Short abstract
Acute kidney injury (AKI) is a common critical clinical disease that is characterized by a rapid decline in renal function and reduced urine output. Ischemia and hypoxia are dominant pathophysiological changes in AKI that are induced by many factors, and the role of the “master” regulator hypoxia-inducible factor-1α (HIF-1α) is well recognized in AKI-related studies. MicroRNAs have been found to act as critical regulators of AKI pathophysiological process. More studies now have reported mutual interactions between HIF-1α and microRNAs in AKI. Therefore, in this brief review, we look into the mutual regulatory mechanisms between HIF-1α and microRNAs and discuss their function in the process of AKI. Recent studies demonstrated that HIF-1α is involved in the regulation of multiple functional microRNAs in AKI, and in turn, the level of HIF-1α is regulated by specific microRNAs. However, the role of the interactions between HIF-1α and microRNAs in AKI are controversial, and whether interventions targeting relevant mechanisms could achieve clinical benefits is not clear. Much work remains to further explore the value of targeting the HIF-1α-microRNA pathway in AKI treatment.
Impact statement
At first, we have discussed the role of hypoxia-inducible factor-1α (HIF-1α) and microRNAs in the acute kidney injury (AKI) pathophysiology. Then we have summarized the interactions between HIF-1α and microRNAs reported by AKI-related studies and concluded their regulatory effects in AKI process. Finally, we have made a vision of HIF-1α/microRNAs pathway’s potential as the intervention target in AKI. The mini review provides a systematic understanding of the crosstalk between HIF-1α and microRNAs in AKI and their effects on AKI pathophysiology and treatment.
Keywords: Acute kidney injury, hypoxia-inducible factor-1α, microRNAs, ischemia, hypoxia, biomarkers
Pathophysiological process of acute kidney injury
Acute kidney injury (AKI) is a clinical syndrome induced by multiple causes, such as ischemia, nephrotoxins, and sepsis. The main characteristics of AKI include a rapid decline in renal function, reduced urine output, and water, electrolyte and acid–base balance disorders.1 As a common complication of critical clinical diseases,2 AKI is easily accompanied with high mortality and poor outcomes for patients.3,4 In addition, patients surviving after AKI are more likely to develop chronic kidney disease5 and end up in renal failure.6 Early identification and therapeutic treatment of AKI is a persistent challenge for clinicians.7 Since no targeted therapy can be applied in the treatment of AKI, it is of high importance to investigate the pathophysiology of AKI and explore clinical strategies for shortened course and better prognosis of AKI patients.
As reported, prerenal causes account for 40–55% of all cases of AKI.8,9 In addition, sepsis10,11 and some nephrotoxic drugs12,13 also induce AKI through ischemic mechanisms. Due to the limited capacity of anaerobic glycolysis and the high oxygen consumption of renal tubular epithelial cells, the kidney is particularly sensitive to ischemia and hypoxia. Therefore, inadequate delivery of oxygen and metabolic substrates easily induces or exacerbates tissue damage in AKI.14 Models of acute ischemia induced by acute occlusion of the renal artery are commonly used to investigate the pathophysiological mechanism of AKI.15–17 In general, the pathophysiological process of ischemic AKI can be divided into three stages: initiation, progression, and repair.14 In the initiation stage, reduced effective arterial volume leads to kidney hypoperfusion, and hypoxia is then induced, especially in the boundary area of the renal cortex and medulla.18 Irreversible mitochondrial damage resulting from hypoxia subsequently results in endothelial damage, tubular epithelial injury, and inflammatory infiltration. In the progression process, tubular epithelial cell injury develops through immunological mechanisms. Cell injuries include dilation, foamy changes, cell polarity changes, loss of brush border, basement membrane denudation, shedding of both necrotic and viable epithelial cells into the tubular lumen, cast formation, and cell death.18 After the peak of tissue damage, surviving tubular epithelial cells start to dedifferentiate, regenerate and proliferate under internal and peripheral regulation.19 After the cytoskeleton and cell polarity are reconstructed, the construction and function of kidney tubules is gradually restored.20,21
Considering the crucial role of ischemia and hypoxia in AKI induced by most causes, here, we focus on the regulatory mechanism and the regulatory effect of hypoxia-inducible factor-1α (HIF-1α), the dominant regulator of cell biological activity under hypoxia.22 MicroRNAs function as regulatory mediators of numerous target proteins by influencing multiple signaling pathways,23,24 and increasing studies now report that the mutual regulatory mechanism existing between HIF-1α and microRNAs plays an important role in AKI.25–27 Therefore, in this review, we examined the crosstalk between HIF-1α and microRNAs in the progression and repair of AKI.
The role of HIF-1α in the progression and kidney repair of AKI
HIF-1α is a nuclear protein that was first discovered in cells cultured in an anoxic environment by Semenza and Wang.22 It is a basic helix-loop-helix (bHLH) transcription factor that is rapidly degraded during normoxia. Under hypoxic conditions, HIF-1α is stabilized and accumulates.28 Upregulated HIF-1α acts as a transcription factor to affect the expression of target genes and activate various downstream signaling pathways, including erythropoietin production, angiogenesis, energy metabolism, and other related pathways, to facilitate cell adaptation to the anoxic environment.29 In recent years, the protective role of HIF-1α in renal injury and repair has drawn increasing attention. With more studies examining HIF-1α-regulation mechanisms, it is now recognized that the expression and function of HIF-1α are regulated at the protein, transcriptional and biological activity levels.
Previous studies have found that two classic enzymes regulate the expression and function of the HIF-1α protein: prolyl hydroxylase domain-containing protein28 and hypoxia-inducible factor 1 subunit alpha inhibitor,30 both of which are oxygen-sensitive HIF-1αhydroxylases. Of late, new findings indicated that phosphorylation and reactive oxygen species (ROS) are also important mechanisms responsible for the regulation of degradation and biological function of HIF-1α under hypoxia.31,32 Other pathways regulating HIF-1α protein expression have also been reported. For example, our recent work found that HIF-1α protein but not mRNA is regulated by microRNA-30c-5p through its downstream target suppressor of cytokine signaling-3 (SOCS3) in human renal tubular epithelial cells.25
Notably, the alteration in HIF-1α mRNA transcription in AKI remains controversial. To determine whether and how the transcription of HIF-1α is regulated in AKI over time, we established a rat model of I/R renal injury and sacrificed the rats at different time points. We observed that levels of not only HIF-1α protein but also HIF-1α mRNA changed over time with ischemia and reperfusion (n = 5–6). Further experiments demonstrated that the transcription of HIF-1α is regulated by inhibitor of DNA binding 1 (ID1), which is also a bHLH transcription factor.33 In contrast, no significant change in HIF-1α mRNA expression was observed by Conde et al.,34 which may be attributed to a short hypoxia time and insufficient stimulation. According to the results of the above studies, we think that HIF-1α protein instantly accumulates at the start of exposure to hypoxia because of decreased degradation. If exposure to hypoxia continues, transcription of HIF-1α mRNA may be activated to ensure its expression.
A number of studies have demonstrated that HIF-1α plays an important role in the AKI process by regulating cell signaling pathways. To test the role of HIF-1α in AKI, pharmacological and genetic mimics and inhibitors of HIF-1α were applied in various AKI models: I/R-induced AKI,35,36 cisplatin-induced AKI,37,38 and rhabdomyolysis-induced AKI.39 In general, the results revealed that the stabilization of HIF-1α exerts a protective effect on the kidney after AKI both in the progression and repair phases. The main protection mechanisms of HIF-1α in the progression stage include helping tubular epithelial cells survive by reducing apoptosis and necrosis,35 improving the cell microenvironment by alleviating macrophage and inflammatory mediator infiltration,40,41 and reducing endothelial injury by upregulating vascular cell adhesion molecule 1 expression.42 Other mechanisms by which HIF-1α reduces kidney injury include inhibiting mitochondrial signaling pathways43 and reducing ROS levels.44 Both mechanisms are closely related to reduced mitochondrial injury. As more evidence revealed the interactions between HIF-1α and mitochondrial injury in AKI progression, we recently performed a series of studies in ischemic AKI animals and in vitro to further understand the mechanisms involved. We found that HIF-1α may protect mitochondria and reduced ROS by promoting mitophagosome formation and fusion with lysosomes (to be published). In addition, we observed that HIF-1α may influence the function of mitochondria by regulating mitochondrial fatty acid oxidation, and more experiments are being carried out to explore the relevant mechanisms. However, in the repair process, it is noteworthy that HIF-1α assists impaired kidney repair through different mechanisms, including inducing tubular epithelial cells to undergo dedifferentiation–regeneration–proliferation45 and promoting angiogenesis.46 The findings of our study that HIF-1α, ID1 (a regulator of cell dedifferentiation), and twist (a master regulator of gastrulation and mesoderm specification) interact in AKI are evidence that HIF-1α has an effect on tubular epithelial cell dedifferentiation–regeneration33 for instance. In addition to the impact on cell signaling pathways, new evidence has shown that an important mechanism that HIF-1α affects the AKI process is by regulating microRNAs.
The role of microRNAs in the progression and kidney repair of AKI
MicroRNAs are noncoding RNAs of approximately 21–23 nucleotides in length that are encoded by specific DNA regions called “mitron”.47 Under the action of RNA polymerase II, premicroRNAs of 60–70 nucleotides are first synthesized. Then, premicroRNAs are transported out of the nucleus and cleaved by dicer enzymes into mature microRNAs. RNA silencing processes are the classic way by which microRNAs regulate the function of target mRNAs.48 The binding of Argonaute proteins to microRNAs is required for forming RNA-silencing complexes (RISCs),49 which are differentially recognized by pairing with the 3'-UTR of the target mRNA.23 The complementary degree of microRNA–mRNA pairing is strongly associated with regulatory mechanisms of microRNAs. Perfect complementarity of microRNA–mRNA pairing activates the endonuclease effect of Argonaute proteins and results in the degradation of the target mRNA while incomplete complementarity of microRNAs and mRNAs results in the blocking of mRNA translation. In humans, the latter mechanism is dominant, but how RISCs inhibit different mRNA translation is still unclear.50 Therefore, microRNAs have been studied by many researches to figure out their regulatory function.
It is acknowledged that microRNAs play a pivotal role in regulating diverse pathophysiological processes, such as inflammation, apoptosis, proliferation, and angiogenesis. In AKI-related studies, microRNAs were found to contribute to AKI early diagnosis, AKI development, and renal repair by regulating these pathophysiological processes. There are various microRNAs and relevant mechanisms involved in AKI induced by different causes. For instance, our previous studies found that the levels of microRNA-30c-5p and microRNA-192-5p were elevated as early as 2 h after surgery in the urine of both ischemic AKI rats and cardiac surgery AKI patients.51 We further investigated the function of these two microRNAs in vivo and in vitro. The results showed that in addition to its early diagnostic value, microRNA-30c-5p also exhibited regulatory capacity in alleviating renal damage and promoting renal repair.25 However, we did not observe the same effect of microRNA-30c-5p and microRNA-192-5p in the cisplatin-induced AKI model (data not shown), while another microRNA, microRNA-140-5p, was found to be protective against cisplatin-induced oxidative stress by activating the NF-E2-related factor 2-dependent antioxidant pathway.52 These findings indicate that regardless of the stability of microRNAs in the samples and species, the expression and function of microRNAs are sensitive to injuries and are divergently affected by different pathogenic factors. Therefore, to ensure the value of a specific microRNA, different experimental AKI models in vivo and in vitro should be conducted to confirm its effect. When analyzing the role of target microRNAs, sample type, source of species, and patient conditions should also be taken into account.
There are many other microRNAs involved in the pathophysiology of AKI apart from microRNA-30c25,51,53 and microRNA-192.51,54 Reviewing published literature, we summarize the important microRNAs involved in the AKI pathophysiology that have been affirmed in more than one model or species. The detailed information of the relevant studies and the roles of microRNAs in AKI are listed in Table 1. Among these microRNAs, microRNA-21 was studied comparatively thoroughly. The results demonstrated that, as an AKI biomarker, microRNA-21 was not only effective in diagnosing AKI induced by I/R or cisplatin but was also significantly associated with severe AKI and other poor postoperative outcomes in cardiac surgery patients, indicating its potential as prognostic markers.59 As a regulator of AKI pathophysiology, microRNA-21 was found to be protective in both the progression and repair phases of AKI. The mechanisms by which microRNA-21 protects the kidney against pathogenic factors include inhibiting inflammatory mediator production,61,64,65 alleviating apoptosis57,61,65 and promoting renal tubular regeneration and proliferation.62 We believe these abundant evidence lay the foundation for future interventions of AKI targeting microRNAs in the clinic.
Table 1.
MicroRNAs involved in AKI pathophysiology that have been confirmed by more than one study.
| miR involved | Species | Sample type | AKI Model/Population | Role in AKI | Reference |
|---|---|---|---|---|---|
| miR-10a | Rat | Plasma | I/R-induced kidney injury | Biomarker of diagnosis | 2455314955 |
| Human | Serum | Critical ill/Cardiac surgery patients | Biomarker of diagnosis | 2607993056 | |
| miR-21 | Mouse | Kidney tissue/Primary RTECs | I/R-induced kidney injury/Low-oxygen gas mixture incubation for 24 h | Involved in ischemia pretreatment protection by targeting PDCD4 | 2278517357 |
| Human | Urine | Critically ill/Cardiac surgery patients/Biopsy-induced tubular injury to the transplanted kidney | Biomarker of diagnosis | 2415325258 | |
| Human | Urine/Plasma | Cardiac surgery patients | Biomarker of diagnosis | 2371741959 | |
| Mouse | Kidney tissue | I/R-induced kidney injury | Biomarker of diagnosis | 2398802060 | |
| Rat | Urine | Cisplatin-induced kidney injury | Biomarker of diagnosis | 2488002554 | |
| Mouse | Kidney tissue | Escherichia coli lipopolysaccharide/Septic kidney injury | Protects kidney against sepsis by inhibiting PDCD4, NF-κB and increasing IL-10 | 2584469961 | |
| Fish | Kidney tissue | Gentamicin-induced kidney injury | Promotes kidney regeneration by proliferative and antiapoptotic effects | 2657727962 | |
| Human | Plasma | Cardiac surgery patients | Biomarker of diagnosis | 2694012463 | |
| Rat | Kidney tissue/NRK-52E cells | I/R-induced kidney injury | Ameliorates kidney injury by inhibiting inflammation and cell apoptosis | 2715276364 | |
| Mouse | Kidney tissue | I/R-induced kidney injury | Involved in ischemia pretreatment protection through targeting MKK3 | 2614964065 | |
| Rat | Plasma | Aristolochic acid-induced kidney injury | Biomarker of diagnosis | 2742229366 | |
| Mouse | Kidney tissue/Exosomes | Ischemic preconditioning of kidney and sepsis-induced organ injury | Involved in protection of local and remote ischemic preconditioning against sepsis-induced organ injury by targeting PDCD4 and NF-κB | 2843737767 | |
| Mouse | Kidney tissue | I/R-induced kidney injury | Promotes angiogenesis in I/R-induced AKI targeting TSP-1 | 2873766068 | |
| Human/Mouse | Kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | Protects against I/R-induced AKI by preventing RTEC apoptosis and inhibiting dendritic cell maturation | 3001348569 | |
| miR-23a | Human | Serum | Septic patients | Biomarker of diagnosis | 2829690470 |
| Human | Serum | Acute myocardial infarction patients | Biomarker of diagnosis | 3103981471 | |
| Mouse | Exosomes/Kidney tissue/Primary RTECs | I/R-induced kidney injury | Attenuates tubulointerstitial inflammation by Inhibiting miRNA-23a prior to I/R injury | 3055189672 | |
| miR-30 family | Human/Rat | Plasma/Kidney tissue | Contrast-induced nephropathy | Biomarker of diagnosis | 2633719073 |
| Human/Rat | Plasma | Contrast-induced kidney injury | Biomarker of diagnosis | 2752840674 | |
| Human/Rat | Urine | I/R-induced kidney injury/Cardiac operation-induced kidney injury | Biomarker of diagnosis | 2805654651 | |
| Human/Rat | Kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | Ameliorates H/R-induced tubular epithelial cell injury via HIF-1α stabilization | 2919095725 | |
| Human | HK2 and NRK-52E cells | Cisplatin-induced injury | Minimizes cisplatin-induced apoptosis and nephrotoxicity by targeting Bnip3L and Hspa5 | 2879626353 | |
| miR-46a | Human/Mouse | Human and mice kidney tissue/Urine/HK-2 cells | I/R-induced kidney injury/Kidney transplant recipients | Ameliorates the development of ischemic AKI by limiting inflammation | 2744456575 |
| miR-126 | Rat | Microvesicles | I/R-induced kidney injury | Protects kidney against I/R-induced injury via pro-angiogenesis | 2249529676 |
| Mouse | Kidney tissue | I/R-induced kidney injury | Protects kidney against I/R-induced injury by promoting vascular integrity | 2461093077 | |
| Human | Serum | Critical ill/Cardiac surgery patients | Biomarker of diagnosis | 2607993056 | |
| miR-127 | Rat | Kidney tissue/NRK-52E cells | I/R-induced kidney injury/H/R model | Protects RTECs against I/R injury by promoting cell adhesion and cytoskeleton organization | 2296260978 |
| Human | Serum | Critical ill/Cardiac surgery patients | Biomarker of diagnosis | 2607993056 | |
| Rat | Kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | Involved in the protection of HIF-1α against maladaptive repair after renal I/R injury | 2810613179 | |
| miR-146 | Rat | Urine | Cisplatin-induced kidney injury | Biomarker of diagnosis | 2488002554 |
| Human | Serum | Critical ill/Cardiac surgery patients | Biomarker of diagnosis | 2607993056 | |
| miR-155 | Mouse | Kidney tissue | Cisplatin-induced kidney injury | Reduces cisplatin-induced nephrotoxicity by targeting c-Fos | 2501565680 |
| Human/Rat | Kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | Promotes renal tubular cell pyroptosis | 2800678581 | |
| miR-192 | Rat | Urine | Cisplatin-induced kidney injury | Biomarker of diagnosis | 2488002554 |
| Human/Rat | Urine | I/R-induced kidney injury/Cardiac operation-induced kidney injury | Biomarker of diagnosis | 2805654651 | |
| miR-210 | Human | Plasma | Critically ill patients | Biomarker of diagnosis | 2170081982 |
| Human | Serum | Critical ill/Cardiac surgery patients | Biomarker of diagnosis | 2607993056 | |
| Human/Rat | Kidney tissue/HK-2 cells | Systemic hypoxia (hypobaric chamber to mimic 9% O2) and I/R induced kidney injury | Attenuates cell injury by decreasing apoptosis induced by hypoxia | 2938786383 | |
| miR-494 | Human/Mouse | Urine/Kidney tissue | I/R-induced kidney injury/Critical ill patients | Biomarker of diagnosis/Contributes to inflammatory or adhesion molecule-induced kidney injury after I/R by inhibiting expression of ATF3 | 2316051384 |
| Human/Mouse | Kidney tissue/HK‐2 cells | Cyclosporine-induced nephrotoxicity | Promotes cyclosporine-induced nephrotoxicity and EMT by inhibiting PTEN | 2585454285 | |
| miR-489 | Rat | Urine | Gentamicin-induced kidney injury | Biomarker of diagnosis | 2707438586 |
| Mouse | Kidney tissue | I/R-induced kidney injury | Protects kidney by targeting PARP1 and apoptosis | 2697543926 | |
| miR-668 | Human/Mouse/Rat | Kidney tissue/RPTCs/Urine/Serum | I/R-induced kidney injury/Hypoxia Treatment/Patients accepting cardiopulmonary surgery | Biomarker of diagnosis; Protects against ischemic kidney injury by repressing MTP18 | 3032574087 |
miR: microRNA; RTECs: renal tubular epithelial cells; PDCD4: programmed cell death protein 4; NF-κB: nuclear factor-κB; IL-10: interleukin-10; MKK3: mitogen-activated protein kinase kinase 3; TSP-1: thrombospondin 1; BCL2: B-cell lymphoma-2; Bnip3L: B-cell lymphoma-2 interacting protein 3 like; Hspa5: heat shock protein family A member 5; ATF3: active transcription factor 3; EMT: epithelial to mesenchymal transition; PTEN: phosphatase and tensin homolog; PARP1: poly (ADP-ribose) polymerase-1; RPTCs: rat proximal tubular cells; MTP18: mitochondrial protein 18 kDa.
The crosstalk between HIF-1α and microRNAs in AKI
As the “master” transcription factor that regulates gene expression under conditions of hypoxia and ischemia, HIF-1α was found to be involved in the regulation of multiple tested and functional microRNAs in AKI. Recently, microRNA-21, microRNA-23a, microRNA-127, microRNA-489, microRNA-668, and microRNA-687 were reported to be HIF-1α-dependent in experimental AKI models. CHIP-Seq and luciferase reporter assays are commonly used methods to confirm the interactions of microRNAs and target genes. Confirmed by CHIP-Seq or luciferase reporter assays, HIF-1α was found to directly bind to the promoter region of these microRNAs, except microRNA-127.78 Regulated microRNAs then influence the pathophysiology of AKI through downstream signaling pathways by controlling the expression of the targets. For example, Jia et al.67 showed that the increased level of microRNA-21 in kidney tissue and serum exosomes induced by ischemic preconditioning was mediated by the binding of HIF-1α to the HRE element of the microRNA-21 promoter region. Elevated microRNA-21 then activated the downstream programmed cell death protein 4 (PDCD4)/NF-κB signaling pathway and resulted in a protective effect against sepsis-induced organ injury. Another group found that the induction of microRNA-48926 and microRNA-66887 by HIF-1α plays a protective role in I/R-induced kidney injury by reducing apoptosis and preserving mitochondrial dynamics, respectively. Although most of the microRNAs induced by HIF-1α are considered protective, injurious microRNAs have also been reported to be induced by HIF-1α in AKI pathogenesis.27 The evidence was recently reported by Bhatt et al. A HIF-1α transcriptional target, microRNA-687, was found to be effective in exacerbating kidney injury by facilitating cell cycle activation and apoptosis, and blocking microRNA-687 attenuates kidney injury by preserving phosphatase and tensin homolog (PTEN) expression.27
In turn, HIF-1α was also reported to be regulated by specific microRNAs. For example, our recent work showed that microRNA-30c-5p elevation induced by ischemia or hypoxia resulted in HIF-1α stabilization via regulating its target gene SOCS3, which is critical for the antiapoptotic effects of microRNA-30c-5p in protecting against ischemic and hypoxic kidney injury.25 However, the mechanisms of the detailed interaction between microRNA-30c-5p and HIF-1α have not been fully understood and should be further investigated. The regulatory effect of microRNA-21 on HIF-1α was reported by two studies. One study found that microRNA-21 led to an increase in HIF-1α after xenon exposure, and the upregulation of HIF-1α was involved in the protection of xenon preconditioning against I/R-induced kidney injury.88 In another study, a feedback interaction was discovered between microRNA-21 and HIF-1α through the PTEN/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway.69 However, because of the lack of exploration of the genetic regulatory mechanism between microRNA-21 and HIF-1α, the significance of the two studies is limited. Another study found that microRNA-210 directly regulates HIF-1α in a systemic and local kidney hypoxia model. Using a luciferase reporter assay, microRNA-210 was found to target the 3'-UTR of HIF-1α mRNA directly in hypoxia. Interestingly, this study reported a conflicting role of HIF-1α in HK2 cell injury induced by hypoxia in which microRNA-210 attenuated hypoxic apoptosis by suppressing HIF-1α activation.83 However, in this study, the authors failed to supply exogenous HIF-1α to reverify their findings. Therefore, the role of microRNA-210-HIF-1α in hypoxia-induced kidney injury should be reconsidered and more thoroughly studied. Recently, a study performed by Mathia et al.89 showed that microRNA-22 was induced to repress HIF-1α in rhabdomyolysis-associated AKI models. The induction of HIF-1α by anti-microRNA-22 molecules was shown by assessing renal gene expression profiles. However, despite HIF-1α upregulation, microRNA-22 antagonism did not attenuate AKI severity, most likely due to the activation of other deleterious genes. In conclusion, the role of the crosstalk between HIF-1α and microRNAs in AKI is complicated and should be further discussed. The detailed information of studies exploring the interactions between HIF-1α and microRNAs in AKI is listed in Table 2, and a brief outlining of the known crosstalk between HIF-1α and various microRNAs in AKI is presented in Figure 1.
Table 2.
The interactions of HIF-1α and microRNAs in AKI.
| Sample type | AKI Model/Population | Regulator | Effector | Effect | Regulatory mechanism | Downstream targets | Role in AKI | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse kidney tissue/RPTCs | I/R-induced kidney injury/Hypoxia treatment | HIF-1α | miR-17-5p | Independent of HIF-1α | / | DR6 | Induced by p53; Protects against renal ischemia-reperfusion injury | 2762299090 |
| Mouse kidney tissue | I/R-induced kidney injury | miR-21 | HIF-1α | Upregulation | / | PDCD4, PTEN | Involving in the protection of xenon preconditioning against I/R-induced kidney injury | 2368114588 |
| Mouse kidney tissue/Exosomes | Ischemic preconditioning of kidney and sepsis-induced organ injury | HIF-1α | miR-21 | Upregulation | Binding to the HRE element of miR-21 promoter region | PDCD4/NF-κB | Involved in protection of local and remote ischemic preconditioning against sepsis-induced organ injury | 2843737767 |
| Mouse kidney tissue/Primary RTECs | I/R-induced kidney injury/Hypoxia | HIF-1α | miR-21 | Upregulation | / | TSP-1 | Promotes angiogenesis in I/R-induced AKI | 2873766068 |
| Mouse kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | miR-21 HIF-1α | HIF-1αmiR-21 | Mutual regulation | Through PTEN/AKT/mTOR pathway | PDCD4/NF-κB | Protects against I/R-induced AKI by preventing RTEC apoptosis and inhibiting dendritic cell maturation | 3001348569 |
| Mouse kidney tissue/DCTs | Rhabdomyolysis-associated AKI/Hypoxia treatment | miR-22 | HIF-1α | Downregulation | / | HIF-1α target genes | Inhibition of miR-22 did not change renal function and injury markers | 2979178189 |
| Mouse exosomes/Kidney tissue/Primary renal tubular epithelial cells | I/R-induced kidney injury | HIF-1α | miR-23a | Upregulation | Binding to miR-23a promoter region | P65, Inflammatory cytokines | miR-23a-enriched exosomes from hypoxic RTECs can activate macrophages to promote tubulointerstitial inflammation | 3055189672 |
| Rat kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | miR-30c-5p | HIF-1α | Upregulation | SOCS3 | C-CASP3, BCL2, BAX | Ameliorates H/R-induced tubular epithelial cell injury via HIF-1α stabilization | 2919095725 |
| Rat kidney tissue/NRK-52E cells | I/R-induced kidney injury/H/R model | HIF-1α | miR-127 | Upregulation | / | KIF3B | Protects RTECs against I/R injury by promoting cell adhesion and cytoskeleton organization | 2296260978 |
| Rat kidney tissue/HK-2 cells | I/R-induced kidney injury/H/R model | HIF-1α | miR-127-3p | Upregulation | / | EMT markers | Involved in the protection of HIF-1α against maladaptive repair after renal I/R injury | 2810613179 |
| Rat kidney tissue/HK-2 cells | Systemic hypoxia and I/R-induced kidney injury | miR-210 | HIF-1α | Downregulation | Directly target 3’-UTR of HIF-1α mRNA | BNIP3, BNIP3L | Attenuates cell injury by decreasing apoptosis induced by hypoxia | 2938786383 |
| Mouse kidney tissue | I/R-induced kidney injury | HIF-1α | miR-489 | Upregulation | Binding to the promoter of calcr site 1 to regulate miR-489 transcription | PARP1 | Protects kidneys and reduces apoptosis | 2697543926 |
| Mouse kidney tissue/RPTCs | I/R-induced kidney injury/Hypoxia treatment | HIF-1α | miR-668 | Upregulation | Binding to miR-668 promoter region | MTP18 | Protects against ischemic kidney injury by preserving mitochondrial dynamics | 3032574063 |
| Mouse kidney tissue/Proximal tubular cells | I/R-induced kidney injury/Hypoxia treatment | HIF-1α | miR-687 | Upregulation | Binding to the miR-687 promoter region | PTEN | Aggravates kidney injury and facilitates cell cycle progression and apoptosis | 2558706827 |
DR6: death receptor 6; AKT: protein kinase B; mTOR: mammalian target of rapamycin; SOCS3: suppressor of cytokine signaling-3; C-CASP3: cleaved caspase 3; BAX: Bcl-2-associated X; KIF3B: kinesin family member 3B; DCTs: distal convoluted tubule cells.
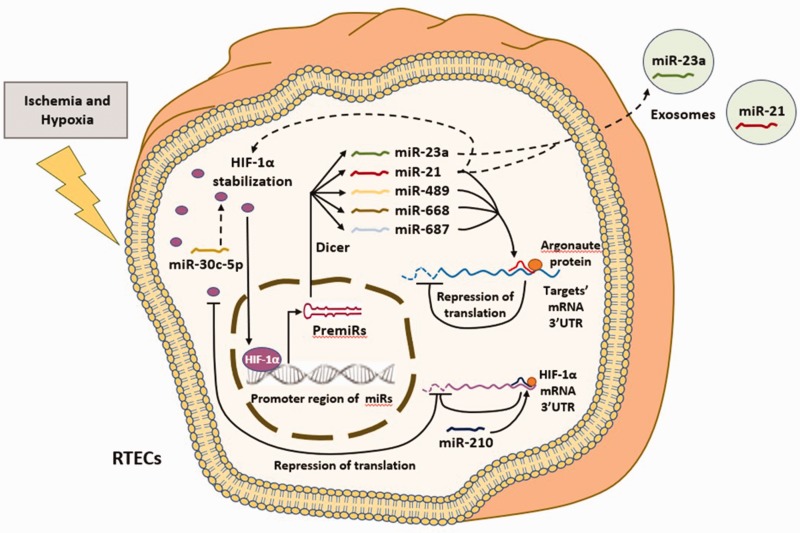
Figure 1.
The known crosstalk between HIF-1α and various microRNAs in AKI. In the presence of ischemia and hypoxia, HIF-1α is stabilized and accumulates in RTECs. Stabilized HIF-1α translocates into nucleus and acts as a transcription factor to bind to the promoter region of target microRNAs (miRs). Under the action of RNA polymerase II, PremiRs are first synthesized. Then PremiRs are transported out of the nucleus and cleaved by dicer enzymes into mature miRs. Known miRs regulated by HIF-1α through the aforementioned mechanisms include miR-21, miR-23a, miR-489, miR-668 and miR-687. MiR-21 and miR-23a could be enriched in exosomes and delivered to target cells. MiR-21, miR-489, miR-668 and miR-687 could affect the expression of target genes through RNA silencing processes with the participation of Argonaute proteins. The binding of Argonaute proteins to these miRs forms RNA-silencing complexes (RISCs) and the pairing of RISCs with the 3'-UTR of the targets’ mRNA results in the blocking of target’s mRNA translation. In turn, HIF-1α could also be regulated by some miRs. The miR-30c-5p and miR-21 could increase the stability of HIF-1α, while miR-210 could target the 3'-UTR of HIF-1α mRNA and decrease the translation level of HIF-1α. (A color version of this figure is available in the online journal.)
Clinical potential of targeting the HIF-1α-microRNA pathway in AKI treatment
Notably, several microRNA-based therapeutics have been tested in other diseases, such as an antagomir (an inhibitor) against microRNA-122 for hepatitis treatment91 and a mimic of microRNA-34 to treat cancer.92 Although similar clinical trials targeting microRNAs in AKI have not yet been reported, carrying out the clinical trials above indicates the clinical prospect of treatments targeting microRNAs. Despite the important role of the interaction between HIF-1α and microRNAs in AKI, whether interventions targeting relevant mechanisms could achieve clinical benefits is not clear. Overall, multiple studies have achieved good results in experimental AKI animals by adopting pharmacological interventions to induce HIF-1α and its downstream microRNAs. However, the problem of eliminating heterogeneity among studies exists. Further studies with larger sample sizes might be useful to resolve this problem.
Recently, more attention has been paid to microRNAs inside exosomes. Studies demonstrated that targeting exosomes mediated by HIF-1α-microRNA pathways may be beneficial for early AKI treatment67 and improve kidney outcomes.72 As newly discovered single membrane vesicles that are secreted by various living cells, exosomes can be transported to recipient cells and organs, acting as regulators of disease pathophysiology through autocrine, paracrine and telecrine mechanisms.93 Due to the structure of the complete monolayer membrane, bioactive components inside the exosomes (mRNAs, proteins and microRNAs) are stable and less susceptible to the external environment. Results showed that HIF-1α-dependent microRNA-enriched exosomes played miscellaneous roles when received by different cells. For instance, Jia et al.67 demonstrated the potential protective role of HIF-1α-dependent microRNA-21-enriched exosomes in sepsis-induced AKI, while Li et al.72 found that HIF-1α-dependent microRNA-23a-enriched exosomes resulted in macrophage activation and tubulointerstitial inflammation of uninjured kidneys. These results indicate the complicated effects of HIF-1α activation on different effector cells in AKI, and the key points of intervention should be concentrated on specific downstream microRNAs in exosomes. In summary, the therapeutic role of HIF-1α-dependent microRNA-enriched exosomes should be explored and tested in more AKI experimental models. In addition, focusing on exosome-target cell-specific communication may better benefit the precise treatment of AKI. Taken together, these studies guide innovative HIF-1α-microRNA-based therapeutics of AKI in the future.
Conclusion
AKI is a common critical clinical disease. Ischemia and hypoxia are dominant pathophysiological changes in AKI that are induced by many causes. In experimental AKI models, HIF-1α and microRNAs are well recognized to act as critical regulators of the pathophysiology of AKI. Recently, increasing studies have reported that mutual regulation mechanisms exist between HIF-1α and microRNAs. Studies have shown that HIF-1α is involved in the regulation of multiple functional microRNAs in AKI, and in turn, the level of HIF-1α can be regulated by some specific microRNAs. However, the role of the interactions between HIF-1α and microRNAs in AKI is controvertible, and whether interventions targeting relevant mechanisms could achieve clinical benefits is not clear. Therefore, much work remains to further explore the value in targeting the HIF-1α-microRNA pathway in AKI treatment.
Authors’ contributions
ZYW and WZ drafted the manuscript. All the authors have read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (No. 81670613).
ORCID iDs
Zhiyu Wang https://orcid.org/0000-0002-4307-8014
Wen Zhang https://orcid.org/0000-0002-9011-361X
References
- 1.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–84 [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012; 380:756–66 [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16:3365–70 [DOI] [PubMed] [Google Scholar]
- 4.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21:345–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan TM, Khan KN. Acute kidney injury and chronic kidney disease. Vet Pathol 2015; 52:441–4 [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and Meta-analysis. Kidney Int 2012; 81:442–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 2015; 10:147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002; 39:930–6 [DOI] [PubMed] [Google Scholar]
- 9.Sesso R, Roque A, Vicioso B, Stella S. Prognosis of ARF in hospitalized elderly patients. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 2004; 44:410–9 [PubMed] [Google Scholar]
- 10.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know?. Crit Care Med 2008; 36:S198–203 [DOI] [PubMed] [Google Scholar]
- 11.Kosaka J, Lankadeva YR, May CN, Bellomo R. Histopathology of septic acute kidney injury: a systematic review of experimental data. Crit Care Med 2016; 44:e897–903 [DOI] [PubMed] [Google Scholar]
- 12.Molitoris BA. Contrast nephropathy: are short-term outcome measures adequate for quantification of long-term renal risk?. Nat Clin Pract Nephrol 2008; 4:594–5 [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg I, Chonchol M, Guetta V. Reversible acute kidney injury following contrast exposure and the risk of long-term mortality. Am J Nephrol 2009; 29:136–44 [DOI] [PubMed] [Google Scholar]
- 14.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011; 7:189–200 [DOI] [PubMed] [Google Scholar]
- 15.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int 2010; 77:9–16 [DOI] [PubMed] [Google Scholar]
- 16.Heyman SN, Lieberthal W, Rogiers P, Bonventre JV. Animal models of acute tubular necrosis. Curr Opin Crit Care 2002; 8:526–34 [DOI] [PubMed] [Google Scholar]
- 17.Lameire N, Biesen WV, Vanholder R. Acute kidney injury. Lancet 2008; 372:1863–5 [DOI] [PubMed] [Google Scholar]
- 18.Kanagasundaram NS. Pathophysiology of ischaemic acute kidney injury. Ann Clin Biochem 2015; 52:193–205 [DOI] [PubMed] [Google Scholar]
- 19.Anglani F, Ceol M, Mezzabotta F, Torregrossa R, Tiralongo E, Tosetto E, Del Prete D, D’Angelo A. The renal stem cell system in kidney repair and regeneration. Front Biosci 2008; 13:6395–405 [DOI] [PubMed] [Google Scholar]
- 20.Wang HL, Liu NM, Li R. Role of adult resident renal progenitor cells in tubular repair after acute kidney injury. J Integr Med 2014; 12:469–75 [DOI] [PubMed] [Google Scholar]
- 21.Bihorac A, Kellum JA. Acute kidney injury in 2014: a step towards understanding mechanisms of renal repair. Nat Rev Nephrol 2015; 11:74–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992; 12:5447–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janas MM, Wang B, Harris AS, Aguiar M, Shaffer JM, Subrahmanyam YV, Behlke MA, Wucherpfennig KW, Gygi SP, Gagnon E, Novina CD. Alternative RISC assembly: binding and repression of microRNA-mRNA duplexes by human ago proteins. RNA 2012; 18:2041–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development 2005; 132:4645–52 [DOI] [PubMed] [Google Scholar]
- 25.Zou YF, Liao WT, Fu ZJ, Zhao Q, Chen YX, Zhang W. MicroRNA-30c-5p ameliorates hypoxia-reoxygenation-induced tubular epithelial cell injury via HIF1alpha stabilization by targeting SOCS3. Oncotarget 2017; 8:92801–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Q, Liu Y, Liu P, Hao J, Liang M, Mi QS, Chen JK, Dong Z. MicroRNA-489 induction by hypoxia-inducible factor-1 protects against ischemic kidney injury. J Am Soc Nephrol 2016; 27:2784–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. MicroRNA-687 induced by hypoxia-inducible factor-1 targets phosphatase and tensin homolog in renal ischemia-reperfusion injury. J Am Soc Nephrol 2015; 26:1588–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nangaku M, Rosenberger C, Heyman SN, Eckardt KU. Regulation of hypoxia-inducible factor in kidney disease. Clin Exp Pharmacol Physiol 2013; 40:148–57 [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Wei Q, Guo C, Dong G, Liu Y, Tang C, Dong Z. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. IJMS 2017; 18:e0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schodel J, Bohr D, Klanke B, Schley G, Schlotzer-Schrehardt U, Warnecke C, Kurtz A, Amann K, Eckardt KU, Willam C. Factor inhibiting HIF limits the expression of hypoxia-inducible genes in podocytes and distal tubular cells. Kidney Int 2010; 78:857–67 [DOI] [PubMed] [Google Scholar]
- 31.Kietzmann T, Mennerich D, Dimova EY. Hypoxia-Inducible factors (HIFs) and phosphorylation: impact on stability, localization, and transactivity. Front Cell Dev Biol 2016; 4:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, Dong Z. Hypoxia and Hypoxia-Inducible factors in kidney injury and repair. Cells 2019; 8:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen D, Zou YF, Gao YH, Zhao Q, Xie YY, Shen PY, Xu YW, Xu J, Chen YX, Feng XB, Shi H, Zhang W. Inhibitor of DNA binding 1 is induced during kidney ischemia-reperfusion and is critical for the induction of hypoxia-inducible factor-1alpha. BioMed Res Int 2016; 2016:e4634386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conde E, Alegre L, Blanco-Sanchez I, Saenz-Morales D, Aguado-Fraile E, Ponte B, Ramos E, Saiz A, Jimenez C, Ordonez A, Lopez-Cabrera M, del Peso L, de Landazuri MO, Liano F, Selgas R, Sanchez-Tomero JA, Garcia-Bermejo ML. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PloS One 2012; 7:e33258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 2008; 19:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Schley G, Turkoglu G, Burzlaff N, Amann KU, Willam C, Eckardt KU, Bernhardt WM. The protective effect of prolyl-hydroxylase inhibition against renal ischaemia requires application prior to ischaemia but is superior to EPO treatment. Nephrol Dial Transplant 2012; 27:929–36 [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Yu X, Zhang Y, Ding G, Zhu C, Huang S, Jia Z, Zhang A. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin Sci 2018; 132:825–38 [DOI] [PubMed] [Google Scholar]
- 38.Wang WW, Li ZZ, Wang W, Jiang Y, Cheng J, Lu S, Zhang JY. Enhanced renoprotective effect of HIF-1alpha modified human adipose-derived stem cells on cisplatin-induced acute kidney injury in vivo. Sci Rep 2015; 5:e10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahling M, Mathia S, Paliege A, Koesters R, Mrowka R, Peters H, Persson PB, Neumayer HH, Bachmann S, Rosenberger C. Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI. J Am Soc Nephrol 2013; 24:1806–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 2003; 14:1825–32 [DOI] [PubMed] [Google Scholar]
- 41.Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-Miller CL, Haase VH. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol 2012; 302:F1172–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, Yao B, Zhang MZ, Harris RC, Duffy KJ, Erickson-Miller CL, Sutton TA, Haase VH. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest 2014; 124:2396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka T, Kojima I, Ohse T, Inagi R, Miyata T, Ingelfinger JR, Fujita T, Nangaku M. Hypoxia-inducible factor modulates tubular cell survival in cisplatin nephrotoxicity. Am J Physiol Renal Physiol 2005; 289:F1123–33 [DOI] [PubMed] [Google Scholar]
- 44.Rosenberger C, Heyman SN, Rosen S, Shina A, Goldfarb M, Griethe W, Frei U, Reinke P, Bachmann S, Eckardt KU. Up-regulation of HIF in experimental acute renal failure: evidence for a protective transcriptional response to hypoxia. Kidney Int 2005; 67:531–42 [DOI] [PubMed] [Google Scholar]
- 45.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet 2013; 382:170–9 [DOI] [PubMed] [Google Scholar]
- 46.Bi LY, Zhao DA, Yang DS, Guo JG, Liang B, Zhang RX, Zhao JL, Bai HT, Li SJ. Effects of autologous SCF- and G-CSF-mobilized bone marrow stem cells on hypoxia-inducible factor-1 in rats with ischemia-reperfusion renal injury. Genet Mol Res 2015; 14:4102–12 [DOI] [PubMed] [Google Scholar]
- 47.Chua JH, Armugam A, Jeyaseelan K. MicroRNAs: biogenesis, function and applications. Curr Opin Mol Ther 2009; 11:189–99 [PubMed] [Google Scholar]
- 48.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–97 [DOI] [PubMed] [Google Scholar]
- 49.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 2008; 9:22–32 [DOI] [PubMed] [Google Scholar]
- 50.Zou YF, Zhang W. Role of microRNA in the detection, progression, and intervention of acute kidney injury. Exp Biol Med 2018; 243:129–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou YF, Wen D, Zhao Q, Shen PY, Shi H, Zhao Q, Chen YX, Zhang W. Urinary microRNA-30c-5p and microRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp Biol Med 2017; 242:657–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao W, Fu Z, Zou Y, Wen D, Ma H, Zhou F, Chen Y, Zhang M, Zhang W. MicroRNA-140-5p attenuated oxidative stress in cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism. Exp Cell Res 2017; 360:292–302 [DOI] [PubMed] [Google Scholar]
- 53.Du B, Dai XM, Li S, Qi GL, Cao GX, Zhong Y, Yin PD, Yang XS. MiR-30c regulates cisplatin-induced apoptosis of renal tubular epithelial cells by targeting Bnip3L and Hspa5. Cell Death Dis 2017; 8:e2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavkovic M, Riefke B, Ellinger-Ziegelbauer H. Urinary microRNA profiling for identification of biomarkers after cisplatin-induced kidney injury. Toxicology 2014; 324:147–57 [DOI] [PubMed] [Google Scholar]
- 55.Wang JF, Zha YF, Li HW, Wang F, Bian Q, Lai XL, Yu G. Screening plasma miRNAs as biomarkers for renal ischemia-reperfusion injury in rats. Med Sci Monit 2014; 20:283–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguado-Fraile E, Ramos E, Conde E, Rodriguez M, Martin-Gomez L, Lietor A, Candela A, Ponte B, Liano F, Garcia-Bermejo ML. A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PloS One 2015; 10:e0127175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, Fang Y, Ding X, Liang M. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int 2012; 82:1167–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramachandran K, Saikumar J, Bijol V, Koyner JL, Qian J, Betensky RA, Waikar SS, Vaidya VS. Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem 2013; 59:1742–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du J, Cao X, Zou L, Chen Y, Guo J, Chen Z, Hu S, Zheng Z. MicroRNA-21 and risk of severe acute kidney injury and poor outcomes after adult cardiac surgery. PloS One 2013; 8:e63390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaucsar T, Revesz C, Godo M, Krenacs T, Albert M, Szalay CI, Rosivall L, Benyo Z, Batkai S, Thum T, Szenasi G, Hamar P. Activation of the miR-17 family and miR-21 during murine kidney ischemia-reperfusion injury. Nucleic Acid Ther 2013; 23:344–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia P, Teng J, Zou J, Fang Y, Wu X, Liang M, Ding X. Xenon protects against septic acute kidney injury via miR-21 target signaling pathway. Crit Care Med 2015; 43:e250–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoppe B, Pietsch S, Franke M, Engel S, Groth M, Platzer M, Englert C. MiR-21 is required for efficient kidney regeneration in fish. BMC Dev Biol 2015; 15:e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaede L, Liebetrau C, Blumenstein J, Troidl C, Dorr O, Kim WK, Gottfried K, Voss S, Berkowitsch A, Walther T, Nef H, Hamm CW, Mollmann H. Plasma microRNA-21 for the early prediction of acute kidney injury in patients undergoing major cardiac surgery. Nephrol Dial Transplant 2016; 31:760–6 [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Shu L. Upregulation of miR-21 by ghrelin ameliorates ischemia/reperfusion-induced acute kidney injury by inhibiting inflammation and cell apoptosis. DNA Cell Biol 2016; 35:417–25 [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Deng X, Kang Z, Wang Y, Xia T, Ding N, Yin Y. Elevation of miR-21, through targeting MKK3, may be involved in ischemia pretreatment protection from ischemia-reperfusion induced kidney injury. J Nephrol 2016; 29:27–36 [DOI] [PubMed] [Google Scholar]
- 66.Pu XY, Shen JY, Deng ZP, Zhang ZA. Plasma-specific microRNA response induced by acute exposure to aristolochic acid I in rats. Archives of Toxicology 2017; 91:1473–83 [DOI] [PubMed] [Google Scholar]
- 67.Jia P, Wu X, Dai Y, Teng J, Fang Y, Hu J, Zou J, Liang M, Ding X. MicroRNA-21 is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit Care Med 2017; 45:e703–e10 [DOI] [PubMed] [Google Scholar]
- 68.Xu X, Song N, Zhang X, Jiao X, Hu J, Liang M, Teng J, Ding X. Renal protection mediated by hypoxia inducible factor-1alpha depends on proangiogenesis function of miR-21 by targeting thrombospondin 1. Transplantation 2017; 101:1811–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y, Hu J, Jia P, Teng J, Ding X. miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Front Physiol 2018; 9:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ge QM, Huang CM, Zhu XY, Bian F, Pan SM. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PloS One 2017; 12:e0173292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan PC, Chen CC, Peng CC, Chang CH, Yang CH, Yang C, Chu LJ, Chen YC, Yang CW, Chang YS, Chu PH. A circulating miRNA signature for early diagnosis of acute kidney injury following acute myocardial infarction. J Transl Med 2019; 17:e139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li ZL, Lv LL, Tang TT, Wang B, Feng Y, Zhou LT, Cao JY, Tang RN, Wu M, Liu H, Crowley SD, Liu BC. HIF-1alpha inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int 2019; 95:388–404 [DOI] [PubMed] [Google Scholar]
- 73.Gutierrez-Escolano A, Santacruz-Vazquez E, Gomez-Perez F. Dysregulated microRNAs involved in contrast-induced acute kidney injury in rat and human. Renal Failure 2015; 37:1498–506 [DOI] [PubMed] [Google Scholar]
- 74.Sun SQ, Zhang T, Ding D, Zhang WF, Wang XL, Sun Z, Hu LH, Qin SY, Shen LH, He B. Circulating MicroRNA-188, -30a, and -30e as early biomarkers for Contrast-Induced acute kidney injury. JAHA 2016; 5:e004138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amrouche L, Desbuissons G, Rabant M, Sauvaget V, Nguyen C, Benon A, Barre P, Rabate C, Lebreton X, Gallazzini M, Legendre C, Terzi F, Anglicheau D. MicroRNA-146a in human and experimental ischemic AKI: CXCL8-dependent mechanism of action. J Am Soc Nephrol 2017; 28:479–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012; 82:412–27 [DOI] [PubMed] [Google Scholar]
- 77.Bijkerk R, van Solingen C, de Boer HC, van der Pol P, Khairoun M, de Bruin RG, van Oeveren-Rietdijk AM, Lievers E, Schlagwein N, van Gijlswijk DJ, Roeten MK, Neshati Z, de Vries AA, Rodijk M, Pike-Overzet K, van den Berg YW, van der Veer EP, Versteeg HH, Reinders ME, Staal FJ, van Kooten C, Rabelink TJ, van Zonneveld AJ. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J Am Soc Nephrol 2014; 25:1710–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aguado-Fraile E, Ramos E, Saenz-Morales D, Conde E, Blanco-Sanchez I, Stamatakis K, del Peso L, Cuppen E, Brune B, Bermejo ML. miR-127 protects proximal tubule cells against ischemia/reperfusion: identification of kinesin family member 3B as miR-127 target. PloS One 2012; 7:e44305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conde E, Gimenez-Moyano S, Martin-Gomez L, Rodriguez M, Ramos ME, Aguado-Fraile E, Blanco-Sanchez I, Saiz A, Garcia-Bermejo ML. HIF-1alpha induction during reperfusion avoids maladaptive repair after renal ischemia/reperfusion involving miR127-3p. Sci Rep 2017; 7:41099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pellegrini KL, Han T, Bijol V, Saikumar J, Craciun FL, Chen WW, Fuscoe JC, Vaidya VS. MicroRNA-155 deficient mice experience heightened kidney toxicity when dosed with cisplatin. Toxicol Sci 2014; 141:484–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu H, Huang T, Ying L, Han C, Li D, Xu Y, Zhang M, Mou S, Dong Z. MiR-155 is involved in renal Ischemia-Reperfusion injury via direct targeting of FoxO3a and regulating renal tubular cell pyroptosis. Cell Physiol Biochem 2016; 40:1692–705 [DOI] [PubMed] [Google Scholar]
- 82.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 2011; 6:1540–6 [DOI] [PubMed] [Google Scholar]
- 83.Liu LL, Li D, He YL, Zhou YZ, Gong SH, Wu LY, Zhao YQ, Huang X, Zhao T, Xu L, Wu KW, Li MG, Zhu LL, Fan M. miR-210 protects renal cell against hypoxia-induced apoptosis by targeting HIF-1 alpha. Mol Med 2017; 23:258–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lan YF, Chen HH, Lai PF, Cheng CF, Huang YT, Lee YC, Chen TW, Lin H. MicroRNA-494 reduces ATF3 expression and promotes AKI. J Am Soc Nephrol 2012; 23:2012–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan J, Benway CJ, Bagley J, Iacomini J. MicroRNA-494 promotes cyclosporine-induced nephrotoxicity and epithelial to mesenchymal transition by inhibiting PTEN. Am J Transplant 2015; 15:1682–91 [DOI] [PubMed] [Google Scholar]
- 86.Zhou X, Qu Z, Zhu C, Lin Z, Huo Y, Wang X, Wang J, Li B. Identification of urinary microRNA biomarkers for detection of gentamicin-induced acute kidney injury in rats. Regul Toxicol Pharmacol 2016; 78:78–84 [DOI] [PubMed] [Google Scholar]
- 87.Wei Q, Sun H, Song S, Liu Y, Liu P, Livingston MJ, Wang J, Liang M, Mi QS, Huo Y, Nahman NS, Mei C, Dong Z. MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury. J Clin Invest 2018; 128:5448–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jia P, Teng J, Zou J, Fang Y, Zhang X, Bosnjak ZJ, Liang M, Ding X. miR-21 contributes to xenon-conferred amelioration of renal ischemia-reperfusion injury in mice. Anesthesiology 2013; 119:621–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mathia S, Rudigier LJ, Kasim M, Kirschner KM. A dual role of miR-22 in rhabdomyolysis-induced acute kidney injury. Acta Physiol 2018; 224:e13102. [DOI] [PubMed] [Google Scholar]
- 90.Hao J, Wei Q, Mei S, Li L, Su Y, Mei C, Dong Z. Induction of microRNA-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int 2017; 91:106–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013; 368:1685–94 [DOI] [PubMed] [Google Scholar]
- 92.Wang R, Ma J, Wu Q, Xia J, Miele L, Sarkar FH, Wang Z. Functional role of miR-34 family in human cancer. Curr Drug Targets 2013; 14:1185–91 [DOI] [PubMed] [Google Scholar]
- 93.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 2010; 78:838–48 [DOI] [PubMed] [Google Scholar]