Abstract
Introduction:
Kidneys from donors with hepatitis C (HCV) infection are traditionally considered to be at risk for poorer survival outcomes, as reflected in the KDPI. Modern direct acting antivirals (DAAs) may modify this risk.
Methods:
Using UNOS data, HCV infected adult first time kidney transplant recipients from 2014–2017 were examined. Graft and patient survival were compared in a propensity matched cohort of recipients of HCV antibody(+) kidneys versus antibody(−) kidneys. Subsequent analysis was performed in a propensity matched cohort of recipients of HCV viremic (RNA positive) vs. HCV naive kidneys.
Results:
There were 379 recipients each in the matched cohort of recipients of HCV antibody(+) vs. HCV antibody(−) kidneys. Despite a higher KDPI (58.2% for HCV antibody(+) vs. 38.8% for HCV antibody(−)), 1 year patient and graft survival were similar in the HCV(+) and HCV(−) groups (95.4% and 94.9% vs 97.9% and 96.0%, p=0.543 and p=0.834, respectively). There were 200 recipients each in the cohort of recipients of HCV viremic vs. HCV naïve kidneys, with the KDPI again higher in the HCV viremic group (56.8% vs 35.2%). Baseline hazard ratios for graft failure (HR 4.69; p=0.009) and death (HR 7.60; p=0.003) were significantly elevated in the viremic group, but crossed 1 at 21 and 24 months, respectively.
Conclusions:
In the modern DAA era, calculated likely KDPI overestimates risk kidneys from HCV antibody(+) donors. Donor viremia conveys an early risk which appears to subside over time. These results suggest that it may be time to revise the kidney donor risk index.
Introduction
Based on studies demonstrating donor hepatitis C virus (HCV) status as an independent risk factor for death and graft loss, kidneys from HCV infected donors have traditionally been considered to have inferior survival outcomes1–5. The kidney donor risk index (KDRI) derived by Rao in 2009 quantified the excess risk of graft loss associated with HCV positive donors, demonstrating a 1.27-fold increased risk for graft loss associated with donor HCV status6. The KDRI derived by Rao has subsequently been mapped to the kidney donor profile index (KDPI), which is meant to rate the kidney on a scale of 0% for kidneys with the longest expected survival to 100% for those with the shortest. A kidney from a HCV positive donor will have a KDPI that is roughly 20% higher than a kidney from an otherwise identical HCV negative donor7,8.
The original KDRI study as well as previous studies on HCV positive donor kidneys were performed in an era where the only treatment for HCV consisted of interferon based regimens, which were typically poorly tolerated and had only limited efficacy9. Since 2014, there has been a revolution in the management of HCV infection with the introduction of direct acting antivirals (DAAs). These new regimens have demonstrated sustained viral response (SVR) rates of over 94% for most genotypes of HCV, with 100% SVR in certain genotypes reported in many instances10–14. In a recent study of Scientific Registry of Transplant Recipients (SRTR) data, Axelrod has demonstrated that DAA treatment significantly improved patient survival in HCV positive recipients of HCV positive donor kidneys9. Sibulesky has further demonstrated kidneys from donors who were HCV nucleic acid testing (NAT) negative/antibody (Ab) positive (as would be the case for a donor who had been successfully treated for HCV) had similar patient and graft survival compared to HCV antibody negative donor organs7.
Given the sea change in HCV treatment in recent years, we hypothesized that the risk associated with HCV positive donor kidneys (whether determined by serology or NAT) in the DAA era would be significantly less than in the pre DAA era in which the KDRI was derived. This study was undertaken to determine whether donor HCV status continues to have a significant effect on post-transplant patient and graft survival in the DAA era. Since kidney allocation is now tied to the KDPI under the new kidney allocation system (KAS)15, these findings have the potential to alter the way in which kidneys are allocated in the United States if the negative effect of donor HCV status has been mitigated in the DAA era.
Methods
Study Population
We performed a retrospective review of HCV Ab positive adult first time recipients of ABO compatible kidney transplant alone from deceased donors contained in the United Network for Organ Sharing (UNOS) standard transplant analysis and research (STAR) file as of March 2019. Recipients with missing values for donor height, weight, and creatinine were excluded, mirroring the methodology used by Rao in the calculation of the original KDRI6. In the first analysis, recipients transplanted with kidneys from HCV Ab positive donors from 2014 through 2017 were compared to a propensity matched group of recipients of kidneys from HCV Ab(−) donors. The timeframe for this analysis was chosen to begin with the widespread introduction of DAAs and ended at a point that would allow at least one year of follow-up for all recipients. HCV positive donors in this analysis were defined according to serologic status, as nucleic acid testing of donors was not available for the entire study period. Donor HCV nucleic acid testing became universally available in the UNOS dataset as of 3/31/2015. Accordingly, a sensitivity analysis was performed in HCV positive recipients transplanted after that date, with comparison made between recipients of HCV NAT (+) donor kidneys and HCV Ab(−)/NAT(−) donor kidneys.
Propensity Matching
The groups in both analyses were matched on the basis of propensity scoring16. The propensity score was derived by multivariable logistic regression modeling which included all the individual factors included in calculation of the KDRI other than donor HCV status, all the factors included in the expected post-transplant survival (EPTS) score, and factors included in the Scientific Registry of Transplant Recipients (SRTR) risk adjustment models for kidney graft survival. The full list of covariates used in the propensity score determination is found in the tables outlining covariate balance after propensity matching. Matching on the KDRI or KDPI themselves was not possible in this analysis because the grouping variable (donor HCV status) strongly influences these scores. Matching on the KDRI or KDPI in this instance would thus have made it impossible to achieve balance on all the other covariates in the model.
After calculation of propensity scores, matching was carried out in a 1:1 nearest neighbor fashion based on the logit of the propensity score. The matching algorithm was “greedy” in that, once a match was made, it was not broken. In order to prevent poor matches from being made, a caliper width equal to 0.2 times the pooled standard deviation of the logit propensity score for the entire cohort was imposed. Matched pairs with a difference in logit propensity score greater than the caliper were discarded. Residual differences in covariates between groups after propensity matching were assessed using the formulas for standardized differences as proposed by Austin17. Standardized differences <0.1 in absolute value are generally considered to be insignificant in terms of introducing residual confounding18. The reason for using standardized differences in this setting is to minimize the effect of the smaller size of the propensity matched cohort compared to the overall cohort, which would reduce the power of traditional significance tests and potentially mask important covariate imbalances. Due to the smaller sample size, propensity matching was not able to achieve adequate balance on all included covariates in the second analysis of HCV NAT(+) vs. NAT(−) donors. To account for residual differences in these covariates in that cohort, multivariable cox proportional hazards analysis of patient and kidney graft survival was performed in the propensity matched cohort, adjusting for factors which remained out of balance after matching.
Missing Data
There was minimal missing data in the variables analyzed for this study. The following variables had missing observations: recipient body mass index (1 missing), cold ischemia time (5 missing), pretransplant dialysis duration (2 missing). Less than 5% missing data is considered inconsequential in terms of introducing bias, so further sensitivity analysis for the effect of missing data was not performed19. Induction immunosuppression data was missing for 363 (15%) of patients in the overall cohort, so it was not included in the propensity score or survival models. Data on induction immunosuppression in the patients for whom it was available is included in supplemental tables 1–4. Data on treatment for rejection within the first year after transplant was also missing in 448 (19%) of patients in the overall cohort, so rejection was not analyzed as an outcome.
Statistical Analysis
Differences in baseline covariates between groups prior to propensity matching were assessed using Student’s t-test for continuous covariates and chi-squared for categorical covariates. After propensity matching, residual differences were assessed using standardized difference as discussed above. The primary endpoint for both analyses was all-cause renal graft survival, which was determined by the Kaplan-Meier method and compared using the log-rank test. Death-censored graft survival was also analyzed. We utilized all-cause and death-censored renal allograft survival rather than a framework where graft loss and death with a functioning graft were treated as competing risks as the cause specific models are viewed to be more appropriate than competing risks for etiologic type research20. The hazard ratio (HR) and 95% confidence interval (CI) for renal graft loss was estimated using a marginal Cox proportional hazards model according to the method of Lee, Wei, and Amato to account for the paired nature of the data21. Patient survival post-transplant was also assessed using the methods described above for analysis of graft survival. Multivariable cox proportional hazards regression analysis of the effect of donor NAT status on survival was performed as noted above.
The proportional hazards assumption was checked by examination of Schoenfeld residuals as well as by checking for a significant interaction between follow-up time and donor HCV status in the model. The proportional hazards assumption held in the analysis of HCV Ab(+) vs. HCV Ab(−) donors. In the analysis restricted to HCV NAT(+) vs. HCV NAT(−)/Ab(−) donors, there was violation of the proportional hazards assumption. As such, an interaction between follow-up time (in months) and donor HCV status was included in the model, allowing the hazard associated with donor HCV NAT positivity to vary with time. The functional form of the time interaction was chosen based on inspection of a plot of scaled Schoenfeld residuals vs. follow-up time. P-values less than 0.05 were considered significant and all were two-tailed. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Analysis of HCV Ab Positive versus HCV Ab Negative Kidneys in the Post-DAA Era
There were 55,203 deceased donor kidney transplant recipients from 2014 through 2017 in the UNOS dataset. Sequentially excluding patients under 18, recipients of previous transplants, recipients of multi-organ transplants, and recipients of ABO incompatible transplants yielded 39,071 patients. Exclusion of recipients with missing donor height, weight, or creatinine yielded a cohort size of 39,035 recipients. Of this cohort, there were 2,348 recipients who were HCV antibody positive, yielding the final cohort for this analysis.
There were 1,218 recipients in the HCV Ab negative group compared to 1,130 recipients in the HCV Ab positive group. Donor and recipient demographic information is presented in table 1. Recipients of HCV Ab positive kidneys were significantly older, more likely to be African American, and more likely to have diabetes. Pre-transplant dialysis duration was 34 months shorter in recipients of HCV Ab(+)kidneys. The HCV Ab(+)donors were significantly younger, had lower creatinine, less likely to be African American, less likely to have hypertension or diabetes, and less likely to have CVA as their cause of death. DCD was significantly more common in the HCV Ab(−)group (21.3% vs. 8.2%; p<0.001). Despite this seemingly more favorable risk factor profile, KDPI was statistically similar in the HCV Ab(+) and HCV Ab(−) groups (50.1% vs. 50.3%; p=0.821).
Table 1:
Baseline comparison of recipients of hepatitis C antibody positive kidneys versus recipients of hepatitis C antibody negative kidneys in the direct acting antiviral era. Continuous covariates are expressed as mean (standard deviation) and categorical covariates are expressed as count (percentage).
| HCV Ab Negative(n=1218) | HCV Ab Positive(n=1130) | P-value | |
|---|---|---|---|
| Recipient Age | 56.6 (10.3) | 59.6 (7.5) | <0.001 |
| Recipient Ethnicity | <0.001 | ||
| Caucasian | 317 (26.0%) | 267 (23.6%) | |
| African American | 627 (51.5%) | 696 (61.6%) | |
| Hispanic | 191 (15.7%) | 125 (11.1%) | |
| Asian | 48 (3.9%) | 23 (2.0%) | |
| Other | 35 (2.9%) | 19 (1.7%) | |
| Female Recipient | 373 (30.6%) | 241 (21.3%) | <0.001 |
| Recipient BMI | 27.7 (4.9) | 27.7 (4.9) | 0.880 |
| Recipient Diabetes | 494 (40.6%) | 614 (54.3%) | <0.001 |
| Current cPRA at Transplant | 21.6 (34.1) | 10.0 (22.8) | <0.001 |
| Recipient Pretransplant Dialysis | 1143 (93.8%) | 1018 (90.1%) | <0.001 |
| Recipient Dialysis Duration (months) | 68.4 (49.1) | 34.5 (31.4) | <0.001 |
| Recipient Education Level | 0.378 | ||
| Less than High School | 73 (6.0%) | 60 (5.3%) | |
| High School | 634 (52.1%) | 573 (50.7%) | |
| Some College | 305 (25.0%) | 290 (25.7%) | |
| Completed College | 147 (12.1%) | 136 (12.0%) | |
| Postgraduate Degree | 41 (3.4%) | 51 (4.5%) | |
| Unknown | 18 (1.5%) | 20 (1.8%) | |
| Recipient Prior Malignancy | 122 (10.0%) | 125 (11.1%) | 0.650 |
| Recipient Peripheral Vascular Disease | 148 (12.2%) | 125 (11.1%) | 0.035 |
| Recipient Primary Insurance | <0.001 | ||
| Private | 154 (12.6%) | 272 (24.1%) | |
| Public | 1060 (87.0%) | 857 (75.8%) | |
| Other | 4 (0.3%) | 1 (0.1%) | |
| Donor Age | 39.0 (15.5) | 33.3 (9.1) | |
| Donor Ethnicity | <0.001 | ||
| Caucasian | 788 (64.7%) | 949 (84.0%) | |
| African American | 236 (19.4%) | 51 (4.5%) | |
| Hispanic | 146 (12.0%) | 107 (9.5%) | |
| Asian | 29 (2.4%) | 8 (0.7%) | |
| Other | 19 (1.6%) | 15 (1.3%) | |
| Female Donor | 496 (40.7%) | 411 (36.4%) | 0.031 |
| Donor Height (cm) | 168.3 (18.0) | 172.6 (9.3) | <0.001 |
| Donor Weight (kg) | 82.1 (26.5) | 78.3 (16.5) | <0.001 |
| Donor BUN | 20.6 (15.6) | 17.2 (10.2) | <0.001 |
| Donor Terminal Creatinine | 1.3 (1.1) | 1.0 (0.6) | <0.001 |
| DCD Donor | 259 (21.3%) | 93 (8.2%) | <0.001 |
| Donor Diabetes | 100 (8.2%) | 20 (1.8%) | <0.001 |
| Donor Hypertension | 362 (29.7%) | 150 (13.3%) | <0.001 |
| Donor Blood Type | <0.001 | ||
| A | 184 (15.1%) | 119 (10.5%) | |
| A1 | 239 (19.6%) | 180 (15.9%) | |
| A1B | 15 (1.2%) | 1 (0.1%) | |
| A2 | 26 (2.1%) | 43 (3.8%) | |
| A2B | 7 (0.6%) | 1 (0.1%) | |
| AB | 15 (1.2%) | 8 (0.7%) | |
| B | 153 (12.7%) | 139 (12.3%) | |
| O | 579 (47.5%) | 639 (56.6%) | |
| Donor Vasodilator Use | 142 (11.7%) | 138 (12.2%) | 0.679 |
| Donor Tattoos | 532 (43.7%) | 906 (80.2%) | <0.001 |
| Donor Cigarette Use | 228 (18.7%) | 268 (23.7%) | 0.003 |
| Donor Cause of Death | <0.001 | ||
| Anoxia | 486 (39.9%) | 745 (65.9%) | |
| CVA | 326 (26.8%) | 103 (9.1%) | |
| Trauma | 370 (30.4%) | 264 (23.4%) | |
| Other | 36 (3.0%) | 18 (1.6%) | |
| Cold Ischemia Time (Hours) | 17.3 (8.3) | 18.2 (8.0) | |
| HLA B Mismatches | <0.001 | ||
| 0 | 68 (5.6%) | 26 (2.3%) | |
| 1 | 276 (22.7%) | 277 (24.5%) | |
| 2 | 874 (71.8%) | 827 (73.2%) | |
| HLA DR Mismatches | <0.001 | ||
| 0 | 163 (13.4%) | 65 (5.8%) | |
| 1 | 575 (47.2%) | 460 (40.7%) | |
| 2 | 480 (39.4%) | 605 (53.5%) | |
| Dual Kidney Transplant | 10 (0.8%) | 5 (0.4%) | 0.250 |
| EnBloc Kidney Transplant | 21 (1.7%) | 1 (0.1%) | <0.001 |
After propensity score matching, there were 379 recipients each in the HCV Ab(+)and HCV Ab(−)groups. Donor and recipient demographics and the standardized differences between covariates in the propensity matched cohort are presented in table 2. The propensity matching algorithm achieved good balance (defined as standardized difference <0.1 in absolute value) for all covariates included in calculation of the propensity scores. Despite being appropriately matched on all other variables used to calculate the KDPI, the mean KDPI was significantly higher in the HCV Ab(+) group (58.2% vs. 38.8%; standardized difference = 0.89).
Table 2:
Comparison of covariates after propensity score matching for recipients of hepatitis C antibody positive kidneys versus recipients of hepatitis C antibody negative kidneys in the direct acting antiviral era. Continuous covariates are expressed as mean (standard deviation) and categorical covariates are expressed as count (percentage). Standardized differences of less than 0.1 in absolute value are considered insignificant.
| HCV Negative(n=379) | HCV Positive(n=379) | Standardized Difference | |
|---|---|---|---|
| Recipient Age | 58.4 (9.8) | 57.6 (8.4) | −0.08 |
| Recipient Ethnicity | |||
| Caucasian | 92 (24.3%) | 99 (26.1%) | −0.04 |
| African American | 220 (58.1%) | 211 (55.7%) | 0.05 |
| Hispanic | 52 (13.7%) | 52 (13.7%) | 0.00 |
| Asian | 7 (1.9%) | 10 (2.6%) | −0.05 |
| Other | 8 (2.1%) | 7 (1.9%) | 0.02 |
| Female Recipient | 85 (22.4%) | 90 (23.8%) | −0.03 |
| Recipient BMI | 27.8 (4.8) | 27.8 (5.0) | 0.00 |
| Recipient Diabetes | 185 (48.8%) | 187 (49.3%) | −0.01 |
| Current cPRA at Transplant | 16.1 (30.8) | 14.9 (28.4) | −0.04 |
| Recipient Pretransplant Dialysis | 349 (92.1%) | 339 (89.5%) | 0.09 |
| Recipient Dialysis Duration (months) | 49.1 (36.9) | 45.6 (39.5) | |
| Recipient Education Level | |||
| Less than High School | 25 (6.6%) | 29 (7.7%) | −0.04 |
| High School | 195 (51.5%) | 190 (50.1%) | 0.03 |
| Some College | 85 (22.4%) | 86 (22.7%) | −0.01 |
| Completed College | 47 (12.4%) | 48 (12.7%) | −0.01 |
| Postgraduate Degree | 20 (5.3%) | 18 (4.8%) | 0.02 |
| Unknown | 7 (1.9%) | 8 (2.1%) | −0.02 |
| Recipient Prior Malignancy | 39 (10.3%) | 34 (9.0%) | 0.04 |
| Recipient Peripheral Vascular Disease | 47 (12.4%) | 46 (12.1%) | 0.01 |
| Recipient Primary Insurance | |||
| Private insurance | 60 (15.8%) | 74 (19.5%) | −0.10 |
| Public | 318 (83.9%) | 304 (80.2%) | 0.10 |
| Other | 1 (0.3%) | 1 (0.3%) | 0.00 |
| Donor Age | 35.7 (14.3) | 57.6 (8.4) | 0.00 |
| Donor Ethnicity | |||
| Caucasian | 299 (78.9%) | 301 (79.4%) | −0.01 |
| African American | 36 (9.5%) | 37 (9.8%) | −0.01 |
| Hispanic | 35 (9.2%) | 34 (9.0%) | 0.01 |
| Asian | 4 (1.1%) | 3 (0.8%) | 0.03 |
| Other | 5 (1.3%) | 4 (1.1%) | 0.02 |
| Female Donor | 137 (36.2%) | 142 (37.5%) | −0.03 |
| Donor Height (cm) | 171.3 (13.2) | 171.5 (9.7) | 0.02 |
| Donor Weight (kg) | 79.9 (22.8) | 79.8 (18.5) | −0.01 |
| Donor BUN | 17.8 (10.7) | 18.0 (11.3) | 0.02 |
| Donor Terminal Creatinine | 1.1 (0.6) | 1.1 (0.8) | 0.03 |
| DCD Donor | 55 (14.5%) | 53 (14.0%) | 0.02 |
| Donor Diabetes | 11 (2.9%) | 16 (4.2%) | −0.07 |
| Donor Hypertension | 79 (20.8%) | 77 (20.3%) | 0.01 |
| Donor Blood Type | |||
| A | 43 (11.4%) | 42 (11.1%) | 0.01 |
| A1 | 71 (18.7%) | 73 (19.3%) | −0.01 |
| A1B | 1 (0.3%) | 1 (0.3%) | 0.00 |
| A2 | 13 (3.4%) | 12 (3.2%) | 0.01 |
| A2B | 0 (0.0%) | 1 (0.3%) | −0.07 |
| AB | 6 (1.6%) | 6 (1.6%) | 0.00 |
| B | 47 (12.4%) | 45 (11.9%) | 0.02 |
| O | 198 (52.2%) | 199 (52.5%) | −0.01 |
| Donor Vasodilator Use | 47 (12.4%) | 45 (11.9%) | 0.02 |
| Donor Tattoos | 245 (64.6%) | 249 (65.7%) | −0.02 |
| Donor Cigarette Use | 86 (22.7%) | 84 (22.2%) | 0.01 |
| Donor Cause of Death | |||
| Anoxia | 194 (51.2%) | 202 (53.3%) | −0.04 |
| CVA | 65 (17.2%) | 63 (16.6%) | 0.01 |
| Trauma | 110 (29.0%) | 104 (27.4%) | 0.04 |
| Other | 10 (2.6%) | 10 (2.6%) | 0.00 |
| Cold Ischemia Time (Hours) | 17.5 (7.9) | 35.7 (10.3) | 0.02 |
| HLA B Mismatches | |||
| 0 | 19 (5.0%) | 16 (4.2%) | 0.04 |
| 1 | 89 (23.5%) | 89 (23.5%) | 0.00 |
| 2 | 271 (71.5%) | 274 (72.3%) | −0.02 |
| HLA DR Mismatches | |||
| 0 | 38 (10.0%) | 35 (9.2%) | 0.03 |
| 1 | 174 (45.9%) | 171 (45.1%) | 0.02 |
| 2 | 167 (44.1%) | 173 (45.7%) | −0.03 |
| Dual Kidney Transplant | 0 (0.0%) | 1 (0.3%) | −0.07 |
| EnBloc Kidney Transplant | 3 (0.8%) | 3 (0.8%) | 0.00 |
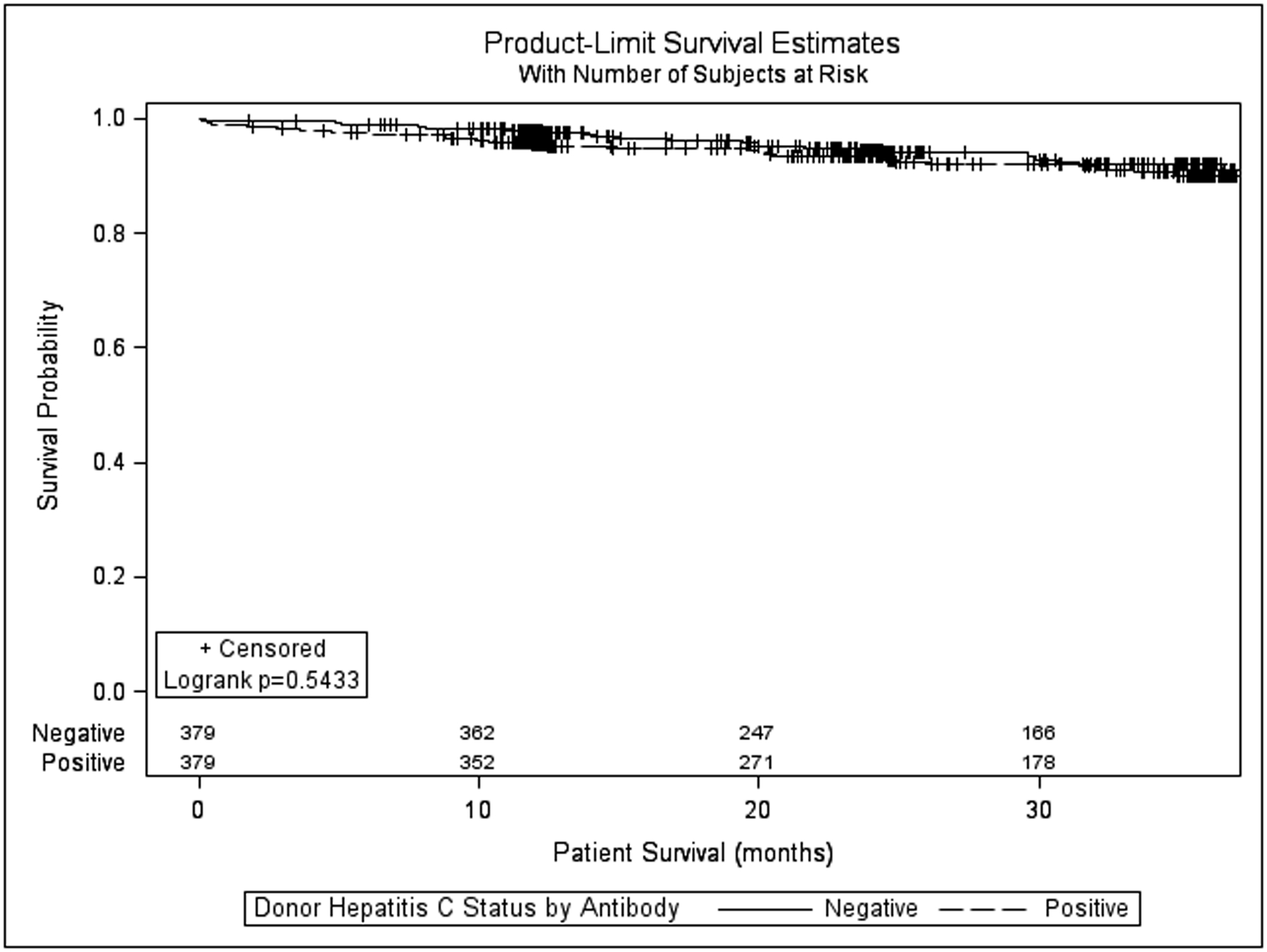
Renal allograft survival (all-cause) at 6, 12, 24, and 36 months (figure 1) in the HCV Ab(−)and HCV Ab(+) groups was similar(97.6%, 96.0%, 91.7%, 87.3% vs. 95.8%, 94.9%, 92.9%, 87.8% p=0.834). The hazard ratio for graft failure associated with HCV Ab(+) kidneys in the matched cohort was also not significant (HR = 1.046, 95% CI 0.690–1.588; p=0.831). Death-censored renal allograft survival at 6, 12, 24, and 36 months in the HCV Ab(−) and HCV Ab(+) groups was also similar (98.7%, 97.8%, 95.3%, and 93.6% vs. 98.7%, 98.7%, 98.0%, and 94.1%; p=0.440, supplemental figure 1). The hazard ratio for death-censored graft failure associated with HCV Ab(+) kidneys was 0.784 (95% CI 0.422–1.457; p=0.442). Patient survival (figure 2) at 6,12,24, and 36 months in the HCV Ab(−)and HCV Ab(+)groups was also similar (98.9%, 97.9%, 94.5%, 89.9% vs. 97.6%, 95.4%, 93.4%, 91.9%; p=0.543)The hazard ratio for death associated with HCV Ab(+) kidneys in this matched cohort was also not significant (HR = 1.168, 95% CI 0.710–1.921; p=0.541).
Figure 1:
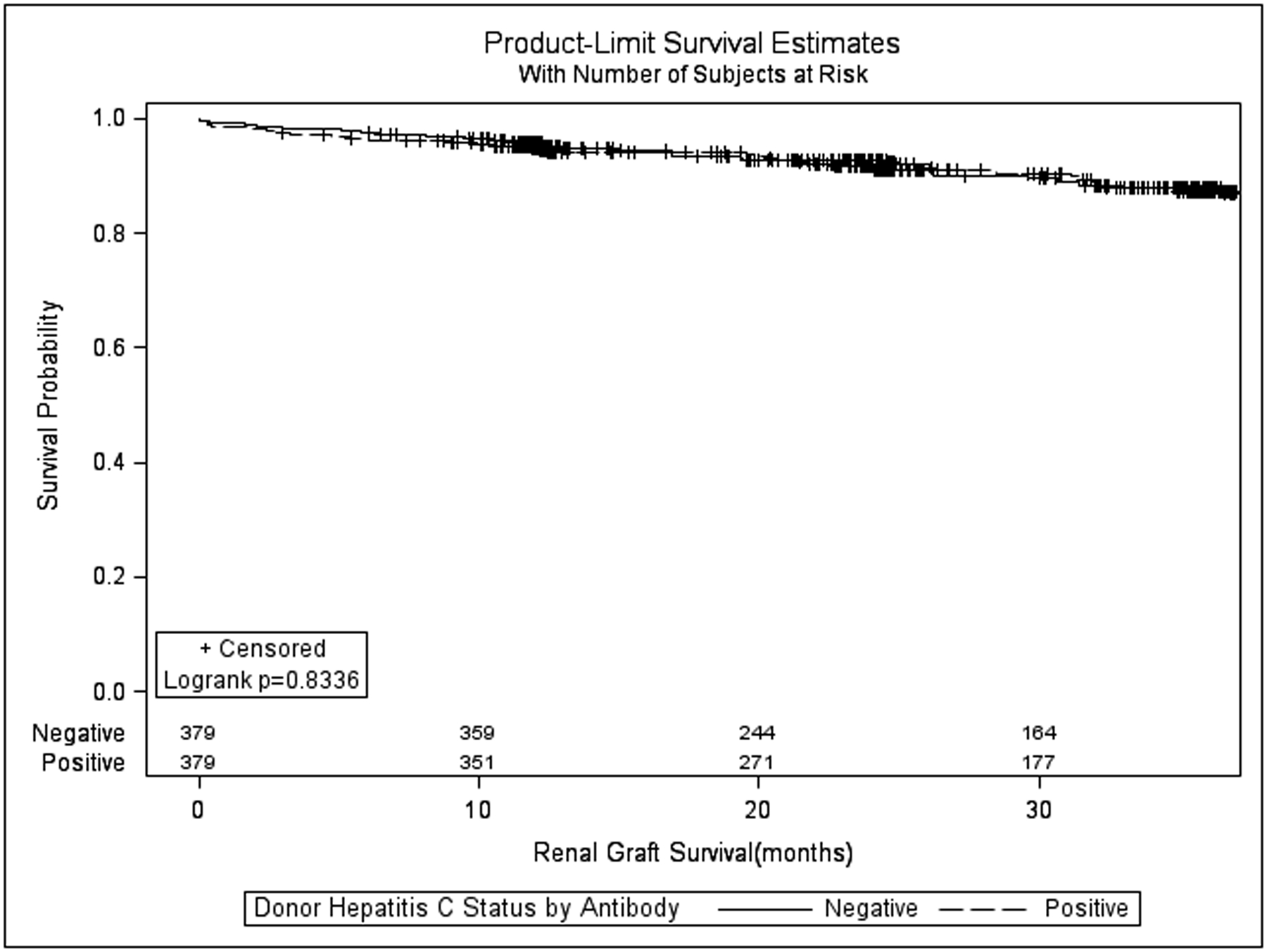
Kaplan-Meier all-cause kidney allograft survival for recipients of hepatitis C virus antibody positive versus hepatitis C antibody negative kidneys after propensity matching.
Figure 2:
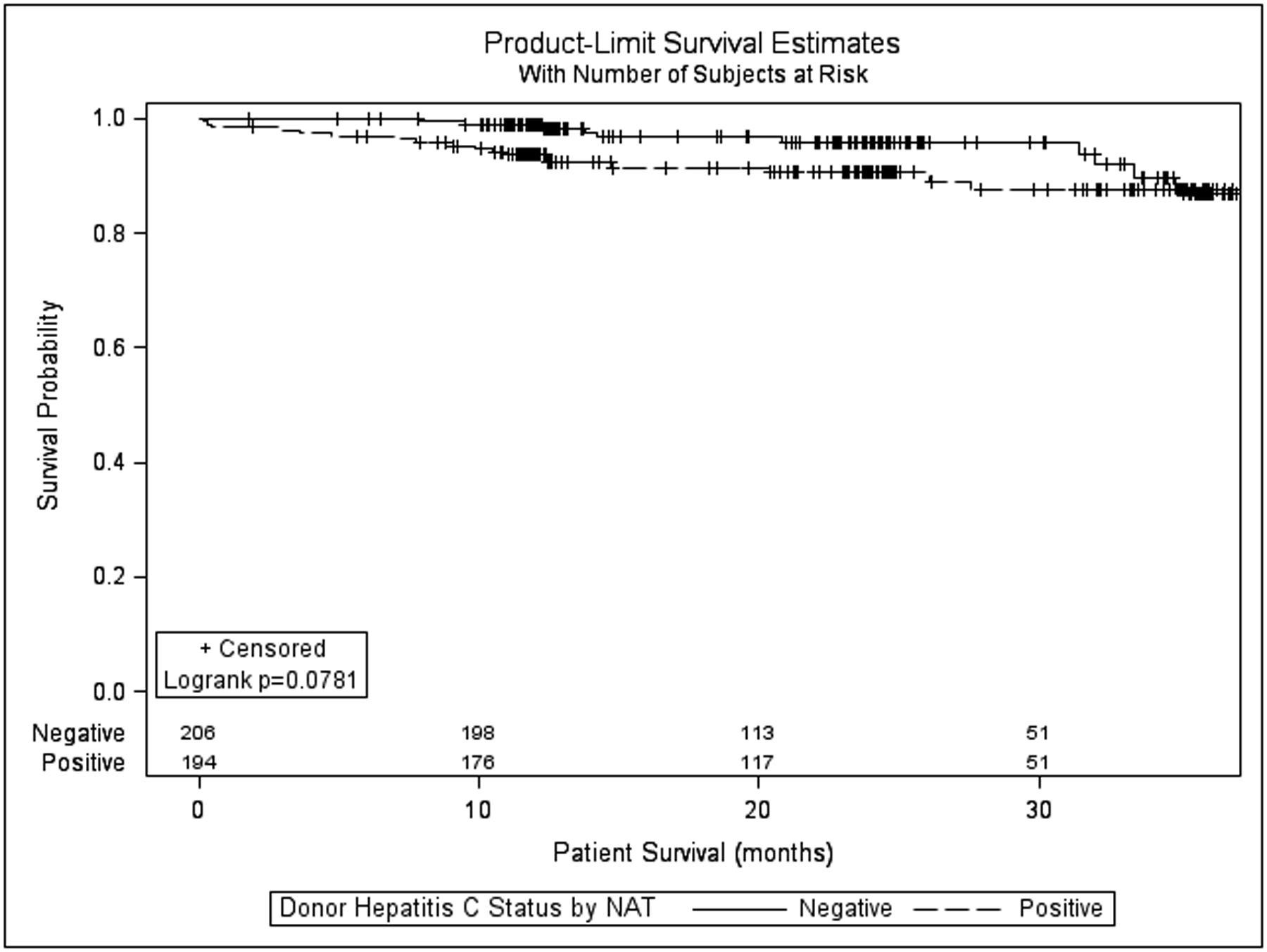
Kaplan-Meier patient survival for recipients of hepatitis C antibody positive versus hepatitis C virus antibody negative kidneys after propensity matching.
Analysis of HCV NAT(+) Kidneys vs. HCV Ab(−) NAT(−) Kidneys
There were 664 recipients of HCV NAT(+) kidneys compared to 874 recipients of HCV Ab(−) NAT(−) kidneys. Incidentally, 25.9% of all HCV Ab(+) patients (n=221) for whom NAT testing was available were NAT (−). Donor and recipient demographic information is presented in table 3. Donors in the HCV NAT(+) tended to be younger, less likely to have hypertension or diabetes, and less likely to die of a cerebrovascular accident (CVA). Donation after cardiac death (DCD) was more common in the HCV Ab(−) NAT(−) group (22.4% vs. 7.8%; p<0.001). KDPI was lower in the HCV NAT(+) vs HCV Ab(−) NAT(−) groups(48.0% vs. 51.1%; p=0.003).
Table 3:
Baseline comparison of recipients of hepatitis C NAT positive versus HCV Ab(−)/NAT(−) kidneys. Continuous covariates are expressed as mean (standard deviation) and categorical covariates are expressed as count (percentage).
| HCV Ab/NAT Negative(n=874) | HCV NAT Positive(n=664) | P-value | |
|---|---|---|---|
| Recipient Age | 56.5 (10.1) | 60.1 (7.4) | |
| Recipient Ethnicity | 0.001 | ||
| Caucasian | 226 (25.9%) | 162 (24.4%) | |
| African American | 447 (51.1%) | 403 (60.7%) | |
| Hispanic | 142 (16.3%) | 73 (11.0%) | |
| Asian | 32 (3.7%) | 15 (2.3%) | |
| Other | 27 (3.1%) | 11 (1.7%) | |
| Female Recipient | 264 (30.2%) | 154 (23.2%) | 0.002 |
| Recipient BMI | 27.6 (4.9) | 27.6 (4.8) | |
| Recipient Diabetes | 347 (39.7%) | 362 (54.5%) | <.0001 |
| Current cPRA at Transplant | 22.0 (34.4) | 11.0 (24.0) | |
| Recipient Pretransplant Dialysis | 824 (94.3%) | 590 (88.9%) | <0.001 |
| Recipient Dialysis Duration (months) | 69.8 (48.2) | 34.8 (32.8) | |
| Recipient Education Level | 0.061 | ||
| Less than High School | 52 (5.9%) | 35 (5.3%) | |
| High School | 462 (52.9%) | 337 (50.8%) | |
| Some College | 224 (25.6%) | 153 (23.0%) | |
| Completed College | 101 (11.6%) | 92 (13.9%) | |
| Postgraduate Degree | 23 (2.6%) | 32 (4.8%) | |
| Unknown | 12 (1.4%) | 15 (2.3%) | |
| Recipient Prior Malignancy | 93 (10.6%) | 74 (11.1%) | 0.889 |
| Recipient Peripheral Vascular Disease | 105 (12.0%) | 70 (10.5%) | 0.044 |
| Recipient Primary Insurance | <0.001 | ||
| Private | 99 (11.3%) | 173 (26.1%) | |
| Public | 771 (88.2%) | 491 (74.0%) | |
| Other | 4 (0.5%) | 0 (0%) | |
| Donor Age | 39.8 (15.1) | 32.3 (8.2) | |
| Donor Ethnicity | <0.001 | ||
| Caucasian | 554 (63.4%) | 556 (83.7%) | |
| African American | 178 (20.4%) | 30 (4.5%) | |
| Hispanic | 105 (12.0%) | 66 (9.9%) | |
| Asian | 21 (2.4%) | 4 (0.6%) | |
| Other | 16 (1.8%) | 8 (1.2%) | |
| Female Donor | 363 (41.5%) | 230 (34.6%) | 0.006 |
| Donor Height (cm) | 168.2 (18.2) | 172.7 (9.3) | |
| Donor Weight (kg) | 82.6 (27.0) | 77.0 (15.6) | |
| Donor BUN | 21.6 (16.7) | 17.0 (9.9) | |
| Donor Terminal Creatinine | 1.3 (1.2) | 0.9 (0.5) | |
| DCD Donor | 196 (22.4%) | 52 (7.8%) | <0.001 |
| Donor Diabetes | 71 (8.1%) | 8 (1.2%) | <0.001 |
| Donor Hypertension | 271 (31.0%) | 68 (10.2%) | <0.001 |
| Donor Blood Type | 0.001 | ||
| A | 145 (16.6%) | 78 (11.8%) | |
| A1 | 166 (19.0%) | 104 (15.7%) | |
| A1B | 12 (1.4%) | 1 (0.2%) | |
| A2 | 20 (2.3%) | 25 (3.8%) | |
| A2B | 5 (0.6%) | 0 (0.0%) | |
| AB | 11 (1.3%) | 3 (0.5%) | |
| B | 104 (11.9%) | 93 (14.0%) | |
| O | 411 (47.0%) | 360 (24.2%) | |
| Donor Vasodilator Use | 101 (11.6%) | 85 (12.8%) | 0.458 |
| Donor Tattoos | 385 (44.1%) | 538 (81.0%) | <0.001 |
| Donor Cigarette Use | 165 (18.9%) | 156 (23.5%) | 0.040 |
| Donor Cause of Death | <0.001 | ||
| Anoxia | 357 (40.9%) | 463 (69.7%) | |
| CVA | 230 (26.3%) | 46 (6.9%) | |
| Trauma | 264 (30.2%) | 143 (21.5%) | |
| CNS Tumor | 3 (0.3%) | 0 (0%) | |
| Other | 20 (2.3%) | 12 (1.8%) | |
| Cold Ischemia Time (Hours) | 17.2 (8.2) | 18.3 (7.8) | |
| HLA B Mismatches | 0.078 | ||
| 0 | 46 (5.3%) | 20 (3.0%) | |
| 1 | 201 (23.0%) | 166 (25.0%) | |
| 2 | 627 (71.7%) | 478 (72.0%) | |
| HLA DR Mismatches | <0.001 | ||
| 0 | 116 (13.3%) | 53 (8.0%) | |
| 1 | 416 (47.6%) | 276 (41.6%) | |
| 2 | 342 (39.1%) | 335 (50.5%) | |
| Dual Kidney Transplant | 5 (0.6%) | 4 (0.6%) | 1.00 |
| EnBloc Kidney Transplant | 15 (1.7%) | 1 (0.2%) | 0.003 |
After propensity score matching, there were 200 recipients each in the HCV NAT(+) and HCV Ab(−) NAT(−) groups, respectively. Donor and recipient demographic information and the standardized differences between covariates in the propensity matched cohort are presented in table 4. Factors which remained out of balance after propensity matching included recipient gender, donor height, proportion of donors with ABO type A1 blood, and the proportion of donors with 0 HLA B mismatches with the recipient. KDPI was higher in the HCV NAT(+) group compared to the HCV Ab(−) NAT(−) group after propensity matching (56.8% vs. 35.2%, standardized difference = 1.09).
Table 4:
Covariate comparison after propensity matching of recipients of HCV NAT positive versus HCV Ab(−)/NAT(−) kidneys. Continuous covariates are expressed as mean (standard deviation) and categorical covariates are expressed as count (percentage). Standardized differences of less than 0.1 in absolute value are considered insignificant.
| HCV NAT Negative/Ab Negative (n=200) | HCV NAT Positive(n=200) | Standardized Difference | |
|---|---|---|---|
| Recipient Age | 57.8 (9.7) | 58.1 (9.0) | 0.03 |
| Recipient Ethnicity | |||
| Caucasian | 51 (25.5%) | 58 (29.0%) | −0.08 |
| African American | 109 (54.5%) | 104 (52.0%) | 0.05 |
| Hispanic | 31 (15.5%) | 26 (13.0%) | 0.07 |
| Asian | 6 (3.0%) | 6 (3.0%) | 0.00 |
| Other | 3 (1.5%) | 6 (3.0%) | −0.10 |
| Female Recipient | 57 (28.5%) | 45 (22.5%) | 0.14 |
| Recipient BMI | 27.6 (4.9) | 27.6 (5.2) | 0.00 |
| Recipient Diabetes | 91 (45.5%) | 91 (45.5%) | 0.00 |
| Current cPRA at Transplant | 19.0 (34.0) | 18.5 (30.6) | −0.01 |
| Recipient Pretransplant Dialysis | 180 (90.0%) | 184 (92.0%) | −0.07 |
| Recipient Dialysis Duration (months) | 50.0 (38.9) | 49.7 (41.8) | −0.01 |
| Recipient Education Level | |||
| Less than High School | 13 (6.5%) | 12 (6.0%) | 0.02 |
| High School | 99 (49.5%) | 94 (47.0%) | 0.05 |
| Some College | 48 (24.0%) | 49 (24.5%) | −0.01 |
| Completed College | 26 (13.0%) | 29 (14.5%) | −0.04 |
| Postgraduate Degree | 10 (5.0%) | 11 (5.5%) | −0.02 |
| Unknown | 4 (2.0%) | 5 (2.5%) | −0.03 |
| Recipient Prior Malignancy | 22 (11.0%) | 22 (11.0%) | 0.00 |
| Recipient Peripheral Vascular Disease | 26 (13.0%) | 20 (10.0%) | 0.09 |
| Recipient Primary Insurance | |||
| Private | 35 (17.5%) | 40 (20.0%) | −0.06 |
| Public | 165 (82.5%) | 160 (80.0%) | 0.06 |
| Donor Age | 34.4 (13.7) | 35.4 (9.2) | |
| Donor Ethnicity | |||
| Caucasian | 159 (79.5%) | 160 (80.0%) | −0.01 |
| African American | 21 (10.5%) | 20 (10.0%) | 0.02 |
| Hispanic | 18 (9.0%) | 18 (9.0%) | 0.00 |
| Asian | 0 (0%) | 1 (0.5%) | −0.10 |
| Other | 2 (1.0%) | 1 (0.5%) | 0.06 |
| Female Donor | 76 (38.0%) | 73 (36.5%) | 0.03 |
| Donor Height (cm) | 170.4 (15.7) | 171.9 (10.3) | 0.12 |
| Donor Weight (kg) | 78.2 (23.7) | 79.1 (17.5) | 0.04 |
| Donor BUN | 18.9 (11.7) | 17.9 (12.1) | −0.08 |
| Donor Terminal Creatinine | 1.0 (0.6) | 1.0 (0.6) | 0.01 |
| DCD Donor | 28 (14.0%) | 23 (11.5%) | 0.08 |
| Donor Diabetes | 6 (3.0%) | 6 (3.0%) | 0.00 |
| Donor Hypertension | 38 (19.0%) | 41 (20.5%) | −0.04 |
| Donor Blood Type | |||
| A | 30 (15.0%) | 25 (12.5%) | 0.07 |
| A1 | 29 (14.5%) | 34 (17.0%) | -0.11 |
| A2 | 7 (3.5%) | 6 (3.0%) | 0.03 |
| AB | 2 (1.0%) | 1 (0.5%) | 0.06 |
| B | 21 (10.5%) | 32 (16.0%) | −0.16 |
| O | 111 (55.5%) | 102 (51.0%) | 0.09 |
| Donor Vasodilator Use | 26 (13.0%) | 29 (14.5%) | −0.04 |
| Donor Tattoos | 140 (70.0%) | 137 (68.5%) | 0.03 |
| Donor Cigarette Use | 43 (21.5%) | 45 (22.5%) | −0.02 |
| Donor Cause of Death | |||
| Anoxia | 109 (54.5%) | 109 (54.5%) | 0.00 |
| CVA | 23 (11.5%) | 26 (13.0%) | −0.05 |
| Trauma | 64 (32.0%) | 59 (29.5%) | 0.05 |
| Other | 4 (2.0%) | 6 (3.0%) | −0.06 |
| Cold Ischemia Time (Hours) | 17.6 (7.8) | 17.9 (7.5) | 0.03 |
| HLA B Mismatches | |||
| 0 | 8 (4.0%) | 13 (6.5%) | -0.11 |
| 1 | 55 (27.5%) | 55 (27.5%) | 0.00 |
| 2 | 137 (68.5%) | 132 (66.0%) | 0.05 |
| HLA DR Mismatches | |||
| 0 | 22 (11.0%) | 22 (11.0%) | 0.00 |
| 1 | 93 (46.5%) | 96 (48.0%) | −0.03 |
| 2 | 85 (42.5%) | 82 (41.0%) | 0.03 |
| Dual Kidney Transplant | 1 (0.5%) | 1 (0.5%) | 0.00 |
| EnBloc Kidney Transplant | 1 (0.5%) | 1 (0.5%) | 0.00 |
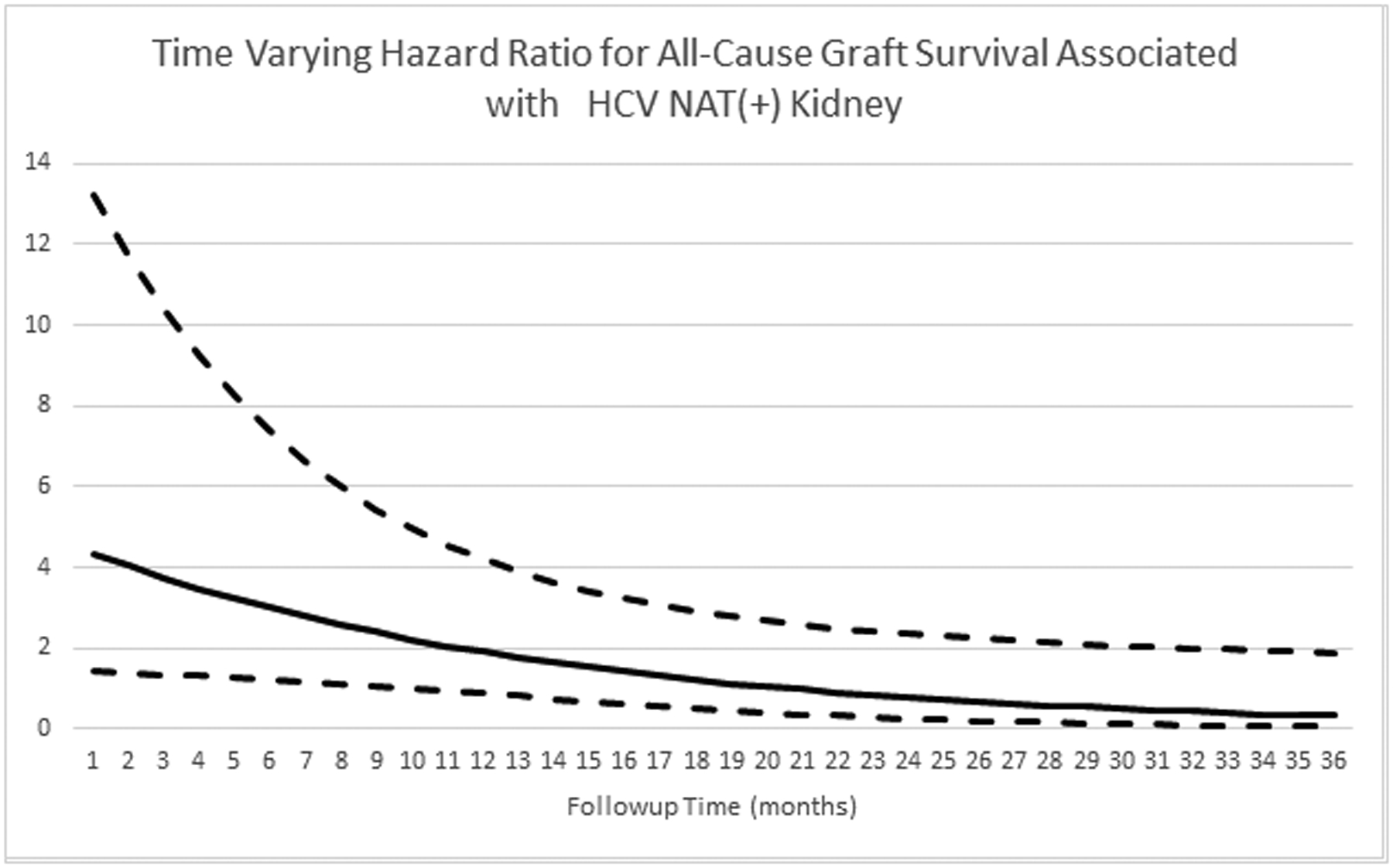
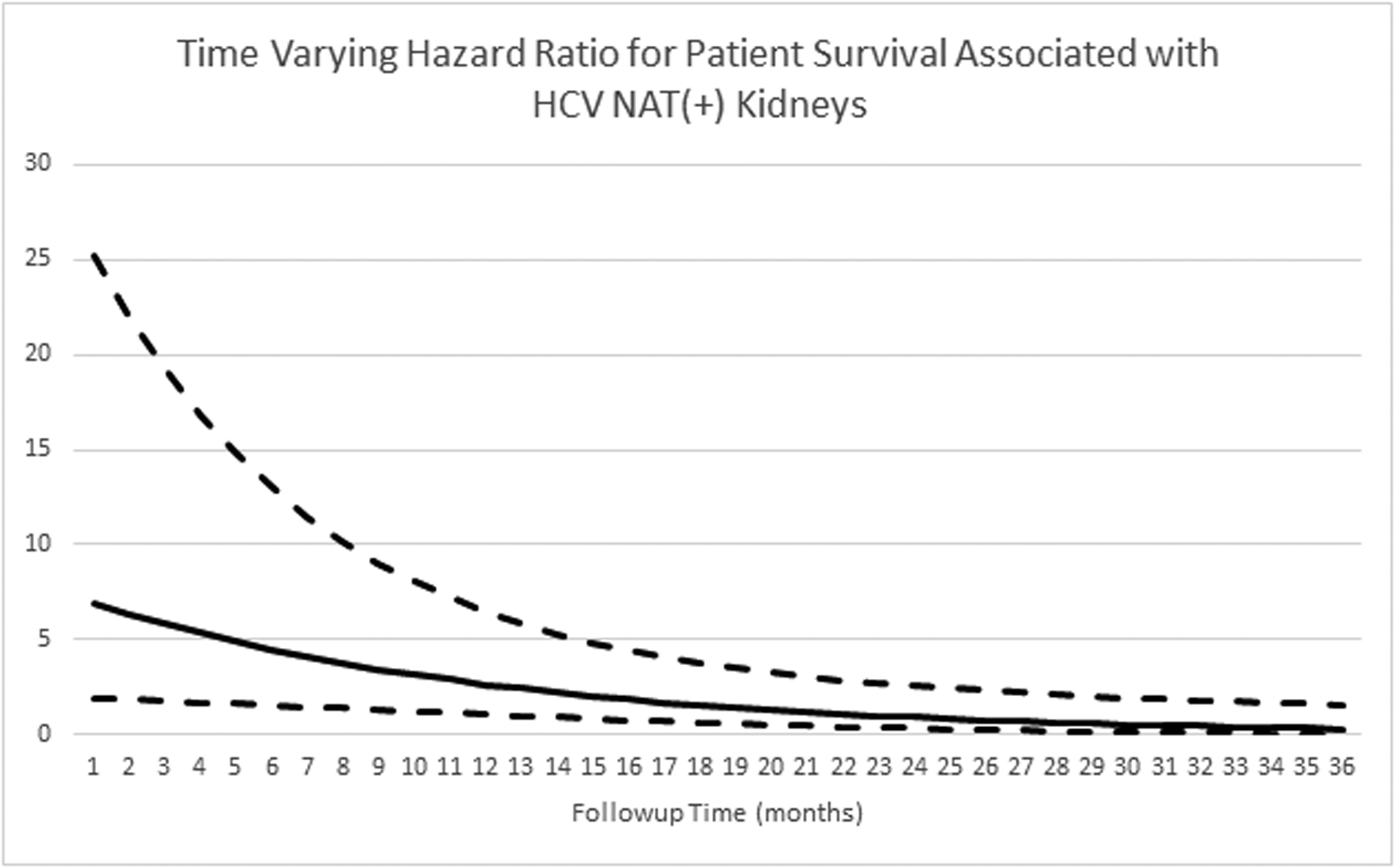
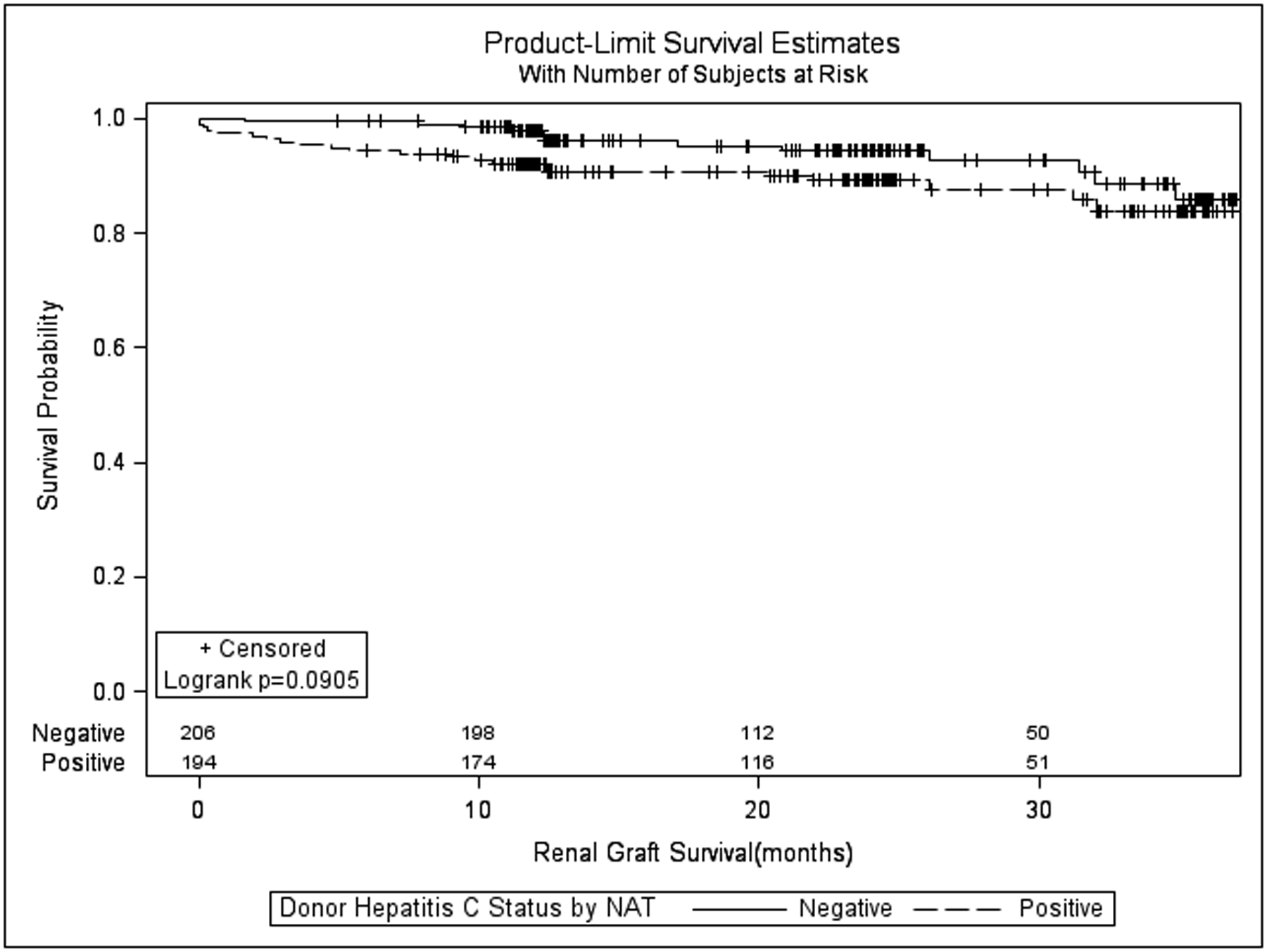
All-cause renal allograft survival and patient survival between the HCV NAT(+) and HCV Ab(−) NAT(−) groups are shown in figure 3 and figure 4, respectively. Death-censored graft survival at 6, 12, 24, and 36 months was also similar between the HCN NAT(+) and HCV Ab(−)/NAT(−) groups (97.0%, 97.0%, 96.1%, and 92.3% vs. 99.5%, 99.0%, 96.7%, and 94.9%; p=0.461, supplemental figure 2). The adjusted hazards for graft failure (figure 5) and patient death (figure 6) associated with donor HCV NAT(+) status demonstrated a declining trend with time. The baseline hazard for all-cause graft failure associated with HCV NAT(+) was 4.692 (95% CI 1.469–14.990; p=0.009). The elevated hazard ratio lost statistical significance by 10 months (HR 2.219; p=0.052). The hazard ratio crossed 1 near 21 months (HR 0.974; p=0.958) although it remained statistically insignificant. The baseline hazard ratio for patient death associated with an HCV NAT(+) donor was 7.595 (95% CI 2.002–28.820; p=0.003). The elevated hazard ratio lost statistical significance at 14 months (HR 2.223; p=0.071). The hazard ratio crossed 1 at 24 months (HR 0.924; p=0.880) although it remained statistically insignificant. The adjusted hazard ratio for death-censored graft failure associated with HCV NAT(+) kidneys was not statistically significant (HR 1.438, 95% CI 0.481–4.296; p=0.516) nor was there a significant interaction with time.
Figure 3:
Kaplan-Meier all-cause kidney allograft survival for recipients of hepatitis C virus NAT positive vs. antibody negative/NAT negative kidneys after propensity matching.
Figure 4:
Kaplan-Meier patient survival for recipients of hepatitis C virus NAT positive vs. antibody negative/NAT negative kidneys after propensity matching.
Figure 5:
Time varying hazard ratio for all-cause graft survival associated with HCV NAT(+) kidneys. Dashed lines represent 95% confidence interval.
Figure 6:
Time varying hazard ratio for patient survival associated with HCV NAT(+) kidneys. Dashed lines represent 95% confidence interval.
Discussion
Large scale registry studies in the United States have consistently demonstrated worse patient and allograft survival with the use of HCV seropositive kidneys1,2,5,22. One of the most recent of these comes from Sawinski’s analysis of UNOS data from 2001 through 20155. In a cohort propensity matched cohort of HCV positive recipients of kidneys from HCV positive versus negative donors, the recipients of HCV positive kidneys faced an increased risk of death (HR 1.43; p<0.001) and graft loss (HR 1.39; p<0.001). Rejection in the two groups was similar (OR 1.16; p=0.35). The potential reasons for inferior graft survival with HCV positive kidneys in the earlier era are likely multifactorial. HCV is known to cause glomerulonephritis in both native and transplanted kidneys23,24. Other cited causes of increased graft loss and mortality with HCV positive kidneys include recipient infection related to over immunosuppression and rapid progression of hepatitis with the onset of immunosuppressive therapy1.
Since the initial approval of interferon-free DAA regimens in December 2013, there have been multiple other effective regimens subsequently approved for all HCV genotypes. In particular, the fixed dose combinations of glecaprevir/pibrentasvir and ledipasvir/sofosbuvir have both been specifically approved and recommended for use in the kidney transplant population25. In the MAGELLAN-2 study, glecaprevir/pibrentasvir achieved a sustained viral response at 12 weeks (SVR12) in 100% of kidney transplant recipients, 20% of whom were treatment experienced26. In the THINKER trial, all 20 HCV negative recipients of HCV viremic kidneys have achieved HCV clearance with DAA therapy and had a similar GFR at 6 and 12 months to matched recipients of HCV negative kidneys27. These and other studies support the hypothesis that the new DAA therapies for HCV have the potential to significantly alter the course of HCV infection post-transplant.
With the ongoing opioid epidemic and concurrent rapid rise in incident HCV infection in the United States28, hepatitis C infected deceased organ donors are becoming increasingly common29,30. Concurrent with the increasing prevalence of HCV positive donors comes an increasing willingness on the part of patients and transplant surgeons to accept HCV positive organs. Bowring has recently shown that kidney transplant candidates are 2.2 times more likely to express willingness to accept an HCV positive organ in the DAA era, and are 1.9 times more likely to be transplanted with an HCV infected organ30. In addition, a few centers are now starting to offer kidneys from hepatitis C infected organs to recipients without HCV27,31. The combination of all these factors means that HCV positive donors will likely become an increasingly important part of the donor pool. As such, the ability to adequately risk stratify these kidneys is crucial.
HCV donors in the current era are now younger and healthier than in prior years, which has been demonstrated in prior studies32 as well as in the data we present in this study. Despite being younger and healthier, the HCV positive donors in the DAA era in this study had similar KDPI in the overall cohort compared to their HCV negative counterparts. The effect of HCV on KDPI became even more apparent after propensity score matching, with an approximately 20% increase in KDPI conferred by HCV status alone in the propensity matched cohort. Despite a higher KDPI, patient and graft survival were similar for recipients of HCV positive and HCV negative donor kidneys when donor HCV status is defined by serology in this study.
Accurate estimation of the risk posed by donor HCV status in the KDPI is particularly important under the current kidney allocation system in which allocation sequence is determined in many instances by the KDPI15. HCV positive kidneys under this allocation scheme, despite having similar longevity to HCV negative organs with much lower calculated KDPI are potentially being unjustifiably excluded from preferential allocation to those with the longest expected post-transplant survival. Conversely, it is likely that some HCV kidneys are classified as marginal (KDPI>85%) when their true expected longevity is more in line with that of more standard risk kidneys. This phenomenon was noted by Sibulesky in an analysis of HCV aviremic kidneys in the DAA era, in which they found 122 high quality kidneys which would have been preferentially allocated to those with the highest post-transplant survival if it were not for the marked effect of donor HCV status on the KDPI7. Our study extends Sibulesky’s findings by including HCV NAT(+)donors, not just those who were HCV Ab(+) but NAT(−)7. Taken together, these studies strengthen the conclusion that donor HCV antibody status is no longer adequate to provide appropriate risk stratification.
Donor HCV NAT positivity does appear to convey an increased risk for inferior patient and graft survival early on, which appears to be mitigated over time. The fact that the death-censored graft survival hazard ratio associated with HCV NAT(+) kidneys was not significant, combined with the much higher initial hazard for patient survival compared to all-cause graft survival suggests that excess early patient deaths rather than graft losses are responsible for the inferior early survival outcomes seen in this study. One possible explanation for this time varying effect could be underutilization of antiviral therapy in the post-transplant setting. Indeed, Axelrod and colleagues found in a recent study that only 12.9% of HCV positive recipients received DAA treatment within 3 years of transplant based on pharmacy claims data9. If antiviral treatment is delayed or withheld until patients develop clinical manifestations of HCV disease, it could produce a pattern similar to what we demonstrate in this study: early worse outcomes that are ameliorated in the later term once viral cure is achieved. We should note that our study can’t conclusively make this determination as we did not have data on antiviral therapy.
In addition, while a universal weakness of propensity matching is the possibility that covariates not included in the derivation of the propensity scores may contribute to residual bias, we believe our choice of covariates to include in the propensity score models are justified as they are the risk stratification factors used by the Organ Procurement and Transplantation Network (OPTN) in the current kidney allocation scheme and the SRTR in its risk adjustment models. Another limitation of propensity matching is the reduction in sample size and power as a result of the matching. We have addressed this partially by use of standardized differences rather than p-values for comparison of balance between the propensity matched groups. There remains the possibility that the lack of significant survival differences in the propensity matched cohorts is simply a reflection of reduced statistical power. Due to the relatively recent introduction of DAA therapy, we only have short term follow-up, so we can’t exclude the possibility that HCV positive kidneys may still have increased risk of graft loss in the later term. Finally, the high proportion of missing and unreliable data regarding immunosuppression and rejection leaves room for residual bias in the results related to differences in immunosuppressive regimens between groups and leaves us unable to determine the contribution of potentially differing rates of rejection to graft and patient survival outcomes.
Results from our study provide evidence that the risk posed by donor HCV infection is different in the current era than it was when the KDRI was originally calculated. We believe consideration should be given to recalibration of the KDRI to reflect the reality of universal NAT testing and the availability of modern curative DAA therapy for HCV.
Supplementary Material
Acknowledgements
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Glossary
- Ab
antibody
- BMI
body mass index
- CI
confidence interval
- Cm
centimeter
- cPRA
calculated panel reactive antibody
- CVA
cerebrovascular accident
- DAA
direct acting antiviral
- DCD
donation after cardiac death
- EPTS
expected post-transplant survival score
- HCV
hepatitis C virus
- HLA
human leukocyte antigen
- HR
hazard ratio
- KDPI
kidney donor profile index
- KDRI
kidney donor risk index
- Kg
kilogram
- NAT
nucleic acid testing
- OPTN
Organ Procurement and Transplant Network
- SRTR
Scientific Registry of Transplant Recipients
- STAR
standard transplant analysis and research
- UNOS
United Network for Organ Sharing
Footnotes
The authors have no conflicts of interest to declare, and there are no sources of funding to report.
This work was presented at the 2019 American Transplant Congress, June 2, 2019, Boston MA.
References
- 1.Abbott KC, Bucci JR, Matsumoto CS, et al. Hepatitis C and renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol. 2003;14(11):2908–2918. [DOI] [PubMed] [Google Scholar]
- 2.Bucci JR, Matsumoto CS, Swanson SJ, et al. Donor hepatitis C seropositivity: clinical correlates and effect on early graft and patient survival in adult cadaveric kidney transplantation. J Am Soc Nephrol. 2002;13(12):2974–2982. [DOI] [PubMed] [Google Scholar]
- 3.Bucci JR, Lentine KL, Agodoa LY, Peters TG, Schnitzler MA, Abbott KC. Outcomes associated with recipient and donor hepatitis C serology status after kidney transplantation in the United States: analysis of the USRDS/UNOS database. Clin Transpl. 2004:51–61. [PubMed] [Google Scholar]
- 4.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10(5):1238–1246. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JB, Eddinger KC, Shelton B, Locke JE, Forde KA, Sawinski D. Effect of kidney donor hepatitis C virus serostatus on renal transplant recipient and allograft outcomes. Clin Kidney J. 2017;10(4):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. [DOI] [PubMed] [Google Scholar]
- 7.Sibulesky L, Kling CE, Blosser C, et al. Are we underestimating the quality of aviremic hepatitis C-positive kidneys? Time to reconsider. Am J Transplant. 2018;18(10):2465–2472. [DOI] [PubMed] [Google Scholar]
- 8.OPTN. KDPI Calculator https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator/. Accessed 10/31/2018.
- 9.Axelrod DA, Schnitzler MA, Alhamad T, et al. The impact of direct-acting antiviral agents on liver and kidney transplant costs and outcomes. Am J Transplant. 2018;18(10):2473–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alqahtani SA, Afdhal N, Zeuzem S, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: Analysis of phase III ION trials. Hepatology. 2015;62(1):25–30. [DOI] [PubMed] [Google Scholar]
- 11.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149(3):649–659. [DOI] [PubMed] [Google Scholar]
- 12.Kwo PY, Poordad F, Asatryan A, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1–6 without cirrhosis. J Hepatol. 2017;67(2):263–271. [DOI] [PubMed] [Google Scholar]
- 13.Heo YA, Deeks ED. Sofosbuvir/Velpatasvir/Voxilaprevir: A Review in Chronic Hepatitis C. Drugs. 2018;78(5):577–587. [DOI] [PubMed] [Google Scholar]
- 14.Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373(27):2618–2628. [DOI] [PubMed] [Google Scholar]
- 15.OPTN. Kidney Allocation Policy https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed 10/31/2018.
- 16.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 17.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. [DOI] [PubMed] [Google Scholar]
- 19.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 20.Sapir-Pichhadze R, Pintilie M, Tinckam KJ, et al. Survival Analysis in the Presence of Competing Risks: The Example of Waitlisted Kidney Transplant Candidates. Am J Transplant. 2016;16(7):1958–1966. [DOI] [PubMed] [Google Scholar]
- 21.Lee EW, Wei LJ, Amato DA. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Dordrecht, Netherlands: Kleuwer Academic; 1992:237–247. [Google Scholar]
- 22.Maluf DG, Archer KJ, Mas VR. Kidney grafts from HCV-positive donors: advantages and disadvantages. Transplant Proc. 2010;42(7):2436–2446. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328(7):465–470. [DOI] [PubMed] [Google Scholar]
- 24.Cruzado JM, Carrera M, Torras J, Grinyo JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1(2):171–178. [PubMed] [Google Scholar]
- 25.AASLD/IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2018; https://www.hcvguidelines.org/unique-populations/kidney-transplant. Accessed 11/1/2018.
- 26.Reau N, Kwo PY, Rhee S, et al. Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection. Hepatology. 2018;68(4):1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reese PP, Abt PL, Blumberg EA, et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med. 2018;169(5):273–281. [DOI] [PubMed] [Google Scholar]
- 28.CDC. Disease Burden from Viral Hepatitis A, B, and C in the United States 2018; https://www.cdc.gov/hepatitis/statistics/index.htm. Accessed 11/1/2018.
- 29.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving Organ Utilization to Help Overcome the Tragedies of the Opioid Epidemic. Am J Transplant. 2016;16(10):2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowring MG, Kucirka LM, Massie AB, et al. Changes in utilization and discard of HCV-antibody positive deceased-donor kidneys in the era of direct-acting antiviral therapy. Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitsky J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 32.Bowring MG, Kucirka LM, Massie AB, et al. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. Am J Transplant. 2017;17(2):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


