Short abstract
While the cortical representation of sensory stimuli is well described for some sensory systems, a clear understanding of the cortical representation of taste stimuli remains elusive. Recent investigations have focused on both spatial and temporal organization of taste responses in the putative taste region of insular cortex. This review highlights recent literature focused on spatiotemporal coding strategies in insular cortex. These studies are examined in the context of the organization and function of the entire insular cortex, rather than a specific gustatory region of insular cortex. In regard to a taste quality-specific map, imaging studies have reported conflicting results, whereas electrophysiology studies have described a broad distribution of taste-responsive neurons found throughout insular cortex with no spatial organization. The current collection of evidence suggests that insular cortex may be organized into a hedonic or “viscerotopic” map, rather than one ordered according to taste quality. Further, it has been proposed that cortical taste responses can be separated into temporal “epochs” representing stimulus identity and palatability. This coding strategy presents a potential framework, whereby the coordinated activity of a population of neurons allows for the same neurons to respond to multiple taste stimuli or even other sensory modalities, a well-documented phenomenon in insular cortex neurons. However, these representations may not be static, as several studies have demonstrated that both spatial representation and temporal dynamics of taste coding change with experience. Collectively, these studies suggest that cortical taste representation is not organized in a spatially discrete map, but rather is plastic and spatially dispersed, using temporal information to encode multiple types of information about ingested stimuli.
Impact statement
The organization of taste coding in insular cortex is widely debated. While early work has focused on whether taste quality is encoded via labeled line or ensemble mechanisms, recent work has attempted to delineate the spatial organization and temporal components of taste processing in insular cortex. Recent imaging and electrophysiology studies have reported conflicting results in regard to the spatial organization of cortical taste responses, and many studies ignore potentially important temporal dynamics when investigating taste processing. This review highlights the latest research in these areas and examines them in the context of the anatomy and physiology of the insular cortex in general to provide a more comprehensive description of taste coding in insular cortex.
Keywords: Gustatory cortex, taste coding, cortical maps, cortical plasticity, review
Introduction
Understanding how sensory stimuli are encoded at the periphery and represented in the mammalian brain have been fundamental questions in the field of neuroscience since its infancy. Despite decades of effort, our understanding of how taste stimuli are represented in the mammalian cortex remains incomplete. This is in part due to uncertainty as to the precise location and extent of putative gustatory cortex within the insular cortex (IC) as well as by the diverse and multimodal nature of the stimuli contained in ingested foods and fluids. While multiple neural tracing studies have demonstrated that IC receives gustatory inputs,1,2 the manner in which neural activity in this cortical area represents taste stimuli, how this information is spatially organized, and the functional relevance of IC for taste-related behaviors have been a focus of intense investigation in recent years. These topics are often discussed in the context of a larger debate within the taste field: Whether the encoding of primary taste qualities is best described by a so-called “labeled line” model, or alternatively, an across-neuron pattern model. This question has been investigated throughout the entire taste pathway, from taste receptor cells to gustatory cortex, and has been recently reviewed.3 Unfortunately, this particular discussion tends to overlook or downplay the potential role of timing of neuronal activity in taste coding. Compelling evidence from a number of physiological studies suggests that central gustatory neurons respond to taste stimuli in a temporally meaningful way, with variation in firing patterns related to stimulus discrimination and intensity.4–7 Moreover, activity in cortical neuronal ensembles can be divided into several sequential phases, each encoding different aspects of taste stimulation in behaving animals.8–10 The current review will not rehash long-standing arguments about whether taste quality is conveyed via the labeled-line or pattern model, but will focus more specifically on recent advances in the understanding of spatial and temporal components of cortical taste coding, including with regard to evidence for or against the presence of a quality-specific or chemotopic map in IC.
General principles of taste coding
Focusing specifically on the physiology of taste coding, there are several fundamental principles to be considered. First, oral stimuli are generally complex and can be characterized by taste quality, hedonic valence,11 and somatosensory features.12–14 Taste stimuli are typically categorized by their chemical composition, with most stimuli perceived by humans and animals as fitting into classes corresponding to basic taste qualities of sweet, sour, salty, bitter, and umami. Once stimuli are introduced to the oral cavity, they activate specialized G protein-coupled receptors and ion channels expressed in taste receptor cells (TRCs) on the tongue.15–21 In general, each quality is linked to distinct transduction mechanisms and receptor cell types, although convergence occurs early in the neural pathway in the peripheral taste neurons innervating TRCs.22–25 The peripheral neurons synapse centrally in the nucleus of the solitary tract (NST), and taste information is subsequently relayed through other CNS areas (including the gustatory thalamus) to the insular cortex.
In addition to being categorized by quality, taste stimuli can also be described by their hedonic valence – i.e. whether the stimulus is perceived as pleasant or aversive. Notably, while taste quality and valence are linked (i.e. mammals have an innate preference for sweet stimuli and an innate aversion to bitter stimuli), the valence of a taste can be modulated by stimulus concentration,23 experience,26 and behavioral state,27 while taste quality generally remains constant. For example, following conditioned taste aversion (CTA) in which an inherently neutral or appetitive stimulus is paired with visceral malaise, the perceived valence of the stimulus changes from appetitive to aversive, even though the taste quality itself is not changed. Finally, most foods and fluids contain mixtures of different taste qualities, and during eating the oral cavity is simultaneously presented with taste and non-taste stimuli, including those that evoke sensations of touch, temperature, and even possibly discomfort and pain. Although particular stimuli may initially activate distinct receptors and cells, these channels rapidly converge along peripheral nerves and central pathways.28
Insular cortex is multimodal and highly interconnected
While so-called gustatory cortex is commonly studied as an independent area, it is located within the larger IC, an area that is interconnected with multiple cortical and subcortical structures, and perhaps best described as having a general role in interoception or homeostatic regulation of the body.29 In rodents, IC is located on the lateral brain surface, along a fairly large portion of the rostral-caudal axis. Adding to its organizational complexity, IC can be delineated based on cytoarchitecture into granular, dysgranular, and agranular regions based on the diminution of the granular cell layer (layer IV), as cortex transitions from six-layered neocortex to three-layered paleocortex. Gustatory input reaches IC via projections primarily from taste thalamus, which is located in the medial parvocellular division of the ventral posteromedial nucleus (VPMpc). Neural tracing studies in rodents demonstrate that terminals from taste thalamic projection cells are densest within dysgranular IC, but taste-responsive neurons have been found in all three divisions.1,2,30–32 Other apparent taste-related inputs appear to originate from the amygdala and parabrachial nucleus of the pons; in turn, neurons in IC project to, and modulate taste responses in, a number of cortical and subcortical targets, including brainstem taste relays.1,33,34
In addition to inputs from taste processing regions, the three divisions of IC receive projections from brain regions involved in somatosensation,35 homeostasis,1 olfaction,36 emotional regulation,1 visceral processing,1,37 and cognition.1 These inputs appear to be somewhat segregated along the rostrocaudal and dorsal-ventral axes of IC. In particular, multiple studies have implicated the posterior insular cortex in the processing of visceral and aversive stimuli, often terming this region “visceral cortex.”37–39 However, there is a high degree of interconnectivity between these regions of insular cortex, with both neural tracing and optical imaging studies showing reciprocal projections between different regions of IC along the rostrocaudal and dorsal-ventral axes, as well as between cortical hemispheres.35,40,41 Thus, individual subregions of IC should not be examined as independent structures, but as part of a larger distributed cortical network. In regards to taste coding, these connections likely carry information about the animal’s behavioral state and satiety, which converges with taste information to generate food or fluid consumption or rejection decisions.
On a functional level, it has been well established that neurons within IC are multimodal. While neurons within classically defined gustatory cortex (the area of IC receiving inputs from VPMpc) respond to taste stimuli, many also respond to olfactory, somatosensory, and visceral stimuli, with single neurons often responding to multiple stimulus modalities.42–46 Recent work has also demonstrated that neurons in the gustatory region will respond to cues predicting taste stimuli following repeated pairings (including visual or auditory cues), suggesting neuronal responses in IC can be modulated by learning and experience.47 As a whole, these findings support the idea that IC, rather than containing a distinct “primary” gustatory cortex, serves to integrate multiple types of information, commensurate with an executive role in regulating behavioral states, consumption decisions, or taste-related learning.29,48–50 We will examine the literature presented here with these general anatomical and functional principles in mind.
Spatial organization of taste coding in insular cortex
As the presence of highly organized topographic “maps” has been documented in other sensory cortices, such as the tonotopic map in auditory cortex and the somatotopic map in the somatosensory cortex,51 recent studies have investigated whether a spatially distinct representation of taste quality might exist within IC, in a so-called chemotopic or “gustotopic” map. The existence of such a map is closely tied to principles of labeled-line coding, including the proposal of a preponderance of narrowly tuned neurons within IC,52 despite a great deal of prior electrophysiological studies demonstrating the presence of broadly tuned and multimodal neurons within IC.
Early physiological studies in rats showed that some degree of oral topography was present in IC, with rough segregation of taste input from different cranial nerves.53 Although responses in individual neurons to different qualities could be found in all regions, there was some bias for sweet-best neurons to be located rostrally and bitter-best more caudal.54 More recently, Accolla et al.55 used an intrinsic imaging preparation to characterize taste-evoked activity (i.e. functional mapping) across the brain’s surface in rats. Although each quality seemed to evoke a distinct spatial pattern, there was a large degree of overlap in taste quality representation, especially at the center of the imaging field. Additionally, positive (sweet) stimuli activated the most rostral portions of the imaging window, while negative (sour and bitter) stimuli activated the most caudal region, suggestive of a hedonic-based organization of IC.
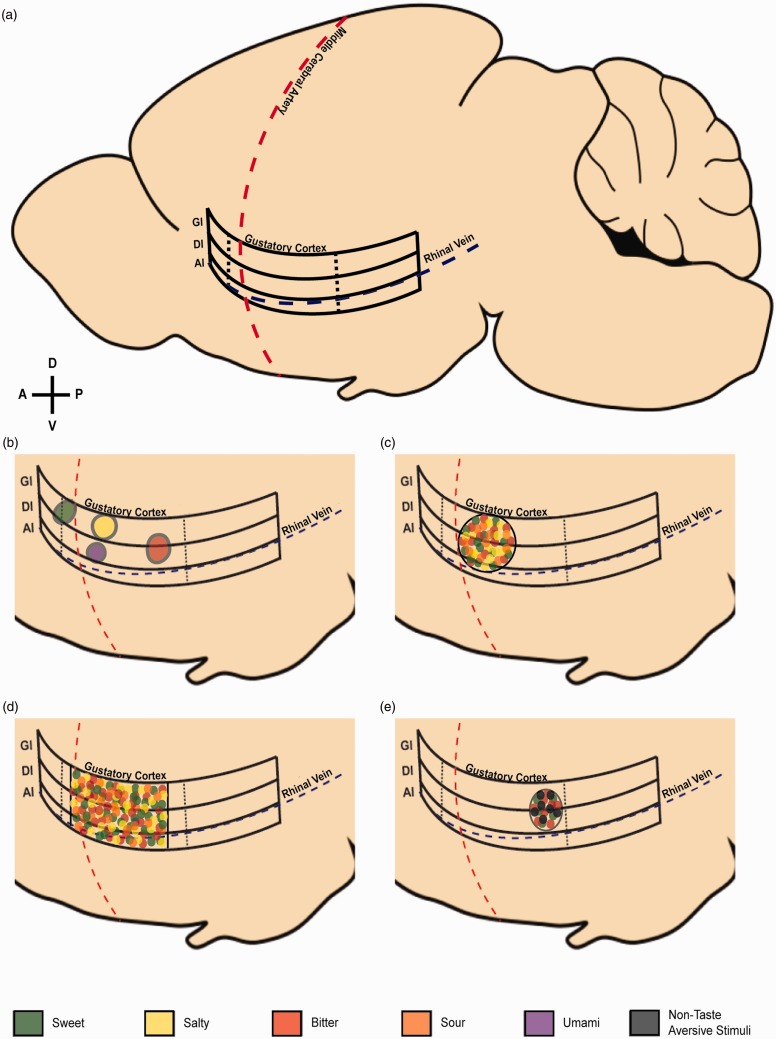
A more recent study used two-photon (2 P) calcium imaging via a bulk-loaded dye to investigate taste quality processing on the surface of IC at single-cell resolution.52 In this study, quality-specific “hot spots” (clusters of responsive cells) of singly tuned neurons were found, with sweet sensitivity located more rostral, bitter caudal, and hot spots for salt and umami located centrally. No overlap among clusters was found, and surprisingly, this study failed to find any evidence of a cortical response to sour stimuli (Figure 1(b)). On the other hand, Fletcher et al.30 used 2 P imaging with the calcium reporter GCaMP6s expressed in IC to investigate responses to basic taste stimuli in neurons in cortical layers 2/3.30 Similar to previous physiological studies, neurons were either broadly or narrowly tuned, and no quality-specific spatial organization of best-responder cells was found (Figure 1(c)).
Figure 1.
(a) Representative sagittal view of the location of insular and gustatory cortex. The boundaries of gustatory cortex as defined by projections of the gustatory thalamus30,52 are denoted with dashed vertical lines. Granular insular cortex (GI), dysgranular insular cortex (DI), agranular insular cortex (AI). (b–e) Representation of the results of gustotopic map studies in mice. Black lines indicate anatomical boundaries of the study. Stimulus type is represented by color: sweet (green), salty (yellow), bitter (red), sour (orange), umami (purple), non-taste aversive stimuli (black). (b) Representation of cortical hotspots of taste quality coding reported in Chen et al.52 Sweet, salty, bitter, and umami stimuli responses are localized to spatially distinct hotspots within gustatory cortex. (c) Representation of spatially overlapping taste quality responses in central gustatory cortex reported in Fletcher et al.30 (d) Representation of overlapping electrophysiological responses to taste stimuli in awake animals along both the dorsal-ventral and rostrocaudal axes of gustatory cortex reported by Levitan et al.10 (e) Representation of cortical responses to sweet, bitter, and aversive non-taste stimuli such as lithium chloride injection and shock in posterior gustatory cortex reported by Gehrlach et al.39 (A color version of this figure is available in the online journal.)
Differences between the results of these two imaging studies may be due to several factors, most notably the difference in sensitivity between the calcium indicators used.56 GCaMP6s is more sensitive than bulk-loaded calcium dyes, potentially allowing for the detection of weaker responses to multiple taste stimuli in the same neurons, which could result in the greater proportion of broadly tuned neurons described by Fletcher et al.30 Additionally, there were differences in the anatomical locations imaged between the two studies, with Chen et al.52 describing taste quality hotspots rostral and caudal to the areas imaged by Fletcher et al.30 However, it is important to point out that a marked lack of spatial organization in IC according to taste quality has been found previously, including electrophysiological studies showing a distribution of broadly tuned neurons throughout IC,54,57,58 and taste-evoked cFos expression indicating almost complete overlap of taste quality in IC.59 A recent study by Levitan et al.10 systematically mapped cortical taste responses across IC by recording the activity of neuronal ensembles in awake, behaving mice. Similar to the findings of Fletcher et al., both broad and narrow tuning was evident among IC neurons, and the proportion of these types was consistent along both the dorsal-ventral and rostral-caudal axes of IC (Figure 1(d)). Moreover, different taste qualities were represented evenly throughout both dimensions and no clustering was found. Interestingly, human imaging studies largely support the idea of overlapping taste quality representation, especially when stimulus concentration is considered.60 Taken together, the evidence overwhelmingly suggests that taste qualities are not organized into a chemotopic map in IC.
While it has become increasingly clear that central, gustatory-related regions of IC lack any chemotopic organization in terms of taste quality, recent studies suggest instead that hedonic valence may be spatially organized along the rostral-caudal axis. For example, optogenetic stimulation of rostral regions of IC results in mice displaying appetitive behaviors, whereas stimulation of caudal regions of IC results in aversive responses.61 Interestingly, these regions were found to project to separate amygdalar subnuclei that have been proposed to be responsible for assigning valence to taste stimuli62,63 with rostral IC projecting to the basolateral amygdala (BLA) and caudal IC projecting to the central nucleus of the amygdala.62,63
Additionally, careful lesion-mapping studies have demonstrated that damage to the caudal (but not central or rostral) IC disrupts taste aversion learning, a type of associative learning requiring the processing of visceral gastrointestinal signaling.38 In line with this, recent literature from outside of the taste field has implicated posterior regions in multimodal aversive processing, including tail-pinch, fear conditioning, and gastric malaise, in addition to the processing of taste stimuli (Figure 1(e)).39 Taken together, these studies support the idea that that IC is organized in a way that reflects a hedonic or valence coding rather than chemotopic coding.
Temporal organization of taste coding in insular cortex
If taste information is not represented by spatially distinct clusters of neurons within the gustatory cortex, how are differences in taste stimuli recognized? One leading hypothesis focuses on specific temporal patterns of neuronal activity representing both taste quality and valence. In this model, the same neuron encodes the presence, quality, and valence of a taste stimulus in temporal pattern of firing. Moreover, a single neuron may respond to multiple taste qualities, and the timing of the neuron’s responses relative to other active cells within the population is proposed to encode taste-specific information.
Awake single-unit responses to taste stimuli in IC neurons have provided evidence for this type of temporal coding strategy in rodent IC.8 Unlike early cortical physiology studies of taste, which quantified taste responses based on changes in averaged spiking activity of a neuron before and during taste delivery, recent studies have implemented a much more fine-scale temporal analysis of taste-evoked activity based on the moving-average of neuronal spiking. This analysis allows for the comparison of a single neuron’s responses to different taste qualities across much shorter timescales and revealed that a neuron’s “best” response varied with the time epoch being examined, suggesting that previous evaluations of a neuron’s taste specificity may have masked time-dependent differences in the response of a single neuron to multiple stimuli.3 Further, this analysis allowed for the characterization of major firing rate changes throughout stimulus delivery and revealed the presence of early (0–200 ms), middle (200 ms to 1 s), and late (> 1 s) epochs of activity across the entire population of neurons.
The authors proposed that these epochs correspond to three distinct aspects of taste coding: with the earliest epoch representing somatosensory coding, or simply the detection of the taste, and aligns well with licking onset, a middle epoch correspond to taste quality coding, and a final epoch encoding taste hedonics.8 While many previous studies using electrophysiology observed variances in neuronal firing between trials, attributing the shifts to noise, these studies were the first to demonstrate that these differences are reflective of population-wide state changes in cortical coding of taste information. While the timing of cortical taste responses appears “noisy” when individual neurons (or even small groups of neurons) are examined, large ensembles of neurons reliably fire through a series of defined sequences that represent specific taste stimuli, even though the timing of transitions between each sequence varies for individual taste trials.9
The importance of temporal epochs in gustatory coding in IC was further demonstrated in a recent study which used specifically timed optogenetic inhibition of IC during taste processing.64 The authors show that inhibiting IC activity specifically in the temporal epoch preceding the palatability or hedonic phase of firing delayed both the onset of palatability-related firing as well as the stereotyped behavioral response to quinine (gaping), but inhibiting IC activity once neurons had entered the palatability phase of firing had no effect on palatability-related firing or gaping behavior. This work is the first to provide evidence suggesting that the temporal epochs observed in IC are due to intrinsic cortical mechanisms encoding taste quality and palatibility rather than simply a relay of these processes from earlier relays in the taste pathway.
The idea of temporal signals encoding multiple characteristics of a stimulus is not limited to IC. Time-dependent stimulus encoding has been suggested in the NST.4,65,66 Interestingly, a typical temporal firing pattern for a tastant such as quinine, delivered to the NST via stimulating electrode while an animal was consuming water, was found to evoke behavioral responses associated with the mimicked taste (in this case aversion).65 Beyond coding for taste quality, the activity of taste neurons in the NST of awake rats is also modulated based on taste intensity66 as well as by licking movements67 and odors.68 Finally, Baez-Santiago et al.69 showed that the temporal codes or epochs found in IC were also present in another brainstem taste relay, the parabrachial nucleus.
Plasticity in cortical taste coding
In sensory systems with clear spatial organization, such as the auditory system, learning can be seen as a reorganization of the cortical representation of the learned stimulus. Similarly, spatial representations of taste stimuli within the IC have been shown to reorganize following associative learning. For example, a CTA study conducted by Accolla et al.26 using intrinsic wide-field imaging indicated that a cortical reorganization of sweet taste responses took place following learning. Here, the authors found a reduction in size of the spatial region of IC that was responsive to saccharin following its pairing with gastric malaise as compared to control animals. This reorganization leads to the cortical representation of the sweet stimulus becoming more similar to the representation of an aversive taste, quinine, suggesting a reorganization of taste valence within the IC. However, it was not possible to draw conclusions in this study regarding how this reorganization happens at the level of individual cells.
To investigate single-cell changes, a study using 2-photon imaging compared differences in the taste responses of BLA projecting and non-BLA projecting IC neurons following CTA to saccharin70 in an attempt to identify how specific IC cell populations are affected by CTA. Following CTA, the number of neurons responding to saccharin and the magnitude of their responses were significantly higher in BLA projection neurons compared to non-projection neurons, suggesting that CTA primarily recruits BLA projecting neurons. On a population level, neuronal responses to saccharin after CTA were more correlated to neuronal responses to naturally aversive quinine in BLA projecting neurons than non-projecting neurons. Interestingly, a more recent study from the same lab has demonstrated that these BLA projecting neurons are required for proper CTA learning and retrieval.71 Together, these studies support the idea that CTA leads to a reorganization of valence coding in specific subpopulations of IC neurons. An important caveat was that single cells could not be followed through the learning, so it is still unclear if taste quality coding is similarly impacted.
Much like plasticity in the spatial representation of taste responses, studies have demonstrated how learning can modulate the timing of cortical taste responses. For example, one study recorded both single neuron and population responses to sucrose before and after CTA as well as after extinction of the CTA.72 While population responses to sucrose remained relatively unchanged in any given experimental period, the majority (∼70%) of single-cell responses changed in both magnitude and temporal structure after both learning and extinction. Furthermore, changes appeared at least 500 ms after taste stimulation during the previously identified palatability phase of cortical neuron taste coding. Lastly, in agreement with other extinction literature, post-extinction responses did not resemble pre-learning responses, reflecting the idea that extinction is new learning rather than “unlearning.” While studies such as this are necessary for determining temporal changes in neuronal activity following learning, they lack spatial precision to identify how specific subpopulations may be changed. Thus, a combination of imaging and electrophysiology will be necessary to fully describe how experience alters the responses of IC neurons in regard to both taste quality and valence.
Recent electrophysiology work has demonstrated that the plasticity of insular cortex neurons is not limited solely to changes in taste quality or valence47 but can also apply to cross-modal stimuli when paired with tastes via associative learning paradigms. For example, unimodal gustatory IC neurons were shown to become responsive to non-gustatory stimuli (somatosensory or olfactory) following the pairing of sucrose with the non-gustatory stimulus.47 Similarly, neurons that were initially characterized as non-gustatory neurons became taste responsive following taste-touch or taste-odor pairing. The findings are interesting in that they provide evidence that modality specificity of IC neurons is also plastic, further calling into question any model espousing “hard-wired” cortical cell responses.
This work built on previous studies that had already demonstrated taste-responsive neurons become tone responsive after taste-tone pairing.27 While this study revealed that neurons in IC responded to the trained auditory cue, it more importantly demonstrated how cortical coding is influenced by the animal’s behavioral state. When comparing neuronal responses of tone-cued and uncued taste stimuli, cued taste stimuli were encoded more quickly as seen by a shift in the temporal coding dynamics described previously. This shift in timing is hypothesized to be due to a decrease in trial-to-trial variability of neuronal taste responses, allowing for IC to more quickly identify taste quality based on neuronal activity patterns.
It is evident that either spatial or temporal analyses alone will be unable to fully characterize taste coding and plasticity in IC, especially when conducted in anesthetized animals.73 Studies using a combined approached will be the most beneficial in continuing to characterize taste coding in insular cortex. With evidence that IC neuronal activity is not only modulated by experience, but also by the animal’s behavioral state, caution must be taken in interpreting IC neuronal responses without proper consideration of behavioral variables. As techniques to isolate specific circuits become more advanced and widely available, these tools will be of great use in determining the organization of IC circuits encoding taste quality and valance, as well as how they relate to the encoding of multiple stimulus types and cognitive states.
Conclusion
Multiple studies using varying techniques have now demonstrated the lack of a taste quality specific or chemotopic map in insular cortex. Overall, findings from numerous studies suggest that cortical taste coding likely arises from spatially distributed neuronal ensembles firing in taste-specific temporal patterns. Future studies combining techniques that allow for both high spatial and temporal resolution will continue to improve our understanding of stimulus coding in insular cortex.
Authors’ contributions
All authors participated in the conception of the manuscript. SMS wrote the manuscript. JDB and MLF edited and reviewed the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a National Institutes of Health grant to JDB and MLF [NIDCD R01 DC016833].
ORCID iD
Stephanie M Staszko https://orcid.org/0000-0002-5376-6398
References
- 1.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 1991; 311:1–16 [DOI] [PubMed] [Google Scholar]
- 2.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res 1986; 379:342–52 [DOI] [PubMed] [Google Scholar]
- 3.Ohla K, Yoshida R, Roper SD, Di Lorenzo PM, Victor JD, Boughter JD, Fletcher M, Katz DB, Chaudhari N. Recognizing taste: Coding patterns along the neural axis in mammals. Chem Senses 2019; 44:237–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol 2003; 90:1418–31 [DOI] [PubMed] [Google Scholar]
- 5.Lemon CH, Katz DB. The neural processing of taste. BMC Neurosci 2007; 8(Suppl 3):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson DM, Boughter JD, Jr., Lemon CH. Bitter taste stimuli induce differential neural codes in mouse brain. PLoS One 2012; 7:e41597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Lorenzo PM, Chen JY, Victor JD. Quality time: representation of a multidimensional sensory domain through temporal coding. J Neurosci 2009; 29:9227–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci 2001; 21:4478–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci USA 2007; 104:18772–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitan D, Lin JY, Wachutka J, Mukherjee N, Nelson SB, Katz DB. Single and population coding of taste in the gustatory cortex of awake mice. J Neurophysiol 2019; 122:1342–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura Y, Okamoto J, Funakoshi M. A role of oral afferents in aversion to taste solutions. Physiol Behav 1968; 3:537–42 [Google Scholar]
- 12.Simon SA, de Araujo IE, Stapleton JR, Nicolelis MA. Multisensory processing of gustatory stimuli. Chemosens Percept 2008; 1:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 2004; 558:147–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol 2003; 90:1865–76 [DOI] [PubMed] [Google Scholar]
- 15.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 2005; 4:143–91 [DOI] [PubMed] [Google Scholar]
- 16.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 2017; 18:485–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 2001; 77:896–903 [DOI] [PubMed] [Google Scholar]
- 18.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 2002; 416:199–202 [DOI] [PubMed] [Google Scholar]
- 19.Kuhn C, Bufe B, Batram C, Meyerhof W. Oligomerization of TAS2R bitter taste receptors. Chem Senses 2010; 35:395–406 [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 1999; 2:1055–62 [DOI] [PubMed] [Google Scholar]
- 21.Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neurosci 2008; 9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 2006; 444:288–94 [DOI] [PubMed] [Google Scholar]
- 23.Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun 2015; 6:8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank M. An analysis of hamster afferent taste nerve response functions. J Gen Physiol 1973; 61:588–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol 1991; 65:1452–63 [DOI] [PubMed] [Google Scholar]
- 26.Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci USA 2008; 105:4010–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontanini A, Katz DB. State-dependent modulation of time-varying gustatory responses. J Neurophysiol 2006; 96:3183–93 [DOI] [PubMed] [Google Scholar]
- 28.Smith DV, Lemon CH. Neural coding in the rNST In: Bradley RM. (ed) The role of the nucleus of the solitary tract in gustatory processing. Boca Raton, FL: CRC Press, 2007, pp.83–99 [PubMed] [Google Scholar]
- 29.Maffei A, Haley M, Fontanini A. Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol 2012; 22:709–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher ML, Ogg MC, Lu L, Ogg RJ, Boughter JD., Jr. Overlapping representation of primary tastes in a defined region of the gustatory cortex. J Neurosci 2017; 37:7595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 1977; 171:157–91 [DOI] [PubMed] [Google Scholar]
- 32.Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res 2000; 36:297–309 [DOI] [PubMed] [Google Scholar]
- 33.Smith DV, Li CS. GABA-mediated corticofugal inhibition of taste-responsive neurons in the nucleus of the solitary tract. Brain Res 2000; 858:408–15 [DOI] [PubMed] [Google Scholar]
- 34.Lundy RF, Jr., Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 2004; 91:1143–57 [DOI] [PubMed] [Google Scholar]
- 35.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 1998; 399:440–68 [DOI] [PubMed] [Google Scholar]
- 36.Shipley MT, Geinisman Y. Anatomical evidence for convergence of olfactory, gustatory, and visceral afferent pathways in mouse cerebral cortex. Brain Res Bull 1984; 12:221–6 [DOI] [PubMed] [Google Scholar]
- 37.Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 1987; 262:27–45 [DOI] [PubMed] [Google Scholar]
- 38.Schier LA, Blonde GD, Spector AC. Bilateral lesions in a specific subregion of posterior insular cortex impair conditioned taste aversion expression in rats. J Comp Neurol 2016; 524:54–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gehrlach DA, Dolensek N, Klein AS, Roy Chowdhury R, Matthys A, Junghanel M, Gaitanos TN, Podgornik A, Black TD, Reddy Vaka N, Conzelmann KK, Gogolla N. Aversive state processing in the posterior insular cortex. Nat Neurosci 2019; 22:1424–37 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Fujita S, Takei H, Song L, Chen S, Suzuki I, Yoshida A, Iwata K, Koshikawa N. Functional mapping of gustatory neurons in the insular cortex revealed by pERK-immunohistochemistry and in vivo optical imaging. Synapse 2010; 64:323–34 [DOI] [PubMed] [Google Scholar]
- 41.Mizoguchi N, Fujita S, Koshikawa N, Kobayashi M. Spatiotemporal dynamics of long-term potentiation in rat insular cortex revealed by optical imaging. Neurobiol Learn Mem 2011; 96:468–78 [DOI] [PubMed] [Google Scholar]
- 42.Samuelsen CL, Fontanini A. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci 2017; 37:244–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanamori T, Kunitake T, Kato K, Kannan H. Neurons in the posterior insular cortex are responsive to gustatory stimulation of the pharyngolarynx, baroreceptor and chemoreceptor stimulation, and tail pinch in rats. Brain Res 1998; 785:97–106 [DOI] [PubMed] [Google Scholar]
- 44.Hanamori T, Kunitake T, Kato K, Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol 1998; 79:2535–45 [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol 1989; 61:1244–58 [DOI] [PubMed] [Google Scholar]
- 46.Maier JX. Single-neuron responses to intraoral delivery of odor solutions in primary olfactory and gustatory cortex. J Neurophysiol 2017; 117:1293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincis R, Fontanini A. Associative learning changes cross-modal representations in the gustatory cortex. Elife 2016; 5:E16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes 2009; 33(Suppl 2):S34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T. Taste responses of cortical neurons. Prog Neurobiol 1984; 23:273–315 [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol 2006; 69:243–55 [DOI] [PubMed] [Google Scholar]
- 51.Thivierge JP, Marcus GF. The topographic brain: from neural connectivity to cognition. Trends Neurosci 2007; 30:251–9 [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science 2011; 333:1262–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol 1980; 44:440–55 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. II. Information processing of taste quality. J Neurophysiol 1985; 53:1356–69 [DOI] [PubMed] [Google Scholar]
- 55.Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci 2007; 27:1396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013; 499:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa H, Hasegawa K, Murayama N. Difference in taste quality coding between two cortical taste areas, granular and dysgranular insular areas, in rats. Exp Brain Res 1992; 91:415–24 [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol 1984; 51:616–35 [DOI] [PubMed] [Google Scholar]
- 59.King MS. Distribution of fos-immunoreactive neurons in the gustatory cortex elicited by intra-oral infusion of taste solutions in conscious rats. Brain Res 2018; 1683:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoenfeld MA, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf JM, Heinze HJ. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience 2004; 127:347–53 [DOI] [PubMed] [Google Scholar]
- 61.Peng Y, Gillis-Smith S, Jin H, Trankner D, Ryba NJ, Zuker CS. Sweet and bitter taste in the brain of awake behaving animals. Nature 2015; 527:512–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, Ryba NJP, Zuker CS. The coding of valence and identity in the mammalian taste system. Nature 2018; 558:127–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, Li B. An insula-central amygdala circuit for guiding tastant-reinforced choice behavior. J Neurosci 2018; 38:1418–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee N, Wachutka J, Katz DB. Impact of precisely-timed inhibition of gustatory cortex on taste behavior depends on single-trial ensemble dynamics. Elife 2019; 8:E45968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Lorenzo PM, Hallock RM, Kennedy DP. Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci 2003; 117:1423–33 [DOI] [PubMed] [Google Scholar]
- 66.Chen JY, Victor JD, Di Lorenzo PM. Temporal coding of intensity of NaCl and HCl in the nucleus of the solitary tract of the rat. J Neurophysiol 2011; 105:697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roussin AT, D’Agostino AE, Fooden AM, Victor JD, Di Lorenzo PM. Taste coding in the nucleus of the solitary tract of the awake, freely licking rat. J Neurosci 2012; 32:10494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Escanilla OD, Victor JD, Di Lorenzo PM. Odor-taste convergence in the nucleus of the solitary tract of the awake freely licking rat. J Neurosci 2015; 35:6284–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baez-Santiago MA, Reid EE, Moran A, Maier JX, Marrero-Garcia Y, Katz DB. Dynamic taste responses of parabrachial pontine neurons in awake rats. J Neurophysiol 2016; 115:1314–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavi K, Jacobson GA, Rosenblum K, Luthi A. Encoding of conditioned taste aversion in cortico-amygdala circuits. Cell Rep 2018; 24:278–83 [DOI] [PubMed] [Google Scholar]
- 71.Kayyal H, Yiannakas A, Kolatt Chandran S, Khamaisy M, Sharma V, Rosenblum K. Activity of insula to basolateral amygdala projecting neurons is necessary and sufficient for taste valence representation. J Neurosci 2019; 39:9369–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moran A, Katz DB. Sensory cortical population dynamics uniquely track behavior across learning and extinction. J Neurosci 2014; 34:1248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lissek T, Obenhaus HA, Ditzel DA, Nagai T, Miyawaki A, Sprengel R, Hasan MT. General anesthetic conditions induce network synchrony and disrupt sensory processing in the cortex. Front Cell Neurosci 2016; 10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]