Abstract
Rotavirus vaccination has substantially reduced the incidence of rotavirus-associated gastroenteritis (RVGE) in high-income countries, but vaccine impact and estimated effectiveness are lower in low-income countries for reasons that are poorly understood. We used mathematical modeling to quantify rotavirus vaccine impact and investigate reduced vaccine effectiveness, particularly during the second year of life, in Malawi, where a monovalent vaccine was introduced in October 2012 with doses at 6 and 10 weeks. We fitted models to twelve years of pre-vaccination data and validated against post-vaccination data to evaluate the magnitude and duration of vaccine protection. The observed rollout of vaccination in Malawi was predicted to lead to a 26–77% decrease in the overall incidence of moderate-to-severe rotavirus-associated gastroenteritis (RVGE) in 2016, depending on assumptions about waning of vaccine-induced immunity and heterogeneity in vaccine response. Vaccine effectiveness estimates were predicted to be higher among 4 to 11-month-olds than 12 to 23-month-olds, even when vaccine-induced immunity did not wane, due to differences in the rate at which vaccinated and unvaccinated individuals acquire immunity from natural infection. We found that vaccine effectiveness during the first and second years of life could potentially be improved by increasing the proportion of infants who respond to vaccination or lowering the rotavirus transmission rate. An additional dose of rotavirus vaccine at 9 months of age was predicted to lead to higher estimated vaccine effectiveness, but only modest (5–16%) reductions in RVGE incidence over the first three years following introduction regardless of assumptions about the waning of vaccine-induced immunity.
One-Sentence Summary:
Administering an additional dose of rotavirus vaccine at 9 months of age in Malawi is predicted to lead to only modest improvements in vaccine impact.
Introduction
Rotavirus is a leading cause of morbidity and death from diarrhea in children worldwide (1, 2). Although the recent introduction of rotavirus vaccines has had a substantial impact on the incidence of rotavirus-associated gastroenteritis (RVGE) in high- and middle-income countries, the impact of vaccination in low-income countries is not as well defined (3). The vast majority of deaths due to rotavirus occur in low-income countries in Asia and Africa (1, 2), but estimates of vaccine efficacy are lower in these settings. Whereas vaccine efficacy was 85–99% in clinical trials conducted in high-income countries (4–7), efficacy was 39–67% in trials conducted in low-income countries for all currently available oral rotavirus vaccines (8–12). Nevertheless, the preventable burden of disease in low-income settings is potentially much greater (13).
The overall impact of a vaccination program results from both the direct and indirect effects of vaccination (14). Two key questions regarding the long-term impact of rotavirus vaccines are whether the direct protection conferred by rotavirus vaccination is reduced in low-income countries due to a poorer initial immune response or waning of vaccine-induced immunity, and whether vaccination will confer sustained indirect (herd) protection similar to that observed in high-income countries (15).
Concerns have been raised that vaccine-induced immunity to rotavirus wanes among infants from low-income settings. These concerns are based on estimates of the observed individual-level direct effect of vaccination. Rotavirus vaccine efficacy, measured under the idealized conditions of randomized controlled trials, has tended to be lower during the second year of follow-up in low-income settings, and correlated with lower titers of serum IgA mounted in response to vaccination for both the Rotarix (RV1) and RotaTeq (RV5) vaccines across multiple countries (16). Lower vaccine effectiveness during the second year of life, measured under the real-world conditions of post-licensure studies, has also been reported in some, but not all, low-income settings (17). However, commonly used estimates of vaccine efficacy and effectiveness on which inferences about waning immunity are based may be biased because they fail to take into account immunity from previous infections (18, 19). Nevertheless, it has been suggested that an additional dose of rotavirus vaccine at 9 months of age can improve vaccine effectiveness and thereby vaccine impact, especially in the second year of life (20). Parenteral rotavirus vaccines are also in development, and may lead to a more robust and durable immune response particularly in low-income settings (21, 22).
In high-income countries, the introduction of rotavirus vaccination was followed by greater reductions in RVGE incidence than expected from the direct protection of the vaccine alone, including among age groups too old to have received the vaccine, providing evidence of indirect (or herd) protection (23–26). Observed patterns of indirect protection in high-income countries are similar to those predicted by mathematical models (27–29). However, similar evidence of indirect protection in low-income countries has been limited (30, 31).
Here we use mathematical models to investigate the potential waning of vaccine-induced immunity and to better understand the role of indirect protection in determining the overall impact of vaccination in a low-income setting. We utilized data from Blantyre, Malawi, where we had over 10 years of pre-vaccination surveillance data (32, 33), as well as data from vaccine efficacy trials and recently conducted vaccine effectiveness and impact studies (10, 30, 34–36). We simulated models with and without waning of vaccine-induced immunity and heterogeneity in vaccine response (Table 1) to explore the concordance between model predictions and the observed impact of vaccination. We examined how common vaccine effectiveness estimates vary depending on model assumptions. Validated models were then used to evaluate strategies to improve vaccine effectiveness measures, including increasing the proportion who respond to vaccination, lowering the rotavirus transmission rate, and administering an additional dose of vaccine at 9 months of age.
Table 1.
Assumptions about vaccine-induced immunity and corresponding models.
| Assumption | Description | Model 1* | Model 2* | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| Waning of vaccine-induced immunity | No | Vaccine-induced immunity is comparable to natural immunity, lowering the risk of reinfection and probability of RVGE given infection; this immunity does not wane | X | X | ||
| Yes | Vaccine-induced immunity provides complete protection against infection with rotavirus; this immunity wanes over time, leaving the infant with the same susceptibility to rotavirus infection and RVGE as prior to vaccination | X | X | |||
| Heterogeneity in vaccine response | No | The probability of responding to each vaccine dose is independent; infants who responded to the first vaccine dose have the same probability of responding to the second vaccine dose as those who failed to respond | X | X | ||
| Yes | Infants who responded to the first dose of the vaccine are more likely to respond to subsequent doses, whereas infants who failed to respond to the first dose are more likely to be “non-responders” (they would fail to respond to rotavirus vaccination regardless of the number of doses administered) | X | X | |||
Models 1 and 2 were not fitted to the post-vaccination data. RVGE, rotavirus-associated gastroenteritis.
Results
Model fit
We modified a previously developed mathematical model of the transmission dynamics of rotavirus (27, 28, 37) to explicitly test hypotheses about the nature of vaccine-induced immunity (Table 1, fig. S1). We fitted our models to pre-vaccination data on the number of RVGE cases among children <5 years of age at Queen Elizabeth Central Hospital (QECH), Blantyre, between July 1997 and December 2009 (Fig. 1, table S1). The pre-vaccination model captured the age distribution of RVGE cases, although it slightly underestimated cases among 5- and 12-month-olds and slightly overestimated cases among 8- to 10-month-olds. In Blantyre the basic reproductive number (R0) of rotavirus, defined as the expected number of secondary infections caused by a primary case in a fully susceptible population, was estimated to be 78.8 (95% credible interval (CrI): 70.5–96.2) (table S1), but this estimate depended on assumptions about the relative infectiousness of subclinical infections and duration of immunity following infection (table S2).
Fig. 1. Model fitted to pre-vaccination rotavirus hospitalization data.
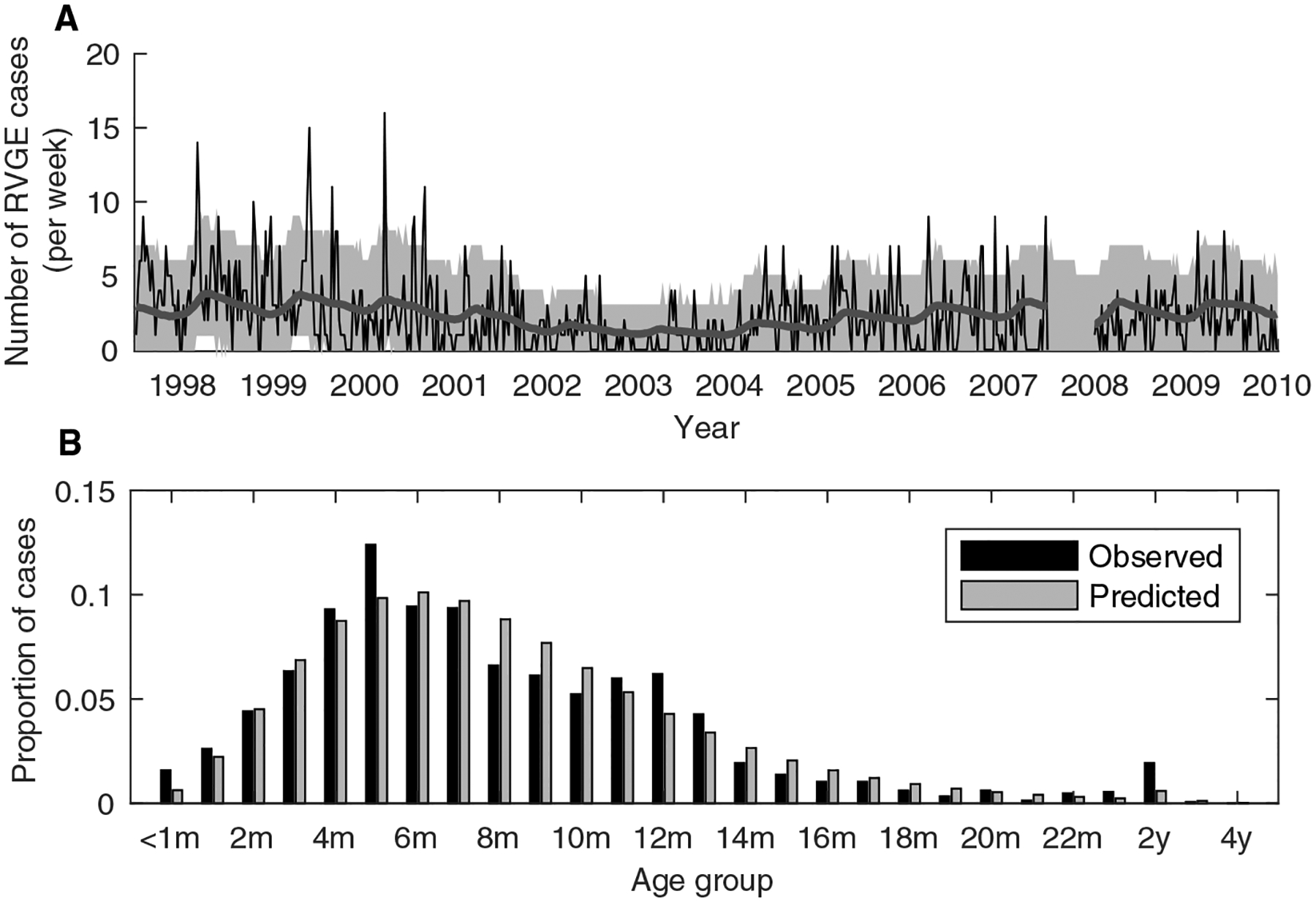
(A) Observed (thin black line) and model-predicted (thick grey line) number of rotavirus-associated gastroenteritis (RVGE) cases per week in children <5 years of age (n=1452). The model prediction corresponds to the average number of cases per week predicted by the best-fit model (that is, the model simulated from the maximum a posteriori parameter estimates). The grey shaded region corresponds to the prediction interval when accounting for parameter uncertainty and the Poisson-distributed observation process (based on 1,000 samples drawn from the posterior distribution of fitted model parameters). (B) Proportion of cases in each age group for the observed data (black bars) and best-fit model (grey bars).
Predicted impact of vaccination
Malawi introduced rotavirus vaccination on October 28, 2012, with two doses of Rotarix administered orally at 6 and 10 weeks of age (36). Vaccine coverage among age-eligible children in Blantyre increased rapidly, with ≥96% of infants receiving at least one dose by June 2014 (fig. S2).
The observed rollout of rotavirus vaccination was predicted to lead to a 12.2–27.0% reduction in the overall incidence of RVGE cases at QECH during the first year following vaccine introduction depending on our assumptions about waning of vaccine-induced immunity and the probability of responding to each dose (Table 2, Fig. 2). By 2016, the predicted reduction in overall incidence was 25.6–76.7%, while the observed incidence of RVGE was 50.8% lower than the pre-vaccination period (Table 2, fig. S3). Models 3 and 4, which assumed vaccination provides temporary complete immunity that wanes over time, predicted the smallest decrease in the overall incidence. In contrast, Model 1, which assumed an equal probability of responding to each vaccine dose and vaccine-induced immunity that provides partial but long-lasting protection (comparable to natural immunity), predicted the largest decrease (Fig. 2, fig. S3). The greatest reduction in incidence was predicted to occur among infants 4–11 months of age, with more modest reductions in incidence predicted among 0- to 3- and 12- to 23-month-olds in all models; a slight increase in cases among 12- to 23-month-olds was predicted when we assumed waning of vaccine-induced immunity (Table 2).
Table 2. Observed and model-predicted vaccine impact.
The observed reduction in annual incidence of rotavirus gastroenteritis (RVGE) cases compared to the incidence predicted in the absence of vaccination is shown. Overall incidence is reported as the number of cases per 100,000 children <5 years of age in Blantyre city, and age-specific incidence is reported as the observed number of cases per year.
| Observed (percent reduction1) | Predicted2 (percent reduction1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-vaccin-ation4 [range] | 123.2 [40.4–267.7] | 18.1 [3–35] | 76.1 [15–156] | 21.4 [8–46] | 122.5 [56.3–192.4] | 16.5 [7.2–25.3] | 77.5 [33.6–117] | 21.6 [12.8–29.8] |
| 20125 | 156.1 (−6.1%) | 15 (46.3%) | 144 (−6.3%) | 46 (−44.0%) | 147.6 (<1%) | 28.7 (<1%) | 136.3 (<1%) | 31.0 (<1%) |
| 2013 | 136.1 (14.6%) | 8 (73.7%) | 101 (31.7%) | 75 (−72.6%) | 116.5–140.2 (12.2–27.0%) | 20.4–27.9 (10.8–34.9%) | 103.2–126.7 (14.9–30.7%) | 38.2–40.8 (3.3–9.4%) |
| 2014 | 87.5 (44.2%) | 5 (83.3%) | 70 (52.1%) | 46 (4.0%) | 61.2–119.6 (23.8–61.0%) | 13.7–24.2 (21.3–55.7%) | 48.8–99.4 (32.4–66.8%) | 23.6–47.6 (−2.3–49.2%) |
| 2015 | 106.2 (26.0%) | 6 (78.3%) | 82 (39.3%) | 55 (−31.9%) | 36.4–105.6 (26.6–74.7%) | 10.3–21.2 (25.4–63.8%) | 27.5–81.7 (40.0–79.8%) | 13.0–43.7 (−7.7–67.9%) |
| 2016 | 56.9 (50.8%) | 6 (73.3%) | 48 (56.5%) | 23 (28.2%) | 27.0–86.1 (25.6–76.7%) | 8.3–17.4 (24.7–64.2%) | 21.3–66.2 (40.4–80.8%) | 8.4–33.9 (−9.0–73.1%) |
| 2013–2016 | 95.8 (32.9%) | 25 (77.4%) | 301 (44.2%) | 199 (−20.5%) | 77.7–119.8 (21.7–58.3%) | 52.7–90.9 (20.1–53.7%) | 200.8–374.0 (31.1–63.0%) | 83.3–165.9 (−3.4–48.1%) |
Percent reduction is relative to the model-predicted incidence (per 100,000 children <5 years old) and annual number of cases in the corresponding years in the absence of vaccination.
Range in best-fit model predictions is given for the four models of response to vaccination.
Cases per 100,000 children <5 years old per year, based on population census estimates for Blantyre city. Population estimates by age were only available through 2014; estimates for 2015 and 2016 (and for the post-vaccination period from 2013–2016) are based on the most recent (2014) data for the size of the population <5 years old.
Mean annual overall incidence and cases by age for 1998–2006 and 2008–2009.
Rotavirus vaccination (RV1) was introduced October 28, 2012, with routine doses administered at 6 and 10 weeks of age.
Fig. 2. Observed and model-predicted vaccine impact by age.
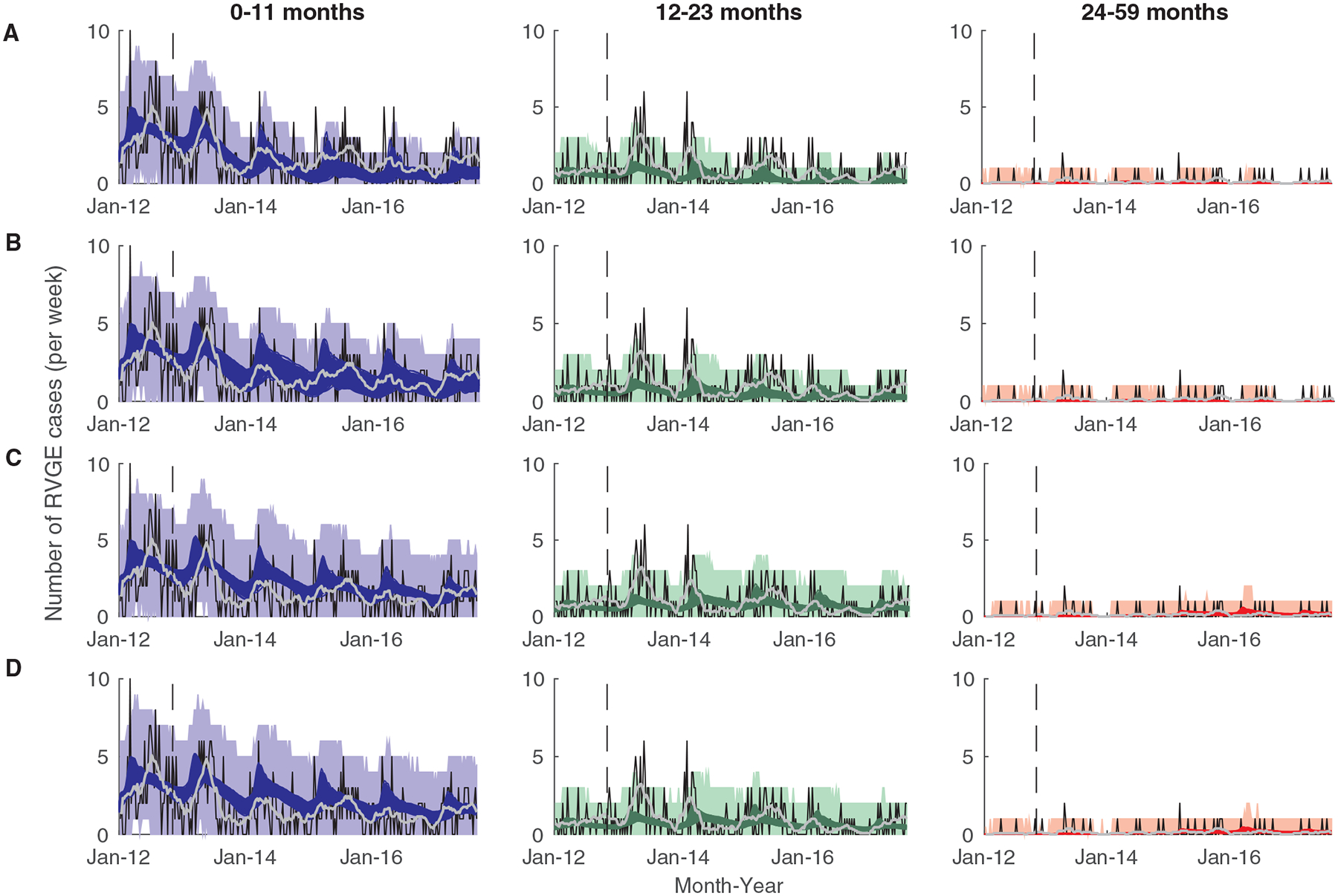
The observed (thin black lines) and model-predicted (colored lines) number of rotavirus-associated gastroenteritis (RVGE) cases per week at QECH are plotted for cases 0 to 11 months (n=539; left, blue), 12 to 23 months (n=269; middle, green), and 24 to 59 months old (n=35; right, red) for (A) Model 1, (B) Model 2, (C) Model 3, and (D) Model 4. The light grey line is the 13-week moving average of the observed number of cases, and the shaded colored regions represent the prediction intervals of the models accounting for parameter uncertainty and observation error. The vertical dashed line represents the time of vaccine introduction.
The range of model predictions was generally consistent with the observed number of cases (Table 2), and with the 33% decline in rotavirus positivity previously reported (30). However, none of our models captured the observed increase in RVGE cases among 12–23-month-olds in the first year following vaccine introduction (Fig. 2, Table 2). Our models also predicted smaller declines in incidence among 0- to 3-month-olds than observed (Table 2, fig. S3), although there may have been less effort to test infants who were too young to have been vaccinated. The model assuming waning of vaccine-induced immunity and homogeneity in vaccine response (Model 3) provided the best fit to the post-vaccination data, whereas Model 1 provided the worst fit (Fig. 2, table S3). However, Models 3 and 4 tended to slightly over-predict cases among vaccinated individuals, whereas Model 1 and (to a lesser extent) Model 2 tended to under-predict cases among vaccinated individuals (fig. S4).
Vaccine effectiveness estimates
To demonstrate how commonly used estimates of vaccine effectiveness based on estimates of relative odds or risk (19) may be biased and not reflect “true” underlying vaccine-conferred protection (as assumed in the models), we compared the predicted vaccine effectiveness estimates from the four models to one another and to those calculated from an ongoing case-control study. The model-predicted effectiveness estimates varied depending on assumptions about the probability of responding to each dose and the waning of vaccine-induced immunity, and were substantially higher during the first versus second year of life regardless of assumptions about waning of vaccine-induced immunity (Fig. 3). For Model 1, estimated vaccine effectiveness was slightly higher than the observed (VEobs,1=77.0%, 95% confidence interval [CI]: 51.8–89.0%; VEobs,2=21.7%, 95% CI: −164.8–76.8%) (Fig. 3). When we assumed heterogeneity in vaccine response (Model 2), the estimated vaccine effectiveness was predicted to be substantially lower during both the first (VE1=59.5%, 95% PI: 44.8–73.6%) and second year of life (VE2=36.8%, 95% PI: 17.0–57.8%), consistent with the observed effectiveness estimates. Predicted vaccine effectiveness estimates were considerably lower than the observed estimates during the first year of life and were negative during the second year of life when we assumed vaccine-induced immunity waned over time (Models 3 and 4) (Fig. 3). The duration of vaccine-induced immunity was estimated to be 32 weeks (95% CrI: 27–41 weeks) when we assumed the probability of responding to each dose was independent (Model 3), or 45 weeks (95% CrI: 32–86 weeks) if we assumed heterogeneity in vaccine response (Model 4).
Fig. 3. Observed and predicted vaccine effectiveness estimates by age.
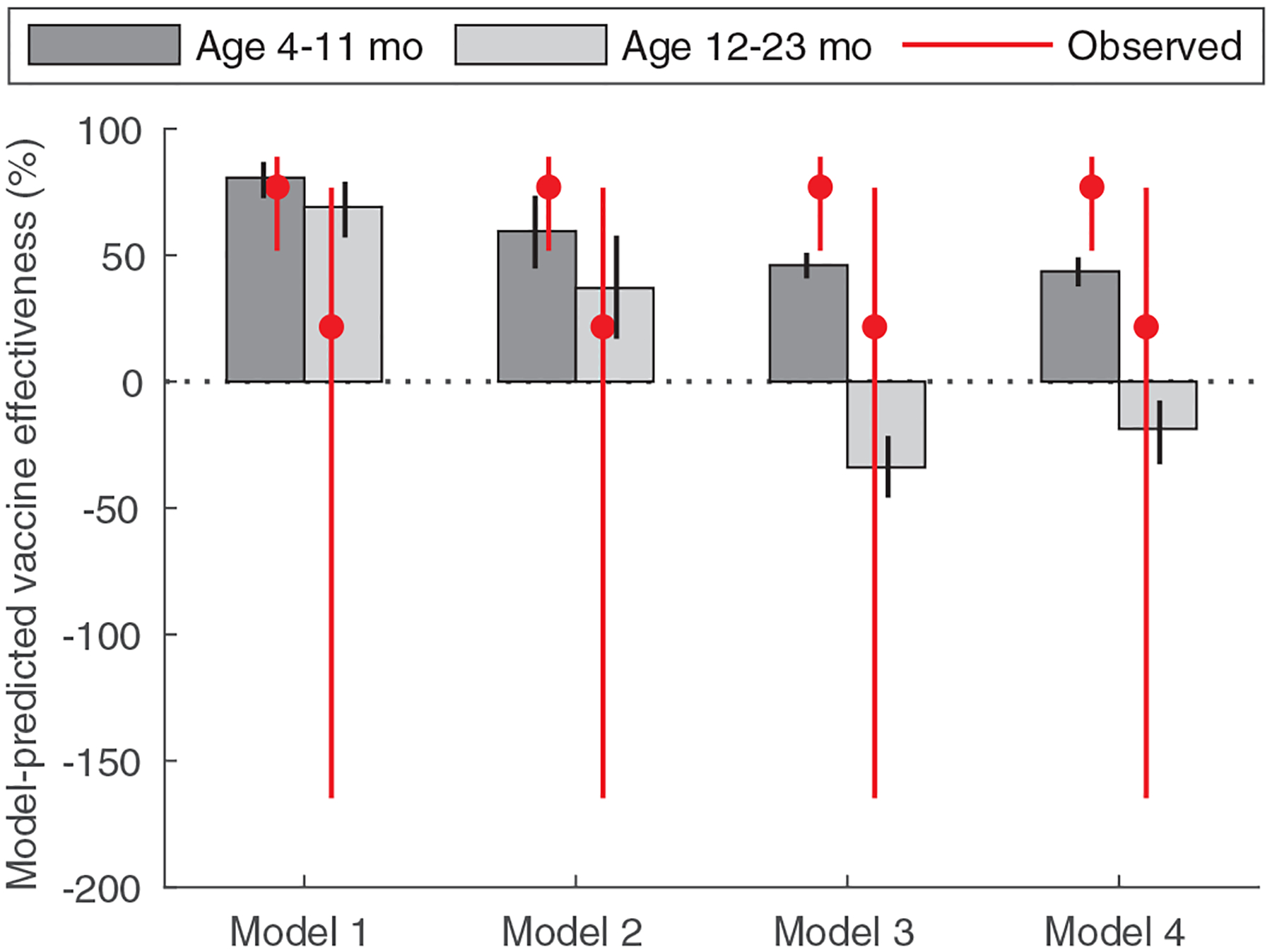
Observed (red lines) and model-predicted (grey bars) vaccine effectiveness estimates are plotted for children 4 to 11 months (n=278 cases, 655 controls) and 12 to 23 months of age (n=113 cases, 291 controls) under the four different model assumptions. The red circles represent the estimated mean vaccine effectiveness, and the red lines show the 95% confidence intervals. The black error bars represent the 95% prediction intervals associated with parameter uncertainty.
Indirect effect estimates
The indirect effect predicted by the models was modest (IE=19.9%, 95% PI: 16.3–21.6% for Model 1), and did not vary substantially based on uncertainty in model parameters or assumptions about heterogeneity in vaccine response and waning of vaccine-induced immunity (table S4). Indirect protection was predicted to be higher among 0- to 11-month-olds (IE1=32.4–35.7% depending on model assumptions) than among 12- to 23-month-olds (IE2=9.8–13.5%) (table S4). The overall incidence of RVGE among <5-year-olds predicted by Models 1 to 4 was similar to that predicted by the corresponding models with direct protection only, but incidence was predicted to be lower among 0- to 3-month-olds and higher among 12- to 23-months-old when accounting for both direct and indirect protection (table S5).
Strategies to improve vaccine effectiveness and impact
We modeled three different scenarios for improving vaccine performance, as measured by estimated vaccine effectiveness and overall impact: (1) increasing the proportion of infants who seroconvert to the first and second dose of vaccine; (2) reducing the transmission rate of rotavirus through non-vaccine interventions; and (3) administering an additional vaccine dose at 9 months of age.
The predicted vaccine effectiveness estimates depended strongly on the proportion who respond to each dose (Fig. 4A,B). Estimates of effectiveness among 12- to 23-month-olds varied from −81.2% when only 40% of individuals responded to each dose to 12.2% when 100% of infants responded to each dose, assuming waning of vaccine-induced immunity (Model 3). Results were similar for the other models (figs. S5–S6).
Fig. 4. Relationship between the vaccine response, the basic reproductive number, and model-predicted vaccine effectiveness estimates.
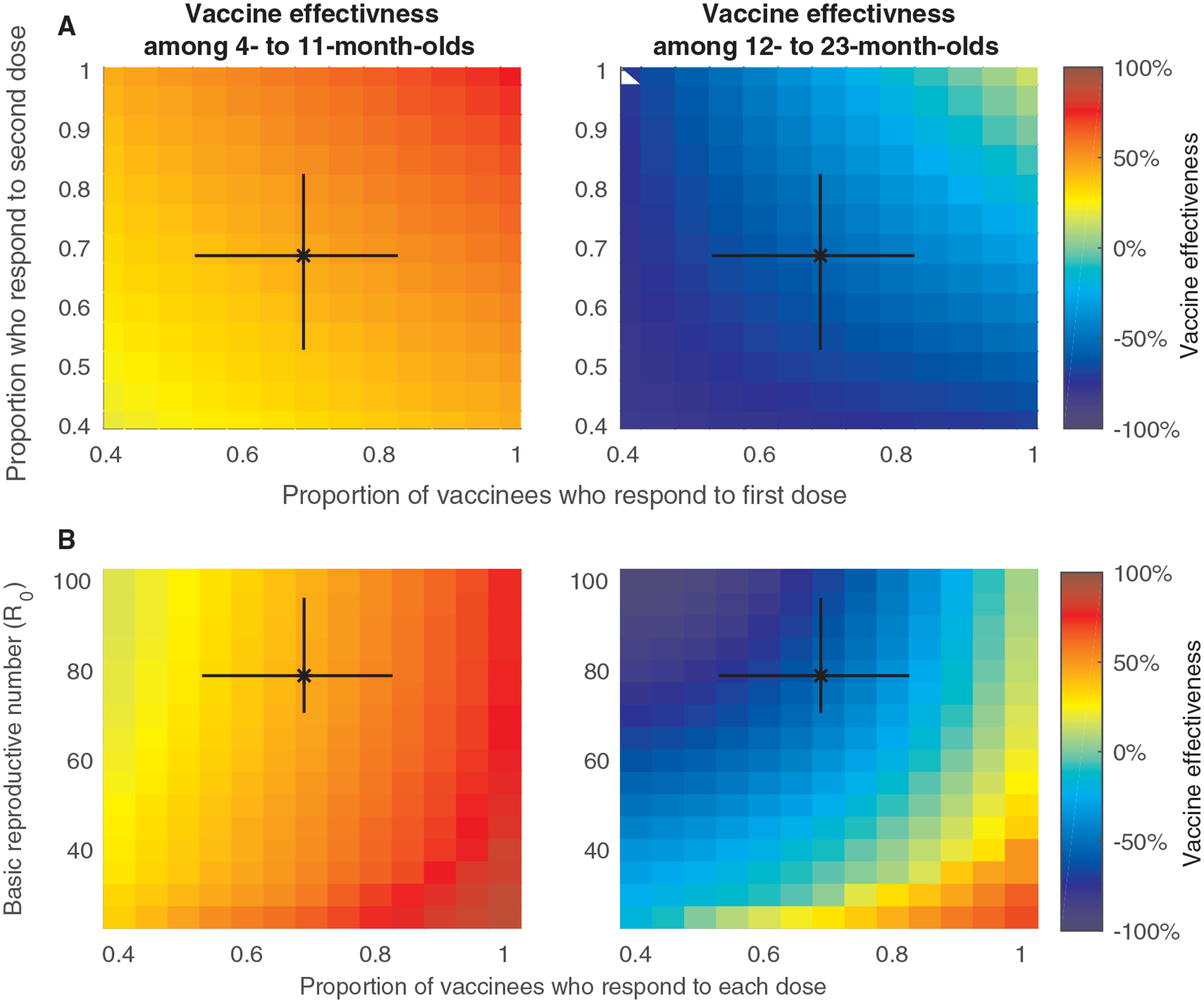
The model-predicted vaccine effectiveness estimates among (A) infants 0 to 11 months old and (B) children 12 to 23 months old is indicated by the color bar for different values of the proportion of infants who respond to the first and second dose for Model 3 (the model assuming waning of vaccine-induced immunity and no heterogeneity in vaccine response). The model-predicted vaccine effectiveness estimates among infants (C) 0 to 11 months old and (D) children 12 to 23 months old is indicated by the color bar for different values of the basic reproductive number (R0) and the proportion of infants who respond to each dose in Model 3. The black bars represent the 95% CrI of the estimated parameters for our model fitted to the pre-vaccination data for Blantyre, Malawi.
The transmission rate of rotavirus, quantified by R0, also influenced the model-predicted vaccine effectiveness estimates (Fig. 4C and D). For any given value of the proportion who respond to each dose, effectiveness estimates were highest when R0 is low, and lower when R0 is high. Estimated vaccine effectiveness during the second year of life was lowest for values of R0>50, similar to that estimated for Blantyre, particularly when the proportion who respond to each dose is <80%. When R0 is low (≤30) and the proportion responding to each dose is high (≥90%), similar to the situation in most high-income countries (27, 28, 38), vaccine effectiveness during the first and second year of life were both predicted to be >60%, even when vaccine-induced immunity wanes. The predicted degree of indirect protection varied between 15.9% and 50.8%, and was higher among 0- to 11-month-olds, particularly when R0 was low and the proportion of infants who responded to each dose was high (fig. S7).
An additional vaccine dose at 9 months of age was predicted to lead to a slightly higher estimated vaccine effectiveness in infants 4 to 11 months of age (VE1 = 82.9%, 95% PI: 75.0–88.6% for Model 1) and considerably higher values during the second year of life (VE2 = 88.3%, 95% PI: 82.0–92.8%) (Fig. 5A). However, estimated effectiveness was predicted to increase over time in all models even without the additional vaccine dose as vaccine coverage stabilized and the force of rotavirus infection declined (figs. S8 to S10). Compared to the modeled impact of the current two-dose schedule, the model-predicted incidence of RVGE was reduced by 32.5% (95% PI: 24.6–39.2%) among 12- to 23-month-olds over the first three years, but the overall incidence of RVGE predicted by Model 1 was only 16.2% (95% PI: 11.8–20.3%) lower than predicted without the additional dose (Fig. 5B and F). The predicted overall impact of an additional 9-month dose of rotavirus vaccine was similar (11.5%, 95% PI: 8.4–14.6%) if we assumed vaccine-induced immunity waned over time (Model 3), but was lower (5.2–8.6%) if we assumed heterogeneity in vaccine response (Models 2 and 4) (Figure 5C to F). A similar reduction in incidence could be achieved by increasing the proportion who respond to each dose of the vaccine by 7–12% (equivalent to a 54–57% seroconversion rate) when assuming homogeneity in vaccine response (Models 1 and 3) or by increasing the proportion who respond to the first dose of vaccine by 3–11% when assuming heterogeneity in vaccine response (Models 2 and 4), or a 20–45% reduction in R0 (fig. S11).
Fig. 5. Model-predicted vaccine effectiveness and impact of an additional dose of rotavirus vaccine administered at 9 months of age.
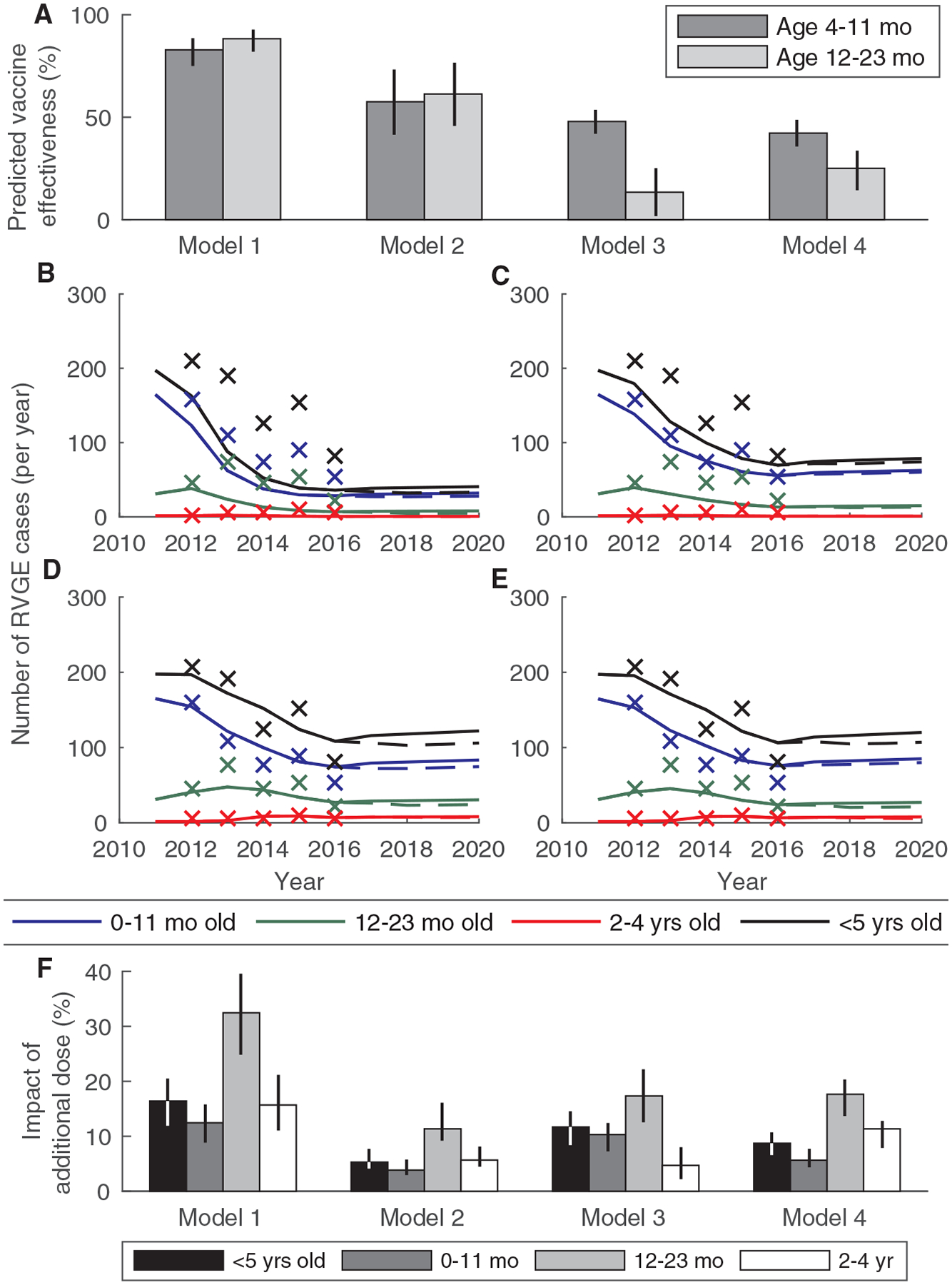
(A) Predicted vaccine effectiveness over two years of follow-up (January 2018 to December 2019) is plotted for infants 4 to 11 months of age and 12 to 23 months of age for the four different models. (B-E) The predicted number of rotavirus-associated gastroenteritis (RVGE) cases presenting to QECH per year for 2011 to 2020 is plotted by age and for all children <5 years of age for the current vaccine schedule (solid lines) and the model including an additional dose at 9 months of age (dashed lines) for (B) Model 1, (C) Model 2, (D) Model 3, and (E) Model 4. The ‘x’s indicate the observed annual number of RVGE cases at QECH in 2012 to 2016. (F) The predicted overall effect (reduction in the relative incidence of RVGE) for the models including an additional dose of vaccine at 9 months of age versus the current vaccine schedule for the 3-year period from January 2018 to December 2020 by age and for all children <5 years old.
Discussion
Using mathematical models fitted to pre-vaccination data on RVGE cases in Blantyre, Malawi, we quantified the overall impact of vaccination resulting from both the direct and indirect effects. We demonstrated that commonly used measures of vaccine effectiveness are expected to be lower for the second year of life even if vaccine-induced immunity does not wane. The observed rotavirus vaccine impact and estimated vaccine effectiveness in Malawi are consistent with either waning of vaccine-induced immunity or heterogeneity in vaccine response. We predicted that an additional dose of rotavirus vaccine at 9 months of age would lead to modest improvements in overall vaccine impact regardless of whether or not vaccine-induced immunity wanes. Similar reductions in RVGE incidence could be achieved with relatively small improvements in the vaccine response rate, since the majority of rotavirus cases still occur during the first year of life.
Estimates of vaccine efficacy and effectiveness during the second year of life will be biased and not reflect the true protection conferred by vaccination, even in clinical trial settings, if they fail to take into account heterogeneity in transmission and the protection conferred by previous infections (19, 39). Vaccinated and unvaccinated infants can no longer be considered randomized after one year of follow-up due to their differential risk of natural infections during the first year of life. Unvaccinated individuals are able to “catch up” to vaccinated individuals as they gain immunity through natural infections, making them more comparable during the second year of life. This will lead to lower estimates of vaccine effectiveness even in the absence of waning immunity, particularly when the rate of (re)infection is high, as it is for rotavirus in Malawi. Therefore, we cannot conclusively determine from the vaccine effectiveness estimates whether or not vaccine-induced immunity is waning. This has been noted previously for other imperfectly immunizing infections, such as malaria (40). Rogawski et al. showed that is possible to partially correct for this bias by controlling for previous episodes of RVGE in the analysis (18). However, infants can also gain partial protection from asymptomatic rotavirus infections (41, 42). Such infections have not been tested for in rotavirus vaccine trials or post-licensure effectiveness studies, and therefore cannot be taken into account using traditional analytical frameworks.
Indirect protection (i.e. herd immunity) is defined as the reduction in incidence among unvaccinated individuals in a population where the vaccine is available compared to the incidence in a completely unvaccinated population (43), and is an important component of overall vaccine impact. Indirect protection has been evident in a number of high-income countries following rotavirus vaccine introduction (23–26, 31), and has played a substantial role in reducing the incidence of RVGE among unvaccinated and age-ineligible children. Estimates of indirect protection in the United States varied from 14–82% during the first four years following vaccine introduction (26). Declines in incidence among age-ineligible children have been noted in Rwanda following vaccine introduction (44). However, no indirect protection was observed during a cluster randomized trial of rotavirus vaccine in Bangladesh (45). In Malawi, some evidence of indirect protection was observed among infants <1 year of age, as noted previously (30), but the number of cases among children 1 year of age and older actually increased relative to the pre-vaccination period, particularly during the first year following vaccine introduction. This may be due in part to variation in reporting effort, as we also observed an increase in the number of rotavirus-negative acute gastroenteritis cases. While our models predict substantial indirect protection among all children <5 years old compared to the incidence rate expected in the absence of vaccination, this was not apparent when comparing the overall impact to that expected from the direct effects of vaccination alone. The latter should not be interpreted as a failure of the vaccine to provide indirect protection, since vaccination is expected to increase the average age of infection (46), and the majority of cases among >1-year-olds have occurred among vaccinated individuals.
Model-predicted estimates of vaccine effectiveness varied depending on both the proportion of infants who respond to each vaccine dose and the rotavirus transmission rate. When the proportion of infants who respond to each dose is high, and when R0 is low (as is the case in most high-income countries (27, 28, 38)), the estimated vaccine effectiveness was predicted to remain high during the second year of life even when vaccine-induced immunity wanes. This suggests other interventions aimed at lowering the rotavirus transmission rate, such as improvements in sanitation and hygiene, could have synergistic benefits for improving vaccine impact. However, when not all infants respond to vaccination, and when R0 is high, estimated vaccine effectiveness is predicted to be lower particularly during the second year of life. Rates of seroconversion to rotavirus vaccination tended to be lower in settings with high under-5 mortality rates (16). The reasons underlying these lower rates of seroconversion are not fully understood, but are likely multifactorial and common to other live oral vaccines (47). Recent studies have suggested interference with oral poliovirus vaccines (48), enterovirus infection (49), and the microbiome (50) may all play a role in explaining the poor response to live oral rotavirus vaccines, which may lead to the development of new strategies to improve vaccine response. Neonatal oral rotavirus vaccines may offer one solution, and demonstrated higher vaccine efficacy than an infant vaccine schedule in one trial (51). Parenteral inactivated rotavirus vaccines are also currently being developed, and may help to overcome the problems associated with live oral vaccines (21, 22).
An additional dose of rotavirus vaccine at 9 months of age has been suggested as a strategy to improve vaccine effectiveness and impact in developing countries. Immunogenicity trials conducted in Bangladesh and Mali have demonstrated that an additional dose of either RV1 (20) or RV5 (52) administered at 9 months of age significantly increased geometric mean titers and rates of IgA and IgG seroconversion. Another recent trial demonstrated significantly higher rates of seroconversion following three doses of RV1 administered at 6, 10, and 14 weeks of age compared to the current two-dose schedule (53). A third dose of vaccine at 14 weeks of age also led to modest improvements in immunogenicity and efficacy in other RV1 trials, including one conducted in Malawi (10, 34, 54), although these trials were underpowered to detect such a difference. However, the potential impact of an additional dose of rotavirus vaccine has yet to be evaluated in the context of larger, programmatic use.
Although an additional dose of rotavirus vaccine at 9 months of age in Malawi was predicted to result in a higher estimated vaccine effectiveness and was associated with a 32% decline in incidence among 12- to 23-month-olds, the predicted reductions in overall incidence were modest (5–16%), since the majority of RVGE cases still occur during the first year of life. This reduction in overall incidence is similar to that predicted by a static model (2–24%) (55). We found the overall impact of an additional dose was lowest when we assumed heterogeneity in vaccine response, since under this assumption, individuals who failed to respond to the first vaccine dose would also be less likely to respond to subsequent vaccine doses. Thus, it will be critical to determine whether an additional dose of rotavirus vaccine at 9 months of age will benefit those who failed to respond to earlier vaccine doses.
There are several limitations of our study that should be considered. As with all modeling studies, we needed to make a number of simplifying assumptions to model the transmission of rotavirus and protection conferred by vaccination. The four models of vaccine protection that we considered here (Table 1) are by no means exhaustive, and represent different extremes with regards to the response to vaccination and waning of vaccine-induced immunity; the reality is likely somewhere in between. We also did not consider potential differences in natural and vaccine-induced immunity against the various rotavirus genotypes. Previous analyses have suggested that the estimated vaccine effectiveness in Malawi is higher against RVGE caused by G1P[8] strains compared to that estimated for G2P[4] and other fully heterotypic strains, but the overall distribution of genotypes causing RVGE in Malawi remains relatively unchanged since vaccine introduction (35). Lastly, we did not have data on previous rotavirus infections and episodes of RVGE among the cases and controls in our vaccine effectiveness study. Therefore, it was not possible to obtain unbiased estimates of vaccine effectiveness by controlling for previous infections (18).
We believe our findings are generalizable to other settings with high rates of rotavirus transmission and low rates of seroconversion following vaccination with oral rotavirus vaccines. Model-predicted vaccine effectiveness estimates varied considerably depending on the basic reproductive number (R0) and proportion of infants who responded to each dose of vaccine. Thus, differences in the rate of transmission of rotavirus may help to explain why estimates of effectiveness have remained high during the second year of life in some low-income settings, but not others (17).
Mathematical modeling provides a valuable tool for understanding the observed impacts of vaccination resulting from both direct and indirect protection, and for evaluating future strategies to improve vaccine impact. Our analysis suggests that future efforts should focus on improving the immune response to vaccination, possibly through the development of novel parenteral rotavirus vaccines or new strategies for the delivery of oral vaccines (for example, a neonatal dose). An additional vaccine dose at 9 months of age was predicted to lead to only modest gains in vaccine impact. However, more studies are needed to gain empirical, unbiased estimates of efficacy and to evaluate the cost-effectiveness of such strategies.
Materials and Methods
Study design
This was a mathematical modeling study with three primary objectives: (1) to evaluate the evidence for waning of vaccine-induced immunity against RVGE, (2) to estimate the level of indirect protection conferred by rotavirus vaccination, and (3) to evaluate strategies to improve rotavirus vaccine effectiveness and impact in a low-income setting. We fitted our model to pre- and post-vaccination data on the weekly number of RVGE cases at QECH in Blantyre, Malawi, and compared to data from a case-control study of vaccine effectiveness in Blantyre. Additional methods and details of the model can be found in the Supplementary Materials and Methods.
Data
The surveillance platform and data have been described previously (32, 33). Briefly, inpatients and outpatients <5 years of age who presented to QECH with acute gastroenteritis (defined as the passage of three or more watery stools in a 24-hour period for <14 days) were enrolled. Written informed consent was obtained from a parent or guardian, and a single stool sample was collected from each child as soon as possible and tested for rotavirus by enzyme immunoassay (Rotaclone; Meridian Diagnostics); positive samples were further characterized by G and P type using reverse-transcription polymerase chain reaction (RT-PCR). During the periods of pre-vaccination surveillance (July 1, 1997 to June 30, 2007 and January 1, 2008 to December 31, 2009), enrollment was restricted to weekdays (Monday through Friday) from 8am to 5pm. Diagnostic stool testing is not a part of routine clinical care at QECH; therefore, data were not available outside of the study periods (July 1 to December 31, 2007 and January 1, 2010 to December 31, 2011).
Enhanced surveillance was initiated at QECH in January 2012 in anticipation of the introduction of rotavirus vaccination (on October 28, 2012) (35, 36); we evaluated the data through August 2017. Enrollment of all children meeting the case definition of acute gastroenteritis occurred Monday through Saturday. A case-control study was initiated on October 29, 2012, to evaluate vaccine effectiveness. Cases were defined as vaccine age-eligible children presenting with acute gastroenteritis who tested positive for rotavirus by enzyme immunoassay, while controls were unmatched vaccine age-eligible children presenting with acute gastroenteritis who tested negative for rotavirus (35, 36). Rotavirus vaccination status was obtained for both rotavirus-positive and negative children from government-issued patient-held “health passports”; those with a missing record (18%) were excluded from estimates of vaccine effectiveness.
Ethical approval for the data collection was obtained from the National Health Sciences Research Committee, Lilongwe, Malawi (867), and from the University of Liverpool Research Ethics Committee (000490).
Statistical analysis
We estimated the observed vaccine effectiveness (VEobs) and corresponding 95% confidence interval (CI) as one minus the odds ratio of receiving two doses of rotavirus vaccine among rotavirus-positive acute gastroenteritis cases versus rotavirus-negative diarrheal controls using logistic regression, adjusting for age and year and month of presentation, as in previous analyses (30, 35).
Model description
We modified a previously developed mathematical model used to describe patterns of RVGE incidence in high-income countries (27, 28, 37), and adapted this model to the low-income-country context. Briefly, the model assumes that individuals are born with maternal immunity that provides protection against rotavirus infection and disease (fig. S1A). Following the waning of maternal immunity, individuals are assumed to be fully susceptible to rotavirus infection and disease. Susceptible individuals are infected at a rate λ(t), also known as the force of infection. Only a fraction of individuals with their first infection will experience moderate-to-severe RVGE. Following infection, we assume individuals gain temporary immunity, then become susceptible to reinfection at a reduced rate. Subsequent infections are assumed to have lower infectiousness, shorter average duration, and lower probability of symptoms. We differentiate among i=1, 2, or 3 or more previous infections, and assume all subsequent infections (i≥3) do not lead to observed cases of moderate-to-severe RVGE. We further divide each state into 1-month age classes for individuals <2 years of age, 1-year age classes for 2- to 4-year-olds, and 5-year age classes for 5- to ≥75-year-olds. The number of individuals in each state is simulated using a series of differential equations.
Modeling vaccination
We simulated the impact of vaccination in two ways to explore assumptions about the potential waning of vaccine-induced immunity (Table 1). First (and in line with previous models (27, 28, 37)), we assumed each vaccine dose mimics one natural infection among those who respond to the vaccine—thus providing partial immunity against reinfection and full protection against moderate-to-severe RVGE following two “successful” vaccine doses and/or natural infections (Models 1 and 2). We included separate compartments for vaccinated individuals, and assumed those who failed to respond to a vaccine dose would stay in their same compartment in the corresponding vaccinated state, while those who responded moved to the next recovered (and vaccinated) compartment (fig. S1A). We also explored the alternative assumption that a single dose of vaccine provided temporary but complete immunity against reinfection with rotavirus among those who responded (Models 3 and 4) (fig. S1B). Following the waning of vaccine-induced immunity, vaccinated infants returned to their previous level of susceptibility, while those who responded to both vaccine doses moved to the next vaccinated (and protected) compartment.
Vaccine coverage in our model was parameterized based on the observed proportion of age-eligible children with rotavirus-negative acute gastroenteritis who had received at least one or two doses of the vaccine collected as part of the case-control study (fig. S2). The numerator consisted of the weekly number of rotavirus-negative controls who were age-eligible and reported having received at least one or two doses of rotavirus vaccine, while the denominator consisted of the weekly number of all age-eligible diarrheal controls who tested negative for rotavirus. We then smoothed the one- and two-dose coverage estimates by calculating the 27-week moving average.
The proportion of individuals who responded following each dose of the vaccine was informed by seroconversion data from the Rotarix vaccine trial conducted in Malawi (34). We assumed that the proportion who seroconverted in the trial (defined as an anti-rotavirus IgA antibody concentration ≥20 U/mL) represents the proportion who received protection equivalent to two natural infections, which in our models would mean the infant was fully protected against moderate-to-severe RVGE. While there is no established correlate of protection for RVGE, a serum IgA concentration ≥20 U/ml has been shown to at least partially predict vaccine efficacy at both the individual and population scale (56). Among those in the two-dose arm of the immunogenicity trial in Malawi, 17/36 (47.2%, 95% CI: 30–64%) of infants seroconverted, while in the three-dose arm, 28/49 (57.1%, 95% CI: 42–72%) of infants seroconverted (34).
We explored two different assumptions for the proportion of infants who responded to each dose (Table 1). First, we assumed the proportion responding to the first and second dose were equal and independent of one another. The probability of responding to each dose (and thus receiving partial protection against RVGE) could be thus estimated by taking the square-root of the rate of seroconversion in the two-dose group. We assumed the probability of responding to a third dose of the vaccine at 9 months of age was the same as the probability of responding to previous doses.
Alternatively, we might assume that there is heterogeneity in vaccine response, such that individuals who failed to respond to the first dose may be less likely to respond to subsequent doses. Thus, we assumed some fraction of infants would fail to seroconvert regardless of the number of doses (“non-responders”), and estimated the proportion of “non-responders” as well as the probability of responding among the remaining segment of the population based on the proportion of infants who seroconverted following two versus three doses during the vaccine trial in Malawi. Uncertainty in the probability of responding to each dose of the vaccine was characterized using beta distributions.
Model-predicted vaccine effects
We calculated three different vaccine effects from our model: (1) the direct effect (that is, vaccine effectiveness), (2) the indirect effect (also called herd protection), and (3) the overall effect (vaccine impact).
The estimated vaccine effectiveness was an output of our model, as determined by the proportion of vaccinated individuals who responded following each dose of the vaccine and the reduction in risk of infection and of RVGE given infection. In line with conventional methods used to estimate vaccine efficacy for rotavirus vaccines (for example (10)), we estimated the vaccine effectiveness predicted by the model as one minus the relative incidence of moderate-to-severe RVGE cases among vaccinated versus unvaccinated by year of age:
where tv is the week of vaccine introduction, tf is the last week of follow-up, Hv,A(t) and Hu,A(t) are the model-predicted incidence of reported RVGE cases among vaccinated and unvaccinated individuals A years of age in week t, respectively, and VA(t) and UA(t) are the total number of vaccinated and unvaccinated individuals A years of age in week t in the model (and hence the sum is the person-time contribution to the vaccinated and unvaccinated states, respectively).
We calculated the model-predicted indirect effect in two ways. First, we estimated the indirect effect in each age group as one minus the relative risk of reported RVGE among unvaccinated children compared to the model-predicted risk in the absence of vaccination:
where Hnovacc,A(t) is the model-predicted incidence of reported RVGE cases among children in age group A in week t in the absence of vaccination, that is, when vaccine coverage is set to zero, and NA(t) is the total number of individuals of in age group A in the population during week t in the model. Next, we simulated the model while fixing λ(t) at the force of infection predicted in the absence of vaccination (i.e. assuming no reduction in transmission following vaccine introduction). We then compared the predicted overall vaccine impact for the full dynamic models to the impact for the corresponding models with direct protection only.
Finally, we estimated the vaccine impact (overall effect) as one minus the model-predicted incidence of RVGE cases with vaccination (accounting for both the direct and indirect effects) divided by the incidence predicted by the model in the absence of vaccination. We estimated the vaccine impact for each year following vaccine introduction.
Model fitting and external validation
To relate the model-predicted incidence of rotavirus infection to the observed incidence of RVGE, we assumed that only a fraction h of individuals with moderate-to-severe RVGE would present to QECH, have a stool sample collected, and test positive for rotavirus. We then assumed that the number of cases of age a in week t was Poisson distributed with a mean equal to the age-specific number of model-predicted incident cases of moderate-to-severe RVGE times the “reporting fraction” h. As the rotavirus testing effort varied through time, we allowed h to vary proportional to the 2-year moving average of the number of rotavirus-negative cases at QECH (fig. S12), and estimated the mean reporting fraction for the pre-vaccination period. We initially fit the model to the pre-vaccination incidence of RVGE cases via maximum a posterior estimation, then used a Markov chain Monte Carlo to obtain posterior distributions for our model parameters (fig. S13).
We validated our models by comparing model predictions for the estimated overall vaccine impact, vaccine effectiveness, and indirect effect to the observed vaccine effects. To predict the incidence of RVGE cases presenting to QECH in the post-vaccination period, we assumed that the rotavirus transmission rate, seasonality, and mean reporting fraction would not change between the 15-year pre-vaccination period and the 5-year post-vaccination period. Thus, in our models, the only thing that should explain changes to RVGE incidence is the introduction of vaccination. To account for changes in surveillance effort over time, we multiplied the model-predicted number of RVGE cases by a scaling factor equal to the moving average of the number of rotavirus-negative cases in each age group divided by average number of rotavirus-negative cases per week during the pre-vaccination period.
For Models 1 and 2, we did not use the post-vaccination data for model fitting. However, for Models 3 and 4, we needed to estimate the duration of vaccine-induced immunity by fitting the model-predicted incidence of RVGE cases to the observed data on rotavirus-positive acute gastroenteritis cases at QECH for the period from January 2012 to August 2017. We also estimated the proportion of infants who responded to each dose, using informative priors for the latter (fig. S14). The fit of the four models to the post-vaccination data is compared in table S3.
Scenarios to improve vaccine performance
We assessed the potential impact of measures aimed at increasing the proportion of infants who seroconvert to the first and second dose of rotavirus vaccine by calculating the estimated vaccine effectiveness for children 4 to 11 months versus 12 to 23 months of age while varying the proportion who respond to each dose between 0.4 and 1. To evaluate the potential impact of non-vaccine interventions that could reduce the transmission rate of rotavirus, and to compare to vaccine effectiveness estimates from higher-income countries, we estimated the vaccine effectiveness for children 4 to 11 months versus 12 to 23 months of age for baseline values of R0 varying from 25 to 100 (consistent with the range of R0 values estimated for different countries (37)), while also varying the proportion who respond to each dose. Finally, we evaluated the impact of an additional vaccine dose administered at 9 months of age by assuming one- and two-dose vaccine coverage would remain at its most recently observed level, and that coverage with the booster dose would be 81% beginning in January 2018, based on the latest available estimate of measles vaccine coverage in Malawi (57). We compared the predicted impact of an additional dose to the other two strategies to determine the level of improvement in immunogenicity or reduction in R0 that would be needed to achieve similar reductions in the incidence of RVGE over the next three years, assuming that each intervention was implemented as a step function in January 2018.
Supplementary Material
Materials and methods
Fig. S1. Diagram of compartmental models for vaccination with and without waning of vaccine-induced immunity.
Fig. S2. Vaccine coverage through time among rotavirus-negative diarrheal cases.
Fig. S3. Observed and model-predicted vaccine impact by age group and year since vaccine introduction.
Fig. S4. Observed and predicted vaccine impact by age and vaccination status.
Fig. S5. Relationship between the proportion of infants who respond to each vaccine dose, the basic reproductive number (R0), and vaccine effectiveness estimates for Model 1.
Fig. S6. Relationship between the proportion of infants who respond to each vaccine dose and the predicted vaccine effectiveness assuming heterogeneity in vaccine response.
Fig. S7. Relationship between the proportion of infants who respond to each vaccine dose, the basic reproductive number (R0), and the model-predicted indirect effect.
Fig. S8. Proportion of the population with natural or vaccine-induced immunity by age.
Fig. S9. Proportion of the population with natural or vaccine-induced immunity by age for the period from January 2018 to December 2019.
Fig. S10. Model-predicted vaccine effectiveness with and without the addition of a third dose of rotavirus vaccine administered at 9 months of age.
Fig. S11. Predicted impact of strategies to improve the proportion of infants responding to vaccination and to reduce the transmission rate of rotavirus.
Fig. S12. Variation in the average number of rotavirus-negative cases through time for the pre- and post-vaccination surveillance periods.
Fig. S13. Prior and posterior distributions of the model parameters estimated by fitting to the pre-vaccination data.
Fig. S14. Trace plots and posterior distributions of estimated vaccination parameters.
Fig. S15. Posterior distributions of estimated vaccination parameters for Models 1 and 2 fitted to the post-vaccination data.
Fig. S16. Relationship between the relative infectiousness of subsequent infections and the estimated basic reproductive number (R0).
Fig. S17. Model-predicted overall vaccine impact and vaccine effectiveness for different assumptions regarding the relative infectiousness of subsequent infections.
Fig. S18. Model-predicted overall vaccine impact and vaccine effectiveness for short-lived complete immunity following infection.
Table S1. Fixed and estimated parameter values.
Table S2. Estimated basic reproductive number for different values of the relative infectiousness of subsequent infections duration of complete immunity.
Table S3. Comparison of model fits to the post-vaccination data.
Table S4. Model-predicted indirect vaccine effectiveness.
Table S5. Model-predicted vaccine impact for the models with and without indirect effects.
Funding:
This work was supported by grant R01AI112970 from the US National Institutes of Health/National Institute of Allergy and Infectious Diseases to V.E.P. Rotavirus surveillance in Blantyre, Malawi was supported by a Wellcome Trust Programme Grant to N.A.C. (number 091909/Z/10/Z). A.B. was the recipient of a Wellcome Trust Clinical PhD Fellowship (number 102466/Z/13/A). K.C.J. was supported by a Wellcome Trust Public Health and Tropical Medicine Training Fellowship (number 201945/Z/16/Z).
Footnotes
Competing interests: V.E.P. is a member of the WHO Immunization and Vaccine-related Implementation Research Advisory Committee (IVIR-AC) and has received reimbursement from Merck for travel expenses to attend a Scientific Input Engagement unrelated to rotavirus vaccines. N.B.Z. and K.C.J. have received research grant support from GlaxoSmithKline Biologicals for work on rotavirus vaccines. B.A.L reports personal fees from Takeda Pharmaceuticals, personal fees from the CDC Foundation, personal fees from Hall Booth Smith, P.C., all unrelated to rotavirus vaccines. J.A.L has received research grants and consulting fees from Merck and Pfizer, unrelated to the current work. N.A.C. has received research grant support and honoraria for participation in rotavirus vaccine Independent Data Monitoring Committee meetings from GlaxoSmithKline Biologicals.
Data and materials availability: All data associated with this manuscript are present in the paper or supplementary materials. Model code and data necessary to reproduce the results of the study are available from https://github.com/vepitzer/rotavirusMalawi (doi: 10.5281/zenodo.3336499).
References and Notes:
- 1.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, 2008 Estimate of Worldwide Rotavirus-Associated Mortality in Children Younger Than 5 Years Before the Introduction of Universal Rotavirus Vaccination Programmes: a Systematic Review and Meta-Analysis., Lancet Infect. Dis 12, 136–141 (2012). [DOI] [PubMed] [Google Scholar]
- 2.GBD Diarrhoeal Diseases Collaborators, Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015, Lancet Infect. Dis 17, 909–948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tate JE, Patel MM, Steele AD, Gentsch JR, Payne DC, Cortese MM, Nakagomi O, Cunliffe NA, Jiang B, Neuzil KM, de Oliveira LH, Glass RI, Parashar UD, Global impact of rotavirus vaccines., Expert Rev. Vaccines 9, 395–407 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, a Karvonen, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe a, Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study., Lancet 370, 1757–1763 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CDC, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O’Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM, Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine., N. Engl. J. Med 354, 23–33 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, López P, Macías-Parra M, Ortega-Barría E, Rivera-Medina DM, Rivera L, Pavía-Ruz N, Nuñez E, Damaso S, Ruiz-Palacios GM, De Vos B, O’Ryan M, Gillard P, Bouckenooghe A, Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study., Lancet 371, 1181–1189 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O’Ryan M, Safety and Efficacy of an Attenuated Vaccine against Severe Rotavirus Gastroenteritis, N. Engl. J. Med 354, 11–22 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Coia ML, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schödel F, Ciarlet M, Neuzil KM, Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial., Lancet 376, 606–614 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TPM, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schödel F, Steele AD, Neuzil KM, Ciarlet M, Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial., Lancet 376, 615–623 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Madhi S, Cunliffe N, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard P, Cheuvart B, Han HH, Neuzil KM, Effect of human rotavirus vaccine on severe diarrhea in African infants, N. Engl. J. Med 362, 289–298 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, Juvekar S, Muliyil J, Arya A, Shaikh H, Abraham V, Vrati S, Proschan M, Kohberger R, Thiry G, Glass R, Greenberg HB, Curlin G, Mohan K, Harshavardhan GVJA, Prasad S, Rao TS, Boslego J, Bhan MK, Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial., Lancet 383, 2136–2143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, McNeal MM, Meyer N, Adehossi E, Djibo A, Jochum B, Grais RF, Efficacy of a Low-Cost, Heat-Stable Oral Rotavirus Vaccine in Niger, N. Engl. J. Med 376, 1121–1130 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Gessner BD, Feikin DR, Vaccine preventable disease incidence as a complement to vaccine efficacy for setting vaccine policy, Vaccine 32, 3133–3138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halloran ME, Longini IM, Struchiner CJ, Design and interpretation of vaccine field studies, Epidemiol. Rev 21, 73–88 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD, Real-world impact of rotavirus vaccination., Pediatr. Infect. Dis. J 30, S1–S5 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Patel MM, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U, A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy., J. Infect. Dis 208, 284–94 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD, Effectiveness of Rotavirus Vaccination: A Systematic Review of the First Decade of Global Postlicensure Data, 2006–2016, Clin. Infect. Dis 65, 840–850 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Rogawski ET, Platts-Mills JA, Colgate ER, Haque R, Zaman K, Petri WA, Kirkpatrick BD, Quantifying the Impact of Natural Immunity on Rotavirus Vaccine Efficacy Estimates: A Clinical Trial in Dhaka, Bangladesh (PROVIDE) and a Simulation Study, J. Infect. Dis 217, 861–868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PG, Rodrigues LC, Fine PE, Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies., Int. J. Epidemiol 13, 87–93 (1984). [DOI] [PubMed] [Google Scholar]
- 20.Zaman K, Fleming JA, Victor JC, Yunus M, Bari TIA, Azim T, Rahman M, Mohammad S, Mowla N, Bellini WJ, Mcneal M, Icenogle JP, Lopman B, Parashar U, Cortese MM, Steele AD, Neuzil KM, Noninterference of Rotavirus Vaccine With Measles-Rubella Vaccine at 9 Months of Age and Improvements in Antirotavirus Immunity: A Randomized Trial, J. Infect. Dis 213, 1686–1693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, Flores J, Cryz S, Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial, Lancet Infect. Dis 17, 843–853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass RI, Jiang B, Parashar U, The future control of rotavirus disease: Can live oral vaccines alone solve the rotavirus problem?, Vaccine 36, 2233–2236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard SL, Malpica-llanos T, Friberg IK, Fischer-Walker C, Ashraf S, Walker N, Estimating the herd immunity effect of rotavirus vaccine, Vaccine 33, 3795–3800 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, Chappell J, Curns AT, Wikswo M, Tate JE, Lopman BA, Parashar UD, Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006–2009., Clin. Infect. Dis 53, 245–253 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Lopman BA, Curns AT, Yen C, Parashar UD, Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States., J. Infect. Dis 204, 980–986 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Panozzo CA, Becker-Dreps S, Pate V, Weber DJ, Funk M. Jonsson, Stürmer T, Brookhart MA, Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007–2010., Am. J. Epidemiol 179, 895–909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitzer VE, Atkins KE, de Blasio BF, Van Effelterre T, Atchison CJ, Harris JP, Shim E, Galvani AP, Edmunds WJ, Viboud C, Patel MM, Grenfell BT, Parashar UD, Lopman BA, Direct and indirect effects of rotavirus vaccination: comparing predictions from transmission dynamic models., PLoS One 7, e42320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitzer VE, Viboud C, Simonsen L, Steiner C, Panozzo CA, Alonso WJ, Miller MA, Glass RI, Glasser JW, Parashar UD, Grenfell BT, Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics., Science 325, 290–294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atchison C, Lopman B, Edmunds WJ, Modelling the seasonality of rotavirus disease and the impact of vaccination in England and Wales., Vaccine 28, 3118–3126 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Bennett A, Pollock L, Jere KC, Pitzer VE, Parashar U, Tate JE, Heyderman RS, Mwansambo C, French N, Nakagomi O, Iturriza-Gomara M, Everett D, Cunliffe NA, Bar-Zeev N, Direct and possible indirect effects of vaccination on rotavirus hospitalisations among children in Malawi four years after programmatic introduction., Vaccine 36, 7142–7148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosettie KL, Vos T, Mokdad AH, Flaxman AD, Khalil I, Troeger C, Weaver MR, Indirect rotavirus vaccine effectiveness for the prevention of rotavirus hospitalization: A systematic review and meta-analysis, Am. J. Trop. Med. Hyg 98, 1197–1201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunliffe NA, Ngwira BM, Dove W, Thindwa BDM, Turner AM, Broadhead RL, Molyneux ME, Hart CA, Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997–2007, J. Infect. Dis 202, S168–S174 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Turner A, Ngwira B, Witte D, Mwapasa M, Dove W, Cunliffe N, Surveillance of rotavirus gastro-enteritis in children in Blantyre, Malawi., Paediatr. Int. Child Health 33, 42–45 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, Chimpeni P, Victor JC, Steele AD, Bouckenooghe A, Neuzil KM, Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life : A randomized, double-blind, placebo controlled trial, Vaccine 4414, 36–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bar-Zeev N, Jere KC, Bennett A, Pollock L, Tate JE, Nakagomi O, Iturriza-Gomara M, Costello A, Mwansambo C, Parashar UD, Heyderman RS, French N, Cunliffe NA, Beard J, Crampin AC, King C, Lewycka S, Mvula H, Phiri T, Verani JR, Whitney CG, Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years after Vaccine Introduction: Ecological and Case-Control Analyses, Clin. Infect. Dis 62, S213–S219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, Mwansambo C, Costello A, Parashar UD, Heyderman RS, French N, Cunliffe NA, Beard J, Crampin AC, King C, Lewycka S, Mvula H, Phiri T, Verani JR, Whitney CG, Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study, Lancet Infect. Dis 15, 422–428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitzer VE, Viboud C, Lopman BA, Patel MM, Parashar UD, Grenfell BT, Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence., J. R. Soc. Interface 8, 1584–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitzer VE, Bilcke J, Heylen E, Crawford FW, Callens M, De Smet F, Van Ranst M, Zeller M, Matthijnssens J, Did Large-Scale Vaccination Drive Changes in the Circulating Rotavirus Population in Belgium?, Sci. Rep 5, 18585 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Struchiner CJ, Halloran ME, Randomization and baseline transmission in vaccine field trials, Epidemiol. Infect 135, 181–194 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White MT, Griffin JT, Drakeley CJ, Ghani AC, Heterogeneity in malaria exposure and vaccine response : implications for the interpretation of vaccine efficacy trials, 9, 82 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velázquez F, Matson D, Calva JJ, Guerrero ML, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM, Rotavirus infection in infants as protection against subsequent infections, N. Engl. J. Med 335, 1022–1028 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DWG, Desselberger U, Crawford SE, John J, Babji S, Estes MK, Kang G, Protective effect of natural rotavirus infection in an Indian birth cohort., N. Engl. J. Med 365, 337–346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halloran M, Struchiner C, Study Designs for Dependent Happenings, Epidemiology 2, 331–338 (1991). [DOI] [PubMed] [Google Scholar]
- 44.Ngabo F, Tate JE, Gatera M, Rugambwa C, Donnen P, Lepage P, Mwenda JM, Binagwaho A, Reine E, Huderf F, Effect of pentavalent rotavirus vaccine introduction on hospital admissions for diarrhoea and rotavirus in children in Rwanda: a time-series analysis, Lancet Glob. Heal 4, e129–e136 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Zaman K, Sack DA, Neuzil KM, Yunus M, Moulton LH, Sugimoto JD, Fleming JA, Hossain I, El Arifeen S, Azim T, Rahman M, Lewis KDC, Feller AJ, Qadri F, Halloran ME, Cravioto A, Victor JC, Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial, PLoS Med. 14, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson RM, May RM, Infectious Diseases of Humans (Oxford University Press, New York, 1991). [Google Scholar]
- 47.Parker EPK, Ramani S, Lopman BA, Church JA, Iturriza-Gómara M, Prendergast AJ, Grassly NC, Causes of impaired oral vaccine efficacy in developing countries, Future Microbiol. 13, 97–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramani S, Mamani N, Villena R, Bandyopadhyay AS, Gast C, Sato A, Laucirica D, Clemens R, Estes MK, O’Ryan ML, Rotavirus serum IgA immune response in children receiving rotarix coadministered with bOPV or IPV, Pediatr. Infect. Dis. J 35, 1137–1139 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Taniuchi M, Platts-Mills JA, Begum S, Uddin MJ, Sobuz SU, Liu J, Kirkpatrick BD, Colgate ER, Carmolli MP, Dickson DM, Nayak U, Haque R, Petri WA, Houpt ER, Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants, Vaccine 34, 3068–3075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris VC, Haak BW, Handley SA, Jiang B, Velasquez DE, Hykes BL, Droit L, Berbers GAM, Kemper EM, van Leeuwen EMM, Boele van Hensbroek M, Wiersinga WJ, Effect of Antibiotic-Mediated Microbiome Modulation on Rotavirus Vaccine Immunogenicity: A Human, Randomized-Control Proof-of-Concept Trial, Cell Host Microbe 24, 197–207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bines JE, At Thobari J, Satria CD, Handley A, Watts E, Cowley D, Nirwati H, Ackland J, Standish J, Justice F, Byars G, Lee KJ, Barnes GL, Bachtiar NS, Viska Icanervilia A, Boniface K, Bogdanovic-Sakran N, Pavlic D, Bishop RF, Kirkwood CD, Buttery JP, Soenarto Y, Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth, N. Engl. J. Med 378, 719–730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haidara FC, Tapia MD, Sow SO, Doumbia M, Coulibaly F, Diallo F, Traoré A, Kodio M, Kelly CL, Fitzpatrick M, Kotloff K, Victor JC, Neuzil K, Evaluation of a booster dose of pentavalent rotavirus vaccine coadministered with measles, yellow fever, and meningitis a Vaccines in 9-Month-old malian infants, J. Infect. Dis 218, 606–613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armah G, Lewis KDCC, Cortese MM, Parashar UD, Ansah A, Gazley L, Victor JC, McNeal MM, Binka F, Steele AD, A Randomized, Controlled Trial of the Impact of Alternative Dosing Schedules on the Immune Response to Human Rotavirus Vaccine in Rural Ghanaian Infants, J. Infect. Dis 213, 1678–1685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madhi S, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD, Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial., Vaccine 30, A44–A51 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Burnett E, Lopman BA, Parashar UD, Potential for a booster dose of rotavirus vaccine to further reduce diarrhea mortality, Vaccine 35, 7198–7203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheuvart B, Neuzil KM, Steele AD, Cunliffe N, Madhi A, Karkada N, Han HH, Vinals C, Cheuvart B, Neuzil KM, Steele AD, Cunliffe N, Cheuvart B, Neuzil KM, Steele AD, Cunliffe N, Madhi SA, Karkada N, Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: Analysis of clinical trials of human rotavirus vaccine, Hum. Vaccines Immunother 5515, 505–511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malawi: WHO and UNICEF estimates of immunization coverage: 2016 revision (World Health Organization, 2017); https://www.who.int/immunization/monitoring_surveillance/data/mwi.pdf. [Google Scholar]
- 58.2008 Malawi Population and Housing Census (National Statistical Office, 2008); http://www.nsomalawi.mw. [Google Scholar]
- 59.World Population Prospects 2017 (United Nations Department of Economic and Social Affairs, Population Division, 2017); https://esa.un.org/unpd/wpp/DataQuery/. [Google Scholar]
- 60.Patel MM, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI, Oral rotavirus vaccines: how well will they work where they are needed most?, J. Infect. Dis 200, S39–S48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kambhampati A, Payne DC, Costantini V, Lopman BA, Host Genetic Susceptibility to Enteric Viruses: A Systematic Review and Metaanalysis, Clin. Infect. Dis 62, 11–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ultsch B, Damm O, Beutels P, Bilcke J, Brüggenjürgen B, Gerber-Grote A, Greiner W, Hanquet G, Hutubessy R, Jit M, Knol M, von Kries R, Kuhlmann A, Levy-Bruhl D, Perleth M, Postma M, Salo H, Siebert U, Wasem J, Wichmann O, Methods for Health Economic Evaluation of Vaccines and Immunization Decision Frameworks: A Consensus Framework from a European Vaccine Economics Community, Pharmacoeconomics 34, 227–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gelman A, Rubin DB, Inference from Iterative Simulation Using Multiple Sequences, Stat. Sci 7, 457–511 (1992). [Google Scholar]
- 64.Lewnard JA, Lopman BA, Parashar UD, Bar-Zeev N, Samuel P, Lourdes Guerrero M, Ruiz-Palacios GM, Kang G, Pitzer VE, Naturally Acquired Immunity Against Rotavirus Infection and Gastroenteritis in Children: Paired Reanalyses of Birth Cohort Studies, J. Infect. Dis 216, 317–326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Effelterre T, Soriano-Gabarró M, Debrus S, Claire Newbern E, Gray J, A mathematical model of the indirect effects of rotavirus vaccination., Epidemiol. Infect 138, 884–897 (2010). [DOI] [PubMed] [Google Scholar]
- 66.de Blasio BF, Kasymbekova K, Flem E, Dynamic model of rotavirus transmission and the impact of rotavirus vaccination in Kyrgyzstan, Vaccine 28, 7923–7932 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Linhares AC, Gabbay YB, Freitas RB, da Rosa ES, Mascarenhas JD, Loureiro EC, Longitudinal study of rotavirus infections among children from Belém, Brazil., Epidemiol. Infect 102, 129–145 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilde J, Yolken R, Willoughby R, Eiden J, Improved detection of rotavirus shedding by polymerase chain reaction, Lancet 337, 323–326 (1991). [DOI] [PubMed] [Google Scholar]
- 69.Ward RL, Bernstein DI, Young EC, Sherwood JR, Knowlton DR, Schiff GM, Human rotavirus studies in volunteers: Determination of infectious dose and serological response to infection, J. Infect. Dis 154, 871–880 (1986). [DOI] [PubMed] [Google Scholar]
- 70.Anderson EJ, Weber SG, Rotavirus infection in adults., Lancet Infect. Dis 4, 91–99 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White LJ, Buttery J, Cooper B, Nokes DJ, Medley GF, Rotavirus within day care centres in Oxfordshire, UK: characterization of partial immunity., J. R. Soc. Interface 5, 1481–1490 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koopman JS, Monto AS, Ira ML, The Tecumseh Study XVI: Family and community sources of rotavirus infection, Am. J. Epidemiol 130, 760–768 (1989). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods
Fig. S1. Diagram of compartmental models for vaccination with and without waning of vaccine-induced immunity.
Fig. S2. Vaccine coverage through time among rotavirus-negative diarrheal cases.
Fig. S3. Observed and model-predicted vaccine impact by age group and year since vaccine introduction.
Fig. S4. Observed and predicted vaccine impact by age and vaccination status.
Fig. S5. Relationship between the proportion of infants who respond to each vaccine dose, the basic reproductive number (R0), and vaccine effectiveness estimates for Model 1.
Fig. S6. Relationship between the proportion of infants who respond to each vaccine dose and the predicted vaccine effectiveness assuming heterogeneity in vaccine response.
Fig. S7. Relationship between the proportion of infants who respond to each vaccine dose, the basic reproductive number (R0), and the model-predicted indirect effect.
Fig. S8. Proportion of the population with natural or vaccine-induced immunity by age.
Fig. S9. Proportion of the population with natural or vaccine-induced immunity by age for the period from January 2018 to December 2019.
Fig. S10. Model-predicted vaccine effectiveness with and without the addition of a third dose of rotavirus vaccine administered at 9 months of age.
Fig. S11. Predicted impact of strategies to improve the proportion of infants responding to vaccination and to reduce the transmission rate of rotavirus.
Fig. S12. Variation in the average number of rotavirus-negative cases through time for the pre- and post-vaccination surveillance periods.
Fig. S13. Prior and posterior distributions of the model parameters estimated by fitting to the pre-vaccination data.
Fig. S14. Trace plots and posterior distributions of estimated vaccination parameters.
Fig. S15. Posterior distributions of estimated vaccination parameters for Models 1 and 2 fitted to the post-vaccination data.
Fig. S16. Relationship between the relative infectiousness of subsequent infections and the estimated basic reproductive number (R0).
Fig. S17. Model-predicted overall vaccine impact and vaccine effectiveness for different assumptions regarding the relative infectiousness of subsequent infections.
Fig. S18. Model-predicted overall vaccine impact and vaccine effectiveness for short-lived complete immunity following infection.
Table S1. Fixed and estimated parameter values.
Table S2. Estimated basic reproductive number for different values of the relative infectiousness of subsequent infections duration of complete immunity.
Table S3. Comparison of model fits to the post-vaccination data.
Table S4. Model-predicted indirect vaccine effectiveness.
Table S5. Model-predicted vaccine impact for the models with and without indirect effects.


