Abstract
Lateral hypothalamus (LH) orexin neuron signaling has been implicated in the motivation to seek and take drugs of abuse. The number of LH orexin neurons has been shown to be upregulated with exposure to drugs of abuse. We sought to determine if the number of LH orexin neurons related to individual differences in motivation (demand) for cocaine in our behavioral economics (BE) paradigm, and whether knockdown of these cells predicted changes in economic demand. We quantified LH orexin cell numbers in animals immediately following our BE paradigm, as well as after a 2-week period of abstinence, to relate the number of LH orexin cells to economic demand for cocaine. We also knocked down LH orexin expression with an orexin morpholino antisense to determine how reduced orexin numbers impacted cocaine demand. We found that animals with greater baseline motivation for cocaine (lower demand elasticity) had more LH orexin neurons. Following a 2-week abstinence from cocaine, the number of LH orexin neurons predicted economic demand for cocaine prior to abstinence, indicating that orexin expression is a persistent marker for demand. Reducing LH orexin cell numbers with antisense decreased motivation for cocaine (increased demand elasticity) without affecting baseline consumption. In addition, the number of spared LH orexin neurons after antisense treatment correlated with individual motivation for cocaine. These studies point to a role for the endogenous number of LH orexin neurons in individual differences in motivation for cocaine.
Keywords: behavioral economics, hypothalamus, motivation
1 |. INTRODUCTION
Orexin A and B (also called hypocretin 1 and 2) are peptides synthesized by a small population of neurons in posterior hypothalamus, ranging mediolaterally from dorsomedial hypothalamic nucleus (DMH), through perifornical hypothalamus (Pef), to lateral hypothalamus (LH).1,2 Orexin A binds both orexin-1 and -2 receptors (OxR1 and OxR2), whereas orexin B preferentially binds OxR2.3 Orexin neurons interact closely with the mesolimbic dopamine (DA) system to drive motivation and addiction.4–10 Many studies now indicate that blockade of OxR1 signaling attenuates cocaine-seeking behaviors, particularly under high effort conditions (for review,11) indicating a role for OxR1 signaling in the motivational properties of cocaine.
A major goal of addiction research is to elucidate the neural substrates that contribute to addiction vulnerability. We previously found that the degree to which drug-associated stimuli activate the LH subpopulation of orexin neurons predicts individual differences in drug seeking. Fos activation of LH, but not Pef/DMH, orexin neurons correlated with reinstatement of extinguished cocaine or morphine preference.12 LH orexin Fos expression also correlated with preference for, and context-induced reinstatement of, ethanol seeking.13 Further, rats that exhibited a multifaceted addiction phenotype following intermittent access (IntA) to cocaine exhibited higher Fos expression selectively in LH orexin neurons following re-exposure to the self-administration context relative to short access control rats.14 OxR1 blockade is particularly effective at reducing drug-seeking in highly motivated animals,14–20 further indicating that individuals with high propensity for drug-seeking may have elevated stimulus-driven LH orexin cell activity.
Although it is clear that the activity of LH orexin neurons is important for drug seeking, emerging evidence indicates that upregulation of orexin expression may also be involved. We reported that the persistent IntA-induced addiction phenotype was associated with higher numbers of orexin-expressing neurons in LH but not Pef/DMH.14 Another recent study found that postmortem tissue from heroin addicts contains higher numbers of orexin-expressing neurons compared with healthy controls, and mice exposed to chronic noncontingent morphine injections had increases in the number of orexin neurons particularly in LH.21 These studies are consistent with an earlier study that reported that chronic alcohol consumption increased the area of prepro-orexin mRNA expression preferentially in LH of alcohol-preferring rats.19 Changes in the number of orexin-expressing LH neurons might reflect individual differences in drug-seeking that result from “state” factors like extended or binge-like drug access. However, it is unclear whether variability in the baseline (nonstimulated) numbers of orexin-expressing LH neurons contributes to trait differences in addiction behavior.
We sought to determine if differences in the numbers of LH orexin neurons contributes to individual differences in baseline or “trait” motivation for cocaine using our within-session behavioral economics (BE) paradigm.22,23 In this paradigm, cocaine consumption is measured at increasing price points. By fitting the resulting data using an exponential demand equation,24 it is possible to calculate demand elasticity (α), or the extent to which consumption changes with price. We previously reported that α predicts several addiction-relevant behaviors, including compulsive (punished) responding for cocaine, drug seeking during initial abstinence, and cue-induced reinstatement of extinguished drug-seeking.20,23 Here, we show that the number of orexin neurons in LH, but not Pef/DMH, correlates with motivation for cocaine (α), and this relationship persists after abstinence. In addition, unilateral LH orexin knockdown with orexin morpholino antisense is sufficient to increase demand elasticity (reduce motivation), and the degree of knockdown predicts the extent of motivation reduction. Collectively, these studies indicate that the number of LH orexin neurons is a potent predictor of addiction vulnerability.
2 |. METHODS
2.1 |. Animals
Adult male Sprague-Dawley rats (300-325 g) were pair-housed on a reverse 12-hour light:dark cycle in a temperature- and humidity-controlled animal facility at Rutgers University or Medical University of South Carolina (MUSC) with ad libitum access to standard rat chow and water. Upon arrival, animals were acclimated to the colony room for 2 days and handled for at least 3 days prior to surgery. All protocols and animal care procedures were approved by the Institutional Animal Care and Use Committee at Rutgers University-New Brunswick or Medical University of South Carolina
2.2 |. Drugs
Cocaine HCl powder was obtained from the National Institute of Drug Abuse (Research Triangle Park, NC) and dissolved in 0.9% sterile saline.
2.3 |. Intravenous catheter surgery
After handling for a minimum of 3 days, rats were anesthetized with a ketamine/xylazine (56.5/8.7 mg/kg, ip, respectively) mixture and also given an analgesic (rimadyl at 5 mg/kg or meloxicam at 1 mg/kg, sc). Rats were implanted with indwelling catheters into the jugular vein for iv infusion of cocaine. For morpholino experiments, cannulae implantation occurred immediately followed catheterization. Cefazolin (0.1 mL; 100 mg/mL) and heparin (0.1 mL; 100 U/mL) were flushed through the iv catheter after surgery and after each self-administration session. Following a 1-week recovery after surgery, animals were trained to self-administer iv cocaine.
2.4 |. Cocaine self-administration
Rats were trained on a fixed ratio-1 (FR-1) cocaine self-administration paradigm (20-s timeout post-infusion) as previously described.25 Sessions occurred in operant chambers contained in sound-attenuating boxes with Med-PC IV software (Med Associates). During training sessions, cocaine infusions (0.19 mg cocaine/infusion) were paired with discrete light and tone cues (white stimulus light above the active lever; 78-dB, 2900-Hz tone). After reaching criteria, (more than or equal to 10 infusions/session for 10 sessions), animals were trained on a BE procedure.
2.5 |. Behavioral economics
Following FR-1 training, rats were trained on a within-session BE procedure, as described previously.22,23 During the 110-minute session, the price of cocaine increased in successive 10-minute intervals on a quarter logarithmic scale (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2, 1.2 μg cocaine per infusion). A demand curve was fit to cocaine consumption data across different prices, as described in a previous paper from our lab.22 From the demand curve, we derived consumption at low effort (Q0) and demand elasticity (α, the rate of decline in consumption as price increases). Q0 is low-effort consumption extrapolated to the y-axis of the demand curve, where cocaine price approaches zero and α is the decay constant (exponential slope, also denoted as demand elasticity) of this curve. Notably, α scales inversely with motivation, so high motivation animals have lower α values. Animals were trained on BE for a minimum of 6 days, and testing occurred when animals displayed stable behavior on the BE paradigm (Qo and α values less than or equal to 30% variability across the last three sessions; range: 6-16 d to reach stability across all tested animals).
2.6 |. Tissue preparation for immunohistochemistry
Animals were deeply anesthetized with a ketamine/xylazine mixture and transcardially perfused with 0.9% sterile saline, then 4% paraformaldehyde. Brains were dissected and postfixed in 4% paraformaldehyde, then 20% sucrose-PBS azide solution. Brains were frozen with dry ice and sectioned at 40 μm on a cryostat. Sections were collected in PBS azide.
2.7 |. Immunohistochemistry
To visualize orexin neurons, hypothalamic tissue was incubated in goat anti-orexin A (1:500, Santa Cruz Biotechnology) in 5% NDS at room temperature overnight. The following day, tissue was incubated in biotinylated donkey anti-goat secondary antibody (1:500, Jackson ImmunoResearch Laboratories) for 1.5 hours, followed by avidin-biotic complex (1:500) for 1.5 hours. After washing, sections were stained with DAB (Sigma) in Tris buffer. Tissue was mounted on slides, dehydrated, and coverslipped with DPX mounting medium (Electron Microscopy Sciences).
2.8 |. Cell quantification
Coronal lateral hypothalamus images were taken across the orexin cell field (2.5-3.8 mm caudal to bregma) with a Zeiss Axio Zoom V16 microscope.26 Tiled photographs were compiled at 20x magnification using Zen 2 imaging software (Carl Zeiss Microscopy). Orexin A-immunopositive neurons were quantified in both hemispheres with Adobe Illustrator by an observer blind to experimental conditions. Three sections were taken per animal, as in our previous studies.13,14,27 The sum of neurons counted in both hemispheres in each section were determined and averaged across the three sections. To determine the extent of orexin knockdown, the percent change in injected vs noninjected hemispheres for each section was averaged across the three sections.
2.9 |. Experiment 1 Relationship between orexin cell numbers and economic demand for cocaine
We first sought to examine the relationship between endogenous orexin cell numbers and baseline economic demand for cocaine. To do this, animals were trained on FR-1 (range: 6-26 d) and the BE paradigm as above until they displayed stable behavior (range: 6-16 d to reach stability, 6-41 d total on BE). The following day, rats were again tested on the BE paradigm and perfused 90 minutes after the point of maximum responding (Pmax), defined as the price at which maximum responding occurred. Tissue was processed for orexin A immunohistochemistry and cell quantification; cell counts were compared with demand measures from the final BE test session.
2.10 |. Experiment 2 Effect of orexin cell knockdown on economic demand for cocaine
2.10.1 |. Stereotaxic surgery
We next investigated the impact of knocking down LH orexin expression using orexin morpholino antisense on demand for cocaine. Immediately following catheter implantation (described above), animals were secured in a stereotactic frame (Kopf, Tujunga, CA, USA) and implanted with a unilateral stainless steel guide cannula (22 gauge, 11 mm, Plastics One, Roanoke, VA, USA) 2 mm dorsal to LH (coordinates relative to bregma: −3.0 mm AP, +/−2.6 mm ML, −6.8 to −7.4 mm DV). We varied the dorsal-ventral cannula coordinates to produce varying degrees of knockdown in morpholino-infused animals. The implanted hemisphere was counterbalanced across all tested animals, such that an equal number of animals were implanted in the left vs right hemisphere. Acrylic cement and jeweler screws were used to secure the cannula to the skull surface.
2.10.2 |. Orexin morpholino antisense microinjections
Animals were trained on FR-1 and BE, as described above (range of FR1 training days for orexin antisense rats: 10-14 d and for control morpholino rats: 10-17 d; range of total days on BE for orexin antisense rats: 11-22 d, and for control morpholino rats: 13-22 days). One day after animals displayed stable behavior on the BE paradigm (orexin antisense: 6-16 d, control morpholino: 7-16 d to reach stability), an injector cannula was unilaterally lowered into LH (2 mm below the guide cannula) to infuse orexin morpholino antisense (Vivo-Morpholino; 150 nmol/0.3 uL in 0.5 mM phosphate buffer, Gene Tools, 5′-GTATCTTCGGTGCAGTGGTCCAAAT-3′) or an inverted orexin control missense (Vivo-Morpholino, 5′-TAAACCTGGTGACGTGGCTTCTATG-3′). The injector cannula was kept in place for 1 minute before infusing the morpholino for 2 minutes. Injectors were then kept in place for 1 minute to reduce diffusion of the morpholino. All animals were infused between 2:00 PM to 3:00 PM, during their active period. Animals were tested on BE for 6 days following morpholino microinjection and perfused on day 6 when peak orexin knockdown occurs.28,29 Animals were perfused with fixative, brains were sectioned, sections were stained for the orexin A protein, and orexin-positive cells were quantified as above. By restricting morpholino injections to a single hemisphere, we were able to simultaneously: (a) confirm the efficacy of the antisense by comparing the number of orexin cells in the injected and uninjected hemispheres; (b) examine the effect of reducing overall orexin numbers on demand for cocaine; and (c) examine the relationship between endogenous orexin cell numbers (in the uninjected hemisphere) with demand prior to antisense injections.
Brain sections adjacent to those used for orexin A staining were dehydrated and Nissl-stained with neutral red. Sections were coverslipped with DPX mounting medium (Electron Microscopy Sciences) to determine cannula location.
2.11 |. Experiment 3 Relationship between LH orexin cell number and demand following abstinence
To determine whether the relationship between LH orexin cell numbers and demand persisted after abstinence, we sacrificed animals with a history of cocaine self-administration 2 weeks after their final BE test. Animals were first trained on FR-1 (range: 7-11 d) and the BE procedure until behavior was stable, as above (range: 6-12 d to reach stability; range of total days on BE: 22-43 d). Cocaine self-administration was then discontinued and rats were tested for locomotor activity in the locomotor chambers (clear acrylic, 40 × 40 × 30 cm), equipped with Digiscan monitors (AccuScan Instruments) in 2-hour sessions, 5 days a week for 2 weeks. During these 2 weeks, rats received ip injections of up to three compounds (propranolol, prazosin, and clonidine) as a control for a separate study (unpublished). At least 2 days washout was given between treatments and before the final sacrifice. Horizontal, vertical, and total locomotor activity was recorded using beam beaks. Rats were perfused immediately after the final habituated locomotor test for orexin cell quantification.
2.12 |. Data analysis
Statistical analyses were performed with GraphPad Prism 7, except for multivariate regressions which were performed using SPSS Statistics (Version 22). Baseline α and Q0 values were determined by averaging performance across the 3-day preceding testing. In Experiment 1, a median split of α was used to determine high demand elasticity vs low demand elasticity animals, and a median split of Q0 identified high takers vs low takers. Unpaired samples t tests were used to compare differences in LH orexin neuron numbers between high and low demand elasticity, or high and low Q0 animals. In Experiment 2, a two-way ANOVA was used to compare BE performance following morpholino treatments. Demand associations with orexin cell counts were determined using multiple linear regression with log10(α) and log10(Q0) set as independent variables with cell count as the dependent variable. In Experiment 3, an unpaired samples t test was used to compare LH orexin neuron numbers in animals subjected to BE vs locomotor testing. Pearson correlations were used for all univariate relations to compare orexin neuron numbers with total locomotor activity or α values. All statistics were two-tailed. A Shapiro-Wilk normality test determined that all data were parametric.
3 |. RESULTS
Across all groups, we observed no relationship between demand elasticity (α) and the number of training days on BE (Pearson correlation; Experiment 1: p=.13; Experiment 2: control, p=.26 and antisense p=.90; Experiment 3: p=.60; data not shown). In addition, the number of LH orexin neurons did not correlate with the number of days of cocaine experience (Pearson correlation, Experiment 1: p=.50; Experiment 2: antisense, p=.85; Experiment 3: p=.57; data not shown) or cocaine intake during FR-1 and BE (Pearson correlation, Experiment 1: p=.08; Experiment 2: antisense, p=.13; Experiment 3: p=.21; data not shown).
3.1 |. Experiment 1 Animals with high motivation for cocaine have more LH orexin-expressing neurons
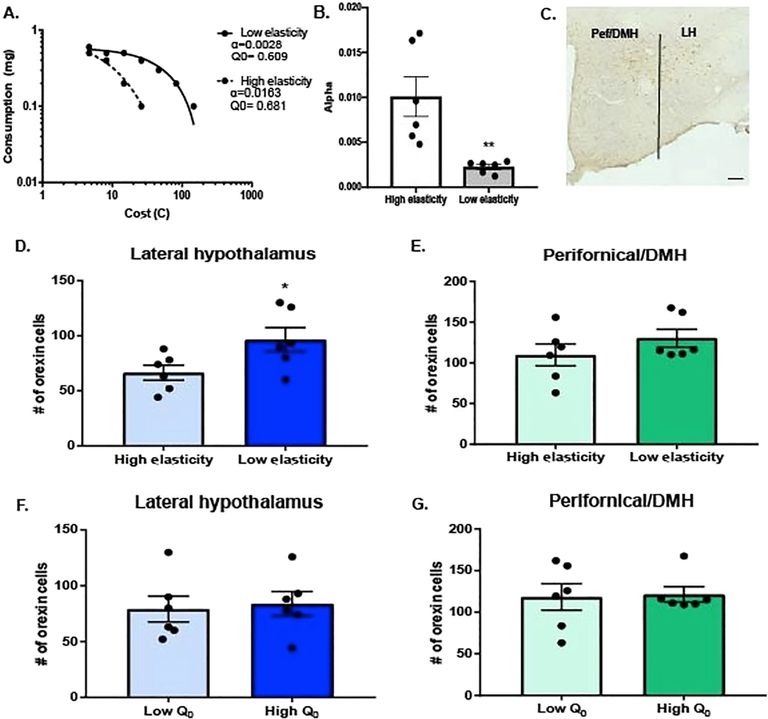
Animals (n = 12) were trained on the BE paradigm and divided into high vs low demand elasticity based on a median split of α values (representative demand curves shown in Figure 1A). Low-demand elasticity (high motivation) animals had significantly lower α values than high demand elasticity (low motivation) animals (Figure 1B, t10 = 3.53, P < .01). Low-demand elasticity animals also had more orexin-immunopositive cells in LH, compared with high-demand elasticity animals (Figure 1D, t10 = 2.32, P < .05). No such differences were observed for perifornical area/dorsomedial hypothalamus (Pef/DMH) orexin neurons (Figure 1E, t10 = 1.20, p= .26). When animals were instead divided based on their preferred level of consumption at low effort (Q0), there were no differences in the numbers of LH (Figure 1F, t10 = 0.29, p=.78) or Pef/DMH (Figure 1G, t10 = 0.17, p= .87) orexin neurons between high- and low-takers.
FIGURE 1.
Number of lateral hypothalamus (LH) orexin neurons is elevated in animals with low-demand elasticity (high motivation). A, Representative demand curves from high (dashed line) and low (solid line) demand elasticity animals with similar baseline consumption (Q0). B, Animals were divided into low and high demand elasticity (low and high α) by a median split. Low-demand elasticity (high motivation) animals had significantly lower mean α values than high-demand elasticity animals (unpaired samples t test, **P < .01). C, Representative frontal section of hypothalamus stained for orexin from an animal sacrificed 90 minutes post-Pmax. Scale bar denotes 200 μm. Midline at left. D, Low demand elasticity (high motivation) animals had more LH orexin neurons compared with high demand elasticity (low motivation) animals (n = 6/group, unpaired samples t test, *P < .05). Bilateral orexin neurons in Pef/DMH and LH were counted separately and averaged across three sections per animal, representing the rostral-caudal extent of the orexin cell field for this panel and for panels E to G. E, High- and low-demand elasticity animals had similar numbers of Pef/DMH orexin cells (unpaired samples t test, ns). F, Animals were divided into low and high Q0 by a median split. No differences were observed in LH orexin numbers in high Q0 compared with low Q0 (unpaired samples t test, ns). G, High and low Q0 animals did not differ in the number of Pef/DMH orexin cells (unpaired samples t test, ns)
3.2 |. Experiment 2 Unilateral knockdown of LH orexin neurons reduces cocaine demand elasticity
We next examined whether the number of LH orexin neurons play a causal role in demand by testing whether LH orexin knockdown would reduce motivation for cocaine (increase α). In a separate cohort of animals, we unilaterally infused an orexin morpholino antisense (n = 9) or control missense (n = 8) into LH after animals displayed stable BE behavior. Injection sites are depicted in Figure 2A.
FIGURE 2.
Knockdown of lateral hypothalamus (LH) orexin-expressing neurons with unilateral morpholino antisense decreases demand for cocaine. A, Schematic representation of LH cannula placements for control missense-infused (n = 8, white circles) and orexin antisense-infused (n = 9, red circles) animals. B, Representative images of hypothalamus stained for orexin (brown) following a unilateral orexin antisense-infusion into LH. The uninjected hemisphere was used as a within-subjects control. The top left panel indicates LH orexin cells in the injected hemisphere, top right LH orexin cells in the uninjected hemisphere. The bottom left panel indicates Pef/DMH cells in the injected hemisphere, whereas the bottom right panel shows those cells in the uninjected hemisphere. Scale bar denotes 200 μm. C, The percent reduction in orexin expression in orexin antisense-infused animals was significantly greater than in the control missense-infused animals (unpaired t test, **P < .01). D, Pef/DMH orexin expression was unaffected in animals microinjected with orexin antisense and control missense-infused into LH, compared with the uninjected hemisphere (unpaired t test, ns). E, Sample demand curves for an animal infused with antisense in LH at baseline (solid line) and following morpholino treatment (dashed line). F, Microinjection of orexin antisense increased α (decreased motivation) compared with baseline (red bars; Sidak’s multiple comparisons test, ***P < .001). No change was observed in control missense-infused animals (white bars; ns). G, There was no effect of treatment on Q0 in animals infused with orexin antisense- (red bars) or control missense- (white bars) into LH (Sidak’s multiple comparisons test, ns)
3.2.1 |. Unilateral infusion of orexin antisense reduced orexin cell numbers
In rats with LH morpholino injection, we observed a significant reduction (approximately 35%) in the number of orexin-expressing neurons in LH (Figure 2B,C; unpaired t test; t15 = 3.55; P < .01), but no change in orexin expression in Pef/DMH (Figure 2B,D; unpaired t test; t15 = 0.49; p=.63). The number of orexin neurons in the uninjected hemisphere of morpholino-infused animals were similar to that observed in animals from Experiment 1, indicating no compensatory effects from morpholino treatment in the contralateral hemisphere (unpaired t test; t19 = 1.084; ns; data not shown). Orexin morpholino antisense microinjections produced varying degrees of knockdown in these animals depending on the location of cannula. We observed that antisense-infused animals with cannula targeted dorsal to the top of the fornix had a knockdown of approximately 25% of orexin cell numbers compared with the uninjected hemisphere, and this was statistically significant (n = 5) (data not shown; unpaired t test; t8 = 2.69; P < .05). This knockdown was proportional to the knockdown observed in our previous publication,14 where injections were made bilaterally into the same dorsal region.
3.2.2 |. Unilateral infusion of antisense reduced motivation
A representative demand curve 6 days following orexin morpholino antisense in LH unilaterally is compared with the baseline demand (prior to antisense injection; within-subject) in Figure 2E. Overall, unilateral orexin antisense into LH significantly increased α (decreased motivation) (Figure 2F, two-way RM ANOVA, main effect of treatment: F 1,16 = 16.61; P < .001; interaction of treatment × morpholino type: F 1,16 = 5.52; P < .05). No changes were observed in Q0 for either orexin antisense or control missense-infused animals (Figure 2G, two-way RM ANOVA, ns). Of note, the change in α observed here following unilateral injections of morpholino was roughly half that reported in our previous study using bilateral LH orexin knockdown, and this trended towards significance (paired samples t test; t4 = 2.67; p=.056; data not shown).
3.2.3 |. The number of LH orexin neurons correlated with motivation for cocaine
Within antisense-infused animals, we observed a negative correlation between overall LH orexin cell numbers (summed across injected and noninjected hemispheres) and α values, such that animals with higher motivation (lower α values) had greater LH orexin + neurons (Figure 3A; β = −.62; P < .05). In contrast, Q0 values were not significantly correlated with the number of LH orexin cells (Figure 3B; β = .41; p=.09). No associations were observed between the number of Pef/DMH orexin neurons and α (Figure 3C; β = −.35; p=.41) or Q0 (Figure 3D; β = .29; p=.49).
FIGURE 3.
Lateral hypothalamus (LH) orexin neuron number predicts cocaine demand elasticity (α) and low-effort consumption (Q0). A,B, The number of LH orexin neurons predicted α (multiple linear regression, P < .05; A) but not Q0 values (multiple linear regression, ns; B). C,D, There was no significant relationship between Pef/DMH orexin cell number and α (C) or Q0 values (D; multiple linear regression)
We found that the percentage change in α correlated with the amount of LH orexin knockdown in antisense-injected animals when compared with the uninjected hemisphere, such that animals with a greater change in baseline α had a larger LH orexin cell knockdown (Figure 4A; β = −.71; P < .05). There was no such association between the change in Q0 and LH orexin knockdown (Figure 4B, β = .09, p=.78).
FIGURE 4.
Lateral hypothalamus (LH) orexin neuron knockdown predicts antisense-induced changes in cocaine demand elasticity. A, The degree of LH orexin knockdown by antisense infusion (expressed as % orexin neurons in the uninjected hemisphere) negatively correlated with the increase in α value compared with baseline (multiple linear regression, *P < .05). B, The degree of LH orexin knockdown did not predict changes in Q0 post-antisense microinjection (multiple linear regression, ns). C, In antisense-infused animals, there was a nonsignificant relationship between the numbers of LH orexin neurons in the uninjected hemisphere and baseline (pre-antisense injection) α values (multiple linear regression, ns). D, The number of LH orexin neurons did not predict baseline Q0 in the uninjected hemisphere (multiple linear regression, ns)
Finally, we correlated baseline (premorpholino injection) cocaine demand elasticity (α) with the number of orexin-expressing neurons in the uninjected hemisphere (as a measure of endogenous orexin levels). Consistent with Experiment 1, there was a strong trend towards a negative correlation between these two measures, although this failed to reach statistical significance (Figure 4C; β = −.40; p=.33). There was no association between baseline Q0 and LH orexin neurons in the uninjected hemisphere (Figure 4D; β = .24; p=.55).
3.3 |. Experiment 3: Relationship between LH orexin cell number and demand following abstinence
To determine if the relationship between LH orexin expression and demand persisted after abstinence or with locomotor activity, we quantified LH and Pef/DMH orexin cells in BE-experienced animals sacrificed immediately after locomotor testing which followed 2 weeks of abstinence from cocaine self-administration (n = 8). LH orexin cell number inversely correlated with animals’ baseline α (measured 2 wk prior to sacrifice; Figure 5A; Pearson correlation; P < .05), whereas Pef/DMH orexin expression did not (Figure 5B; Pearson correlation; p=.77). This demonstrates that LH orexin cell numbers during abstinence reflected individual differences in motivation for cocaine. We also observed that the number of LH orexin cells did not correlate with total locomotor activity in the test prior to sacrifice (Figure 5C; Pearson correlation; p=.98). This reveals that individual differences in numbers of orexin-expressing neurons in Experiments 1 and 2 were not likely because of differences in general arousal/motor activity. We also found no difference in the number of LH orexin neurons in animals perfused following locomotor testing and animals Experiment 1 (Figure 5D; unpaired samples t test; t18 = 0.12; p=.90).
FIGURE 5.
Numbers of lateral hypothalamus (LH) orexin neurons predict individual differences in α values following abstinence from cocaine. A, LH orexin cell numbers at 2 weeks of cocaine abstinence correlated with animals’ baseline α values measured 2 weeks previously (Pearson correlation, P < .05). B, No relationship was observed between baseline α values and the number of Pef/DMH orexin cells (Pearson correlation, ns). C, The number of LH orexin neurons in locomotor-tested animals did not predict habituated locomotor activity (Pearson correlation, ns). D, Animals sacrificed immediately following locomotor testing (locomotor, 2 wk after cocaine experience) had similar numbers of LH orexin neurons compared with animals sacrificed 90 minutes post-Pmax, indicating that total number of orexin neurons after abstinence did not change from that immediately following cocaine exposure (unpaired samples t test, ns)
4 |. DISCUSSION
We show that the number of orexin cells in LH predicts motivation (demand elasticity) for cocaine. A similar relationship was observed when tissue was collected from rats that underwent 2 weeks abstinence from cocaine. Differences in LH orexin number were not because of variability in arousal state, as orexin expression did not correlate with general locomotor activity. In addition, knockdown of orexin signaling reduced demand (α) but not low effort consumption of cocaine (Q0), and degree of knockdown correlated with the change in α following knockdown, consistent with other studies from our lab.14,30 These studies are consistent with the view that orexin mediates the motivational properties of cocaine without influencing the hedonic value of the drug.9,11 Taken together, our results indicate that the number of orexin-expressing neurons in LH is a determinant of individual differences in cocaine motivation.
4.1 |. High motivation animals have greater orexin expression in lateral hypothalamus
Across all three experiments, animals with more LH orexin cells had higher motivation for cocaine. In Experiment 1, high motivation (low demand elasticity) animals had greater LH orexin expression than low motivation animals (high demand elasticity) animals. In Experiment 2, the number of remaining LH orexin cells following orexin morpholino antisense knockdown correlated with demand elasticity for cocaine, and the change in LH orexin cell number correlated with the change in α value. The relationship between demand elasticity and LH orexin neuron number does not require the presence of the drug or drug-paired context because in Experiment 3, the number of LH orexin cells in animals sacrificed immediately after general locomotor testing (after 2 wk of abstinence) correlated inversely with cocaine demand elasticity 2 weeks prior. Importantly, across all experiments, there was no relationship between total cocaine intake and LH orexin cell number. Our results extend prior findings that prolonged drug exposure can increase numbers of LH orexin-expressing cells,14,19,21 by indicating that the number of LH orexin neurons per se predicts drug demand, independent of the amount of prior consumption. Taken together, these results are consistent with our hypothesis that orexin signaling is particularly important in highly motivated individuals,20 and that the number of LH orexin neurons could serve as a biomarker of addiction susceptibility.
We proposed that orexin signaling translates motivational drive into behavioral output,9 particularly in response to drug-associated cues and contexts.30–33 Our lab has shown that LH orexin neurons in particular mediate reinstatement of drug-seeking elicited by drug-associated stimuli.10–12,29 Thus, higher expression and/or activation of these cells may attach greater motivational significance to cocaine-associated stimuli and result in greater motivation (demand) for cocaine. A recent study in hypocretin/orexin knockout mice demonstrated that animals lacking the neuropeptide have reduced incubation of cocaine craving and do not reinstate drug-seeking in response to cocaine-paired cues.34 Greater numbers of cells promoting cue reactivity in high motivation animals may also explain our previous finding that animals with low α measured in BE have greater cue-induced reinstatement of cocaine seeking.23 Therefore, a greater number of LH orexin-expressing cells may result in higher cue reactivity and greater drug-seeking in highly motivated animals.
Although we did not directly assess the pathways through which orexin mediates motivation for cocaine, other studies indicate that VTA is likely an important target region. Significant data indicates that orexin neurons drive motivational processes via projections to ventral tegmental area (VTA).9 Orexin potentiates cocaine-induced plasticity of VTA DA neurons in vitro,4,35 and orexin-glutamate interactions in VTA are necessary for cue-associated cocaine seeking.5 Blocking orexin-1 receptor signaling in VTA reduces cocaine-seeking5,8 or effortful self-administration,7 whereas infusion of orexin A into VTA reinstates extinguished cocaine seeking.12,36 Orexin input to VTA increases DA release in nucleus accumbens (NAc),37–39 which is associated with goal-directed behavior.40 Greater LH orexin input to the mesolimbic dopamine system may drive greater cocaine-taking in high motivation animals and future experiments could seek to determine if greater LH orexin input to VTA circuit specifically mediates motivation for cocaine.
Consistent with a selective role for the orexin system in motivated responding for cocaine, we generally saw no relationship between orexin cell numbers and low-effort cocaine intake. We did not observe a difference in the number of orexin cells between high and low takers (high and low Q0) in Experiment 1. Also, we observed no change in Q0 following antisense injections (Experiment 2), indicating no causal relationship between orexin cell numbers and low-effort cocaine intake. These findings are consistent with previous studies from our lab and others indicating that blockade of orexin-1 receptor signaling has no effect on low effort (FR1) cocaine consumption.7,31,38,41,42
4.2 |. Unilateral LH orexin knockdown is sufficient to reduce cocaine demand
Unilateral orexin knockdown with orexin morpholino antisense was sufficient to reduce motivation for cocaine (increase demand elasticity), but not low effort consumption (Q0). Peak selective knockdown of orexin A protein occurs 6 days post-antisense without affecting expression of interdigitated neurons containing melanin-concentrating hormone.14,28,29 Although other studies have shown no effect of unilateral orexin inhibition or knockdown on behavior,5,29 ours is the first to examine unilateral orexin knockdown during BE performance requiring high levels of motivation. It may be that orexin neurons bilaterally project to reward-associated areas like VTA, and unilateral knockdown reduces orexin input to both hemispheres in the target brain area. Orexin collateralization in reward-associated areas has largely been unexplored. Thus, future anatomical studies should investigate the extent to which orexin inputs collateralize in reward-associated regions like VTA.
4.3 |. Lateral hypothalamus orexin neuron number correlates with motivational but not arousal state
Our findings revealed that the number of orexin neurons in LH, but not in Pef/DMH, predicted motivation for cocaine as assessed by the BE paradigm (demand elasticity). Our lab also recently determined that knockdown of orexin in LH, but not in Pef/DMH, reduced motivation for cocaine.14 We also observed that the number of LH orexin neurons did not correlate with locomotor activity, indicating that differences in cell number were not related to nonspecific changes in, eg, arousal state. Taken together, these results support our lab’s hypothesis that LH orexin cells are preferentially involved in regulating motivational processes and are less important for arousal/stress regulation.11,43,44
Our results are consistent with several studies from our lab and others indicating that individual differences in the LH orexin system are associated with reward/motivation levels. LH orexin neurons and mRNA expression is upregulated in animals with high motivation for drugs.14,19,21 In addition, Fos expression, specifically in LH orexin cells, correlates with preference for both morphine and cocaine.12,29,45–47 The preferential involvement of LH orexin cells in drug-seeking may be mediated by distinct inputs or outputs compared with Pef/DMH cells,6,48 though anatomical evidence thus far remains unclear.
Collectively, these studies demonstrate that the number of LH orexin neurons is a significant factor contributing to individual differences in motivation for cocaine and may be an important element in addiction pathology.
ACKNOWLEDGEMENTS
We would like to thank Mr. Griffin Poole for assistance with behavioral procedures and histology and Ms. Ami Gadhia for her assistance with the behavioral component of the cocaine self-administration experiments. These experiments were supported by the US Public Health Service awards from the National Institute of Drug Abuse to G.A.J. (R01 DA006214), C.B.P. (F31DA042588), B.S.B (F30DA035065), M.H.J (K99DA045765), and C.J. Martin Fellow from the National Health and Medical Research Council of Australia to M.H.J (No. 1072706).
Funding information
C.J. Martin Fellowship from the National Health and Medical Research Council of Australia, Grant/Award Number: 1072706; U.S. Public Health Service, Grant/Award Numbers: NIDA F30DA035065, NIDA F31DA042588 and NIDA R01DA006214
REFERENCES
- 1.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Miwa Y, Yamanaka A, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92(3):259–266. [DOI] [PubMed] [Google Scholar]
- 4.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. [DOI] [PubMed] [Google Scholar]
- 5.Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl). 2013;226(4):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. [DOI] [PubMed] [Google Scholar]
- 7.Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR.The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31(2):336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James MH, Charnley JL, Levi EM, et al. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14(5):684–690. [DOI] [PubMed] [Google Scholar]
- 9.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James MH, Mahler SV, Moorman DE, Aston-Jones G. A decade of orexin/hypocretin and addiction: where are we now? Curr Top Behav Neurosci. 2017;33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. [DOI] [PubMed] [Google Scholar]
- 13.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci. 2016;43(5):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 2018;85(11):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez MF, Moorman DE, Aston-Jones G, Becker HC. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016;1636:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats. Alcohol. 2009;43(5):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 2017;1654(Pt A:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol. 2011;162(4):880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James MH, Bowrey HE, Stopper CM, Aston-Jones G. Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci. 2018; In press. 10.1111/ejn.14166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thannickal TC, John J, Shan L, et al. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med. 2018;10(447): eaao4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl). 2013;226(1):113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A. 2014;111(32):11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–198. [DOI] [PubMed] [Google Scholar]
- 25.McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to accumbens core pathway is recruited in a dopamine-dependent manner to drive cued reinstatement of cocaine seeking. J Neurosci. 2016;36(33):8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. 2007. Academic Press: London, UK. [Google Scholar]
- 27.Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32(38):13309–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reissner KJ, Sartor GC, Vazey EM, Dunn TE, Aston-Jones G, Kalivas PW. Use of vivo-morpholinos for control of protein expression in the adult rat brain. J Neurosci Methods. 2012;203(2):354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32(13):4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41(9):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30(3):493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L, Ghee SM, Chan C, et al. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340(3):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58(1):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner N, Rossetti C, Sakurai T, et al. Hypocretin/orexin deficiency decreases cocaine abuse liability. Neuropharmacology. 2018; 133:395–403. [DOI] [PubMed] [Google Scholar]
- 35.Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28(8):1545–1556. [DOI] [PubMed] [Google Scholar]
- 36.Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65(10):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baimel C, Lau BK, Qiao M, Borgland SL. Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep. 2017;18(6):1346–1355. [DOI] [PubMed] [Google Scholar]
- 38.Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl). 2011;214(2):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prince CD, Rau AR, Yorgason JT, Espana RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Nerosci. 2015;6(1):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front Biosci (Elite Ed). 2013;5:273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borgland SL, Chang SJ, Bowers MS, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aston-Jones G, Smith RJ, Sartor GC, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29(10):571–577. [DOI] [PubMed] [Google Scholar]
- 45.Richardson KA, Aston-Jones G. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J Neurosci. 2012;32(11):3809–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasheras MC, Laorden ML, Milanes MV, Nunez C. Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology. 2015;95:168–180. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur J Neurosci. 2012;35(9):1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]