Abstract
Alport syndrome is a hereditary disease affecting Type IV collagen characterized by hematuria, progressive renal failure, sensorineural hearing loss, and ocular abnormalities. Most cases are X-linked and involve the COL4A5 gene with a minority of patients having autosomal recessive mutations in the COL4A3 or COL4A4 genes encoding the α3(IV) or α4(IV) chain respectively. Here, we describe the case of a 31-year-old woman who presented during pregnancy with hematuria and proteinuria and was diagnosed with autosomal recessive Alport syndrome (ARAS) post-partum. Her biopsy was notable for findings of segmental glomerulosclerosis with some collapsing features, in addition to thin basement membranes and rare “splitting”. Genetic testing identified two novel mutations in the COL4A4 gene: a truncating frame shift mutation c.3861delinsCTC and a missense mutation c.4708G>A (p.Glu1570Lys), both of which we assert to be pathogenic. She had normal full-term delivery without complications. This case has several unique features including the relatively mild disease phenotype and the findings of glomerular scarring with collapsing features on renal biopsy. The successful pregnancy outcome and her clinical presentation add to the growing body of evidence that ARAS can have a variable phenotype.
Keywords: Autosomal recessive Alport syndrome, COL4A4, pregnancy
Introduction
Alport syndrome is a hereditary disease affecting Type IV collagen characterized by hematuria, progressive renal failure, sensorineural hearing loss, and ocular abnormalities [1]. Approximately 85% of cases are X-linked, caused by mutations in the COL4A5 gene encoding the α5(IV) chain [2]. Autosomal recessive Alport syndrome (ARAS), responsible for about 15% of cases, is caused by homozygous or compound heterozygous mutations in the COL4A3 or COL4A4 genes on chromosome 2 encoding the α3(IV) or α4(IV) chain respectively [3,4]. Autosomal dominant inheritance is rare [5].
While improved mutation detection has led to the identification of hundreds of likely pathogenic variants, and kindred analyses have broadened the spectrum of reported phenotypes, the features of Alport syndrome in women remains poorly described [6]. Little is known about maternal and fetal outcomes of pregnancy in women with Alport syndrome. We describe the case of a woman who presented during pregnancy with hematuria and proteinuria and was diagnosed with ARAS. This report highlights a case of ARAS with a relatively mild phenotype and favorable pregnancy outcome, both of which enhance our understanding of collagen IV related kidney diseases.
Case Presentation
A 31-year-old female G1P0 presented for evaluation of proteinuria during pregnancy. She had conceived via in vitro fertilization. Routine urinalysis at 11 weeks 2 days gestation showed 3+ protein and 3+ blood. Spot urine protein was 1.6 grams per gram of creatinine. A 24 hour urine collection at 14 weeks 4 days gestation showed 2,064mg of protein. Serum creatinine was 0.77 mg/dL. She denied a history of hematuria, proteinuria, or kidney disease. Prior urinalyses were not available, but she reported never being told about urinary abnormalities by prior physicians. She reported mild nausea. Her medical history was notable only for infertility. There was no family history of kidney disease or hearing loss. Both of her parents have had normal urinalyses. On examination, she was well-appearing with a blood pressure of 108/54 mmHg. Her lungs were clear to auscultation, the uterus was not palpable, and she had no peripheral edema. Urine sediment showed 6 red blood cells per high powered field, half of which were acanthocytes. Ultrasound revealed symmetric kidneys without hydronephrosis, cysts, or masses. Additional testing demonstrated negative ANA, ANCA, anti-GBM antibody, HIV antibody, hepatitis B surface and core antigens, hepatitis C antibody, and normal HbA1C, C3 and C4 levels.
She was closely monitored, and during the third trimester, the proteinuria rose rapidly to 6.94 g/g creatinine, serum albumin dropped to 2.5 g/dL, and serum creatinine increased to 1.09 mg/dL. Her blood pressure remained normal, and she had no edema. Labor was induced at 38 weeks gestation and she delivered a healthy baby girl weighing 3201 grams.
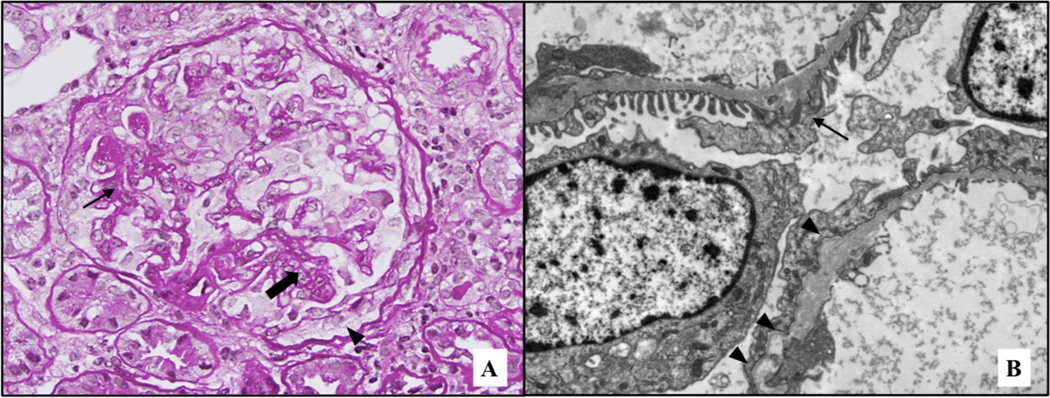
At 7 weeks postpartum, the proteinuria decreased to 2.3 g/g creatinine, serum albumin increased to 3.6 g/dL, and serum creatinine decreased to 0.84 mg/dL. A percutaneous renal biopsy was performed. The specimen contained 24 glomeruli, 7 of which were globally sclerotic and 6 of which showed segmental or global collapsing features with sclerosis (Figure 1, Panel A). There was moderate interstitial fibrosis and tubular atrophy and interstitial foam cells. Immunofluorescence was negative for immunoglobulins, light chains, C3, and C1q. The α1, α3, and α5 chains of Type IV collagen stained normally. Electron microscopy of two glomeruli revealed only focal foot process effacement. There was widespread thinning of the basement membrane (mean 224 nm, SD 47 nm). Occasional thickening with splitting of the basement membrane was present (Figure 1, Panel B).
Figure 1: Representative images of renal biopsy.
(A) Glomerulus showing foci of segmental sclerosis (arrow), as well as some capillary collapse (thick arrow). In addition, a focus of prominent epithelial cells (pseudocrescent) is present (arrowhead). PAS stain, original magnification 40X. (B) Ultrastructural examination revealed only focal foot process effacement, together with diffuse thinning of the glomerular basement membranes (mean 224 nm, SD 47 nm), as seen in the upper capillary wall (arrow). Rare foci of thickening, together with splitting and scalloping of the outer contour of the lamina densa were identified (arrowheads), as seen in the lower capillary wall. Original magnification 8,000X.
With features suggesting Alport syndrome, after obtaining consent from the patient, we performed DNA sequencing of the COL4A3, COL4A4, and COL4A5 genes using Sanger sequencing (Complete Alport Syndrome Evaluation Panel, Athena diagnostics, Marlborough, MA, USA). Sequencing revealed a heterozygous c.3861delinsCTC frameshift mutation and a heterozygous c.4708G>A p.(Glu1570Lys) missense mutation, both in the in the COL4A4 gene (NM_000092.4). Both variants were predicted to be pathogenic by SIFT and PolyPhen. According to the Leiden Open Variation Database (https://databases.lovd.nl/shared/genes/COL4A4), neither mutation has been reported previously in Alport Syndrome. Given the presence of two COL4A4 mutations along with her clinical presentation and renal biopsy findings, she was diagnosed with ARAS. There were no ocular defects on ophthalmologic examination except for mild myopia. Ten months post-partum, serum creatinine was 0.9 mg/dL and serum albumin was 3.8 g/dL while proteinuria persisted at 3,216 mg/g creatinine.
Both variants were submitted to NCBI ClinVar (accession numbers SCV000920872 and SCV000920873).
Discussion
We describe a 31-year-old woman with hematuria and proteinuria during the first trimester of pregnancy. During the third trimester the proteinuria increased to nephrotic range with a decrease in serum albumin and rise in creatinine. Post-partum, proteinuria persisted. A renal biopsy was notable for glomerular scarring and widespread thinning of the glomerular basement membranes with rare foci of thickening and splitting. Genetic testing revealed compound heterozygous mutations in COL4A4, most consistent with ARAS.
With the increased availability of genetic testing, including next generation and whole exome sequencing, our understanding of the molecular basis and diverse phenotypes of collagen IV related renal diseases has expanded. It was previously proposed that heterozygous mutations in COL4A3 and COL4A4 cause thin basement membrane nephropathy (TBMN) and lead to benign hematuria [5], while homozygous or compound heterozygous mutations lead to ARAS. Autosomal dominant inheritance has also been described [7, 8]. It is now apparent that mutations in COL4A3 and COL4A4 result in a spectrum of renal disease, from hematuria to progressive chronic kidney disease, with the clinical phenotype influenced by the molecular consequences of the patient’s genotype [9]. Our patient’s genotype suggests ARAS, but she presented without ocular or hearing abnormalities, at a relatively older age, and with preserved GFR. In a series of 30 genetically proven ARAS females, the average age at diagnosis was 17.7 +/− 8.1 years, while only 40% had hearing loss and 10% had ocular abnormalities. The median age of ESRD was 21 years, although only 43% had developed ESRD at the time of analysis [10]. In another series of 40 patients with ARAS, 29% of adults had normal renal function. In cases where renal failure occurred, COL4A3/4 variants were more commonly nonsense mutations [11].
The intermediate phenotype in our case is likely explained by the specific COL4A4 variant identified. She has a truncating mutation that predicts altered protein function, and another mutation that may have a less deleterious effect. Additionally, variants in modifier genes may interact with COL4A4 mutations to alter the expressed phenotype. Such modifier loci have been suggested in other renal diseases [12–14].
A significant feature of our patient’s biopsy is the segmental and global glomerulosclerosis with collapsing features. We postulate that the glomerular scarring is a result of Alport syndrome with the additive effect of hyperfiltration of pregnancy. While the glomerulosclerosis in this case was not focal, it is of interest that several reports have described a link between COL4 mutations and focal and segmental glomerulosclerosis (FSGS) [15–18]. Voskarides et al. first described a link between heterozygous COL4A4 and COL4A3 mutations and FSGS on biopsy in a study of Greek-Cypriot families [15]. Of 82 patients with heterozygous COL4A4/COL4A3 mutations, 47 (57.3%) developed chronic renal failure or ESRD. Among the families segregating hematuria and COL4A4/COL4A3 mutations, 15 kidney biopsies from 15 patients were available, all showing FSGS. Using targeted next generation sequencing, Gast et al. identified COL4 mutations in 38% of patients with presumed familial FSGS [13]. Whole exome sequencing of a diverse cohort of 3315 adults with chronic kidney disease identified 91 patients (2.7%) with COL4A variants, 15 of which (16%) had a clinical diagnosis of FSGS [19]. These findings raise the possibility that COL4 nephropathies are not infrequently misclassified as FSGS. Identification of thinning or splitting of the glomerular basement membrane on electron microscopy is essential for accurate diagnosis.
It has been generally understood that the glomerular basement membrane in ARAS shows absent α5 staining due to failure of the α5 chain to be deposited in the GBM in the absence of α3 or α4 chains. However, the GBM α5 expression pattern was normal in our patient. Normal α5 expression has been reported in other cases of ARAS. In a systematic review of ARAS, among individuals with collagen α staining available on biopsy, 8 of 34 (24%) had a normal α5 expression pattern [20]. Furthermore, 7 of 8 with normal α5 expression had at least one missense mutation. Our case is consistent with this observation. These findings highlight the variable functional consequences of COL4 mutations on the assembly of the collagen IV network. In our patient, the c.4708G>A mutation is in the non-collagenous C-terminal domain responsible for the assembly of α chains into heterotrimers. Heterotrimer assembly may not be impaired by this mutation, but other characteristics of the collagen network may be. The c.3861delinsCTC mutation is in the collagenous domain, which might affect the integrity of the collagen network, but not necessarily the α5 component. Our case emphasizes that intact collagen staining does not exclude variants of Alport Syndrome.
Finally, it is particularly notable that our patient had a favorable pregnancy outcome. Pregnancies in XLAS and ADAS are generally characterized by worsening proteinuria and preterm delivery, but most have return of proteinuria to pre-pregnancy levels and no worsening of renal function [21]. There are three reported cases of ARAS pregnancies, all in women diagnosed with AS prior to conception. Crovetto et al. describe pregnancies in two sisters with ARAS with deafness and proteinuria prior to conception [22]. Both developed nephrotic syndrome, delivered preterm, and proteinuria reverted to pre-pregnancy levels with stable renal function. Nishizawa et al. describe a woman with a homozygous COL4A4 mutation (c.3307G>A) with proteinuria but normal renal function prior to conception [23]. She developed nephrotic syndrome and proteinuria improved after delivery. Unlike these cases, our patient did not have the nephrotic syndrome, but she did develop significant proteinuria that persisted after delivery, likely the result of glomerulosclerosis accelerated by pregnancy. Our case suggests that in ARAS, normal renal function, the absence of hypertension, and mild proteinuria pre-conception probably favor a good pregnancy outcome as is seen in other forms of chronic kidney disease [24].
Limitations include the lack of genetic sequencing in our patient’s parents. However, with ARAS, her parents are obligate carries as mutation rates are low [11]. We also do not have laboratory testing prior to pregnancy which may have identified hematuria and/or proteinuria prior to conception and accelerated the diagnosis.
In conclusion, our case contributes to our knowledge understanding of pregnancy outcomes in ARAS. It supports the current understanding that normal renal function, mild proteinuria, and lack of hypertension predict favorable pregnancy outcomes. Additionally, it illustrates that ARAS phenotypes vary widely as the result of the specific genetic variants present. As sequencing technologies become more widely-available to the practicing clinician, we posit that the clinical spectrum of ARAS will continue to become more diverse.
Footnotes
Statement of Ethics: Informed consent was obtained from the patient prior to submission of the manuscript. This study did not require review/approval by the appropriate Ethics Committee.
Disclosure Statement: The authors declare that they have no relevant conflicts of interest to disclose.
References
- 1.WILLIAMSON DA. Alport’s syndrome of hereditary nephritis with deafness. Lancet. 1961;2(7216):1321–3. [DOI] [PubMed] [Google Scholar]
- 2.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248(4960):1224–7. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994;8(1):77–81. [DOI] [PubMed] [Google Scholar]
- 4.Feingold J, Bois E, Chompret A, Broyer M, Gubler MC, Grünfeld JP. Genetic heterogeneity of Alport syndrome. Kidney Int. 1985;27(4):672–7. [DOI] [PubMed] [Google Scholar]
- 5.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013;24(3):364–75. [DOI] [PubMed] [Google Scholar]
- 6.Savige J, Colville D, Rheault M, Gear S, Lennon R, Lagas S, et al. Alport Syndrome in Women and Girls. Clin J Am Soc Nephrol. 2016;11(9):1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jefferson JA, Lemmink HH, Hughes AE, Hill CM, Smeets HJ, Doherty CC, et al. Autosomal dominant Alport syndrome linked to the type IV collage alpha 3 and alpha 4 genes (COL4A3 and COL4A4). Nephrol Dial Transplant. 1997;12(8):1595–9. [DOI] [PubMed] [Google Scholar]
- 8.Fallerini C, Dosa L, Tita R, Del Prete D, Feriozzi S, Gai G, et al. Unbiased next generation sequencing analysis confirms the existence of autosomal dominant Alport syndrome in a relevant fraction of cases. Clin Genet. 2014;86(3):252–7. [DOI] [PubMed] [Google Scholar]
- 9.Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, et al. Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018;93(5):1045–51. [DOI] [PubMed] [Google Scholar]
- 10.Oka M, Nozu K, Kaito H, Fu XJ, Nakanishi K, Hashimura Y, et al. Natural history of genetically proven autosomal recessive Alport syndrome. Pediatr Nephrol. 2014;29(9):1535–44. [DOI] [PubMed] [Google Scholar]
- 11.Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA. COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol. 2013;24(12):1945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee R, Hoffman M, Cliften P, Seshan S, Liapis H, Jain S. Targeted exome sequencing integrated with clinicopathological information reveals novel and rare mutations in atypical, suspected and unknown cases of Alport syndrome or proteinuria. PLoS One. 2013;8(10):e76360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gast C, Pengelly RJ, Lyon M, Bunyan DJ, Seaby EG, Graham N, et al. Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2016;31(6):961–70. [DOI] [PubMed] [Google Scholar]
- 14.Voskarides K, Papagregoriou G, Hadjipanagi D, Petrou I, Savva I, Elia A, et al. COL4A5 and LAMA5 variants co-inherited in familial hematuria: digenic inheritance or genetic modifier effect? BMC Nephrol. 2018;19(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voskarides K, Damianou L, Neocleous V, Zouvani I, Christodoulidou S, Hadjiconstantinou V, et al. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol. 2007;18(11):3004–16. [DOI] [PubMed] [Google Scholar]
- 16.Pierides A, Voskarides K, Kkolou M, Hadjigavriel M, Deltas C. X-linked, COL4A5 hypomorphic Alport mutations such as G624D and P628L may only exhibit thin basement membrane nephropathy with microhematuria and late onset kidney failure. Hippokratia. 2013;17(3):207–13. [PMC free article] [PubMed] [Google Scholar]
- 17.Voskarides K, Patsias C, Pierides A, Deltas C. COL4A3 founder mutations in Greek-Cypriot families with thin basement membrane nephropathy and focal segmental glomerulosclerosis dating from around 18th century. Genet Test. 2008;12(2):273–8. [DOI] [PubMed] [Google Scholar]
- 18.Voskarides K, Pierides A, Deltas C. COL4A3/COL4A4 mutations link familial hematuria and focal segmental glomerulosclerosis. glomerular epithelium destruction via basement membrane thinning? Connect Tissue Res. 2008;49(3):283–8. [DOI] [PubMed] [Google Scholar]
- 19.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. N Engl J Med. 2019;380(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JM, Nozu K, Choi DE, Kang HG, Ha IS, Cheong HI. Features of Autosomal Recessive Alport Syndrome: A Systematic Review. J Clin Med. 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunini F, Zaina B, Gianfreda D, Ossola W, Giani M, Fedele L, et al. Alport syndrome and pregnancy: a case series and literature review. Arch Gynecol Obstet. 2018;297(6):1421–31. [DOI] [PubMed] [Google Scholar]
- 22.Crovetto F, Moroni G, Zaina B, Acaia B, Ossola MW, Fedele L. Pregnancy in women with Alport syndrome. Int Urol Nephrol. 2013;45(4):1223–7. [DOI] [PubMed] [Google Scholar]
- 23.Nishizawa Y, Takei T, Miyaoka T, Kamei D, Mochizuki T, Nitta K. Alport syndrome and pregnancy: Good obstetric and nephrological outcomes in a pregnant woman with homozygous autosomal recessive Alport syndrome. J Obstet Gynaecol Res. 2016;42(3):331–5. [DOI] [PubMed] [Google Scholar]
- 24.Alkhunaizi A, Melamed N, Hladunewich MA. Pregnancy in advanced chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2015;24(3):252–9. [DOI] [PubMed] [Google Scholar]