Abstract
Objective:
To assess which patients respond best following cytoreductive nephrectomy for renal cell carcinoma (RCC) with sarcomatoid dedifferentiation (sRCC) and whether outcomes are improving over time.
Methods:
We identified 562 patients with metastatic RCC treated between 1989–2018 with cytoreductive nephrectomy. We reviewed baseline clinical and pathological characteristics, including the presence of sRCC, and metastatic sites at time of nephrectomy. The primary study endpoint was overall survival (OS). Univariate and multivariate Cox-regression analyses were used to identify significant predictors of OS.
Results:
The study cohort had 192 sRCC patients, with a median age of 59 years. Frequently involved metastatic locations were lung (n=115), retroperitoneal nodes (n=63) and axial skeleton (n=43). Lung metastasis were more prevalent in clear cell histology (p=0.0017) whereas nodal involvement was associated with non-clear cell subtypes (p=0.0064).
Median follow-up was 14 months. Estimated 2- and 5-year OS were 34.1% and 14.8%, respectively. On multivariate analysis, metastases to the liver (HR=1.64; 95% CI 1.02–2.63; p=0.04), lung (HR=1.50; 95% CI 1.05–2.14; p=0.03), retroperitoneal nodes (HR=1.52; 95% CI 1.03–2.25; p=0.04) and non-clear cell histology (HR=1.61; 95% CI 1.10–2.35; p=0.01) were associated with worse OS in the sRCC cohort.
Conclusion:
OS after cytoreductive nephrectomy for sRCC and non-sRCC is improving over time. In patients with sRCC, presentations with unifocal metastasis not involving the liver or lung, clear cell histology and node negative disease have better outcomes following cytoreductive nephrectomy and may yield greater benefit from the procedure.
Keywords: Cytoreductive nephrectomy, Sarcomatoid Dedifferentiation, Histology, Metastatic locations, Renal Cell Carcinoma
Introduction
Renal cell carcinoma (RCC) with sarcomatoid dedifferentiation (sRCC) is an aggressive tumor variant, occurring in approximately 5% of all RCC and 15–20% of patients presenting with metastatic RCC 1–3. Typically presenting as advanced disease across multiple histological subtypes 4, 5, sRCC is an independent predictor of worse outcomes including disease progression, cancer-specific and overall mortality 6, 7. Prior to the introduction of targeted therapy, Shuch et al. reported that cytoreductive nephrectomy in sRCC had a median survival of 5–6 months compared with 17.7–22 months for non-sRCC 2. With the expansion of newer, more effective systemic therapy options since 2006, outcomes following cytoreductive nephrectomy for sRCC compared with non-sRCC may have improved.
The CARMENA trial has recently questioned the benefit of cytoreductive nephrectomy in general, emphasizing the importance of appropriate patient selection for the procedure 8, 9. Despite the aggressive nature of sRCC and poor survival associated with it, the CARMENA study did not report the impact of sRCC on the trial’s survival outcomes. In patients with sRCC undergoing cytoreductive nephrectomy, clear cell histology and a lower percentage of sarcomatoid features predict for improved overall survival 2. Studies not restricted to sRCC also found that metastatic disease in retroperitoneal nodes, liver and supra-diaphragmatic nodes at the time of nephrectomy predict worse overall outcomes 10, 11. However, whether the sites of metastases at the time of cytoreductive nephrectomy has a similar effect on outcomes in sRCC requires further exploration.
Therefore, we aimed to determine whether outcomes of cytoreductive nephrectomy for sRCC have improved over time and whether granular details including sites of metastases are associated with survival outcomes.
Methods
Following Institutional Review Board approval, we searched our prospectively collated kidney cancer database and identified 562 patients with metastatic RCC treated with a cytoreductive nephrectomy between the years 1989 and 2018. Of these patients, 192 had sarcomatoid features within their pathology reports and were included in the final study cohort.
Baseline clinical characteristics including age, sex, race, body mass index (BMI), Karnofsky Performance Status (KPS), smoking history and preoperative systemic therapies were collected for all patients. All surgical specimens were reviewed by dedicated genitourinary pathologists to verify the presence of sarcomatoid differentiation, although the percentage of sarcomatoid features was not available. Tumor histology, size and stage were noted. The total number and location of metastatic sites at the time of surgery, identified by imaging studies with or without histological confirmation, were systematically annotated. Disease involving the vertebrae, skull, facial bones, ribs and sternum were defined as involving the “axial skeleton”; all other bone metastases were classified as “appendicular bone”. Nodal involvement was assessed as a whole and categorized into supra-diaphragmatic, retroperitoneal and atypical nodal sites for metastatic site evaluation. Sites which were involved in <10% of patients were categorized as “other”.
Baseline clinicopathological characteristics were summarized with the median and interquartile range (IQR) for continuous variables and the frequency and percentage for categorical variables. These variables were compared by the presence of sarcomatoid features across the 562 patients and by histology (clear cell vs. non-clear cell) within the sRCC cohort using the Fisher Exact test for categorical variables and Mann-Whitney U test for continuous variables. We further evaluated the association of metastatic sites with tumor histology, and the presence of multiple metastases, using the Fisher Exact test. Patient outcomes within the sRCC cohort and across the entire sRCC and non-sRCC groups were compared between therapeutic eras: cytokine (pre-2005), targeted (2006 – 2014) and immunotherapy (2015 – 2018).
The primary study endpoint was overall survival (OS) defined as the time from nephrectomy to death. The Kaplan-Meier method was used to estimate overall survival with survivors censored at their last follow-up date. The log-rank test was used for between group comparisons. Univariate Cox regression analyses of clinicopathological factors including metastatic sites was undertaken to identify significant predictors of OS. A multivariate analysis of significant findings from the univariate model was performed to identify independent predictors of outcome. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using R version 3.5.1 (R Core Development Team, Vienna, Austria).
Results
From the initial 562 patients, the cohort included 192 patients with sRCC, 153 of whom were men (80%) (Supplemental Table 1). The median age at the time of nephrectomy was 59 years (IQR, 52–66). Approximately half the sRCC cohort had a history of previous cigarette smoking and most patients (85%) had a KPS ≥80. Clear cell histology was apparent in 139 patients (72%). Fourteen patients received systemic therapy prior to consolidative nephrectomy. Patients with clear cell RCC were more often males (84% vs. 70%, p=0.045) and patients with non-clear cell histology were more likely to have nodal involvement (64% vs. 42%, p=0.016). Baseline clinicopathological characteristics stratified by tumor histology are outlined in Table 1.
Table 1.
Baseline clinicopathological characteristics, stratified by histology
| Clear Cell (n=139) | Non-Clear Cell (n=53) | p-value | |
|---|---|---|---|
| Gender; Male (%) | 116 (83.5) | 37 (69.8) | 0.045 |
| Race (%) | 0.167 | ||
| Asian | 6 (4.3) | 0 (0.0) | |
| Black | 2 (1.4) | 3 (5.7) | |
| White | 112 (80.6) | 44 (83.0) | |
| Unknown | 19 (13.7) | 6 (11.3) | |
| BMI (median [IQR]) | 26.60 [24.22, 30.71] | 24.80 [21.92, 29.95] | 0.075 |
| Age (median [IQR]) | 60.25 [51.82, 67.41] | 58.67 [49.65, 63.89] | 0.182 |
| Smoking History; Yes (%) | 77 (55.4) | 26 (50.0) | 0.519 |
| No. of Metastatic Organs (median [IQR]) | 2.00 [1.00, 3.00] | 2.00 [1.00, 3.00] | 0.528 |
| Primary Tumor Stage (%) | 0.474 | ||
| T1 / T2 | 21 (14.5) | 5 (9.4) | |
| T3 / T4 | 118 (85.5) | 48 (90.6) | |
| Tumor Size (median [IQR]) | 10.00 [8.00, 11.90] | 9.40 [6.50, 14.00] | 0.871 |
| Node Status (%) | 0.016 | ||
| Involved | 59 (42.4) | 34 (64.2) | |
| Nx | 39 (28.1) | 12 (22.6) | |
| Not Involved | 41 (29.5) | 7 (13.2) | |
| Pretreated; Yes (%) | 11 (7.9) | 3 (5.7) | 0.761 |
| KPS < 80 | 16 (14.4) | 4 (9.8) | 0.593 |
There were 392 metastatic sites at the time of nephrectomy, with a median of 2 involved sites per patient (range 1–5, IQR 1–3). Seventy-two (37.5%) patients had unifocal metastases (one site of metastatic disease) and 120 (62.5%) patients had multifocal metastases (>1 site). There was no significant difference in the number of metastatic sites between clear cell and non-clear cell tumors (p=0.74). The lung (n=115, 59.9%), retroperitoneal lymph nodes (n=64, 33.3%) and axial skeleton (n=42, 21.9%) were the most common metastatic sites. Distinct patterns of metastatic distribution were noted in the different histological sub-groups; clear cell tumors preferentially metastasized to the lung (OR 2.83, p=0.0017) and non-clear cell to retroperitoneal nodes (OR 2.04, p=0.040) (Figure 1).
Figure 1.
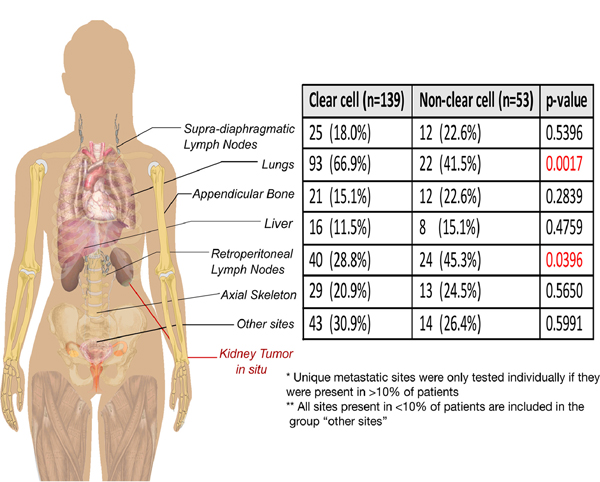
Distribution of sRCC metastatic sites
Median follow up of survivors was 14 months (IQR 6.7 – 38.6) (Supplemental Figure 1). Of the total cohort, 142/192 patients died, 136 (95.8%) from cancer specific causes, with a median time for any-cause mortality of 11 months. The median OS was 13.5 months (95% CI: 11.8 – 19.1) and the estimated 2- and 5-year OS was 34.1% (95% CI: 27.5–42.3%) and 14.8% (95% CI: 9.7–22.6%), respectively. The sRCC cohort had significantly worse survival compared with non-sRCC patients (p<0.0001) (Figure 2A), even after adjusting for differences among patients with complete baseline characteristics (HR 1.96; 95% CI: 1.50 – 2.57 p<0.001) (Supplementary Figure 2).
Figure 2.
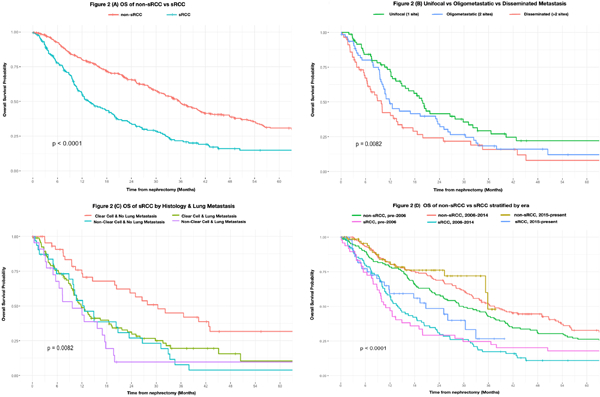
Survival plots of:
A) OS of sRCC by histology and lung metastases
B) OS of sRCC by metastatic focality
C) OS of non-sRCC vs sRCC
D) OS of non-sRCC vs sRCC stratified by era
Non-clear cell histology and multifocal metastases were predictive for worse OS. The number of disease sites was a significant predictor when evaluated as a continuous variable or categorized into unifocal vs multifocal (Figure 2B). Metastatic disease involving the liver, retroperitoneal nodes and the lung also conferred inferior survival outcomes. When all significant variables were combined into a multivariable analysis, the locations and histology, but not metastatic focality, were statistically significant (Table 2).
Table 2.
Univariate and multivariate factors predicting OS for sRCC
| Univariate OS Analysis | Multivariable OS Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Category (Ref.) | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Age at Nephrectomy | Age (Years) | 1.0049 | 0.99–1.02 | 0.5392 | |||
| Gender | Male (Ref. Female) | 1.0444 | 0.69–1.57 | 0.8356 | |||
| Karnofsky Performance Status | <80 (Ref. ≥80) | 1.3476 | 0.78–2.32 | 0.2822 | |||
| Smoking Status | Smoking History (Ref. Non-smoker) | 1.0598 | 0.76–1.48 | 0.7327 | |||
| Prior Systemic Therapy | Pretreated (Ref. Treatment naïve) | 0.8359 | 0.45–1.55 | 0.5691 | |||
| Tumor Size | Maximum Size (cm) | 1.0320 | 0.99–1.07 | 0.1130 | |||
| Tumor Stage | T3/T4 (Ref. T1/T2) | 1.5475 | 0.94–2.55 | 0.0855 | |||
| Histology | Non-Clear Cell (Ref. Clear Cell) | 1.5196 | 1.06–2.17 | 0.0216 | 1.6116 | 1.10–2.35 | 0.0134 |
| Metastatic Focality | Multifocality (Ref.Unifocality) | 1.6257 | 1.15–2.31 | 0.0066 | 1.265 | 0.84–1.91 | 0.2623 |
| Retroperitoneal Adenopathy | Involved (Ref. Not Involved) | 1.8286 | 1.29–2.60 | 0.0008 | 1.5211 | 1.03–2.25 | 0.0350 |
| Supra-diaphragmatic Adenopathy | Involved (Ref. Not Involved) | 1.1621 | 0.78–1.74 | 0.4664 | |||
| Lung Metastases | Involved (Ref. Not Involved) | 1.4308 | 1.02–2.01 | 0.0393 | 1.4974 | 1.05–2.14 | 0.027 |
| Appendicular Bone Metastases | Involved (Ref. Not Involved) | 0.9472 | 0.61–1.48 | 0.8119 | |||
| Axial Skeleton Metastases | Involved (Ref. Not Involved) | 0.9532 | 0.64–1.41 | 0.8117 | |||
| Liver Metastases | Involved (Ref. Not Involved) | 1.9121 | 1.21–3.02 | 0.0055 | 1.6383 | 1.02–2.63 | 0.0413 |
| Rare Site Metastases | Involved (Ref. Not Involved) | 1.1763 | 0.82–1.69 | 0.3833 | |||
Nodal involvement was more common in non-clear cell histology, driven by a non-clear cell propensity for retroperitoneal nodal metastases (p=0.041). Nodal involvement was also significantly associated with OS, however when analyzing the association between the different metastatic sites and outcome, retroperitoneal nodal involvement was the only nodal site that independently predicted for worse OS. We therefore included retroperitoneal nodal involvement in the multivariate analysis.
Lung metastases were significantly associated with multifocal disease (p=0.0024), however after adjusting for the number of metastatic sites as a continuous variable or as unifocal vs multifocal, the presence of lung metastases remained a significant predictor of worse OS. Lung metastases also significantly potentiated clear cell histology outcomes, with the absence of lung involvement and clear cell histology otherwise representing the most favorable presentation for lengthened survival following cytoreductive nephrectomy (Figure 2C).
In terms of preoperative management, there was no survival benefit for the 14 patients who received systemic therapy prior to cytoreduction. Preoperative biopsy occurred in 44 patients, with 63.6% sensitivity for sRCC. Undertaking a preoperative biopsy was not associated with a statistically significant difference in OS (p=0.19). Furthermore, of the patients with a preoperative biopsy, a positive biopsy finding was not predictive for worse OS (p=0.52).
There was a significant improvement in survival between the sRCC groups and non-sRCC groups when separated by therapeutic eras (p<0.0001) (Figure 2D). Within just the sRCC cohort, despite advancements in technology and therapy, there has not been a statistically significant improvement in patient outcomes across the cytokine (n=48), targeted (n=92) and immunotherapy eras (n=52) (p=0.45). Nonetheless, the median OS has increased for sRCC across each of the therapeutic eras, from 10.9 months pre-2006 to 20.7 months in patients treated from 2015–2018.
Discussion
In the current study, we report the results of the largest single institution series evaluating outcomes from cytoreductive nephrectomy for sRCC. Similar to previous publications, we observed that patients with sRCC experienced generally poor outcomes, with a median survival of 13.5 months, and 95.8% of deaths due to cancer-specific causes. However, outcomes from cytoreductive nephrectomy for sRCC and non-sRCC improved over time. Within the sRCC cohort, non-clear cell histology and multifocal disease were associated with worse OS, as were retroperitoneal, lung and liver metastases.
Studies evaluating patients with metastatic RCC in the cytokine therapy era showed a benefit from utilizing a cytoreductive nephrectomy 12–14. Retrospective studies in the targeted era also found a benefit 15, 16. However, the recently published CARMENA trial, a randomized control trial, showed non-inferiority for Sunitinib alone vs upfront nephrectomy followed by Sunitinib (HR 0.89; 95% CI 0.71 – 1.10) 8. This outcome, possibly reflecting the high proportion of poor risk patients recruited to the trial, emphasizes the importance of patient selection for cytoreductive nephrectomy 9. Moreover, the CARMENA trial did not explore the impact of sRCC on their results. Several studies evaluating the outcome of cytoreductive nephrectomy have included sarcomatoid dedifferentiation as a reportable variable. Most studies showed a significantly elevated risk for worse survival on multivariate analysis, with a hazard ratio ranging from 1.69 – 2.14 1, 17–19. In two other studies, sRCC was significant in univariate, but not multivariate analysis 20, 21. One study found a reduced hazard ratio on multivariate analysis, although not statistically significant, which may be due to including tumor grade in the same model 22.
Shuch et al directly compared 62 sRCC cytoreductive nephrectomies with 355 non-sRCC cases, demonstrating a significantly worse overall survival of 4.9 vs 17.7 months (HR 1.66; 95% CI 1.31 – 2.11). In addition to sarcomatoid features, the paper reported that gender, performance status, the number of metastatic sites and tumor size also impacted outcomes 2. Notably, patients in the study with sRCC were less likely to receive any systemic therapy than patients with non-sRCC and those that did mostly received either Interleukin-2 or Gemcitabine/Doxyrubicin. Thomas et al also explored matched sRCC patients undergoing cytoreductive nephrectomy with and without metastasectomy 23. The researchers demonstrated that positive lymph nodes on pathology doubles risk of death (HR 2.1 95% CI 1.1 – 4.0, p=0.03) from sRCC, but did not see a statistically significant benefit from metastasectomy in this population. The median overall survival was 8.3 months. Our sRCC cohort had a median survival of 13.5 months, with the difference possibly explained by a higher proportion of patients with a better performance status (KPS ≥90: 51% vs 23% vs 23%) and a more contemporary cohort. Nonetheless, we also noted a significant difference between postoperative outcomes of patients with sRCC compared with non-sRCC.
In order to improve patient selection for cytoreductive nephrectomy, Culp et al evaluated predictors of survival among all patients undergoing cytoreductive nephrectomy 10, 11. A preoperative risk criteria was reported consisting of low serum albumin, elevated serum lactate dehydrogenase, liver metastasis, retroperitoneal adenopathy, supra-diaphragmatic adenopathy, clinical stage ≥T3 disease and symptomatic metastasis. Despite the fact that sarcomatoid dedifferentiation was associated with outcome, it was not included in the final criteria due to the difficulties in reliably detecting sRCC on the preoperative biopsy. Similarly, in the current cohort focused on patients with sRCC, we found an association between liver and retroperitoneal metastatic sites and adverse outcome. While we did not find an association between supra-diaphragmatic metastases and outcome, lung metastases were associated with inferior survival. This may be the result of a different coding system.
We found a high rate of lung (60%) and nodal (48%) metastases in our cohort. Previous studies of metastatic sRCC have reported similar rates of lung (63% – 70%) and nodal disease (27% – 55%) 23, 24. However, in addition to this finding, we observed an association between these metastatic sites and histology, with clear cell histology associated with lung metastases and non-clear cell histology associated with retroperitoneal metastases. This association has not been described in patients with sRCC before. Interestingly, a similar pattern has been reported between primary tumor histology and the location of asynchronous metastases after nephrectomy for localized RCC 25. Potentially, patients with non-clear cell histology may benefit from lymph node dissection at the time of surgery. Furthermore, when combining location with histology there was an effect on OS. Patients with clear cell histology without lung metastases had significantly greater survival after cytoreductive nephrectomy.
While outcomes for sRCC are poor with systemic therapy or surgery 24, 26, the optimal timing for surgery in metastatic sRCC remains equivocal 27. The argument against upfront cytoreductive nephrectomy is that not all patients will be able to receive systemic therapy following surgery. However, a population-based study demonstrated that patients with metastatic RCC receiving an upfront cytoreductive nephrectomy are more likely to ultimately receive targeted therapy plus surgery and this was subsequently associated with an improved OS 28. We did not find an association between treatment sequence and outcome, however only 14 patients were treated with systemic therapy prior to surgery limiting the power of this analysis.
Despite not directly testing the systemic therapy used, we explored outcomes between patients operated on in the cytokine, targeted and early stages of the immunotherapy eras and observed an increase in the median OS. Although this did not reach statistical significance in the sRCC group alone, when comparing the sRCC and non-sRCC cohort there was a statistically significant improvement in survival across these eras. This improvement may be due to better perioperative care, systemic treatment, or patient selection. We did not explore changes in patients determined as non-operative candidates. Researching whether patient selection for cytoreductive nephrectomy has changed over this period is important to understand potential reasons for improved outcomes. In terms of systemic therapy, previous publications have shown a high rate of PD-1 and PD-L1 receptor expression in tumors with sarcomatoid dedifferentiation, suggesting that they may be more sensitive to immunotherapy 29. With new immunotherapy options available, the question will arise as to whether cytoreductive nephrectomy should be delayed until after systemic therapy.
There are limitations to this analysis. Due to the retrospective design of the study, we did not have accurate data regarding percentage of sarcomatoid features, the presence of rhabdoid features nor hematological parameters of patients, which were included in some previous studies. When available, these parameters should be included in future studies. Furthermore, we could not accurately account for all systemic treatments given to the patients. The evaluation of sites of metastases does not address the volume of disease burden; this has previously been demonstrated to be important 30 and was not explored in this analysis.
Conclusion
Our study confirmed a poorer survival outcome of patients with sRCC undergoing cytoreductive nephrectomy compared with non-sRCC, with a 2-year overall survival of 34%. Among these patients, those with clear cell histology and non-lung metastases had significantly greater OS. This information could help select patients for cytoreductive nephrectomy in the presence of sarcomatoid histology.
While outcomes for sRCC and non-sRCC have improved over time, the approval of new immunotherapy agents will necessitate a re-evaluation of how to optimally deliver multimodal care for the management of metastatic sRCC.
Supplementary Material
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Glossary
Abbreviations
- CARMENA
Cancer du Rein Metastatique Nephrectomie et Antiangiogéniques
- CI
Confidence interval
- HR
Hazard ratio
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
- IQR
Interquartile range
- KPS
Karnofsky Performance Status
- OS
Overall survival
- RCC
Renal cell carcinoma
- sRCC
Renal cell carcinoma with sarcomatoid dedifferentiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gu L, Li H, Wang H, et al. Presence of sarcomatoid differentiation as a prognostic indicator for survival in surgically treated metastatic renal cell carcinoma. Journal of Cancer Research and Clinical Oncology. 2017;143:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuch B, Said J, La Rochelle JC, et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology--is up-front resection indicated and, if not, is it avoidable? J Urol. 2009;182:2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebacle C, Pooli A, Bessede T, Irani J, Pantuck AJ, Drakaki A. Epidemiology, biology and treatment of sarcomatoid RCC: current state of the art. World Journal of Urology. 2018. [DOI] [PubMed] [Google Scholar]
- 4.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome . The American journal of surgical pathology. 2004;28:435–441. [DOI] [PubMed] [Google Scholar]
- 5.Shuch B, Bratslavsky G, Shih J, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU international. 2012;109:1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Wu B, Zha Z, Zhao H, Feng Y. The prognostic value and clinicopathological features of sarcomatoid differentiation in patients with renal cell carcinoma: a systematic review and meta-analysis. Cancer management and research. 2018;10:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang BY, Thompson RH, Lohse CM, et al. A novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU international. 2015;115:405–411. [DOI] [PubMed] [Google Scholar]
- 8.Méjean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. 2018;379:417–427. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Russo P. Cytoreductive Nephrectomy - Patient Selection Is Key. N Engl J Med. 2018;379:481–482. [DOI] [PubMed] [Google Scholar]
- 10.Culp SH, Karam JA, Wood CG. Population-based analysis of factors associated with survival in patients undergoing cytoreductive nephrectomy in the targeted therapy era. Urologic oncology. 2014;32:561–568. [DOI] [PubMed] [Google Scholar]
- 11.Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010;116:3378–3388. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. [DOI] [PubMed] [Google Scholar]
- 13.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. The New England journal of medicine. 2001;345:1655–1659. [DOI] [PubMed] [Google Scholar]
- 14.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet (London, England). 2001;358:966–970. [DOI] [PubMed] [Google Scholar]
- 15.Bhindi B, Abel EJ, Albiges L, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma . European Urology. 2019;75:111–128. [DOI] [PubMed] [Google Scholar]
- 16.Heng DYC, Wells JC, Rini BI, et al. Cytoreductive Nephrectomy in Patients with Synchronous Metastases from Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium.European Urology. 2014;66:704–710. [DOI] [PubMed] [Google Scholar]
- 17.Abel EJ, Spiess PE, Margulis V, et al. Cytoreductive Nephrectomy for Renal Cell Carcinoma with Venous Tumor Thrombus. The Journal of urology. 2017;198:281–288. [DOI] [PubMed] [Google Scholar]
- 18.Carrasco A, Thompson RH, Leibovich BC, Lohse CM, Cheville JC, Boorjian SA. The impact of histology on survival for patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy. Indian journal of urology : IJU : journal of the Urological Society of India. 2014;30:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda H, Takagi T, Kondo T, et al. Prognostic value of the Glasgow Prognostic Score for patients with metastatic renal cell carcinoma treated by cytoreductive nephrectomy. International Journal of Clinical Oncology. 2018;23:539–546. [DOI] [PubMed] [Google Scholar]
- 20.Capitanio U, Abdollah F, Matloob R, et al. Effect of number and location of distant metastases on renal cell carcinoma mortality in candidates for cytoreductive nephrectomy: implications for multimodal therapy. International journal of urology : official journal of the Japanese Urological Association. 2013;20:572–579. [DOI] [PubMed] [Google Scholar]
- 21.Sakai I, Miyake H, Hinata N, Fujisawa M. Improved survival in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy in the era of targeted therapy. International Journal of Clinical Oncology. 2014;19:674–678. [DOI] [PubMed] [Google Scholar]
- 22.Corcoran AT, Kaffenberger SD, Clark PE, et al. Hypoalbuminaemia is associated with mortality in patients undergoing cytoreductive nephrectomy. BJU international. 2015;116:351–357. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AZ, Adibi M, Slack RS, et al. The Role of Metastasectomy in Patients with Renal Cell Carcinoma with Sarcomatoid Dedifferentiation: A Matched Controlled Analysis. The Journal of urology. 2016;196:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korenbaum C, Pierard L, Thiery A, et al. Treatments, Outcomes, and Validity of Prognostic Scores in Patients With Sarcomatoid Renal Cell Carcinoma: A 20-Year Single-Institution Experience. Clinical genitourinary cancer. 2018;16:e577e–586. [DOI] [PubMed] [Google Scholar]
- 25.Narayan V, Puligandla M, Haas NB, Subramanian P, DiPaola RS, Uzzo R. Patterns of Relapse and Implications for Post-Nephrectomy Surveillance in Patients with High Risk Nonclear Cell Renal Cell Carcinoma: Subgroup Analysis of the Phase 3 ECOG-ACRIN E2805 Trial. The Journal of urology. 2019;201:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyriakopoulos CE, Chittoria N, Choueiri TK, et al. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clinical genitourinary cancer. 2015;13:e79–85. [DOI] [PubMed] [Google Scholar]
- 27.Bex A, Mulders P, Jewett M, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA oncology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhindi B, Habermann EB, Mason RJ, et al. Comparative Survival following Initial Cytoreductive Nephrectomy versus Initial Targeted Therapy for Metastatic Renal Cell Carcinoma. The Journal of urology. 2018;200:528–534. [DOI] [PubMed] [Google Scholar]
- 29.Joseph RW, Millis SZ, Carballido EM, et al. PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation . Cancer immunology research. 2015;3:1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbastefano J, Garcia JA, Elson P, et al. Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU international. 2010;106:1266–1269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


