Short abstract
NANOG is an important stem cell transcription factor involved in human development and cancerogenesis. Its expression is complex and regulated on different levels. Moreover, NANOG protein might regulate hundreds of target genes at the same time. NANOG is crucial for preimplantation development phase and progressively decreases during embryonic stem cells differentiation, thus regulating embryonic and fetal development. Postnatally, NANOG is undetectable or expressed in very low amounts in the majority of human tissues. NANOG re-expression can be detected during cancerogenesis, already in precancerous lesions, with increasing levels of NANOG in high grade dysplasia. NANOG is believed to enable cancer cells to obtain stem-cell like properties, which are believed to be the source of expanding growth, tumor maintenance, metastasis formation, and tumor relapse. High NANOG expression in cancer is frequently associated with advanced stage, poor differentiation, worse overall survival, and resistance to treatment, and is therefore a promising prognostic and predictive marker. We summarize the current knowledge on the role of NANOG in cancerogenesis and development, including our own experience. We provide a critical overview of NANOG as a prognostic and diagnostic factor, including problems regarding its regulation and detection.
Impact statement
NANOG has emerged as a key stem cell transcription factor in normal development and cancerogenesis. It is generally regarded as a good prognostic and predictive factor in various human cancers. It is less known that it is expressed already at precancerous stages in various organs, suggesting that finally an ideal candidate diagnostic marker has been discovered, enabling to distinguish between true dysplasia and reactive atypia. NANOG regulation is complex, and new insights into our understanding of its regulation might provide important information for future development in a broad field of two entirely different processes, i.e. normal development and cancerogenesis, showing how a physiologic mechanism can be used and abused, transforming itself into a key mechanism of disease development and progression.
Keywords: Nanog, stem cells, development, cancerogenesis, precancerosis, cancer
Introduction
NANOG was originally described in 2003 and named after the mythological Celtic land, ‘Tir Na Nog’, land of the ever-young.1,2 NANOG is an official gene symbol that corresponds to full gene name Nanog homebox and it has two alias symbols FLJ12581 and FLJ40451.3 It is a stem cell transcription factor that plays a major role in regulation of human development; it is involved in cell fate determination, proliferation, and apoptosis.4–7 In embryonic stem cells (ESCs), it is crucial for the maintenance of the pluripotency.8 After birth, NANOG expression is very low or it is silenced and remains in that state in physiological conditions in most tissues through life-span.9 However, NANOG expression might be detectable in cancer cells exhibiting stem cell-like properties (cancer stem cells, CSCs) which are believed to be the source of malignant transformation, progression of cancer and development of metastases. Cancer of various origin show detectable levels of NANOG.8–11
Regulation of NANOG
NANOG expression is complex and regulated on different levels, including DNA (e.g. copy number variation, methylation), mRNA (e.g. miRNAs), and protein level (e.g. protein regulators). Moreover, it has been suggested that NANOG as a protein might regulate expression of hundreds of target genes by binding to their promoter regions.7,9,12–15
Various pathways and consequences of tumor development (e.g. hypoxia) have been identified as modulators of NANOG expression. OCT4 and SOX2 are one of the most important and investigated protein regulators of NANOG; they form complex with KLF4 and bind to the OCT4/SOX2 motif upstream of the transcription start site of NANOG promoter.8 In addition, NANOG expression may be directly regulated by TCF3 and P53, in a negative manner, while BM-1 and SNAIL regulate it in a positive manner. Phosphorylation of NANOG protein by PKCε or FAK enhances its activity.5,6
Along the protein regulators, NANOG expression is also regulated by epigenetic mechanisms, e.g. miRNAs and methylation, as well as by various post-translational modifications.8,9,16 Despite extensive research, molecular regulation of NANOG in cancer cells is not completely understood yet.7
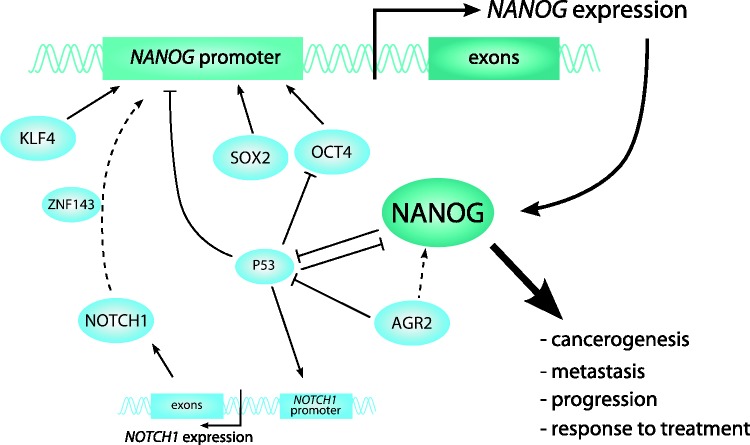
The results of our previous study provided further evidence about regulation of NANOG expression in cancer (Figure 1). In oral squamous cell carcinoma (OSCC), NANOG was in positive correlation with its protein regulators, OCT4, SOX2, KLF4, AGR2, NOTCH1, and miR-34a. Our results also suggest that copy number variation, methylation of its promoter and other tested miRNAs have a minor, if any, role in the regulation of NANOG.17
Figure 1.
Regulation of NANOG expression in carcinoma. Dashed arrows show predicted regulatory functions. (A color version of this figure is available in the online journal.)
NANOG in development
The detailed description of embryogenesis is beyond the scope of the current review and can be found elsewhere.4,5,18 Development of multicellular organisms from a single-cell zygote (fertilized oocytes) is precisely regulated by many genes, including NANOG.18 NANOG is a core transcription factor for preimplantation development phase and in embryonic and fetal development. During this process, NANOG maintains pluripotency, regulates other pluripotent-related genes, and progressively decreases as ESCs differentiate.5–9,19
Mouse models
Data on gene expression during development are available mainly from mouse models, whereas they are limited in human. Expression of Nanog and Oct4 in preimplantation phase in mouse is crucial for inner cell mass (ICM) and trophectoderm (TE) formation. During this process, level of Nanog mRNA increases at the 4- and 8-cell stage, is the highest at the morula stage, where it is polarized in the center of the morula, and then decreases. Furthermore, Nanog is restricted to ICM.1,18–20 Precise localization and regulation of these transcription factors are very important for competent blastocyst formation.18 Nevertheless, although Oct4 is believed as one of the major Nanog regulators, Nanog in ICM is not dependent on Oct4 expression.21 It is also interesting that maternal Nanog and Oct4 proteins are maintained until the 4-cell stage and start decreasing at the 8-cell stage; after that only embryonic proteins are expressed.20
In post implantation period of a mouse embryo development, Nanog and Oct4 are both crucial, as suggested from the Nanog and/or Oct4 knockout mouse models, in which embryos developed into a normal blastocyst, but thereafter, developmental arrest occurred. Similarly, Nanog-null embryos models were not able to develop into viable epiblast.20,22
Human development
There are limited data on gene expression in human development. In contrast to mouse models, studies on humans showed that NANOG mRNA expression was absent in 2-cell, 4-cell, and 8-cell stages of preimplantation phase. However, NANOG mRNA was detected from the 8-cell stage in some cells of compacted morula (eccentric localization), in the epiblast cells during their transformation from ICM, and in the ICM of a blastocyst. Moreover, it was absent in the TE.5 In contrast, another study showed that NANOG and OCT4 cDNA expression was detected in all blastomeres from the 5-, 6- and 8- cell stage embryos, regardless of the developmental stage.23
There are also limited data about NANOG expression in fetal development. During ovary development, NANOG was expressed from 5.5 to 15 weeks post fertilization, although the expression level decreased in the later stages.4 NANOG was also strongly expressed in most of the fetal gonocytes in testes up to gestational week 20, after that only single positive cells were observed and in week 42 no NANOG was detected.6,16 NANOG protein was also detected in the cells of a tooth germ of fetuses of both genders and at different gestational periods.19 Cardiac mesenchymal stem cells (MSCs) isolated from 14 to 16 week old fetuses also expressed NANOG, OCT4, and SOX2.24
Maternal NANOG expression during pregnancy has also been analyzed. NANOG mRNA and protein expression were detected in MSCs, isolated from different parts of the umbilical cord, from amniotic fluid, placenta, and chorionic villi.25,26 Human amniotic fluid stem cells express NANOG mRNA in the 1st trimester, but not in the 2nd trimester.27
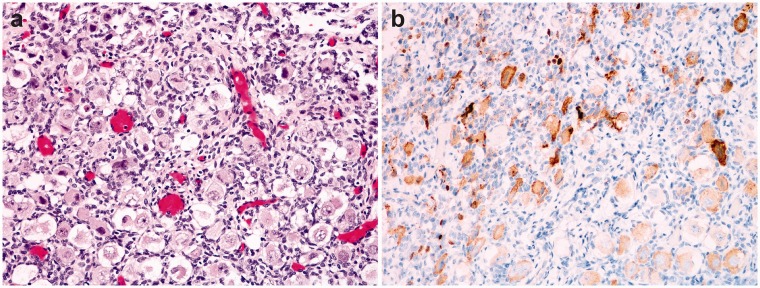
We analyzed immunohistochemical expression of NANOG in various organs and tissues of 15 human fetuses and found NANOG expression only in the testes and ovaries (Figure 2), regardless of the gestational age.
Figure 2.
Fetal ovary, 36 weeks of gestation (a). Immunohistochemistry for NANOG shows positive reaction in oocytes (b). (A color version of this figure is available in the online journal.)
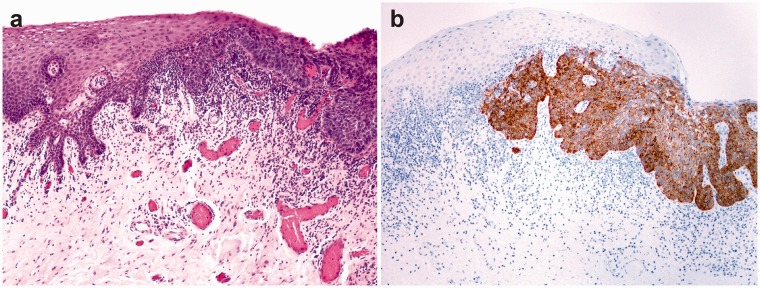
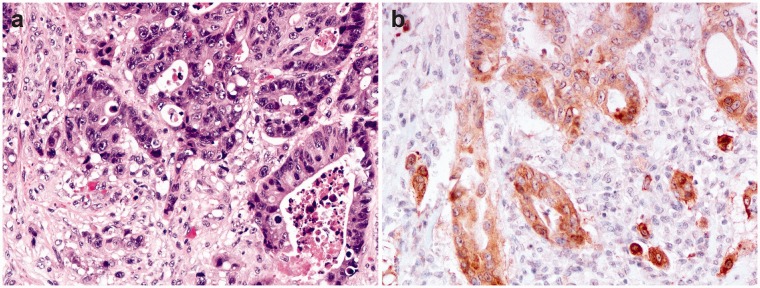
Figure 3.
Dysplasia of the oral mucosa – transition of the normal squamous epithelium to dysplastic epithelium (a). Dysplastic squamous epithelium stains immunohistochemically for NANOG, there is no staining in the normal epithelium (b). (A color version of this figure is available in the online journal.)
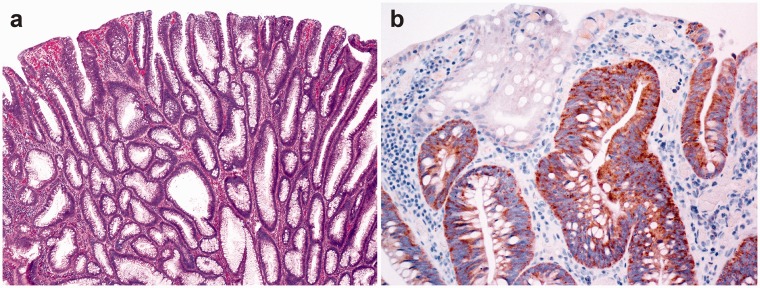
Figure 4.
Tubular adenoma of the colon (a). Focally positive immunohistochemical reaction for NANOG in dysplastic glands of adenoma (b). (A color version of this figure is available in the online journal.)
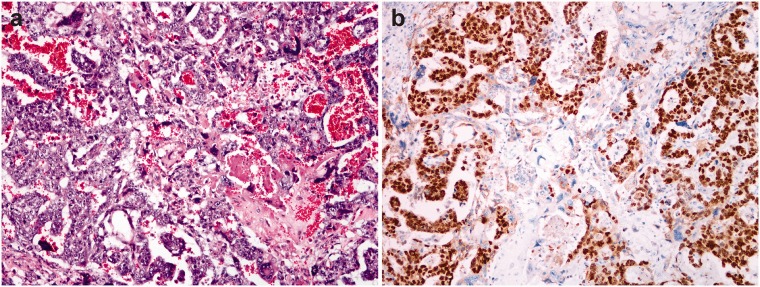
Figure 5.
Adenocarcinoma of the colon – neoplastic glands show marked cellular atypia and focal necrosis (a). Immunohistochemical reaction for NANOG is positive in the majority of tumor cells (b). (A color version of this figure is available in the online journal.)
Detection of NANOG in human tissues
After birth, NANOG is expressed in a limited number of tissues and cells. NANOG detection has been described in ESCs and MSCs. NANOG protein can be detected in healthy testis after birth; however, mRNA showed weak NANOG expression in adult testis. Some weakly positive single cells for NANOG protein have been described in small intestine, thyroid gland, and in glandular cell of uterine cervix. However, in most of the normal adult tissue, NANOG protein cannot be detected.5,6,8,9 Similarly, we did not find NANOG protein in normal mucosa of the head and neck. However, we did detect some mRNA expression of NANOG.17 We found expression of NANOG in the ovary and testis, regardless of age (Figure 2).
The discrepancies between certain studies might be also explained by differences in expression of mRNA and protein of NANOG. First, different regulatory mechanisms influence mRNA (transcription level) and protein (translational level) expression. Second, there are numerous methodological differences in detecting protein (e.g. detection systems, clones of antibodies, and scoring) as well as in detecting mRNA (sample processing, e.g. formalin-fixed paraffin-embedded or fresh tissue, primers or probes, normalization). Different function of NANOG protein might result in either cytoplasmic (translated protein with minor influence on other genes) or nuclear reaction (fully activated NANOG influencing the transcription of other genes). Both cytoplasmic and nuclear staining were detected in our study using immunohistochemistry (Figures 2 to 6). Differences in sensitivity and specificity of detection methods are another issue, besides the inter- and intratumor heterogeneity.28
Figure 6.
Mixed germ cell tumor of the testis, consisting of embryonal carcinoma and choriocarcinoma (a). Immunohistochemical reaction for NANOG is positive in embryonal carcinoma and negative in choriocarcinoma (b). (A color version of this figure is available in the online journal.)
NANOG in human cancerogenesis
NANOG is believed to be one of the crucial transcriptional factors enabling cancer cells to obtain stem-cell like properties. CSCs are similar to normal stem cells, they can differentiate and have abilities for self-renewal. CSCs manifest stem cell-like properties from oncogenic reprogramming of different self-renewal and other stem cell-related genes, miRNAs, cell surface proteins, and transcription factors. Moreover, CSCs are immortal and as such persist in tumors, usually in pools, representing the source for expanding growth of the tumor, tumor maintenance, metastasis formation, and tumor relapse.7,8,29
NANOG in precancerosis
NANOG can be expressed already in precancerous lesions suggesting that it should be considered as a diagnostic marker, enabling to distinguish between true dysplasia and reactive lesions. In the head and neck mucosa, for example, NANOG cytoplasmic protein was expressed in 60% of laryngeal dysplasias; in 27% of lesions, the expression was strong and it was negligible in normal adjacent epithelia and in stromal cells. Importantly, 55% of patients with laryngeal precancerous lesions with strong cytoplasmic NANOG reaction developed laryngeal cancer in comparison to only 20% of patients with negative to moderate NANOG expression, five years after the initial diagnosis. Similarly, in the oral mucosa, epithelial dysplasia expressed cytoplasmic and nuclear NANOG in 16% and 4% of cases, respecitvely.10,28 NANOG expression increased with the grade of dysplasia. Significant correlation with a higher risk of progression to invasive carcinoma was observed in oral dysplasia with a positive NANOG expression. Interestingly, strong cytoplasmic NANOG expression was in significant correlation with a higher cancer incidence in comparison to nuclear NANOG expression.10,28,29
In the uterine cervix, NANOG expression progressively increased from normal mucosa to cervical intraepithelial neoplasia, and it was significantly higher in cervical carcinomas.30
In the stomach, NANOG protein was detected in dysplasia (67%) and intestinal metaplasia (60%) but not in the normal mucosa.31 A higher NANOG mRNA expression was also found in colorectal adenoma in comparison to normal colon mucosa.32
NANOG in cancer
NANOG expression has been described in various human cancers. High NANOG expression was frequently associated with more advanced stage, poor differentiation, and a worse overall survival. In some studies, correlation of NANOG expression with resistance to therapy was found. NANOG is therefore believed to be a prognostic and predictive marker. The significant role of NANOG in cancer development has been further supported by an experimental finding of NANOG inhibition resulting in inhibition of tumor initiation.6–9,11,16
In head and neck squamous cell carcinoma (HNSCC), studies showed a negligible or absent NANOG protein expression in normal adjacent epithelia and in stromal cells. In OSCC, NANOG mRNA expression was significantly higher in tumor samples and non-tumoral margins (expressed in 100% and 80%, respectively) compared to healthy normal mucosa. NANOG protein was detected in 71–100% of tested OSCCs with various intensity of reaction (strong in 32–59%, moderate in 16–35% and weak or negative in 31–35%). Expression of both, protein and mRNA was associated with stage, grade, lymph node metastasis, and worse prognosis.7,10–13,33–35 Significant up-regulation of NANOG expression was observed in cisplatin-resistant patients with OSCC after therapy.36
In salivary gland mucoepidermoid carcinoma, NANOG was mainly expressed in ductal structures and associated with perineural invasion and desmoplasia when compared with normal salivary gland. Two studies showed strong NANOG protein expression in 26–46%, weak to moderate expression in 29–42%, and negative expression in 25–30% of all mucoepidermoid carcinomas.37,38
In gliomas, expression of NANOG was up-regulated compared to normal brain tissue and showed a positive correlation with malignancy, and expression was significantly higher in high‐grade compared to low‐grade gliomas. NANOG mRNA and protein expression showed positive reaction in nuclei and cytoplasm in 95% of primary glioma samples. NANOG protein expression was detected in 95% of astrocytomas, in 96% of oligodendrogliomas, in 94% of oligoastrocytomas, and in 100% of glioblastomas. Patients with astrocytomas and glioblastomas showed significantly shorter survival rate when high levels of NANOG protein were detected. Another study showed that in a group of patients that did not respond to chemoradiotherapy, mean expression of NANOG mRNA was higher, compared to responders, after three months of therapy.39–41
In lung cancer, overexpression of NANOG was significantly related to TNM stage and differentiation, to the presence of pleural and vascular invasion, and a decreased overall and disease-free survival.42–44 Various studies showed NANOG expression in 30–93% of lung cancer specimens.44–46 In squamous carcinoma, low NANOG expression was found in 72% and high in 27% of patients. In adenocarcinoma, low and high expression was found in 67% and 33% of cases, respectively.45 In another study, cytoplasmic NANOG protein was observed in 43% of adenocarcinoma and in 92% of squamous cell carcinoma. No expression was found in healthy lung tissue.43 In poorly differentiated lung cancers, nuclear NANOG expression was high.44 Another study showed NANOG overexpression as a predictive marker, since NSCLC patients treated with platinum-based chemotherapy with NANOG overexpression showed association with short overall survival and poor prognosis.47
In urinary system, NANOG was analyzed in renal cell carcinoma (RCC) and in urothelial carcinoma. In RCC, NANOG mRNA expression was significantly higher in RCC compared to tumor-adjacent tissue and was also in correlation to higher TNM stage, histological grade, and significantly lower survival rates.48–50 NANOG protein was expressed in 80–98% of all RCCs, and in only 9% of normal-adjacent tissue samples.48,49 In another study, significant differences in NANOG expression were found in RCC subtypes. NANOG cytoplasmic expression was suggested to be a prognostic predictor of RCC.49 In urothelial carcinoma, NANOG mRNA was expressed in 90% of cases.51 All urothelial carcinomas showed NANOG protein expression; 85% showed high and 15% showed low expression. NANOG was detected in the nuclei and cytoplasm in 53%, only in the cytoplasm in 36%, in the nuclear membrane in 6%, and only in nuclei in 5% of samples. Percentage of cancer cells with high NANOG expression was 91% in muscle invasive bladder cancer and in non-muscle invasive bladder cancer 74%.50
In colorectal, gastric, and esophageal carcinomas, meta-analysis on NANOG expression showed positive correlation to TNM stage, differentiation, gender, depth of infiltration, and poor overall survival. In gastric cancer, mRNA expression of NANOG was significantly higher in tumor tissue compared with paired adjacent normal tissue, and was associated with TNM stage, tumor grade, and shortened overall survival. Similarly, NANOG protein expression was positive in 10–26% of gastric cancers.52–55 In 83% of tumor-adjacent tissue samples, NANOG expression was low or absent. NANOG was correlated with advanced clinical stage, with lymph node status, tumor differentiation and poorer prognosis. However, NANOG protein was absent in gastric signet-ring cell carcinoma.56 In colorectal carcinoma, NANOG mRNA expression was detected in all cancer samples and was absent in all tumor-adjacent normal tissue samples.51 NANOG protein was mainly expressed in the cytoplasm and had significantly higher expression in tumor samples (40%) compared to tumor-adjacent tissue samples (18%).57
In pancreatic carcinoma, the intensity of NANOG immunoreactivity was significantly stronger in the metaplastic ducts than in normal acini or in pancreatic carcinoma tissue, where only weak expression was observed. NANOG protein was detected in the nuclei and cytoplasm in 38–54% cancer samples and in 19% of tumor-adjacent tissue samples.58,59 NANOG up-regulation was correlated with TNM stage in pancreatic duct adenocarcinoma. NANOG protein was expressed in 81% of cancer tissue; expression was strong in 26%, moderate in 32%, weak in 23% and it was absent in 19% of samples.60 NANOG might thus be associated with the early stages of pancreatic cancer and might be used as a potential prognostic marker.58,59
In hepatocellular carcinoma (HCC), NANOG expression showed positive correlation with TNM stage, vascular invasion (when co-expressed with OCT4) and differentiation, and predicted a worse clinical outcome. NANOG protein was expressed in 32% and was co-expressed with OCT4 in 14% of HCC cases in the nucleus and cytoplasm of HCC cells. Nanog expression was significantly higher in HCC tissue compared to tumor-adjacent tissue.61,62
In breast cancer, NANOG protein expression was mainly detected in the nuclei, and in lower amount in the cytoplasm, too. It was higher in the majority of tumor tissue samples compared with paired adjacent normal tissue and was significantly related to stage of the disease, lymph node metastasis, poor differentiation, and a worse overall survival. NANOG protein was detected in 28–56% cases of breast carcinoma. Expression was high in 9–36% and low or absent in 72–91% of tumors. Tumor-adjacent tissue expressed NANOG in 14% of cases.63–66
In ovarian cancer, NANOG mRNA and protein were increased and in positive association with clinical stage, differentiation, and with poor overall survival.67–69 Ovarian serous carcinoma expressed NANOG protein in cytoplasm in 22% and in cytoplasm and/or nucleus in 70%.67,68 NANOG-positive carcinomas are usually high-grade, whereas low-grade, borderline, and benign tumors do not express NANOG.68 Another study on ovarian tumors showed NANOG expression in 64% of samples: in 49% of normal ovarian tissue, in 65% of borderline serous cystadenomas, and in 85% of serous cystadenocarcinomas.69 In the ovarian serous cystadenoma, results on NANOG expression are controversial, from completely negative to detected expression in 48% of samples.67,69
There are very limited data on NANOG expression in prostatic and cervical cancer. NANOG mRNA expression was detected in 80% of the prostate cancer samples.51 Needle biopsy tissue containing prostatic adenocarcinoma and benign prostatic hyperplasia expressed nuclear and cytoplasmic NANOG protein.70 Squamous cancer of the cervix also expressed NANOG protein in 33% of patients; moderate to strong expression was detected in 24% of positive samples.71
Testicular germ cell tumors also express NANOG mRNA and protein; NANOG protein and mRNA were expressed in all seminomas and embryonal carcinomas, but not in teratoma, yolk sac tumor, and choriocarcinoma.16
NANOG as a treatment target
NANOG is a potential candidate for gene therapy due to its involvement in a variety of oncogenic pathways. Since there is no expression of NANOG in most healthy tissues, targeting NANOG has potentially very limited off-target effects. Specific suppression of NANOG when re-expressed in precancerosis and different tumors could affect different NANOG downstream effectors, prevent chemo-resistance, reduce metastasis and tumor growth, and enhance immune surveillance. There is evidence demonstrating therapeutic effect of targeting NANOG using different approaches in cell lines and mouse models of different cancer types. Other studies investigated synergistic therapeutic effect of targeting NANOG with cancer vaccination or with chemotherapeutic drugs, where an increased sensitivity for drugs and an induced cell apoptosis was observed.72–75 Information regarding Nanog-targeting therapy in human cancer is limited, i.e. there is an ongoing clinical trial (phase 2) with NANOG inhibitor in combination with sorafenib in adult patients with hepatocellular carcinoma.76
Conclusions
NANOG is an important stem cell transcription factor with a complex regulation, and it is involved in human development and cancerogenesis. Being associated with an aggressive course, poor prognosis, and resistance to therapy, it can be used as prognostic and predictive factor in various cancers. The fact that it is also re-expressed in precancerosis but not in the majority of the normal adult tissues and in other pathologic conditions makes it a perfect candidate marker for distinguishing between precancerosis and reactive conditions in ambiguous cases.
Authors’ contribution
All authors contributed to the manuscript preparation.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Slovenian Research Agency (ARRS) under PhD thesis grant for young researcher Gašper Grubelnik and under research core funding No. P3-0054.
ORCID iDs
Gašper Grubelnik https://orcid.org/0000-0001-5783-3327
Marina Kos https://orcid.org/0000-0002-9179-6096
Nina Zidar https://orcid.org/0000-0001-6866-3220
References
- 1.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003; 113:631–42 [DOI] [PubMed] [Google Scholar]
- 2.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003; 113:643–55 [DOI] [PubMed] [Google Scholar]
- 3.Yates B, Braschi B, Gray KA, Seal RL, Tweedie S, Bruford EA. Genenames.org: the HGNC and VGNC resources in 2017. Nucleic Acids Res 2017; 45:D619–d25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of pluripotent stem cell markers in the human fetal ovary. Hum Reprod 2008; 23:589–99 [DOI] [PubMed] [Google Scholar]
- 5.Hambiliki F, Strom S, Zhang P, Stavreus-Evers A. Co-localization of NANOG and OCT4 in human pre-implantation embryos and in human embryonic stem cells. J Assist Reprod Genet 2012; 29:1021–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology 2005; 47:48–56 [DOI] [PubMed] [Google Scholar]
- 7.Yu SS, Cirillo N. The molecular markers of cancer stem cells in head and neck tumors. J Cell Physiol 2020; 235:65–73 [DOI] [PubMed] [Google Scholar]
- 8.Gong S, Li Q, Jeter CR, Fan Q, Tang DG, Liu B. Regulation of NANOG in cancer cells. Mol Carcinog 2015; 54:679–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mato Prado M, Frampton AE, Stebbing J, Krell J. Gene of the month: NANOG. J Clin Pathol 2015; 68:763–5 [DOI] [PubMed] [Google Scholar]
- 10.Rodrigo JP, Villaronga MA, Menendez ST, Hermida-Prado F, Quer M, Vilaseca I, Allonca E, Pedregal Mallo D, Astudillo A, Garcia-Pedrero JM. A novel role for nanog as an early cancer risk marker in patients with laryngeal precancerous lesions. Sci Rep 2017; 7:11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Liu J, Chen S, Fang C, Zhang X, Luo Z. Prognostic significance of NANOG expression in solid tumors: a meta-analysis. Onco Targets Ther 2018; 11:5515–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues M, Xavier FCA, Andrade NP, Lopes C, Miguita Luiz L, Sedassari BT, Ibarra AMC, Lopez RVM, Kliemann Schmerling C, Moyses RA, Tajara da Silva EE, Nunes FD. Prognostic implications of CD44, NANOG, OCT4, and BMI1 expression in tongue squamous cell carcinoma. Head Neck 2018; 40:1759–73 [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Fan H, Xu J, Zhao E. Prognostic implication of NOTCH1 in early stage oral squamous cell cancer with occult metastases. Clin Oral Investig 2018; 22:1131–8 [DOI] [PubMed] [Google Scholar]
- 14.van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol 2018; 71:88–91 [DOI] [PubMed] [Google Scholar]
- 15.Nettersheim D, Biermann K, Gillis AJ, Steger K, Looijenga LH, Schorle H. NANOG promoter methylation and expression correlation during normal and malignant human germ cell development. Epigenetics 2011; 6:114–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer 2005; 104:2092–8 [DOI] [PubMed] [Google Scholar]
- 17.Grubelnik G, Boštjančič E, Zidar N. Expression of NANOG and its regulation in oral squamous cell carcinoma. Submitted. [DOI] [PMC free article] [PubMed]
- 18.Cui W, Mager J. Transcriptional regulation and genes involved in first lineage specification during preimplantation development. Adv Anat Embryol Cell Biol 2018; 229:31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Cunha JM, da Costa-Neves A, Kerkis I, da Silva MC. Pluripotent stem cell transcription factors during human odontogenesis. Cell Tissue Res 2013; 353:435–41 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Nozawa Y, Tsukamoto S, Kaneko T, Manabe I, Imai H, Minami N. CHD1 acts via the Hmgpi pathway to regulate mouse early embryogenesis. Development 2015; 142:2375–84 [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Scholer HR. Role of Oct4 in the early embryo development. Cell Regen 2014; 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell 2009; 138:722–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galan A, Montaner D, Poo ME, Valbuena D, Ruiz V, Aguilar C, Dopazo J, Simon C. Functional genomics of 5- to 8-cell stage human embryos by blastomere single-cell cDNA analysis. PLoS One 2010; 5:e13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garikipati VNS, Singh SP, Mohanram Y, Gupta AK, Kapoor D, Nityanand S. Isolation and characterization of mesenchymal stem cells from human fetus heart. PLoS One 2018; 13:e0192244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharti D, Shivakumar SB, Park JK, Ullah I, Subbarao RB, Park JS, Lee SL, Park BW, Rho GJ. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res 2018; 372:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim J, Razi ZR, Law J, Nawi AM, Idrus RB, Ng MH. MSCs can be differentially isolated from maternal, Middle and fetal segments of the human umbilical cord. Cytotherapy 2016; 18:1493–502 [DOI] [PubMed] [Google Scholar]
- 27.Moschidou D, Drews K, Eddaoudi A, Adjaye J, De Coppi P, Guillot PV. Molecular signature of human amniotic fluid stem cells during fetal development. Curr Stem Cell Res Ther 2013; 8:73–81 [DOI] [PubMed] [Google Scholar]
- 28.de Vicente JC, Rodriguez-Santamarta T, Rodrigo JP, Allonca E, Vallina A, Singhania A, Donate-Perez Del Molino P, Garcia-Pedrero JM. The emerging role of NANOG as an early cancer risk biomarker in patients with oral potentially malignant disorders. J Clin Med 2019; 8:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo P, Gao A, Zhang G, Han H, Zhou Q. Decoding the knots of initiation of oncogenic epithelial-mesenchymal transition in tumor progression. Curr Cancer Drug Targets 2013; 13:996–1011 [DOI] [PubMed] [Google Scholar]
- 30.Ye F, Zhou C, Cheng Q, Shen J, Chen H. Stem-cell-abundant proteins nanog, nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC Cancer 2008; 8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Wang X, Chen B, Xiao Z, Li W, Lu Y, Dai J. The human pluripotency gene NANOG/NANOGP8 is expressed in gastric cancer and associated with tumor development. Oncol Lett 2010; 1:457–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osama A, Sabry D, Hassany SM, Abdelmoneim SS, Sabry A. SIRT-1expression is associated with expression of NANOG in patients with colorectal adenocarcinoma. Cancer Biomark 2016; 17:155–63 [DOI] [PubMed] [Google Scholar]
- 33.Ravindran G, Sawant SS, Hague A, Kingsley K, Devaraj H. Association of differential beta-catenin expression with oct-4 and nanog in oral squamous cell carcinoma and their correlation with clinicopathological factors and prognosis. Head Neck 2015; 37:982–93 [DOI] [PubMed] [Google Scholar]
- 34.Kim HM, Kang YH, Byun JH, Jang SJ, Rho GJ, Lee JS, Park BW. Midkine and NANOG have similar immunohistochemical expression patterns and contribute equally to an adverse prognosis of oral squamous cell carcinoma. Int J Mol Sci 2017; 18:2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Kang YH, Lee JS, Byun JH, Kim UK, Jang SJ, Rho GJ, Park BW. Positive expression of NANOG, mutant p53, and CD44 is directly associated with clinicopathological features and poor prognosis of oral squamous cell carcinoma. BMC Oral Health 2015; 15:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY. Markedly increased Oct4 and nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med 2011; 40:621–8 [DOI] [PubMed] [Google Scholar]
- 37.Destro Rodrigues MF, Sedassari BT, Esteves CM, de Andrade NP, Altemani A, de Sousa SC, Nunes FD. Embryonic stem cells markers Oct4 and nanog correlate with perineural invasion in human salivary gland mucoepidermoid carcinoma. J Oral Pathol Med 2017; 46:112–20 [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Wang Y, Qi X, Xie J, Wei Z, Yin X, Wang Z, Meng J, Han W. Prognostic factors of palatal mucoepidermoid carcinoma: a retrospective analysis based on a double-center study. Sci Rep 2017; 7:43907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, Zhang Y, Ling EA, Gao J, Hao A. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and nanog in human gliomas. Histopathology 2011; 59:763–75 [DOI] [PubMed] [Google Scholar]
- 40.Soni P, Qayoom S, Husain N, Kumar P, Chandra A, Ojha BK, Gupta RK. CD24 and nanog expression in stem cells in glioblastoma: correlation with response to chemoradiation and overall survival. Asian Pac J Cancer Prev 2017; 18:2215–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsir T, Edqvist PH, Carlson J, Ribom D, Bergqvist M, Ekman S, Popova SN, Alafuzoff I, Ponten F, Nister M, Smits A. A study of embryonic stem cell-related proteins in human astrocytomas: identification of nanog as a predictor of survival. Int J Cancer 2014; 134:1123–31 [DOI] [PubMed] [Google Scholar]
- 42.Huang G, Zhang J, Wang X, Chen Y, Liu D, Guo S. Clinicopathological and prognostic significance of nanog expression in non-small cell lung cancer: a Meta-analysis. Onco Targets Ther 2019; 12:3609–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park E, Park SY, Sun P-L, Jin Y, Kim JE, Jheon S, Kim K, Lee CT, Kim H, Chung J-H. Prognostic significance of stem cell-related marker expression and its correlation with histologic subtypes in lung adenocarcinoma. Oncotarget 2016; 7:42502–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slawek S, Szmyt K, Fularz M, Dziudzia J, Boruczkowski M, Sikora J, Kaczmarek M. Pluripotency transcription factors in lung cancer-a review. Tumour Biol 2016; 37:4241–9 [DOI] [PubMed] [Google Scholar]
- 45.Li XQ, Yang XL, Zhang G, Wu SP, Deng XB, Xiao SJ, Liu QZ, Yao KT, Xiao GH. Nuclear beta-catenin accumulation is associated with increased expression of nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med 2013; 11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gialmanidis IP, Bravou V, Petrou I, Kourea H, Mathioudakis A, Lilis I, Papadaki H. Expression of Bmi1, FoxF1, nanog, and gamma-catenin in relation to hedgehog signaling pathway in human non-small-cell lung cancer. Lung 2013; 191:511–21 [DOI] [PubMed] [Google Scholar]
- 47.Chang B, Park MJ, Choi SI, In KH, Kim CH, Lee SH. NANOG as an adverse predictive marker in advanced non-small cell lung cancer treated with platinum-based chemotherapy. Onco Targets Ther 2017; 10:4625–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu B, Cai H, Xu Z, Xu T, Zou Q, Gu M. Expressions of stem cell transcription factors nanog and Oct4 in renal cell carcinoma tissues and clinical significance. Artif Cells Nanomed Biotechnol 2016; 44:1818–23 [DOI] [PubMed] [Google Scholar]
- 49.Rasti A, Mehrazma M, Madjd Z, Abolhasani M, Saeednejad Zanjani L, Asgari M. Co-expression of cancer stem cell markers OCT4 and NANOG predicts poor prognosis in renal cell carcinomas. Sci Rep 2018; 8:11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Md Akhir MKA, Hussin H, Veerakumarasivam A, Choy CS, Abdullah MA, Abd Ghani F. Immunohistochemical expression of NANOG in urothelial carcinoma of the bladder. Malays J Pathol 2017; 39:227–34 [PubMed] [Google Scholar]
- 51.Amini S, Fathi F, Mobalegi J, Sofimajidpour H, Ghadimi T. The expressions of stem cell markers: Oct4, nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, Colon, and prostate cancer, and certain cancer cell lines. Anat Cell Biol 2014; 47:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, Sawada T, Ohira M, Hirakawa K. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res 2012; 174:130–5 [DOI] [PubMed] [Google Scholar]
- 53.Li N, Deng W, Ma J, Wei B, Guo K, Shen W, Zhang Y, Luo S. Prognostic evaluation of nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol 2015; 32:433. [DOI] [PubMed] [Google Scholar]
- 54.Basati G, Mohammadpour H, Emami Razavi A. Association of high expression levels of SOX2, NANOG, and OCT4 in gastric cancer tumor tissues with progression and poor prognosis. J Gastrointest Cancer 2019. Jan 9. doi:10.1007/s12029-018-00200-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Liang C, Zhao T, Ge H, Xu Y, Ren S, Yue C, Li G, Wu J. The clinicopathological and prognostic value of NANOG in human gastrointestinal luminal cancer: a Meta-analysis. Int J Surg 2018; 53:193–200 [DOI] [PubMed] [Google Scholar]
- 56.Lin T, Ding YQ, Li JM. Overexpression of nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol 2012; 29:878–85 [DOI] [PubMed] [Google Scholar]
- 57.You L, Guo X, Huang Y. Correlation of cancer stem-cell markers OCT4, SOX2, and NANOG with clinicopathological features and prognosis in operative patients with rectal cancer. Yonsei Med J 2018; 59:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas 2010; 39:622–6 [DOI] [PubMed] [Google Scholar]
- 59.Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X, Lu J, Fan X, Zhu S, Wang Y, Guo Q, Wang L, Huang Y, Zhu M, Wang Z. Knockdown of Oct4 and nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett 2013; 340:113–23 [DOI] [PubMed] [Google Scholar]
- 60.Gao S, Pan Y, Song L, Dong L, Weng LI, Wang P, Hua Y, Chen Z, Liu L. Nanog predicts poor prognosis in human pancreatic cancer and is downregulated by QingyihuaJi formula in pancreatic cancer stem cells. Evid Based Complement Alternat Med 2016; 2016:7028289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin X, Li YW, Zhang BH, Ren ZG, Qiu SJ, Yi Y, Fan J. Coexpression of stemness factors Oct4 and nanog predict liver resection. Ann Surg Oncol 2012; 19:2877–87 [DOI] [PubMed] [Google Scholar]
- 62.Liang C, Zhang K, Ge H, Li W, Li G, Wu J. Prognostic and clinicopathological value of nanog in hepatocellular carcinoma: a meta-analysis. Clin Chim Acta 2018; 477:24–31 [DOI] [PubMed] [Google Scholar]
- 63.Emadian Saravi O, Naghshvar F, Torabizadeh Z, Sheidaei S. Immunohistochemical expression of nanog and its relation with clinicopathologic characteristics in breast ductal carcinoma. Iran Biomed J 2019; 23:184–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F, Zhang J, Yang H. OCT4, SOX2, and NANOG positive expression correlates with poor differentiation, advanced disease stages, and worse overall survival in HER2(+) breast cancer patients. Onco Targets Ther 2018; 11:7873–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagata T, Shimada Y, Sekine S, Hori R, Matsui K, Okumura T, Sawada S, Fukuoka J, Tsukada K. Prognostic significance of NANOG and KLF4 for breast cancer. Breast Cancer 2014; 21:96–101 [DOI] [PubMed] [Google Scholar]
- 66.Jin C, Zhang X, Sun M, Zhang Y, Zhang G, Wang B. Clinical implications of the coexpression of SRC1 and NANOG in HER-2-overexpressing breast cancers. Onco Targets Ther 2016; 9:5483–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee M, Nam EJ, Kim SW, Kim S, Kim JH, Kim YT. Prognostic impact of the cancer stem cell-related marker NANOG in ovarian serous carcinoma. Int J Gynecol Cancer 2012; 22:1489–96 [DOI] [PubMed] [Google Scholar]
- 68.Kenda Suster N, Frkovic Grazio S, Virant-Klun I, Verdenik I, Smrkolj S. Cancer stem cell-related marker NANOG expression in ovarian serous tumors: a clinicopathological study of 159 cases. Int J Gynecol Cancer 2017; 27:2006–13 [DOI] [PubMed] [Google Scholar]
- 69.Pan Y, Jiao J, Zhou C, Cheng Q, Hu Y, Chen H. Nanog is highly expressed in ovarian serous cystadenocarcinoma and correlated with clinical stage and pathological grade. Pathobiology 2010; 77:283–8 [DOI] [PubMed] [Google Scholar]
- 70.Jiang MY, Lee TL, Hao S-S, Mahooti S, Baird SM, Donoghue DJ, Haas M. Visualization of early prostatic adenocarcinoma as a stem cell disease. Oncotarget 2016; 7:76159–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chopra S, Deodhar K, Pai V, Pant S, Rathod N, Goda JS, Sudhalkar N, Pandey P, Waghmare S, Engineer R, Mahantshetty U, Ghosh J, Gupta S, Shrivastava S. Cancer stem cells, CD44, and outcomes following chemoradiation in locally advanced cervical cancer: results from a prospective study. Int J Radiat Oncol Biol Phys 2019; 103:161–8 [DOI] [PubMed] [Google Scholar]
- 72.Wang ML, Chiou SH, Wu CW. Targeting cancer stem cells: emerging role of nanog transcription factor. Onco Targets Ther 2013; 6:1207–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuciak M, Mas C, Borges I, Sanchez-Gomez P, Ruiz I. Chimeric NANOG repressors inhibit glioblastoma growth in vivo in a context-dependent manner. Sci Rep 2019; 9:3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia Y, Xu T, Wang C, Li Y, Lin Z, Zhao M, Zhu B. Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy. Int J Nanomed 2018; 13:143–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang CE, Yu CC, Hu FW, Chou MY, Tsai LL. Enhanced chemosensitivity by targeting nanog in head and neck squamous cell carcinomas. Int J Mol Sci 2014; 15:14935–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Annett S, Robson T. Targeting cancer stem cells in the clinic: current status and perspectives. Pharmacol Ther 2018; 187:13–30 [DOI] [PubMed] [Google Scholar]