoma. J Nucl Med 2014; 55:22324434294 [Google Scholar]
- 187.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA 2012; 109:11717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jasgit CS, Michael LM, Manish S, Victor M, Valentina B, Shivaani K, Erica Michelle S-R, Andres F-T, Nehal JL, Brenda G, Dawei X, Tenshang J, Eric LP, Amy J-F, Xiaohua X, Anthony WT, Emiliano C. PF-06647020 (PF-7020), an antibody-drug conjugate (ADC) targeting protein tyrosine kinase 7 (PTK7), in patients (pts) with advanced solid tumors: results of a phase I dose escalation and expansion study. J Clin Oncol 2018; 36:5565–65 [Google Scholar]
- 189.Gang EJ, Hsieh YT, Pham J, Zhao Y, Nguyen C, Huantes S, Park E, Naing K, Klemm L, Swaminathan S, Conway EM, Pelus LM, Crispino J, Mullighan CG, McMillan M, Muschen M, Kahn M, Kim YM. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene 2014; 33:2169–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Robert RM, Andrew HK, Chiorean EG, Eunice Lee K, Heinz-Josef L, Paul IN, Debra LW, Masamoto F, Kohei M, Tetsuhi I, Hiroyuki K. A phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol 2015; 33:e15270–e70 [Google Scholar]