Abstract
Recent work using novel neuroimaging methods has revealed shorter white matter fiber bundle length (FBL) in older compared to younger adults. Shorter FBL also corresponds to poorer performance on cognitive measures sensitive to advanced age. However, it is unclear if individual factors such as cognitive reserve (CR) effectively moderate the relationship between FBL and cognitive performance. This study examined CR as a potential moderator of cognitive performance and brain integrity as defined by FBL. Sixty-three healthy adults underwent neuropsychological evaluation and 3T brain magnetic resonance imaging. Cognitive performance was measured using the Repeatable Battery of Assessment of Neuropsychological Status (RBANS). FBL was quantified from tractography tracings of white matter fiber bundles,derived from the diffusion tensor imaging. CR was determined by estimated premorbid IQ. Analyses revealed that lower scores on the RBANS were associated with shorter whole brain FBL (p = 0.04) and lower CR (p = 0.01) CR moderated the relationship between whole brain FBL and RBANS score (p < 0.01). Tract-specific analyses revealed that CR also moderated the association between FBL in the hippocampal segment of the cingulum and RBANS performance (p = 0.03). These results demonstrate that lower cognitive performance on the RBANS is more common with low CR and short FBL. On the contrary, when individuals have high CR, the relationship between FBL and cognitive performance is attenuated. Overall, CR protects older adults against lower cognitive performance despite age-associated reductions in FBL.
Keywords: Cognition, Aging, Neuropsychological assessment, Diffusion tensor imaging, RBANS
Introduction
The aging brain exhibits extensive micro and macroscopic changes that can lead to cognitive and functional impairment. A number of recent studies on aging have focused on the role of white matter in this relationship. The cerebral white matter undergoes age-related degenerative changes including damage to myelin and loss of axonal fibers (Peters 2002; Marner et al. 2003; Schmidt et al. 2011). These anatomical changes are readily quantified at a macroscopic, gross level using structural magnetic resonance imaging (MRI; Beaulieu 2002; Hagman et al. 2006; Soares et al. 2013; Sun et al. 2005), and correlate with reduced cognitive performance (Peters 2002; Madden et al. 2009; Salat et al. 2005; Voineskos et al. 2012).
Diffusion tensor imaging (DTI) provides an ideal method to study white matter integrity (Filippi et al. 2001; Jeong et al. 2011) at a microstructural level. Many DTI studies utilize scalar metrics, such as fractional anisotropy (FA) and mean diffusivity (MD) to characterize water diffusion within a voxel. The values of these and other scalar metrics in each image voxel reflect the speed of water diffusion and the extent to which it is directionally restricted by the microstructural anatomy of the underlying tissue. DTI tractography can be used to measure the orientation of white matter fiber bundles reflecting neuronal fiber pathways (Conturo et al. 1999; Mori et al. 1999).
More recently, quantitative tractography based on DTI (qtDTI) has been developed to assess cerebral white matter integrity using both scalar metrics and tractography. qtDTI metrics such as fiber bundle length (FBL) are effective in detecting tract specific alterations that may be distributed anywhere along the tractography model (Correia et al. 2008). FBL represents the length of coherent bundles of nerve fibers and can beused to detect specific diffusivity properties along a fiber bundle. This method is sensitive to to reductions in white matter microstructural integrity (Correia et al. 2008). Shorter FBL is believed to reflect axonal loss with possible contributions from demyelination, gliosis, or other pathological processes that commonly occur in older adults (Correia et al. 2008).
Previous studies utilizing qtDTI have observed significant negative associations between age and FBL in healthy older individuals (Baker et al. 2014; Bolzenius et al. 2013; Salminen et al. 2013). These findings are consistent with post-mortem data demonstrating reduced fiber lengths with advanced age (Marner et al. 2003; Tang et al. 1997). A prior study also revealed a significant relationship between FBL and cognitive performance in healthy adults over the age of 50 (Behrman-Lay et al. 2014). However, it is unclear whether individual factors protect against lower cognitive function in the context of reduced FBL in older adults.
Prior studies have identified important variables that moderate the relationship between neuroimaging abnormalities and neurocognitive performance, with a particular focus on cognitive reserve (CR; Kesler et al. 2003; Sánchez et al. 2002; Satz 1993; Lane et al. 2011; Sawrie et al. 2000; Stern et al. 1996). CR theory has been proposed to explain the discrepancy between individuals with similar degrees of neuropathology, but with varying levels of cognitive performance (Stern 2002). The role of CR has been widely studied in relation to neuropsychological manifestations of subcortical white matter hyperintensities (WMH) defined as T2 hyperintense markers and believed to represent vascular dysfunction in healthy older adults (Brickman et al. 2011; Dufouil et al. 2003; Nebes et al. 2006; Murray et al. 2011). Studies have reported that CR moderates the relationship between subcortical WMH and cognition in older individuals (Brickman et al. 2011; Dufouil et al. 2003; Nebes et al. 2006; Lane et al. 2011; Murray et al. 2011). Yet, little research has focused on the impact of CR on the relationship between cognition and the microstructural integrity of cerebral white matter independent of WMH in healthy adults (Arenaza-Urquijo et al. 2011). Importantly, no studies have examined the impact of CR on the relationship between cognition and FBL. This is an important gap in the literature as aging exerts both vascular and nonvascular effects on the brain.
The purpose of the current study was to investigate the relationships between CR, FBL, and cognitive performance in a sample of healthy adults over age 50. Specifically, we examined whether CR moderated the relationship between FBL and cognitive performance. We hypothesized individuals with higher CR would demonstrate better cognitive performance despite similar whole brain FBL, compared to individuals with lower CR. As a secondary aim, we evaluated the relationships between CR, cognitive performance, and FBL in specific white matter association tracts including the cingulate gyrus segment of the cingulum (CGC), hippocampal segment of the cingulum (CGH), inferior fronto-occipital fasiculus (IFOF), and uncinate fasiculus (UF). Premorbid IQ (using the Wide Range Achievement Test-4- reading subtest; WRAT-4) was used as our measure of CR.
Methods
Participants
Sixty-three individuals enrolled in a study of healthy cognitive aging were included in the present study. Participants were recruited from the local community using print, radio, and direct outreach. Participants were also recruited from the Research Participant Registry of the Washington University Institute of Clinical and Translational Sciences (ICTS).
The study was approved by the affiliated Institutional Review Board and all participants provided informed consent. Data included in this manuscript were obtained in compliance with the Helsinki Declaration. Participants were required to be English speaking and over the age of 50. Exclusion criteria consisted of a history of neurological disease including dementia, stroke, Parkinson’s disease, or other conditions that could impact mental status. Participants with diabetes requiring treatment, head injury with loss of consciousness greater than 5 min, alcohol or drug abuse, or a significant Axis I or II psychiatric condition (e.g., schizophrenia, bipolar disorder, current severe depression) were also excluded. Individuals with scores below 24 on the Mini-Mental State Examination were excluded. A physician evaluated all imaging scans to exclude individuals with gross radiological abnormalities.
Neuroimaging acquisition
MRI acquisitions were obtained using a head-only Magnetom Allegra 3T MRI scanner at Washington University in St. Louis. The scanner is optimized for head imaging, with high-performance head-only gradients capable of 400 T/m/s slew rate, and 40 mT/m gradient strength on each axis simultaneously, with 100 % duty cycle. The gradients enable rapid echoplanar readout and efficient diffusion encoding with relatively short echo time (TE) and scan time, without signal-to-noise (SNR) tradeoffs (e.g., parallel imaging). Quality assurance was conducted daily to ensure data fidelity. Movement was limited by application of surgical tape across the forehead, and headphones that closely fit in head coil. Each scanning session began with a scout scan consisting of three orthogonal planes to confirm head positioning. For quality assurance, identical pulse sequences and movement minimization tactics (e.g., tape across the forehead) were used throughout the course of the study. Automated high-order shimming was utilized.
Structural MRI acquisitions
Whole brain structural scans were obtained using a T1-weighted magnetization-prepared rapid-acquisition gradient echo (MP-RAGE) sequence (Mugler and Brookeman 1990); a double-echo proton-density (PD)/T2-weighted turbo spin echo (TSE) sequence; and a T2-weighted fluid-attenuated inversion-recovery (FLAIR) TSE sequence (Hajnal et al. 1992).
Diffusion-Weighted Imaging (DWI) acquisition
Axial DWI was acquired using a customized single-shot multislice echo-planar tensor-encoded pulse sequence designed and implemented in-house. Thirty-one non-collinear diffusion-encoded directions were used in the acquisition consisting of 24 main directions (diffusion weighting of b = 996 s/mm2) (Conturo et al. 1996). Additionally, we used a “core” of tetrahedral- perpendicular directions (b = 1412 and 680 s/mm2, respectively) and 5 I0 acquisitions (b ~ 0) for wide directional coverage and signal-to-noise (SNR) efficiency to cancel eddy-current effects. Pulse sequence and acquisition parameters were optimized for tractography, and attention was paid to high SNR to prevent track foreshortening (Lori et al. 2002). The TE was 86.2 ms with full-Fourier acquisition (maximizing SNR and minimizing artifact). In a TR of 7.82 s, 64 contiguous 2.0-mm slices were acquired for each contrast. The acquisition matrix was 128 × 128 with a 256 × 256 mm FOV (isotropic 2.0 × 2.0 × 2.0 mm voxels). Signal averaging (72 total acquisitions) was acquired over two scan repeats, and all unedited data were included in the analyses. Raw data were saved to the operating system disk, and floating-point DWI images were custom reconstructed using a SunFire V880 computer server.
Quantitative diffusion tractography
Each individual’s DWI images and b-vectors were registered to the I0 image using FSL’s FLIRT (mutual information metric) (Jenkinson et al. 2002) to correct for subject motion. Brain tissue was extracted with FSL’s Brain Extract Tool (Smith 2002). Tensors and fractional anisotropy (FA) values were reconstructed using linear least squares fitting. Whole brain tractography was obtained by streamline integration with trilinear interpolation of the DWI and linear least squares fitting of the diffusion tensor. Tracking was performed with the tensors’s principal eigenvector with the following parameters: one random seed per voxel, second-order Runge-Kutta integration, an angle threshold of 35 degrees, an FA threshold of 0.15, and a minimum-length threshold of 10 mm.
FA images were registered with the Johns Hopkins University (JHU) white matter atlas using affine registration and FSL FLIRT to examine anatomical features of specific tracts. Tracts including the CGC, CGH, IFOF, and UF were selected due to previously established associations with global cognition (Baek et al. 2013; Booth et al. 2013; Cremers et al. 2016). Each tract was modeled separately by hemisphere and streamlines were selected for inclusion in each bundle using the JHU atlas regions-of-interest. Fibers were included in the bundle if at least 80 % of the arc length was contained in the bundle mask, and streamlines were culled within 0.8 mm of an existing tract were used to reduce redundancy (Zhang et al. 2003). FBL was computed from the total length of the streamlines included in the bundle. Eight participants failed processing during tract-specific analysis and therefore were only included in the whole brain analysis. FBL was normalized by dividing by total intracranial volume (Correia et al. 2008).
Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)
The RBANS (Randolph 1998) yields five scores including: (1) Immediate Memory-learning verbal information (2) Language-confrontation naming and semantic fluency (3) Visuospatial/Construction- construction of a complex figure and judgment of line orientation (4) Attention- digit span and coding (5) Delayed Memory- recall of verbal learning tasks and complex figure after delay. Raw subtest scores are scaled for each cognitive domain to create index scores. The outcome measures for each subtest are converted to an index score based on standardized performance scores by age. Index scores are combined to create an overall standard score with a mean of 100 and standard deviation of 15. Higher scores indicate better cognitive functioning.
Wide Range Achievement Test-4 (WRAT-4)
The WRAT-4 (Glutting and Wilkinson 2005) reading subtest score provides a measure of reading achievement and premorbid IQ. Participants read aloud a list of 55 words that increase in complexity throughout the task, and one point is awarded for each correctly pronounced word. Total words correctly pronounced serves as the outcome measure and were converted to age adjusted standard scores. Standard scores with a mean of 100 and standard deviation of 15 were reported. The WRAT-4 standardized score served as the CR variable in the analyses. Previous studies have successfully utilized premorbid IQ as a measure of CR (Bleecker et al. 2007; Brickman et al. 2011; Foley et al. 2012).
Statistical analyses
Primary anlayses
All statistical analyses were conducted using R version 2.14.0. Demographic information is listed in Table 1. An independent samples t-test was utilized to determine potential differences in whole brain FBL, RBANS score, and/or CR between males and females. Males had significantly longer FBL (t = 2.52, p = 0.01), therefore sex was included as a covariate in the primary regression models. No other differences were noted between males and females. Two multiple regressions (main effects and interaction) were completed to determine the relationship between total RBANS score and whole brain FBL, and whether the relationship was moderated by Cr. Prior to regression analyses, whole brain FBL and CR were mean-centered to reduce multicollinearity (Aiken et al. 1991). In the main effects regression model, total RBANS score was regressed onto the mean-centered whole brain FBL and the mean-centered CR variable. An interaction term (FBL*CR) was then added to the regression as a moderator in a second model to determine if the relationship between whole brain FBL and total RBANS score was dependent on the level of CR. An analysis of variance (ANOVA) was utilized to determine the relationship between the two regression models (Chambers 1992). Individuals were then separated into groups based on low (< 1 SD below the mean; low CR group), moderate (−1 SD ≤ CR ≤ 1 SD away from mean; moderate CR group), and high levels of CR (> 1 SD above the mean; high CR group) for display purposes. These cut-offs are arbitrary and sample dependent, but this method of illustrating the effects is well documented (Aiken et al. 1991; Cohen et al. 2003). General linear models were completed to examine the simple slopes for each CR group.
Table 1.
Demographic Information
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Total Sample | ||||
| N = 63 participants (20 males, 43 females) | ||||
| Age (years) | 63.13 | 8.23 | 51 | 81 |
| Education (years)a | 15.52 | 2.53 | 12 | 20 |
| WRAT-4 Standard Score | 107.11 | 9.71 | 88 | 135 |
| RBANS Standard Score | 101.90 | 11.97 | 72 | 129 |
| Whole Brain FBL (mm) sum | 1,105,049 | 193,781 | 748,708 | 1,570,893 |
| Sample with tract specific FBL | ||||
| N = 55 participants (16 males, 39 females) | ||||
| Age (years) | 62.60 | 8.18 | 51 | 81 |
| Education (years)a | 15.20 | 2.54 | 12 | 20 |
| WRAT-4 Standard Score | 106.89 | 9.97 | 88 | 135 |
| RBANS Standard Score | 102.11 | 12.50 | 72 | 129 |
| Cingulate gyrus segment of cingulum FBL (mm) sum | 17,123 | 3930 | 9069 | 25,984 |
| Hippocampal segment of cingulum FBL (mm) sum | 4552 | 1391 | 2292 | 8824 |
| Inferior fronto-occipital fasciculus FBL (mm) sum | 66,318 | 15,410 | 37,658 | 105,177 |
| Uncinate fasciculus FBL (mm) sum | 5892 | 1545 | 2887 | 9239 |
Definitions: RBANS Repeatable Battery for the Assessment of Neuropsychological Status, WRAT-4 Wide Range Achievement Test-4, FBL Fiber bundle length, SD standard deviation
The years of education was determined by the highest level of education completed, in years. Continuing education, trade school credits, and additional degrees at the same level (i.e., two bachelor’s degrees) were not counted toward the final number of education years
Secondary analyses
Secondary analyses were conducted in a subset of participants (n = 55) with FBL quantification in white matter tracts (Table 1). Four independent samples t-tests were utilized to determine potential sex differences in specific white matter tract FBL (CGC, CGH, IFOF, UF) and RBANS performance. Males had significantly longer FBL in the CGC (t = 4.20, p < 0.01) therefore, sex was included as a covariate in the regression models examining the relationships between the CGC, RBANS, and CR. However, there were no differences in FBL between males and females in other white matter tracts (ps > 0.05). The same analytic process from the primary analyses was uiltized to address the tract-specific analyses.
Results
Primary analyses
Primary analyses examined the impact of whole brain FBL and CR on RBANS performance. The main effects regression model of total RBANS score onto whole brain FBL and CR was statistically significant (F(3,59) = 3.88; p = 0.01, R2 = 0.16, f2 = 0.19), with FBL and CR accounting for 16 % of the variance in RBANS performance. Both longer whole brain FBL (t = 2.13, p = 0.04) and higher CR (t = 2.52, p = 0.01) were significantly related to better performance on the RBANS. The overall interaction (FBL*CR) was also statistically significant (F(4,58) = 4.35 p < 0.01, R2 = 0.23, f2 = 0.30), suggesting that the effect of FBL on RBANS differed as a function of CR (simple effects and interaction values listed in Table 2). This model accounted for 23 % of the variance in RBANS performance. The higher R2 (additional 11 % of the variance in RBANS performance) demonstrated that the regression that included the FBL*CR interaction term was a better fit of the data than the model without the interaction and this was confirmed by an ANOVA contrasting the two regression models (F(1,58) = 4.99, p = 0.03, Cohen’s d = 0.56).
Table 2.
Summary of primary interaction regression analysis for the moderating effects of CR on whole brain FBL and RBANS scores
| Variable | Estimate | Std. Error | t value | p value |
|---|---|---|---|---|
| Intercept | 1.04e + 02 | 5.39e + 00 | 19.32 | < 2e–16 |
| Sex | −1.39 + 00 | 3.10e + 00 | −0.45 | 0.655 |
| FBL | 1.42e–05 | 7.54e–06 | 1.89 | 0.064 |
| CR | 4.26e–01 | 1.45e–01 | 2.94 | 0.005** |
| FBL*CR | 1.65e–06 | 7.36e–07 | −2.24 | 0.029* |
N=63. FBL is mean-centered. CR is mean-centered. FBL*CR is the interaction of mean-centered FBL and mean-centered CR
Defintions: FBL Fiber bundle length, CR Cognitive Reserve
p <. 05
p < .01
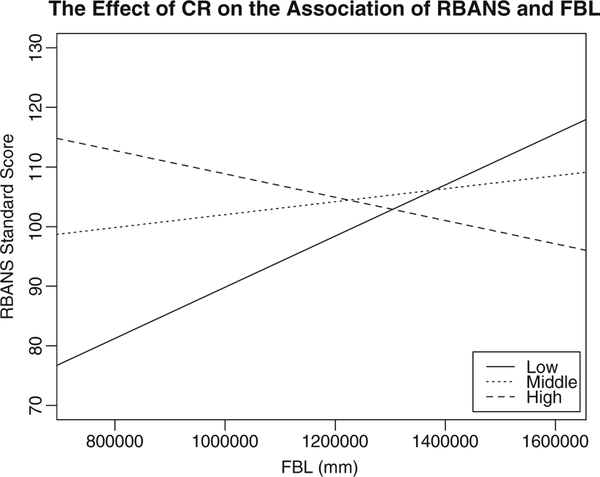
An important point to note for this model is the form of the interaction. Figure 1 illustrates the simple slopes for the relationship between RBANS with varying levels of CR. Furthermore, results of the simple slope analyses (Table 3) revealed that individuals with the lowest CR demonstrated a strong positive relationship between RBANS and whole brain FBL (t = 3.25, p = 0.01). That is, when individuals with lower CR have shorter FBL, they are more likely to demonstrate lower cognitive performance on the RBANS. On the contrary, when individuals have moderate (t = 1.23, p = 0.22) or high CR (t = −0.78, p = 0.48), the relationship between FBL and RBANS scores is attenuated.
Fig. 1.
The effect of CR in the association of RBANS and FBL. Low Cognitive Reserve: solid line (< 1 SD below the mean) Moderate Cognitive Reserve: dotted line (−1 SD ≤ CR ≤ 1 SD from the mean) High Cognitive Reserve: dashed line (> 1 SD above the mean) Definitions: RBANS Repeatable Battery for the Assessment of Neuropsychological Status, FBL Fiber Bundle Length, CR Cognitive Reserve
Table 3.
Simple slope analyses for CR groups
| Variable | Estimate | Sth. Error | t value | p value | 95 % CI | Coefficient |
|---|---|---|---|---|---|---|
| Low CR | 4.29e-05 | 1.32e-05 | 3.25 | 0.01* | 1.30e-05, 7.28e-05 | 4.29e-05 |
| Moderate CR | 1.09e-05 | 8.80e-06 | 1.233 | 0.22 | 6.88e-06, 2.86e-05 | 1.09e-05 |
| High CR | −1.96e-05 | 2.51e-05 | −0.78 | 0.48 | −8.92e-05, 5.01e-05 | −1.95e-05 |
N=63. FBL is mean-centered. FBL*CR is the interaction of mean-centered FBL and trichotomized CR groups
Definitions: CR Cognitive Reserve, CI Confidence Interval
p < 0.05
Secondary analyses
The main effects regression model of total RBANS score onto the CGH and CR demonstrated that FBL in the CGH predicted RBANS performance at a trend level F(2,52) = 2.64; p = 0.08, R2 = 0.09, f2 = 0.09, accounting for 9 % of the variance in RBANS performance. Results indicated that higher CR was associated with better RBANS performance at a trend level (t = 1.77, p = 0.08). However, longer FBL was not significantly associated with better RBANS performance in the CGH (t = 1.00, p = 0.32). The regression with an interaction included (CGH FBL*CR) was significant (F(3, 51) = 3.13 p = 0.03, R2 = 0.16, f2 = 0.19), accounting for 16 % of the variance in RBANS performance. The higher adjusted R2 in the interaction model demonstrated that the regression that included the interaction term was a better fit of the data and this was confirmed by an ANOVA (F(1, 51) = 3.82, p = 0.06, Cohen’s d = 0.53). There were no significant main effects or interactions observed in the CGC, IFOF, UF regression models (p’s > 0.05).
Discussion
In the present study we examined the impact of CR, as estimated by premorbid IQ, on cognition and FBL in healthy older adults. Neuropsychological assessment and neuroimaging were utilized to examine the relationship between cognitive performance and FBL in healthy older adults over the age of 50. Results of the present study confirm prior findings that longer FBL is associated with better cognitive performance (Behrman-Lay et al. 2014). Current findings also demonstrate a positive relationship between cognitive performance and FBL in the CGH. Importantly, these data suggest that CR moderates the relationship between cognition and whole brain FBL. CR also moderates the relationship between cognition and FBL in the CGH. Our study revealed that FBL and cognitive relationships were pronounced among individuals with lower CR, while individuals with higher CR show a more attenuated relationship between cognition and FBL. Specifically, individuals with higher levels of CR had less cognitive impact due to shorter FBL, suggesting that they are better protected against reduced cognitive performance associated with shorter FBL. In contrast, individuals with lower CR had significantly lower cognitive performance with shorter FBL.
Relationships between CR and cognitive aging have been well documented. Engagement in mentally stimulating activities protects against cognitive decline due to aging and disease by promoting synaptic growth early in life (Kesler et al. 2003; Mortimer et al. 2003). The enhancement of neural connections creates efficient cognitive processes that withstand neuropathology and/or the early establishment of these connections may enable an individual to complete a cognitive task by utilizing alternative cognitive processes (Stern 2009). It is also possible that individuals with higher CR engage in more cognitively stimulating activities throughout the lifetime (e.g., occupational attainment, social engagement, or physical activity), which may then lead to promotion of compensatory brain mechanisms.
Conclusions regarding the relationship between CR, FBL, and cognitive performance should be tempered by several limitations of the current study. First, prior research has utilized variables other than premorbid IQ as a proxy of CR, therefore this variable may not be inclusive of all factors that could potentially influence CR. While examination of a variety of factors may provide a more comprehensive understanding of the role of CR on cognition and presumed nonvascular white matter changes with age, premorbid IQ has been widely used as a proxy of reserve in previous studies for several reasons. Specifically, it has been suggested that IQ may reflect cognitive skills acquired in early childhood leading to increased neuronal connections (Stern 2002). Although education level has been widely used in studies of CR, an advantage of using estimated premorbid IQ (i.e., WRAT-4 reading subtest) as a moderator is that this proxy indicator of CR is associated with broader life experiences than formal education. Lastly, the current study is limited in the assessment of executive function. However, we successfully identified relationships between whole brain and tract specific imaging with cognition measured with the RBANS, suggesting sufficient sensitivity of the cognitive battery. Nevertheless, future studies examining the moderation of CR on neuropsychological performance and FBL utilizing a broader range of cognitive tests are warranted, particularly since individual cognitive domains may provide additional understanding of the relationships between CR and neuropsychological performance. Such analyses could also be utilized to assess the relationships between cognitive performance and individual tract integrity to provide specificity to the relationships between FBL, cognitive performance, and CR.
Overall the results of the present study confirm a positive association between whole brain and tract specific FBL and cognitive performance in older adults. Further, findings suggest that individuals with a higher CR are able to sustain greater amounts of white matter alterations than individuals with lower CR. These results support the role of the CR in the protection against lower cognitive performance in the context of reduced white matter integrity among older adults.
Acknowledgments
Funding Supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant number R01 NS052470 and R01 NS039538, National Institutes of Health/National Institute of Mental Health grantR21 MH090494 and R21 MH105822.Recruitmentdatabase searches were supported in part by National Institutes of Health/National Center for Research Resources grant UL1 TR000448.
Footnotes
Compliance with ethical standards
Informed consent All procedures followed were in accordance with the ethnical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinksi Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Conflict of interest Laurie M Baker, David H Laidlaw, Ryan Cabeen, Erbil Akbudak, Thomas E Conturo, Stephen Correria, David F Tate, Jodi M Heaps-Woodruff, Matthew R Brier, Jacob Bolzenius, Lauren E Salminen, Elizabeth M Lane, Amanda R McMichael, and Robert H Paul declare no conflicts of interest.
References
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Arenaza-Urquijo EM, Bosch B, Sala-Llonch R, Solé-Padullés C, Junqué C, Fernández-Espejo D, & Bartrés-Faz D. (2011). Specific anatomic associations between white matter integrity and cognitive reserve in normal and cognitively impaired elders. The American Journal of Geriatric Psychiatry, 19(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Baek SO, Kim OL, Kim SH, Kim MS, Son SM, Cho YW, & Jang SH (2013). Relation between cingulum injury and cognition in chronic patients with traumatic brain injury; diffusion tensor tractography study. NeuroRehabilitation, 33(3), 465–471. [DOI] [PubMed] [Google Scholar]
- Baker LM, Laidlaw DH, Conturo TE, Hogan J, Zhao Y, Luo X, & Paul RH (2014). Impact of advanced age on fiber bundle lengths utilizing diffusion MRI in white matter tracts. Neurology, 83(3), 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. (2002). The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine, 15(7–8), 435–455. [DOI] [PubMed] [Google Scholar]
- Behrman-Lay AM, Usher C, Conturo TE, Correia S, Laidlaw DH, Lane EM, & Paul RH (2014). Fiber bundle length and cognition: a length-based tractography MRI study. Brain Imaging and Behavior, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker ML,Ford DP,Celio MA,Vaughan CG,&Lindgren KN (2007). Impact of cognitive reserve on the relationship of lead exposure and neurobehavioral performance. Neurology, 69(5), 470–476. [DOI] [PubMed] [Google Scholar]
- Bolzenius JD, Laidlaw DH, Cabeen R, Conturo TE, McMichael AR, Lane EM, & Paul RH (2013). Impact of body mass index on fiber bundle lengths among healthy adults. Brain Imaging and Behavior, 7(3), 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T,Bastin ME,Penke L,Maniega SM,Murray C,Royle NA, & Starr JM (2013). Brain white matter tract integrity and cognitive abilities in community-dwelling older people: the Lothian birth cohort, 1936. Neuropsychology, 27(5), 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, & Stern Y. (2011). White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiology of Aging, 32(9), 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JM, Freeny A, & Heiberger RM (1992). Chapter 5 of statistical models in S. Analysis of Variance; Designed Experiments. Wadsworth & Brooks/Cole, Pacific Grove. [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Hillsdale: Erlbaum. [Google Scholar]
- Conturo TE, McKinstry RC, Akbudak E, & Robinson BH (1996). Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magnetic Resonance in Medicine, 35(3), 399–412. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, & Raichle ME (1999). Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy Of Sciences, 96(18), 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia S, Lee SY, Voorn T, Tate DF, Paul RH, Zhang S, & Laidlaw DH (2008). Quantitive tractography metrics of white matter integrity in diffusion-tensor MRI. NeuroImage, 42, 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers LG, de Groot M, Hofman A, Krestin GP, van der Lugt A, Niessen WJ, & Ikram MA (2016). Altered tract-specific white matter microstructure is related to poorer cognitive performance. The Rotterdam Study. Neurobiology of Aging, 39, 108–117. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alperovitch A, & Tzourio C. (2003). Influence of education on the relationship between white matter lesions and cognition. Neurology, 60(5), 831–836. [DOI] [PubMed] [Google Scholar]
- Filippi M, Cercignani M, Inglese M, Horsfield MA, & Comi G. (2001). Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology, 56(3), 304–311. [DOI] [PubMed] [Google Scholar]
- Foley JM, Ettenhofer ML, Kim MS, Behdin N, Castellon SA, & Hinkin CH (2012). Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Applied Neuropsychology: Adult, 19(1), 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glutting J, & Wilkinson G. (2005). Wide range achievement test (WRAT-4). Austin: Pro-Ed. [Google Scholar]
- Hagman P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, & Meuli R. (2006). Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics, 26(suppl_1), S205–S223. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, Bryant DJ, Kasuboski L, Pattany PM, De Coene B, Lewis PD, et al. (1992). Use of fluid attenuated inversion recovery (FLAIR) pulse sequences in MRI of the brain. Journal of Computer Assisted Tomography, 16, 841–844. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S. (2002). Improved optimization for the robust and acute linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jeong H, Kim J, Choi HS, Kim ES, Kim DS, Shim KW, & Lee SK (2011). Changes in integrity of normal-appearing white matter in patients with moyamoya disease: a diffusion tensor imaging study. American Journal of Neuroradiology, 32(10), 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Adams HF, Blasey CM, & Bigler ED (2003). Premorbid intellectual functioning, education, and brain size in traumatic brain injury: An investigation of the cognitive reserve hypothesis. Applied Neuropsychology, 10(3), 153–162. [DOI] [PubMed] [Google Scholar]
- Lane EM, Paul RH, Moser DJ, Fletcher TD, & Cohen RA (2011). Influence of education on subcortical hyperintensities and global cognitive status in vascular dementia. Journal of the International Neuropsychological Society, 17(03), 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori NF, Akbudak E, Shimony JS, Cull TS, Snyder AZ, Guillory RK, & Conturo TE (2002). Diffusion tensor fiber tracking of human brain connectivity: acquisition methods, reliability analysis and biological results. NMR in Biomedicine, 15, 494–515. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, & Song AW (2009). (2009). cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychology Review, 19, 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, & Pakkenberg B. (2003). Marked loss of myelinated nerve fibers in the human brain with age. The Journal of Comparative Neurology, 462(2), 144–152. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, & van Zijl PC (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45(2), 265–269. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Snowdon DA, & Markesbery WR (2003). Head circumference, education and risk of dementia: findings from the nun study. Journal of Clinical and Experimental Neuropsychology, 25(5), 671–679. [DOI] [PubMed] [Google Scholar]
- Mugler JP, & Brookeman JR (1990). Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magnetic Resonance in Medicine, 15(1), 152–157. [DOI] [PubMed] [Google Scholar]
- Murray AD, Staff RT, McNeil CJ, Salarirad S, Ahearn TS, Mustafa N, & Whalley LJ (2011). The balance between cognitive reserve and brain imaging biomarkers of cerebrovascular and Alzheimer’s diseases. Brain, 134(Pt 12), 3687–3696. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, Saxton JA, … & DeKosky ST (2006). The relation of white matter hyperintensities to cognitive performance in the normal old: education matters. Aging, Neuropsychology, and Cognition, 13(3–4), 326–340. [DOI] [PubMed] [Google Scholar]
- Peters A. (2002). The effects of normal aging on myelin and nerve fibers: a review. Journal of Neurocytology, 31(8–9), 581–593. [DOI] [PubMed] [Google Scholar]
- Randolph C. (1998). Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). San Antonio: Psychological Corporation. [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, & Dale AM (2005). Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences, 1064(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Salminen LE, Schofield PR, Lane EM, Heaps JM, Pierce KD, Cabeen R, & Paul RH (2013). Neuronal fiber bundle lengths in healthy adult carriers oftheApoE4 allele: A quantitative tractography DTI study. Brain Imaging and Behavior, 7(3), 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez JL, Rodríguez M, & Carro J. (2002). Influence of cognitive reserve on neuropsychologic functioning in Alzheimer’s disease type sporadic in subjects of Spanish nationality. Cognitive and Behavioral Neurology, 15(2), 113–122. [PubMed] [Google Scholar]
- Satz P. (1993). Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology, 7(3), 273. [Google Scholar]
- Sawrie SM, Martin RC, Faught RE, Maton B, Hugg JW, & Kuzniecky RI (2000). Nonlinear trends in hippocampal metabolic function and verbal memory: evidence of Cognitive Reserve in temporal lobe epilepsy? Epilepsy & Behavior, 1(2), 106–111. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M, & Jellinger K. (2011). Heterogeneity in age-related white matter changes. Acta Neuropathologica, 122(2), 171–185. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, & Sousa N. (2013). A hitchhiker’s guide to diffusion tensor imaging. Frontiers in Neuroscience, 7(31), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(03), 448–460. [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, & Evans DL (1996). Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Archives of Neurology, 53(2), 148–153. [DOI] [PubMed] [Google Scholar]
- Sun SW, Neil JJ, Liang HF, He YY, Schmidt RE, Hsu CY, & Song SK (2005). Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magnetic Resonance in Medicine, 53(6), 1447–1451. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, & Gundersen HJG (1997). Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of Aging, 18(6), 609–615. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, & Mulsant BH (2012). Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiology of Aging, 33(1), 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Demiralp C, & Laidlaw DH (2003). Visualizing diffusion tensor MR images using streamtubes and streamsurfaces. Visualization and Computer Graphics, IEEE Transactions on, 9(4), 454–462. [Google Scholar]