Abstract
Erysipelas, caused by the bacterium Erysipelothrix rhusiopathiae, is re-emerging in swine and poultry production systems worldwide. While the global genomic diversity of this species has been characterized, how much of this genomic and functional diversity is maintained at smaller scales is unclear. Specifically, while several key immunogenic surface proteins have been identified for E. rhusiopathiae, little is known about their presence among field strains and their divergence from vaccines, which could result in vaccine failure. Here, a comparative genomics approach was taken to determine the diversity of E. rhusiopathiae strains in pigs in Great Britain over nearly three decades, as well as to assess the field strains’ divergence from the vaccine strain most commonly used in British pigs. In addition, the presence/absence and variability of 13 previously described immunogenic surface proteins was determined, including SpaA which is considered a key immunogen. We found a high diversity of E. rhusiopathiae strains in British pigs, similar to the situation described in European poultry but in contrast to swine production systems in Asia. Of the four clades of E. rhusiopathiae found globally, three were represented among British pig isolates, with Clade 2 being the most common. All British pig isolates had one amino acid difference in the immunoprotective domain of the SpaA protein compared to the vaccine strain. However, we were able to confirm using in silico structural protein analyses that this difference is unlikely to compromise vaccine protection. Of 12 other known immunogenic surface proteins of E. rhusiopathiae examined, 11 were found to be present in all British pig isolates and the vaccine strain, but with highly variable degrees of conservation at the amino acid sequence level, ranging from 0.3 to 27% variant positions. Moreover, the phylogenetic incongruence of these proteins suggests that horizontal transfer of genes encoding for antigens is commonplace for this bacterium. We hypothesize that the sequence variants in these proteins could be responsible for differences in the efficacy of the immune response. Our results provide the necessary basis for testing this hypothesis through in vitro and in vivo studies.
Keywords: antigen, Erysipelothrix rhusiopathiae, genomics, Great Britain, pigs (Sus domesticus), vaccine, whole genome sequencing
Introduction
Erysipelothrix rhusiopathiae, the causative agent of erysipelas, remains a persistent challenge for swine and poultry production systems worldwide. Although well-controlled for decades through vaccination, erysipelas is re-emerging in several European and Asian countries (Eriksson et al., 2013; Kwok et al., 2014; Janßen et al., 2015; Ogawa et al., 2017). E. rhusiopathiae was also recently implicated in large-scale mortalities and population declines in muskoxen and other wild ungulates in North America (Kutz et al., 2015; Forde T.L. et al., 2016). While Erysipelothrix spp. infection can impact all pig production stages and is a common and significant cause of carcass condemnation, its true economic burden is largely unknown. The E. rhusiopathiae genome is comprised of a single chromosome of about 1.8 million base pairs (MB), with an average GC content of 36.5% (Ogawa et al., 2011; Kwok et al., 2014; Tang et al., 2016). Comparative genomic analysis of a diverse collection of E. rhusiopathiae isolates from a wide range of host species recently led to a better understanding of the global diversity and population structure of this species (Forde T. et al., 2016), including its propensity for homologous recombination. Strains can be broadly divided into Clades 1, 2, and 3, as well as a clade phylogenetically “intermediate” to Clades 2 and 3. The genomic diversity of E. rhusiopathiae at smaller (e.g. national) scales has only begun to be explored. However, studies conducted to date illustrate that this may range from highly related clonal strains (e.g. as seen in pigs in Japan; Ogawa et al., 2017), to phylogenetically diverse strains, as observed in poultry in Germany (Janßen et al., 2015). The degree of genomic diversity present could affect the ability of vaccines to provide protection against the full spectrum of circulating field strains.
An important component of E. rhusiopathiae diversity that has remained relatively unstudied is that of its surface proteins. Because of their high propensity for host-pathogen interactions, surface proteins often play an important role in virulence mechanisms, as well as in eliciting a host immune response, thereby representing potential vaccine candidates (Gamberini et al., 2005). One of the most critical immunogens for E. rhusiopathiae identified to date is the surface protective antigen (Spa)A protein (Makino et al., 1998; Imada et al., 1999; Shimoji et al., 1999). Of the three different Spa types (A, B, and C) that have been found in Erysipelothrix spp. (To and Nagai, 2007), SpaA is by far the most widely prevalent Spa type in E. rhusiopathiae. In a global collection of E. rhusiopathiae isolates examined from various host species, the spaA gene was found in more than 90% of isolates (79/86), including all those from pigs and poultry (Forde T. et al., 2016). This corroborates several international studies where the spaA gene was the exclusive Spa type found, including from pig outbreaks in Japan (n = 83) (To et al., 2012), isolates from pigs in Australia (n = 44) (Eamens et al., 2006), and a large study of 165 predominantly poultry isolates from Germany (Janßen et al., 2015). It has been shown that the N-terminal immunoprotective domain of this surface protein – also referred to as the hypervariable domain – is the component responsible for eliciting protective immunity (Figure 1; Imada et al., 1999; Shimoji et al., 1999). SpaA also plays an important role in pathogenesis by increasing resistance to phagocytosis and promoting endothelial adherence (Harada et al., 2014; Borrathybay et al., 2015; Zhu et al., 2017a, b). Several variants (groups) of the SpaA protein have already been described, based on amino acid differences within the immunoprotective domain (Uchiyama et al., 2014; Janßen et al., 2015; Ogawa et al., 2017). While tests have been conducted on both mice and pigs to assess cross-protection among different Spa types using strains representing multiple serotypes (To et al., 2010), it remains unclear whether amino acid variants within the SpaA protein can result in differences in protection. Moreover, the genetic sequence of spaA of E. rhusiopathiae strains used in vaccines has not yet been characterized.
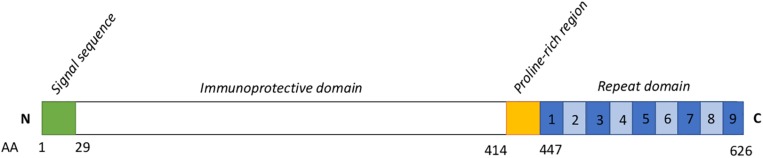
FIGURE 1.
Schematic representation of the SpaA protein sequence. Based on To and Nagai, 2007. Numbers below indicate amino acid (AA) positions. Numbers within the blue boxes represent the typical number of repeat units. The signal sequence (AA 1-29) is thought to be associated with the secretion mechanism for this protein. The immunoprotective domain (AA 30-413) is the component conferring immunogenicity, and upon which typing schemes for SpaA have been developed. The role of the proline-rich region (415–447) has not been well described. Seven to 13 repeats of a 20-AA sequence containing a GW module make up the C-terminal domain of SpaA; these have a role in binding the SpaA protein to the bacterial cell surface.
Additional surface proteins that have a demonstrated role in immunogenicity of E. rhusiopathiae include rhusiopathiae surface protein A (RspA) (Shimoji et al., 2003), choline binding protein B (cbpB) (Shi et al., 2013; Zhu et al., 2018) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Zhu et al., 2018); these proteins have all elicited protection during challenge studies in mice, and the latter two also in pigs. An additional eight putative surface proteins were recently found to give rise to varying degrees of protective immunity in mice (Hu et al., 2017; Table 1). As with SpaA variants, the exact role and the extant diversity of these different surface proteins among E. rhusiopathiae strains have yet to be determined.
TABLE 1.
Surface proteins of Erysipelothrix rhusiopathiae with a suspected role in immunogenicity.
| Locus tag | Nucleotide | Amino acid | |||||
| Gene | Name/Description | (former) | Positionsa | length | length | References | |
| 1 | spaAb | Choline-binding protein | ERH_RS00500 (ERH_0094) | 112,931–114,811 | 1881 | 626 | Imada et al., 1999; Shimoji et al., 1999 |
| 2 | rspA | Rhusiopathiae surface protein A; B-type domain-containing protein; LPXTG-motif cell wall anchor | ERH_RS03410 (ERH_0668) | 701,546–707,524 | 5979 | 1992 | Shimoji et al., 2003 |
| 3 | CbpB | Choline-binding protein | ERH_RS03905 (ERH_0768) | 813,020– 814,840 | 1821 | 606 | Shi et al., 2013; Zhu et al., 2018 |
| 4 | GAPDH (gapA) | type I glyceraldehyde-3-phosphate dehydrogenase | ERH_RS07885 (ERH_1534) | 1,618,401–1,619,405 (rev) | 1005 | 334 | Shi et al., 2013; Zhu et al., 2018 |
| 5 | Da (dap2) | Dipeptidyl aminopeptidase; S9 family peptidase | ERH_RS00295 (ERH_0059) | 62355–64328 (rev) | 1974 | 657 | Hu et al., 2017 |
| 6 | CbpA | Collagen-binding protein; cell wall anchor | ERH_RS00405 (ERH_0075) | 89263–94584 | 5322 | 1773 | Hu et al., 2017 |
| 7 | Plp | Pectin lyase fold-containing protein | ERH_RS00860 (ERH_0165) | 196,260–200,819 | 4560 | 1519 | Hu et al., 2017 |
| 8 | Atsp | ABC transporter, extracellular solute-binding protein | ERH_RS01215 (ERH_0228) | 262,816–264,261 | 1446 | 481 | Hu et al., 2017 |
| 9 | Neu (nanH) | Neuraminidase; LPXTG-motif cell wall anchor domain protein | ERH_RS01540 (ERH_0299) | 331,513–335,127 (rev) | 3615 | 1204 | Hu et al., 2017 |
| 10 | Bga | Beta-galactosidase; DUF4982 domain-containing protein | ERH_RS03965 (ERH_0780) | 825,779–832,054 | 6276 | 2091 | Hu et al., 2017 |
| 11 | Bml | Basic membrane lipoprotein; BMP family ABC transporter substrate-binding protein | ERH_RS05080 (ERH_1005) | 1,061,289–1,062,380 (rev) | 1092 | 363 | Hu et al., 2017 |
| 12 | CwpA | LPXTG-motif cell wall anchor domain protein | ERH_RS07320 (ERH_1428) | 1,500,578–1,506,982 (rev) | 6405 | 2134 | Hu et al., 2017 |
| 13 | CwpB | LPXTG-motif cell wall anchor domain protein | ERH_RS07450 (ERH_1454) | 1,537,616–1,540,657 (rev) | 3042 | 1013 | Hu et al., 2017 |
All listed proteins have experimentally been shown to be involved in eliciting a host immune response. aPositions are in relationship with reference strain Fujisawa (NC_015601). bspaB and spaC sequences, with a role in immunogenicity of Erysipelothrix spp., were not found in any of the isolates in this study.
The objectives of this study were to (1) characterize the overall genomic diversity of E. rhusiopathiae in British pigs and assess the amount of genetic divergence between field strains and a commonly used vaccine strain in the United Kingdom (UK), and (2) to quantify the amount of genetic diversity in Spa and other immunogenic surface proteins.
Materials and Methods
Bacterial Isolates and Vaccine Strain
Archived E. rhusiopathiae isolates from clinical pig erysipelas cases from across England and Wales were provided by the UK Animal and Plant Health Agency (APHA; Supplementary Table S1). A total of 48 isolates were initially selected to uniformly represent as a wide a time frame as possible; dates of isolation ranged from 1987 to 2014. Isolates were also selected for geographic representation, including isolates submitted to 10 different regional Veterinary Investigation Centers (Supplementary File S1, Supplementary Figure S1). To minimize the inclusion of epidemiologically linked isolates (i.e. stemming from a common outbreak), efforts were made to select isolates from different years when originating from the same investigation center. Isolates were serotyped prior to this study using previously described methods (Opriessnig et al., 2013); those selected for inclusion in this study aimed to represent a mix of serotypes 1a, 1b and 2, which encompass 90% of the isolates recovered from clinical erysipelas in British pigs (McNeil et al., 2017). DNA was extracted from isolates regrown on blood agar plates using the DNeasy Blood & Tissue kit (Qiagen). With the same kit, DNA was also extracted directly from one of the most commonly used inactivated E. rhusiopathiae vaccines in the UK (referred to hereafter as the vaccine strain; see Commercial Products Used). We also endeavored to perform DNA extraction from a second commercial vaccine, however, this vaccine did not test positive by E. rhusiopathiae probe-based qPCR, and DNA of sufficient quantity (as measured by Qubit) could not be extracted despite multiple attempts.
Library Preparation, Sequencing and Assembly
Library preparation, sequencing and de novo assembly were performed by MicrobesNG (Birmingham, UK). Libraries were prepared using the Nextera XT v2 kit and sequenced on the Illumina HiSeq2500 platform, generating 250 base pair paired-end reads. Reads were trimmed using Trimmomatic (Bolger et al., 2014), and assembled de novo using SPAdes v. 3.7.0 (Bankevich et al., 2012) with default settings. Assembly quality metrics (i.e. number of contigs, N50) were obtained using QUAST (Gurevich et al., 2013). Three of the isolates submitted had poor sequencing quality indicative of contamination (i.e. unexpected total length and GC content) and were excluded from further analyses, resulting in 45 whole genome E. rhusiopathiae sequences (WGS) from British pigs. Since key questions in this study were related to surface antigens and their relationship with a current vaccine strain, the vaccine extract was submitted for sequencing twice on two independent runs for variant confirmation.
Population Structure
To place the newly sequenced isolates (i.e. the vaccine strain and the 45 British pig isolates from which high quality sequence data were obtained) within the broader global population structure of E. rhusiopathiae, a phylogenetic tree was estimated that included an additional 75 isolates from Clades 2, 3 and the intermediate clade (Forde T. et al., 2016) (Supplementary Table S1). The Fujisawa reference genome (NC_015601.1), the first complete high-quality genome for E. rhusiopathiae (Ogawa et al., 2011), was also included; this is a virulent serotype 1a strain isolated from a pig in Japan prior to 1985. Serotype was already known for 10/75 of the global isolates. In silico serotype testing was also done to detect isolates of serotypes 1a, 1b, 2, and 5. In brief, BLAST searches of all de novo assemblies were done using primer pairs described for each serotype (Shiraiwa et al., 2018). A BLAST search was considered positive for a given serotype if there were matches for both forward and reverse primers across their full length with a maximum of one SNP difference, and that yielded the expected amplicon length (i.e. distance between primers). The phylogeny – based on core single nucleotide polymorphisms (SNPs) – was built using Nullarbor1 (Seemann et al.) implemented through the CLIMB computing platform for microbial genomics (Connor et al., 2016). The Nullarbor pipeline uses the program Freebayes v1.1.0 for variant calling across all isolates with respect to a reference genome. It subsequently builds a core SNP alignment using Snippy v3.2, where “core sites” are genomic positions that are present in all the included isolates. This core alignment comprised 3490 SNPs. FastTree v2.1.10 was then called within Nullarbor to infer the phylogeny using maximum-likelihood based on the GTR + G4 model of substitution.
Variability of Spa and Other Surface Protein Sequences
Spa genes were searched for in all newly sequenced isolates by performing BLASTn searches using a custom database of spaA, spaB, and spaC nucleotide sequences from different serotypes available on GenBank (Supplementary Tables S1, S2), implemented within Geneious v. 11.0.5 (Kearse et al., 2012). A BLAST hit was considered positive for a particular Spa type if it had greater than 95% pairwise identity; in practice, homologous Spa types had ∼98% pairwise identity, while heterologous types generally had ∼90% identity or lower. Nucleotide sequences of the spaA gene based on the BLAST hits were extracted from all de novo assemblies (n = 121). spaA sequences were similarly extracted from seven whole genome sequences of E. rhusiopathiae available on GenBank: Fujisawa, SY1027 (NC_021354.1), GXBY-1 (NZ_CP014861.1), WH13013 (NZ_CP017116.1), NCTC7999 (NZ_UFYF01000001.1), NCTC8163 (NZ_LR134439.1), and ML101 (NZ_CP029804.1). Finally, to further explore the variability of this surface protein, an additional 215 publicly available spaA nucleotide sequences were downloaded (Supplementary Table S2), resulting in a total of 343 spaA sequences. Translations were performed either using the transeq program from EMBOSS (Rice et al., 2000), or within Geneious, using translation Table 11 for bacteria. Amino acid sequences were aligned using MUSCLE (Edgar, 2004), implemented within Geneious, and any amino acid variants were recorded in comparison with the Fujisawa reference sequence. A phylogenetic tree was estimated from the protein alignment using a Neighbor-Joining method implemented in Geneious Tree Builder, using the Jukes-Cantor model.
The amino acid variability within 12 additional surface proteins that have been shown to play a role in E. rhusiopathiae immunogenicity was also examined (Table 1). A custom BLAST database of the nucleotide sequences of these genes was created based on the sequences from the Fujisawa reference genome within the program Geneious. Each de novo assembly from British pig isolates and the vaccine strain was queried to identify and extract the homologous gene sequences, which were then translated to amino acid sequences and aligned within Geneious along with the Fujisawa reference sequence. Phylogenies for each protein were estimated as described for SpaA. Variants of these different surface proteins were identified based on clustering observed in mid-point rooted phylogenies (Supplementary File S2).
Conformation of SpaA Protein Variants
To assess the potential structural impact of SpaA protein variants identified, the 3-D protein structure of a typical group 1 SpaA (Figure 4) was initially modeled using the I-TASSER platform (Roy et al., 2010). I-TASSER uses LOMETS, a meta-threading server that combines several threading programs to detect structural templates from the Protein Data Bank (PDB) using threading or fold recognition. The threading-aligned regions of these templates provide the building blocks and spatial restraints for the prediction of the target protein, with unaligned areas predicted ab initio. Full-length structural models were predicted by I-TASSER, and the best structural model – identified through comparison of C-scores – was used for further analysis. C-scores are typically bounded between −5 and 2 with higher values indicating greater confidence in the predicted model and −1.5 acting as a useful threshold above which more than 90% of predictions are correct (Roy et al., 2010). To assess the structural similarity of the SpaA structural model to existing PDB structures, template modeling score (TM-score) and root-mean-square deviation (RMSD) were calculated across aligned Cα atoms using TM-align v20170708 (Zhang and Skolnick, 2005). TM-scores are bounded between 0 and 1 where 1 indicates perfect structural alignment, scores above 0.5 assume roughly the same fold while scores below 0.2 correspond to randomly chosen unrelated proteins (Zhang and Skolnick, 2005). Mutations were introduced using the PyMOL molecular graphics system (Schrödinger2), where the most common rotamer was selected and energy minimization was performed in the locality of the mutated site.
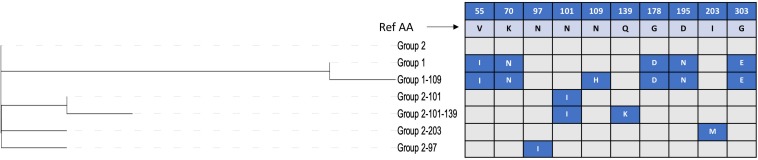
FIGURE 4.
Discriminatory amino acid positions within the immunoprotective domain of the SpaA protein of Erysipelothrix rhusiopathiae. Amino acid (AA) variant positions with respect to the Fujisawa reference sequence are listed in the top row, with the amino acid found in the reference sequence shown in the second row. The phylogenetic tree shown to the left shows the relatedness among the SpaA sequence variants. Variants in each group are shown in blue boxes, with the AA change indicated.
Epitope scores were predicted per-residue based on amino acid identity, surface exposure and side chain orientation using BEpro (Sweredoski and Baldi, 2008). Amino acid diversity was calculated using an alignment of all available E. rhusiopathiae SpaA protein sequences (n = 343) (including residues 1–447 of the N-terminal region). Diversity at each amino acid position was calculated as the true diversity at q = 2 (Inverse Simpson index), whereby 1 indicates a fully conserved position and numbers greater than 1 indicate increasing diversity. Amino acid diversity was visualized on the structural model using PyMOL.
Results
Sequencing, Population Structure and Serotype
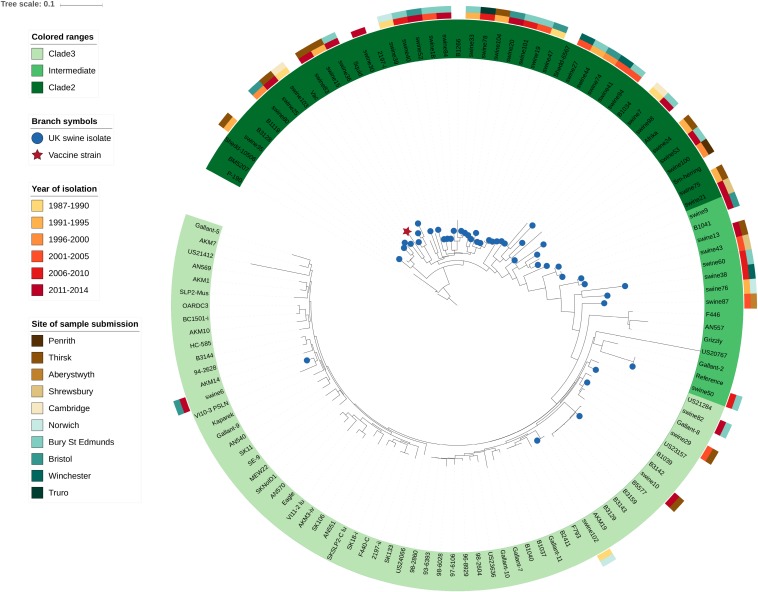
Among the 45 newly sequenced E. rhusiopathiae isolates from British pigs and the vaccine strain, a minimum mean sequencing depth of 26X was achieved, with a mean of 82X. The majority of the newly sequenced strains from British pigs fell within Clade 2 (32/45 = 71.1%), as did the vaccine strain (Figure 2). Five isolates (11.1%) were in Clade 3, while the remaining 17.8% (8/45) were within the intermediate clade. None of the isolates belonged to Clade 1. The average number of core SNP differences separating the pig isolates from one-another was 435 (median = 377, range 0–767). This was comparable to the number of core SNPs separating the vaccine strain from the field strains (median = 413, range 328–655). The core SNP profile of the vaccine strain was 100% identical across the two sequencing rounds. No clear geographic clustering was evident based on submitting veterinary center. Similarly, no obvious temporal clustering was apparent, indicating that rather than strain turnover, multiple divergent strains have remained in circulation throughout the study period (Figure 2). The genetic distance (based on core SNPs) of the British pig isolates from the vaccine strain did not change appreciably over time, thus providing no evidence for potential vaccine-induced selection (Supplementary File S1, Supplementary Figure S2). The phenotypically determined serotypes for the British pig isolates and vaccine were confirmed in silico in 40 of 46 isolates, with six discrepancies (Supplementary Table S1). However, upon re-testing, the phenotypic serotype of these isolates matched that predicted by in silico testing. Among the global collection, the Fujisawa reference strain was confirmed as serotype 1a in silico, while 19 isolates were designated as serotype 1b, 27 as serotype 2, 17 as serotype 5, and 12 had no BLAST hits for any of the primer pairs, suggestive of belonging to a serotype other than 1a, 1b, 2 or 5. This corroborated with the previously determined serotype for eight isolates, including one serotype 9 for which none of these primer pairs matched. Two mismatches between phenotypic and in silico serotype were found: 1. Isolate HC-585, previously classified as serotype 1a, did not have any primer pair matches; 2. Isolate Mew22, a serotype “N” (i.e. does not react with any of the panel of antisera), was classified as a serotype 1b. The reverse primer sequence for serotype 5 was found to consistently have one SNP difference with all serotype 5 isolates. The distribution of different serotypes within the phylogeny is shown in Figure 3, where there was limited correlation with Clade.
FIGURE 2.
Diversity of Erysipelothrix rhusiopathiae in British pigs as shown within a global phylogenetic context. This maximum likelihood phylogeny is based on core single nucleotide polymorphisms (SNPs) identified through the Nullarbor pipeline. British pig isolates (n = 45) are represented by blue circles at branch tips, while the vaccine strain is represented by a red star. Branches with no symbol are isolates from a broad global collection (n = 75) from various host species and geographic locations representing Clades 2, 3 and the intermediate clade. An isolate from Clade 1 was used to determine the appropriate rooting position (not shown). The Fujisawa strain was used as the reference genome. Spatio-temporal diversity of the British pig isolates is illustrated by color bars to the outside of the phylogeny, with the inner color representing the year range of isolation, and the outer color bar representing the veterinary center to which the isolate was submitted.
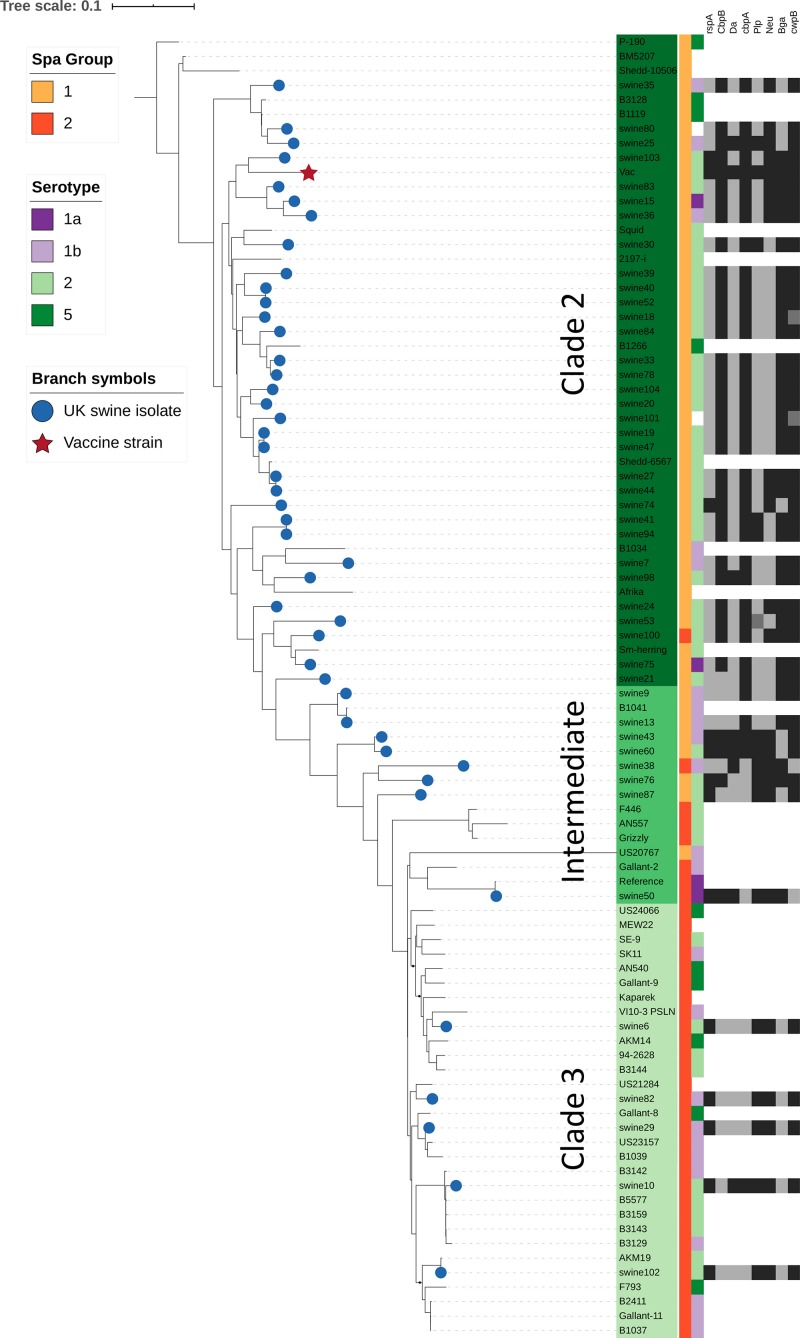
FIGURE 3.
Relationship of SpaA group, serotype, and immunogenic surface proteins to phylogenetic population structure. This phylogenetic tree is based on core single nucleotide polymorphisms (SNPs), and was estimated using the Nullarbor pipeline (same sequence data as in Figure 2). British pig isolates are represented by blue circles at branch tips, while the vaccine strain is represented by a red star. SpaA group (1 or 2) is shown by the yellow/orange color strip. Serotype (1a, 1b, 2, 5) as determined in silico is shown by the purple/green color strip. Those where phenotypic testing was not performed or where in silico test results differed are not shown. Variants of 8 different immunogenic surface proteins are shown by the black/gray color strips for all British pig isolates. Those shown in black are those which cluster most closely with the variant found in the vaccine strain; isolates in dark gray represent isolates with surface protein variants in outlier groups (Supplementary Table S1, Supplementary File S1).
Occurrence and Variability of Spa Proteins
The spaA gene was present in all the newly sequenced isolates, while spaB and spaC genes were not detected. In the translated SpaA protein sequences, substantial diversity was found within the immunoprotective or “hypervariable” domain, which spans amino acids 30–413 (Figure 1). All SpaA amino acid sequences (n = 343) were initially classified into the five SpaA groups previously described (Janßen et al., 2015) (Supplementary Table S3), which are based upon amino acid variants in this domain. Beyond the previously reported positions, three additional variable amino acid sites were present in at least five isolates, and thus considered discriminatory. These were variants at position 97 (N/I), position 109 (N/H), and position 139 (Q/K). There were also several amino acid variants (n = 53) present in fewer than five isolates (Supplementary Table S4). One isolate (20767, from a wild bird) had a highly divergent SpaA protein, with 19 unique amino acids. If these sites are not considered, there were 34 variable sites, four of which had two different amino acid variants (3 alleles).
The phylogenetic tree of the different SpaA protein variants suggests that there are two main groups (Figure 4), and we found that these groups are correlated with the overall population structure of E. rhusiopathiae (Figures 3, 5). We therefore propose a simplified nomenclature for SpaA groups, where Group 1 has amino acid variants at positions 55 (V/I), 70 (K/N), 178 (G/D), 195 (D/N) and 303 (G/E) in relation with the Fujisawa reference, consistent with the previous scheme proposed by Janßen et al. (2015). All other previously described groups are minor variants of what we now refer to as Group 2. These variants appear to arise multiple times throughout the phylogeny (Supplementary File S1, Supplementary Figure S3); this is suggestive of either parallel evolution (homoplasy) or recombination. A detailed description of how this SpaA classification relates to previously described groups is found in Supplementary Table S3 and Supplementary File S3.
FIGURE 5.
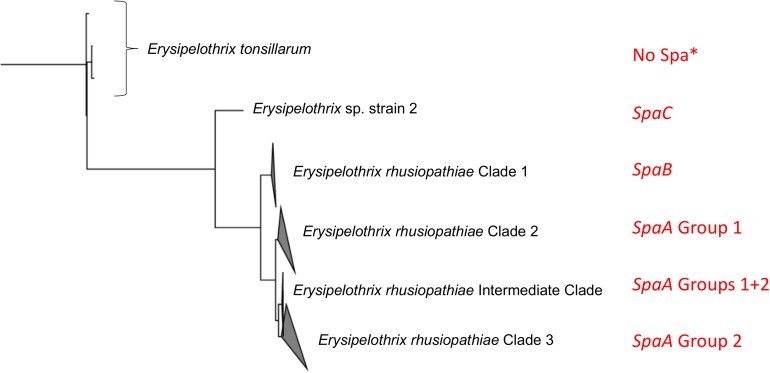
Relationship of Spa type and SpaA group to the population structure of Erysipelothrix spp. This figure is adapted from Forde T. et al., 2016. The tree is rooted to other genera of the family Erysipelotrichaceae. Spa type and SpaA group are indicated in red. SpaA group is based on discriminatory amino acid variants (Figure 4). ∗spaA and spaB have been reported only once each in Erysipelothrix tonsillarum (Pomaranski et al., 2017).
All isolates within the SpaA group 1 belonged to Clade 2 or the intermediate clade. All Clade 2 isolates possessed SpaA group 1 except for a single British pig isolate (swine100, Figure 3). Since the majority of the British pig isolates belonged to Clade 2, SpaA group 1 was the variant found in the majority of these strains (82%; Supplementary Table S1). The vaccine strain was a variant of Group 1, with an additional amino acid difference at position 109 (N/H). This variant was also found in seven other sequences from GenBank; however, none of the British pig isolates investigated in this study shared this difference.
Predicted Structure of SpaA
We aimed to assess the potential functional implications of variant residues detected within SpaA. However, given that the structure of SpaA is unknown and its function poorly understood, its structure was predicted as an exploratory exercise. A full-length structural model was produced using I-TASSER, a program that predicts structure using a combination of threading to existing protein structures and ab initio modeling. The majority of the highest ranked structural templates for the SpaA protein detected by I-TASSER were surface-located choline-binding proteins of Streptococcus pneumoniae, either phosphorylcholine esterase (Pce) also known as choline-binding protein E (CbpE) (PDB ID: 2BIB; Hermoso et al., 2005) or choline-binding protein F (CbpF) (PDB IDs: 2V04, 2V05; Molina et al., 2009). Other Firmicutes templates from Clostridium difficile and Leuconostoc mesenteroides were also identified. The highest-ranked structural model predicted using I-TASSER had a C-score of −1.30 indicating a good degree of confidence in the predicted structure (Roy et al., 2010).
Comparison with solved PDB structures showed that the structural model of E. rhusiopathiae SpaA most closely resembled the Pce protein of S. pneumoniae (Hermoso et al., 2005) (TM-score = 0.82, RMSD = 1.50 Å for 524 aligned Cα atoms, 17.6% amino acid sequence identity in structurally aligned region). The S. pneumoniae Pce comprises an N-terminal catalytic module (300 residues) and a C-terminal tail (228 residues) responsible for choline-binding (Hermoso et al., 2005). The hypervariable domain of the SpaA structural model (residues 30–413) aligned to the Pce catalytic module (TM-score = 0.75, RMSD = 1.88 Å for 303 aligned Cα atoms), while the C-terminal domain (residues 448–626) aligned to the Pce choline-binding domain (TM-score = 0.98, RMSD = 0.37 Å for 177 aligned Cα atoms).
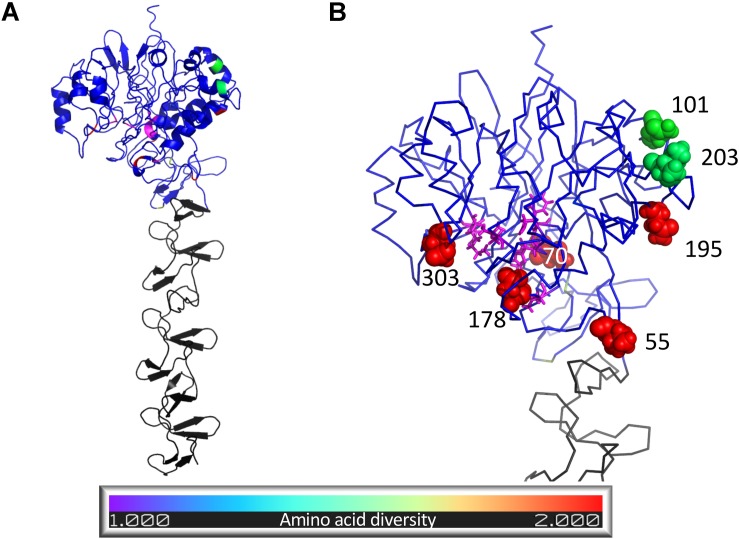
While the function of the N-terminal domain is unknown, we hypothesized that it might be similar to that of its closest homolog, which we found to be the Pce protein of S. pneumoniae. As such, we examined the proximity of variable SpaA positions to the residues of the structural model homologous to those comprising the Pce catalytic site. Residues of the S. pneumoniae Pce protein catalytic domain active site were mapped to the SpaA structural model using TM-align so that the proximity of variable SpaA sites to the potential active site could be calculated. The structural model of the E. rhusiopathiae SpaA protein is shown in Figure 6, colored according to amino acid diversity calculated across the alignment of available sequences. Of the residues with the highest amino acid diversity (55, 70, 101, 178, 195, 203, 303, 426, and 435, Inverse Simpson index > 1.3), positions 70, 178 and 303 were closest to residues of the potential active site with distances of under 10 Å (for comparison, the distance between covalently bound residues was 3.5–4 Å). For position 109, the position at which most E. rhusiopathiae sequences from British pigs differ relative to the vaccine (Asn – His), the lowest distance to a residue aligned to the Pce protein catalytic domain active site was 13.3 Å which is beyond the range expected to be structurally impacted by a mutation between His and Asn at that position.
FIGURE 6.
Structural model of Erysipelothrix rhusiopathiae SpaA protein predicted using I-TASSER. Residues structurally aligned to the active site of the S. pneumoniae Pce protein are colored magenta while the remaining residues of the N-terminal domain (residues 1–447) are colored following the legend according to amino acid diversity (Inverse Simpson index) calculated from 343 SpaA sequences sourced from newly sequenced isolates and public sequence databases. Residues of the C-terminal domain (residues 448–626) are colored gray. (A) Cartoon representation of whole structural model showing secondary structure. (B) Close up ribbon representation of N-terminal domain with spheres highlighting the nine residues with highest amino acid diversity (Inverse Simpson index > 1.3) and with residues structurally aligned to the active site of the S. pneumoniae Pce protein (magenta) shown in stick format. Figure created using Pymol (Schrödinger).
The extent to which SpaA is recognized by antibodies of the humoral immune system is not known. To investigate the potential for variant residues to influence antibody recognition, the degree to which their location within the structural model showed epitope characteristics was assessed. Thus, to evaluate the potential of the variant residues influencing recognition by the humoral immune system, the structural model was used to generate epitope scores that reflect the likelihood of each residue belonging to a B-cell epitope. Epitope scores, which reflect the likelihood of each residue belonging to a B-cell epitope, were calculated for each residue within the SpaA structural model based on surface exposure, amino acid identity and side chain orientation (Sweredoski and Baldi, 2008). Of the nine high-diversity positions, 55 and 435 were both placed among the highest predicted epitope scores, at the 94th and 93rd percentile, respectively, but there was no consistent relationship between diversity and epitope scores. The epitope score associated with position 109 on the SpaA was at only the 8th percentile calculated across all positions, suggesting the position is unlikely to be important for antibody recognition. For all positions, amino acid diversity, predicted secondary structure, epitope score, and aligned S. pneumoniae Pce protein active site residues are detailed in Supplementary Table S6.
Occurrence and Variability of Other Surface Proteins
Of the 12 other E. rhusiopathiae surface proteins examined, 11 were present in all isolates from British pigs and the vaccine (i.e. were core genes). However, the degree to which amino acid sequences were conserved varied greatly among proteins (Table 2; Supplementary Table S5; Supplementary File S2). Of the surface proteins examined, GAPDH was the most conserved, with only a single variant across the 334 amino acid protein, found in a single isolate (swine74). Atsp and Da were also highly conserved, with 6 and 13 variant positions, respectively. It is noteworthy that three of the six variant positions in Atsp were unique to the vaccine strain. Of the 52 variant positions identified in Bga, three of these were unique to the vaccine strain. One isolate (swine98) also had a mutation conferring a premature stop codon for this protein. A slightly higher proportion of sites varied in Neu (3.4%), Bml (3.8%) and rspA (4.8%); the latter had four sites unique to the vaccine. CbpA showed much higher diversity, with variants at 162 of 1773 amino acid positions (9.1%), but none of which were unique to the vaccine.
TABLE 2.
Amino acid (AA) sequence diversity in surface proteins related to immunogenicity of Erysipelothrix rhusiopathiae.
| Protein | BLAST hit results where coding | ||||
| length | # Variant AAs* | # variant AAs | Insertions/deletions and | sequence was spread across | |
| Surface protein | (AAs) | (% of total AAs) | unique to vaccine | premature stop codons | multiple contigs+ |
| rspA | 1992 | 96 (4.8%) | 4 | swine40 (3 contigs) swine94 (3 contigs) | |
| cbpB | 606 | 27 (4.5%) | 1 | Deletion 20AAs swine78 (1 repeat unit) | |
| GAPDH (gapA) | 334 | 1 (0.3%) | 0 | ||
| Dipeptidyl aminopeptidase (Da, dap2) | 657 | 13 (2%) | 0 | ||
| CbpA | 1773 | 162 (9.1%) | 0 | ||
| Plp | 1519–1531 | 91 (6%) | 0 | Insertion of either 4 AAs (16 strains) or 12 AAs (2 strains) | swine40 (2 contigs) |
| Atsp | 481 | 6 (1.2%) | 3 | swine50 (2 contigs) | |
| Neuraminidase (Neu, nanH) | 1204 | 41 (3.4%) | 1 | ||
| Beta-galactosidase (Bga) | 2091 | 52 (2.6%) | 3 | swine98 stop codon at AA Pos 658 | swine40 (3 contigs) swine50 (2 contigs) swine94 (3 contigs) |
| Bml | 364 | 14 (3.8%) | 0 | swine40 (2 contigs) | |
| CwpA | 2134 | N/A | N/A | Full length BLAST hits in 13 isolates (5322 bp) Missing stop codon in 6 isolates Remaining 27 isolates with BLAST hits ranging from 758 – 3116 bp (median = 2610) | |
| CwpB | 1009–1020 | 276 (27%) | 1 | Insertion 7AAs swine18 Deletion 4AAs swine103 | swine40 (2 contigs) |
Isolates were examined from British pigs (n = 45) and a commercially available vaccine strain in comparison with the Fujisawa reference strain. * Comparisons are made in relationship with reference strain Fujisawa (NC_015601). +Complete coding sequences were found in all isolates (based on BLASTn searches of the de novo assembled nucleotide sequences) unless otherwise stated. bp = base pairs.
The only proteins with any insertions or deletions identified among the British pig isolates were CbpB, Plp and CwpB. In CbpB, one isolate (swine78) had a deletion of 20 amino acids, corresponding to the loss of a repeat unit. In Plp, an insertion of either 4 amino acids (16 strains) or 12 amino acids (two strains) was present. In CwpB, a single isolate (swine103) had a deletion of 4 amino acids (positions 971–974), while another isolate (swine18) had an insertion of 7. This protein also had the highest proportion of variant positions (27%), although the majority of the variant sites (83%) were due to two isolates whose sequences differed greatly from the others (swine18 and swine101; Supplementary Table S5 and Supplementary File S2). Finally, one surface protein examined was variably present among the isolates. Complete nucleotide sequences for CwpA (5322 bp) were present in only 13 isolates, as well as in an additional 6 isolates that were missing a stop codon. The remaining 27 isolates had BLAST hits for this sequence ranging from 758 – 3116 bp (median = 2610); the vaccine strain had a hit length of 3116 bp. Some of the BLAST hits for the surface proteins investigated were only partial sequences spread across multiple contigs (i.e. had not been completely assembled) (Table 2). The affected isolates had lower quality assemblies overall (swine94, 50, and 40), as shown by the lowest N50 values among the newly sequenced isolates (Supplementary Table S1).
The phylogenetic trees generated for the different surface proteins (Supplementary File S2) did not show concordance with the core SNP phylogeny. This is illustrated in Figure 3, where for each surface protein, the isolates that cluster most closely with the protein sequence of the vaccine strain are shown. There were no major changes to the distribution of the different surface protein variants over the timeframe of the study period (Supplementary File S1, Supplementary Figure S4).
Discussion
Diversity of Circulating E. rhusiopathiae Strains in Pigs in Great Britain and Their Relation to a Commonly Used Vaccine Strain
While the global genomic diversity of E. rhusiopathiae has been characterized, how much of this genomic and functional diversity is maintained at smaller scales was unclear. This study provides a valuable contribution to our understanding of E. rhusiopathiae genomics, and in particular provides novel data on the diversity of surface antigens. Moreover, fully sequencing a commercial vaccine strain of E. rhusiopathiae has provided valuable insights into its relationship to circulating field strains.
Throughout the 27-year time period from which samples were collected for this study (1987 – 2014), phylogenetically diverse strains of E. rhusiopathiae were isolated from British pigs (Figure 2). Similarly high diversity was previously observed in poultry isolates from Germany based on multi-locus sequence typing (Janßen et al., 2015), while the diversity of E. rhusiopathiae circulating in pigs in Asia has been shown to be restricted to strains within the intermediate clade (Ogawa et al., 2017). The lower diversity of E. rhusiopathiae in Asian countries could be due a more recent introduction of the pathogen, and/or differences in animal trade, pig breeding, herd management or biosecurity. The majority of the British pig isolates belong to Clade 2, which differs from what was previously found in North America, where all pig and poultry isolates investigated (n = 14) belonged to Clade 3 (Forde T. et al., 2016), suggesting different E. rhusiopathiae clades have become dominant on these continents. The vaccine strain also belonged to Clade 2. We found no evidence that E. rhusiopathiae strains in circulation in Great Britain have diverged from the vaccine strain over the study period. It would have been interesting to investigate the strains associated with other commercially available vaccines, however, we were unable to detect and extract E. rhusiopathiae DNA from the second vaccine tested. Whether there is a component of the vaccine adjuvant (e.g. aluminum hydroxide) that inhibited detection of DNA by Qubit and PCR is unknown. None of the isolates we sequenced from British pigs in this study belonged to E. rhusiopathiae Clade 1, emphasizing the rarity of strains from this clade causing disease in the major production species worldwide; in a global collection of isolates previously sequenced (Forde T. et al., 2016), only 8% (7/86) belonged to Clade 1, and none of these were from pigs or poultry.
Spa Type and Correlation With Population Structure and Clinical Disease
No spaB or spaC genes were found among the E. rhusiopathiae isolates from British pigs. Given the lack of Clade 1 isolates, this finding was expected and is consistent with previous observations that Clade 1 isolates carry the spaB gene, whereas Clade 2, 3 and intermediate isolates carry the spaA gene (Figure 5; Forde T. et al., 2016). To date, spaC has only been associated with Erysipelothrix sp. strain 2, and not E. rhusiopathiae. The predominance of the spaA gene in isolates associated with clinical erysipelas has been supported in several studies (Eamens et al., 2006; To et al., 2012; Janßen et al., 2015). It therefore seems that spaB and spaC (i.e. isolates belonging to E. rhusiopathiae Clade 1 and E. sp. Strain 2) are less relevant when considering vaccine design for production species. However, given that E. rhusiopathiae Clade 1 is more commonly isolated from marine mammals in captivity (Opriessnig et al., 2013; Forde T. et al., 2016), and Erysipelothrix spp. carrying the spaC gene has recently been associated with disease in fish (Pomaranski et al., 2017), protecting against Erysipelothrix spp. carrying these Spa types could be of greater relevance for aquatic species. It was previously suggested that Spa proteins are likely important virulence factors associated with the pathogenic potential of E. rhusiopathiae in comparison to the less pathogenic E. tonsillarum (To and Nagai, 2007), and until recently, no Spa-related genes or proteins had been found in E. tonsillarum (To and Nagai, 2007; Shen et al., 2010). To our knowledge, the occurrence of spaA and spaB genes in E. tonsillarum has only been documented in one paper, among isolates from ornamental fish (Pomaranski et al., 2017). However, given that the species was defined based only on sequencing of the gyrB gene, it is conceivable that this isolate may have been misclassified.
SpaA Diversity, Correlation With Population Structure, and Relevance for Vaccination and Pathogenicity
This study represents the largest examination of the diversity of SpaA protein sequences in E. rhusiopathiae conducted to date, based on a collection of publicly available sequences and de novo whole genome sequence assemblies (n = 343). With this extensive collection of sequences, we were able to identify additional amino acid variants that could further discriminate SpaA protein sequences in comparison to previous typing schemes (Uchiyama et al., 2014; Janßen et al., 2015). We were also able to assess for correlation of these groups with population structure (Figure 5). SpaA group 1 was only present in Clade 2 and the intermediate clade, but was absent from the Clade 3 isolates examined (Figure 3). Janßen et al. (2015) found a strong correlation between SpaA group 1 and their ST complex 9. It is very likely that ST-9 corresponds with Clade 2, since we found that all Clade 2 isolates had this SpaA variant with one exception. We propose amalgamating all other previously described SpaA groups into a single group, forthwith referred to as SpaA group 2, since variants within this group are limited to only one or two amino acid differences. Moreover, these variants appear to be unconstrained by the core phylogeny (Supplementary File S1). Such incongruent patterns could arise either through parallel evolution (homoplasy), or, more likely, through horizontal gene transfer, given the apparent propensity for recombination observed in this species.
Whether the observed differences within the SpaA protein could be responsible for differential immune protection – or for differences in virulence – remains an important area for future study. For instance, the vaccine strain tested in this study belonged to Group 1 SpaA along with the majority of British pig isolates, but had an amino acid change from N→H at position 109. Whether this could result in differential immune response is unknown; however, based on the predicted structure of SpaA, we found that this position is unlikely to represent an epitope.
Isolates with methionine at position 203 (i.e. Janßen Group 5, Uchiyama Group 1) were rare among the isolates examined in this study. This variant was present in one British pig isolate (Supplementary Tables S1, S3) and in three WGS available on GenBank (GXBY-1, WH13013 and ML101), but in none of the global collection of isolates (n = 75). It was previously found in a handful of pig isolates from Germany (n = 4) (Janßen et al., 2015). Otherwise, this variant has been mostly limited to strains from China (Supplementary Table S2) and Japan, where it is widespread and has been isolated from pigs with acute, subacute and chronic infections (To et al., 2012; Uchiyama et al., 2017) and where it appears to be increasing in prevalence (Ogawa et al., 2017). There is, however, not yet any evidence of this variant being associated with an increase in virulence (Zou et al., 2015). One particular variant that would be worthy of further investigation is that of alanine at position 195 (previously classified as Group 3 by Uchiyama et al., 2014). This is the variant carried by the pathogenic reference strain SY1027 from China (Kwok et al., 2014), and was the variant carried by the E. rhusiopathiae strain associated with large-scale muskox die-offs in northern Canada (Kutz et al., 2015; Forde T.L. et al., 2016). This variant has also been reported among pigs in Japan, although is apparently being replaced by newer lineages (Ogawa et al., 2017). Whether this amino acid change confers increased virulence would be valuable to explore.
Predicted SpaA Protein Structure
We found that the amino acid sequence of the E. rhusiopathiae SpaA protein predicts a protein structure most closely resembling that of choline-binding proteins of Streptococcus pneumoniae. Indeed, the homology in the C-terminal region of these proteins had previously been described (Borrathybay et al., 2015). This region – containing tandem repeats including the GW motif – is a motif conserved across Gram-positive bacteria that allows proteins to bind to choline residues of techoic acid on the cell surface (Jedrzejas, 2001). It is believed that this motif is what allows SpaA to bind to phosphorylcholine of the of E. rhusiopathiae capsule (Harada et al., 2014), facilitating binding to host endothelial cells. To our knowledge, no studies have previously examined the structure of the N-terminal region of SpaA, which is the portion shown to be involved in its protective immunogenicity. Among proteins for which the structure has been determined, we found the N-terminal portion of the S. pneumoniae Pce protein was the closest match, although with lower homology in comparison with the C-terminal portion. S. pneumoniae Pce modifies the distribution of phosphoryl choline on the bacterial surface, impairing the ability of the host immune system to efficiently bind, providing a mechanism for immune escape (Hermoso et al., 2005). Given the predicted structural similarity, it is possible that SpaA has a similar function, though this hypothesis would need to be further explored through functional assays.
Working with the hypothesis that SpaA may have a similar function to S. pneumoniae Pce, we explored whether SpaA positions with high amino diversity, including those differing between groups 1 and 2, were located near to a possible active site. The N-terminal module of the Pce protein contains a binuclear Zn2 + catalytic center (Hermoso et al., 2005). Within this active center, particular residues have been shown to be involved in substrate binding and catalysis, facilitating teichoic acid hydrolysis which releases phosphoryl choline moieties reducing recognition by the immune system. In SpaA, the variant amino acid residues at positions 70, 178 and 303 were those closest to potential active site, as based on homology with the S. pneumoniae Pce protein. Since these are all variants that distinguish between SpaA groups 1 and 2, it is possible that these variants confer functional differences between these groups.
Alternatively, we considered that variable positions may have played a role in recognition by the humoral immune system. To do so, we assessed whether diverse amino acid sites tended to occupy locations within the structural model that exhibited signatures of epitope regions. With the notable exception of position 55, a variant that differs between groups 1 and 2, and position 435 that possessed very high predicted epitope scores, the diverse positions did not show a general tendency toward high epitope scores, suggesting pressure from the humoral immunity may not be a major driver of the observed genetic diversity. The role of SpaA in immune recognition has not been characterized in great detail. In vitro experiments and challenge studies to assess cross-protection, wherein isolates from different SpaA groups are included, would be valuable.
Variability in Immunogenic Surface Proteins
Our study provides novel information about the sequence diversity of different immunogenic surface proteins of E. rhusiopathiae. The degree of variability differed substantially among the 12 proteins we investigated, ranging from proteins that were nearly completely conserved across all 45 isolates and the vaccine strain (GAPDH) to 27% of the amino acids showing variation (CwpB), as well as some proteins with small insertions and deletions (Plp and CwpB). Coding sequences for these proteins were found to be core genes, with the exception of CwpA which was variably present. Based on the phylogenetic trees estimated for each protein (Supplementary File S1), variants of the different proteins did not correlate with the phylogenetic relationship among isolates (Figure 3), suggesting that horizontal transfer of genes encoding for antigens is commonplace for E. rhusiopathiae.
We investigated whether there was any evidence of antigenic divergence (Bart et al., 2014) between field strains of E. rhusiopathiae and a common vaccine strain, as this could potentially be of concern for vaccine efficacy. However, we did not observe any such temporal shift in surface protein variants for any of the antigens we examined (Supplementary File S1). The vaccine strain had unique amino acids in 5 of the 12 surface proteins examined – rspA, Bga, Atsp, Neu and CwpB (i.e. that were not present among the 45 pig isolates). Of particular note is in Atsp, where this included three of only six variants across the protein and among all isolates. The impact of these differences – if any – would be valuable to explore. The most conserved surface protein – GAPDH – has been confirmed to be expressed at the cell surface (Shi et al., 2013), and like SpaA, RspA and RspB, has a role in endothelial adhesion (Zhu et al., 2017c). It was recently found to provide good protection in both murine and porcine challenge models (Zhu et al., 2018). These authors suggested that given its proven role as a protective antigen, in addition to being a housekeeping gene and thus highly conserved – as confirmed by this study – GAPDH could make a good candidate for a subunit vaccine. Indeed, subunit vaccines based on surface-exposed proteins have been proposed as a potential alternative to traditional live or bacterin-based vaccines (Hu et al., 2017).
Serotype as an Epidemiological and Immunological Marker
E. rhusiopathiae isolates have historically been described by their serotype, which is based on testing for agglutination of an isolate against a panel of serotype-specific antisera using a double agar-gel diffusion method (Kucsera, 1973). It was recently determined that a chromosomal region encoding a putative pathway for polysaccharide biosynthesis is responsible for defining the antigenicity of the major serotypes 1a, 1b, 2 and 5, and that these can be distinguished based on their genetic composition at this locus (Ogawa et al., 2018; Shiraiwa et al., 2018). However, this present study lends further support to the fact that serotype is inappropriate for assessing the genetic relatedness among isolates at larger scales (e.g. at a national level). It has even been reported that the same serotypes can be found across multiple Erysipelothrix species (Takahashi et al., 2008; McNeil et al., 2017). Given the lack of phylogenetic informativeness of serotype, it may not be the most relevant feature by which to primarily classify isolates. Rather, a hierarchical approach should be taken (i.e. Figure 5), wherein Erysipelothrix spp. isolates are described by species, clade and/or Spa type, followed by SpaA group and serotype. The value of serotype as an epidemiological marker at smaller scales (e.g. within or between farm spread) would be valuable to assess.
Whether serotype is a relevant trait for predicting cross-protection among strains remains unknown and warrants further study. It has been suggested that vaccines based on serotype 2 strains confer protection against serotype 1 and 2 strains in pigs, as well as other serotypes to a variable degree (Wood et al., 1981; Takahashi et al., 1984; Sawada and Takahashi, 1987; Kitajima et al., 1998). Since serotypes 1 and 2 are those most commonly associated with clinical disease globally (Wood and Harrington, 1978; Cross and Claxton, 1979; Takahashi et al., 1996; Opriessnig et al., 2004; Coutinho et al., 2011; McNeil et al., 2017), these challenge studies suggest that currently available vaccines should confer protective immunity against a variety of strains. However, since these pig challenge studies have only included one representative strain per serotype, the potential for confounding is very high (i.e. the possibility that these findings are based on immunogenic features other than serotype), making it difficult to assess the likelihood or relevance of serotype-associated cross protection.
The newly reported PCR scheme (Shiraiwa et al., 2018) for distinguishing among serotypes 1a, 1b, 2 and 5 was successfully applied in silico to determine the serotype of sequenced isolates for which serotype was not previously known. Initial discrepancies between phenotypic and in silico results for some British pig isolates were resolved upon re-running the agglutination tests on fresh subcultures, suggesting that the in silico method may produce more accurate and reproducible results than standard methods. However, as the authors of this PCR scheme previously noted and as we found for global isolate MEW22, one limitation is that certain serovars that would be phenotypically untypeable (“N”) using the double agar-gel diffusion method may be assigned to a serotype. This likely represents the original serotype that no longer yields serotype-specific antigen due to only minor genetic differences that result in changes in antigen-antibody reactions. Further studies will be valuable to expand our understanding of the genetic basis for other serotypes and the molecular tools available for distinguishing them. We found that the reverse primer sequence for serotype 5 had one nucleotide discrepancy with all isolates of this serotype in the global collection (n = 17); this sequence should therefore be updated to 5′-GAAATAATGCCAATAGATGGAGCACC-3′.
Vaccine Cross Protection
Vaccination success is multifactorial, and includes factors related to delivery (e.g. maintenance of cold chain), host-related factors (e.g. age, health status, genetic factors), and factors related to the pathogen. This study highlights important knowledge gaps associated with pathogen-related factors for E. rhusiopathiae vaccination, namely what are the determinants of protective immunity, and what features confer cross-protection. Protection induced by E. rhusiopathiae vaccination is via both cell-mediated and humoral immunity (Shimoji, 2000; Pomorska-Mól et al., 2012). Protective immunity is more difficult to elucidate, as it can only be assessed by challenge studies. It is likely stimulated via a complex array of antigens, including proteins and polysaccharides. Ultimately, it would be valuable for producers and clinicians to know what vaccine(s) would be suitable to provide protection against the specific strains found on a farm, but information to make such a judgment is currently lacking. The surface proteins explored in this study have all been shown to be immunogenic, but whether differences in their structure or their presence or absence can impact a strain’s ability to confer a protective immune response against different strains remains unknown. In most challenge studies that have been conducted in both mice and pigs, the majority of E. rhusiopathiae vaccines have provided a protective immune response against a range of different strains. The key question for the field may therefore be ‘What genetic and/or antigenic differences between strains would result in a failure to provide cross protection?’. There is evidence that the most common vaccine strains are not consistently protective against strains assigned to serotypes 9 or 10 (Wood, 1979; Wood et al., 1981; Takahashi et al., 1984), however, these strains are likely sufficiently rare in field conditions to make this a minor concern (Haesebrouck et al., 2004). It has been well recognized that gram-positive bacterial cell wall antigens, including lipoteichoic acid, have immunomodulating properties (Shimoji et al., 2019). It is possible that serotype differences in E. rhusiopathiae result in different immunostimulatory potencies (i.e. have a role as adjuvants), but this requires further studies for confirmation. Whether phylogenetic distance (e.g. differences in clade) impacts the ability of a vaccine to confer protective immunity is an important area of study that has yet to be explored. For instance, it is not known whether the vaccine strain (Clade 2) would be protective against more divergent isolates from Clade 3. Similarly, the relevance of surface protein variants – including SpaA groups – for immune cross-protection is unknown. This could initially be explored using serum from immunized pigs, and testing for antibody responses against different strains through ELISA. A further complication is the possibility of mixed infection, which we previously showed to be quite common for E. rhusiopathiae infection, at least in wild ungulates (Forde T.L. et al., 2016). If a host were infected with multiple strains, particular variants might gain hold if they are undetected by the immune system. The inclusion of multiple strains in a single vaccine could potentially overcome some of these issues.
Conclusion
In this study, we show that diverse E. rhusiopathiae strains have been circulating in pigs in Great Britain over the past few decades, and that the average genetic distance of these field strains from the vaccine strain has remained relatively stable over time. This study provides further support that SpaA is the most relevant Spa type associated with clinical erysipelas in production species (i.e. pigs and poultry); suggestions to update the nomenclature related to SpaA groups based on amino acid variants are provided. We provide novel data on the degree of conservation of various immunogenic surface proteins, and demonstrate that horizontal gene transfer has likely contributed to the observed diversity. Research into which variables are relevant for conferring cross-protection will be critical for understanding the relevance of this diversity for vaccine development. As the agricultural industry is working to reduce the use of antibiotics due to increasing concerns related to antimicrobial resistance, the availability and selection of effective vaccines is increasingly important. Variants identified in this study could serve as a basis for guiding the selection of strains to be included in future in vitro and in vivo challenge studies.
Commercial Products Used
The sequenced vaccine was Porcilis Ery®, MSD Animal Health, which contains serotype 2 strain M2. This strain, either as a monovalent vaccine or in combination with vaccines against parvovirus ± Leptospira spp., reportedly accounts for more than half of the total market in United Kingdom vaccines against E. rhusiopathiae (R. Warin, Hipra, personal communication). The second vaccine from which DNA was not successfully extracted was Eryseng®, Hipra.
Data Availability Statement
The sequence data generated for this study can be found in the European Nucleotide Archive under accession number PRJEB34953.
Author Contributions
TF and TO conceived, designed, and coordinated the study. JT and SW provided sample collections and associated metadata. TF performed laboratory work and drafted the manuscript. TF, NK, WH, and RB performed and/or advised on data analysis. All authors contributed to editing the manuscript and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Seymour Lopez for assistance with some of the preliminary data analyses.
Funding. This study was supported by the John Robertson Bequest Fund. During the time of this study, TLF was supported by a Marie Skłodowska-Curie fellowship (659223 – MEBA) from the European Union’s Horizon 2020 Research and Innovation Program and by a Biotechnology and Biological Sciences Research Council (BBSRC) Future Leader Fellowship (FORDE/BB/R012075/1). TO was supported by BBSRC Roslin Institute Strategic Program Control of Infectious Diseases funding (BBS/E/D/20002173 and BBS/E/D/20002174). WH was supported by the Medical Research Council and BBSRC under grant MR/R024758/1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00418/full#supplementary-material
References
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart M. J., Harris S. R., Advani A., Arakawa Y., Bottero D., Bouchez V., et al. (2014). Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 5:e01074. 10.1128/mBio.01074-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrathybay E., Gong F.-J., Zhang L., Nazierbieke W. (2015). Role of surface protective antigen A in the pathogenesis of Erysipelothrix rhusiopathiae strain C43065. J. Microbiol. Biotechnol. 25 206–216. 10.4014/jmb.1407.07058 [DOI] [PubMed] [Google Scholar]
- Connor T. R., Loman N. J., Thompson S., Smith A., Southgate J., Poplawski R., et al. (2016). CLIMB (the Cloud Infrastructure for Microbial Bioinformatics): an online resource for the medical microbiology community. BioRxiv [preprint] 10.1101/064451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho T. A., Imada Y., Barcellos D. E. S. N., Oliveira S. J., Moreno A. M. (2011). Phenotypic and molecular characterization of recent and archived Erysipelothrix spp. isolated from Brazilian swine. Diagn. Microbiol. Infect. Dis. 69 123–129. 10.1016/j.diagmicrobio.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Cross G. M., Claxton P. D. (1979). Serological classification of Australian strains of Erysipelothrix rhusiopathiae isolated from pigs, sheep, turkeys and man. Aust. Vet. J. 55 77–81. 10.1111/j.1751-0813.1979.tb15170.x [DOI] [PubMed] [Google Scholar]
- Eamens G. J., Forbes W. A., Djordjevic S. P. (2006). Characterisation of Erysipelothrix rhusiopathiae isolates from pigs associated with vaccine breakdowns. Vet. Microbiol. 115 329–338. 10.1016/j.vetmic.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson H., Nyman A.-K., Fellström C., Wallgren P. (2013). Erysipelas in laying hens is associated with housing system. Vet. Rec. 173:18. 10.1136/vr.101388 [DOI] [PubMed] [Google Scholar]
- Forde T., Biek R., Zadoks R., Workentine M. L., De Buck J., Kutz S., et al. (2016). Genomic analysis of the multi-host pathogen Erysipelothrix rhusiopathiae reveals extensive recombination as well as the existence of three generalist clades with wide geographic distribution. BMC Genomics 17:461. 10.1186/s12864-016-2643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde T. L., Orsel K., Zadoks R. N., Biek R., Adams L. G., Checkley S. L., et al. (2016). Bacterial genomics reveal the complex epidemiology of an emerging pathogen in arctic and boreal ungulates. Front. Microbiol. 7:1759 10.3389/fmicb.2016.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberini M., Gómez R. M., Atzingen M. V., Martins E. A. L., Vasconcellos S. A., Romero E. C., et al. (2005). Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol. Lett. 244 305–313. 10.1016/j.femsle.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Gurevich A., Saveliev V., Vyahhi N., Tesler G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29 1072–1075. 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesebrouck F., Pasmans F., Chiers K., Maes D., Ducatelle R., Decostere A. (2004). Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 100 255–268. 10.1016/j.vetmic.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Harada T., Ogawa Y., Eguchi M., Shi F., Sato M., Uchida K., et al. (2014). Phosphorylcholine and SpaA, a choline-binding protein, are involved in the adherence of Erysipelothrix rhusiopathiae to porcine endothelial cells, but this adherence is not mediated by the PAF receptor. Vet. Microbiol. 172 216–222. 10.1016/j.vetmic.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Hermoso J. A., Lagartera L., González A., Stelter M., García P., Martínez-ripoll M., et al. (2005). Insights into pneumococcal pathogenesis from the crystal structure of the modular teichoic acid phosphorylcholine esterase Pce. 12 533–538. 10.1038/nsmb940 [DOI] [PubMed] [Google Scholar]
- Hu Y.-F., Zhao D., Yu X.-L., Hu Y.-L., Li R.-C., Ge M., et al. (2017). Identification of bacterial surface antigens by screening peptide phage libraries using whole bacteria cell-purified antisera. Front Microbiol 8:82 10.3389/fmicb.2017.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada Y., Goji N., Ishikawa H., Kishima M., Sekizaki T. (1999). Truncated surface protective antigen (SpaA) of Erysipelothrix rhusiopathiae serotype 1a elicits protection against challenge with serotypes 1a and 2b in pigs. Infect. Immun. 67 4376–4382. 10.1128/iai.67.9.4376-4382.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janßen T., Voss M., Kühl M., Semmler T., Philipp H.-C., Ewers C. (2015). A combinational approach of multilocus sequence typing and other molecular typing methods in unravelling the epidemiology of Erysipelothrix rhusiopathiae strains from poultry and mammals. Vet. Res. 46:84. 10.1186/s13567-015-0216-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrzejas M. J. (2001). Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65 187–207. 10.1128/MMBR.65.2.187-207.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T., Oishi E., Amimoto K., Ui S., Nakamura H., Okada N., et al. (1998). Protective effect of NaOH-extracted Erysipelothrix rhusiopathiae vaccine in pigs. J. Vet. Med. Sci. 60 9–14. 10.1292/jvms.60.9 [DOI] [PubMed] [Google Scholar]
- Kucsera G. (1973). Proposal for standardization of the designations used for serotypes of Erysipelothrix rhusiopathiae (Migula) Buchanan. Int. J. Syst. Bacteriol. 23 184–188. 10.1099/00207713-23-2-184 [DOI] [Google Scholar]
- Kutz S., Bollinger T., Branigan M., Checkley S., Davison T., Dumond M., et al. (2015). Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian arctic. Can. Vet. J. 56 560–563. [PMC free article] [PubMed] [Google Scholar]
- Kwok A. H., Li Y., Jiang J., Jiang P., Leung F. C. (2014). Complete genome assembly and characterization of an outbreak strain of the causative agent of swine erysipelas – Erysipelothrix rhusiopathiae SY1027. BMC Microbiol. 14:176. 10.1186/1471-2180-14-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Yamamoto K., Murakami S., Shirahata T., Uremura K., Sawada T., et al. (1998). Properties of repeat domain found in a novel protective antigen. SpaA, of Erysipelothrix rhusiopathiae. Microb. Pathog. 25 101–109. 10.1006/mpat.1998.0216 [DOI] [PubMed] [Google Scholar]
- McNeil M., Gerber P. F., Thomson J., Williamson S., Opriessnig T. (2017). Serotypes and Spa types of Erysipelothrix rhusiopathiae isolates from British pigs (1987 to 2015). Vet. J. 225 13–15. 10.1016/j.tvjl.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Molina R., González A., Stelter M., Inmaculada P., Kahn R., Morales M., et al. (2009). Crystal structure of CbpF, a bifunctional choline-binding protein and autolysis regulator from Streptococcus pneumoniae. EMBO Rep. 10 246–251. 10.1038/embor.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Ooka T., Shi F., Ogura Y., Nakayama K., Hayashi T., et al. (2011). The genome of Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas, reveals new insights into the evolution of firmicutes and the organism’s intracellular adaptations. J. Bacteriol. 193 2959–2971. 10.1128/JB.01500-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Shiraiwa K., Nishikawa S., Eguchi M., Shimoji Y. (2018). Identification of the chromosomal region essential for serovar-specific antigen and virulence of serovar 1 and 2 strains of Erysipelothrix rhusiopathiae. Infect. Immun. 86:e00324-18. 10.1128/IAI.00324-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Shiraiwa K., Ogura Y., Ooka T., Nishikawa S., Eguchi M., et al. (2017). Clonal lineages of Erysipelothrix rhusiopathiae responsible for acute swine erysipelas in japan identified by using genome-wide single-nucleotide polymorphism analysis. Appl. Environ. Microbiol. 83:e00130-17. 10.1128/AEM.00130-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opriessnig T., Hoffman L. J., Harris D. L., Gaul S. B., Halbur P. G. (2004). Erysipelothrix rhusiopathiae: genetic characterization of midwest US isolates and live commercial vaccines using pulsed-field gel electrophoresis. J. Vet. Diagn. Invest. 16 101–107. 10.1177/104063870401600202 [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Shen H. G., Bender J. S., Boehm J. R., Halbur P. G. (2013). Erysipelothrix rhusiopathiae isolates recovered from fish, a harbour seal (Phoca vitulina) and the marine environment are capable of inducing characteristic cutaneous lesions in pigs. J. Comp. Pathol. 148 365–372. 10.1016/j.jcpa.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Pomaranski E. K., Reichley S. R., Yanong R., Shelley J., Pouder D. B., Wolf J. C., et al. (2017). Characterization of spaC-type Erysipelothrix sp. isolates causing systemic disease in ornamental fish. J. Fish Dis. 41 49–60. 10.1111/jfd.12673 [DOI] [PubMed] [Google Scholar]
- Pomorska-Mól M., Markowska-Daniel I., Pejsak Z. (2012). Effect of age and maternally-derived antibody status on humoral and cellular immune responses to vaccination of pigs against Erysipelothrix rhusiopathiae. Vet. J. 194 128–130. 10.1016/j.tvjl.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. (2000). EMBOSS: the european molecular biology open software suite. Trends Genet. 16 276–277. 10.1016/s0168-9525(00)02024-2 [DOI] [PubMed] [Google Scholar]
- Roy A., Kucukural A., Zhang Y. (2010). I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5 725–738. 10.1038/nprot.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T., Takahashi T. (1987). Cross protection of mice and swine inoculated with culture filtrate of attenuated Erysipelothrix rhusiopathiae and challenge exposed to strains of various serovars. Am. J. Vet. Res. 48 239–242. [PubMed] [Google Scholar]
- Shen H. G., Bender J. S., Opriessnig T. (2010). Identification of surface protective antigen (spa) types in Erysipelothrix reference strains and diagnostic samples by spa multiplex real-time and conventional PCR assays. J. Appl. Microbiol. 109 1227–1233. 10.1111/j.1365-2672.2010.04746.x [DOI] [PubMed] [Google Scholar]
- Shi F., Ogawa Y., Sano A., Harada T., Hirota J., Eguchi M., et al. (2013). Characterization and identification of a novel candidate vaccine protein through systematic analysis of extracellular proteins of Erysipelothrix rhusiopathiae. Infect. Immun. 81 4333–4340. 10.1128/IAI.00549-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji Y. (2000). Pathogenicity of Erysipelothrix rhusiopathiae: virulence factors and protective immunity. Microbes Infect. 2 965–972. 10.1016/s1286-4579(00)00397-x [DOI] [PubMed] [Google Scholar]
- Shimoji Y., Mori Y., Fischetti V. A. (1999). Immunological characterization of a protective antigen of Erysipelothrix rhusiopathiae: identification of the region responsible for protective immunity. Infect. Immun. 67 1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji Y., Ogawa Y., Osaki M., Kabeya H., Maruyama S., Mikami T., et al. (2003). Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagens. J. Bacteriol. 185 2739–2748. 10.1128/jb.185.9.2739-2748.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji Y., Ogawa Y., Tsukio M., Shiraiwa K., Nishikawa S., Eguchi M. (2019). Genome-wide identification of virulence genes in Erysipelothrix rhusiopathiae: use of a mutant deficient in a tagF homolog as a safe oral vaccine against swine erysipelas. Infect. Immun. 18:e673-19. 10.1128/IAI.00673-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa K., Ogawa Y., Nishikawa S., Eguchi M., Shimoji Y. (2018). Identification of serovar 1a, 1b, 2, and 5 strains of Erysipelothrix rhusiopathiae by a conventional gel-based PCR. Vet. Microbiol. 225 101–104. 10.1016/j.vetmic.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Sweredoski M. J., Baldi P. (2008). PEPITO: improved discontinuous B-cell epitope prediction using multiple distance thresholds and half sphere exposure. Bioinformatics 24 1459–1460. 10.1093/bioinformatics/btn199 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Fujisawa T., Umeno A., Kozasa T., Yamamoto K., Sawada T. (2008). A taxonomic study on Erysipelothrix by DNA-DNA hybridization experiments with numerous strains isolated from extensive origins. Microbiol. Immunol. 52 469–478. 10.1111/j.1348-0421.2008.00061.x [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nagamine N., Kijima M., Suzuki S., Takagi M., Tamura Y., et al. (1996). Serovars of Erysipelothrix strains isolated from pigs affected with erysipelas in Japan. J. Vet. Med. Sci. 58 587–589. 10.1292/jvms.58.587 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Takagi M., Sawada T., Seto K. (1984). Cross protection in mice and swine immunized with live erysipelas vaccine to challenge exposure with strains of Erysipelothrix rhusiopathiae of various serotypes. Am. J. Vet. Res. 45 2115–2118. [PubMed] [Google Scholar]
- Tang H.-B., Xie J., Wang L., Liu F., Wu J. (2016). Complete genome sequence of Erysipelothrix rhusiopathiae strain GXBY-1 isolated from acute swine erysipelas outbreaks in south China. Genom Data 8 70–71. 10.1016/j.gdata.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To H., Nagai S. (2007). Genetic and antigenic diversity of the surface protective antigen proteins of Erysipelothrix rhusiopathiae. Clin. Vaccine Immunol. 14 813–820. 10.1128/CVI.00099-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To H., Sato H., Tazumi A., Tsutsumi N., Nagai S., Iwata A., et al. (2012). Characterization of Erysipelothrix rhusiopathiae strains isolated from recent swine erysipelas outbreaks in Japan. J. Vet. Med. Sci. 74 949–953. 10.1292/jvms.11-0533 [DOI] [PubMed] [Google Scholar]
- To H., Someno S., Nagai S., Koyama T., Nagano T. (2010). Immunization with truncated recombinant protein SpaC of Erysipelothrix rhusiopathiae strain 715 serovar 18 confers protective immunity against challenge with various serovars. Clin. Vaccine Immunol. 17 1991–1997. 10.1128/CVI.00213-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M., Shimazaki Y., Isshiki Y., Kojima A., Hirano F., Yamamoto K., et al. (2017). Pathogenic characterization of Erysipelothrix rhusiopathiae Met-203 type SpaA strains from chronic and subacute swine erysipelas in Japan. J. Vet. Med. Sci. 79 18–21. 10.1292/jvms.16-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M., Yamamoto K., Ochiai M., Yamamoto T., Hirano F., Imamura S., et al. (2014). Prevalence of Met-203 type spaA variant in Erysipelothrix rhusiopathiae isolates and the efficacy of swine erysipelas vaccines in Japan. Biologicals 42 109–113. 10.1016/j.biologicals.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Wood R. L. (1979). Specificity in response of vaccinated swine and mice to challenge exposure with strains of Erysipelothrix rhusiopathiae of various serotypes. Am. J. Vet. Res. 40 795–801. [PubMed] [Google Scholar]
- Wood R. L., Booth G. D., Cutlip R. C. (1981). Susceptibility of vaccinated swine and mice to generalized infection with specific serotypes of Erysipelothrix rhusiopathiae. Am. J. Vet. Res. 42 608–614. [PubMed] [Google Scholar]
- Wood R. L., Harrington R., Jr. (1978). Serotypes of Erysipelothrix rhusiopathiae isolated from swine and from soil and manure of swine pens in the United States. Am. J. Vet. Res. 39 1833–1840. [PubMed] [Google Scholar]
- Zhang Y., Skolnick J. (2005). TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33 2302–2309. 10.1093/nar/gki524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Cai C., Wang Y., Li J., Wu C., Kang C., et al. (2017a). Characterization of roles of SpaA in Erysipelothrix rhusiopathiae adhesion to porcine endothelial cells. Microb. Pathog. 113 176–180. 10.1016/j.micpath.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Zhu W., Wang Y., Cai C., Li J., Chao W., Jin M. (2017b). Erysipelothrix rhusiopathiae recruit host plasminogen via the major protective antigen SpaA. FEMS Microbiol. Lett. 364:fnx036 10.1093/femsle/fnx036 [DOI] [PubMed] [Google Scholar]
- Zhu W., Wu C., Kang C., Cai C., Wang Y., Li J., et al. (2018). Evaluation of the protective efficacy of four newly identified surface proteins of Erysipelothrix rhusiopathiae. Vaccine 36 8079–8083. 10.1016/j.vaccine.2018.10.071 [DOI] [PubMed] [Google Scholar]
- Zhu W., Zhang Q., Li J., Wei Y., Cai C., Liu L., et al. (2017c). Glyceraldehyde-3-phosphate dehydrogenase acts as an adhesin in Erysipelothrix rhusiopathiae adhesion to porcine endothelial cells and as a receptor in recruitment of host fibronectin and plasminogen. Vet. Res. 48:16. 10.1186/s13567-017-0421-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Zhu X., Muhammad H. M., Jiang P., Li Y. (2015). Characterization of Erysipelothrix rhusiopathiae strains isolated from acute swine erysipelas outbreaks in Eastern China. J. Vet. Med. Sci. 77 653–660. 10.1292/jvms.14-0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data generated for this study can be found in the European Nucleotide Archive under accession number PRJEB34953.