Short abstract
The process of cancer development and progression is driven by distinct subsets of cancer stem cells (CSCs) that contribute the self-renewal capacity as the major impetus to the metastatic dissemination and main impediments in cancer treatment. Given that CSCs are so scarce in the tumor mass, there are debatable points on the metabolic signatures of CSCs. As opposed to differentiated tumor progenies, CSCs display exquisite patterns of metabolism that, depending on the type of cancer, predominately rely on glycolysis, oxidative metabolism of glutamine, fatty acids, or amino acids for ATP production. Metabolic heterogeneity of CSCs, which attributes to differences in type and microenvironment of tumors, confers CSCs to have the plasticity to cope with the endogenous mitochondrial stress and exogenous microenvironment. In essence, CSCs and normal stem cells are like mirror images of each other in terms of metabolism. To achieve reprogramming, CSCs not only need to upregulate their metabolic engine for self-renewal and defense mechanism, but also expedite the antioxidant defense to sustain the redox homeostasis. In the context of these pathways, this review portrays the connection between the metabolic features of CSCs and cancer stemness. Identification of the metabolic features in conferring resistance to anticancer treatment dictated by CSCs can enhance the opportunity to open up a new therapeutic dimension, which might not only improve the effectiveness of cancer therapies but also annihilate the whole tumor without recurrence. Henceforth, we highlight current findings of potential therapeutic targets for the design of alternative strategies to compromise the growth, drug resistance, and metastasis of CSCs by altering their metabolic phenotypes. Perturbing the versatile skills of CSCs by barricading metabolic signaling might bring about plentiful approaches to discover novel therapeutic targets for clinical application in cancer treatments.
Impact statement
This minireview highlights the current evidence on the mechanisms of pivotal metabolic pathways that attribute to cancer stem cells (CSCs) with a special focus on developing metabolic strategies of anticancer treatment that can be exploited in preclinical and clinical settings. Specific metabolic inhibitors that can overwhelm the properties of CSCs may impede tumor recurrence and metastasis, and potentially achieve a permanent cure of cancer patients.
Keywords: Cancer stem cell, metabolic reprogramming, p53, Myc, HIF
Introduction
To date, many lines of research suggest that cancer can be considered as a metabolic disease characterized by defective respiration or enhanced glycolysis. Genomic alterations and other hallmarks of cancers are nowadays conceived as downstream epiphenomena of underlying causes of metabolic disorders in cancer cells.1 Dysregulation of glucose metabolism, fatty acid synthesis, and glutaminolysis that satisfy the bioenergetic and biosynthetic needs of cancer cells can cause their resistance to therapies.2 As such, it is evident that cancer cells can modulate their metabolism to optimize energy demands3 and induce immune escape,4 which could be exploited as therapeutic targets for cancer treatment in light of the evolving metabolic landscape. Given that cancer stem cells (CSCs) embrace many malignant traits conferring therapeutic resistance, recurrence, and metastasis, eradication of CSCs can hold a promise of permanent cure for cancer patients.
CSCs manage to retain stem cell properties embracing the capacity of self-renewal and differentiation into progenies to form the epigenetically defined intra-clonal bulk tumors. It is reminiscent of normal stem cells and transit-amplification of cell populations with differentiation capability,5,6 which is called “stemness” of cancer cells. Metaphorically, the majority of heterogeneous tumor cells resemble various kinds of worker bees and CSCs are like the queen bees that establish the bee colony and heterogeneity of the hierarchy. Without the queen, the whole colony will collapse. Likewise, CSCs sustain the entire cancer population in a similar fashion. Unfortunately, conventional cancer therapies kill only differentiated tumor cells but spare CSCs, an extremely rare subset of resistant cells within the tumor mass, which is the main cause of recurrence and metastasis.7 This is due to the fact that CSCs are able to expel anticancer drugs via ABC-transporters.8 Peculiar properties of CSCs, such as manifesting quiescent phenotype, maintaining reactive oxygen species (ROS) at low levels, and residing in hypoxic region within tumors may also compromise the effectiveness of anticancer therapies.9 These underlie the observation that CSCs are refractory to treatment and have a high incidence of relapse even after therapy has almost eradicated the tumors. Finding CSCs may provide an effective direction and a major leap toward a cure of cancers. Spherogenesis, side population, cellular markers, and aldehyde dehydrogenase (ALDH) activity of CSCs are currently major avenues to characterize and isolate CSCs.10 However, there are a lot of challenges of isolating and enriching such rare CSCs from heterogeneous tumor bulk in the absence of knowing their specific biomarkers. An alternative characterization of CSCs from a metabolic perspective provides a new direction to identify and target this rare subpopulation. Therefore, the metabolism-based therapy has become a dawning strategy to halt cancer progression, and substantial efforts have been made to discover novel targets for anticancer treatment. In this review, we highlight the key players of metabolic pathways that characterize CSCs with insights in metabolic therapeutic strategies that can be exploited in preclinical and clinical settings. Specific metabolic inhibitors that can overwhelm stem cell properties may halt disease recurrence and attenuate the dissemination of CSCs and the capacity of spawning metastases of cancer cells.
Metabolic reprogramming in the CSCs
The metabolic hallmarks of CSCs in different types or status of tumors like proliferative vs. quiescent or normoxic vs. hypoxic have been mired in controversy, and the molecular mechanism governing the stemness properties of CSCs remains unclear.11 Differences in tissue oxygenation and genetic background may be one of the causative factors in the development of metabolic complexity, plasticity and heterogeneity in different types of CSCs.12 Remaining controversies may be related to diverse surface markers and isolation techniques of CSCs in different tumor types.13 Notwithstanding the divergent findings in the metabolism of CSCs, a corpus of evidence is emerging to show that these unique metabolic signatures are pivotal to the function of CSCs. The discoveries of stem cells spark heated debate over whether CSCs employ central metabolic pathways as seen in normal stem cells, which are quite dissimilar to the differentiated bulk of tumor cells. Even though bioenergetic signature of CSCs has remained elusive, many lines of evidence have accumulated to demonstrate that metabolic pathways in CSCs are like those in tissue stem cells. The metabolic signature of stem cell-like osteosarcoma resembles that of somatic stem cells characterized by an upregulation of glycolytic flux.14 This metabolic shift of CSCs encompasses a decrease in oxidative phosphorylation (OXPHOS) but a pronounced increase in lactate production, expression of glycolytic enzymes and glucose consumption compared with their differentiated counterparts. Although glycolysis is enhanced in the CSCs of osteosarcoma,14 breast cancer,15 nasopharyngeal,16 colorectal,17 and hepatocellular carcinoma,18 many studies have shown that CSCs of glioma,19 pancreatic ductal adenocarcinoma,20 leukemia21 rely mainly on mitochondrial respiration for the major supply of energy (Table 1). These oxidative CSCs are less glycolytic and have increased levels of mitochondrial mass, membrane potential, ATP, and ROS concentrations as compared with their differentiated counterparts. Pancreatic cancer cells with oncogene ablation acquired stemness traits to display active mitochondrial function and rely less on glucose and glutamine metabolism, but more on pyruvate and palmitate to fuel the tricarboxylic acid cycle.22 However, the reliance on glycolysis or OXPHOS is still ambiguous in glioblastoma, lung, breast, and ovarian CSCs. These diverse observations may stem from the fact that the definition and isolation methods of CSCs were not identical among these studies, and hence different CSCs came from a variety of sources with divergent characteristics. In any case, metabolic heterogeneity of different subclones of CSCs can bear different metabolic phenotypes because of differences in genetic or microenvironmental factors of cancers (Figure 1).13,23
Table 1.
Cancer stem cells with different metabolic characteristics in various cancer types.
| Cancer type | Metabolic phenotype | Model of study | CSCs/tumor cells | Methods |
|---|---|---|---|---|
| Acute myeloid leukemia (AML) | OXPHOS21 | Primary cultures from human samples | Primary AML and normal hematopoietic cells | ABT-263 and Seahorse extracellular flux analyzer |
| Brain cancer | Glycolysis24–26 | In vitro | LC26-R, LC26-RTL (170), LCAS-R, and LCAS-RTL(138) cells with BTIC features | Seahorse extracellular flux analyzer |
| In vitro (xenograft) and in vitro | Human glioblastomaU87 cells and athymic mice | Clark-type oxygen electrode | ||
| In vitro | NCH421k, NCH441, and NCH644 cells | Gene expression analysis | ||
| In vitro | GSC11 and GSC23 from human primary glioblastoma tissues, U87, and non-malignant human astrocytes (NHAs) | Clark-type oxygen electrode | ||
| OXPHOS19 | Human tumors, PDXIn vivo (xenograft) | CD133+ cells, Gliomaspheres | Seahorse extracellular flux analyzer | |
| PPP27 | In vivo | Glioblastoma stem-like (GS) cell lines | Gene expression profiling and isotope tracing | |
| Purine metabolism25 | In vivo (xenograft) and in vitro | Brain tumor initiating cells (BTICs) | Metabolomics by isotope tracing | |
| Breast cancer | Glycolysis15,28,29 | In vitro | CD44+CD24low EPCAM+ breast cancer cells | Seahorse extracellular flux analyzer and isotope tracing |
| In vitro | Human breast cancer cells | Proteomics and targeted metabolomics | ||
| In vitro | Human breast cells MDA-MB 231 (ER−) and MCF-7 (ER+) | Glucose uptake, glutamate, glutamine, NAD+/NADH ratio determination, and proteomics | ||
| OXPHOS26 | In vitro | Breast cancer cell lines MCF-7 and T47D | Seahorse extracellular flux analyzer and label-free quantitative analysis | |
| FAO30 | In vitro | ErbB2-MCF-10A, BT-474 cells, an ErbB2 expressing breast cancer cell lines | Isotope tracing | |
| Mitochondrial biogenesis and FAO31 | In vitro | MCF-7 breast cancer cells | Seahorse extracellular flux analyzer and label-free quantitative analysis | |
| PPP32 | In vitro | Breast cancer cell lines SUM149 and SUM159 and ALDH+ cells | Glucose uptake and lactate production assays, NADPH, and glucose 6-phosphate measurements | |
| Ketone bodies29,33 | In vitro | MCF-7 breast cancer cells | Seahorse extracellular flux analyzer | |
| In vitro and in vivo (xenograft) | MDA-MB-231 (GFP+) cells | 3-OH-butyrate effects on tumor growth, migration, and quantitation of tumor angiogenesis | ||
| Cervical cancer | TCA cycle34 | In vitro | Human cervical cancer cell line SiHa and ovarian clear cell adenocarcinoma cell line OVTOKO | Measurement of metabolites |
| Colorectal cancer | Glycolysis17 | In vitro | Human colon cancer LoVo cell line and non-small cell lungcarcinoma (NSCLC) A549 and NCI-H460 cell lines | Clark-type oxygen electrode and proteomics |
| Glycolysis, TCA cycle, and cysteine/methionine metabolism35 | In vitro | CD110+ cells | Metabolomics | |
| Lysine catabolism36 | In vivo (xenograft) | CD110+ cells and primary CRC cells (CRC102, CRC105, and CRC108) | Transcriptomics and proteomics | |
| Hepatocellular carcinoma | Glutamine37,38 | In vitro | V138/H1299 | BD Oxygen biosensor system |
| In vitro | H1299 cells infected with a recombinant adenovirus expressing p53 and HCT116 cells (p53+/+ and p53−/−) | Glutathione assay, determination of glutamate and glutamine concentrations | ||
| Glycolysis18 | In vitro | CD133+ cells PLC/PRF/5 | Seahorse extracellular flux analyzer, gene expression profiling, and proteomics | |
| Glycolysis and FAO in sh-Nanog-TICs39 | In vitro (xenograft) | CD133+/CD49f+ TICs from HCC tissues and HCV NS5A Tg mice | Isotope tracing, metabolomics, and seahorse extracellular flux analyzer | |
| Leukemia | OXPHOS21 | Primary cultures from human samples | CD34+ cells primary AML and normal hematopoietic cells | ABT-263 and Seahorse extracellular flux analyzer |
| FAO40 | In vivo (xenograft) | Bulk of tumor cellsOCI-AML3 cells | Clark-type oxygen electrode and isotope tracing | |
| FAO41 | In vivo (xenograft) | CD150+CD48–CD41-Flt3–CD34- KSL cells sorted from Pml+/+ or Pml–/– mice | Isotope tracing and Seahorse extracellular flux analyzer | |
| Lung cancer | Glycolysis17 | In vitro | Human non-small cell lung carcinoma (NSCLC) A549 and NCI-H460 cell lines and colon cancer LoVo cell line | Clark-type oxygen electrode and proteomics |
| OXPHOS42 | In vivo (xenograft) and in vitro | SP+ cells, A549 lung cancer cell line | Clark-type oxygen electrode | |
| Nasopharyngeal carcinoma | Glycolysis16,43 | In vitro | Radioresistant human NPC cell lines TW01 and HONE-1, sphere-forming and SP+ cells | Morphological subtyping of mitochondria |
| In vitro and in vivo (xenograft) | Radioresistant human NPC cell lines TW01, TW06, and HONE-1, sphere-forming and SP+ cells | Seahorse extracellular flux analyzer | ||
| Osteosarcoma | Glycolysis14 | In vitro | MG63 cells and 3AB‐OS stem cells | Seahorse extracellular flux analyzer and D-glucose and lactate measurement |
| Ovarian cancer | Glycolysis44 | In vivo (xenograft and serial in vivo passaging) and in vitro | Mouse ovarian surface epithelium cells (MOSE) C57BL/6 mice | Isotope tracing and Seahorse extracellular flux analyzer |
| Glycolysis45 | In vitro | Epithelial ovarian cancer (EOC) | Isotope tracing | |
| OXPHOS and PPP46 | Fresh human samples and in vivo (xenograft) | CD44+CD117+ cells | Oligomycin, antimycin A, rotenone, metformin by Annexin V/PI staining | |
| Pancreatic cancer | Glutamine47 | In vitro | ABCG2high cells | ATP, NADP+/NADPH, and glutathione |
| Glutamine (non-canonical pathway of glutamine48 metabolism) | In vivo (xenograft) and in vitro | AsPC-1 cells | Gene expression, enzymatic activity assays, and NADP+/NADPH ratio determination | |
| OXPHOS22 | In vivo (inducible mouse model of mutated KRAS2) and in vitro | Sphere-forming cells | Isotope tracing, metabolomics, and Seahorse extracellular flux analyzer | |
| OXPHOS20 | In vivo (xenograft) and in vitro | CD133+ cells and CD44+ CD133+ Primary human PDAC cells | Seahorse extracellular flux analyzer | |
| Papillary thyroid carcinoma | OXPHOS49 | In vitro | PTC-derived TPC-1 and B-CPAP cell lines | GC/MS spectrometry measurement of metabolites |
| Teratocarcinoma | Glycolysis60 | In vitro | P19SCs and P19dCs | Clark-type oxygen electrode |
FAO: fatty acid oxidation; OXPHOS: oxidative phosphorylation; PDX: patient-derived xenograft; PPP: pentose phosphate pathway.
Figure 1.
Metabolic heterogeneity of CSCs. Instead of dwelling in a solitary place, CSCs reside in a diversified ecosystem encircled by extracellular matrix, endothelial cells, immune cells, and cancer-associated fibroblasts (CAFs). In contrast to their differentiated counterparts, CSCs display an exquisite metabolic characteristics according to the type of cancer, which may predominately rely on glycolysis, catabolism of glutamine, fatty acids, and certain amino acids. Heterogeneity of CSC metabolism across different types and microenvironment of tumors confers CSCs to have the plasticity to cope with the endogenous mitochondrial stress and/or exogenous microenvironment. (A color version of this figure is available in the online journal.)
In addition to glucose metabolism, recent reports have disclosed the peculiarity of CSCs metabolism including lipid metabolism, redox state, and utilization of alternative fuels including amino acids and ketone bodies (Figure 1 and Table 1). Leukemia-initiating cells and hematopoietic stem cells may depend on mitochondrial fatty acid oxidation (FAO) for the production of ATP and NADPH.30,41 Glioblastoma CSCs can switch from higher pentose phosphate pathway (PPP) activity for elevated proliferation rate under acute oxygenation to a glucose-dependent status under hypoxia and a migrating process to protect cells against hypoxic cell damage.27 Besides, oxidative stem-like cells may be fueled by more differentiated glycolytic somatic cells through a “reverse Warburg effect” under normoxic conditions in breast cancer.51 Epithelial-mesenchymal transition (EMT) contributes stemness to CSCs with the capabilities to catabolize pyruvate, lactate, amino acids such as glutamine, glutamate, and alanine, as well as ketone bodies from the microenvironment to support mitochondrial respiration in CSCs.52 Intriguingly, tumor cells with impaired respiration can regain mitochondrial function by receiving mitochondrial DNA (mtDNA) from the surrounding cells in tumor microenvironment, which renders tumor-initiating capacity and therapeutic resistance.53,54 Cancer-associated fibroblasts (CAFs)-derived exosomes are laden with mtDNA, which can be released into CSC niches and consumed by dormant CSCs.55 This horizontal transfer of mtDNA promotes respiration-proficient CSCs with higher OXPHOS potential and the development of hormonal therapy resistance.55 The horizontal transfer of mtDNA recapitulates the pivotal metabolic interaction between CSCs and the tumor microenvironment (Figure 1).
As for the mitochondrial distribution, the mitochondria are widely scattered over the cell periphery in well-differentiated cells, whereas these organelles are clustered in a peri-nuclear fashion in CSCs.16 Similarly, the mitochondria are peri-nuclear clustered in embryonic stem cells (ESCs) and are rearranged to disperse throughout the cytoplasm upon differentiation.56 The peri-nuclear accumulation of mitochondria supports the transportation of mitochondrial transit peptide to strengthen mitochondrial function.57 It may also indicate that ATP would be efficiently transported from mitochondria to the nucleus, which is refrained from fluctuations in Ca2+ levels that often occur in the cytoplasm.57
Likewise, reduction-oxidation (redox) homeostasis and mitochondrial membrane potential (Δψm) are imperative hallmarks for a balance between differentiation and sustaining stemness status of stem cells and CSCs.58–60 In contrast to differentiated tissue cells, normal hemopoietic and mammary epithelial stem/progenitor cells reside in a low oxygen niche, that is essential to self-renew and deter from differentiation.58,61 Stem cells launch the expression of antioxidant enzymes to maintain ROS at low levels for sheltering the cells from oxidative damage.62 Oppositely, raising the ROS levels can compel stem cells to a lineage-specific differentiation.63 In conformity with normal stem cells, CSCs with augmented antioxidant defense systems keep ROS levels at bay and may confer CSCs with survival advantage and therapeutic resistance.9,16,46 Since mitochondrial respiration is a notorious intracellular ROS sources, preferential dependence on glycolysis in certain types of CSCs is considered a way to shield themselves from accumulating too much ROS. Glycolytic pathway can consolidate the antioxidant defensive mechanism through generating NADPH by glucose 6-phosphate dehydrogenase (G6PD) for the recycling of the low molecular weight antioxidants including GSH (reduced glutathione), thioredoxin and peroxiredoxin through glutathione reductase. It was shown that ROS generation results in mitochondrial depolarization,64 inferring that CSCs with low ROS levels may retain the mitochondrial membrane integrity and normal Δψm. Impairment or downregulation of mitochondrial uncoupling proteins may also result in low ROS but high Δψm levels, which sustain the malignancy of CSCs.60 The Δψm is also regarded as a prerequisite factor for ESCs to differentiate or initiate teratoma formation.65 ESCs with a high Δψm tend to develop teratoma, while a decrease of the Δψm in ESCs is able to stagnate tumor growth.65
In essence, it seems that the CSCs are equipped with an extraordinary bioenergetic machinery to cope with oxidative stress, hypoxia, and challenge of the microenvironment. This dynamic and multidimensional Warburg effect of CSCs compared with differentiated tumor counterparts is specifically referred to as “the Warburg effect 2.0” to signify the upgraded and pellucid version of CSC pathophysiology regarding the CSC heterogeneity across tissue-specific variations, distinct histologic tumor subtypes, as well as the CSCs niche.66
Metabolic regulators wheel CSC properties
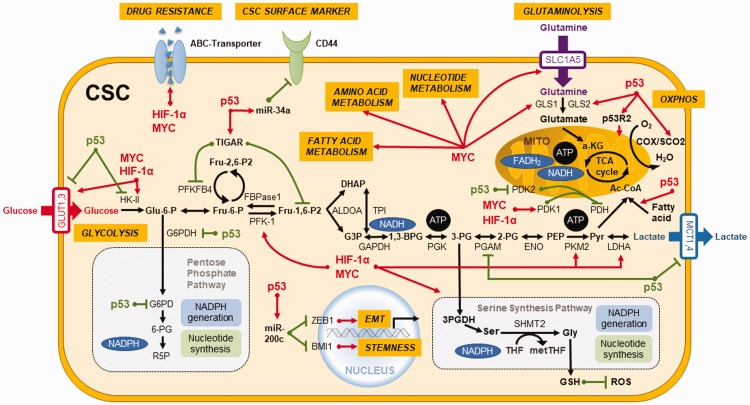
Accumulating evidence reveals that pluripotency transcription factors such as NANOG, MEIS1, Wnt, or OCT4 are inextricably intertwined with metabolic regulators such as MYC, p53, K-Ras, and HIF1α, and their link with metabolic reprogramming dictates the stemness properties of CSCs.67,68 Certain metabolic signatures of CSCs act as a coordinator of stemness to perform several intricate functions in sustaining the stemness characteristics. Therefore, we highlight the therapeutic potential of these critical metabolic regulators that may govern the metabolic plasticity and redox homeostasis in CSCs (Figure 2).68
Figure 2.
Key regulators orchestrate metabolic plasticity and stemness features in CSCs. Critical transcription factors such as MYC, HIF-1α, and p53 not only govern metabolic plasticity and redox homeostasis in CSCs but also serve as coordinators in regulating the stemness traits and differentiation capabilities of CSCs. Activation is represented by red lines and suppression is denoted by green lines. (A color version of this figure is available in the online journal.)
p53
TP53 is an extensively studied tumor suppressor gene in oncology research.69 Occurrence of p53 mutations represents an early event in more than 50% of human malignancies.70 p53 plays a crucial role in regulating glycolytic pathway through TIGAR (TP53-induced glycolysis and apoptosis regulator) and phosphoglycerate mutase (PGM) to suppress glycolytic enzymes and downregulate the transcriptional levels of glucose transporter 1 (GLUT1) and GLUT4 (Figure 2).71 Additionally, p53 upregulates mitochondrial metabolism by enhancing the biosynthesis of synthesis of cytochrome c oxidase 2 (SCO2) and glutaminase 2 (GLS2) (Figure 2).37,72 Also, p53 abrogates the biosynthesis of nucleotides and lipids in tumor cells by attenuating the PPP (Figure 2).73 Moreover, p53 reduces the biosynthesis of fatty acids but boosts FAO, thereby promoting OXPHOS by the increase of metabolic products of FAO in mitochondria of cancer cells (Figure 2).74 In 2007, it was reported that adult human fibroblasts can be reprogrammed into ESC-like induced pluripotent stem cells (iPSCs) by overexpressing defined factors.75 Upregulation of glycolytic genes was unveiled to precede the expression of stemness markers in the reprogramming process.76 In addition, p53 pathway attenuates the reprogramming efficiency of somatic cells to iPSCs77 and CSCs.78 The absence of p53 imparts the resistance of colon CSCs to paclitaxel because of increased autophagy and decreased apoptosis.79 Moreover, p53 was found to upregulate miR-34a to dampen the expression of CD44, which is a CSC surface marker that participates in the metastasis of CSCs.80 In addition, p53 is able to upregulate miR-200c to reverse the stemness and EMT of CSCs (Figure 2).81 We demonstrated that the reactivation of p53 by resveratrol could impede the stemness, EMT process, and metabolic remodeling of CSCs in nasopharyngeal carcinoma.43 Taken together, p53 may be considered as an important target for CSCs-targeting therapy.
MYC
MYC regulates cancer metabolism and redox balance by boosting glycolysis82 and glutaminolysis by upregulation of glutamine transporters and mitochondrial GLS through microRNAs (Figure 2).83,84 Glutamine fuels ATP production and intermediates in the biosynthesis of amino acids, nucleic acids, fatty acids, and glutathione, which bears powerful antioxidant and other biological activities.85 In line with the findings in iPSCs, overexpression of MYC acts as a bridge between glycolysis and stemness in cancers.67 MYC upregulates stemness and differentiation, as well as signaling pathways that result in the chemotherapy resistance of CSCs.67,86 Silencing of c-MYC was found to re-sensitize colon CSCs to chemotherapeutic agents through down-regulation of ABC transporters (Figure 2).87 In CSCs, metabolic reprogramming driven by MYC is linked to CD44 variant isoforms (CD44v)-mediated redox homeostasis, tumor suppressor FBW7-driven c-MYC degradation, and ubiquitination, which are associated with chemoresistance.88
HIF
HIF-1α and HIF-2α have a pivotal role in cancer cell progression for CSCs to trigger the metabolic reprogramming from an oxidative to a glycolytic phenotype to cope with low levels of oxygen, pH, and nutrients in the stringent tumor microenvironment (Figure 2).89 HIF-1α regulates cancer migration, angiogenesis, and cell survival pathways,90 and it also upregulates carbonic anhydrases for controlling pH gradient between intracellular and extracellular environments.91,92 This pH shift affects the drug absorption and metabolism and suppresses cytoplasmic retention of anticancer drugs.93 Overexpression of HIF in CSCs enhances cancer progression through upregulation of PKM2 (pyruvate kinase muscle isozyme), ABC transporters (Figure 2), vascular endothelial growth factor, angiogenesis, recruitment of tumor-associated macrophages, and CD8+/− T cells, as well as attenuation of natural killer cells.94–96
Other metabolic regulators
Aside from a rapid ATP generation, glycolytic flux can supply metabolites to furnish PPP to produce NADPH and biosynthetic building blocks to support anabolic processes and protect CSCs from oxidative stress.97 In breast CSCs, glucose transporters GLUT1 and GLUT4 and key glycolytic enzymes including HK (hexokinase), PKM2, and lactate dehydrogenase A (LDHA) control the central pathway of glucose catabolism and display increased activities. However, treatment with 2-DG (2-deoxyglucose), a glucose analogue that suppresses HK2, preferentially interferes with the viability of CSCs compared with differentiated tumor counterparts, demonstrating the crucial roles of glycolysis in the maintenance and growth of breast CSCs.98 Additionally, PKM2 and PFKFB4 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4) were corroborated to be critical stemness regulators in glioma stem cells.99 The interaction of nuclear PKM2 and OCT4 can upregulate stemness genes to enrich the subset of CSCs under metabolic stress and thereby promote cancer metastasis.100 LDHA generates lactate to adjust pH and direct USP28-mediated de-ubiquitination and stabilization of MYC and activation of SLUG promoter, which endows breast cancer cells with stemness features.101 Inhibition of G6PD, the gatekeeper of the PPP, decreased spherogenesis and the ALDH activity in breast CSCs.32 Besides, phosphorylation of Bcl-2 associated death promotor (BAD) by kinases like Akt activates glucokinase to promote glycolysis and support the growth and proliferation of CSCs.2 Dephosphorylated BAD directs cell death and dampens the metabolic pathways required for an elevated glycolysis to confer survival advantage of CSCs.102
Metabolism-based therapy fires arrow at Achilles’ heel of CSCs
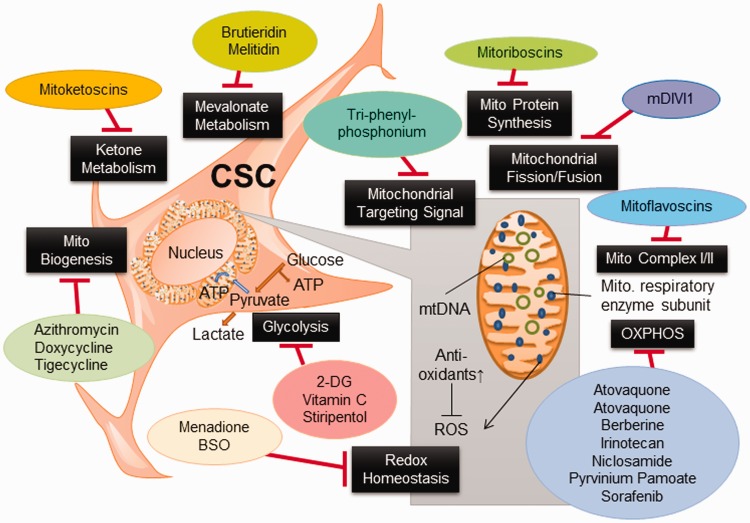
Through collective efforts dedicated to the development of new anticancer drugs targeting the metabolism of cancer cells, a variety of metabolism-based drugs have been tested in preclinical and clinical studies (Figure 3 and Table 2). Glucose deprivation by 3-bromopyruvate (3-BrPA) and analog 3-BrOP induced the in vitro elimination of CSCs and compromised in vivo tumorigenicity.17,103 2-DG could competitively inhibit glycolysis and block the generation of glucose 6-phosphate and inhibit HK2, and preferentially suppress the propagation of CSCs.16 Pyruvate dehydrogenase kinase (PDK) inhibitor such as dichloroacetic acid (DCA) can induce the formation of PKM2/OCT4 complex and attenuate the transcriptional activity of OCT4, which modulates the apoptosis in glioma stem cells.99 Vitamin C suppresses glycolysis104 and miR-302/367 to compromise the reprogramming of breast CSCs through downregulation of TET1 gene.105 It also inhibits stress-induced epinephrine-activated LDHA, and thereby decreases the lactate generation to dampen the USP28/MYC/SLUG pathway in CSCs.101
Figure 3.
Metabolism-based therapy compromises CSC properties. Metabolism-based therapeutic strategies targeting pivotal metabolic pathways that can be exploited in preclinical and clinical settings. Specific metabolic inhibitors that can overwhelm stem cell properties may halt disease recurrence, attenuate CSC dissemination, and dampen the capacity of spawning metastasis of cancer cells. (A color version of this figure is available in the online journal.)
Table 2.
Metabolic inhibitors in different metabolic phenotypes.
| Metabolic phenotype | Target | Metabolic inhibitor |
|---|---|---|
| Glycolysis | Glucose transporter 1 (GLUT1) | WZB117 |
| Hexokinase 2 (HK2) | Dihydronaringenin phloretol | |
| 2-Deoxyglucose (2-DG) | ||
| 3-Bromopyruvate (3-BrPA) | ||
| Lonidamine (LND) | ||
| Pyruvate dehydrogenase kinase (PDK) | Dichloroacetic acid (DCA) | |
| Lactate dehydrogenase A (LDHA) | FX11 and Oxamate | |
| Pyruvate kinase (PKM) | TLN232 | |
| Monocarboxylate transporters (MCT) | SR13800 and SR13801 | |
| Hypoxia inducible factor 1α (HIF-1α) | Echinomycin, Topotecan (Hycamtin), Digoxin, and PX-478 | |
| Autophagy | Hydroxychloroquine (HCQ) | |
| N-glycosylation | 2-Deoxyglucose (2-DG) | |
| ATP synthase | Vitamin C | |
| GABAergic activity | Stiripentol | |
| OXPHOS | PDH and α-keto dehydrogenase | CPI-613 (devimistat) |
| Mitochondrial complex I | Metformin | |
| Mitochondrial division inhibitor 1 | Mdivi-1 | |
| 3-Hydroxy-3-methylglutaryl-CoA-reductase (HMG-CoA reductase) | Brutieridin | |
| Melitidin | ||
| Mito-ribosome | Mitoriboscins | |
| OXCT1/ACAT1 | Mitoketoscins | |
| Mitochondrial complex I/II | Mitoflavoscins | |
| Mitochondria | Decyl (triphenyl) phosphonium (DTPP) | |
| Dihydroorotate dehydrogenase (quinone), mitochondrial | Atovaquone | |
| DNA replication and transcription | Irinotecan (Campto) | |
| Vascular endothelial growth factor | Sorafenib | |
| Glucose uptake | Niclosamide | |
| Mitochondrial biogenesis | Doxycycline (Doxymycin capsule) | |
| Tigecycline | ||
| Azithromycin (Zithromax) | ||
| OXPHOS/complex II | Pyrvinium pamoate | |
| OXPHOS/complex III | Atovaquone | |
| Mitochondrial Complex V | Bedaquiline | |
| Cyclin-dependent kinase 4/6 (CDK4/6) | Palbociclib | |
| Lipogenesis | Fatty acid synthase (FASN) | Cerulenin, C75 and Orlistat |
| Stearoyl-CoA desaturase 1 (SCD1) | A37062 | |
| Glutaminolysis | Glutaminase | BPTES and CB-839 |
| Transaminase | Amino-oxyacetic acid (AOA) | |
| SLC1A5 | GPNA |
OXPHOS: oxidative phosphorylation.
As increased mitochondrial biogenesis and OXPHOS are observed in certain types of CSCs, mitochondrial inhibitors such as oligomycin, rotenone, antimycin A, metformin, or phenformin can lead to apoptosis of CSCs.106 FAO inhibitor etomoxir can suppress CPT1 (carnitine palmitoyl-transferase 1) and re-sensitize CSCs to cytotoxic agents.106 Based on the “endosymbiotic theory,” mitochondria in eukaryotic cells were initially derived from endocytosis of aerobic bacteria. Consequently, mitochondria still share some characteristics similar to bacteria, explaining the potential use of some FDA-approved antibiotics to target at mitochondria.107 These antibiotics such as doxycycline and azithromycin were reported to impede the spherogenesis of CSCs through inhibition of mitochondrial function (Table 2).107
Combination therapies encompassing conventional chemotherapies and chemo-sensitizing agents would be the most effective way to enhance the efficacy of CSCs-targeted therapy. 5-fluorouracil (5-FU)-refractory cells showed increased levels of PKM2 and acquisition of stemness in colon cancer. Co-treatment of 5-FU and metformin was found to dampen the respiratory chain Complex I activity, abolish spherogenesis of colon CSCs, and diminish the stemness markers.108 Doxycycline resistant-CSCs are relatively more sensitive to metabolism-based drugs including OXPHOS inhibitors including atovaquone and irinotecan, glycolysis inhibitors such as vitamin C and stiripentol, as well as an autophagy inhibitor chloroquine (Table 2).107 Emerging mitochondrial inhibitors “mitoketoscins” with special focus on ketone metabolism mimic the structure of coenzyme A, which functionally inhibit the activity of CSCs through binding to OXCT1 and ACAT1 catalytic sites within the binding sites of coenzyme A (Table 2).109 In addition, diphenyleneiodonium chloride (DPI) halts mitochondrial respiration through the suppression of flavin-dependent enzymes, which constitute respiratory chain complexes I and II. Accordingly, DPI-induced chemoquiescence significantly reduces CSC subpopulation.33 Moreover, a mitochondria-targeting compound, tri-phenyl phosphonium (TPP), can selectively annihilate both bulk tumor mass and CSCs but spare normal fibroblasts (Table 2).110 TPP seems to be able to distinguish metabolically the mitochondria in normal cells from those in malignant cells because bulk tumor mass and CSCs likely have mitochondria with a higher Δψm.111 Besides, targeting DRP1 protein by mDIVI1 compromised the mitochondrial fission–fusion cycles and mitochondrial function, cell migration, and CSC signaling (Table 2).111 Naturally occurring mitochondrial inhibitors, Brutieridin and Melitidin, can suppress mevolonate metabolism to inhibit the propagation of CSCs (Table 2).112 GLS1 inhibitors such as 968 or BPTES can attenuate CSC properties in hepatocellular carcinoma via increased ROS levels and impaired Wnt/β-catenin signaling (Table 2).113 Glutaminase inhibitors, including Zaprinast (an anti-asthma drug) and BPTES, were able to effectively compromise the stemness and sensitize pancreatic CSCs to radiotherapy and enhance apoptosis in vitro and in vivo caused by redox imbalance.48
Glycolysis and OXPHOS are two main metabolic engines in CSCs, which are not necessarily mutually exclusive or disjointed. It is worth mentioning that some CSCs manifest metabolic plasticity by switching to glycolytic phenotype when respiration pathway is blocked.28,114,115 In addition, the metabolic compensation with OXPHOS or other microenvironmental nutrient supply in CSCs may also render their resistance to inhibition of glycolysis.13 Sequential or combinational treatment of two or more metabolic inhibitors would block the development of drug resistance and completely eradicate CSCs. For instance, “two metabolic hit strategy” proposes that the utilization of mitochondria-interfering agent like doxycycline drastically reverses the metabolism of CSCs toward an inflexible glycolytic pathway, rendering a second metabolic hit that completely halts the biochemical machinery of CSCs.116 Metformin combined with either bromodomain and extraterminal motif (BET) or inhibitor JQ-1 for the treatment of pancreatic cancer or PI3K inhibitor for treating ovarian cancer can simultaneously halt OXPHOS and indirectly suppress glycolysis.20,117 Furthermore, CSCs do not become resistant to drugs upon administration of a mitochondrial ROS inducer menadione, implying that accumulated ROS levels to a toxic level may be an alternative strategy to increase the effectiveness of anticancer therapy to eradicate CSCs.20 As a consequence, blocking glutathione synthesis by buthionine sulfoximine could disturb the redox homeostasis in CSCs, and subsequently decrease the clonogenicity and facilitating the radiotherapy responses of cancer patients.9,118
Because of the fact that some metabolic features of CSCs are common to those of normal stem cells, an accurate distinction between them is awaited. Once these metabolic differences can be identified, a novel therapy is able to be established to kill only CSCs but spare tissue stem cells. In such a scenario, simultaneous or sequential block of glycolytic flux and OXPHOS may be a new treatment to concurrently eliminate glycolytic and oxidative CSCs. These metabolic targets expose unrecognized Achilles’ heel in CSCs amendable for therapeutic intervention to prevent recurrence and thereby achieve long-term remission in cancer patients.
Future perspectives
CSCs may choose either glycolysis or OXPHOS as the major metabolic engine in response to environmental factors including tumor size, hypoxia, and the sequence of activation of oncogenes. The metabolic plasticity of the CSC subpopulation can be one of the major obstacles to eradicate CSCs. Therefore, a comprehensive portrait of the plasticity still awaits to be deciphered in CSCs. Instead of dwelling in a solitary place, CSCs reside in a diversified ecosystem encircled by extracellular matrix, immune cells, endothelial cells, and CAFs. Given that CSC–stromal interactions are quintessential for determining treatment response, limitations pertaining to the differences of tumor microenvironment can be minimized by devising better niche-targeting strategies. Thorough scrutiny of the singularities of mitostemness can provide clues to tackle CSCs by metabolic intervention. We envision that metabolic therapeutic interventions would eventually be practiced as an add-on to standard cytotoxic regimens, especially in halting cancer recurrence and metastasis to achieve more effective long-term disease remission. Lastly, discovering the metabolic distinction between CSCs and tissue stem cells is a prerequisite to develop new therapies that can aim at metabolically distinct CSCs but spare the normal tissue cells.
Authors’ contributions
YAS: conception and design, article writing. SCP: conception and design, first draft of article writing. IC and RYL: first draft of article writing. YHW: conception and design, article writing and revision, and administrative support.
DECLARATION OF CONFLICTING INTERESTS
The author(s) have declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review has been prepared on the basis of the results obtained from research projects sponsored by grants (MOST104-2314-B-715–003-MY3, MOST104-2627-M-715–002, MOST104-2320-B-715–006-MY2, MOST105-2627-M-715–001, MOST106-2627-M-371–001, MOST106-2320-B-371–002, and MOST107-2320-B-371–002) from the Ministry of Science and Technology, Taiwan. The work in the laboratory of YA Shen was supported by a TMU grant 108–5403-003–112. The research work carried out in the laboratory of YH Wei that are included in this article have been supported by the above-mentioned grants and partly supported by intramural grants (Nos. 107-CCH-NPI-052 and No. 108-CCH-IST-149) from Changhua Christian Hospital, Changhua City, Taiwan.
ORCID iD
Yau-Huei Wei https://orcid.org/0000-0002-6429-2546
References
- 1.Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014; 35:515–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. Cancer stem cell metabolism: a potential target for cancer therapy. Mol Cancer 2016; 15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman BJ, Stine ZE, Dang CV. From krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 2016; 16:619–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marijt KA, Sluijter M, Blijleven L, Tolmeijer SH, Scheeren FA, van der Burg SH, van Hall T. Metabolic stress in cancer cells induces immune escape through a PI3K-dependent blockade of IFNγ receptor signaling. J Immunother Cancer 2019; 7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414:105–11 [DOI] [PubMed] [Google Scholar]
- 6.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1:313–23 [DOI] [PubMed] [Google Scholar]
- 7.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol 2007; 23:675–99 [DOI] [PubMed] [Google Scholar]
- 8.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res 2006; 312:3701–10 [DOI] [PubMed] [Google Scholar]
- 9.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009; 458:780–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbaszadegan MR, Bagheri V, Razavi MS, Momtazi AA, Sahebkar A, Gholamin M. Isolation, identification, and characterization of cancer stem cells: a review. J Cell Physiol 2017; 232:2008–18 [DOI] [PubMed] [Google Scholar]
- 11.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells – what challenges do they pose? Nat Rev Drug Discov 2014; 13:497–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae YC, Kim JH. Cancer stem cell metabolism: target for cancer therapy. BMB Rep 2018; 51:319–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer 2016; 114:1305–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem 2014; 115:368–79 [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh A, Arfuso F, Newsholme P, Dharmarajan A. Regulation of cancer stem cell metabolism by secreted frizzled-related protein 4 (sFRP4). Cancers 2018; 10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle 2015; 14:86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH, Huang P. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ 2014; 21:124–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, Wu T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget 2015; 6:40822–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V, Radovanovic I, Rheinbay E, Provero P, Stamenkovic I. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev 2012; 26:1926–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Grana O, Viera CR, Yuneva M, Sainz B, Jr, Heeschen C. MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 2015; 22:590–605 [DOI] [PubMed] [Google Scholar]
- 21.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013; 12:329–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014; 514:628–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gammon L, Biddle A, Heywood HK, Johannessen AC, Mackenzie IC. Sub-sets of cancer stem cells differ intrinsically in their patterns of oxygen metabolism. PLoS One 2013; 8:e62493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malchenko S, Sredni ST, Boyineni J, Bi Y, Margaryan NV, Guda MR, Kostenko Y, Tomita T, Davuluri RV, Velpula K, Hendrix MJC, Soares MB. Characterization of brain tumor initiating cells isolated from an animal model of CNS primitive neuroectodermal tumors. Oncotarget 2018; 9:13733–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Yang K, Xie Q, Wu Q, Mack SC, Shi Y, Kim LJY, Prager BC, Flavahan WA, Liu X, Singer M, Hubert CG, Miller TE, Zhou W, Huang Z, Fang X, Regev A, Suva ML, Hwang TH, Locasale JW, Bao S, Rich JN. Purine synthesis promotes maintenance of brain tumor initiating cells in glioma. Nat Neurosci 2017; 20:661–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb R, Fiorillo M, Chadwick A, Ozsvari B, Reeves KJ, Smith DL, Clarke RB, Howell SJ, Cappello AR, Martinez-Outschoorn UE, Peiris-Pages M, Sotgia F, Lisanti MP. Doxycycline down-regulates DNA-PK and radiosensitizes tumor initiating cells: implications for more effective radiation therapy. Oncotarget 2015; 6:14005–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kathagen A, Schulte A, Balcke G, Phillips HS, Martens T, Matschke J, Gunther HS, Soriano R, Modrusan Z, Sandmann T, Kuhl C, Tissier A, Holz M, Krawinkel LA, Glatzel M, Westphal M, Lamszus K. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol 2013; 126:763–80 [DOI] [PubMed] [Google Scholar]
- 28.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013; 23:316–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010; 9:3506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009; 461:109–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Francesco EM, Maggiolini M, Tanowitz HB, Sotgia F, Lisanti MP. Targeting hypoxic cancer stem cells (CSCs) with doxycycline: implications for optimizing anti-angiogenic therapy. Oncotarget 2017; 8:56126–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debeb BG, Lacerda L, Larson R, Wolfe AR, Krishnamurthy S, Reuben JM, Ueno NT, Gilcrease M, Woodward WA. Histone deacetylase inhibitor-induced cancer stem cells exhibit high pentose phosphate pathway metabolism. Oncotarget 2016; 7:28329–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozsvari B, Bonuccelli G, Sanchez-Alvarez R, Foster R, Sotgia F, Lisanti MP. Targeting flavin-containing enzymes eliminates cancer stem cells (CSCs), by inhibiting mitochondrial respiration: vitamin B2 (riboflavin) in cancer therapy. Aging (Albany NY) 2017; 9:2610–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Takahashi J, Eguchi S, Yamashita A, Tomio K, Wada-Hiraike O, Oda K, Nagamatsu T, Osuga Y, Fujii T. Spheroid cancer stem cells display reprogrammed metabolism and obtain energy by actively running the tricarboxylic acid (TCA) cycle. Oncotarget 2016; 7:33297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen KY, Liu X, Bu P, Lin CS, Rakhilin N, Locasale JW, Shen X. A metabolic signature of colon cancer initiating cells. Conf Proc IEEE Eng Med Biol Soc 2014; 2014:4759–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Wei D, Gao W, Xu Y, Hu Z, Ma Z, Gao C, Zhu X, Li Q. TPO-induced metabolic reprogramming drives liver metastasis of colorectal cancer CD110+ tumor-initiating cells. Cell Stem Cell 2015; 17:47–59 [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, Sugano S, Sato E, Nagao T, Yokote K, Tatsuno I, Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A 2010; 107:7461–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A 2010; 107:7455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, Hess S, Machida K. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab 2016; 23:206–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, Andreeff M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest 2010; 120:142–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML-PPAR-Delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med 2012; 18:1350–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW, Yu SC, Qian GS. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer 2011; 129:820–31 [DOI] [PubMed] [Google Scholar]
- 43.Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, Chen YJ. Resveratrol impedes the stemness, epithelial-mesenchymal transition, and metabolic reprogramming of cancer stem cells in nasopharyngeal carcinoma through p53 activation. Evid Based Complement Alternat Med 2013; 2013:590393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson AS, Roberts PC, Frisard MI, Hulver MW, Schmelz EM. Ovarian tumor-initiating cells display a flexible metabolism. Exp Cell Res 2014; 328:44–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One 2014; 9:e84941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasto A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, Nicoletto MO, Manicone M, Indraccolo S, Amadori A. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget 2014; 5:4305–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao J, Liu PP, Hou G, Shao J, Yang J, Liu K, Lu W, Wen S, Hu Y, Huang P. Regulation of stem-like cancer cells by glutamine through beta-catenin pathway mediated by redox signaling. Mol Cancer 2017; 16:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Fu Z, Chen R, Zhao X, Zhou Y, Zeng B, Yu M, Zhou Q, Lin Q, Gao W, Ye H, Zhou J, Li Z, Liu Y, Chen R. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget 2015; 6:31151–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caria P, Tronci L, Dettori T, Murgia F, Santoru ML, Griffin JL, Vanni R, Atzori L. Metabolomic alterations in thyrospheres and adherent parental cells in papillary thyroid carcinoma cell lines: a pilot study. Int J Mol Sci 2018; 19:2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vega-Naredo I, Loureiro R, Mesquita KA, Barbosa IA, Tavares LC, Branco AF, Erickson JR, Holy J, Perkins EL, Carvalho RA, Oliveira PJ. Mitochondrial metabolism directs stemness and differentiation in P19 embryonal carcinoma stem cells. Cell Death Differ 2014; 21:1560–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon N, Skinner AM, Pommier RF, Schillace RV, O’Neill S, Peckham JL, Muller P, Condron ME, Donovan C, Naik A, Hansen J, Pommier SJ. Gene expression signatures of breast cancer stem and progenitor cells do not exhibit features of Warburg metabolism. Stem Cell Res Ther 2015; 6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuyas E, Corominas-Faja B, Menendez JA. The nutritional phenome of EMT-induced cancer stem-like cells. Oncotarget 2014; 5:3970–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med 2013; 11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, Pesdar EA, Sobol M, Filimonenko A, Stuart S, Vondrusova M, Kluckova K, Sachaphibulkij K, Rohlena J, Hozak P, Truksa J, Eccles D, Haupt LM, Griffiths LR, Neuzil J, Berridge MV. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab 2015; 21:81–94 [DOI] [PubMed] [Google Scholar]
- 55.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafe M, Cricca M, Gasparre G, Lyden D, Bromberg J. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A 2017; 114:e9066–e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn 1996; 205:64–72 [DOI] [PubMed] [Google Scholar]
- 57.Lonergan T, Bavister B, Brenner C. Mitochondria in stem cells. Mitochondrion 2007; 7:289–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee BWL, Ghode P, Ong D. Redox regulation of cell state and fate. Redox Biol 2018; 25:101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dayem AA, Choi HY, Kim JH, Cho SG. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers (Basel) 2010; 2:859–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye XQ, Wang GH, Huang GJ, Bian XW, Qian GS, Yu SC. Heterogeneity of mitochondrial membrane potential: a novel tool to isolate and identify cancer stem cells from a tumor mass? Stem Cell Rev Rep 2011; 7:153–60 [DOI] [PubMed] [Google Scholar]
- 61.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 2008; 10:1883–94 [DOI] [PubMed] [Google Scholar]
- 62.Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, von Zglinicki T, Lako M. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells 2008; 26:455–64 [DOI] [PubMed] [Google Scholar]
- 63.Crespo FL, Sobrado VR, Gomez L, Cervera AM, McCreath KJ. Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells 2010; 28:1132–42 [DOI] [PubMed] [Google Scholar]
- 64.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 2008; 18:165–73 [DOI] [PubMed] [Google Scholar]
- 65.Schieke SM, Ma M, Cao L, McCoy JP, Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem 2008; 283:28506–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menendez JA, Joven J, Cufí S, Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Martin-Castillo B, López-Bonet E, Alarcón T, Vazquez-Martin A. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle 2013; 12:1166–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med 2014; 4:a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jagust P, de Luxan-Delgado B, Parejo-Alonso B, Sancho P. Metabolism-based therapeutic strategies targeting cancer stem cells. Front Pharmacol 2019; 10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell 2009; 137:413–31 [DOI] [PubMed] [Google Scholar]
- 70.Perri F, Pisconti S, Della Vittoria Scarpati G. P53 mutations and cancer: a tight linkage. Ann Transl Med 2016; 4:522–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Y, Liu J, Feng Z. The regulation of cellular metabolism by tumor suppressor p53. Cell Biosci 2013; 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wanka C, Brucker DP, Bahr O, Ronellenfitsch M, Weller M, Steinbach JP, Rieger J. Synthesis of cytochrome C oxidase 2: a p53-dependent metabolic regulator that promotes respiratory function and protects glioma and colon cancer cells from hypoxia-induced cell death. Oncogene 2012; 31:3764–76 [DOI] [PubMed] [Google Scholar]
- 73.Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 2011; 13:310–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simabuco FM, Morale MG, Pavan ICB, Morelli AP, Silva FR, Tamura RE. p53 and metabolism: from mechanism to therapeutics. Oncotarget 2018; 9:23780–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–72 [DOI] [PubMed] [Google Scholar]
- 76.Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 2011; 14:264–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 2009; 460:1132–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shetzer Y, Solomon H, Koifman G, Molchadsky A, Horesh S, Rotter V. The paradigm of mutant p53-expressing cancer stem cells and drug resistance. Carcinogenesis 2014; 35:1196–208 [DOI] [PubMed] [Google Scholar]
- 79.Wu S, Wang X, Chen J, Chen Y. Autophagy of cancer stem cells is involved with chemoresistance of Colon cancer cells. Biochem Biophys Res Commun 2013; 434:898–903 [DOI] [PubMed] [Google Scholar]
- 80.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17:211–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, Liu M, Chen CT, Yu D, Hung MC. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 2011; 13:317–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massihnia D, Avan A, Funel N, Maftouh M, van Krieken A, Granchi C, Raktoe R, Boggi U, Aicher B, Minutolo F, Russo A, Leon LG, Peters GJ, Giovannetti E. Phospho-Akt overexpression is prognostic and can be used to tailor the synergistic interaction of akt inhibitors with gemcitabine in pancreatic cancer. J Hematol Oncol 2017; 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 2008; 105:18782–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458:762–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi Y-K, Park K-G. Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul) 2018; 26:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elbadawy M, Usui T, Yamawaki H, Sasaki K. Emerging roles of c-Myc in cancer stem cell-related signaling and resistance to cancer chemotherapy: a potential therapeutic target against colorectal cancer. Int J Mol Sci 2019; 20:2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H-L, Wang P, Lu M-Z, Zhang S-D, Zheng L. c-Myc maintains the self-renewal and chemoresistance properties of colon cancer stem cells. Oncol Lett 2019; 17:4487–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida GJ, Saya H. Inversed relationship between CD44 variant and c-Myc due to oxidative stress-induced canonical Wnt activation. Biochem Biophys Res Commun 2014; 443:622–7 [DOI] [PubMed] [Google Scholar]
- 89.Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer 2010; 102:789–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3:721–32 [DOI] [PubMed] [Google Scholar]
- 91.Sawayama H, Ishimoto T, Sugihara H, Miyanari N, Miyamoto Y, Baba Y, Yoshida N, Baba H. Clinical impact of the Warburg effect in gastrointestinal cancer (review). Int J Oncol 2014; 45:1345–54 [DOI] [PubMed] [Google Scholar]
- 92.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 2002; 62:3387–94 [PubMed] [Google Scholar]
- 93.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker BCRP/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 2004; 279:24218–25 [DOI] [PubMed] [Google Scholar]
- 94.Peng G, Liu Y. Hypoxia-inducible factors in cancer stem cells and inflammation. Trends Pharmacol Sci 2015; 36:374–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uribe D, Torres A, Rocha JD, Niechi I, Oyarzun C, Sobrevia L, San Martin R, Quezada C. Multidrug resistance in glioblastoma stem-like cells: role of the hypoxic microenvironment and adenosine signaling. Mol Aspects Med 2017; 55:140–51 [DOI] [PubMed] [Google Scholar]
- 97.Wong TL, Che N, Ma S. Reprogramming of Central carbon metabolism in cancer stem cells. Biochim Biophys Acta Mol Basis Dis 2017; 1863:1728–38 [DOI] [PubMed] [Google Scholar]
- 98.Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D’Agostino D, Forlì F, D’Aguanno S, Todaro M, Stassi G, Di Ilio C, De Laurenzi V, Urbani A. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis 2014; 5:e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morfouace M, Lalier L, Oliver L, Cheray M, Pecqueur C, Cartron PF, Vallette FM. Control of glioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis 2014; 5:e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang YC, Chien MH, Liu HY, Chang YC, Chen CK, Lee WJ, Kuo TC, Hsiao M, Hua KT, Cheng TY. Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett 2018; 421:28–40 [DOI] [PubMed] [Google Scholar]
- 101.Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, Su Q, Liu B, Yu J, Luo X, Yin L, Cheng W, An F, He B, Liang D, Wu S, Chu P, Song L, Liu X, Luo H, Xu J, Pan Y, Wang Y, Li D, Huang P, Yang Q, Zhang L, Zhou BP, Liu S, Xu G, Lam EWF, Kelley KW, Liu Q. Stress-induced epinephrine enhances lactate dehydrogenase a and promotes breast cancer stem-like cells. J Clin Invest 2019; 129:1030–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sastry KS, Al-Muftah MA, Li P, Al-Kowari MK, Wang E, Ismail Chouchane A, Kizhakayil D, Kulik G, Marincola FM, Haoudi A, Chouchane L. Targeting proapoptotic protein BAD inhibits survival and self-renewal of cancer stem cells. Cell Death Differ 2014; 21:1936–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Attia YM, El-Abhar HS, Al Marzabani MM, Shouman SA. Targeting glycolysis by 3-bromopyruvate improves tamoxifen cytotoxicity of breast cancer cell lines. BMC Cancer 2015; 15:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Francesco EM, Bonuccelli G, Maggiolini M, Sotgia F, Lisanti MP. Vitamin C and doxycycline: a synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017; 8:67269–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramezankhani B, Taha MF, Javeri A. Vitamin C counteracts miR-302/367-induced reprogramming of human breast cancer cells and restores their invasive and proliferative capacity. J Cell Physiol 2019; 234:2672–82 [DOI] [PubMed] [Google Scholar]
- 106.Peixoto J, Lima J. Metabolic traits of cancer stem cells. Dis Model Mech 2018; 11:112–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sotgia F, Ozsvari B, Fiorillo M, De Francesco EM, Bonuccelli G, Lisanti MP. A mitochondrial based oncology platform for targeting cancer stem cells (CSCs): MITO-ONC-RX. Cell Cycle 2018; 17:2091–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denise C, Paoli P, Calvani M, Taddei ML, Giannoni E, Kopetz S, Kazmi SMA, Pia MM, Pettazzoni P, Sacco E, Caselli A, Vanoni M, Landriscina M, Cirri P, Chiarugi P. 5-fluorouracil resistant Colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget 2015; 6:41706–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ozsvari B, Sotgia F, Simmons K, Trowbridge R, Foster R, Lisanti MP. Mitoketoscins: novel mitochondrial inhibitors for targeting ketone metabolism in cancer stem cells (CSCs). Oncotarget 2017; 8:78340–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozsvari B, Sotgia F, Lisanti MP. Exploiting mitochondrial targeting signal(s), TPP and bis-TPP, for eradicating cancer stem cells (CSCs). Aging (Albany NY) 2018; 10:229–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peiris-Pagès M, Bonuccelli G, Sotgia F, Lisanti MP. Mitochondrial fission as a driver of stemness in tumor cells: mDIVI1 inhibits mitochondrial function, cell migration and cancer stem cell (CSC) signalling. Oncotarget 2018; 9:13254–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fiorillo M, Peiris-Pages M, Sanchez-Alvarez R, Bartella L, Di Donna L, Dolce V, Sindona G, Sotgia F, Cappello AR, Lisanti MP. Bergamot natural products eradicate cancer stem cells (CSCs) by targeting mevalonate, Rho-GDI-signalling and mitochondrial metabolism. Biochim Biophys Acta Bioenerg 2018; 1859:984–96 [DOI] [PubMed] [Google Scholar]
- 113.Li B, Cao Y, Meng G, Qian L, Xu T, Yan C, Luo O, Wang S, Wei J, Ding Y, Yu D. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine 2019; 39:239–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, Reue K, Christofk H, Mischel PS, Pajonk F. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci U S A 2011; 108:16062–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, Weil RJ, Nakano I, Sarkaria JN, Stringer BW, Day BW, Li M, Lathia JD, Rich JN, Hjelmeland AB. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci 2013; 16:1373–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Francesco EM, Sotgia F, Lisanti MP. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem J 2018; 475:1611–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li C, Liu VW, Chan DW, Yao KM, Ngan HY. LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells. Int J Gynecol Cancer 2012; 22:15–22 [DOI] [PubMed] [Google Scholar]
- 118.Rodman SN, Spence JM, Ronnfeldt TJ, Zhu Y, Solst SR, O’Neill RA, Allen BG, Guan X, Spitz DR, Fath MA. Enhancement of radiation response in breast cancer stem cells by inhibition of thioredoxin- and glutathione-dependent metabolism. Radiat Res 2016; 186:385–95 [DOI] [PMC free article] [PubMed] [Google Scholar]