Abstract
Background
The increased activity of regulatory B cells (Breg) is known to be involved in immunosuppression during helminth infection, which is characterized by inducing IL-10-producing Breg cells. However, the current knowledge of B cell subsets differentiation and IL-10-independent immunoregulatory mechanisms of B cells in schistosomiasis is insufficient.
Methods
BALB/c mice were percutaneously infected with cercariae for investigating the profile of B cell subsets during Schistosoma japonicum infection. B cells isolated from the spleen or peritoneal cavity were analyzed for the regulatory phenotype after stimulation with soluble egg antigens (SEA) in vitro. CD4+ T cells were then cocultured with B cells pretreated with or without anti-PD-L1 antibody for investigating the role of B cells from infected mice on regulating CD4+ T cells. Furthermore, the in vivo administration of anti-PD-L1 antibody was conducted to investigate the role of PD-L1 in regulating host immunity during infection.
Results
The percentages of peritoneal and splenic B-1a cells, as well as marginal zone B (MZB) cells were decreased at eight and twelve weeks after infection compared to those from uninfected mice. In splenic B cells, TGF-β expression was increased at eight weeks but declined at twelve weeks of infection, and PD-L1 expression was elevated at both eight and twelve weeks of infection. In addition, SEA stimulation in vitro significantly promoted the expression of IL-10 in peritoneal B cells and CD5 in splenic B cells, and the SEA-stimulated splenic and peritoneal B cells preferentially expressed PD-L1 and TGF-β. The splenic B cells from infected mice were able to suppress the function of Th1 and Th2 cells in vitro but to expand the expression of Tfh transcription factor Bcl6, which was further enhanced by blocking PD-L1 of B cells before co-cultivation. Moreover, Th2 response and Bcl6 expression in CD4+ T cells were also increased in vivo by blocking PD-L1 after infection, although the hepatic pathology was slightly influenced.
Conclusions
Our findings revealed that S. japonicum infection modulates the differentiation of B cell subsets that have the capability to affect the CD4+ T cell response. This study contributes to a better understanding of B cells immune response during schistosomiasis.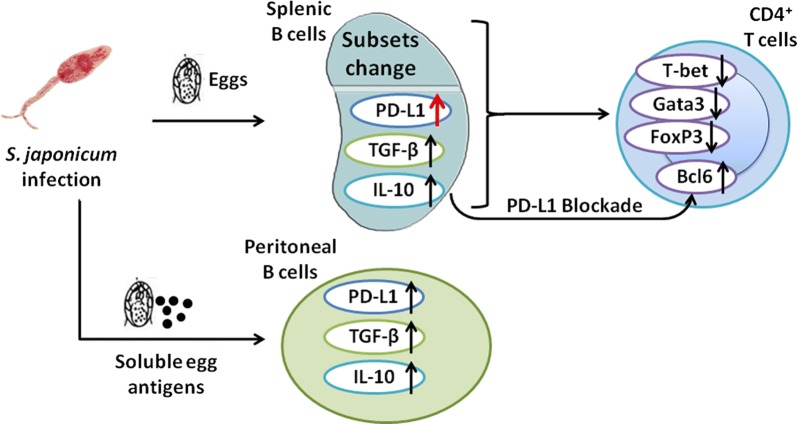
Keywords: Schistosoma japonicum, Regulatory B cells, Th responses, PD-L1, IL-10
Background
Schistosomes are pathogens that preferentially stimulate Th2 cells after the deposition of eggs in tissues [1]. Chronic schistosome infection is characterized by the downregulation of the immune system, which is widely considered to be associated with a decreased prevalence of inflammatory diseases in their hosts [2, 3]. Recent studies have revealed that apart from regulatory T cells (Treg), B cells can also be involved in this process. Most experimental studies on schistosome-induced regulatory B cells have focused on Schistosoma mansoni [4, 5]. However, there are remarkable differences in the development and progression of immunopathology and immune modulation between S. mansoni and S. japonicum [6].
B cells are conventionally considered to participate in immune responses by producing antibodies. Recently, the regulatory property of B cells has been reported in many inflammatory diseases [7]. The phenotypes of Breg cells have been studied, and B cell subsets with regulatory functions include transitional 2 marginal zone B precursor (T2-MZP) cells [8], MZB cells [5, 9], B10 cells and B-1a cells [10–12]. In addition to IL-10, Breg cells have been increasingly reported to play immunomodulatory role through other mechanisms. More recently, TGF-β-producing Breg cells have been reported to control the inflammation in autoimmune diabetes [13]. Besides, Breg cells characterized by elevated PD-L1 suppress autoimmune disease and anti-tumor immune response through inhibiting antibody production and T cells activation [14–17]. PD-L1 is constitutively expressed in various murine immune cells, including macrophages, dendritic cells, B cells, and T cells [18]. A previous study has shown that through increasing the PD-L1 expression of macrophages, schistosome worms induce T cells anergy [19].
Breg cells exhibit immunosuppressive function via diverse regulatory mechanisms. In addition to schistosome-induced splenic B cells, B-1a cells from the peritoneal cavity (PerC) also have the regulatory capability to induce Treg cells [20]. Moreover, it has been suggested that B cells are essential for Th2 response during infection with S. mansoni [21]. In contrast, several studies have indicated that B cells from schistosome-infected mice can inhibit ovalbumin-specific Th2 responses in a partially IL-10-dependent way [8, 22]. Furthermore, in the course of secondary type 2 response to stimulation with soluble egg antigens (SEA), expansion of Tfh cell population is dependent on B cells, since the diminished expansion and activation of Tfh cells are found in the mice administrated with anti-CD20 antibody [23]; whereas other studies have revealed that PD-Lhi B cells are critical regulators in Tfh cell programing [14, 24, 25]. All of the above suggest that B cells play a pleiotropic role in regulating immunity to schistosome infection. However, the role of B cells from S. japonicum-infected mice in the regulation of CD4+ T cell activity has not yet been completely defined.
In order to better understand the characteristics of B cells at different stages of S. japonicum infection, we conducted this study and found that the percentages of B-1a and MZB cells decreased and the expression of PD-L1, IL-10, TGF-β and IFN-γ in splenic B cells was upregulated during acute and/or chronic infection. B cells in mice with acute infection had the capability to affect cytokine responses of CD4+ T cells, and blocking PD-L1 on B cells from infected mice resulted in a recovery of IL-4-producing CD4+ T cells. Moreover, to clarify the role of SEA in inducing regulatory phenotypes of B cells, we performed SEA stimulation in vitro and in vivo and found that SEA of S. japonicum induced higher levels of CD5 in B cells from the spleen but not in PerC B cells. SEA-stimulated splenic and PerC B cells preferentially expressed PD-L1 and TGF-β. Overall, S. japonicum-induced B cells exhibit multiple regulatory phenotypes, and they are capable of altering the CD4+ T cell response.
Methods
Mice and infection
Eight-week-old female BALB/c mice were purchased from the Hubei Provincial Center for Disease Control and Prevention (Wuhan, China). Mice were maintained in a specific pathogen-free condition, and percutaneously infected with 16 ± 2 cercariae of S. japonicum shed from infected Oncomelania hupensis snails acquired from Nanjing Institute of Schistosomiasis Prevention and Control (Nanjing, China). Mice were sacrificed by CO2 asphyxiation at the indicated time for further studies.
Preparation of soluble antigens
Adult S. japonicum were obtained by portal perfusion of mice infected for 8 weeks. The male and female worms were separated under a stereomicroscope. Soluble worm antigen (SWA) was prepared by homogenizing male worms in phosphate-buffered saline (PBS), as previously described [26]. Eggs of S. japonicum were isolated from livers of infected mice, placed in PBS and sonicated as previously described for harvesting the soluble fraction used as soluble egg antigens (SEA) [27]. The concentration of antigens was determined by bicinchoninic acid (BCA) assay.
Cell isolation
Peritoneal cavity cells were obtained by an intraperitoneal injection of 8 ml PBS supplemented with 2% fetal calf serum and 2 ml air, and then passed through a 27 G needle. Single cell suspensions from the spleen were prepared by dispersion through a 70 μm cell strainer (BD Biosciences, San Jose, CA, USA), and erythrocytes depleted by lysis with 0.87% ammonium chloride solution. Total cell numbers were recorded, and cell viability was determined by trypan blue. Splenic B cells and CD4+ T cells were isolated using the Mouse Pan-B Cell Isolation Kit (Miltenyi Biotec, Auburn, CA, USA) and the Mouse CD4 T Lymphocyte Enrichment Set (BD Biosciences) following the manufacturer’s instructions, respectively. Briefly, pan B cells were obtained by negative selection (purity 90–94%) using magnetic beads against CD4, CD11c, CD49b, CD90.2, Gr-1 and Ter119. CD4+ T cells were enriched by negative selection (purity 92–96%) using magnetic beads against CD8, CD11b, CD45R, CD49b and Ter119.
Stimulation of murine B cells with soluble antigens in vitro
Mouse splenic B cells and PerC washout cells (1.5 × 106 cells per ml) were cultured in medium (RPMI 1640 glutamax; Gibco, Grand Island, NY) containing 5% fetal bovine serum (FBS; Gibco, Grand Island, NY), 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Sigma-Aldrich). For investigating the reaction of B cells to SEA in vitro, these cells were stimulated with SEA (20 μg/ml, 20 μl SEA in 1 ml culture system), lipopolysaccharide (LPS, 10 μg/ml) or PBS as a control. After cultivation at 37 °C for 24 h, the cells were employed for surface molecules detection.
Soluble antigens treatment in vivo
Mice were intraperitoneally injected with two doses of 100 μg SEA, 100 μg SWA or PBS every seven days. At day 14 after the first injection, peritoneal cavity washout cells and single-cell suspensions from the spleen were harvested for surface staining.
Antibody blocking and co-culture in vitro
Antibody blocking was performed similarly to the method described by Robert et al. [28]. Briefly, isolated splenic B cells (1 × 106 cells per ml) were pre-treated with 10 µg/ml anti-PD-L1 antibody (10F.9G2; Bioxcell, West Lebanon, NH, USA), or isotype antibody (LTF-2; Bioxcell) for 4 h at 37 °C, washed, and then co-cultured with enriched isogenic CD4+ T cells at a 1:1 ratio in the presence of rIL-2 (20 ng/ml) (Novus Biologicals, Littleton, CO, USA) for another 24 h. Expression of intracellular cytokines and transcription factors in CD4+ T cells was determined as described above.
Antibody treatment in vivo
To block PD-L1 in vivo, 100 μg of anti-PD-L1 antibody (10F.9G2; Bioxcell), 100 μg of rat IgG2a isotype control (LTF-2; Bioxcell), or PBS was intraperitoneally injected twice a week, starting at 29 days post-infection (dpi). At 56 dpi, all the mice were sacrificed. Mice were antibody-treated for 4 weeks in total. Splenic cells were prepared for flow cytometry (FCM) analysis, and livers were isolated for pathological examination. Livers were digested at 37 °C with 10% KOH for 20 min and the infection intensity was analyzed. Serum samples were isolated and stored at – 80 °C for cytokine determination.
Flow cytometry
Single cell suspension was prepared and washed in staining buffer (PBS with 0.02% sodium azide and 2% FBS). Fc receptors were blocked by incubating with anti-CD16/32 antibody (2.4G2; BD Biosciences) for 15 min. For surface marker analysis, cells were incubated with the following monoclonal antibodies: CD19-FITC/APC-Cy7 (1D3); CD5-PE-Cy5 (53–7.3); TGF-β1-PE (TW7-16B4); CD4-FITC/APC/APC-Cy7 (RM4-5); CD62L-APC-Cy7 (MEL-14); CD44-PE (IM7); PD-L1-PE (MIH5) (BD Biosciences); CD21-APC (7E9); and CD23-FITC (B3B4) (Biolegend, San Diego, CA, USA).
For all intracellular cytokine staining, cells were stimulated with 1 μg/ml ionomycin (Sigma-Aldrich), 100 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 10 μg/ml Brefeldin A (BD Biosciences) for 4 h at 37 °C. Cells were then washed, stained extracellularly, fixed and permeabilized with the intracellular cytokine detection kit (BD Biosciences) according to the manufacturer’s instructions, and then stained with IL-10-PE/APC/BV421 (JES5-16E3), IL-4-PE/APC (11B11) and IFN-γ-PE/PF-Cy7 (XMG1.2) (BD Biosciences).
For transcription factor staining, cells were fixed and permeabilized with the transcription factor staining kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions, and then stained with Foxp3-APC/PE (FJK-16s), T-bet-eFlour660 (4B10), Gata3-PE-Cy7 (TWAJ) and Bcl6-PE (K112-91) (eBioscience).
Dead cells were excluded by staining with the fixable viability dye eFluor 506 (eBioscience). All antibodies were used at an optimal concentration after titration. Gating of cells was based on the specific isotype control as well as fluorochrome minus one (FMO) setting when needed. Flow cytometry was performed using FACSVerse or LSR II (BD Biosciences). Data were analyzed with FlowJo software (BD Biosciences).
Cytokine measurements
The concentration of cytokines in serum from mice was measured by the BD Biosciences mouse cytokine bead array (CBA) kit as per the manufacturer’s instructions. Briefly, 50 μl of each sample was incubated with mixed capture beads and mouse PE detection reagent for 2 h at room temperature. One ml wash buffer was added into the sample tube followed by centrifugation at 200×g for 5 min. The bead pellet was then resuspended with 300 μl wash buffer for FCM analysis. The cytokine concentration in each sample was calculated from the fitting curve with a dilution factor applied.
Liver pathology
The right lobe of liver from each mouse was fixed in 4% paraformaldehyde and then embedded in paraffin. Five µm sections were dewaxed and stained with Masson’s trichrome. The Masson’s trichrome stain identified the collagen fibers as light blue. Six random (magnification of 100×) digital images were captured from each sample using the Mshot Image Analysis System (Guangzhou Micro-shot Technology Co., Ltd, Guangzhou, China), and the ratio of the collagen fiber area to the total captured area of each section was quantified using ImageJ software in a blinded fashion. Representative images were acquired at a magnification of 200×.
Real-time PCR
Total RNA was extracted from liver tissues with Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using a reverse transcription kit (TOYOB, Tokyo, Japan) according to the manufacturer’s instructions. Real-time PCR was performed using SYBR green master mix (TOYOB) on a MyiQTM2 thermal cycler (Bio-Rad, Hercules, CA, USA). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize the real-time PCR data, and the primers used for quantitative PCR were as follows: GAPDH (5ʹ-GTG TTT CCT CGT CCC GTA G-3ʹ and 5ʹ-ATG GCA ACA ATC TCC ACT TT-3ʹ); a-SMA/Acta2 (5ʹ-GAG CGT GAG ATT GTC CG-3ʹ and 5ʹ-GCT GTT ATA GGT GGT TTC G-3ʹ); and Col1a1 (5ʹ-ACA TGT TCA GCT TTG TGG ACC-3ʹ and 5ʹ- TAG GCC ATT GTG TAT GCA GC-3ʹ).
Statistical analysis
All data are presented as the mean ± standard error (SE) of the mean. A horizontal line with a symbol representing the P-value indicates a statistical comparison. We used two-sided Student’s unpaired t-test or Mann–Whitney U-test for comparison of two groups, and Kruskal–Wallis test with Dunn’s post-hoc test or one-way ANOVA with Bonferroni’s post-hoc test for multiple comparisons, depending on the data distribution (Shapiro–Wilk’s test) and homogeneity of variance (Levene’s test). Specific statistical tests are described in the figure legends. A P-value of < 0.05 was considered significant. All statistical analyses were performed using SPSS software (SPSS, Chicago, Illinois, USA).
Results
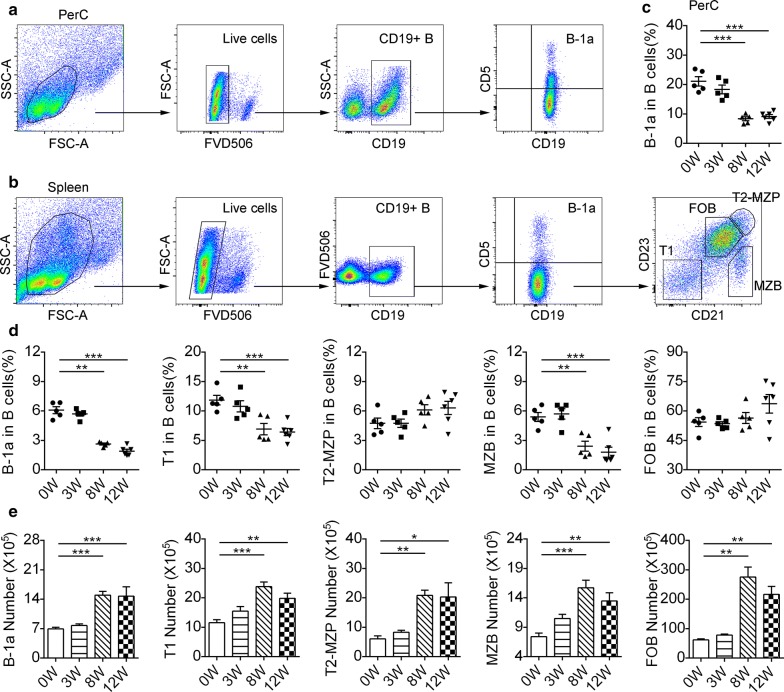
Schistosoma japonicum infection modulates B cell differentiation
We first investigated the B cell subsets in the PerC and spleen that have been confirmed to play a regulatory role in mice, such as B-1a cells, MZB cells and T2-MZP cells, at different time points after S. japonicum infection (Fig. 1a, b). Flow cytometric analysis showed that, at three weeks post-infection (early stage) representing a time point prior to oviposition, the proportions of PerC B-1a cells (Fig. 1c) and the indicated splenic B cell subsets (Fig. 1d) were not significantly different from those in uninfected mice. Likewise, there were no changes in the percentages of T2-MZP cells (F(3, 17) = 2.323, P = 0.1115) and follicular B (FOB) cells (F(3, 17) = 2.15, P = 0.1315) at eight (acute stage) or twelve weeks (chronic stage) of infection (Fig. 1d). Besides, the proportions of T1 B cells (F(3, 17) = 10.94, P < 0.001) and MZB cells (F(3, 17) = 16.2, P < 0.001) in the infected mice at acute and chronic stage were significantly lower than those of uninfected mice (Fig. 1d). Similarly, the proportions of PerC B-1a cells (F(3, 17) = 31.22, P < 0.001) (Fig. 1c) and splenic B-1a cells (F(3, 17) = 86.9, P < 0.001) (Fig. 1d) decreased at acute and chronic stage. The numbers of splenic B cell subsets increased gradually when the infection became acute (Fig. 1e).
Fig. 1.
Infection with Schistosoma japonicum alters splenic and PerC B-cell composition. Splenic and PerC lymphocytes were isolated from mice at zero (uninfected mice, 0W), three (3W), eight (8W), and twelve weeks (12W) post-infection. Gating schemes for PerC B-1a cells (a) and splenic B-cell subsets (b) within the CD19+ gated B-cell population are shown. Splenic B-cell subpopulations (b) are shown: B-1a cells (CD19+CD5+), T1 B cells (CD19+CD5−CD21−CD23−), T2-MZP cells (CD19+CD5−CD21+CD23+), MZB cells (CD19+CD5−CD23−CD21+) and FOB cells (CD19+CD5−CD23+CD21−). The percentages of PerC B-1a cells (c) were measured. The percentages (d) and the absolute numbers (e) of splenic B cell subsets were measured from at least five mice of each time point after infection. All data are representative of at least two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
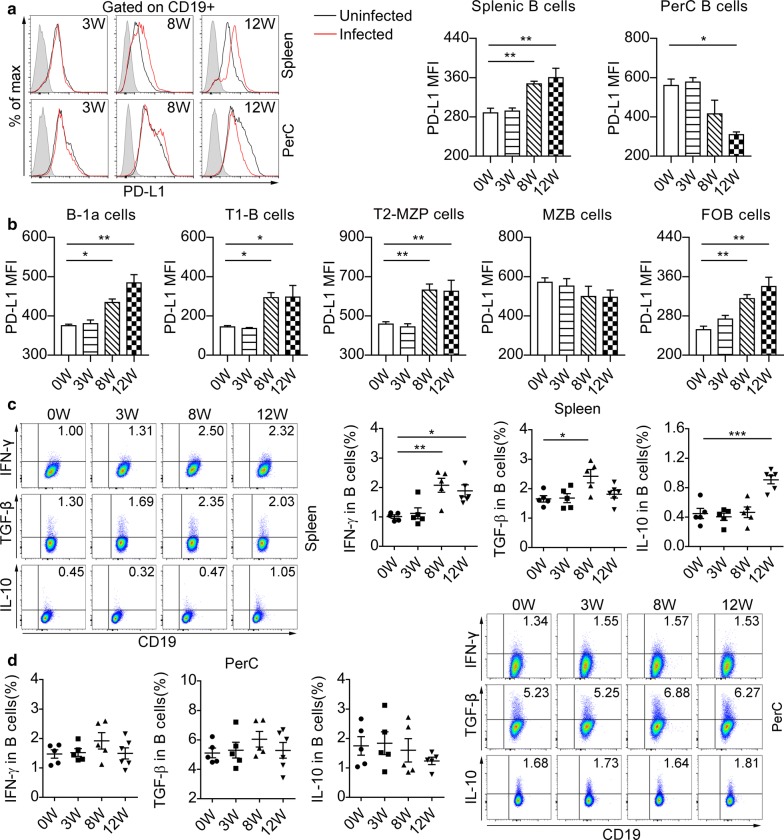
Characterization of splenic and PerC B cells during S. japonicum infection
To explore the potential of B cells to induce hyporesponsiveness during infection with S. japonicum, we investigated B cells from the spleen and peritoneal cavity for immune-regulatory markers. The expression of PD-L1 in splenic B cells was increased at eight and twelve weeks after infection, compared to those from uninfected mice (F(3, 16) = 10.6, P < 0.001) (Fig. 2a). Interestingly, the expression of PD-L1 in PerC CD19+ B cells was similar between uninfected mice and mice infected for either three or eight weeks, but was downregulated in mice at twelve weeks post-infection (F(3, 16) = 9.83, P < 0.01) (Fig. 2a). We further analyzed the expression of PD-L1 in splenic B cell subsets (Fig. 2b). We did not observe the change of PD-L1 expression in MZB cells (F(3, 16) = 0.976, P = 0.4285) during S. japonicum infection, but acute and chronic infection enhanced the expression of PD-L1 in B-1a cells (F(3, 16) = 16.6, P < 0.001), T1 B cells (F(3, 16) = 7.78, P < 0.01), T2-MZP B cells (F(3, 16) = 9.5, P < 0.001) and FOB cells (F(3, 16) = 12.1, P < 0.001).
Fig. 2.
Infection with Schistosoma japonicum elevates PD-L1 expression and modulates cytokines profile of splenic B cells. a Flow cytometric histograms represent cell surface expression of PD-L1 in splenic and PerC B cells from uninfected mice (black line) and infected mice (red line) at each time point. The shaded histograms represent the FMO control. Significance levels from statistical analysis of mean fluorescence intensity (MFI) of PD-L1 in splenic and PerC B cells are indicated. b The MFI of PD-L1 in splenic B cell subsets are summarized. The splenic (c) and PerC (d) lymphocytes were stimulated with PMA and ionomycin in the presence of Brefeldin A for 4 h and followed by extracellular and intracellular staining. The percentages of IFN-γ+, TGF-β+ or IL-10+ cells gated on CD19+ B cells are shown. All data are representative results of two independent experiments with at least 5 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001
Next, we examined the cytokine production of B cells after S. japonicum infection. The percentage of splenic B cells expressing IL-10 was increased at twelve weeks post-infection (F(3, 17) = 15.84, P < 0.001), while TGF-β in splenic CD19+ B cells was elevated at eight weeks after infection but declined thereafter (F(3, 17) = 4.979, P < 0.01) (Fig. 2c). Schistosome infection induced elevated percentages of IFN-γ-expressing splenic B cells at both acute and chronic stages (F(3, 17) = 7.954, P < 0.001) (Fig. 2c). Moreover, there were no differences in IL-10, TGF-β, or IFN-γ expression of PerC CD19+ B cells between infected and uninfected mice (Fig. 2d).
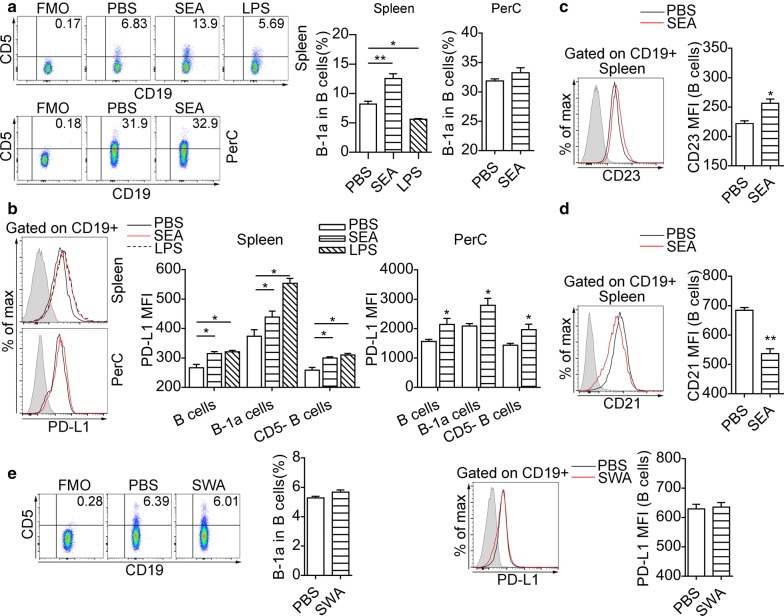
SEA-stimulated B cells acquire a regulatory phenotype
We next tested whether SEA could induce a regulatory phenotype in B cells. To test the induction of PD-L1 in B cells by SEA in vitro, purified B cells from uninfected mice were cultured for 24 h with the stimulation of SEA (20 μg/ml) (designated SEA-B) or LPS (10 μg/ml) (LPS-B), and negative control B cells were cultured with PBS (PBS-B). We found that splenic SEA-B cells expressed higher level of CD5 compared to PBS-B cells (F(2, 9) = 41.6, P < 0.001), whereas the expression of CD5 was not significantly increased in PerC SEA-B cells (t(6) = 1.54, P = 0.1882) (Fig. 3a). LPS failed to induce an increase in the percentage of CD5+ B cells (Fig. 3a). The splenic and PerC SEA-B cells expressed higher levels of surface PD-L1 (Fig. 3b). The level of CD23 (t(6) = 3.96, P < 0.05) was increased in splenic SEA-B cells (Fig. 3c), but the expression of CD21 in splenic SEA-B cells (t(6) = 5.341, P < 0.01) was decreased (Fig. 3d). Besides, SWA failed to affect the expression of CD5 and PD-L1 in splenic B cells in vitro (Fig. 3e, f).
Fig. 3.
SEA drives CD5 and PD-L1 expression of B cells in vitro. Splenic B cells and PerC washout cells from uninfected mice were cultured for 24 h in the presence of PBS or indicated stimuli. a Representative dot plots indicate the expression of CD5 on gated CD19+ B cells from spleen and peritoneal cavity and significance levels from statistical analyses are indicated. b Flow cytometric histograms represent cell surface expression of PD-L1 in splenic and PerC B cells, and significance levels from statistical analyses of PD-L1 MFI of splenic and PerC B-cell are indicated. The expression of CD23 (c) and CD21 (d) in splenic B cells was measured at the end of culture. The shaded histograms represent the FMO control. In e and f, splenic B cells were cultured for 24 h in the presence of PBS or SWA (20 μg/ml). e Representative dot plots indicate the expression of CD5 on gated CD19+ B cells from spleen and significance levels from statistical analyses are indicated. f Flow cytometric histograms represent cell surface expression of PD-L1 in splenic B cells, and significance from statistical analyses of PD-L1 MFI of splenic B-cell are indicated. All data are representative results of two independent experiments with at least 4 mice per group. *P < 0.05, **P < 0.01
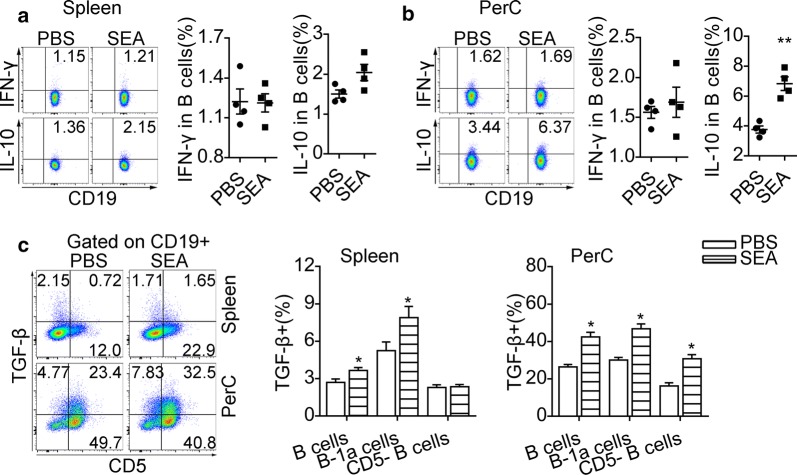
Additionally, SEA stimulation for 24 h did not significantly enhance IL-10 expression in splenic B cells (t(6) = 2.384, P = 0.0545) (Fig. 4a), but drove the expression of IL-10 in PerC B cells (t(6) = 5.974, P < 0.01) (Fig. 4b). Our data also showed that SEA treatment in vitro did not affect the intracellular IFN-γ expression in splenic and PerC B cells (Fig. 4a, b). After the stimulation with SEA, splenic B-1a cells (t(6) = 4.603, P < 0.05) but not splenic CD5- B cells (t(6) = 0.369, P = 0.7248) showed significantly increased surface expression of TGF-β (Fig. 4c). Both B-1a cells (t(6) = 11.23, P < 0.05) and CD5- B cells (t(6) = 10.16, P < 0.05) in PerC had significantly increased expression of TGF-β after SEA treatment in vitro (Fig. 4c).
Fig. 4.
SEA-stimulated B cells acquire elevated expression of IL-10 and TGF-β in vitro. Splenic B cells and PerC washout cells from uninfected mice were stimulated with SEA for 24 h. Intracellular IL-10 and IFN-γ staining were performed after the restimulation with PMA and ionomycin in the presence of Brefeldin A. Representative flow cytometric plots and significance from statistical analyses of the percentages of IL-10+ B cells and IFN-γ+ B cells in splenic B cells (a) and PerC B cells (b) are shown. c Representative plots of CD5 and TGF-β expression on CD19+ B cells and the percentages of B cell subsets expressing TGF-β. All data are representative results of two independent experiments with at least 4 mice per group. *P < 0.05, **P < 0.01
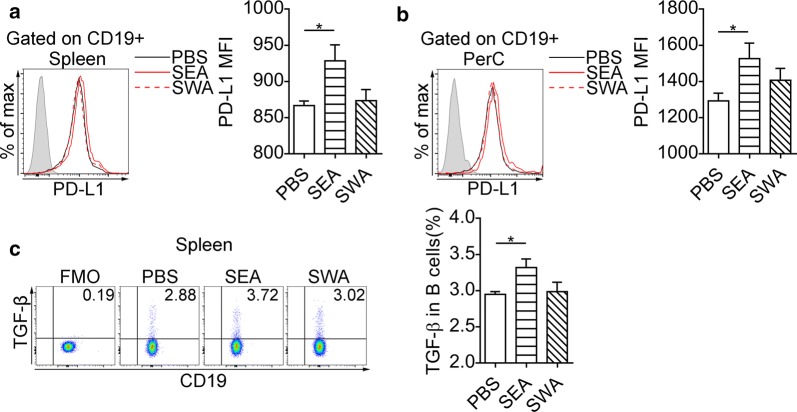
We further examined if intraperitoneal injection of SEA was sufficient to induce expression of PD-L1 in B cells. Intraperitoneal injection of SEA into mice increased the expression of PD-L1 in splenic B cells (F(2, 10) = 4.18, P < 0.05) (Fig. 5a) and PerC B cells (F(2, 10) = 4.43, P < 0.05) (Fig. 5b), as well as the expression of TGF-β (F(2, 12) = 3.98, P < 0.05) in splenic B cells (Fig. 5c). In contrast, SWA injection did not affect the expression of PD-L1 in B cells (Fig. 5a, b).
Fig. 5.
SEA induces expression of PD-L1 and TGF-β on B cells in vivo. Flow cytometry histograms represent cell surface expression of PD-L1 in splenic B cells (a) and PerC B cells (b), and significance levels from statistical analysis of PD-L1 MFI of splenic (a) and PerC B cells (b) are shown. The shaded histograms represent the FMO control. c Representative plots of TGF-β surface expression in CD19+ B cells and the percentages of TGF-β expressing B cells. The data are representative results of two independent experiments with at least 4 mice per group. *P < 0.05
B cells from S. japonicum-infected mice modulate CD4+ T cell response
We performed splenic CD4+ T cell and B cell co-cultures to investigate the suppressive functions of B cells from mice at eight weeks post-infection. The frequencies of T-bet+ (F(3, 18) = 74.8, P < 0.01), Gata3+ (F(3, 16) = 236, P < 0.01) and FoxP3+ (F(3, 20) = 639, P < 0.01) in CD4+ T cells (Fig. 6a), as well as the percentages of CD4+ T cells producing IFN-γ (F(3, 16) = 43, P < 0.01), IL-4 (F(3, 14) = 14.9, P < 0.01) and IL-10 (F(3, 16) = 37.1, P < 0.01) (Fig. 6b), significantly decreased after co-culture with B cells from infected mice. In contrast, B cells from infected mice enhanced the expression of Bcl6 in CD4+ T cells (F(3, 18) = 240, P < 0.01) (Fig. 6c). Additionally, B cells from infected mice were prone to generate fewer CD4+ T effector memory (TEM, CD44+CD62L−) cells than B cells from uninfected mice (F(3, 20) = 198, P < 0.01) (Fig. 6d).
Fig. 6.
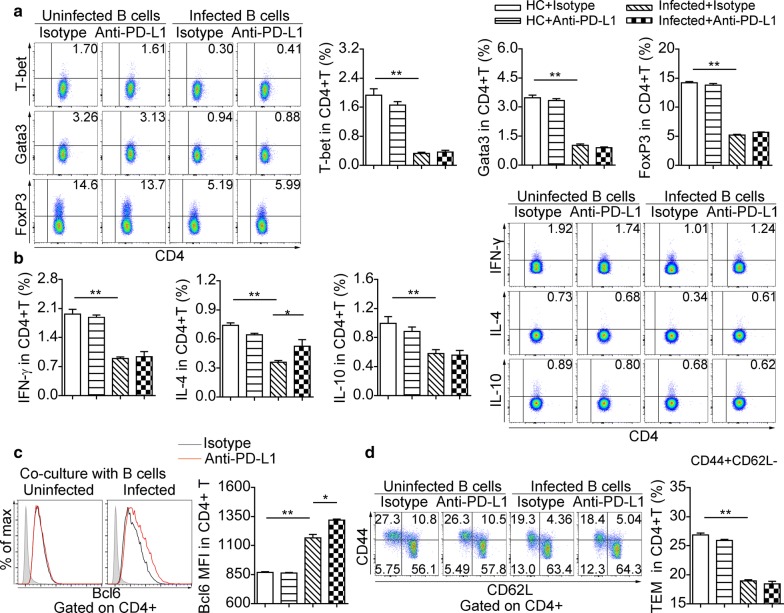
B Cells from infected mice modulate the phenotype of CD4+ T Cells in vivo. Purified splenic B cells from uninfected mice and infected mice (eight weeks) were treated with isotype antibody or anti-PD-L1 antibody respectively prior to co-culture with CD4+ T cells. a Representative dot plots of T-bet, Gata3, and FoxP3 in CD4+ T cells and significance levels from statistical analysis. b Representative flow cytometric plots of IL-10, IL-4 and IFN-γ in CD4+ T cells and significance levels from statistical analyses. c Representative flow cytometric histograms of Bcl6 expression in CD4+ T cells and significance levels from statistical analysis. The shaded histograms represent the FMO control. d Representative dot plots of CD44 versus CD62L gated on CD4+ T cells (CD44+CD62L− T effector memory, TEM) and significance levels from statistical analysis. All data are representative results of two independent experiments with at least 4 mice per group. *P < 0.05, **P < 0.01
Based on the elevated expression of PD-L1 in splenic B cells at eight weeks of experimental S. japonicum infection (Fig. 2b), we blocked PD-L1 on B cells before co-culture with CD4+ T cells. Anti-PD-L1 antibody pre-treatment did not recover the expression of T-bet, Gata3, FoxP3 (Fig. 6a), IFN-γ and IL-10 (Fig. 6b) in CD4+ T cells, whereas the expression of IL-4 and Bcl6 was significantly increased in CD4+ T cells (Fig. 6b, c). Also, anti-PD-L1 antibody treatment did not affect suppression in CD4+ T effector memory cells mediated by B cells from infected mice (Fig. 6d).
PD-L1 blocking partially expands the Th2 response in S. japonicum-infected mice
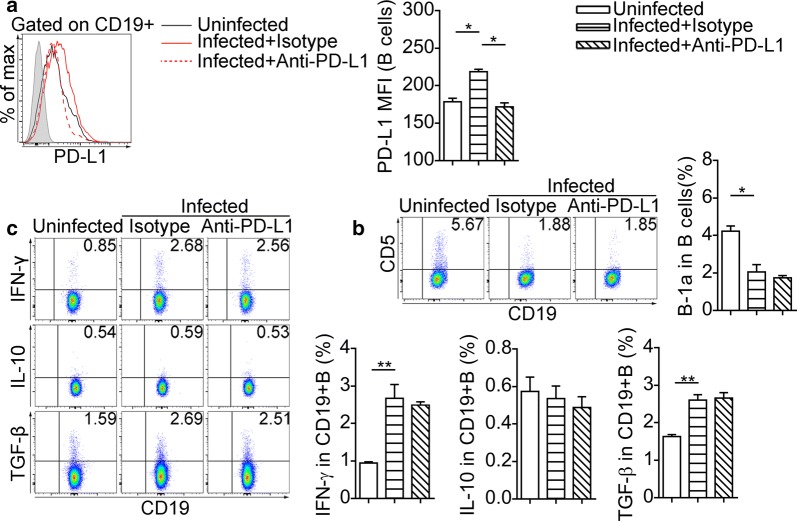
To further understand the role of PD-L1 in regulating host immunity of schistosomiasis, we used anti-PD-L1 antibody in the S. japonicum infection model. The PD-L1 MFI of splenic CD19+ B cells from infected mice decreased after anti-PD-L1 antibody treatment (F(2, 12) = 29.5, P < 0.05) (Fig. 7a). Nevertheless, the splenic CD19+ B cells from infected mice treated with anti-PD-L1 antibody and isotype antibody were similar in the proportion of B-1a cells (Fig. 7b) and the expression of IFN-γ, IL-10 and TGF-β (Fig. 7c).
Fig. 7.
PD-L1 blocking fails to alter the regulatory molecules in splenic B-cell during infection. a Overlay of representative histograms show PD-L1 expression in splenic CD19+ B cells. Representative dot plots show the expression of CD5 (b), IFN-γ, IL-10 and TGF-β (c) in splenic CD19+ B cells. Bar graphs indicate the results from one of two independent experiments with ≥ 5 mice in each group. *P < 0.05, **P < 0.01
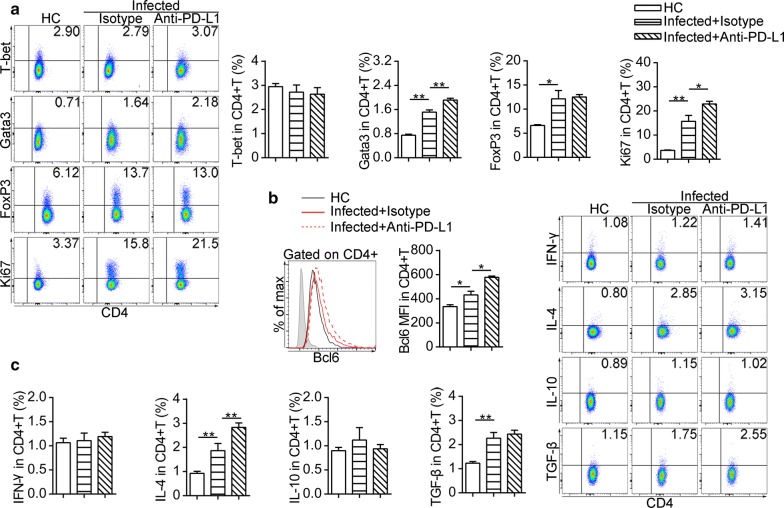
However, PD-L1 blocking significantly increased the proportion of Gata3+CD4+ T cells (F(2, 13) = 72.1, P < 0.01) and the proliferation of CD4+ T cells in the spleen (Fig. 8a). Also, with anti-PD-L1 antibody treatment, CD4+ T cells from infected mice exhibited a higher level of Bcl6 (F(2, 12) = 32.6, P < 0.01) (Fig. 8b) and secreted more IL-4 (F(2, 13) = 26.3, P < 0.01) (Fig. 8c). The expression of T-bet, FoxP3 (Fig. 8a), IFN-γ, IL-10 and TGF-β (Fig. 8c) in CD4+ T cells from infected mice with anti-PD-L1 antibody treatment were similar to those found in infected mice treated with the isotype antibody.
Fig. 8.
In vivo blocking of PD-L1 partially expands Th2 response after S. japonicum infection. a Flow cytometric dot plots represent expression of T-bet, Gata3, FoxP3 and Ki67 in splenic CD4+ T cells. b Flow cytometric histograms represent Bcl6 expression in splenic CD4+ T cells from uninfected mice (black line) and infected mice treated with isotype antibody (red line) and infected mice treated with anti-PD-L1 antibody (dashed line). The shaded histograms represent the FMO control. c Flow cytometric dot plots showing expression of IFN-γ, IL-4, IL-10 and TGF-β in splenic CD4+ T cells. All bar graphs indicate results from one of two independent experiments with ≥ 5 mice in each group. Experiments were performed two times with n = 10–12 per group. *P < 0.05, **P < 0.01
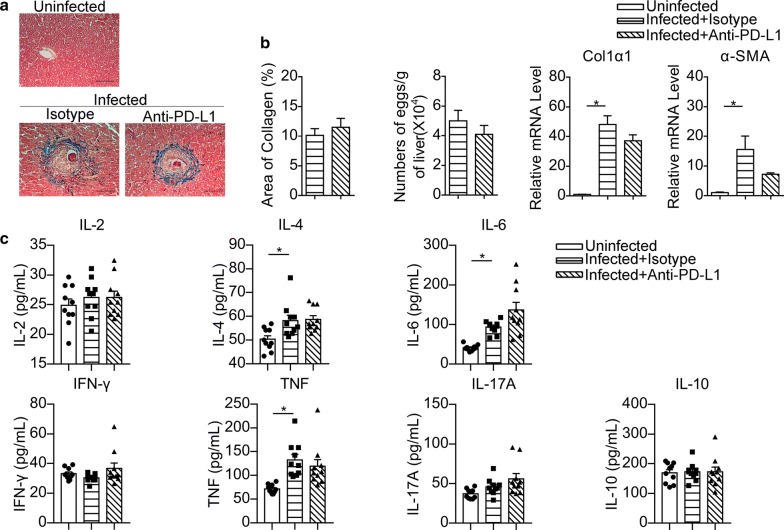
In addition, hepatic pathology indicated by collagen and a-SMA expression, as well as hepatic egg burden, were not significantly affected by anti-PD-L1 antibody treatment in schistosome-infected mice (Fig. 9a, b). No significant differences were found in the levels of IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ and TNF in serum between infected mice treated with anti-PD-L1 antibody and isotype antibody (Fig. 9c).
Fig. 9.
PD-L1 blocking fails to affect hepatic pathology and serum cytokines during infection. a Representative images of Masson’s trichrome staining for hepatic fibrosis analysis (original magnification of 200×). b Quantification of hepatic collagen deposition and egg burden are shown, and total RNA was extracted from livers and analyzed by RT-PCR for the expression of Col1a1 and a-SMA. In a and b, the data are representative results of two independent experiments with at least 4 mice per group. c The serum levels of IL-2, IL-4, IL-6, IL-10, IL-17A, TNF and IFN-γ were assayed by CBA. The data represent the cumulative results of two independent experiments. *P < 0.05. Scale-bars: a, 100 μm
Discussion
It has been well accepted that various helminth infections lead to a strong and comprehensive immune modulation coming down to the whole process of immune response, including almost all the natural and adaptive immunity of their hosts [2]. Enhanced effective CD4+ Th cell response has been clearly illustrated in human infection and experimental schistosome models, which is initiated as a Th1 response before abundant oviposition by female worms. At six to eight weeks post-infection, this response switches to Th2, and downmodulation of the Th2 and Th1 response is observed generally at ten weeks, as an essential mediation to favor the survival of both hosts and parasites [1, 29].
Apart from commonly known Treg cells that predominate during the chronic stage of schistosomiasis, regulatory functions of B cells are also demonstrated in the process of schistosomiasis. Previous studies [30, 31] have shown that the deficiency of B cell or B-1a cell has led to a higher mortality in schistosome-infected mice, as well as that B-1a cells regulate the IL-5 and IFN-γ production of Th cells and control the size of ova granulomas during infection. Besides, CD1dhi B cells from S. mansoni-infected mice have been shown to function in dampening inflammation in allergic airway inflammation [5]. Murine S. japonicum infection has been reported to downregulate allergic airway inflammation through reducing Th17 and Th2 effector cytokines in the lungs [32], but few studies focus on the immune regulatory role of B cells during S. japonicum infection. Moreover, Rosser et al. [12] have proposed that the immunosuppression of B cells is not achieved by a specific Breg subset with a particular phenotype, but by the outcome of the interaction between multiple B cell populations and other cells in the immune system, although the Breg subsets have been described as T2-MZP cells [8] and MZB cells [5, 9] in S. mansoni-infected mice. Here we found that the proportions of B cell populations with regulatory potential, such as B-1a and MZB cells, were markedly reduced during S. japonicum acute and chronic infection. The percentages of B cell subsets with regulatory features were not upregulated in S. japonicum infection models, but the numbers of splenic B-1a cells, T2-MZP cells and MZB cells increased progressively when the infection became acute, and these increases were likely due to the development of splenomegaly in schistosome-infected mice [33] and B cell expansion [34].
A previous study has shown that B-1a cells in the peritoneal cavity spontaneously express IL-10, which is further increased in LPS-stimulated PerC B-1a cells [35]. The changes of PerC B-1a cells during early S. mansoni infection remain controversial [36–38]. Velupillai et al. [37] have reported that B-1a cell outgrowth depends on the mouse strain during S. mansoni infection, and at early and acute infection, the frequencies of PerC B-1a cells change differently among various strains of infected mice. Besides, the percentages of splenic B-1a cells increase in C3H/HeN mice during acute and chronic S. mansoni infection [37, 39]. Here, we observed that the proportions of PerC and splenic B-1a cells remained relatively constant at three weeks of S. japonicum infection, whereas these proportions were significantly reduced during acute and chronic schistosomiasis. Overall, the outgrowth of B-1a cells in murine schistosomiasis was probably not only related to mouse strain, but also associated with schistosome species. Since B cells are crucial in egg-induced granulomatous response during schistosomiasis [6], our findings suggest that the immunoregulatory mechanism of B cells in S. japonicum-infected mice might be different from that in S. mansoni infections, although the change of hepatic fibrosis led by B cell-depletion in S. mansoni-infected mice was similar to that in mice infected with S. japonicum [30].
It has been reported that Breg cells can suppress the immune response by secreting inhibitory cytokines and interacting with surface molecules of the target cells [40]. LPS-stimulated B cells can inhibit Th1 response and prevent autoimmune diabetes by secreting TGF-β [13]. IFN-γ may play diverse roles at different stages of the immune response since it has the capabilities of both inhibiting and stimulating Treg cells [41, 42]. In the present study, IL-10 expression in splenic B cells was enhanced during the chronic stage, which is consistent with a previous report in S. mansoni infection models [5]. Expression of TGF-β in splenic B cells was increased in the acute stage of infection but declined after twelve weeks of infection. Schistosome infection also elevated IFN-γ production in splenic B cells in both acute and chronic stages, but there was no change in the expression of IL-10, TGF-β, or IFN-γ in PerC CD19+ B cells during schistosome infection, which could be explained by the heterogeneity and distinct composition of B cells derived from the spleen and peritoneal cavity [43]. These B cells responded differentially to parasite infection and a previous report [44] supports this finding by showing that PerC B cells activated by anti-CD40 and LPS secreted more IL-10 and IL-6 compared to splenic B cells. PD-L1 expression in splenic B cells was elevated during acute and chronic infection, whereas the expression of PD-L1 in PerC B cells was decreased during chronic schistosomiasis. The difference in PD-L1 expression of B cells during infection might be due to the higher basic level of PD-L1 in PerC B cells compared to that in splenic B cells [45] and the infection-induced migration of PerC B-1a cells to the liver [46].
Moreover, previous studies have confirmed that SEA can directly interact with B cells to enhance their regulatory potential shown as increasing IL-10 expression [9, 47]. CD23 is the IgE Fc receptor which can regulate allergen-induced airway inflammation [48], and splenic B cells can be subdivided into T2-MZP cells and MZB cells based on the expression of CD21 and CD23. CD23 expression levels of Breg cells are diverse in different disease models [22]. In our study, we found that S. japonicum SEA stimulation enhanced the expression of IL-10, TGF-β, CD5, CD23 and PD-L1 in PerC B cells or splenic B cells, which have been identified as the markers of Breg cells. Although SEA had the capability of increasing CD5 and IL-10 expression in PerC B cells in vitro, a reduced proportion of PerC B-1a cells and a slightly decreased IL-10 expression in PerC B cells were observed during schistosome infection in our study. This differential effect on the expression of CD5 and IL-10 in PerC B cells resulting from SEA stimulation in vitro and infection in vivo might be due to the fact that PerC B-1a cells identified by surface marker CD5 were competent to secrete a large amount of IL-10 [35], and that PerC B-1a cells migrated into the liver to improve hepatic fibrosis induced by schistosome infection [46]. Beyond that, the findings from in vivo and in vitro experiments have suggested that the dynamic changes of these cytokines and surface molecules in B cells during schistosome infection might be influenced not only by the stimulation of SEA but also the internal dynamics of the immune system disturbed by infection [49].
We also found the suppression of Th1 and Th2 cells by splenic B cells from acutely infected mice in co-cultures with CD4+ T cells. A previous study has reported that S. japonicum infection promotes the expansion of Tfh cells [50]. The interactions between B cells and naïve T cells are essential for the generation of Tfh cells [51, 52]. Nevertheless, several studies have reported that PD-L1-expressing B cells regulate Tfh cells [14, 24, 25]. Herein, we demonstrated that blocking PD-L1 on B cells from acutely infected mice enhanced the expansion of Bcl6 that is described as being the critical transcription factor for Tfh cell differentiation [53]. Additionally, anti-PD-L1 treatment of splenic B cells from acutely infected mice before co-culture partially restored the expression of IL-4 in T cells, whereas Th2 transcription factor Gata3 expression was unchanged. These might be related to the fact that Tfh cells are the primary IL-4-producing cells during helminth infection [54, 55].
Although PD1 signaling in B cells has been demonstrated to inhibit the proliferation and cytokine production of activated B cells [56], we did not find the effects of systematic PD-L1 blocking on the proportion of B-1a cells and cytokine production in splenic B cells during S. japonicum infection. PD-L1 blocking in mice after infection only reduced PD-L1 MFI of splenic B cells from infected mice, and this might be because treatment with anti-PD-L1 antibody in vivo affected the binding of fluorescent antibodies to PD-L1. Studies in mice with schistosome and Litomosoides sigmodontis infection have indicated that Th2 hypo-responsiveness is not related to PD-1 and PD-L1 interaction [22, 57, 58]. However, other studies have suggested that PD-1 is necessary for the Th2 hyporesponsiveness during schistosome infection [19, 59]. Our findings from the PD-L1 blocking experiment in vivo were consistent with the latter, as we observed that PD-L1 blocking not only enhanced the expression of IL-4 and Bcl6 in splenic CD4+ T cells but also increased the expression of Th2 transcription factor Gata3. These were caused not just by blocking PD-L1 on B cells, since a previous study reported that S. mansoni worms selectively increased the expression of PD-L1 in macrophages [19]. Deficiency of PD-L1 specific in B cells would be useful to clarify the role of PD-L1 of B cells during schistosome infection.
The intervention of anti-PD-L1 antibody after oviposition did not exert an influence on hepatic egg burden, and there was no appreciable pathological change indicated by hepatic collagen deposition. It is well known that various resident or recruited inflammatory cells are responsible for the activation of hepatic stellate cells and contribute to liver fibrosis [60]. Our data might imply that only the intervention of PD-L1 could not impact on the pathogen-associated fibrosis in the liver, although the CD4+ T cell response was modulated by PD-L1 blockade.
Conclusions
Our study revealed that different schistosome species might result in the distinct differentiation of B cell subsets. Schistosoma japonicum infection could induce the regulatory function of B cells that interact with T cells in an intricate way and display multiple regulatory phenotypes. Further investigation of the role of PD-L1 specific in B cells in modulating T cell activation in schistosome infection should be helpful for the control of other T cell-mediated diseases.
Acknowledgements
Not applicable.
Abbreviations
- Breg
regulatory B cells
- CBA
cytokine bead array
- dpi
days post-infection
- FBS
fetal Bovine Serum
- FCM
Flow cytometry
- FMO
fluorochrome minus one
- FOB
follicular B cells
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- MZB
marginal zone B cells
- PerC
peritoneal cavity
- PMA
phorbol 12-myristate 13-acetate
- SE
standard error
- SEA
soluble egg antigens
- SWA
soluble worm antigen
- T2-MZP
transitional 2 marginal zone B precursor cells
- TEM
T effector memory cells
- Treg
regulatory T cells
Authors’ contributions
JX and WL designed the study. JX, FG, LS, YZ, XZ and SL acquired the experimental data. JX and WL contributed to data analyses and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (http://www.nsfc.gov.cn/) (Nos. 81471979 and 81672047) to WL, and (No. 81772221) to SL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
All animal experiments were carried out strictly according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China. This study was approved by the Ethics Committee of Animal Experimentation of Tongji Medical College and the Institutional Animal Care and Use Committee (IACUC) of Hubei Province, China (Permit Number: SYXK 2016-0057).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Junli Xiao, Email: 471856746@qq.com.
Fei Guan, Email: 327252637@qq.com.
Li Sun, Email: 540010607@qq.com.
Yijie Zhang, Email: 448187035@qq.com.
Xiaoyan Zhang, Email: 745021533@qq.com.
Shengjun Lu, Email: lushengjun@hust.edu.cn.
Wenqi Liu, Email: liu_wq2002cn@hotmail.com.
References
- 1.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 2.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 3.King CL, Medhat A, Malhotra I, Nafeh M, Helmy A, Khaudary J, et al. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–4721. [PubMed] [Google Scholar]
- 4.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(1114–24):e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 5.van der Vlugt LE, Labuda LA, Ozir-Fazalalikhan A, Lievers E, Gloudemans AK, Liu KY, et al. Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS ONE. 2012;7:e30883. doi: 10.1371/journal.pone.0030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014;30:141–150. doi: 10.1016/j.pt.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Smits HH, Hammad H, van Nimwegen M, Soullie T, Willart MA, Lievers E, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–940. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Haeberlein S, Obieglo K, Ozir-Fazalalikhan A, Chaye MAM, Veninga H, van der Vlugt L, et al. Schistosome egg antigens, including the glycoprotein IPSE/alpha-1, trigger the development of regulatory B cells. PLoS Pathog. 2017;13:e1006539. doi: 10.1371/journal.ppat.1006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Lundy SK, Boros DL. Fas ligand-expressing B-1a lymphocytes mediate CD4(+)-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10. Infect Immun. 2002;70:812–819. doi: 10.1128/IAI.70.2.812-819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 14.Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, et al. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol. 2016;28:423–433. doi: 10.1093/intimm/dxw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan H, Wan Y, Lan J, Wang Q, Wang Z, Li Y, et al. PD-L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci Rep. 2016;6:35651. doi: 10.1038/srep35651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 19.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 20.Hsu LH, Li KP, Chu KH, Chiang BL. A B-1a cell subset induces Foxp3(-) T cells with regulatory activity through an IL-10-independent pathway. Cell Mol Immunol. 2015;12:354–365. doi: 10.1038/cmi.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez HJ, Wang Y, Stadecker MJ. In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation. J Immunol. 1997;158:4832–4837. [PubMed] [Google Scholar]
- 22.van der Vlugt L, Obieglo K, Ozir-Fazalalikhan A, Sparwasser T, Haeberlein S, Smits HH. Schistosome-induced pulmonary B cells inhibit allergic airway inflammation and display a reduced Th2-driving function. Int J Parasitol. 2017;47:545–554. doi: 10.1016/j.ijpara.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, et al. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med. 2017;23:601–610. doi: 10.1038/nm.4315. [DOI] [PubMed] [Google Scholar]
- 25.Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L, et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186:5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 26.Li YS, Ross AG, Sleigh AC, Li Y, Waine GJ, Williams GJ, et al. Antibody isotype responses, infection and re-infection for Schistosoma japonicum in a marshland area of China. Acta Trop. 1999;73:79–92. doi: 10.1016/S0001-706X(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 27.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 28.Schaut RG, Lamb IM, Toepp AJ, Scott B, Mendes-Aguiar CO, Coutinho JF, et al. Regulatory IgDhi B cells suppress T cell function via IL-10 and PD-L1 during progressive visceral leishmaniasis. J Immunol. 2016;196:4100–4109. doi: 10.4049/jimmunol.1502678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundy SK, Lukacs NW. Chronic schistosome infection leads to modulation of granuloma formation and systemic immune suppression. Front Immunol. 2013;4:39. doi: 10.3389/fimmu.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheever AW, Byram JE, Hieny S, von Lichtenberg F, Lunde MN, Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985;7:399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 31.Gaubert S, Viana da Costa A, Maurage CA, Lima EC, Fontaine J, Lafitte S, et al. X-linked immunodeficiency affects the outcome of Schistosoma mansoni infection in the murine model. Parasite Immunol. 1999;21:89–101. doi: 10.1046/j.1365-3024.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 32.Qiu S, Fan X, Yang Y, Dong P, Zhou W, Xu Y, et al. Schistosoma japonicum infection downregulates house dust mite-induced allergic airway inflammation in mice. PLoS ONE. 2017;12:e0179565. doi: 10.1371/journal.pone.0179565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, et al. Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection. Infect Immun. 2008;76:2212–2218. doi: 10.1128/IAI.01433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes LM, Pereira MA, Gerken SE, Vaz N. Polyclonal activation of B lymphocytes during experimental infection with Schistosoma mansoni. Parasitology. 1990;100:83–91. doi: 10.1017/S0031182000060145. [DOI] [PubMed] [Google Scholar]
- 35.O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 36.Velupillai P, Sypek J, Harn DA. Interleukin-12 and -10 and gamma interferon regulate polyclonal and ligand-specific expansion of murine B-1 cells. Infect Immun. 1996;64:4557–4560. doi: 10.1128/IAI.64.11.4557-4560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velupillai P, Secor WE, Horauf AM, Harn DA. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J Immunol. 1997;158:338–344. [PubMed] [Google Scholar]
- 38.el-Cheikh MC, Bonomo AC, Rossi MI, Pinho Mde F, Borojevic R. Experimental murine schistosomiasis mansoni: modulation of the B-1 lymphocyte distribution and phenotype expression. Immunobiology. 1998;199:51–62. doi: 10.1016/S0171-2985(98)80063-6. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira FL, Aguiar AM, Borojevic R, El-Cheikh MC. IgE expression on the surface of B1 and B2 lymphocytes in experimental murine schistosomiasis. Braz J Med Biol Res. 2005;38:1033–1042. doi: 10.1590/S0100-879X2005000700006. [DOI] [PubMed] [Google Scholar]
- 40.Floudas A, Amu S, Fallon PG. New insights into IL-10 dependent and IL-10 independent mechanisms of regulatory B cell immune suppression. J Clin Immunol. 2016;36(Suppl. 1):25–33. doi: 10.1007/s10875-016-0263-8. [DOI] [PubMed] [Google Scholar]
- 41.Chang JH, Kim YJ, Han SH, Kang CY. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25− T cells to CD4+ Tregs. J Clin Investig. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 44.Margry B, Kersemakers SC, Hoek A, Arkesteijn GJ, Wieland WH, van Eden W, et al. Activated peritoneal cavity B-1a cells possess regulatory B cell properties. PLoS ONE. 2014;9:e88869. doi: 10.1371/journal.pone.0088869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirose T, Tanaka Y, Tanaka A, Sakai H, Sasaki Y, Shinohara N, et al. PD-L1/PD-L2-expressing B-1 cells inhibit alloreactive T cells in mice. PLoS ONE. 2017;12:e0178765. doi: 10.1371/journal.pone.0178765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yong L, Tang Y, Ren C, Liu M, Shen J, Hou X. B1 cells protect against Schistosoma japonicum-induced liver inflammation and fibrosis by controlling monocyte infiltration. PLoS Negl Trop Dis. 2019;13:e0007474. doi: 10.1371/journal.pntd.0007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian F, Hu X, Xian K, Zong D, Liu H, Wei H, et al. B10 cells induced by Schistosoma japonicum soluble egg antigens modulated regulatory T cells and cytokine production of T cells. Parasitol Res. 2015;114:3827–3834. doi: 10.1007/s00436-015-4613-x. [DOI] [PubMed] [Google Scholar]
- 48.Haczku A, Takeda K, Hamelmann E, Loader J, Joetham A, Redai I, et al. CD23 exhibits negative regulatory effects on allergic sensitization and airway hyperresponsiveness. Am J Respir Crit Care Med. 2000;161:952–960. doi: 10.1164/ajrccm.161.3.9905046. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho A, Forni L, Holmberg D, Ivars F, Vaz N. From an antigen-centered, clonal perspective of immune responses to an organism-centered, network perspective of autonomous activity in a self-referential immune system. Immunol Rev. 1984;79:151–168. doi: 10.1111/j.1600-065X.1984.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Yang X, Li Y, Zhu J, Zhou S, Xu Z, et al. Follicular helper T cells promote liver pathology in mice during Schistosoma japonicum infection. PLoS Pathog. 2014;10:e1004097. doi: 10.1371/journal.ppat.1004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebert LM, Horn MP, Lang AB, Moser B. B cells alter the phenotype and function of follicular-homing CXCR5+ T cells. Eur J Immunol. 2004;34:3562–3571. doi: 10.1002/eji.200425478. [DOI] [PubMed] [Google Scholar]
- 52.Deenick EK, Ma CS, Brink R, Tangye SG. Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr Opin Immunol. 2011;23:111–118. doi: 10.1016/j.coi.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Lu L, Qian S, Fung JJ, Lin F. Hepatic stellate cells directly inhibit B cells via programmed death-ligand 1. J Immunol. 2016;196:1617–1625. doi: 10.4049/jimmunol.1501737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J Clin Investig. 2009;119:1019–1028. doi: 10.1172/JCI36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Werf N, Redpath SA, Azuma M, Yagita H, Taylor MD. Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection. PLoS Pathog. 2013;9:e1003215. doi: 10.1371/journal.ppat.1003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou S, Jin X, Li Y, Li W, Chen X, Xu L, et al. Blockade of PD-1 signaling enhances Th2 cell responses and aggravates liver immunopathology in mice with schistosomiasis japonica. PLoS Negl Trop Dis. 2016;10:e0005094. doi: 10.1371/journal.pntd.0005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.