Abstract
Systemic sclerosis (SSc) is an autoimmune disease that can cause fibrosis in vital organs, often resulting in damage to the skin, blood vessels, gastrointestinal system, lungs, heart, and/or kidneys. Patients with SSc are also likely to develop microstomia, which can render dental treatment difficult and painful, thereby necessitating advanced anesthetic management. This is a case report of a 61-year-old woman with a history of SSc with microstomia, interstitial pneumonia, and gastroesophageal reflux disease in whom intravenous moderate sedation was performed using a combination of dexmedetomidine and ketamine for dental extractions. Both anesthetic agents are known to have analgesic effects while minimizing respiratory depression. Consequently, the increased discomfort caused by opening the patient's mouth and stretching the buccal mucosa was sufficiently managed, permitting an increase in maximum interincisal opening and completion of treatment without complications. Patients with SSc present with serious comorbidities that can negatively impact anesthetic management, so the implementation of an anesthetic plan that takes such risks into account is required. Furthermore, emergency airway management is likely to be difficult in patients with microstomia. For intravenous moderate sedation, combined use of dexmedetomidine and ketamine, which have analgesic effects while minimizing respiratory depression, may be particularly effective in patients with SSc and microstomia.
Key Words: Systemic sclerosis, Microstomia, Interstitial pneumonia, Dexmedetomidine, Ketamine
Systemic sclerosis (SSc) is an immune-mediated rheumatic disease involving the overproduction of collagen and other extracellular components in various tissues. The diffuse fibrosis and vascular damage can be widespread, impacting the skin, blood vessel walls, musculoskeletal system, and vital internal organs, such as the lungs, kidneys, and heart. Potential comorbidities or complications associated with SSc include sclerema, gastroesophageal reflux disease (GERD), pulmonary fibrosis, pulmonary hypertension, chronic renal disease, and arrhythmias associated with myocardial fibrosis.1 Interstitial pneumonia due to pulmonary fibrosis develops in approximately 80% of patients with SSc,2 so restrictive lung disease is commonly found in these patients. Currently, the most common cause of death in patients with SSc is pulmonary fibrosis.3 Seventy percent of patients with SSc also develop microstomia secondary to fibrosis of the facial skin and oral mucosa. These patients can have increased discomfort during dental treatment because of the microstomia, which can necessitate excessive stretching of the oral tissues, particularly the buccal mucosa, for adequate surgical access. Often the use of sedation or general anesthesia may be required for these patients to tolerate dental treatment.4,5 Taking these factors into consideration is crucial when creating an anesthetic plan for managing patients with SSc.
Unlike most anesthetic agents, including opioids, benzodiazepines, and propofol, dexmedetomidine is a selective α2 adrenergic agonist capable of producing sedative and analgesic effects without notable respiratory depression.6 However, use of dexmedetomidine as a solo agent during intravenous sedation for potentially stimulating dental procedures such as third molar extractions has been reported to lack a consistent amnestic effect.7 Moreover, when used alone dexmedetomidine has demonstrated a limited ability to provide suitable analgesia during painful or stimulating surgical procedures, and often can cause hypotension and bradycardia.8,9 To overcome these potential issues, several anesthetic agents have been combined with dexmedetomidine to provide sedation during noxious or invasive surgical procedures, one of which is ketamine.
Ketamine is an N-methyl-d-aspartate receptor antagonist often used to provide sedation and/or augment analgesia in painful surgical procedures.10 Ketamine causes an increase in sympathetic tone, leading to an increased heart rate (HR), cardiac output, and blood pressure (BP). In addition, ketamine preserves the airway reflexes and produces minimal respiratory depression.11,12 However, its cardiostimulatory effects and unwanted side effects, which include hypersalivation,13 limit its use as a single intravenous agent for dental procedures. By combining ketamine and dexmedetomidine, it is possible to prevent the bradycardia and hypotension often associated with dexmedetomidine when used as a solo agent, while also enhancing the sedative and analgesic effects expected with either agent alone.14 Therefore, a combination of ketamine and dexmedetomidine may be useful for patients receiving intravenous moderate sedation for dental or oral surgical procedures.15
This case report describes the anesthetic management of a patient with SSc, who has notable restrictive lung disease and microstomia, using a combination of dexmedetomidine and ketamine to provide intravenous moderate sedation for dental extractions. Informed consent to publish the details of this case was obtained from the patient.
CASE PRESENTATION
The patient was a 61-year-old woman (weight 64 kg, height 169 cm, body mass index 22.4 kg/m2) who had been diagnosed 8 years earlier with SSc. Complications associated with her history of SSc included interstitial pneumonia due to pulmonary fibrosis, Raynaud phenomenon, GERD, and a remote history of pericardial effusion.
She was previously scheduled to undergo extraction of the maxillary left second and third molars with local anesthesia. However, she had a masklike face and microstomia due to cutaneous fibrosis of her face and oral mucosa, which limited her oral opening to 20 mm. Use of an oral retractor to further increase her mouth opening failed because of substantial discomfort. Although intraoral administration of local anesthesia (2% lidocaine with 1:80,000 epinephrine) was attempted, the significant microstomia and limited mouth opening greatly inhibited visibility of the posterior maxillary anatomy, preventing adequate access for the local anesthetic injections. The patient subsequently expressed considerable fear and anxiety pertaining to the surgical procedure and potential discomfort, prompting termination of the visit and a discussion of alternative anesthetic management options, such as intravenous sedation or general anesthesia.
PREOPERATIVE ASSESSMENT
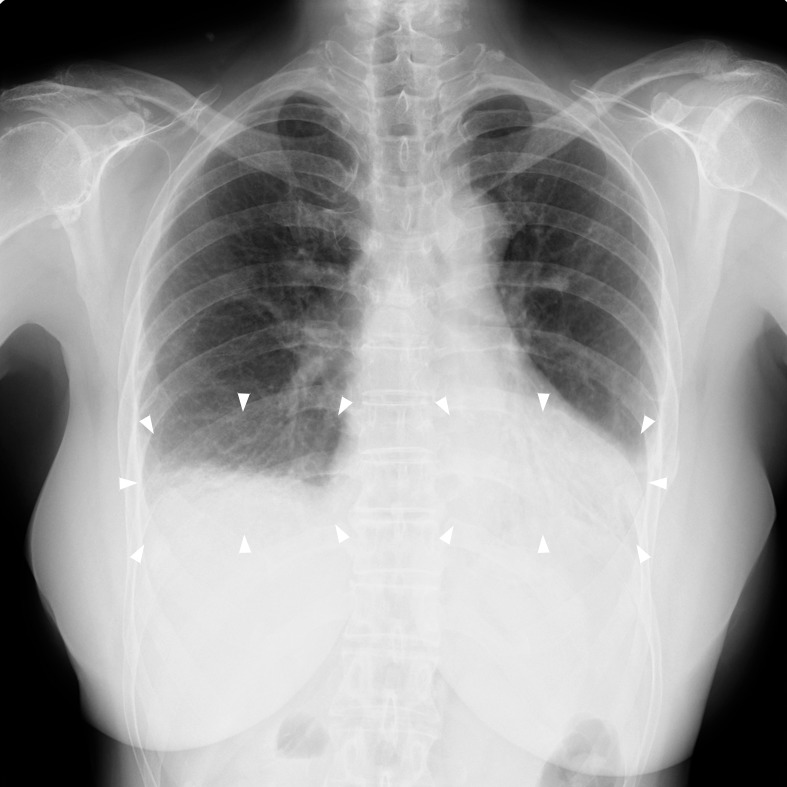
Preanesthetic consultation involved a thorough reassessment of the patient's medical history and physical status to determine the ideal anesthetic plan. Physical evaluation revealed her airway to be Mallampati class III, with a maximum interincisal opening of 20 mm limited by the patient's comfort, and with no limitations to her cervical range of motion. Thyromental distance was not assessed. The patient reported that her pulmonary fibrosis and resulting interstitial pneumonia had stabilized, and she denied being febrile; however, she did report the presence of a dry cough and exertional dyspnea. She was class III on the Hugh-Jones dyspnea scale and demonstrated shortness of breath even when walking on level ground. Preoperative pulmonary function tests revealed a reduced forced vital capacity of 980 mL and a forced vital capacity as percentage of predicted of 36.9%, both consistent findings expected with restrictive lung disease (Table 1). Chest radiographs demonstrated reticular shadows in both lower lung fields, consistent with pulmonary fibrosis (Figure). Sclerema was observed in her upper extremities, and there were flexion contractures of her fingers, with the noted inability to make a fist bilaterally. Her hands were cold and pallid because of Raynaud phenomenon. As a result, percutaneous oxygen saturation (Spo2) measurements using her fingers were unreliable so the probe was placed on her right foot instead, producing a preoperative Spo2 measurement of 96% in room air. Preoperative arterial blood gas values were obtained to assess baseline pulmonary function and were all within the normal range. Additionally, a noninvasive BP cuff was placed on the upper right arm. Her baseline BP was 112/78 mm Hg, with HR of 75 bpm.
Table 1.
Preoperative Pulmonary Function Tests
|
Variable* |
Actual Measurement |
Expected or Normal Value Ranges |
| FVC, mL | 980 | 3500–4000 |
| %FVC | 36.9 | ≥80 |
| FEV1, mL | 910 | 2300–2500 |
| FEV1% | 90.5 | ≥70 |
FVC indicates forced vital capacity; %FVC, forced vital capacity as percentage of predicted; FEV1, forced expiratory volume in 1 second; and FEV1%, forced expiratory volume in 1 second as percentage of forced vital capacity.
Preoperative chest radiograph showing reticular shadows (arrowheads) in both lower lung fields.
The patient reported a history of severe GERD, with significant habitual reflux of gastric contents. In fact, she reported a history of gastric contents filling her oral cavity nearly to the point of overrun when she merely lay down after eating. It was unclear how severe the patient's GERD was on an empty stomach; however, no alterations to the customary NPO instructions were deemed necessary.
She reported a remote history of a pericardial effusion; however, a recent echocardiographic examination indicated that her cardiac function was preserved, with resolution and no recurrence of the pericardial effusion. Consultation with her attending internal medicine physician confirmed that she did not have pulmonary hypertension. There were no abnormal findings on the preoperative electrocardiogram or blood tests, which consisted of a complete blood cell count, serum chemistry panel, and coagulation profile. Her medications included the immunosuppressants azathioprine (50 mg/d) and tacrolimus (2 mg/d), prednisolone (5 mg/d), beraprost sodium (20 mcg/d), lansoprazole (30 mg/d), and nifedipine (20 mg/d), which was prescribed for the Raynaud phenomenon.
Given her medical history, preoperative test results, and current physical status, namely her significantly impaired respiratory status, an intubated general anesthetic was considered to pose a substantially high risk for perioperative respiratory complications, particularly difficulty extubating or weaning the patient off the ventilator and/or subsequent worsening of her interstitial pneumonia. Therefore, after discussion with the treating oral surgeon, the decision was made to treat this patient in the dental hospital setting using intravenous moderate sedation with the additional anesthetic goals of avoiding respiratory depression, pulmonary aspiration, and hypothermia.
DAY OF SURGERY
The patient presented to the dental hospital on the day of surgery, having appropriately followed the NPO instructions (8 h fasting; 2 h no clear fluids). The intraoperative depth of sedation was assessed using the Observer's Assessment of Alertness/Sedation (OAA/S) scale with the target score set for 3–4, which correlates with minimal to moderate sedation. The temperature in the treatment room was adjusted to 26°C (78.8°F) while preparing the patient in order to minimize peripheral vasoconstriction due to triggering her Raynaud phenomenon. When the patient entered the treatment room, supplemental oxygen was delivered at a rate of 3 L/min via a nasal cannula equipped to permit capnography capabilities. The patient was placed in the semi-Fowler position, after which additional appropriate anesthetic monitors were placed. Intraoperative anesthetic monitoring consisted of a pulse oximeter placed on the right big toe, a 3-lead electrocardiogram, a noninvasive BP cuff placed on the right upper arm, capnography via the nasal cannula, and a pretracheal stethoscope, which was placed to help monitor ventilation. Prior to induction the patient's vital signs were BP 126/82 mm Hg, HR 88 bpm, respiratory rate (RR) 18 breaths/min, and Spo2 100% on 3 L/min of O2. The patient's hands were warmed before intravenous access was secured with a 22-gauge intravenous catheter placed in the back of her left hand. Metoclopramide 10 mg, famotidine 20 mg, and hydrocortisone 300 mg were administered intravenously, after which a continuous infusion of dexmedetomidine was initiated at a rate of 6 mcg/kg/h. Five minutes later, the patient's OAA/S score was 4 (minimal sedation level), with BP 107/74 mm Hg, HR 71 bpm, RR 17 breaths/min, and Spo2 100% on 3 L/min via nasal cannula. At this point, a bolus of ketamine 0.6 mg/kg (38 mg) was administered intravenously and a continuous infusion of ketamine 0.5 mg/kg/h was initiated. When dexmedetomidine at 6 mcg/kg/h had been continuously administered for 10 minutes, the patient's OAA/S score was 3 (moderate sedation) and her vital signs were BP 120/67 mm Hg, HR 73 bpm, and Spo2 100%. The dosage of dexmedetomidine was subsequently reduced to 0.7 mcg/kg/h. Thereafter, an oral retractor (Almighty mouth gag, YDM, Tokyo, Japan) was placed, after which the patient gave no indication of pain and her vital signs remained stable with no noted respiratory depression. By this time her mouth opening was noted to be 32 mm. Twelve minutes from the start of sedation, the fibrotic buccal mucosa was gently stretched using a dental mirror, causing the patient to complain of pain, start to move, and have an increase in BP (141/82 mm Hg). Therefore, an additional bolus of ketamine (10 mg) was administered intravenously. Local anesthesia, which consisted of 3.6 mL of 2% lidocaine (72 mg) with 1:80,000 epinephrine (0.045 mg), was administered via buccal and palatal infiltration in the left posterior maxillary region. The surgical procedure began 18 minutes after the initiation of the sedation, at which point her vital signs were BP 130/67 mm Hg, HR 85 bpm, RR 17 breaths/min, and Spo2 100%. Because of the overly fibrotic nature of her facial skin and oral mucosa, surgical access to the maxillary molars with the dental instruments and extraction forceps was rather inhibited. The dental extractions were completed despite being complicated and requiring removal of bone, which the oral surgeon performed successfully with the aid of a bone chisel. The patient had no complaints of pain during the actual extractions and her OAA/S score remained at 3–4 (minimal to moderate sedation). At 35 minutes from the start of the sedation, her OAA/S score was 3 (moderate sedation), with the following vital signs: BP 88/42 mm Hg, HR 55 bpm, RR 16 breaths/min, and Spo2 100%. At this point, the dexmedetomidine infusion was further reduced to 0.4 mcg/kg/h. Fifty minutes after the start of the sedation, acetaminophen 1000 mg was administered intravenously for postoperative analgesia, after which the patient's OAA/S score remained at 3–4. At 58 minutes after the start of sedation, the surgical procedure concluded, the infusions of ketamine and dexmedetomidine were stopped, and her vital signs were BP 95/45 mm Hg, HR 65 bpm, RR 17 breaths/min, and Spo2 100%. The total doses of dexmedetomidine and ketamine used were 88 mcg and 72 mg, respectively. The patient experienced hypotension during treatment, which was quickly corrected, but no significant respiratory depression was noted. She opened her eyes and emerged from the anesthesia 16 minutes later. There were no postoperative complications, such as emergence delirium, hallucinations, nausea, or vomiting, noted. She was able to walk 150 minutes after cessation of anesthesia and was subsequently discharged at that time. Her OAA/S scores, vital signs (BP, HR, Spo2, and RR), and total doses of anesthetic agents are summarized in Table 2. The total operating time was 40 minutes, the total anesthesia time was 58 minutes, the emergence time was 74 minutes, and the recovery time was 150 minutes.
Table 2.
OAA/S Scale, Vital Signs, and Total Dosages of Anesthetic Agents at Key Times in the Case*
|
After Entering Operating Room |
Before Local Anesthesia |
Start of Surgery |
At Time of Intraoperative Hypotension |
End of Surgery |
|
| Time point, min† | 0 | 12 | 18 | 35 | 58 |
| OAA/S scale | 5 | 3 | 3 | 3 | 4 |
| Blood pressure, mm Hg | 126/82 | 120/67 | 130/67 | 88/42 | 95/45 |
| Heart rate, bpm | 88 | 73 | 85 | 55 | 65 |
| Percutaneous oxygen saturation, % | 100 | 100 | 100 | 100 | 99 |
| Respiratory rate, breaths/min | 18 | 16 | 17 | 16 | 17 |
| Total doses of dexmedetomidine, mcg | 0 | 66 | 70 | 82 | 88 |
| Total doses of ketamine, mg | 0 | 42 | 55 | 64 | 72 |
OAA/S indicates Observer's Assessment of Alertness/Sedation.
Time point 0 denotes the time at which administration of the intravenous anesthetic agents was initiated.
During follow-up on the following day, the patient could not recall any part of the surgical procedure and reported being satisfied with her sedation. The patient did not have any exacerbations of interstitial pneumonia in the month following her dental treatment.
DISCUSSION
The present case involved a patient with SSc with resulting microstomia, restrictive lung disease, and GERD, all of which can have serious complications related to anesthesia management. Development of an anesthetic plan for this patient required consideration of the following additional concerns or goals: (a) likelihood for increased anesthetic and analgesic requirements due to the prolonged opening and stretching of the oral soft tissues during surgical procedure, (b) avoiding respiratory depression due to the patient's restrictive lung disease, and (c) mitigating the risk of pulmonary aspiration secondary to GERD. Therefore, the developed anesthetic plan included use of anesthetic agents that do not typically cause substantial dose-dependent respiratory depression and preoperative administration of a histamine blocker and metoclopramide to reduce the risk of pulmonary aspiration secondary to GERD. Dexmedetomidine does not cause notable respiratory depression, but often causes hypotension and bradycardia and has limited ability to provide suitable analgesia.6–9 Ketamine also produces little respiratory depression, but has sympathomimetic properties leading to modest tachycardia and hypertension and provides adequate analgesia during painful surgical procedures.10–12 Both dexmedetomidine and ketamine cause minimal respiratory depression when used alone and have additive benefits of sedative and analgesic effects. Therefore, a combination of dexmedetomidine and ketamine was selected for intravenous moderate sedation for this patient.
There was no significant decrease in the patient's Spo2 or RR, and no manual airway management was required. Given the SSc-related anatomical deformities impacting this patient's airway, including tightening of the facial skin and microstomia, emergency management of the airway was anticipated to be difficult. Propofol and midazolam are anesthetic agents that are commonly used for intravenous sedation. However, propofol is a potent respiratory depressant that can produce significant intraoperative apnea. Similarly, midazolam poses a risk of respiratory depression when combined with other central nervous system depressants or administered at high doses.16 Furthermore, propofol and midazolam lack any analgesic effects. It was considered that intravenous moderate sedation by combined continuous infusions of dexmedetomidine and ketamine posed little risk of significant respiratory depression and could be effective in patients with SSc. However, respiratory complications can still occur even with intravenous moderate sedation, necessitating proper precautions, such as ensuring the presence of a video laryngoscope, a laryngeal mask airway, and/or a fiberoptic bronchoscope for emergency management of the airway.1 Therefore, the same emergency airway management preparations were made in this case.
High-dose dexmedetomidine has been reported to cause soft tissue obstruction in the airway as a result of posterior displacement of the tongue and collapse of the soft tissue musculature of the airway.17 Therefore, in the present case, a continuous low-dose infusion of dexmedetomidine was administered rather than a high bolus dose. A high-dose bolus of dexmedetomidine can also cause hypotension and bradycardia. Ketamine preserves the airway reflexes and produces minimal respiratory depression, but also is known to cause psychomimetic effects (hallucinations, memory defects, emergence delirium), nausea, and vomiting.11,12 Although guaranteed prevention of ketamine's psychomimetic effects is not possible, reducing the risks of these effects is feasible using coadministration of a benzodiazepine or an α2 adrenoreceptor agonist.18 A recent study by Gupta et al19 has shown that premedication with dexmedetomidine can effectively and safely attenuate the ketamine-induced psychomimetic effects. In the present case, the lack of any appreciable psychomimetic effects induced by ketamine was deemed due to the coadministration of dexmedetomidine. The increase in mouth opening with the dental retractor following induction of anesthesia was attributed in part to the analgesic effects of ketamine and dexmedetomidine. Although the patient demonstrated an increase in BP thought to be due to the pain and discomfort from stretching of the buccal mucosa with a dental mirror during administration of local anesthesia, the increase in BP was successfully managed by administering an additional small bolus of ketamine. Moreover, after the subsequent bolus of ketamine the patient did not complain of pain despite the fibrotic nature of the facial skin impeding access of dental instruments and forceps to the maxillary molars. This suggests that the small bolus dose of ketamine provided an effective analgesic action during painful oral surgery procedure for this patient.
Dexmedetomidine can prevent the tachycardia, hypertension, and salivation associated with ketamine, whereas ketamine can prevent the bradycardia and hypotension associated with dexmedetomidine.14,20 However, as the plasma concentrations of dexmedetomidine increase, there is a resulting decrease in HR, a progressive reduction in cardiac output, and a biphasic (high then low) dose-response relationship for BP and vascular resistance.21 Therefore, in the present case, dexmedetomidine was administered first, followed by ketamine, after confirming a decrease in the patient's BP and HR had occurred. As a result, induction of anesthesia was achieved without any significant fluctuations in hemodynamics. On the other hand, the patient experienced hypotension during the surgical procedure, so continuous administration of dexmedetomidine was subsequently reduced. This hypotension was primarily attributed to the end of the most stimulating part of the dental extraction procedure. Consequently, the pain caused by opening the patient's mouth and stretching the buccal mucosa might have been substantially reduced, leading to the development of hypotension. Although the hemodynamic effects of dexmedetomidine and ketamine were relatively balanced in this case, the optimal dosage of each agent has yet to be identified.
The absence of pulmonary hypertension in this patient was also a factor in selecting ketamine. Patients with SSc are susceptible to pulmonary arterial hypertension secondary to interstitial pneumonia and pulmonary fibrosis.22 Ketamine has been reported to enable safe management of anesthesia in patients with pulmonary hypertension without increasing pulmonary vascular resistance.23,24 Moreover, intravenous sedation with dexmedetomidine has been reported not to increase pulmonary vascular resistance in patients who have undergone a Fontan procedure.25 Therefore, the combination of dexmedetomidine and ketamine may be effective even in patients with SSc complicated by pulmonary arterial hypertension.
GERD is a common finding in patients with SSc, as they are likely to have a dilated lower esophageal sphincter that increases their susceptibility to reflux of the gastric contents. Additionally, many intravenous anesthetics, including dexmedetomidine, may reduce lower esophageal sphincter tone, which can increase the risk of pulmonary aspiration.26,27 A previous report indicated that even though dexmedetomidine decreased lower esophageal sphincter tone, the reductions were small even at higher concentrations.28 It was felt that use of dexmedetomidine would be unlikely to promote gastroesophageal reflux during sedation. Therefore, dexmedetomidine was selected as an appropriate agent for this case. However, when developing an anesthetic plan for a patient with SSc and severe GERD, the benefits and risks of nonintubation versus intubation should be considered. Because of this patient's impaired baseline respiratory status, the decision to proceed with a nonintubated intravenous moderate sedation was made, leaving the airway essentially unsecured. Given that reduced lower esophageal sphincter tone increases the risk of reflux and aspiration, steps were made to mitigate the damage from any potential pulmonary aspiration by decreasing gastric volume and increasing gastric fluid pH. Roberts et al1 recommended increasing the gastric fluid pH with histamine blockers in patients with SSc prior to induction of anesthesia. Additional recommendations included the use of metoclopramide to promote gastric motility, lowering gastric volume and increasing esophageal sphincter tone.1 This patient did not demonstrate any signs or symptoms of pulmonary aspiration during case nor any worsening of her interstitial pneumonia postoperatively. Therefore, preoperative administration of the histamine blockers and metoclopramide may have been an effective preventive strategy.
SSc is often associated with fibrosis and thickening of the skin, flexion contractures, and vasoconstriction, all of which may inhibit ease of peripheral intravenous access, as in the present case. To improve success rates, anesthesiologists should maintain an operating room ambient temperature of ≥21°C29,30 and consider use of warm intravenous fluids before administration to minimize complications related to peripheral vasoconstriction. In the present case, the BP cuff was placed on the upper right arm and BP was measurable noninvasively. However, noninvasive BP monitoring may be difficult in areas involving dermal thickening, flexion contractures, and vasoconstriction, necessitating consideration of invasive monitoring such as an arterial line.1
The bispectral index (BIS) is a commonly used anesthetic monitor that processes electroencephalographic data to help assess the depth of anesthesia and guide subsequent administration of anesthetic agents.31 However, ketamine acts as a dissociative anesthetic agent, which has been shown to increase the θ activity on the electroencephalogram and is known to increase BIS values in anesthetized patients, essentially limiting the reliability of the BIS monitor.32 Therefore, rather than using the BIS to assess the level of sedation, we chose to use the OAA/S scale instead. Although the results obtained depend on the assessor, the sedation scoring system was considered effective for this intravenous moderate sedation case using the combination of ketamine and dexmedetomidine.
CONCLUSION
In conclusion, dental or oral surgery may be difficult and painful in patients with SSc and microstomia, necessitating advanced anesthetic management. For patients with severe SSc, pulmonary fibrosis, and pulmonary arterial hypertension the risks associated with intubated general anesthesia may be considered too high. Therefore, intravenous minimal to moderate sedation may be considered an acceptable alternative. However, microstomia may make emergency airway management and/or intubation quite difficult, even during intravenous sedation.1 Combined intravenous moderate sedation with dexmedetomidine and ketamine, which produces minimal respiratory depression and has analgesic effects, may be considered an effective option for patients with SSc and microstomia.
REFERENCES
- 1.Roberts JG, Sabar R, Gianoli JA, Kaye AD. Progressive systemic sclerosis: clinical manifestations and anesthetic considerations. J Clin Anesth. 2002;14:474–477. doi: 10.1016/s0952-8180(02)00380-x. [DOI] [PubMed] [Google Scholar]
- 2.Bolster MB, Silver RM. Assessment and management of scleroderma lung disease. Curr Opin Rheumatol. 1999;11:508–513. [PubMed] [Google Scholar]
- 3.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology 2nd ed. Philadelphia, Pa: WB Saunders Co;; 2002. p. 137. [Google Scholar]
- 5.Albilia JB, Lam DK, Blanas N, Clokie CM, Sándor GK. Small mouths ... big problems? A review of scleroderma and its oral health implications. J Can Dent Assoc. 2007;73:831–836. [PubMed] [Google Scholar]
- 6.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 7.Ustun Y, Gunduz M, Erdogan O, Benlidayi ME. Dexmedetomidine vs. midazolam in outpatient third molar surgery. J Oral Maxillofac Surg. 2006;64:1353–1358. doi: 10.1016/j.joms.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Jalowiecki P, Runder R, Gonciarz M, Kawecki P, Petelenz M, Dziurdzik P. Sole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopy. Anesthesiology. 2005;103:269–273. doi: 10.1097/00000542-200508000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115:171–182. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 10.Miller AC, Jamin CT, Elamin EM. Continuous intravenous infusion of ketamine for maintenance sedation. Minerva Anestesiol. 2011;77:812–820. [PubMed] [Google Scholar]
- 11.Tweed WA, Minuck M, Mymin D. Circulatory responses to ketamine anesthesia. Anesthesiology. 1972;37:613–619. doi: 10.1097/00000542-197212000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Bourke DL, Malit LA, Smith TC. Respiratory interactions of ketamine and morphine. Anesthesiology. 1987;66:153–156. doi: 10.1097/00000542-198702000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Haas DA, Harper DG. Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–68. [PMC free article] [PubMed] [Google Scholar]
- 14.Tobias JD. Dexmedetomidine and ketamine: an effective alternative for procedural sedation? Pediatr Crit Care Med. 2012;13:423–427. doi: 10.1097/PCC.0b013e318238b81c. [DOI] [PubMed] [Google Scholar]
- 15.Hasan MS, Dexmedetomidine Chan L. and ketamine sedation for dental extraction in children with cyanotic heart disease. J Oral Maxillofac Surg. 2014;72:1920.e1–e4. doi: 10.1016/j.joms.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Giovannitti JA., Jr Pharmacology of intravenous sedative/anesthetic medications used in oral surgery. Oral Maxillofac Surg Clin North Am. 2013;25:439–451. doi: 10.1016/j.coms.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–790. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77:357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta K, Gupta A, Gupta PK, Rastogi B, Agarwal S, Lakhanpal M. Dexmedetomidine premedication in relevance to ketamine anesthesia: a prospective study. Anesth Essays Res. 2011;5:87–91. doi: 10.4103/0259-1162.84193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levänen J, Mäkelä ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82:1117–1125. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 23.Williams GD, Philip BM, Chu LF, et al. Ketamine does not increase pulmonary vascular resistance in children with pulmonary hypertension undergoing sevoflurane anesthesia and spontaneous ventilation. Anesth Analg. 2007;105:1578–1584. doi: 10.1213/01.ane.0000287656.29064.89. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell BG, Jackson E. Role of ketamine in the management of pulmonary hypertension and right ventricular failure. J Cardiothorac Vasc Anesth. 2012;26:e24–e25. doi: 10.1053/j.jvca.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Tokuhira N, Atagi K, Shimaoka H, Ujiro A, Otsuka Y, Ramsay M. Dexmedetomidine sedation for pediatric post-Fontan procedure patients. Pediatr Crit Care Med. 2009;10:207–212. doi: 10.1097/PCC.0b013e31819a3a3e. [DOI] [PubMed] [Google Scholar]
- 26.Smith G, Dalling R, Dougan LR, Williams TI. The effect of thiopentone and suxamethonium on gastrooesophageal pressure gradients. Br J Anaesth. 1978;50:76–27. [PubMed] [Google Scholar]
- 27.D'Angelo R, Miller R. Pregnancy complicated by severe preeclampsia and thrombocytopenia in a patient with scleroderma. Anesth Analg. 1997;85:839–841. doi: 10.1097/00000539-199710000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Turan A, Wo J, Kasuya Y, et al. Effects of dexmedetomidine and propofol on lower esophageal sphincter and gastroesophageal pressure gradient in healthy volunteers. Anesthesiology. 2010;112:19–24. doi: 10.1097/01.anes.0000365963.97138.54. [DOI] [PubMed] [Google Scholar]
- 29.Black CM, Stevens WM. Scleroderma. Rheum Dis Clin North Am. 1989;15:193–212. [PubMed] [Google Scholar]
- 30.Thompson J, Conklin KA. Anesthetic management of a patient with scleroderma. Anesthesiology. 1983;59:69–71. doi: 10.1097/00000542-198307000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Hans P, Dewandre PY, Brichant JF, Bonhommonee V. Comparative effects of ketamine on bispectral index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–340. doi: 10.1093/bja/aei047. [DOI] [PubMed] [Google Scholar]