Abstract
Background
This study was conducted to analyze the activity of masticatory muscles depending on the presence of temporomandibular joint disorders (TMD) when foods of various hardness are masticated.
Material/Methods
We enrolled 20 subjects (13 men and 7 women) who met our selection criteria, and they were divided into 3 groups (the Disorder Group, the Malalignment Group, and the Control Group) based on surveys and screening inspection. The average of reference voluntary contraction (RVC) was used to measure masticatory muscle activity. Using surface electromyography (SEMG) for each group during masticatory activity, the activities of the masseter muscle and temporalis muscle were measured based on the hardness of 3 different foods (soft, sticky, and hard).
Results
Characteristics of these 3 groups prior to the experiment were identical, and subsequent findings were as follows: First, when masticating sticky food, the Disorder Group and Malalignment Group showed significant differences from the Control Group in activities of the masseter muscle and temporalis muscle (p<0.05). Second, when masticating hard food, the Malalignment Group and Control Group showed significant differences from the Disorder Group in the masseter muscle and temporalis muscle activities (p<0.05). Based on these findings, the study showed that malalignment affects movement of the temporomandibular joint during mastication of sticky food, and the temporomandibular joint movement was affected by the presence of pain during mastication of hard food.
Conclusions
Our results provide basic data useful in the diagnosis of temporomandibular joint disorder, as well as guiding future studies on this topic.
MeSH Keywords: Electromyography, Mastication, Masticatory Muscles, Temporomandibular Joint Dysfunction Syndrome
Background
With the growing awareness of the importance of oral health, temporomandibular joint disorder (TMD) has received increased public interest [1]. TMD includes a wide variety of functional disorders involving the temporomandibular joint and masticatory muscle, and is associated with general symptoms such as pain, limited movement, muscle stress, and joint soundness [2]. It is a pain syndrome commonly affecting the head and face [3], and 75% of all people experience at least 1 general symptoms of TMD during their lifetime [4]. Its prevalence is highest in people ages 20–50 years, and women are twice as likely to be affected than men [5].
The main symptoms of TMD are muscle pain, pain at and functional deterioration of the temporomandibular joint, limited movement of the mandible, and movement of the mandible with pain [1]. Pain may also be experienced in other parts of the body, such as the ear, temple, forehead, larynx, spine, and shoulder girdle [6], and associated symptoms include joint unsoundness and mandibular deviation [7]. Most of all, as deteriorated masticatory function is prone to lead to food deprivation, it could eventually cause deterioration of quality of life [8]. Etiological causes of TMD may widely vary, in addition to biological, environmental, sociological, emotional, and cognitive etiologies [9]. Emotional tension, loss of teeth, occlusal interferences, masticatory muscle disorder, incorrect posture, and variation of temporomandibular joint structure are closely associated with TMD [10].
Anatomical structure problems of the TMD can be diagnosed by CT, MRI, or X-ray images [11]. MRI is an optimal method to comprehensively evaluate a patient with signs and symptoms of TMD [12]. This examination is, however, expensive, not easily accessible, and somewhat limited in assessing clinical symptoms [11]. In addition, other available technologies for diagnosis of TMD include mandibular Kinesiography (MKG) [13], electromyography [14], and ultrasonography [15]. Sforza et al. [16] suggested that mandibular kinematic and surface electromyography analysis are very useful in diagnosing TMD.
The study conducted by Tosato and Caria [17] showed there were significant difference between the articular disorder group and the asymptomatic group during isometric contraction by measuring muscle activity after dividing the temporomandibular joint disorder group into 4 groups (articular disorder group, myogenic disorder group, mixed disorder group, and asymptomatic group) based on surveys and clinical trials. The study by De Felicio et al. [8] found no difference between the temporomandibular joint disorder group and the control group, other than the fact that the temporomandibular joint disorder group had lower average amplitude than the control group, based on the comparison of masticatory muscle activity between these 2 groups during mastication of sugar-free gum. Mapelli et al. [18] found that the masseter muscle activity of the severe disorder group was lower than in the control group, based on comparison of masseter muscle activities among 3 groups (severe disorder group, moderate disorder group, and control group) during mastication of sugar-free gum.
Previous studies, however, did not classify the subjects with malalignment of temporomandibular joint but without pain. This is because it is difficult to diagnose TMD, since the evaluation of movements of the temporomandibular joint and joint noise is conducted through surveys or from an objective perspective [19–21], and patients describe symptoms in many different ways [22]. Consequently, it shows that treatments are not being delivered properly, because the criteria for TMD are comprehensive and a definite mechanism is yet to be established [23].
Moreover, previous studies made measurements by mastication of chewing gum or by maximum voluntary contraction. These methods fail to reflection the actual situations in which foods of various degrees of hardness are being masticated, and further studies need to be performed concerning mastication of food under diverse conditions.
Therefore, the present study sought to establish criteria for diagnosis of TMD through surveys and screening tests, and also analyzed the activities of the masseter muscle and temporalis muscle that occur during mastication of food depending on presence of TMD. We hope our results will provide a basis for development of diagnostic criteria for TMD by using the EMG information obtained during mastication.
Material and Methods
Subjects
We enrolled 20 adult men and women currently studying at our university located in Seoul. The inclusion criteria were: all permanent teeth without any prior history of orthodontic therapy [1], and without any skeletal or dental anomaly and without any prosthetic treatments and maxillofacial operations.
Exclusion criteria were: currently undergoing orthodontic therapy, suffering mental illness or debilitating neurological diseases, cutaneous disorders or trauma at the measurement sites, and incapable of filling out the study-related forms.
All subjects for this study were chosen only after they fully understood the experiment, were given a complete explanation concerning the procedures and anticipated effects, and signed the consent form to participate in this study. The study was approved by the Clinical Trial Review Committee of Sahmyook University.
Procedure
The study had a cross-sectional design. There were 3 study groups: a Temporomandibular Dysfunction Group (DG), a Temporomandibular Joint Malalignment Group (MG), and a Control Group (CG), and subjects were assigned to groups based on the results of surveys and screening tests. The questionnaire used prior to the experiment was the Helkimo index, which consists of 2 parts. The first part is an anamnestic component asking 3 questions about name, age, and gender, and 8 true-false questions concerning the temporomandibular joint. The second part is an examination to assess clinical dysfunctions such as mandibular opening, deviation during lowering, pain and dysfunction of the temporomandibular joint, and muscle pain. This index has been used to measure the severity and pain of patients with TMD [1]. In this study, the anamnestic component was used to categorize the subjects. The activities of the masseter muscle and temporalis muscle were assessed based on the hardness of foods and temporomandibular joint condition.
The screening test consists of 6 categories examining crepitus, pain, and presence of left/right deviations. To minimize errors from the screening test, all subjects were examined by a physical therapist. Participants with crepitus or lateral deviation of the mandible with pain were classified into the DG group, and participants with no symptom (pain and/or crepitus) but with mandible deviation were classified into the MG group. The subjects in the Control Group (CG) were healthy without any problem with their temporomandibular joint.
Surface electromyography (SEMG: Telemyo 2400 GT Telemetry EMG system, Noraxon, USA, 2007) was performed to assess masseter muscle and temporalis muscle activities of subjects. Muscle activities were measured while the subjects put the food in their mouth and chewed. To prevent errors of measurement, electrodes were attached to the most developed muscle belly, while the distance between electrodes were maintained at 2 cm after hairs were removed and the sites were thoroughly cleaned with alcohol. The RVC was measured when subjects bit down strongly for 5 s on a 10-mm-thick cotton pad positioned between the lower and upper jaws, and this procedure was repeated 3 times to gain average values.
Subjects masticated foods with 3 different levels of hardness (marshmallows for soft, jelly for sticky, and candy for hard) in a random order, and they were measured for muscle activities at the same time. The average values summing the values of left/right muscle activities measured were evaluated as results. Subjects were instructed to put each of the food items in their mouth and masticate them as naturally as possible. For the following experiments, subjects cleansed their mouth in order to remove any remaining food by sipping water and were allowed to take a short break [24].
Measurements
Surface electromyography (SEMG: Telemyo 2400 GT Telemetry EMG system, Noraxon, USA, 2007) was performed to assess activities of the masseter muscle and temporalis muscle.
Collected data was stored and analyzed using software for electromyography (MyoResearch Master 1.06 XP). A sampling rate was set at 1000 Hz and frequency bandwidth was 10~450Hz. The EMG signals measured from the muscle were processed through a root mean square (RMS), which provides a value close to actual values of EMG signals, following rectification, and an ECG filter was used to minimize the effects from electrical signals of trunk heartbeat. EMG signals from each muscle were normalized through a percentage and used for analysis. During the EMG analysis, measurements of RVC were conducted for 5 s with consideration of individual effects of study subjects. For the average activity, measured values for 3 s during movements excluding the first 1 s and the final 1 s were used, and the average value summing the values of left/right muscle activities measured were evaluated as results.
Statistical analysis
SPSS 21.0 was used for statistical analysis. Normality tests were conducted on all data with Shapiro-Wilk test, and all data were assessed for normality of distribution. Because 3 groups were compared to each other, one-way repeated-measures ANOVA was performed. To assess differences between groups, post hoc tests were conducted with the Duncan multiple range test. The level of statistical significance was set at 0.05.
Results
A total of 20 participants were recruited in this study. No significant differences were found in general characteristics of participants in each group (Table 1).
Table 1.
General characteristics of subjects. (n=20).
| General characteristics | Values |
|---|---|
| Sex (Male/Female) | 13/7 |
| Age (years) | 22.8±2.4 |
| Height (cm) | 170.13±5.99 |
| Weight (kg) | 64.18±10.12 |
Values are expressed as mean±standard deviation.
The values of masseter muscle activity measured during mastication of soft food were 34.14% RVC from the DG, 38.96% RVC from the MG, and 60.25% RVC from the CG. The values of temporalis muscle activity were 28.19% RVC from the DG, 40.77% RVC from the MG, and 61.35% RVC from the CG. Based on the experiment findings, the values of masseter muscle activity from the DG and MG showed statistically significant differences from that of the CG (p<0.05), and the values of temporalis muscle activity showed a statistically significant difference from the DG and CG (p<0.05) (Figure 1, Table 2).
Figure 1.
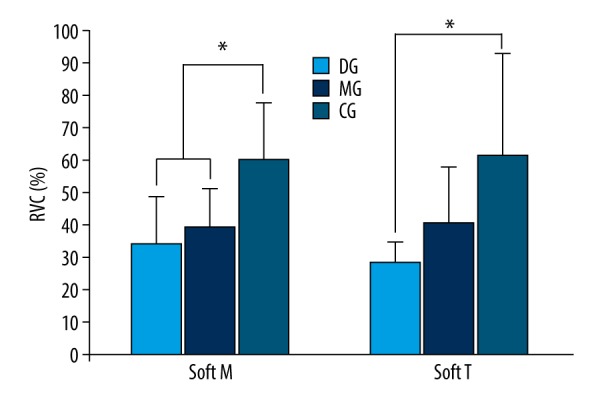
Comparison of muscle activity during mastication of soft food (* p<0.05). Soft M – masseter muscle activity during mastication of soft food; Soft T – temporalis muscle activity during mastication of soft food; DG – Temporomandibular Dysfunction Group; MG – Temporomandibular Joint Malalingment Group; CG – Control Group.
Table 2.
Comparison of muscle activity during mastication of soft food.
| Muscle activity | F | p-Value | Post hoc | ||
|---|---|---|---|---|---|
| Masseter | DG (n=7) | 34.14±14.48 | 5.255 | 0.017 | AB|C |
| MG (n=8) | 38.96±12.15 | ||||
| CG (n=5) | 60.25±17.40 | ||||
| Temporalis | DG | 28.19±6.12 | 4.465 | 0.028 | A|C |
| MG | 40.77±16.75 | ||||
| CG | 61.35±31.36 |
Values are expressed as mean±standard deviation. DG – Temporomandibular Dysfunction Group; MG – Temporomandibular Joint Malalignment Group; CG – Control Group.
The values of masseter muscle activity measured during mastication of sticky food were 48.05% RVC from the DG, 46.04% RVC from the MG, and 79.95% RVC from the CG. The values of temporalis muscle activity were 37.5% RVC from the DG, 79.95% RVC from the MG, and 83.55% RVC from the CG. Based on the experiment findings, the values of masseter muscle activity from the DG and MG showed statistically significant differences from that of the CG (p<0.05), and the values of temporalis muscle activity also showed statistically significant differences from the both disorder groups and the CG (p<0.05) (Figure 2, Table 3).
Figure 2.
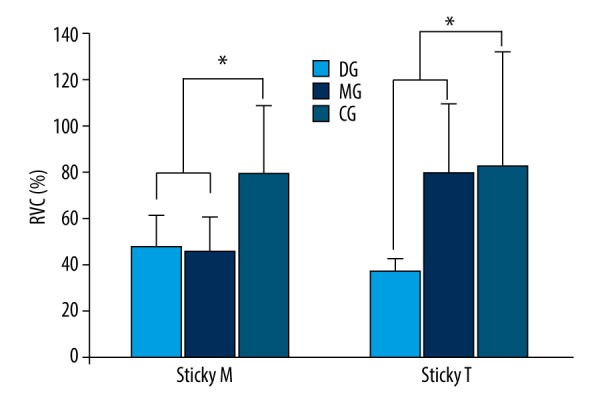
Comparison of muscle activity during mastication of sticky food (* p<0.05). Sticky M – masseter muscle activity during mastication of sticky food; Sticky T – temporalis muscle activity during mastication of sticky food; DG – Temporomandibular Dysfunction Group; MG – Temporomandibular Joint Malalingment Group; CG – Control Group.
Table 3.
Comparison of muscle activity during mastication of sticky food.
| Muscle activity | F | p-Value | Post hoc | ||
|---|---|---|---|---|---|
| Masseter | DG (n=7) | 48.05±13.69 | 5.824 | 0.012 | AB|C |
| MG (n=8) | 46.04±14.55 | ||||
| CG (n=5) | 79.95±29.01 | ||||
| Temporalis | DG | 37.50±5.00 | 5.010 | 0.019 | A|C |
| MG | 79.95±29.01 | ||||
| CG | 83.55±48.39 |
Values are expressed as mean±standard deviation. DG – Temporomandibular Dysfunction Group; MG – Temporomandibular Joint Malalignment Group; CG – Control Group.
The values of masseter muscle activity measured during mastication of hard food were 38.81% RVC from the DG, 58.74% RVC from the MG, and 66.28% RVC from the CG. The values of temporalis muscle activity were 25.02% RVC from the DG, 61.5% RVC from the MG, and 70.63% RVC from the CG. Based on the experiment findings, the values of masseter muscle and temporalis muscle activities from the MG and CG showed statistically significant differences from that of the DG (p<0.05) (Figure 3, Table 4).
Figure 3.
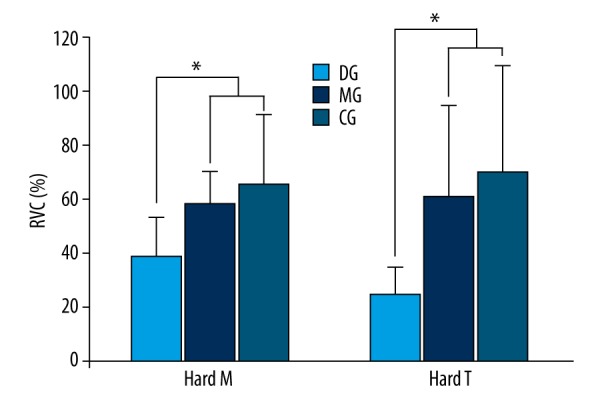
Comparison of muscle activity during mastication of hard food (* p<0.05). Hard M – masseter muscle activity during mastication of hard food; Hard T – temporalis muscle activity during mastication of hard food; DG – Temporomandibular Dysfunction Group; MG – Temporomandibular Joint Malalingment Group; CG – Control Group.
Table 4.
Comparison of muscle activity during mastication of hard food.
| Muscle activity | F | p-Value | Post hoc | ||
|---|---|---|---|---|---|
| Masseter | DG (n=7) | 38.81±14.48 | 4.465 | 0.028 | AB|C |
| MG (n=8) | 58.74±11.77 | ||||
| CG (n=5) | 66.28±25.59 | ||||
| Temporalis | DG | 25.03±15.18 | 4.279 | 0.031 | A|C |
| MG | 61.50±33.69 | ||||
| CG | 70.63±39.97 |
Values are expressed as mean±standard deviation. DG– Temporomandibular Dysfunction Group; MG – Temporomandibular Joint Malalignment Group; CG – Control Group.
Discussion
This study was conducted to compare activities of masseter muscle and temporalis muscle depending on the presence of TMD during mastication of food with diverse textures.
From prior studies, Mapelli et al. [18] suggested that TMD hinders maximum contraction of muscles by affecting activities of muscles, and patients with TMD do not increase masticatory force in order to reduce masticatory movements and muscle pain. Ardizone et al. [25] reported that patients with TMD showed maximum voluntary contraction from the masseter muscle and temporalis muscle, and Peck et al. [26] found that changes in masticatory movements were evident from patients with TMD for a long period of time due to accompanying pain.
The present study was performed determine whether not only TMD, but also malalignment of temporomandibular joint, affects muscle activities. According to the findings from this study, the DG and MG both showed significant differences (p<0.05) in activities of masseter muscle and temporalis muscle during mastication of sticky food, and the MG and CG both showed significant differences (p<0.05) from the DG in activities of masseter muscle and temporalis muscle during mastication of hard food. As a result, it was confirmed that malalignment alone in the temporomandibular joints affected the movements of masseter muscle and temporalis muscle during mastication of sticky food; and it was also verified that activities of the masseter muscle and temporalis muscle were affected by the presence of pain during mastication of hard food.
In previous studies, the groups with TMD were classified through surveys and screening tests of patients. Tosato and Caria [17] subdivided subjects into 4 groups based on RDC-TMD questionnaire and clinical trials, De Felicio et al. [8] established a group with TMD based on a few criteria in addition to the ProTMD multi-part II questionnaire, and Mapelli et al. [18] divided subjects into 3 groups based on results of the ProTMD multi-questionnaire and clinical trials. However, these previous studies did not classify patients with malalignment of temporomandibular joint but without pain. Hence, the present study used the Helkimo index and screening tests to classify TMD. In the present study, the classification methods for TMD used by previous studies were supplemented through 8 question within Helkimo index and 6 categories in the screening test, which enabled our study to classify patients with malalignment of temporomandibular joint but without pain as a Malalignment Group.
Mapelli et al. [18] measured the maximum voluntary mastication of the masseter muscle and temporalis muscle during mastication of sugar-free gum, and De Felicio et al. [18] measured the maximum voluntary contraction of the masseter muscle and temporalis muscle during mastication of sugar-free gum, but these previous studies failed to assess mastication of food with a variety of hardness in daily life. Therefore, the present study focused on measuring activities of the masseter muscle and temporalis muscle during mastication of food with 3 different levels of hardness.
Among some subjects in this study, RVC% data exceeded 100% RVC when muscle activities were measured, showing that subjects did not bite the rubber tube with maximum force during the test of voluntary contraction references. This may be because the changeability among study subjects from all variables of SEMG is caused by the diversity of SMEG characteristics as a result of anatomical and physical factors, such as the thickness of muscle fibers or hypodermal layers, distribution and number of fibers inside the muscle, length of muscle fibers, and timing of muscle contraction [24].
While conducting this study, some participants did not experience any pain despite having malalignment of temporomandibular joints. These participants did not originally plan to go through treatments because of not feeling any pain; however, it was believed that they were able to actually check their conditions of temporomandibular joints through this study, which led them to develop a positive approach toward possible treatments. If further improved diagnostic procedures were developed based on the findings above, we believe that they could serve as a dependable framework for diagnosis of TMD.
Conclusions
This study was conducted to investigate the differences in activities of masseter muscle and temporalis muscle depending on the presence of TMD during mastication of food with various textures.
We found that muscle activities in the masseter muscle and temporalis muscle during mastication of sticky food showed significant differences from the DG and MG compared to that of the CG (p<0.05); and during mastication of hard food, it was also confirmed that muscle activities manifesting from masseter muscle and temporalis muscle displayed significant differences from both the MG and CG compared to the DG (p<0.05).
Our findings show that malalignment affects the movements of temporomandibular joints during mastication of sticky food and affects the movements of temporomandibular joints during mastication of hard food depending on the presence of pain. Therefore, this study presents information on muscle activities manifesting during mastication of food with diverse textures depending on the presence of TMD, and we hope our results will be of use in future related studies and in diagnosis of TMD. To generalize this finding, further studies with larger sample sizes are needed.
Footnotes
Source of support: This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (no. 2017R1C1B5076661)
Conflict of interest
None.
References
- 1.Rani S, Pawah S, Gola S, Bakshi M. Analysis of Helkimo index for temporomandibular disorder diagnosis in the dental students of Faridabad city: A cross-sectional study. J Indian Prosthodont Soc. 2017;17:48–52. doi: 10.4103/0972-4052.194941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okeson JP. Etiology of functional disturbances in the masticatory system. Management of temporomandibular disorders and occlusion. 2013:130–63. [Google Scholar]
- 3.Speciali JG, Dach F. Temporomandibular dysfunction and headache disorder. Headache. 2015;55(Suppl 1):72–83. doi: 10.1111/head.12515. [DOI] [PubMed] [Google Scholar]
- 4.De Kanter RJ, Truin GJ, Burgersdijk RC, et al. Prevalence in the Dutch adult population and a meta-analysis of signs and symptoms of temporomandibular disorder. J Dent Res. 1993;72:1509–18. doi: 10.1177/00220345930720110901. [DOI] [PubMed] [Google Scholar]
- 5.Maixner W, Diatchenko L, Dubner R, et al. Orofacial pain prospective evaluation and risk assessment study – the OPPERA study. J Pain. 2011;12:T4–11.e1–2. doi: 10.1016/j.jpain.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walczynska-Dragon K, Baron S. The biomechanical and functional relationship between temporomandibular dysfunction and cervical spine pain. Acta Bioengin Biomech. 2011;13:93–98. [PubMed] [Google Scholar]
- 7.Calixtre LB, Gruninger BL, Haik MN, et al. Effects of cervical mobilization and exercise on pain, movement and function in subjects with temporomandibular disorders: A single group pre-post test. J Appl Oral Sci. 2016;24:188–97. doi: 10.1590/1678-775720150240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Felício CM, Mapelli A, Sidequersky FV, et al. Mandibular kinematics and masticatory muscles EMG in patients with short lasting TMD of mild-moderate severity. J Electromyogr Kinesiol. 2013;23:627–33. doi: 10.1016/j.jelekin.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359:2693–705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 10.Modi P, Shaikh SS, Munde A. A cross sectional study of prevalence of temporomandibular disorders in university students. Int J Sci Res Publ. 2012;2:1–3. [Google Scholar]
- 11.Choi KH, Kwon OS, Jerng UM, et al. Development of electromyographic indicators for the diagnosis of temporomandibular disorders: A protocol for an assessor-blinded cross-sectional study. Integr Med Res. 2017;6:97–104. doi: 10.1016/j.imr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauer RL, Semidey MJ. Diagnosis and treatment of temporomandibular disorders. Am Fam Physician. 2015;91:378–86. [PubMed] [Google Scholar]
- 13.Sato S, Nasu F, Motegi K. Analysis of kinesiograph recordings and masticatory efficiency after treatment of non-reducing disk displacement of the temporomandibular joint. J Oral Rehabil. 2003;30:708–13. doi: 10.1046/j.1365-2842.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- 14.Santana-Mora U, Cudeiro J, Mora-Bermudez MJ, et al. Changes in EMG activity during clenching in chronic pain patients with unilateral temporomandibular disorders. J Electromyogr Kinesiol. 2009;19:e543–49. doi: 10.1016/j.jelekin.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Ho KY, Ho S, Colletti PM. Use of ultrasonography for assessing treatment efficacy in a case with ankylosis of the temporomandibular joint. J Orthop Sports Phys Ther. 2016;46:225. doi: 10.2519/jospt.2016.0404. [DOI] [PubMed] [Google Scholar]
- 16.Sforza C, Tartaglia GM, Lovecchio N, et al. Mandibular movements at maximum mouth opening and EMG activity of masticatory and neck muscles in patients rehabilitated after a mandibular condyle fracture. J Craniomaxillofac Surg. 2009;37:327–33. doi: 10.1016/j.jcms.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Tosato JDP, Caria PHF. Electromyographic activity assessment of individuals with and without temporomandibular disorder symptoms. J Appl Oral Sci. 2007;15:152–55. doi: 10.1590/S1678-77572007000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mapelli A, Zanandrea Machado BC, Giglio LD, et al. Reorganization of muscle activity in patients with chronic temporomandibular disorders. Arch Oral Biol. 2016;72:164–71. doi: 10.1016/j.archoralbio.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Park M-H, Ko M-Y. Screening evaluation and predicting prognosis of craniomandibular disorder patients with the Solberg questionnaire. Journal of Oral Medicine and Pain. 1984;19:111–23. [Google Scholar]
- 20.Ko M-Y, Kim M-E. Clinical features of the patients with craniomandibular disorders. Journal of Oral Medicine and Pain. 1993:18. [Google Scholar]
- 21.Sohn D-E, Ahn Y-W, Park J-S, Ko M-Y. An epidemiological study of temporomandibular disorders patients by screening questionnaire. Journal of Oral Medicine and Pain. 2000;29:341–51. [Google Scholar]
- 22.Wieckiewicz M, Boening K, Wiland P, et al. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J Headache Pain. 2015;16:106. doi: 10.1186/s10194-015-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mcneill C. Management of temporomandibular disorders: Concepts and controversies. J Prosthet Dent. 1997;77:510–22. doi: 10.1016/s0022-3913(97)70145-8. [DOI] [PubMed] [Google Scholar]
- 24.Remijn L, Groen BE, Speyer R, et al. Reproducibility of 3D kinematics and surface electromyography measurements of mastication. Physiol Behav. 2016;155:112–21. doi: 10.1016/j.physbeh.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Ardizone I, Celemin A, Aneiros F, et al. Electromyographic study of activity of the masseter and anterior temporalis muscles in patients with temporomandibular joint (TMJ) dysfunction: Comparison with the clinical dysfunction index. Med Oral Patol Oral Cir Bucal. 2010;15:e14–19. doi: 10.4317/medoral.15.e14. [DOI] [PubMed] [Google Scholar]
- 26.Peck CC, Murray GM, Gerzina TM. How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust Dent J. 2008;53:201–7. doi: 10.1111/j.1834-7819.2008.00050.x. [DOI] [PubMed] [Google Scholar]


