Abstract
Background
Despite conflicting results, considerable evidence suggests the association between single nucleotide polymorphisms in MTHFR, XRCC1 and OGG1 genes and, risk of developing breast cancer. Here a case-control study is reported, including 135 breat cancer patients and 112 healthy women, all representative of Northern Sardinian population.
Methods
Polymerase chain reaction/restriction fragment length polymorphism method was used to determine the genotypes of five polymorphisms: MTHFR C677T (rs1801133) and A1298C (rs1801131), XRCC1 Arg194Trp (rs1799782) and Arg399Gln (rs25487) and OGG1 Ser326Cys (rs1052133). Allelic, genotypic and haplotype association analyses with disease risk and clinicopathological parameters were performed.
Results
A nominally significant association with breast cancer risk was observed for MTHFR C677T polymorphism heterozygous genotype in the codominant model (OR: 0.57, 95% CI: 0.32–1.00, p = 0.049) and for Cys/Cys genotype of the OGG1 Ser326Cys polymorphism in the recessive model (OR: 0.23, 95% CI: 0.05–1.11, p = 0.0465). No significant differences were found at genotype-level for A1298C polymorphism of the MTHFR gene and Arg194Trp and Arg399Gln of the XRCC1 gene. Furthermore, the OGG1 and XRCC1 rs25487 polymorphisms were nominally associated with PgR, Her2 status and with sporadic breast cancer, respectively.
Conclusions
Based on genetic characteristics of individuals included in this study, results suggest that MTHFR CT and OGG1 Cys/Cys genotypes have a protective effect that may have an influence on breast cancer risk in a representative Northern Sardinian population.
Keywords: Mediterranean population, MTHFR SNPs, XRCC1 SNPs, OGG1 SNPs
Background
Breast cancer (BC) is the most common malignancy and remains the main cause of cancer-related deaths in women worldwide [1]. Although mechanisms of breast carcinogenesis have not been completely elucidated, it is known that genetic factors can interact with numerous environmental factors thus determining an overall individual susceptibility. The study of BC susceptibility in isolated populations - where evolutionary forces and genetic features might have amplified the effect of specific risk alleles - offers the opportunity to shed further light on the pivotal role of genetic alterations in the occurrence of cancer. In such a context, the Mediterranean island of Sardinia shows a unique high incidence of several diseases with monogenic and multifactorial inheritance [2–4] and represents an important case-study population for human geneticists interested in inferring the complex mapping of traits and diseases. The relatively homogeneous genetic composition reported for Sardinian population has shown to be useful in depicting genetic factors of predisposition to BC [5–7]. A gap in BC incidence is observed in Italy between areas in northern Italy compared to central and southern areas; Sardinia shows the highest incidence rate (134,4 new cases/100.000 inhabitants) among southern regions, and the lowest among areas in northern Italy [8]. Here we reported a pilot case-control study carried out on a cohort of 135 northern Sardinian BC patients and 112 healthy controls, who were genotyped for polymorphisms in three restriction length molecular markers with frequency in Sardinian population > 5%. Specifically, the present study aimed to evaluate the role as risk factors of five genetic variants in three genes involved in methylation and DNA synthesis and in DNA repair mechanisms: the Methylenetetrahydrofolate reductase gene (MTHFR), the x-ray repair cross-complementing group 1 gene (XRCC1) and the 8-Oxoguanine glycosylase gene (OGG1). We also proposed to evaluate the possible association between these Single nucleotides polymorphisms (SNPs) of these genes and the main clinicopathological features.
The MTHFR is a key enzyme in the folate metabolic pathway and catalyses the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the circulating form of folate and the methyl donor in biological processes including methylation of proteins and nucleic acids. Furthermore, MTHFR plays an essential role in de novo synthesis of purines and pyrimidine nucleoside, but also in DNA biosynthesis, repair and maintenance of DNA stability. Two common polymorphisms in the MTHFR gene, C677T-rs1801133 and A1298C-rs1801131, have been associated with decreased enzyme activity and increased levels of plasma homocysteine [9–11]. The C677T allelic variant involves a cytosine to thymine substitution at position 677, a consequence of transformation from an alanine to a valine at codon 222 in the N-terminal catalytic domain, and it is correlated with increased thermolability and reduced enzyme activity. The A1298C allelic variant results into the change from a glutamic acid to alanine residue at codon 429 in the C-terminal regulatory domain of the protein but the reduction in enzymatic activity remains still controversial. Public eQTL data (GTEX catalog, release V8, accessed 26 Nov 2019), show that the rs1801133 minor allele causes a decreased expression of MTHFR gene in skin cells and cultured fibroblasts, while the minor allele of rs1801131 is associated with higher expression of the gene in many different tissues. However, an evident linkage between the polymorphic variants of the MTHFR gene and the increased risk of BC has not been clearly established. Functional relevance of rs1801133 has been confirmed by GWAS data about regulation of serum folate levels [https://www.ebi.ac.uk/gwas/variants/rs1801133] and other haematological traits (https://genetics.opentargets.org/variant/1_11796321_G_A), as supported by studies about high-altitude adaptation in Tibetans [www.ncbi.nlm.nih.gov/pubmed/28373541]; rs1801131 is associated with pressure related traits and hypertension (https://genetics.opentargets.org/variant/1_11794419_T_G).
The XRCC1 gene is located on chromosome 19q13.2 and contains 17 exons that encodes a 70 kDa protein consisting of 633 amino acids. Shen et al. [12] have identified two polymorphisms resulting in nonconservative amino acid substitution variants in the XRCC1 gene. In this study C → T substitution at codon 194 in exon 6 (Arg to Trp, rs1799782) and G → A substitution at codon 399 in exon 10 (Arg to Gln, rs25487) were analysed. The Arg194Trp polymorphism is close to a modified residue (phosphothreonine in position 198), and it is located in the linker region between the NH2-terminal domain and the BRCT1 (BRCA1 C-terminus) domain (positions 315–403); the Arg399Gln polymorphism occurs in important biologically interaction domains with PARP (poly ADP-ribose polymerase). The Trp derived allele has been associated with an increased Base Excision Repair (BER) activity, while the 399Gln allele with its reduction [13]. Public eQTL data show that the effect of rs25487 on the expression of XRCC1 is strictly tissue dependent, showing instead for example opposite effects of the minor allele in nerve tibial cells and testis derived cells; the rs1799782 minor allele causes a minor gene expression in thyroid cells.
OGG1 gene, which is located on chromosome 3p26.2 and also involved in BER, encodes a DNA glycosylase and apurinic/apyrimidinic lyase activities to excise 8-oxoguanine (8-OH-G), a mutagenic base which occurs as a result of Reactive oxigen species (ROS) exposure. Ser326Cys in exon 7 (rs1052133) is the most frequent and most studied polymorphism in the OGG1 gene. Amino acid replacement of serine with cysteine causes a reduced OGG1 DNA repair activity [14]. The effect on gene activity is confirmed by eQTL data, with the minor allele being associated with lower expression of OGG1.
These polymorphisms have been previously correlated with various cancer types [15–20] and differences in their allelic variant effects have been reported for different ethnic groups [21]. However, they have never been investigated so far within the Sardinian population with the scope to find, if any, a possible evident correlation between polymorphisms occurrence and BC risk, clinicopathological traits and lifestyle exposures.
Methods
Sampling plan
In this case-control study, conducted between 2017 and 2019, a total of 247 unrelated women were included: 135 BC patients enrolled at the Medical Oncology Unit in Sassari University Hospital and a control group consisting of 112 healthy women, who had never previously been affected by any tumour, enrolled at the Unit of Occupational Medicine of the University of Sassari.
A further control group, formed by 109 women who had voluntarily accessed to Lega Italiana Lotta contro i Tumori (LILT, Sassari) prevention facilities for investigations, was also analyzed. However, LILT volunteers’ sample was then excluded from the study as a self-selection bias was highlighted (data not shown), being such a group of individuals exposed to a higher level of familiarity compared to the other healthy controls.
In order to recruit only individuals representative of Sardinian population, the participants were selected on the basis of their surname (which had to be typically Sardinian) and place of birth of their grandparents (which had to be located in Northern Sardinia). All women taking part to the study were asked to fill in a questionnaire regarding a wide range of all known risk factors for BC development: i.e. demographic characteristics, body weight and Body mass index (BMI), reproductive-hormone profile, family history of cancer, passive and active cigarette smoking (pack-years were calculated as years smoked multiplied by the current number of cigarettes smoked per day divided by 20) [17], lifetime alcohol use (only the frequency was evaluated and not the quantity introduced), physical activity (at least two to three times a week), fruit and vegetable intake (classified as high at least two portions per day), diet composition and more. In completing the questionnaire regarding lifestyle, the participants were invited to consider the widest possible time span and not only the period close to the enrolment.
The questionnaire collected all information needed to identify the familial forms of BC compared to sporadic ones. In summary, the inclusion criteria adopted for identified family forms were: one case with BC diagnosis before 39 years of age and/or bilateral diagnosis of BC before the age of 40 in two first or second degree relatives; presence of 3 or more cases of BC in first or second degree relatives in the same side of the family tree; cases of ovarian cancer; male BC cases; presence of multiple primary cancers in any organ in the same individual or family [22].
Polymorphisms genotyping
EDTA blood samples were obtained from all study participants at the moment of enrolment and were stored at − 20 °C until further use.
Genomic DNA was extracted from 200 μl of peripheral blood by QIAmp DNA Blood Mini Kit (Qiagen, Germany) following manufacturer’s instructions.
The following reported allelic variants for three genes were investigated by Polymerase chain reaction/restriction fragment length polymorphism (PCR-RFLP) assay: MTHFR C677T (rs1801133) and A1298C (rs1801131), XRCC1 Arg194Trp (rs1799782) and Arg399Gln (rs25487) and OGG1 Ser326Cys (rs1052133).
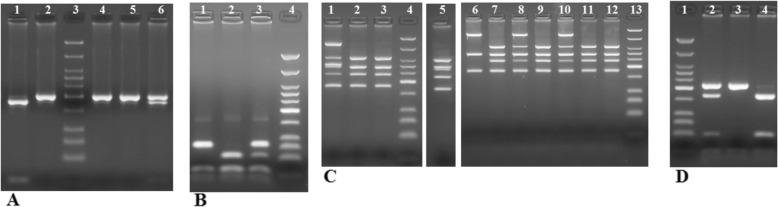
MTHFR C677T and A1298C: genotyping was performed as previously reported [23] (Fig. 1a-b).
Fig. 1.
Electrophoretic separation of MTHFR, XRCC1 and OGG1 digestion in 3% Metaphor gel. Marker of molecular weight: Low Molecular Weight DNA Ladder (NEB). aMTHFR C677T: lane 1 TT (178, 23 bp), lanes 2, 4, 6 CC (201 bp), lane 6 CT (201, 178, 23 bp), lane 3 marker molecular weight; bMTHFR A1298C: lane 1 CC (84, 31, 30, 28, 18 bp), lanes 2 AA (56, 31, 30, 28, 18 bp), lane 3 AC (84, 56, 31, 30, 28, 18 bp), lane 4 marker molecular weight; cXRCC1 Arg194Trp (bold) and Arg399Gln PCR-multiplex: lane 1 arg/trp-arg/gln (615, 374, 313, 292, 241, 174 bp), lanes 2, 3, 7, 9, 11, 12 arg/arg-arg/arg (374, 292, 241, 174 bp), lane 5 arg/trp-arg/arg (374, 313, 292, 241, 174 bp), lane 6 arg/arg-gln/gln (615, 292, 174 bp), lanes 8,10 arg/arg-arg/gln (615, 374, 292, 241, 174 bp), lanes 4, 13 marker molecular weight. d. OGG1 Ser326Cys: lane 1 marker molecular weight, lane 2 ser/cys (213, 164, 49, 21 bp), lane 3 ser/ser (213, 21 bp), lane 4 cys/cys (164, 49, 21 bp)
XRCC1 Arg194Trp and Arg399Gln: a multiplex PCR was used to amplify 491 and 615 bp fragments containing the 194 and 399 polymorphisms respectively [24]. PCR was performed in a total reaction volume of 25 μl containing 100 ng DNA, 1x PCR buffer, 2 mM of MgCl2, 0.2 mM of each dNTP, 1.5 units of Taq Gold DNA polymerase (Applied Biosystems, USA), 0.6 μM for codon 194 of each primer (5′ GCC CCG TCC CAG GTA 3′ and 3′ AGC CCC AAG ACC CTT TCA CT 5′), and 0.8 μM for codon 399 (5′ TTG TGC TTT CTC TGT GTC CA 3′ and 3′ TCC TCC AGC CTT TTC TGA TA 5′). Thermal cycling conditions consisted of an initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 45 s, 56.4 °C for 45 s, 72 °C for 60 s with a final extension at 72 °C for 5 min. The Arg alleles at codons 194 and 399 create MspI restriction sites. The multiplex PCR products were digested with MspI (New England Biolabs, USA) in a volume of 25 μl at 37 °C for 3 h. Among the genotypes for codon 194, the Arg/Arg resulted in 292, 174 and 21 bp, Arg/Trp in 313, 292, 174 and 21 bp, Trp/Trp in 313 and 174 bp digestion products. A 174 bp fragment was present in all the samples because of an invariant MspI site that was used as an internal control for complete digestion. Among the genotypes for codon 399, the Arg/Arg resulted in 374 and 241 bp, Arg/Gln in 615, 374 and 241 bp, Gln/Gln in 615 bp digestion products. (Fig. 1c).
OGG1 Ser326Cys: PCR was performed with the final volume of 20 μl of reaction mixture containing 100 ng DNA, 5x PCR buffer, 0.2 mM of each dNTP, 2.5 units of Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs, USA) and 0.6 μM of each primer (5′ CCC AAC CCC AGT GGA TTC TCA TTG C 3′ and 5′ GGT GCC CCA TCT AGC CTTGCG GCC CTT 5′). Thermal cycling conditions consisted of an initial denaturation at 98 °C for 30 s, followed by 35 cycles of 98 °C for 30 s, 67 °C for 30 s, 72 °C for 30s with a final extension at 72 °C for 5 min. The 234 bp PCR product was digested using Fnu4HI (New England Biolabs, USA) in a volume of 25 μl for 3 h at 37 °C. A mismatch was incorporated in the reverse primer to create an invariant restriction site of 21 bp which produced a 21 bp fragment in all the samples [25]. The C → G cysteine variant creates a Fnu4HI restriction enzyme site and produces fragments of 213, 164, 49 and 21 bp (Fig. 1d). All the PCR products were electrophoresed in a 3% Metaphor agarose gel stained with ethidium bromide and visualized by UV transillumination.
Statistical analysis
Minor allele frequency of the five polymorphisms here considered has been verified in the public resource SardiNIA Pheweb (https://pheweb.irgb.cnr.it/).
The allelic status of each individual was inferred based on the SNPs analysis performed by PCR-RFLP.
Agreement on genotype frequencies to Hardy-Weinberg equilibrium (HWE) expectation was assessed independently among controls for each SNP, using a 1-degree-of-freedom chi-square test.
Student’s t-test and Fisher’s exact test were used to evaluate the differences in descriptive variables at diagnosis between the cases and controls, such as age or BMI.
For each SNP, the genotypic and allelic association were tested considering multiple inheritance models (additive, dominant, codominant and over-dominant, and recessive), using a custom R script, to compare BC patients with the group of healthy controls enrolled in the present study. The R package fsmb was used to calculate odds ratios (OR) and 95% confidence intervals (95% CI) according to Woolf method. When a 0 count was observed a Gart-adjusted logit interval was calculated.
Before association, potential confounders (as age and BMI) were tested by including them as covariates in the association models; none of them showed a significant impact, so no covariate was added in the analyses.
Haplotype analysis was restricted to polymorphisms located on the same chromosome: i) the haplotype rs1801133/rs1801131 (MTHFR) and ii) the haplotype rs1799782/rs25487 (XRCC1). Measures of Linkage disequilibrium (LD) between each pair of SNPs (D’ and r2 statistics) and haplotype reconstruction were obtained in 1000 genomes European population with the LDpair web tool (https://ldlink.nci.nih.gov/?tab=ldpair). The most common haplotype was selected as the reference. OR and 95% CI were calculated to estimate the degree of the association between haplotypes and the risk of breast cancer.
A threshold of p ≤ 0.05 was used to assess statistical significance for each genetic association. A Bonferroni correction was also applied to adjust for multiple testing, obtaining a significant threshold of p < 0.0027 for differences in descriptive variables at diagnosis between the cases and controls (Table 1, numb. of tests = 18) and p < 0.01 for association analyses (Suppl. Table 1A, numb. tests = 5).
Table 1.
Descriptive questionnaire data of cases and controls
| Variables | Cases X | Cases n. | Cases % | Contr. X | Contr. n. | Contr. % | P value |
|---|---|---|---|---|---|---|---|
| Age | 57.00 | 50.03 | 9.0E-07a | ||||
| Height | 159.24 | 160.50 | 0.1 | ||||
| Weight | 64.50 | 60.20 | 0.002a | ||||
| BMI | 25.40 | 23.40 | |||||
| < 25 | 72 | 53.33 | 80 | 71.43 | 0.004 | ||
| 25–30 | 38 | 28.15 | 25 | 22.32 | |||
| ≥ 30 | 25 | 18.52 | 7 | 6.25 | |||
| Age at menarche | 12.44 | 12.72 | 0.1 | ||||
| 7–10 | 12 | 8.89 | 8 | 7.14 | 0.7 | ||
| 11–13 | 90 | 66.67 | 72 | 64.28 | |||
| 14–17 | 33 | 24.44 | 32 | 28.58 | |||
| Menopause | |||||||
| natural menopause | 62 | 45.93 | 55 | 49.11 | 0.6 | ||
| age | 49.90 | 48.60 | 0.1 | ||||
| HRT regimen | 11 | 17.74 | 8 | 14.54 | 0.6 | ||
| Parity | 97 | 71.85 | 71 | 63.39 | 0.2 | ||
| first full-term pregnancy | 27.59 | 29.56 | 0.1 | ||||
| number of live birth | 2.05 | 1.82 | 0.1 | ||||
| Breastfed | 78 | 80.41 | 63 | 88.73 | 0.2 | ||
| months of breastfeeding | 11.83 | 12.72 | 0.9 | ||||
| Oral contraceptive use | 78 | 57.77 | 87 | 77.67 | 0.001a | ||
| duration of employment: | |||||||
| ≤ 5 y | 20 | 25.64 | 33 | 37.93 | 0.2 | ||
| 6–14 y | 35 | 44.87 | 33 | 37.93 | |||
| ≥ 15 y | 23 | 29.49 | 21 | 24.14 | |||
| Benign breast lesions | 29 | 21.48 | 12 | 10.71 | 0.03 | ||
| BC family | |||||||
| in first degree relatives | 1.34 | 0.74 | 8.8E-05a | ||||
| 0 cases | 47 | 34.81 | 62 | 55.36 | 4.0E-04a | ||
| 1–2 cases | 58 | 42.96 | 42 | 37.50 | |||
| ≥ 3 | 30 | 22.22 | 8 | 7.14 | |||
| Tumor family | |||||||
| in first degree relatives: | 3.42 | 2.81 | 0.03 | ||||
| 0–2 cases | 51 | 37.78 | 57 | 50.90 | 0.05 | ||
| 3–5 | 57 | 42.22 | 43 | 38.39 | |||
| ≥ 6 | 27 | 20.00 | 12 | 10.71 | |||
| Alcohol intake | |||||||
| daily-often | 31 | 22.96 | 13 | 11.61 | 0.03 | ||
| occasionally- nondrinkers | 104 | 77.04 | 99 | 88.39 | |||
| Smoking status (smokers at recruitment) | 19 | 14.07 | 18 | 16.07 | 0.7 | ||
| Smokers (at recruitment+ex smokers) | 68 | 50.37 | 56 | 50.00 | 0.2 | ||
| cigarette packs/year: | |||||||
| < 10 | 30 | 44.12 | 25 | 44.64 | 0.3 | ||
| 10–19 | 16 | 23.53 | 19 | 33.93 | |||
| ≥ 20 | 22 | 32.35 | 12 | 21.43 | |||
| passive smoke: | 100 | 74.07 | 87 | 77.68 | 5.0E-04a | ||
| little | 22 | 22.00 | 41 | 47.13 | |||
| moderately | 46 | 46.00 | 27 | 31.03 | |||
| strong | 32 | 32.00 | 19 | 21.84 | |||
| Osteoporosis | 41 | 30.37 | 22 | 19.64 | 0.06 | ||
| Chemical exposure | 43 | 31.85 | 18 | 16.07 | 0.04 | ||
| Sport activity (2–3 times a week) | 45 | 33.33 | 43 | 38.39 | 0.4 | ||
| Adolescence sport activity | 36 | 26.66 | 56 | 50.00 | 2.0E-04a | ||
aSignificant after Bonferroni correction
To assess whether the cohort from Northern Sardinia enrolled in this study may be considered as representative of the whole Sardinian population and consequently to evaluate the reliability of our sample in being representative of whole Sardinian population genetic variation, we performed a 1-df chi square test to compare allelic frequencies between our groups and the whole Sardinia population.
The public eQTL data used for the analyses described in this manuscript were obtained from the GTEx Portal on 12/01/2019 (https://gtexportal.org/home/).
To evaluate the interaction between personal habits and SNPs with significant association with BC risk, a logistic regression model was performed where the multiplicative term of alcohol consumption, dietary folate intake and smoke and the SNPs were considered.
Results
Patient’s lifestyle and behaviour characteristics
Table 1 summarizes data emerged from the questionnaire analysis that were related to demographic, hormonal-reproductive and lifestyle characteristics of all participants in our case-control study. The mean age of cases and controls at the time of enrolment was 57.0 and 50.03, respectively (p = 9 × 10− 7). No significant differences emerged between BC patients and healthy women regarding menarche and menopausal age, parity and breastfeeding. There was an increase in BMI in patients (p = 0.004) compared to controls, and an increase in the use of oral contraceptives in the control group compared to cases (p = 0.001). The BC patients had benign lesions of the breast (p = 0.03) and a familiarity of BC (p = 8.8 × 10–5) and tumours in general (p = 0.03) higher than controls. A greater number of affected women claimed to take alcohol daily/often (p = 0.03), to be subjected to passive smoking (p = 5 × 10− 4) and to be exposed to environmental toxic substances for work or other reasons (p = 0.04). Finally, a greater amount of practice in competitive physical activity during adolescence was significantly highlighted in the control group compared to the cases (p = 2 × 10− 4). There were no significant differences in the introduction of fruit and vegetable in the diet, as well as white meat, milk, eggs, fish, etc. While it emerged that in women affected by BC the following were introduced more frequently in their diet: red meat, cured meat, cheese, bread, pasta, potatoes, legumes, butter and lard and less frequently cereals (data not shown).
Clinicopathological data related to BC patients
Data on clinicopathological characteristics of BC patients enrolled in this study are shown in Table 2. Most of the tumours were invasive ductal carcinoma (IDC) (85.18%) and early stage tumours (65.91%). According to the Nottingham prognostic index for BC combined to the histology grade, 12 patients were classified as G1 (8.89%), 79 as G2 (58.52%) and 43 as G3 (31.85%). Finally, molecular subtypes of the 135 BC were: 57 Luminal A (42.22%), 21 Luminal B-like Her-2 neg (15.55%), 21 Luminal B-like Her-2 pos (15.55%), 21 Her-2 overexpressing (15.55%) and 15 Triple negative (11.11%). The table also shows data concerning age at diagnosis and laterality (43.70 and 56.30% respectively).
Table 2.
Clinicopathological features of BC patients
| Variables of BC (n. = 135) | n. | (%) | |
|---|---|---|---|
| Age at diagnosis (mean years) | 52.15 | ||
| ≤ 40 | 22 | 16.30 | |
| > 40 | 113 | 83.70 | |
| Family BC | |||
| Sporadic | 76 | 56.30 | |
| Familial | 59 | 43.70 | |
| Laterality | |||
| Bilateral | 11 | 8.15 | |
| Unilateral | 124 | 91.85 | |
| Menopausal status (at diagnosis) | |||
| premenopausal | 72 | 53.33 | |
| postmenopausal | 63 | 46.66 | |
| Histologic type | |||
| IDC | 115 | 85.18 | |
| ILC | 10 | 7.40 | |
| D or L in situ | 7 | 5.18 | |
| Other | 3 | 2.22 | |
| Histologic grade | |||
| G1 | 12 | 8.89 | |
| G2 | 79 | 58.52 | |
| G3 | 43 | 31.85 | |
| missing | 1 | 0.74 | |
| Hormonal receptor | |||
| ER | pos | 98 | 72.59 |
| neg | 37 | 27.41 | |
| PgR | pos | 91 | 67.40 |
| Neg | 44 | 32.60 | |
| Her-2 | |||
| pos | 42 | 31.11 | |
| neg | 88 | 65.18 | |
| missing | 5 | 3.70 | |
| Ki67 | |||
| ≤ 30% | 103 | 76.30 | |
| > 30% | 31 | 22.96 | |
| missing | 1 | 0.74 | |
| Tumor size (T) | |||
| T0-Tis | 7 | 5.18 | |
| T1-T2 | 96 | 71.11 | |
| T3-T4 | 32 | 23.70 | |
| Lymph nodes grade (N) | |||
| N0 | 59 | 43.70 | |
| N1 | 40 | 29.62 | |
| N2 | 19 | 14.08 | |
| N3 | 7 | 5.18 | |
| Nx | 10 | 7.41 | |
| Distant Metastasis | |||
| M0 | 111 | 82.22 | |
| M1 | 22 | 16.30 | |
| missing | 2 | 1.48 | |
| Clinical stages | |||
| 0 | 12 | 8.88 | |
| I | 43 | 31.85 | |
| II | 34 | 25.18 | |
| III | 19 | 14.10 | |
| IV | 21 | 15.55 | |
| missing | 6 | 4.44 | |
| Luminal A | 57 | 42.22 | |
| Luminal B-like Her-2 neg | 21 | 15.55 | |
| Luminal B-like Her-2 pos | 21 | 15.55 | |
| Her-2 overexpressing | 21 | 15.55 | |
| Triple negative | 15 | 11.11 | |
As regards the 59 familial BC reported in Table 2, a total of eleven families (8.15%) were reported with cases of BC insurgents at a young age and concomitant presence of cases of ovarian cancer, two families (1.50%) with cases of BC in the male, 26 (19.25%) families with three cases of BC, twenty-six (19.25%) with four cases and nine (6.66%) with five or more cases. Finally, the highly familiar BC had an average of BC cases of 4.82 and tumours in general of 2.06 compared to 2.47 and 1.93 of sporadic BC, respectively (data not shown).
Association with disease risk
The distribution of allelic frequencies in the whole Sardinian population [26–28] for the five SNPs here considered, is reported in Table 3.
Table 3.
Genotype and allele distribution of MTHFR, XRCC1 and OGG1 polymorphisms
| Gene (SNP) | Model | Genotype | Cases n.135 (n. %) |
Controls n.112 (n. %) |
OR (95% CI) | p | ||
|---|---|---|---|---|---|---|---|---|
|
MTHFR(rs1801133) Ref. Allele = C Alt. Allele = T |
Codominant | C/C | 53 | 39.25 | 31 | 27.68 | 1.00 | |
| C/T | 60 | 44.44 | 62 | 55.36 | 0.57 (0.32–1) | 0.049 | ||
| T/T | 22 | 16.30 | 19 | 16.96 | 0.68 (0.32–1.44) | 0.31 | ||
| Dominant | C/C | 53 | 39.25 | 31 | 27.68 | 1.00 | ||
| C/T-T/T | 82 | 60.74 | 81 | 72.32 | 0.59 (0.35–1.02) | 0.06 | ||
| Recessive | C/C-C/T | 113 | 83.70 | 93 | 83.03 | 1.00 | ||
| T/T | 22 | 16.30 | 19 | 16.96 | 0.95 (0.49–1.87) | 0.89 | ||
| Overdominant | C/C-T/T | 75 | 55.60 | 50 | 44.64 | 1.00 | ||
| C/T | 60 | 44.44 | 62 | 55.36 | 0.65 (0.39–1.07) | 0.09 | ||
| Allele | C | 166 | 61.48 | 124 | 55.36 | 1.00 | ||
| T | 104 | 38.52 | 100 | 44.64 | 0.78 (0.54–1.11) | 0.17 | ||
| Hardy Weinberg eq. | p = 0.47 | p = 0.25 | ||||||
|
MTHFR(rs1801131) Ref. Allele = A Alt. Allele = C |
Codominant | A/A | 66 | 48.88 | 56 | 50.00 | 1.00 | |
| A/C | 55 | 40.74 | 49 | 43.75 | 0.95 (0.56–1.61) | 0.86 | ||
| C/C | 14 | 10.37 | 7 | 6.25 | 1.7 (0.64–4.5) | 0.28 | ||
| Dominant | A/A | 66 | 48.88 | 56 | 50.00 | 1.00 | ||
| A/C-C/C | 69 | 51.11 | 56 | 50.00 | 1.05 (0.63–1.73) | 0.86 | ||
| Recessive | A/A-A/C | 121 | 89.63 | 105 | 93.75 | 1.00 | ||
| C/C | 14 | 10.37 | 7 | 6.25 | 1.74 (0.68–4.46) | 0.25 | ||
| Overdominant | A/A-C/C | 80 | 59.26 | 63 | 56.2 | 1.00 | ||
| A/C | 55 | 40.74 | 49 | 43.75 | 0.88 (0.53–1.47) | 0.63 | ||
| Allele | A | 187 | 69.26 | 168 | 75.00 | 1.00 | ||
| C | 83 | 30.74 | 63 | 28.12 | 1.18 (0.8–1.74) | 0.39 | ||
| Hardy Weinberg eq. | p = 0.69 | p = 0.49 | ||||||
|
XRCC1(rs1799782) Ref. Allele = C Alt. Allele = T |
Codominant | C/C | 128 | 94.81 | 102 | 91.07 | 1.00 | |
| C/T | 7 | 5.18 | 10 | 8.93 | 0.56 (0.21–1.52) | 0.25 | ||
| Allele | C | 263 | 97.40 | 214 | 95.53 | 1.00 | ||
| T | 7 | 5.18 | 10 | 4.46 | 0.57 (0.21–1.52) | 0.26 | ||
| Hardy Weinberg eq. | p = 1.00 | p = 1.00 | ||||||
|
XRCC1(rs25487) Ref. Allele = G Alt. Allele = A |
Codominant | G/G | 64 | 47.41 | 53 | 47.32 | 1.00 | |
| G/A | 55 | 40.74 | 43 | 38.39 | 1.06 (0.62–1.82) | 0.83 | ||
| A/A | 16 | 11.85 | 16 | 14.28 | 0.83 (0.38–1.81) | 0.64 | ||
| Dominant | G/G | 64 | 47.40 | 53 | 47.32 | 1.00 | ||
| G/A-A/A | 71 | 52.59 | 59 | 52.68 | 1 (0.6–1.65) | 0.99 | ||
| Recessive | G/G-G/A | 119 | 88.15 | 96 | 85.71 | 1.00 | ||
| A/A | 16 | 11.85 | 16 | 14.28 | 0.81 (0.38–1.7) | 0.57 | ||
| Overdominant | G/G-A/A | 80 | 59.26 | 69 | 61.61 | 1.00 | ||
| G/A | 55 | 40.74 | 43 | 38.39 | 1.1 (0.66–1.84) | 0.71 | ||
| Allele | G | 183 | 67.78 | 149 | 66.52 | 1.00 | ||
| A | 87 | 64.44 | 75 | 33.48 | 0.94 (0.65–1.38) | 0.77 | ||
| Hardy Weinberg eq. | p = 0.56 | p = 0.14 | ||||||
|
OGG1(rs1052133) Ref. Allele = C Alt. Allele = G |
Codominant | C/C | 87 | 64.44 | 70 | 62.50 | 1.00 | |
| C/G | 46 | 34.07 | 35 | 31.25 | 1.06 (0.62–1.82) | 0.84 | ||
| G/G | 2 | 1.48 | 7 | 6.25 | 0.23 (0.05–1.14) | 0.05 | ||
| Dominant | C/C | 87 | 64.44 | 70 | 62.50 | 1.00 | ||
| C/G-G/G | 48 | 35.55 | 42 | 37.50 | 0.92 (0.55–1.55) | 0.75 | ||
| Recessive | C/C-C/G | 133 | 98.52 | 105 | 93.75 | 1.00 | ||
| G/G | 2 | 1.48 | 7 | 6.25 | 0.23 (0.05–1.11) | 0.0465 | ||
| Overdominant | C/C-G/G | 89 | 65.92 | 77 | 68.75 | 1.00 | ||
| C/G | 46 | 34.07 | 35 | 31.25 | 1.14 (0.67–1.94) | 0.64 | ||
| Allele | C | 220 | 81.48 | 175 | 78.12 | 1.00 | ||
| G | 50 | 18.52 | 49 | 21.87 | 0.81 (0.52–1.26) | 0.35 | ||
| Hardy Weinberg eq. | p = 0.24 | p = 0.4 | ||||||
None of the 1-df chi square tests used to compare allelic frequencies between our groups and the whole Sardinian population showed statistical significance. All SNPs were successfully genotyped in all participants to the present study and were in accordance with HWE in healthy controls (p > 0.05).
Using the chi-square test, the association between the SNPs tested and the BC risk under five gene models (codominant, dominant, recessive, overdominant and additive) was analysed. Minor allele frequencies in cases and controls were showed.
Genotype analysis of MTHFR polymorphisms showed that CT genotype frequency of rs1801133 polymorphism revealed a nominal association with lower risk of BC in the codominant model (CT vs. CC, OR: 0.57, 95% CI: 0.32–1.00, p = 0.049). This trend was confirmed by dominant and overdominant models (p = 0.055 and 0.088 respectively).
Furthermore, the analysis of combined effect of C677T and A1298C polymorphisms showed that no subject presented with homozygous mutant allele in both polymorphic sites.
Likewise, no significant effect of BC susceptibility was highlighted in this study for SNPs studied in XRCC1. No homozygous case was found neither in Arg194Trp nor in concurrent heterozygous allele Trp with Arg399Gln homozygous mutant.
Furthermore, the GG genotype frequency of OGG1-rs1052133 polymorphism revealed a nominal association with lower risk of breast cancer in the recessive model (GG vs. CC/CG, OR: 0.23, 95% CI: 0.05–1.11, p = 0.0465, with the trend confirmed in the codominant model for GG genotype (p = 0.05).
Association analysis between the risk of BC and haplotypes of MTHFR gene (rs1801133 - rs1801131) and XRCC1 gene (rs1799782 - rs25487) were also performed. Haplotypes were reconstructed from the 1000G data and results of their distribution among BC cases and controls were summarized in Table 4. The pairwise LD is given for each pair of SNPs, and r2 measurements indicate that LD is low. Among the MTHFR haplotypes, rs1801133-T / rs1801131-A is the most frequent in both cases and controls, while rs1801133-T / rs1801131-C is absent (Table 4). Among the XRCC-1 haplotypes, rs1799782 -C / rs25487 -G is the most frequent, while rs1801133-T / rs1801131-A is absent. Overall, none of the considered haplotypes is significantly associated with BC risk.
Table 4.
Aplotype frequencies of MTHFR and XRCC1 in BC patiens and controls D’ and r2 statistics calculated in 1000 genomes European population
| Gene | Haplotype | Cases | Controls | OR (95% CI) | p | |
|---|---|---|---|---|---|---|
|
MTHFR D’ = 0.9999 r2 = 0.262 |
rs1801133 | rs1801131 | ||||
| T | A | 95 (38.52%) | 110 (44.64%) | 1.00 | ||
| C | C | 77 (31.11%) | 70 (28.12%) | 1.27 (0.83–1.95) | 0.27 | |
| C | A | 75 (30.37%) | 67 (27.23%) | 1.30 (0.84–1.99) | 0.24 | |
| T | C | 0.00 | 0.00 | – | – | |
|
XRCC1 D’ = −0.9997 r2 = 0.031 |
rs1799782 | rs25487 | ||||
| C | G | 160 (64.81%) | 153 (62.05%) | 1.00 | ||
| C | A | 81 (32.59%) | 83 (33.48%) | 0.92 (0.63–1.34) | 0.67 | |
| T | G | 6 (2.59%) | 11 (4.46%) | 0.52 (0.19–1.45) | 0.21 | |
| T | A | 0.00 | 0.00 | |||
Association between genotypes, clinical features and lifestyles
In this study the possible relationship between some clinicopathological parameters such as estrogen receptor (ER) and progesterone receptor (PgR) status, Her-2 and Ki67 expression and lymph node involvement (Suppl. Table 1A) and other parameters such as age at diagnosis, BMI, pre/post menopause diagnosis and family history of BC cases and the distribution of SNP genotypes was explored (Suppl. Table 1B).
Regarding the clinical features, only the OGG1-rs1052133 polymorphism is associated at the nominal level with PgR status: G allele is significantly more frequent among PgR- cases (allelic model: OR: 0.09, 95% CI: 0.04–2.04, p = 0.0439). Furthermore, it is significantly associated with Her2 status, being more frequent among Her2+ cases (recessive model: OR: 10.92, 95% CI: 0.51–232.81, p = 0.0399). None of these associations is confirmed after Bonferroni correction (Table 5).
Table 5.
Association of OGG1 polymorphisms and PgR, Her-2 status in BC patients
| Variable | Model |
OGG1 rs1052133 |
OR (95% CI) | p |
|---|---|---|---|---|
|
PgR+/PgR- (n. 90/45) |
Recessive | C/C-C/G 90/43 | 1.00 | – |
| G/G 0/2 | 0.09 (0.04–2.04)a | 0.0439 | ||
| Her2+/Her2 (n. 42/88) | Recessive | C/C-C/G 40/88 | 1.00 | – |
| G/G 2/0 | 10.92 (0.51–232.81)a | 0.0399 |
aGart adjusted logit interval
Moreover, a nominal association of the XRCC1-rs25487 polymorphism was found with family history of BC, with G/A genotype associated in both the codominant and overdominant models (OR: 2.19; 95% CI: 1.04–4.62, p = 0.0393 and OR: 2.16; 95% CI: 1.06–4.41, p = 0.0337) respectively, no other significant association was found with the other clinicopathological features (Table 6).
Table 6.
Association of XRCC1 polymorphism rs25487 with BC family history status in BC patients
| Variable | Model |
XRCC1 (rs25487) |
OR (95% CI) | p |
|---|---|---|---|---|
|
BC family history Spor/Famil (n.76/59) |
Codominant | G/G 31/33 | 1.00 | – |
| G/A 37/18 | 2.19 (1.04–4.62) | 0.0393 | ||
| A/A 8/8 | 1.06 (0.36–3.18) | 0.91 | ||
| Overdominant | G/G-A/A 39/41 | 1.00 | – | |
| G/A 37/18 | 2.16 (1.06–4.41) | 0.0337 |
We also explored the effect of polymorphisms in relation to smoking status (67 current smokers+ex-smokers versus 68 never smokers), alcohol intake (89 daily-often intake versus 46 non-drinkers) and fruit and vegetables intake (88 high versus 47 low intake, where intakes are classified as high if the individual consumed at least two portions of fruit and vegetables per day). It was not found any significant evidence of interaction between MTHFR-rs1801133 and OGG1-rs1052133 (SNPs with significant association with BC risk) polymorphisms and these lifestyles in cases and controls (data not shown).
Discussion
BC is a complex and heterogeneous disease in which many genetic factors and lifestyle-related factors are combined, resulting into a multifactorial aetiology. Through the questionnaires we aimed to investigate the various risk factors and the effects of changes in lifestyle on mammary carcinogenesis.
In our study no significant differences between BC patients and controls were found in regards with hormonal-reproductive factors. However, the stratification in age groups of the menarche onset and the duration of oral contraceptive use shows a tendency towards an increased risk in the case of early onset of menarche and prolonged use of oral contraceptives, respectively. Similarly, a slight trend is observed for the average age of menopause and hormone replacement therapy regimen, while in our sample parity and breastfed are not aligned with most of the results in the literature [29–32]. Furthermore, our case-control study has confirmed that increase in BMI, the previous onset of benign lesions and genetic factors determining a high familiarity of BC and other tumors act as strong risk factors.
Several strong epidemiological and clinical studies support the correlation between mammary tumorigenesis, tumour progression and obesity. The main mechanisms underlying this link are capacity of conversion of androgenic precursors to estrogen through peripheral aromatization in adipose tissue, the alteration of physiological levels of insulin, insulin-like growth factor (IGF), adipokines, changes in the adipose tissue microenvironment and chronic low-grade inflammation [33–38]. Juarez-Cruz JC et al. [39] have recently shown that the leptin, a hormone secreted by adipocytes, through the FAK and Src kinases activation, contributes to BC progression. Physical activity is closely related to obesity. A meta-analysis study investigating the relationship with BC risk showed a 20% reduction if physical activity is performed in adolescence and within 24 years old [40], by delaying the onset of menarche and reducing the bioavailable hormones levels [41]. Our study has significantly confirmed these data in relation to obesity and physical activity.
In our case-control study we found a significant increase in the frequency of alcoholic drink intake in affected women compared to controls (daily-often 22.96% against 11.61 p = 0.03 respectively), even though quantity was not evaluated. Over 100 studies support a positive association between alcohol consumption and BC risk in all age groups. Alcohol increases the risk of BC in a dose-dependent manner, but unfortunately the awareness of alcohol as a risk factor is low as well as the ability of women to estimate the amount of alcohol contained in the drinks taken daily. It is clear that information campaigns are needed on this topic and other modifiable risk factors [42]. No difference was found between the two groups for what concerns fruit and vegetables intake.
Our findings revealed that affected women are strong smokers, in fact 32.35% of them have a packs/year value≥20 compared to 21.43% in controls. In addition, a strong impact of exposure to passive smoking emerged, in agreement with other studies that showed that exposure to passive smoking contributes to an increased risk in particular of Er+/PgR+ BC [43]. Finally, 31.85% BC women compared to 16.07% of controls, reported having come into contact throughout their lifetime with chemicals such as paints, solvents, pesticides, herbicides, environmental pollutants, textile industry products, etc.
Often the questionnaire procedure can produce selection bias that can, however, be minimized when the study is appropriately conducted. All women participating to our study completed the questionnaire after receiving the necessary information from the same operator. In completing the questionnaire regarding lifestyle, the participants were invited to consider the widest possible time span and not their conduct in the period close to enrolment. We noticed that many women tended to minimize, perhaps for cultural reasons, some factors such as alcohol consumption, the use of oral contraceptive and other avoidable factors, whose role in cancerogenesis is known, as the excessive introduction of foods such as cold cuts, red meat, dessert. Therefore, we believe that these data could be underestimated. On the contrary, the affected women showed excessive attention and evaluation towards possible contacts with chemicals.
In addition to identifying mutations in predisposition genes, several studies have been carried out on the association of gene polymorphisms involved in various cellular processes, with the risk and clinicopathological features of BC. Accordingly, we have investigated if some SNPs of MTHFR, XRCC1 and OGG1 genes affect the pathogenesis in a cohort of Northern Sardinian BC patients and healthy controls.
It is known that folate plays a crucial role in regulating DNA repair and DNA methylation. The MTHFR enzyme belongs to a complicated metabolic pathway which also involves other enzymes such as methionine synthase (MTR), methionine synthase reductase (MTRR). It is biologically plausible that genetic polymorphism of these genes altering the pool of methyl group level can have effects on DNA synthesis, repair and methylation, consequent gene instability and cancer development. MTHFR wild-type C genotype can lead to an increased risk due to aberrant DNA methylation leading to activation of proto-oncogenes by hypomethylation of their promoter region and transcriptional silencing of tumor suppressor genes in case of hypermethylation of CpG islands. While the allelic T variant leads to high plasma levels of homocysteine that influences DNA methylation and can lead to changes in the availability of nucleotides for DNA synthesis and repair [44, 45] due to folate-dependent reactions. Balance in these extremely complex processes is therefore strongly influenced not only by gene polymorphisms, but also by the amount of folates introduced through diet, and other factors, including alcohol consumption, and by currently well-known ethnic differences and variable genetic background. For this reason, literature findings show controversial data [46] especially for rs1801133: on the one hand the TT genotype is associated to an increased cancer risk [47–53] on the other hand a relatively large number of studies support a null or protective effect [54–58]. In agreement with the latter, our results have shown that the CT allele of MTHFR-rs1801133 is associated with lower risk of BC in the codominant model, while no significant difference was found for the rs1801131 polymorphism in any model examined.
Our study compared to the previous one [23], with the study groups carefully selected regarding ethnicity, greater sample size and with a control group without the auto-selection bias, showed no evidence of association between the MTHFR-rs1801133 and rs1801131 and the clinicopathological variables.
Regarding lifestyle variables, some authors report an association between MTHFR polymorphisms and some lifestyle approaches such as cigarette smoking status, alcohol intake or high or low folate introduction through diet [59–62]. It is known that cigarette smoking is a major preventable risk factor for lung cancer but also for many other cancers including BC, being able to interfere and modify genetic and epigenetic pathways and the expression of many proinflammatory interleukins [63]. The association between alcohol intake and the risk of developing BC in pre- and postmenopausal women has been demonstrated. Alcohol can act indirectly through the mutagenic and promoting action of its first metabolite acetaldehyde, also by increasing the level of estrogen, causing alterations of the immune system and finally causing nutritional deficiencies including folate and other vitamins [64]. We did not find any significant evidence of interaction between the polymorphisms analyzed and these lifestyles approaches.
It is clear how complex is the study of the relationships existing between genetic factors (we cannot exclude the still unknown interaction with other genes) and lifestyle, especially as regards the folate and alcohol introduction, and higher BC susceptibility. This leads to the conflicting results existing in the literature that can be minimized, within the same population, if the studies are conducted with standardized and rigorous criteria.
It is well-known that gene alterations in components of DNA repair systems, deriving from cumulative exogenous and endogenous mutagens, play a key-role in abnormal growth and malignant transformation. Some common polymorphisms in the gene repair systems may influence the ability of repairing damaged DNA and could be involved in carcinogenesis and tumour progression. The XRCC1 protein plays a central role in BER system, which is responsible for the repair of single-strand DNA break resulting from exposure to X-rays, ROS and alkylating agents [65]. XRCC1 protein acts as a scaffolding protein interacting with 8-oxoguanine DNA glycosylase, DNA ligase III, DNA polymerase β, and poly (ADP-ribose) polymerase at the site of damaged DNA.
In our study no association with the risk of BC was demonstrated for XRCC1-rs1799782 (Arg to Trp) and rs25487 (Arg to Gln). The distribution of Arg194Trp and Arg399Gln polymorphisms are significantly influenced by ethnicity: the frequency of the Trp allele is higher in Asian populations than in African and Caucasian populations [66]. Furthermore, it has been observed that patients with the Arg/Arg genotype exhibited significantly higher levels of chromosomal breaks than those with the Trp allele [67]. While the Gln allele of rs25487 leads to a reduction in BER DNA repair activity [24]. Also in this case, the results about the frequency of genotypes of XRCC1-rs1799782 (Arg to Trp) and rs25487 (Arg to Gln) and the BC susceptibility are contrasting [21, 68–77]. In our study, the XRCC1-rs25487 polymorphism is nominally associated with family history of BC in two genetic models. In particular, the G/A genotype is significantly associated with sporadic cases in both codominant and overdominant models. Most BC are sporadic forms, which develop in the general population in the absence of familiarity and are mainly related to the action of sex hormones and environmental factors such as smoking, alcohol and nutrition. The transformation into a malignant phenotype depends on the accumulation of random genetic mutations in somatic cells. Such mutations often appear and are/or stabilized during cellular division. The hormones can modulate the growth control mechanisms, cell division frequency, differentiation and, in this way, have the possibility of favoring the onset and accumulation of random genetic errors. In this context, the association we observed, causing as reported a reduction in BER activity, could determine a greater susceptibility to transformation from chemical carcinogens in the mammary gland.
The OGG1 gene, another component of the BER pathway, is a key enzyme which removes 8-oxoguanine considered as a marker of oxidative stress and highly mutagenic. Our study shows an association between the OGG1-rs1052133: the GG (Cys) genotype frequency revealed, indeed a nominal association with lower risk of BC in the recessive model, thus exercising a protective role in BC. In the literature, many functional studies suggest that the 326Cys allele is associated with the reduced DNA repair activity and may contribute to mammary carcinogenesis [78, 79] and other types of cancer [80–82]. However, some studies report conflicting results [74, 83–86]. Weiguang et al. [78] in a meta-analysis study, suggested that the OGG1 Ser326Cys polymorphism is significantly associated with BC in both premenopausal and postmenopausal European women and the 326Cys allele play a protective role in BC carcinogenesis. Synowiec et al. [84] studied the association of polymorphisms of some DNA repair genes with BC by the modulation of the cellular response to oxidative stress. They also observed a protective effect of some genotypes, including the OGG1 Cys allele, against BC occurrence.
This missense variant has been shown to influence the expression level of TTLL3 (Tubulin Tyrosine Ligase Like 3) and OGG1; more in details, [https://gtexportal.org/home/snp/rs1052133], CC genotype is associated with lower mRNA expression of TTLL3 in transformed fibroblasts (p = 7.4e-8) and tibial artery (p = 0.0000034), and with lower expression of OGG1 in transformed fibroblasts (p = 0.000035) [87]. Several previous studies have assessed the role of the OGG1 polymorphism rs1052133 in human tumors. A statistically significant association was observed between the genotype distribution of rs1052133 and chronic myelogenous leukaemia patients in an African population [88]. The same polymorphism has been nominally associated also with the risk of squamous cell carcinomas of the head and neck (SCCHN) among north Indians [89]; in the same study, the mutant (G) allele has been proposed to be a protective factor for SCCHN among north Indian subpopulations. Furthermore, OGG1-rs1052133 polymorphism has been implicated in the pathogenesis of nasopharyngeal carcinoma (NPC), especially among women: genotype GG at rs1052133 was associated with significantly lower NPC risk than genotypes GC + CC OR: 0.770, 95% CI: 0.595–0.996, p = 0.012) [90].
Regarding the clinical features, only the OGG1-rs1052133 polymorphism is associated at the nominal level with PgR-status: G allele is significantly more frequent among PgR- cases. Furthermore, it is significantly associated with Her2 status, being more frequent among Her2+ cases. This suggests an association between the G allele and the molecular subtype PgR- and Her2 overexpressing BC in our cohort. Amplification or overexpression of Her-2 occurs in approximately 15–30% of BC. Ali K. et al. [91] in a meta-analysis study showed higher OGG1 mutation frequencies in association with IDC, ER-, PgR-, and HER-2/neu status and other clinicopathological features in Pakistani populations and worldwide.
Conclusion
Our results suggest a possible association between CT genotype in the MTHFR-rs1801133 and the GG genotype in the OGG1-rs1052133 and a decreasing BC risk in the Northern Sardinia population. Furthermore, some interesting associations were found between some allelic variants and clinical features. However, we believe that latter data should be taken with caution because of the reduction in the sample size that subdivision into sub-categories involves.
Due to its relatively homogeneous genetic composition, the Mediterranean island of Sardinia represents an interesting population for inferring the genetic risk factors in complex diseases such as BC. Here we reported a pilot case-control study carried out on a cohort of Northern Sardinia BC patients and healthy controls, aimed to evaluate the disease risk of genetic variants in three genes involved in DNA methylation and repair mechanisms. Mutational analyses and gene expression profiles allowed us to establish that BC is a complex and heterogeneous disease with marked characteristics of biological variability and response to treatments determined by an equally complex network of aetiological factors. Therefore, it is clear that several studies with larger sample size are warranted both on our and on other ethnic groups to validate the risk factors and to clarify the complex relationships between different genes and their interaction with environmental factors in BC risk and disease progression. Overall, even if our findings should be treated with caution because of the relatively small sample size and heterogeneity, they might be helpful in early detection of BC through identification of population at risk in light of the growing need of decision tools for personalized medicine.
Supplementary information
Additional file 1: Table S5 A. Association of MTHFR, XRCC1, OGG1 polymorphisms and ER, PgR, Her-2, Ki67 and Lymph Node status in BC patients. B. Association of MTHFR, XRCC1, OGG1 polymorphisms and Age at diagnosis, BMI, Menopause, BC family history status in BC patients.
Acknowledgements
We thank the volunteers of “Lega Italiana Lotta contro i Tumori” (LILT) for the great collaboration shown.
Abbreviations
- BC
Breast cancer
- MTHFR
Methylenetetrahydrofolate reductase
- XRCC1
X-ray repair cross-complementing group 1
- OGG1
8-Oxoguanine glycosylase
- SNP
Single nucleotide polymorphism
- BER
Base excision repair
- ROS
Reactive oxigen species
- BMI
Body mass index
- PCR-RFLP
Polymerase chain reaction restriction fragment length polymorphism
- HWE
Hardy-Weinberg equilibrium
- OD
Odd ratio
- CI
Confidence interval
- LD
Linkage disequilibrium
- IDC
Invasive ductal carcinoma
- ER
Estrogen receptor
- PgR
Progesterone receptor
Authors’ contributions
MRM and MF conceived, coordinated the study and drafted manuscript. PC, MF, and FC performed statistical analysis. MS, PC, DS and AA performed manuscript revision. MRM, MRDM, FS and GP carried out SNPs genotyping and contributed to clinical data management. MRM, EL, AS, CC and MB were involved in the recruitment of the patients and controls. CP, VS and AP provided the clinical data. All authors approved the final manuscript.
Funding
This work was supported in part by grants from Fondazione Banco di Sardegna, “Salute Pubblica, Medicina Preventiva e Riabilitativa”, Anno 2018. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This experimental study was approved by ASL Sassari Bioethical Committee and written informed consent was obtained from each participant, according to Italian Legislation, 2468/CE 14/03/2017. All data are treated according to the EU General Data Protection Regulation 2016/679.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matteo Floris and Daria Sanna contributed equally to this work.
Contributor Information
Matteo Floris, Email: mfloris@uniss.it.
Maria Rosaria Muroni, Email: mrmuroni@uniss.it.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-06749-w.
References
- 1.Ban KA, Godellas CV. Epidemiology of breast cancer. Surg Oncol Clin N Am. 2014;23:409–422. doi: 10.1016/j.soc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Rosatelli MC, Dozy A, Faà V, et al. Molecular characterization of beta-thalassemia in the Sardinian population. Am J Hum Genet. 1992;50:422–426. [PMC free article] [PubMed] [Google Scholar]
- 3.Loudianos G, Dessi V, Lovicu M, Angius A, Figus A, et al. Molecular characterization of Wilson disease in the Sardinian population–evidence of a founder effect. Hum Mutat. 1999;14:294–303. doi: 10.1002/(SICI)1098-1004(199910)14:4<294::AID-HUMU4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Rosatelli MC, Meloni A, Meloni A, Devoto M, Cao A, Scott HS, Peterson P, Heino M, Krohn KJ, Nagamine K, Kudoh J, Shimizu N, Antonarakis SE. A common mutation in Sardinian autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. Hum Genet. 1998;103:428–434. doi: 10.1007/s004390050846. [DOI] [PubMed] [Google Scholar]
- 5.Karvonen M, Tuomilehto J, Libman I, La Porte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND project group. Diabetologia. 1993;36:883–892. doi: 10.1007/BF02374468. [DOI] [PubMed] [Google Scholar]
- 6.Pugliatti M, Rosati G, Carton H, Riise T, Drulovic J, et al. The epidemiology of multiple sclerosis in Europe. Eur J Neurol. 2006;13:700–722. doi: 10.1111/j.1468-1331.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CWK, Marcus JH, Sidore C, et al. Genomic history of the Sardinian population. Nat Genet. 2018;50:1426–1434. doi: 10.1038/s41588-018-0215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AIRTUM . Associazione Italiana Registri Tumori. Italy: Milan; 2018. [Google Scholar]
- 9.Scazzone C, Bono A, Tornese F, Arsena R, Schillaci R, Butera D, Cottone S. Correlation between low folate levels and hyperhomocysteinemia, but not with vitamin B12 in hypertensive patients. Ann Clin Lab Sci. 2014;44:286–290. [PubMed] [Google Scholar]
- 10.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 11.Lievers KJ, Boers GH, Verhoef P, den Heijer M, Kluijtmans LA, van der Put NM, Trijbels FJ, Blom HJ. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl) 2001;79:522–528. doi: 10.1007/s001090100253. [DOI] [PubMed] [Google Scholar]
- 12.Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- 13.Duell EJ, Millikan RC, Pittman GS, et al. Polymorphisms in the DNA repair gene XRCC1 and breast cancer. Cancer Epidemiol Biomark Prev. 2001;10:217–222. [PubMed] [Google Scholar]
- 14.Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, Sugimura H, Nohmi T, Kasai H, Yokota J. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16:3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 15.Hosseini M, Houshmand M, Ebrahimi A. MTHFR polymorphisms and breast cancer risk. Arch Med Sci. 2011;7:134–137. doi: 10.5114/aoms.2011.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izmirli M. A literature review of MTHFR (C677T and A1298C polymorphisms) and cancer risk. Mol Biol Rep. 2013;40:625–637. doi: 10.1007/s11033-012-2101-2. [DOI] [PubMed] [Google Scholar]
- 17.Boccia S, Gianfagna F, Persiani R, La Greca A, Arzani D, Rausei S, D'ugo D, Magistrelli P, Villari P, Van Duijn CM, Ricciardi G. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and susceptibility to gastric adenocarcinoma in an Italian population. Biomarkers. 2007;12:635–644. doi: 10.1080/13547500701546766. [DOI] [PubMed] [Google Scholar]
- 18.Hu Z, Ma H, Chen F, Wei Q. Shen H.XRCC1 polymorphisms and cancer risk: a meta-analysis of 38 case-control studies. Cancer Epidemiol Biomark Prev. 2005;14:1810–1818. doi: 10.1158/1055-9965.EPI-04-0793. [DOI] [PubMed] [Google Scholar]
- 19.Vodicka P, Stetina R, Polakova V, Tulupova E, Naccarati A, Vodickova L, Kumar R, Hanova M, Pardini B, Slyskova J, Musak L, De Palma G, Soucek P, Hemminki K. Association of DNA repair polymorphisms with DNA repair functional outcomes in healthy human subjects. Carcinogenesis. 2007;28:657–664. doi: 10.1093/carcin/bgl187. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: a review of the functional and epidemiologic literature. Mol Carcinog. 2005;42:127–141. doi: 10.1002/mc.20067. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Ha TC, Tai BC. XRCC1 gene polymorphisms and breast cancer risk in different populations: a meta-analysis. Breast. 2009;18:183–191. doi: 10.1016/j.breast.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Hardi H, Melki R, Boughaleb Z, El Harroudi T, Aissaoui S, Boukhatem N. Significant association between ERCC2 and MTHR polymorphisms and breast cancer susceptibility in Moroccan population: genotype and haplotype analysis in a case-control study. BMC Cancer. 2018;18:292. doi: 10.1186/s12885-018-4214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castiglia P, Sanna V, Azara A, De Miglio MR, Murgia L, Pira G, Sanges F, Fancellu A, Carru C, Bisail M, Muroni MR. Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms in breast cancer: a Sardinian preliminary case-control study. Int J Med Sci. 2019;16:1089–1095. doi: 10.7150/ijms.32162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin a variant frequency. Cancer Res. 1999;59:2557–2561. [PubMed] [Google Scholar]
- 25.Cornetta T, Festa F, Testa A, Cozzi R. DNA damage repair and genetic polymorphisms: assessment of individual sensitivity and repair capacity. Int J Radiat Oncol Biol Phys. 2006;66:537–545. doi: 10.1016/j.ijrobp.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Sidore C, Busonero F, Maschio A, et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat Genet. 2015;47:1272–1281. doi: 10.1038/ng.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danjou F, Zoledziewska M, Sidore C, et al. Genome-wide association analyses based on whole-genome sequencing in Sardinia provide insights into regulation of hemoglobin levels. Nat Genet. 2015;47:1264–1271. doi: 10.1038/ng.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoledziewska M, Sidore C, Chiang C, et al. Height-reducing variants and selection for short stature in Sardinia. Nat Genet. 2015;47:1352–1356. doi: 10.1038/ng.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press) 2019;11:151–164. doi: 10.2147/BCTT.S176070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakur P, Seam RK, Gupta MK, Gupta M, Sharma M, Fotedar V. Breast cancer risk factor evaluation in a Western Himalayan state: a case-control study and comparison with the Western world. South Asian J Cancer. 2017;6:106–109. doi: 10.4103/sajc.sajc_157_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clavel-Chapelon F, Launoy G, Auquier A, et al. Reproductive factors and breast cancer risk. Effect of age at diagnosis. Ann Epidemiol. 1995;5:315–320. doi: 10.1016/1047-2797(95)00099-S. [DOI] [PubMed] [Google Scholar]
- 32.Freund C, Mirabel L, Annane K, Mathelin C. Allaitement maternel et cancer du sein [breastfeeding and breast cancer] Gynecol Obstet Fertil. 2005;33:739–744. doi: 10.1016/j.gyobfe.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Andò S, Gelsomino L, Panza S, Giordano C, Bonofiglio D, Barone I, Catalano S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers. 2019;11:62. [DOI] [PMC free article] [PubMed]
- 34.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–83. [DOI] [PubMed]
- 35.Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2017;26:355–365. doi: 10.1158/1055-9965.EPI-16-0602. [DOI] [PubMed] [Google Scholar]
- 36.Zimta AA, Tigu AB, Muntean M, Cenariu D, Slaby O, Berindan-Neagoe I. Molecular links between central obesity and breast Cancer. Int J Mol Sci. 2019;20:5364. [DOI] [PMC free article] [PubMed]
- 37.Hadrup S, Donia M, Thor SP. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6:123–133. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Y, Qian H, Liu Y, Duan L, Li Y, Shi G. J the roles of regulatory B cells in cancer. J Immunol Res. 2014;2014:215471. doi: 10.1155/2014/215471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juárez-Cruz JC, Zuñiga-Eulogio MD, Olea-Flores M, Castañeda-Saucedo E, Mendoza-Catalán MA, Ortuño-Pineda C, Moreno-Godinez ME, Villegas-Comonfort S, Padilla-Benavides T, Navarro-Tito N. Leptin induces cell migration and invasion in a FAK-Src- dependent manner in breast cancer cells. Endocr Connect. 2019. [DOI] [PMC free article] [PubMed]
- 40.Lagerros YT, Hsieh SF, Hsieh CC. Physical activity in adolescence and young adulthood and breast cancer risk: a quantitative review. Eur J Cancer Prev. 2004;13:5–12. doi: 10.1097/00008469-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6:213–218. doi: 10.1186/bcr921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair J, McCann M, Sheldon E, Gordon I, Brierley-Jones L, Copson E. The acceptability of addressing alcohol consumption as a modifiable risk factor for breast cancer: a mixed method study within breast screening services and symptomatic breast clinics. BMJ Open. 2019;9:e027371. [DOI] [PMC free article] [PubMed]
- 43.Tong JH, Li Z, Shi J, Li HM, Wang Y, Fu LY, Liu YP. Passive smoking exposure from partners as a risk factor for ER+/PR+ double positive breast cancer in never-smoking Chinese urban women: a hospital-based matched case control study. PLoS One. 2014;9:e97498. [DOI] [PMC free article] [PubMed]
- 44.Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 45.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 46.Bravatà V. Controversial roles of methylenetetrahydrofolate reductase polymorphisms and folate in breast cancer disease. Int J Food Sci Nutr. 2015;66:43–49. doi: 10.3109/09637486.2014.959896. [DOI] [PubMed] [Google Scholar]
- 47.Meneses-Sanchez P, Garcia-Hernandez SC, Porchia LM, Pérez-Fuentes R, Torres-Rasgado E, Del Angel SA, Gonzalez-Mejia ME. C677T and A1298C methylenetetrahydrofolate reductase polymorphisms and breast cancer susceptibility among Latinos: a meta-analysis. Breast Cancer. 2019;26:602–611. doi: 10.1007/s12282-019-00961-8. [DOI] [PubMed] [Google Scholar]
- 48.Mo W, Ding Y, Zheng Y, Zou D, Ding X. Associations between folate metabolism enzyme polymorphisms and breast cancer: A meta-analysis. Breast J. 2019. [DOI] [PubMed]
- 49.Huang CY, Chang WS, Shui HA, et al. Evaluation of the contribution of methylenetetrahydrofolate reductase genotypes to Taiwan breast cancer. Anticancer Res. 2014;34:4109–4115. [PubMed] [Google Scholar]
- 50.Ramos-Silva A, Figuera LE, Soto-Quintana OM, Puebla-Pérez AM, Ramírez-Patiño R, Gutiérrez-Hurtado I, Carrillo-Moreno DI, Zúñiga-González GM, Dávalos-Rodríguez IP, Gallegos-Arreola MP. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with breast cancer in a Mexican population. Genet Mol Res. 2015;14:4015–4026. doi: 10.4238/2015.April.27.16. [DOI] [PubMed] [Google Scholar]
- 51.Macis D, Maisonneuve P, Johansson H, Bonanni B, Botteri E, Iodice S, Santillo B, Penco S, Gucciardo G, D'Aiuto G, Rosselli Del Turco M, Amadori M, Costa A, Decensi A. Methylenetetrahydrofolate reductase (MTHFR) and breast cancer risk: a nested-case-control study and a pooled meta-analysis. Breast Cancer Res Treat. 2007;106:263–271. doi: 10.1007/s10549-006-9491-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhong S, Chen Z, Yu X, Li W, Tang J, Zhao J. A meta-analysis of genotypes and haplotypes of methylenetetrahydrofolate reductase gene polymorphisms in breast cancer. Mol Biol Rep. 2014;41:5775–5785. doi: 10.1007/s11033-014-3450-9. [DOI] [PubMed] [Google Scholar]
- 53.Zhang XF, Liu T, Li Y, Li S. Association between MTHFR 677C/T and 1298A/C gene polymorphisms and breast cancer risk. Genet Mol Res. 2015;14:16425–16430. doi: 10.4238/2015.December.9.12. [DOI] [PubMed] [Google Scholar]
- 54.Hekim N, Ergen A, Yaylim I, Yilmaz H, Zeybek U, Oztürk O, Isbir T. No association between methylenetetrahydrofolate reductase C677T polymorphism and breast cancer. Cell Biochem Funct. 2007;25:115–117. doi: 10.1002/cbf.1274. [DOI] [PubMed] [Google Scholar]
- 55.Chou YC, Wu MH, Yu JC, Lee MS, Yang T, Shih HL, Wu TY, Sun CA. Genetic polymorphisms of the methylenetetrahydrofolate reductase gene, plasma folate levels and breast cancer susceptibility: a case-control study in Taiwan. Carcinogenesis. 2006;27:2295–2300. doi: 10.1093/carcin/bgl108. [DOI] [PubMed] [Google Scholar]
- 56.Batschauer AP, Cruz NG, Oliveira VC, Coelho FF, Santos IR, Alves MT, Fernandes AP, Carvalho MG, Gomes KB. HFE, MTHFR, and FGFR4 genes polymorphisms and breast cancer in Brazilian women. Mol Cell Biochem. 2011;357:247–253. doi: 10.1007/s11010-011-0895-1. [DOI] [PubMed] [Google Scholar]
- 57.Justenhoven C, Hamann U, Pierl CB, Rabstein S, Pesch B, Harth V, Baisch C, Vollmert C, Illig T, Brüning T, Ko Y, Brauch H. One-carbon metabolism and breast cancer risk: no association of MTHFR, MTR, and TYMS polymorphisms in the GENICA study from Germany. Cancer Epidemiol Biomark Prev. 2005;14:3015–3018. doi: 10.1158/1055-9965.EPI-05-0592. [DOI] [PubMed] [Google Scholar]
- 58.Pooja S, Carlus J, Sekhar D, Francis A, Gupta N, Konwar R, Kumar S, Kumar S, Thangaraj K, Rajender S. MTHFR 677C>T polymorphism and the risk of breast cancer: evidence from an original study and pooled data for 28031 cases and 31880 controls. PLoS One. 2015;10:e0120654. [DOI] [PMC free article] [PubMed]
- 59.Sellers TA, Vierkant RA, Cerhan JR, et al. Interaction of dietary folate intake, alcohol, and risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Cancer Epidemiol Biomark Prev. 2002;11(10 Pt 1):1104–1107. [PubMed] [Google Scholar]
- 60.Beilby J, Ingram D, Hähnel R, Rossi E. Reduced breast cancer risk with increasing serum folate in a case-control study of the C677T genotype of the methylenetetrahydrofolate reductase gene. Eur J Cancer. 2004;40:1250–1254. doi: 10.1016/j.ejca.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Rohan TE, Jain MG, Howe GR, Miller AB. Dietary folate consumption and breast cancer risk. J Natl Cancer Inst. 2000;92:266–269. doi: 10.1093/jnci/92.3.266. [DOI] [PubMed] [Google Scholar]
- 62.Zhang SM, Willett WC, Selhub J, Hunter DJ, Giovannucci EL, Holmes MD, Colditz GA, Hankinson SE. Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst. 2003;95:373–380. doi: 10.1093/jnci/95.5.373. [DOI] [PubMed] [Google Scholar]
- 63.Pezzuto A, Citarella F, Croghan I, Tonini G. The effects of cigarette smoking extracts on cell cycle and tumor spread: novel evidence. Future Sci OA. 2019;5:FSO394. doi: 10.2144/fsoa-2019-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pöschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 65.Duell EJ, Wiencke JK, Cheng TJ, Varkonyi A, Zuo ZF, Ashok TD, Mark EJ, Wain JC, Christiani DC, Kelsey KT. Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis. 2000;21:965–971. doi: 10.1093/carcin/21.5.965. [DOI] [PubMed] [Google Scholar]
- 66.Takeshita H, Fujihara J, Yasuda T, Kimura-Kataoka K. Worldwide distribution of four SNPs in X-ray and Repair and cross-complementing group 1 (XRCC1) Clin Transl Sci. 2015;8:347–350. doi: 10.1111/cts.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tuimala J, Szekely G, Wikman H, Järventaus H, Hirvonen A, Gundy S, Norppa H. Genetic polymorphisms of DNA repair and xenobiotic-metabolizing enzymes: effects on levels of sister chromatid exchanges and chromosomal aberrations. Mutat Res. 2004;554:319–333. doi: 10.1016/j.mrfmmm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Guo S, Mao X, Ming L. XRCC1 Arg399Gln polymorphism is not associated with breast cancer in Chinese. Int J Clin Exp Med. 2015;8:10429–10436. [PMC free article] [PubMed] [Google Scholar]
- 69.Breast Cancer Association C Commonly studied single-nucleotide polymorphisms and breast cancer: results from the breast Cancer association consortium. J Natl Cancer Inst. 2006;98:1382–1396. doi: 10.1093/jnci/djj374. [DOI] [PubMed] [Google Scholar]
- 70.Qian Y, Zhang JP, Dong J, Wang FR, Lin YD, Xu M, Wu LL, Shi P, Shen HB. Relationship between polymorphisms of X-ray repair cross-complementing group 1 gene Arg194Trp, Arg399Gln and susceptibility of breast cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:242–246. [PubMed] [Google Scholar]
- 71.Shadrina AS, Ermolenko NA, Boyarskikh UA, Sinkina TV, Lazarev AF, Petrova VD, Filipenko ML. Polymorphisms in DNA repair genes and breast cancer risk in Russian population: a case-control study. Clin Exp Med. 2016;16:21–28. doi: 10.1007/s10238-014-0329-y. [DOI] [PubMed] [Google Scholar]
- 72.Sterpone S, Mastellone V, Padua L, Novelli F, Patrono C, Cornetta T, Giammarino D, Donato V, Testa A, Cozzi R. Single-nucleotide polymorphisms in BER and HRR genes, XRCC1 haplotypes and breast cancer risk in Caucasian women. J Cancer Res Clin Oncol. 2010;136:631–636. doi: 10.1007/s00432-010-0791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moullan N, Cox DG, Angèle S, Romestaing P, Gérard JP, Hall J. Polymorphisms in the DNA repair gene XRCC1, breast cancer risk, and response to radiotherapy. Cancer Epidemiol Biomark Prev. 2003;12(11 Pt 1):1168–1174. [PubMed] [Google Scholar]
- 74.Sanjari Moghaddam A, Nazarzadeh M, Noroozi R, Darvish H, Mosavi JA. XRCC1 and OGG1 gene polymorphisms and breast Cancer: a systematic review of literature. Iran J Cancer Prev. 2016;9:e3467. doi: 10.17795/ijcp-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smolarz B, Bryś M, Forma E, Zadrożny M, Bieńkiewicz J, Romanowicz H. Data on single nucleotide polymorphism of DNA repair genes and breast Cancer risk from Poland. Pathol Oncol Res. 2019;25:1311–1317. doi: 10.1007/s12253-017-0370-8. [DOI] [PubMed] [Google Scholar]
- 76.Özgöz A, Hekimler Öztürk K, Yükseltürk A, Şamlı H, Başkan Z, Mutlu İçduygu F, Bacaksız M. Genetic variations of DNA repair genes in breast Cancer. Pathol Oncol Res. 2019;25:107–114. doi: 10.1007/s12253-017-0322-3. [DOI] [PubMed] [Google Scholar]
- 77.Smolarz B, Michalska MM, Samulak D, Romanowicz H, Wójcik L. Polymorphism of DNA repair genes inbreast cancer. Oncotarget. 2019;10:527–535. doi: 10.18632/oncotarget.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan W, Xu L, Feng Y, Yang Y, Chen W, Wang J, Pang D, Li D. The hOGG1 Ser326Cys polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;122:835–842. doi: 10.1007/s10549-009-0722-5. [DOI] [PubMed] [Google Scholar]
- 79.Sangrajrang S, Schmezer P, Burkholder I, Waas P, Boffetta P, Brennan P, Bartsch H, Wiangnon S, Popanda O. Polymorphisms in three base excision repair genes and breast cancer risk in Thai women. Breast Cancer Res Treat. 2008;111:279–288. doi: 10.1007/s10549-007-9773-7. [DOI] [PubMed] [Google Scholar]
- 80.Kim JI, Park YJ, Kim KH, Kim JI, Song BJ, Lee MS, Kim CN, Chang SH. hOGG1 Ser326Cys polymorphism modifies the significance of the environmental risk factor for colon cancer. World J Gastroenterol. 2003;9:956–960. doi: 10.3748/wjg.v9.i5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xing DY, Tan W, Song N, Lin DX. Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer in a Chinese population. Int J Cancer. 2001;95:140–143. doi: 10.1002/1097-0215(20010520)95:3<140::AID-IJC1024>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 82.Wikman H, Risch A, Klimek F, Schmezer P, Spiegelhalder B, Dienemann H, Kayser K, Schulz V, Drings P, Bartsch H. hOGG1 polymorphism and loss of heterozygosity (LOH): significance for lung cancer susceptibility in a caucasian population. Int J Cancer. 2000;88:932–937. doi: 10.1002/1097-0215(20001215)88:6<932::AID-IJC15>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 83.Ding DP, Zhang Y, He XF. Lack of association between hOGG1 Ser326Cys polymorphism and breast cancer susceptibility in European population. Breast Cancer Res Treat. 2011;129:1023–1026. doi: 10.1007/s10549-011-1571-6. [DOI] [PubMed] [Google Scholar]
- 84.Synowiec E, Stefanska J, Morawiec Z, Blasiak J, Wozniak K. Association between DNA damage, DNA repair genes variability and clinical characteristics in breast cancer patients. Mutat Res. 2008;648:65–72. doi: 10.1016/j.mrfmmm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Newcomb PA, Egan KM, Titus-Ernstoff L, Chanock S, Welch R, Brinton LA, Lissowska J, Bardin-Mikolajczak A, Peplonska B, Szeszenia-Dabrowska N, Zatonski W, Garcia-Closas M. Genetic polymorphisms in base-excision repair pathway genes and risk of breast cancer. Cancer Epidemiol Biomark Prev. 2006;15:353–358. doi: 10.1158/1055-9965.EPI-05-0653. [DOI] [PubMed] [Google Scholar]
- 86.Gu D, Wang M, Zhang Z, Chen J. Lack of association between the hOGG1 Ser326Cys polymorphism and breast cancer risk: evidence from 11 case-control studies. Breast Cancer Res Treat. 2010;122:527–531. doi: 10.1007/s10549-009-0723-4. [DOI] [PubMed] [Google Scholar]
- 87.Gamazon ER, Segrè AV, van de Bunt M, Wen X, Xi HS, et al. Using an atlas of gene regulation across 44 human tissues to inform complex disease- and trait-associated variation. Nat Genet. 2018;50:956–967. doi: 10.1038/s41588-018-0154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hassan FM. OGG1 rs1052133 polymorphism and genetic susceptibility to chronic Myelogenous Leukaemia. Asian Pac J Cancer Prev. 2019;20:925–928. doi: 10.31557/APJCP.2019.20.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitra AK, Singh SV, Garg VK, Sharma M, Chaturvedi R, Rath SK. Protective association exhibited by the single nucleotide polymorphism (SNP) rs1052133 in the gene human 8-oxoguanine DNA glycosylase (hOGG1) with the risk of squamous cell carcinomas of the head & neck (SCCHN) among north Indians. Indian J Med Res. 2011;133:605–612. [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Y, Wu Y, Zhou X, Yao M, Ning S, Wei Z. Association of polymorphisms hOGGI rs1052133 and hMUTYH rs3219472 with risk of nasopharyngeal carcinoma in a Chinese population. Onco Targets Ther. 2016;9:755–760. doi: 10.2147/OTT.S95944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ali K, Mahjabeen I, Sabir M, Mehmood H, Kayani MA. OGG1 mutations and risk of female breast Cancer: meta-analysis and experimental data. Dis Markers. 2015;2015:690878. doi: 10.1155/2015/690878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S5 A. Association of MTHFR, XRCC1, OGG1 polymorphisms and ER, PgR, Her-2, Ki67 and Lymph Node status in BC patients. B. Association of MTHFR, XRCC1, OGG1 polymorphisms and Age at diagnosis, BMI, Menopause, BC family history status in BC patients.
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.