Abstract
Background
The 2018 Global Initiative for Chronic Obstructive Lung Disease Report reveals that the blood eosinophil count could forecast the risk of flare-ups. This study explored the correlations of blood eosinophils with fractional exhaled nitric oxide (FeNO) and pulmonary function parameters in acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Material/Methods
The data of patients with AECOPD at our hospital admitted between July 2018 and June 2019 were retrospectively analyzed. All patients were stratified into an eosinophilic group (≥2%) or a noneosinophilic group (<2%) based on the peripheral eosinophil count per centum. Cross-sectional analysis was performed to compare clinical characteristics, percentage of eosinophils, FeNO, and pulmonary function between the 2 groups.
Results
After applying the inclusion/exclusion criteria, 247 patients were included. FeNO values were higher in eosinophilic group (n=97) than in noneosinophilic group (n=150) (P=0.005). The forced expiratory volume in 1 second% predicted (FEV1% predicted), FEV1, and forced vital capacity (FVC) were higher in the eosinophilic group than in the noneosinophilic group (P=0.043; P=0.040; and P=0.011, respectively). Blood eosinophilia showed positive correlations with FeNO (P=0.004) and spirometry variables (FEV1 [% predicted], P=0.003; FEV1, P<0.001; and FVC, P<0.001). An FeNO level of 22.5 ppb was the best cutoff value to predict blood eosinophilia (P=0.000).
Conclusions
Blood eosinophil count is a likely biomarker that can predict positive relationship with FeNO values and pulmonary function parameters.
MeSH Keywords: Disease Progression; Eosinophils; Nitric Oxide; Pulmonary Disease, Chronic Obstructive; Respiratory Function Tests
Background
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide, and to has been predicted to rise to the third leading cause of death by 2020 [1]. It is a heterogeneous disease, characterized by incomplete reversible progressive airflow limitation, which is consistent with chronic inflammation-induced permanent structural changes to airways and pulmonary blood vessels [1,2]. Acute exacerbation of COPD (AECOPD) is characterized by rapid aggravation of clinical manifestations, flare-up of airway inflammation, rapid decrease in pulmonary function, and increased risk of death; thus, it also increases socio-economic pressure and reduces the life quality of patients [1,3].
The 2018 Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report suggests that the sputum or blood acidophilic leukocytes per centum could forecast the risk of flare-ups [1]. Based on former research, 28% of AECOPD patients present with hypereosinophilia in the sputum, which could forecast flare-up risk and the effects of glucocorticoid treatment [4]. Despite its simplicity and reliability in evaluating respiratory tract inflammatory reactions, the sputum specimen collection also has some disadvantages in clinical use. For instance, 1) testing can become complicated and cannot be applied at the bedside; 2) specific requirements for patients’ physical conditions; 3) high professional and technical ability of the operator required; and 4) it may pose other complications.
Therefore, simple, easy methods of clinical examination, including blood peripheral eosinophilic evaluation and fractional exhaled nitric oxide (FeNO), have attracted increasing attention [5,6]. The FeNO, a noninvasive detection technique, could safely, quickly, and simply determine the eosinophilic respiratory tract inflammatory response [7]. Previous studies have shown that the FeNO aids in the distinction of eosinophilic and noneosinophilic airway inflammation, and may play potential roles in COPD, asthma, and interstitial lung diseases [8–10]. Chou et al. [11] showed that FeNO is positively associated with sputum eosinophils in patients with COPD. However, whether a relationship exists between FeNO and blood eosinophils in patients with AECOPD is unknown.
Accordingly, peripheral blood eosinophil counts/ratio have also been gaining increasing research attention as a potential diagnostic alternative, given their ease of evaluation in clinical practice. Several studies have investigated the influence of eosinophils on airway inflammation in COPD [11–14]. A cutoff point of 2% was used as the defined threshold for blood eosinophilia stratification, which is a very sensitive approach to predict eosinophil-related airway inflammation and eosinophil-related flare-up [4,15]. The study of Choi et al. [16] revealed that the level of forced expiratory volume in 1 second (FEV1) in an eosinophilic group (% of blood eosinophils ≥2%) was markedly higher than that in a noneosinophilic group (% of blood eosinophils <2%) of AECOPD patients. Nevertheless, the aim of their research was to explore the association of blood eosinophil per centum and bacterial infection, but not pulmonary function [16].
Consequently, based on the foregoing information, this cross-sectional project was designed to assess the association of blood eosinophil per centum with FeNO values in with AECOPD patients. We also investigated the correlation between the percentage of blood eosinophils and pulmonary function parameters (i.e., FEV1% predicted, FEV1 and forced vital capacity [FVC]).
Material and Methods
Protocol design
The present project was a cross-sectional research (Figure 1). The included patients with AECOPD were stratified into an eosinophilic group (≥2%) and a noneosinophilic group (<2%), based on their peripheral eosinophil cell counts. The data, which linked real-world clinical and medical administrative records, were derived from routine clinical practice and medical procedures, respectively.
Figure 1.
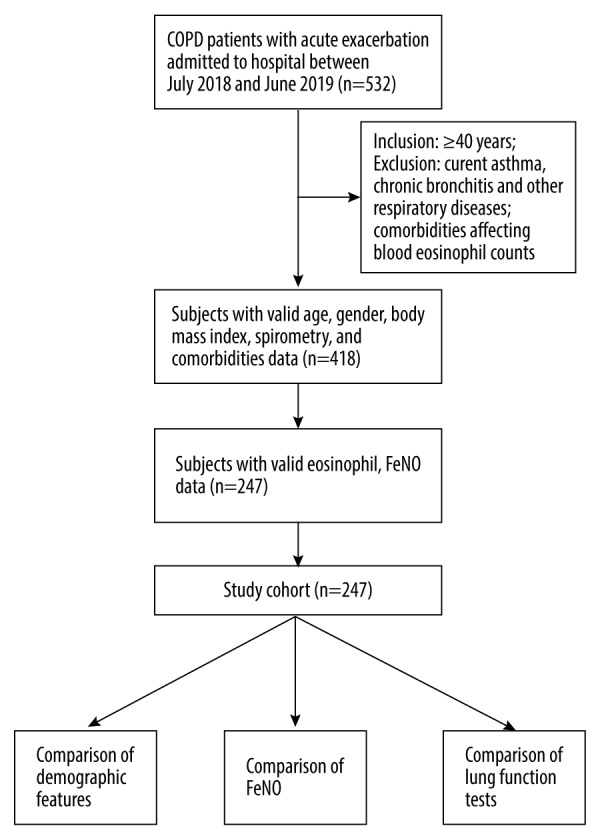
Flow chart showing the screening procedure for the participants.
Data sources and patients
We made the data extraction of the study based on the electronic medical database at Jiangxi Provincial People’s Hospital. We retrospectively reviewed and analyzed the medical demographic features and baseline clinical records of subjects involved in the study. We also collected the following postbronchodilator values of the pulmonary function test (PFT): % predicted FEV1, FEV1, FVC, and FEV1/FVC (%). Tests for FeNO, other laboratory tests, and spirometry measurements were conducted within 24 hours of admission, all on the same day.
Study patients
Inclusion and exclusion criteria
All patients hospitalized for AECOPD (diagnosed according to the 2018 GOLD guidelines [1]) from July 2018 to June 2019 were included. Patients were no younger than 40 years. Patients were excluded if: 1) valid data on demographic and/or clinical characteristics or outcome records (including FeNO levels, PFT results, and peripheral blood cell counts/ratios) were absent; 2) they had one or more of the following diseases, e.g., acute pulmonary edema, pulmonary tuberculosis, interstitial lung disease, pneumothorax, lung cancer, asthma-COPD, acute cardiac dysfunction, or amnesia; 3) they had a comorbidity that could affect the blood eosinophilic granulocyte per centum, such as allergic diseases, parasitic infections/diseases, cancer, or any dermatological, hematological, infectious, or autoimmune disease; or 4) they had undergone any steroid or antibiotic treatments 4 weeks prior to hospitalization.
Assessments and study outcomes
Blood collection and analysis: peripheral venous blood eosinophilic count/ratio analysis was performed using an Automated Hematology Blood Analyzer (Abbott Cell-Dyn3700; Abbott, USA) and its imported high-quality matching reagents.
FeNO
Measurements of FeNO levels were performed before the PFT, using an exhaled nitric oxide determination system (NIOX MINO, sensor model TK300/500, Aerocrine AB, Solna, Sweden), in accordance with the instruction for standardized FeNO procedures [17]. Participants were required no eating, drinking, or smoking for 1 hour or longer, before the FeNO tests. Briefly, patients were required to breathe in exogenous nitric oxide-free air to the maximum lung capacity, after which they had to breathe out with a velocity of 50 mL/s, which lasted for 6–10 seconds. The expiratory pressure was controlled at 10–20 cmH2O, and the nitric oxide analyzer automatically calculated the required values.
Spirometry
Airflow limitation was calculated using spirometry (MasterScreen Pneumo PC spirometer; JAEGER; Germany) to measure post-bronchodilator FEV1, FVC, and FEV1/FVC, according to the manufacturer’s instructions [18]. In addition, based on the reported reference equations, we computed the prediction percentage values (% predicted) [19]. All patients were required to undergo PFT in a reproducible manner, to obtain optimal results.
Ethics approval
We performed the present protocol in compliance with the tenets of the Helsinki Declaration, and got endorsement from our hospital’s ethics committee. Considering the retrospective design of the study, the hospital’s ethics committee exempted the need for informed consent; however, all identifying patient data were anonymized.
Statistical analysis
Data analysis was conducted by SPSS 25. We described the normally distributed data of continuous variables as mean±standard deviation (SD) and conducted t-tests for comparisons. We expressed nonparametric data in median (interquartile range) and employed Mann-Whitney U test for comparisons. The comparisons between classification variables using chi-squared test. The relationship of blood eosinophilic cell per centum with FeNO and lung function was calculated by means of the Spearman’s rank correlation coefficient. The correlation of FeNO and blood eosinophil per centum was determined by performing receiver operating characteristic (ROC) curve calculation. The optimum cutoff point was identified by the maximum sum of the sensitivity and specificity. For all analyses, statistical difference was defined at P<0.05.
Results
Clinical features of participants
This present study flowchart is shown in Figure 1. The demographic and clinical characteristic data of all participants enrolled in this project are shown in Table 1. In our retrospective cross-sectional study, 247 eligible participants with AECOPD were included. The median age was 72.0 years (67.0–79.0 years). Approximately 78.1% (193 patients) were male, and almost all participants were either a current-smoker or an ex-smoker (78 out of 169 patients) with a median pack-years of 30.0 (range: 0.0–40.0 pack-years). As expected, patients had several comorbidities such as coronary artery disease (15.8%), high blood pressure (32.0%), and diabetes (6.5%).
Table 1.
Demographic features and baseline characteristics of present research population.
| Variable | Total (n=247) | Noneosinophilic* (n=150) | Eosinophilic** (n=97) | P-value# |
|---|---|---|---|---|
| Age (years) | 72.0 (67.0, 79.0) | 72.0 (67.0, 79.0) | 72.0 (65.0, 80.0) | 0.814 |
| Males, n (%) | 193 (78.1) | 119 (79.3) | 74 (76.3) | 0.572 |
| Body mass index, kg/m2, Mean (SD) | 21.5 (3.7) | 22.4 (3.4) | 22.3 (4.0) | 0.860 |
| Smokers, n (%) | 173 (70.0) | 108 (72.0) | 65 (67.0) | 0.403 |
| Pack years | 30.0 (0.0, 40.0) | 23.0 (0.0, 40.0) | 30.0 (0.0, 40.0) | 0.115 |
| FeNO, levels, ppb, mean (SD) | 27.2 (20.7) | 23.9 (15.2) | 32.3 (26.3) | 0.005 |
| Postbronchodilator FEV1 (% predicted) | 37.0 (27.0, 49.0) | 34.5 (26.0, 48.3) | 40.0 (30.0, 49.0) | 0.012 |
| Postbronchodilator FEV1 (L) | 0.74 (0.57, 0.98) | 0.72 (0.56, 0.97) | 0.82 (0.63, 1.04) | 0.010 |
| Postbronchodilator FVC (L) | 1.39 (1.07, 1.76) | 1.30 (1.06, 1.69) | 1.52 (1.19, 1.82) | 0.011 |
| Postbronchodilator FEV1/FVC (%) | 53.0 (49.0, 65.0) | 53.0 (48.0, 65.0) | 53.0 (50.0, 66.0) | 0.563 |
| Comorbidities | ||||
| Coronary artery disease, n (%) | 39 (15.8) | 21 (14.0) | 18 (18.6) | 0.338 |
| Hypertension, n (%) | 79 (32.0) | 52 (34.7) | 27 (27.8) | 0.261 |
| Diabetes, n (%) | 16 (6.5) | 9 (6.0) | 7 (7.2) | 0.704 |
Refers to blood eosinophil count <2%;
Refers to blood eosinophil count ≥2%.
P-value is for eosinophilic group vs. noneosinophilic group.
SD – standard deviation; FeNO – fractional exhaled nitric oxide; ppb – parts per billion; FEV1 – forced expiratory volume in one second; FVC – forced vital capacity.
Spirometry measurements are presented in Table 1. All patients had severe lung impairment, and the median postbronchodilator FEV1 (% predicted), FEV1 (L), FVC (L), and FEV1/FVC (%) values were 37.0 (range: 27.0–49.0); 0.74 (range: 0.57–0.98); 1.39 (range: 1.07–1.76); and 53.0 (range: 49.0–65.0), respectively. The patient population had mean ± SD FeNO levels of 27.2 ± 20.7 parts per billion (ppb).
Considering a threshold value of ≥2% for the per centum of eosinophillic granulocytes in blood, 97 patients (39.2%) were classified as having peripheral blood eosinophilia, and the remaining 150 patients (60.8%) with peripheral blood noneosinophilia.
FeNO and lung function parameters
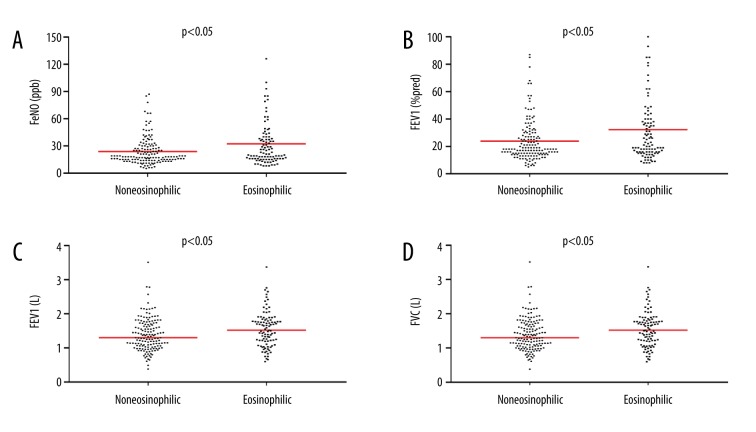
We compared the primary parameters using 2% as peripheral blood eosinophil percentage. The eosinophilic group showed significantly higher FeNO values than the noneosinophilic group (mean [SD] was 32.3 [26.3] ppb versus 23.9 [15.2] ppb, P=0.005) (Table 1, Figure 2A).
Figure 2.
Scatter plots for comparison between the noneosinophilic group (<2% blood eosinophils) and eosinophilic group (≥2% blood eosinophils). (A) Comparison of FeNO levels. (B) Comparison of FEV1 (% predicted) levels. (C) Comparison of FEV1 levels. (D) Comparison of FVC levels. FeNO – fractional exhaled nitric oxide; FEV1 – forced expiratory volume in one second; FVC – forced vital capacity.
The postbronchodilator% predicted FEV1 levels in eosinophilic patients were markedly higher in comparison with those in noneosinophilic patients (40.0% [range: 30.0–49.0%] versus 34.5% [range: 26.0–48.3%], P = 0.043) (Table 1, Figure 2B). Furthermore, eosinophilic patients had significantly higher levels of FEV1 and FVC than noneosinophilic patients (0.82 L (range: 0.63–1.04 L) versus 0.72 L (range: 0.56–0.97 L), P=0.040; 1.52 L (range: 1.19–1.82 L) versus 1.30 L (range: 1.06–1.69 L), P=0.011, respectively) (Table 1, Figure 2C, 2D). However, no significant differences were noted in FEV1/FVC (P=0.563) between the 2 groups (Table 1).
Correlations among blood eosinophilic granulocyte numbers, FeNO levels, and pulmonary function parameters
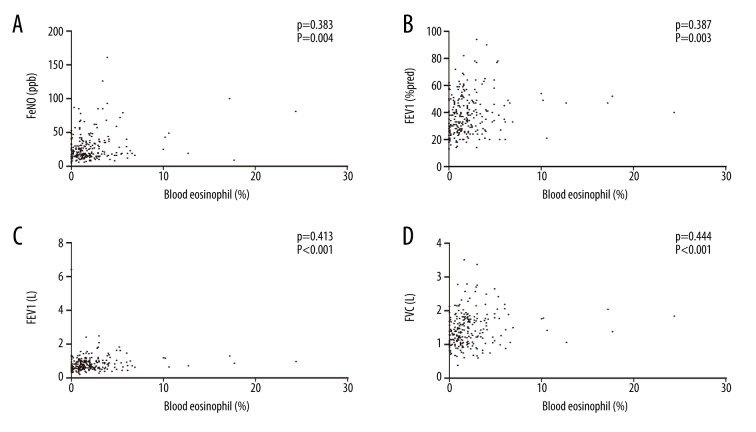
A markedly positive correlation was observed between the number of eosinophilic granulocytes in blood and FeNO values (P=0.383; P=0.004) (Figure 3A). We also observed a significant correlation of blood eosinophilia and FEV1 (% predicted) in AECOPD (P=0.387; P=0.003) (Figure 3B). Similarly, blood eosinophilic counts were associated with both FEV1 (P=0.413; P<0.001) (Figure 3C) and FVC (P=0.444; P<0.001) (Figure 3D).
Figure 3.
Scatter plots for correlations among blood eosinophil count (%), FeNO levels, and pulmonary function parameters. (A) Correlation of blood eosinophil per centum and FeNO values (ppb). (B) Correlation of blood eosinophil per centum and FEV1 (% predicted). (C) Correlation of blood eosinophil per centum and FEV1 (L). (D) Correlation of blood eosinophil per centum and FVC (L). FeNO – fractional exhaled nitric oxide; ppb – parts per billion. FEV1 – forced expiratory volume in one second; FVC – forced vital capacity.
However, no significant correlation was observed between the blood eosinophilic granulocyte count and the FEV1/FVC (P=0.092; P=0.148). The ROC curve analysis showed that an FeNO level of 22.5 ppb was the best cutoff value to predict blood eosinophilia, the area under the curve (AUC) = 0.73; 95% confidence interval=0.67–0.79, P=0.000). The sensitivity and specificity for predicting blood eosinophilia at an FeNO level of 22.5 ppb were 77.3% and 60.0%, respectively (Figure 4).
Figure 4.
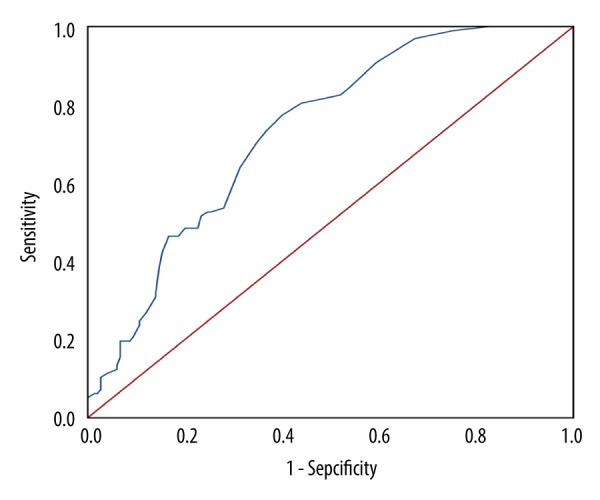
Receiver operating characteristic curve to predict blood eosinophilia (≥2% blood eosinophils) using FeNO values. FeNO – fractional exhaled nitric oxide.
Discussion
The current study was conducted using a real-world electronic medical database and retrospectively analyzed the relationship among peripheral blood eosinophils, lung function parameters, and FeNO values (n=247). We analyzed detailed information regarding patients with AECOPD. The following are the 3 main findings of the study: first, 97 patients (39.2%) had a peripheral blood eosinophilic percentage ≥2%. This may provide important information for future large-scale surveys of blood eosinophil levels. Second, the results revealed that FeNO levels were significantly higher among eosinophilic patients than noneosinophilic patients. This indicates that FeNO may be a good marker to identify eosinophilic inflammation in patients with AECOPD. Third, patients in the eosinophilic group showed better pulmonary function (FEV1 [% predicted], FEV1 [L], FVC [L]), which suggests that different levels of blood eosinophils may independently influence the PFT results.
Previous studies on peripheral blood eosinophils have been mainly focused on stable COPD [20–23]. Hence, studies evaluating the latent role of blood eosinophilic granulocytes in patients with AECOPD are scarce. Several studies have reported that up to 40% of patients with AECOPD show ≥2% blood eosinophils at admission [24–27]. Bélanger et al. [28] reported that 36% of AECOPD patients have confirmed blood eosinophilia, which is consistent with our present findings.
In contrast, DiSantostefano et al. [29], in their cross-sectional study based on an American COPD population survey cohort (n=948), showed that up to 70% of adults with COPD in the United States have >2% blood eosinophil levels. The disparity in these results may be related to differences in the inclusion/exclusion criteria employed by the studies. For instance, unlike the National Health and Nutrition Examination Survey 2007–2010, DiSantostefano et al. [29] based their findings on a study population comprising patients with spirometry-defined COPD, including patients with asthma. However, our study participants did not show the asthma-COPD overlap syndrome.
The ECLIPSE study [30] reported that an eosinophilic group tends to have higher FEV1, which is consistent with our findings. Furthermore, our study evaluated a greater number of pulmonary function parameters. However, the observation of elevated FEV1 in eosinophilia is not consistent with the findings of the Copenhagen General Population Study, which reported no differences in FEV1 among individuals with different blood eosinophil counts [31]. Considering the Copenhagen General Population Study used 3.3% (340 cells/μL) as the blood eosinophil cutoff point for patient stratification, it is likely that different cutoff points could affect the correlations observed with PFT parameters.
According to the 2015 recommendation, the FeNO level is significantly increased if its value is ≥32 ppb [32]. The mean FeNO level of the eosinophilic group in the present study was 32.3 ppb. This suggests a considerably high level of FeNO in patients with eosinophilia, and a positive correlation between blood eosinophilic granulocyte count and FeNO values.
Furthermore, the present results also provide evidence of a positive correlation between peripheral blood eosinophilic percentage and FeNO in AECOPD. However, Gao et al. [33] reported that blood eosinophil percentage has no significant correlation with FeNO in patients with AECOPD. The disparity in these results might be related to different eosinophil thresholds. In the Gao et al. study, eosinophilia was defined as the percentage of eosinophils in the blood ≥1%, while the threshold for eosinophilia in the present study was ≥2% [33].
This study has several limitations. First, strict inclusion and exclusion criteria were applied before finalizing the study design, to ensure reliable results. Only patients with AECOPD and complete available data were included, leading to a relatively small sample size. In addition, our findings should be cautiously extrapolated to patients with AECOPD worldwide, considering that all our patients were Chinese. Finally, considering the single-center, retrospective nature of the study, there were no consecutive measurements of any study variable. However, this limitation may have had only a negligible effect on the results.
Larger, more appropriate multicenter prospective surveys should be undertaken in the future to more reliably and comprehensively evaluate the clinical significance of peripheral blood eosinophils in the AECOPD phenotype. These studies should be specifically designed to evaluate the clinical relevance of peripheral blood eosinophils to lung function and FeNO levels.
Conclusions
This study using real-world medical data provides useful and comprehensive evidence that blood eosinophil count may be a biomarker to predict a positive relationship with FeNO values and lung function variables (i.e., FEV1% predicted, FEV1, and FVC) in patients with AECOPD. The findings are clinically significant and will likely aid in the advancement of future therapy and management of AECOPD. Larger, long-term trials are warranted in the future.
Footnotes
Source of support: This study was supported by a grant from the Jiangxi Province Science and Technology Support Plan (Grant No. 20141BBG70045)
Conflicts of interest
None.
References
- 1.Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. Available from URL: http://www.goldcopd.org.
- 2.Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:478–85. doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman EK. Exacerbations in chronic obstructive pulmonary disease: Do they contribute to disease progression? Proc Am Thorac Soc. 2007;4:586–90. doi: 10.1513/pats.200706-068TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–71. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 5.Pavord ID, Bafadhel M. Exhaled nitric oxide and blood eosinophilia: Independent markers of preventable risk. J Allergy Clin Immunol. 2013;132:828–29. doi: 10.1016/j.jaci.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, Fe(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70:115–20. doi: 10.1136/thoraxjnl-2014-205634. [DOI] [PubMed] [Google Scholar]
- 7.Donohue JF, Herje N, Crater G, Rickard K. Characterization of airway inflammation in patients with COPD using fractional exhaled nitric oxide levels: A pilot cut-study. Int J Chron Obstruct Pulmon Dis. 2014;16:745–51. doi: 10.2147/COPD.S44552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavord ID, Shaw DE, Gibson PG, Taylor DR. Inflammometry to assess airway diseases. Lancet. 2008;372:1017–19. doi: 10.1016/S0140-6736(08)61421-X. [DOI] [PubMed] [Google Scholar]
- 9.Ricciardolo FL, Sorbello V, Ciprandi G. FeNO as biomarker for asthma phenotyping and management. Allergy Asthma Proc. 2015;36:e1–8. doi: 10.2500/aap.2015.36.3805. [DOI] [PubMed] [Google Scholar]
- 10.Cameli P, Bargagli E, Bergantini L, et al. Evaluation of multiple-flows exhaled nitric oxide in idiopathic and non-idiopathic interstitial lung disease. J Breath Res. 2019;13 doi: 10.1088/1752-7163/ab0233. 026008. [DOI] [PubMed] [Google Scholar]
- 11.Chou KT, Su KC, Huang SF, et al. Exhaled nitric oxide predicts eosinophilic airway inflammation in COPD. Lung. 2014;192:499–504. doi: 10.1007/s00408-014-9591-8. [DOI] [PubMed] [Google Scholar]
- 12.Soter S, Barta I, Antus B. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation. 2013;36:1178–85. doi: 10.1007/s10753-013-9653-8. [DOI] [PubMed] [Google Scholar]
- 13.Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: A surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–504. doi: 10.2147/COPD.S100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brightling CE. Clinical applications of induced sputum. Chest. 2006;129:1344–48. doi: 10.1378/chest.129.5.1344. [DOI] [PubMed] [Google Scholar]
- 15.Landis S, Suruki R, Maskell J, et al. Demographic and clinical characteristics of COPD patients at different blood eosinophil levels in the UK clinical practice research datalink. COPD. 2018;15:177–84. doi: 10.1080/15412555.2018.1441275. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Oh JY, Lee YS, et al. The association between blood eosinophil percent and bacterial infection in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:953–59. doi: 10.2147/COPD.S197361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 18.Standardization of spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Park HY, Lee H, Koh WJ, et al. Association of blood eosinophils and plasma periostin with FEV1 response after 3-month inhaled corticosteroid and long-acting beta2-agonist treatment in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;11:23–30. doi: 10.2147/COPD.S94797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eltboli O, Mistry V, Barker B, Brightling CE. Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology. 2015;20:667–70. doi: 10.1111/resp.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: A randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–42. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 24.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:39–47. doi: 10.2147/copd.2006.1.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bafadhel M, Davies L, Calverley PM, et al. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: A further analysis. Eur Respir J. 2014;44:789–91. doi: 10.1183/09031936.00062614. [DOI] [PubMed] [Google Scholar]
- 26.Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151:366–73. doi: 10.1016/j.chest.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa K, Camargo CA. Prevalence of blood eosinophilia in hospitalized patients with acute exacerbation of COPD. Respirology. 2016;21:761–64. doi: 10.1111/resp.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bélanger M, Couillard S, Courteau J, et al. Eosinophil counts in first COPD hospitalizations: A comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045–54. doi: 10.2147/COPD.S170743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. 2016;112:88–96. doi: 10.1016/j.rmed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 31.Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in COPD: The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193:965–74. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 32.The Chinese national guidelines on diagnosis and management of cough (2015) Chin Med J. 2016;39:321–39. [PubMed] [Google Scholar]
- 33.Gao J, Zhang M, Zhou L, et al. Correlation between fractional exhaled nitric oxide and sputum eosinophilia in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1287–93. doi: 10.2147/COPD.S134998. [DOI] [PMC free article] [PubMed] [Google Scholar]